Antikinetoplastid Activity of Sesquiterpenes Isolated from the Zoanthid Palythoa aff. clavata
Abstract
1. Introduction
2. Results and Discussion
2.1. Source of Natural Metabolites
2.2. In Vitro Activity
2.2.1. Leishmanicidal and Trypanocidal Activity
2.2.2. Cytotoxic Activity and Selectivity
2.3. Mechanisms of Action of Compounds 2, 3 and 11
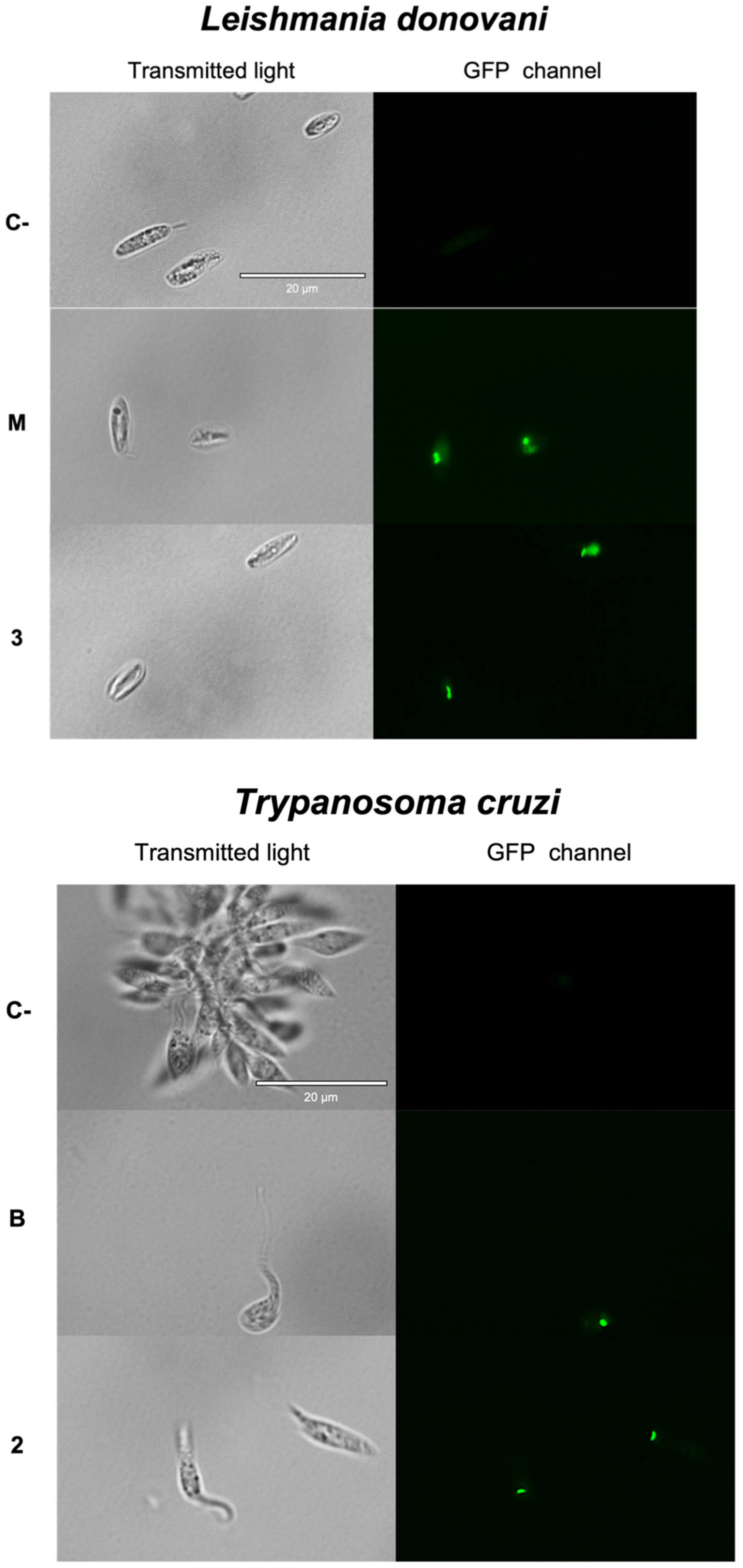
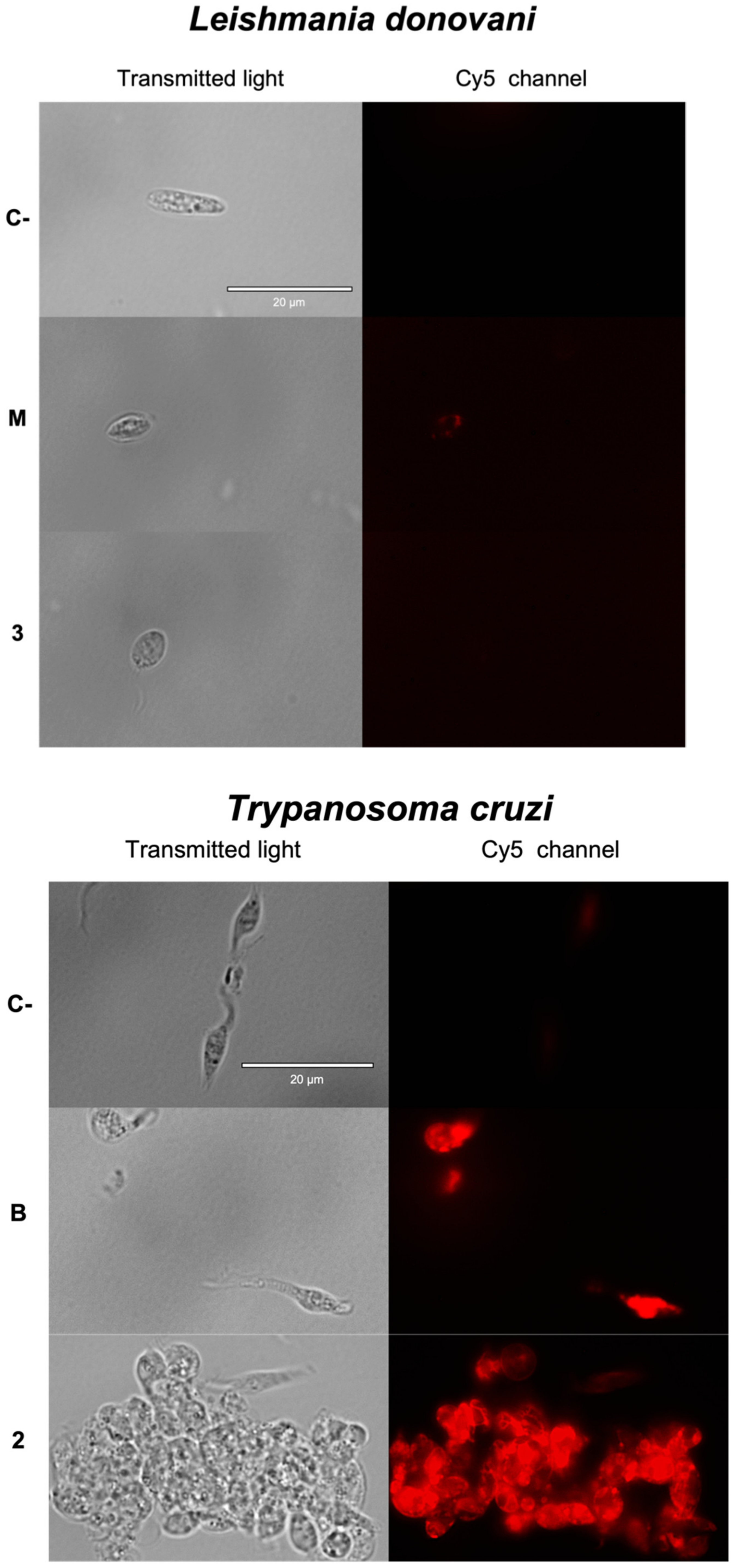
2.3.1. Chromatin Condensation Analysis
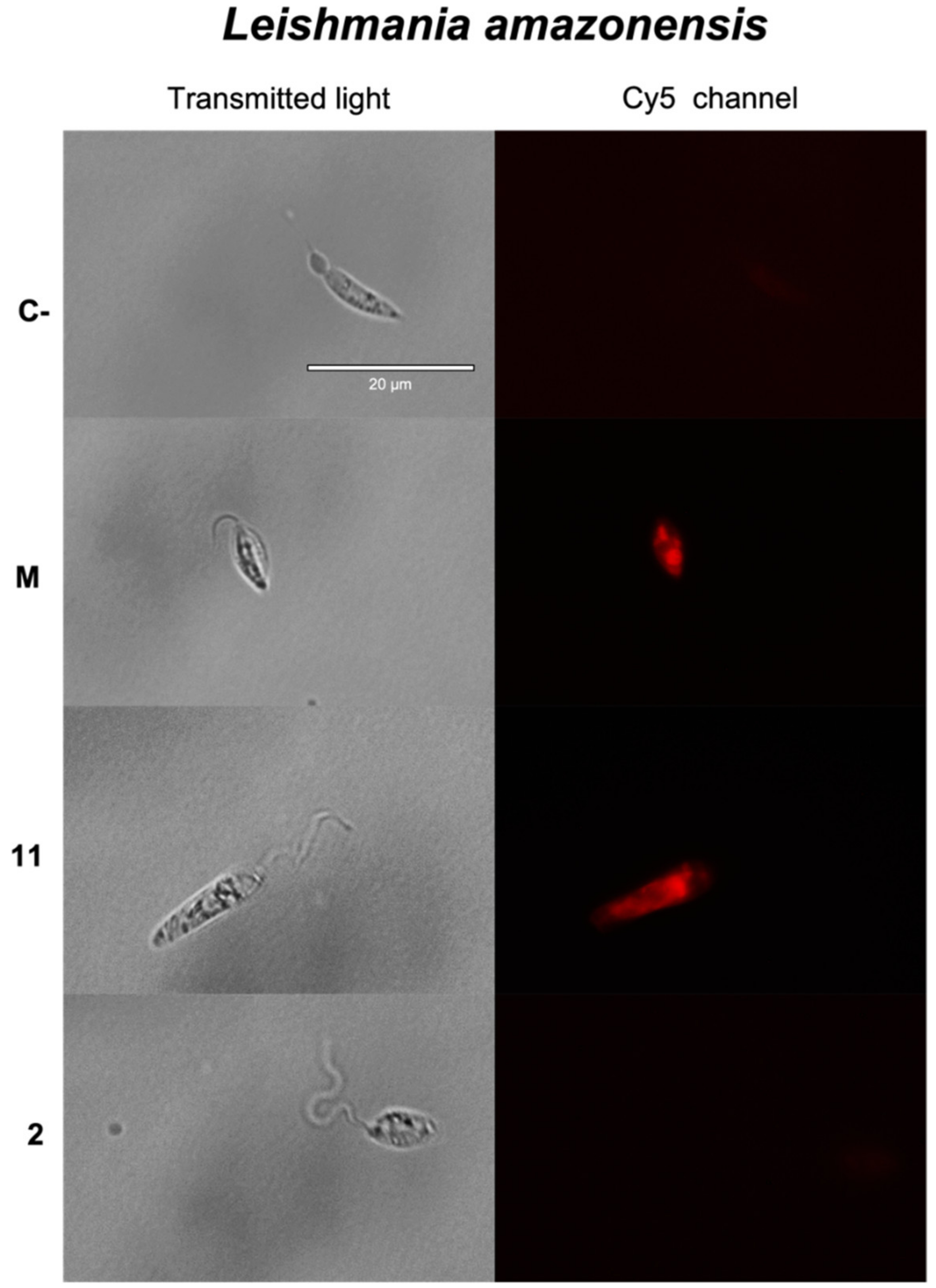
2.3.2. Plasmatic Membrane Permeability Analysis
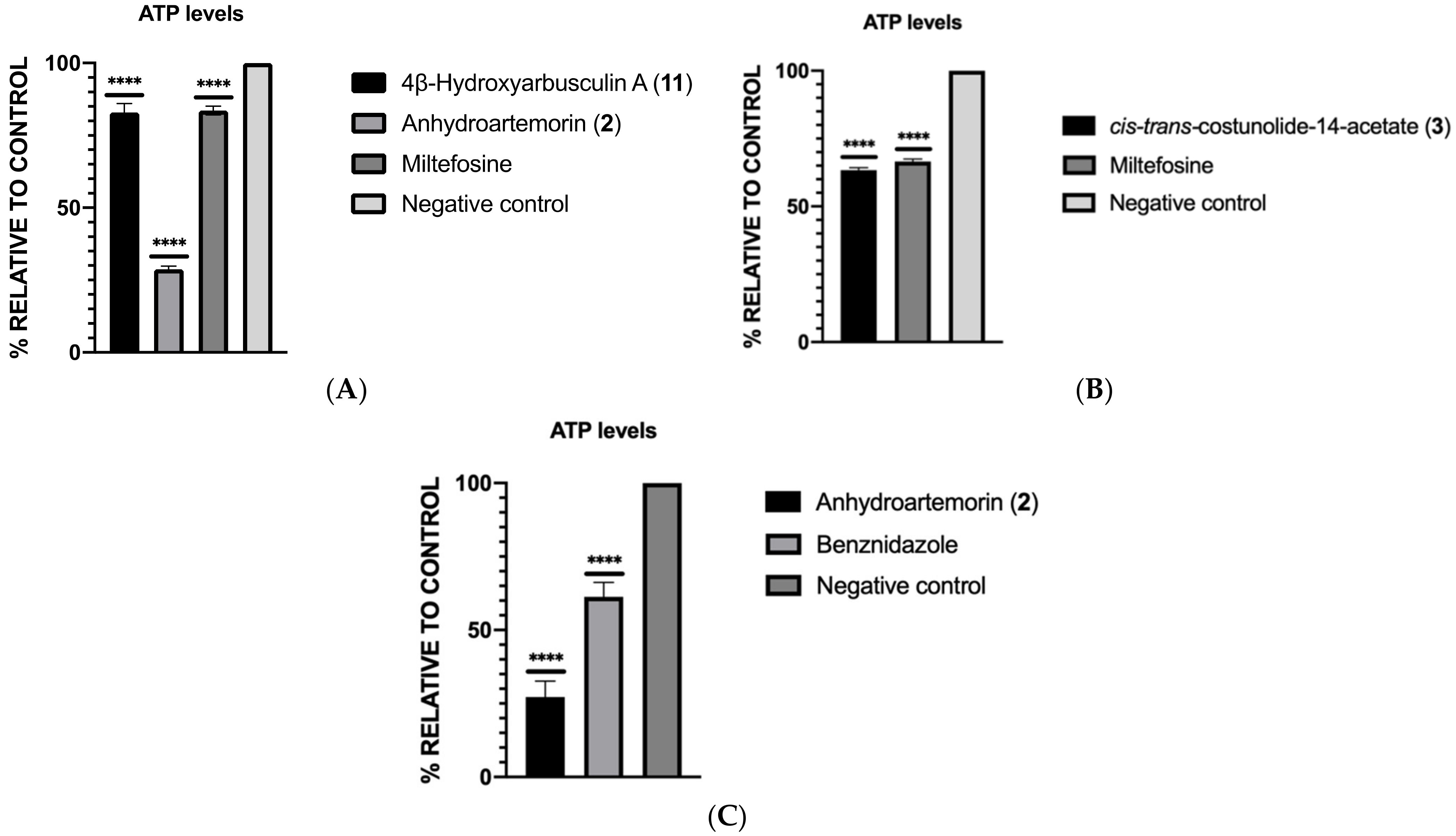
2.3.3. ATP Levels Analysis
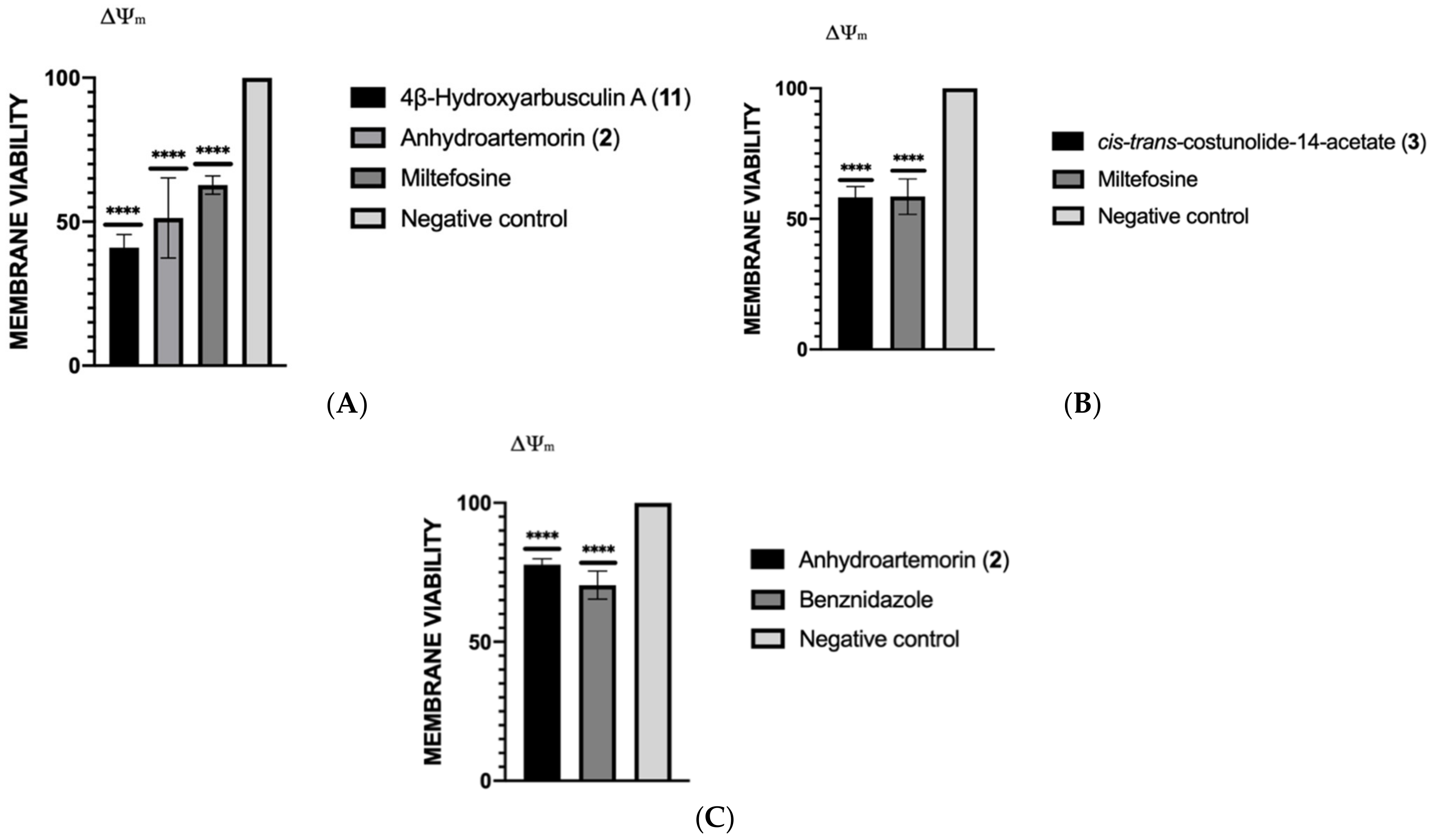
2.3.4. Mitochondrial Membrane Potential Analysis
2.3.5. Reactive Oxygen Species (ROS) Detection
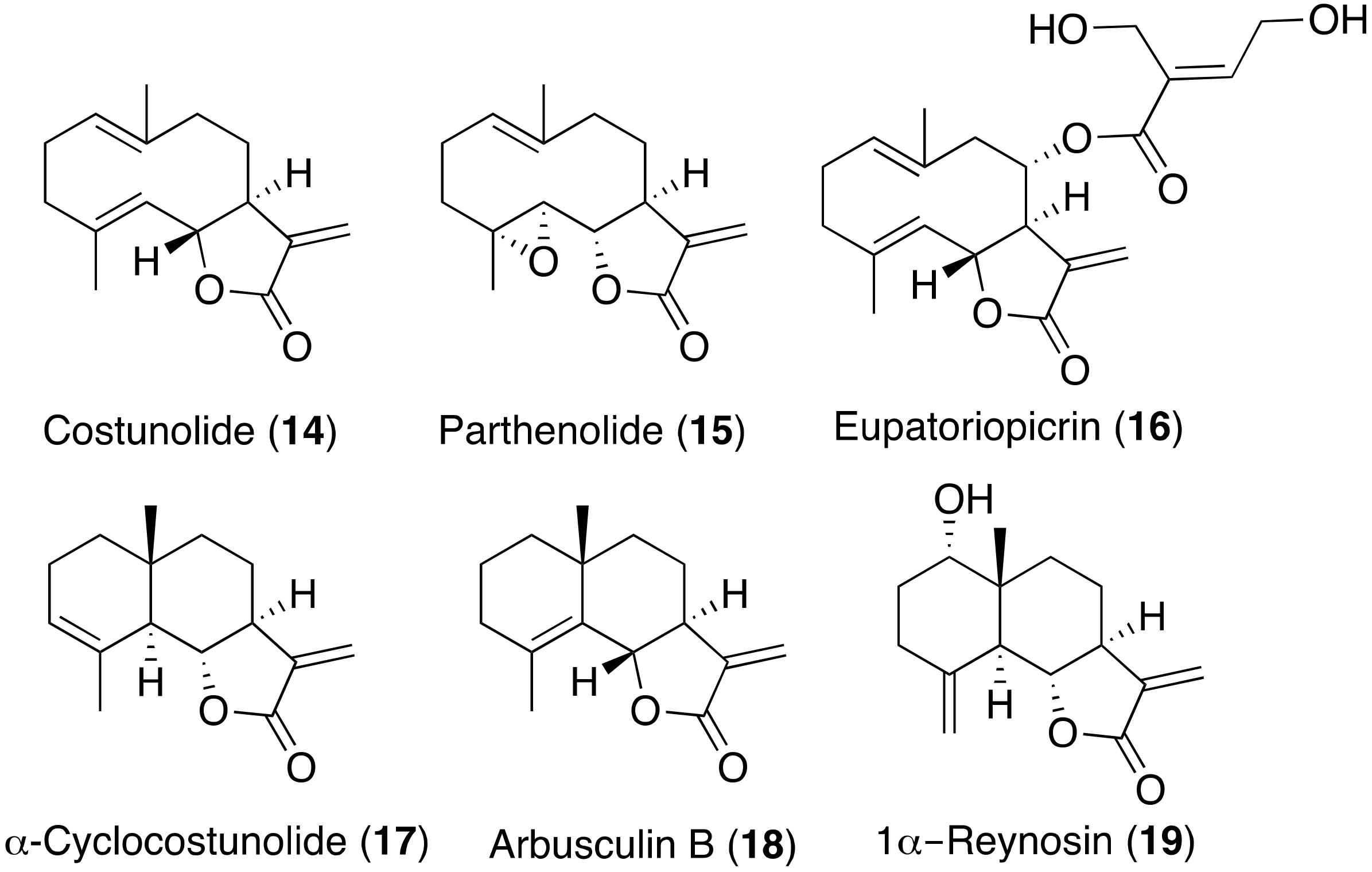
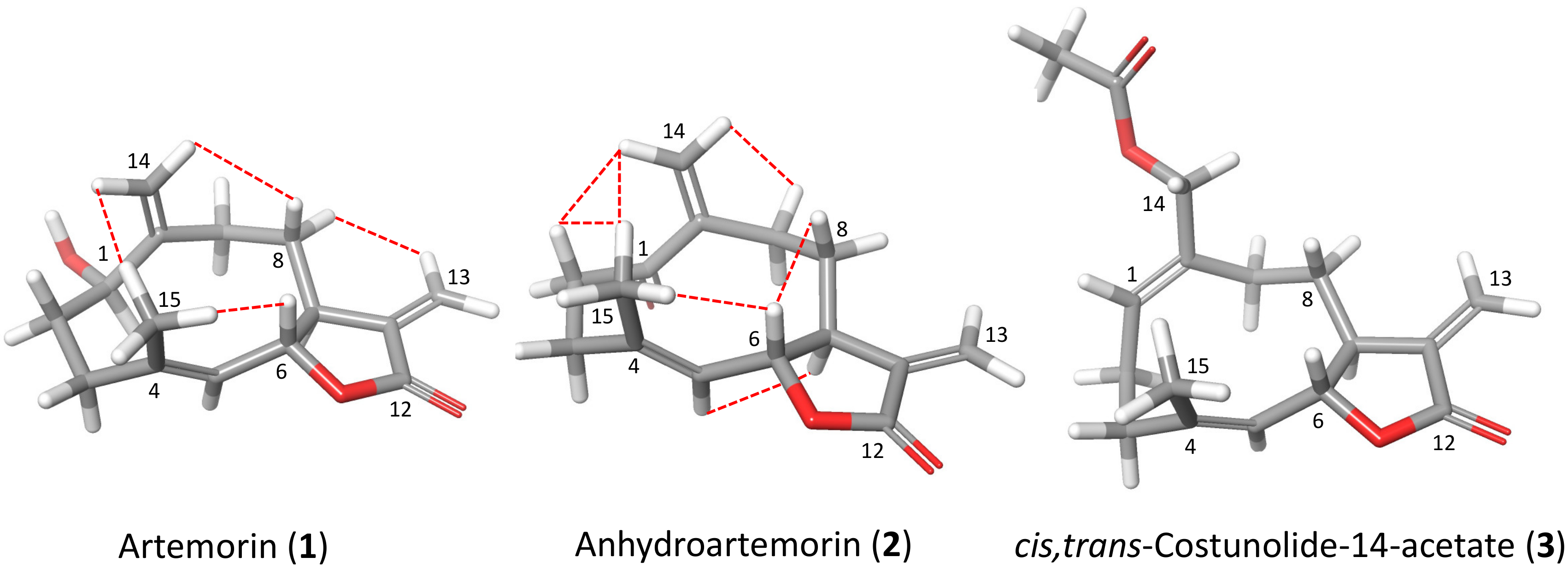
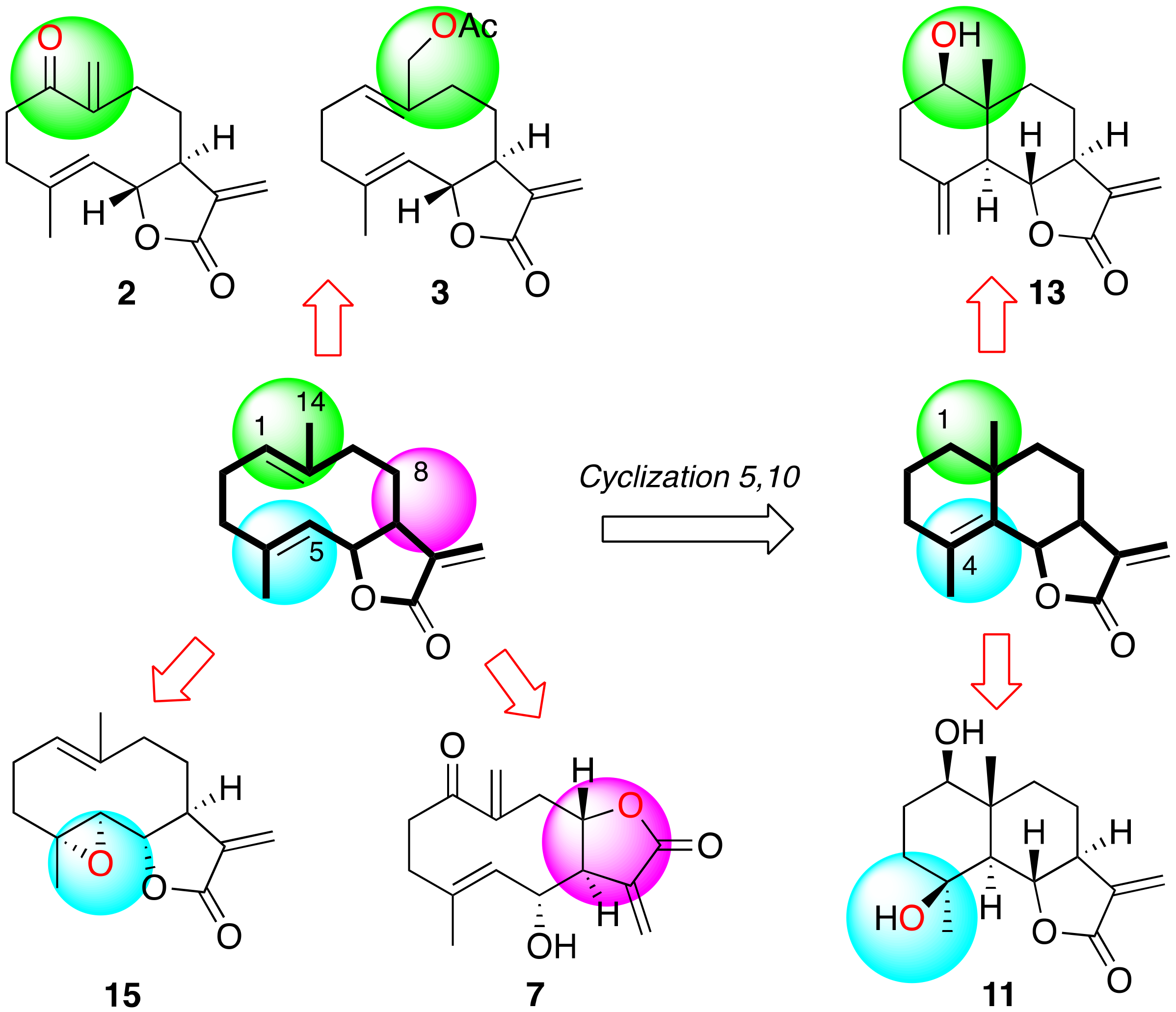
2.4. Structure–Activity Relationship
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Isolation of Sesquiterpene Lactones
3.4. Strains
3.5. Anti-Kinetoplastid Activity
3.6. Cytotoxic Activity
3.7. Chromatin Condensation Analysis
3.8. Plasmatic Membrane Permeability Analysis
3.9. ATP Levels Analysis
3.10. Mitochondrial Membrane Potential Analysis
3.11. Reactive Oxygen Species (ROS) Detection
3.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stolk, W.A.; Kulik, M.C.; Le Rutte, E.; Jacobson, J.; Richardus, J.H.; de Vlas, S.J.; Houweling, T.A. Between-Country Inequalities in the Neglected Tropical Disease Burden in 1990 and 2010, with Projections for 2020. PLoS Negl. Trop. Dis. 2016, 10, e0004560. [Google Scholar] [CrossRef]
- Hotez, P.J.; Molyneux, D.H.; Fenwick, A.; Kumaresan, J.; Sachs, S.E.; Sachs, J.D.; Savioli, L. Control of Neglected Tropical Diseases. N. Engl. J. Med. 2007, 357, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Investing to Overcome the Global Impact of Neglected Tropical Diseases: Third WHO Report on Neglected Tropical Diseases 2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Kourbeli, V.; Chontzopoulou, E.; Moschovou, K.; Pavlos, D.; Mavromoustakos, T.; Papanastasiou, I. An Overview on Target-Based Drug Design against Kinetoplastid Protozoan Infections: Human African Trypanosomiasis, Chagas Disease and Leishmaniases. Molecules 2021, 26, 4629. [Google Scholar] [CrossRef] [PubMed]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martínez, A.F.; Newman, S.; Ramanan, P.; A Suarez, J. A Review of Leishmaniasis: Current Knowledge and Future Directions. Curr. Trop. Med. Rep. 2021, 8, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, C.M. Clinical Manifestations of Visceral Leishmaniasis (American Visceral Leishmaniasis). In The Epidemiology and Ecology of Leishmaniasis; Claborn, D., Ed.; IntechOpen: London, UK, 2017; pp. 17–30. [Google Scholar] [CrossRef]
- Handler, M.Z.; Patel, P.A.; Kapila, R.; Al-Qubati, Y.; Schwartz, R.A. Cutaneous and mucocutaneous leishmaniasis: Clinical perspectives. J. Am. Acad. Dermatol. 2015, 73, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Okwor, I.; Uzonna, J. Social and Economic Burden of Human Leishmaniasis. Am. J. Trop. Med. Hyg. 2016, 94, 489–493. [Google Scholar] [CrossRef]
- Moncayo, A. Chagas disease: Current epidemiological trends after the interruption of vectorial and transfusional transmission in the Southern Cone countries. Memórias Inst. Oswaldo Cruz 2003, 98, 577–591. [Google Scholar] [CrossRef]
- Lewis, M.D.; Kelly, J.M. Putting Infection Dynamics at the Heart of Chagas Disease. Trends Parasitol. 2016, 32, 899–911. [Google Scholar] [CrossRef]
- Bonney, K.M. Chagas disease in the 21st Century: A public health success or an emerging threat? Parasite 2014, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.P.S.; Barrett, M.P.; Dranoff, G.; Faraday, C.J.; Gimpelewicz, C.R.; Hailu, A.; Jones, C.L.; Kelly, J.M.; Lazdins-Helds, J.K.; Mäser, P.; et al. Drug Discovery for Kinetoplastid Diseases: Future Directions. ACS Infect. Dis. 2019, 5, 152–157. [Google Scholar] [CrossRef]
- Das, P.; Saha, S.; BoseDasgupta, S. The ultimate fate determinants of drug induced cell-death mechanisms in Trypanosomatids. Int. J. Parasitol. Drugs Drug Resist. 2021, 15, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Menna-Barreto, R.F.S. Cell death pathways in pathogenic trypanosomatids: Lessons of (over)kill. Cell Death Dis. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Gannavaram, S.; Debrabant, A. Programmed cell death in Leishmania: Biochemical evidence and role in parasite infectivity. Front. Cell. Infect. Microbiol. 2012, 2, 95. [Google Scholar] [CrossRef] [PubMed]
- Moujir, L.; Callies, O.; Sousa, P.M.C.; Sharopov, F.; Seca, A.M.L. Applications of Sesquiterpene Lactones: A Review of Some Potential Success Cases. Appl. Sci. 2020, 10, 3001. [Google Scholar] [CrossRef]
- Adekenov, S. Sesquiterpene lactones with unusual structure. Their biogenesis and biological activity. Fitoterapia 2017, 121, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Shulha, O.; Zidorn, C. Sesquiterpene lactones and their precursors as chemosystematic markers in the tribe Cichorieae of the Asteraceae revisited: An update (2008–2017). Phytochemistry 2019, 163, 149–177. [Google Scholar] [CrossRef] [PubMed]
- Kaleem, S.; Ge, H.; Yi, W.; Zhang, Z.; Wu, B. Isolation, structural elucidation, and antimicrobial evaluation of the metabolites from a marine-derived fungus Penicillium sp. ZZ1283. Nat. Prod. Res. 2021, 35, 2498–2506. [Google Scholar] [CrossRef] [PubMed]
- Ngokpol, S.; Suwakulsiri, W.; Sureram, S.; Lirdprapamongkol, K.; Aree, T.; Wiyakrutta, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Drimane Sesquiterpene-Conjugated Amino Acids from a Marine Isolate of the Fungus Talaromyces minioluteus (Penicillium minioluteum). Mar. Drugs 2015, 13, 3567–3580. [Google Scholar] [CrossRef]
- Ma, M.; Ge, H.; Yi, W.; Wu, B.; Zhang, Z. Bioactive drimane sesquiterpenoids and isocoumarins from the marine-derived fungus Penicillium minioluteum ZZ1657. Tetrahedron Lett. 2020, 61, 151504. [Google Scholar] [CrossRef]
- Guella, G.; Pietra, F.; Dini, F. Rarisetenolide, Epoxyrarisetenolide, and Epirarisetenolide, New-Skeleton Sesquiterpene Lactones as Taxonomic Markers and Defensive Agents of the Marine Ciliated Morphospecies Euplotes rariseta. Helvetica Chim. Acta 1996, 79, 2180–2189. [Google Scholar] [CrossRef]
- Suzuki, M.; Kowata, N.; Kobayashi, H.; Tanaka, I. The Structure of a Germacrane-Type Sesquiterpene Alcohol, a Possible Precursor of Guaiane-Type Sesquiterpenes from the Brown Alga Dictyopteris divaricata. Chem. Lett. 1990, 19, 2187–2190. [Google Scholar] [CrossRef]
- Luo, X.; Li, P.; Wang, K.; de Voogd, N.J.; Tang, X.; Li, G. Cytotoxic sesquiterpenoid quinones from South China Sea sponge Dysidea sp. Nat. Prod. Res. 2021, 35, 2866–2871. [Google Scholar] [CrossRef] [PubMed]
- Cuc, N.T.; Anh, H.L.T.; Hang, D.T.T.; Nhiem, N.X.; Dang, N.H.; Nam, N.H.; Yen, P.H.; Thung, D.C.; Thu, V.K.; Van Minh, C.; et al. Sesquiterpenes from the Vietnamese Marine Sponge Dysidea Fragilis. Nat. Prod. Commun. 2015, 10, 1341–1342. [Google Scholar] [CrossRef]
- Diep, C.N.; Lyakhova, E.G.; Berdyshev, D.V.; Kalinovsky, A.I.; Tu, V.A.; Cuong, N.X.; Nam, N.H.; Van Minh, C.; Stonik, V.A. Structures and absolute stereochemistry of guaiane sesquiterpenoids from the gorgonian Menella woodin. Tetrahedron Lett. 2015, 56, 7001–7004. [Google Scholar] [CrossRef]
- Ghan, W.R.; Tinto, W.F.; Mooreb, R. New germacrane derivatives from gorgonian octocorals of the genus Pseudopterogorgia. Tetrahedon 1990, 46, 1499–1502. [Google Scholar]
- Sung, P.-J.; Kao, S.-Y.; Kao, C.-Y.; Chang, Y.-C.; Chen, Y.-H.; Su, Y.-D. Seco-germacrane anhydride: Occurrence of a sesquiterpene lactone in the gorgonian coral Menella sp. (Plexauridae). Biochem. Syst. Ecol. 2012, 40, 53–55. [Google Scholar] [CrossRef]
- Zhang, S.; Won, Y.-K.; Ong, C.-N.; Shen, H.-M. Anti-Cancer Potential of Sesquiterpene Lactones: Bioactivity and Molecular Mechanisms. Curr. Med. Chem. Agents 2005, 5, 239–249. [Google Scholar] [CrossRef]
- Matos, M.S.; Anastácio, J.D.; Dos Santos, C.N. Sesquiterpene lactones: Promising natural compounds to fight inflammation. Pharmaceutics 2021, 13, 991. [Google Scholar] [CrossRef]
- Matejić, J.; Šarac, Z.; Ranđelović, V. Pharmacological Activity of Sesquiterpene Lactones. Biotechnol. Biotechnol. Equip. 2010, 24, 95–100. [Google Scholar] [CrossRef]
- Rodríguez-Expósito, R.L.; Nocchi, N.; Reyes-Batlle, M.; Sifaoui, I.; Suárez-Gómez, B.; Díaz-Marrero, A.R.; Souto, M.L.; Piñero, J.E.; Fernández, J.J.; Lorenzo-Morales, J. Antiamoebic effects of sesquiterpene lactones isolated from the zoanthid Palythoa aff. clavata. Bioorganic Chem. 2021, 108, 104682. [Google Scholar] [CrossRef]
- Tabatabaei, S.M.; Ebrahimi, S.N.; Salehi, P.; Sonboli, A.; Tabefam, M.; Kaiser, M.; Hamburger, M.; Farimani, M.M. Antiprotozoal Germacranolide Sesquiterpene Lactones from Tanacetum sonbolii. Planta Medica 2019, 85, 424–430. [Google Scholar] [CrossRef]
- López, C.; Reimer, J.D.; Brito, A.; Simón, D.; Clemente, S.; Hernández, M. Diversity of zoantharian species and their symbionts from the Macaronesian and Cape Verde ecoregions demonstrates their widespread distribution in the Atlantic Ocean. Coral Reefs 2019, 38, 269–283. [Google Scholar] [CrossRef]
- Julianti, T.; Hata, Y.; Zimmermann, S.; Kaiser, M.; Hamburger, M.; Adams, M. Antitrypanosomal sesquiterpene lactones from Saussurea costus. Fitoterapia 2011, 82, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Izumi, E.; Morello, L.G.; Ueda-Nakamura, T.; Yamada-Ogatta, S.F.; Filho, B.P.D.; Cortez, D.A.G.; Ferreira, I.C.P.; Morgado-Díaz, J.A.; Nakamura, C.V. Trypanosoma cruzi: Antiprotozoal activity of parthenolide obtained from Tanacetum parthenium (L.) Schultz Bip. (Asteraceae, Compositae) against epimastigote and amastigote forms. Exp. Parasitol. 2008, 118, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Nour, A.M.M.; Khalid, S.A.; Kaiser, M.; Brun, R. Quantitative Structure—Antiprotozoal Activity Relationships of Sesquiterpene Lactones. Molecules 2009, 14, 2062–2076. [Google Scholar] [CrossRef]
- Schmidt, T.J. Structure-Activity Relationships of Sesquiterpene Lactones. In Studies in Natural Products Chemistry; Rahman, A.-U., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 33, pp. 309–392. [Google Scholar]
- Arberas-Jiménez, I.; García-Davis, S.; Rizo-Liendo, A.; Sifaoui, I.; Reyes-Batlle, M.; Chiboub, O.; Rodríguez-Expósito, R.L.; Díaz-Marrero, A.R.; Piñero, J.E.; Fernández, J.J.; et al. Laurinterol from Laurencia johnstonii eliminates Naegleria fowleri triggering PCD by inhibition of ATPases. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Zeouk, I.; Sifaoui, I.; López-Arencibia, A.; Reyes-Batlle, M.; Bethencourt-Estrella, C.J.; Bazzocchi, I.L.; Bekhti, K.; Lorenzo-Morales, J.; Jiménez, I.A.; Piñero, J.E. Sesquiterpenoids and flavonoids from Inula viscosa induce programmed cell death in kinetoplastids. Biomed. Pharmacother. 2020, 130, 110518. [Google Scholar] [CrossRef]
- Jimenez, V.; Kemmerling, U.; Paredes, R.; Maya, J.; Sosa, M.; Galanti, N. Natural sesquiterpene lactones induce programmed cell death in Trypanosoma cruzi: A new therapeutic target? Phytomedicine 2014, 21, 1411–1418. [Google Scholar] [CrossRef]
- Kumar, R.; Tiwari, K.; Dubey, V.K. Methionine aminopeptidase 2 is a key regulator of apoptotic like cell death in Leishmania donovani. Sci. Rep. 2017, 7, 95. [Google Scholar] [CrossRef][Green Version]
- Cartuche, L.; Sifaoui, I.; López-Arencibia, A.; Bethencourt-Estrella, C.J.; Nicolás-Hernández, D.S.; Lorenzo-Morales, J.; Piñero, J.E.; Díaz-Marrero, A.R.; Fernández, J.J. Antikinetoplastid Activity of Indolocarbazoles from Streptomyces sanyensis. Biomolecules 2020, 10, 657. [Google Scholar] [CrossRef]
- Rizo-Liendo, A.; Sifaoui, I.; Reyes-Batlle, M.; Chiboub, O.; Rodríguez-Expósito, R.L.; Bethencourt-Estrella, C.J.; Nicolás-Hernández, D.S.; Hendiger, E.B.; López-Arencibia, A.; Rocha-Cabrera, P.; et al. In Vitro Activity of Statins against Naegleria fowleri. Pathogens 2019, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- López-Arencibia, A.; Nicolás-Hernández, D.S.; Bethencourt-Estrella, C.J.; Sifaoui, I.; Reyes-Batlle, M.; Rodríguez-Expósito, R.L.; Rizo-Liendo, A.; Lorenzo-Morales, J.; Bazzocchi, I.L.; Piñero, J.E.; et al. Withanolides from Withania aristata as Antikinetoplastid Agents through Induction of Programmed Cell Death. Pathogens 2019, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Marrero, A.R.; López-Arencibia, A.; Bethencout-Estrella, C.J.; Cen-Pacheco, F.; Sifaoui, I.; Creus, A.H.; Duque-Ramírez, M.C.; Souto, M.L.; Daranas, A.H.; Lorenzo-Morales, J.; et al. Antiprotozoal activities of marine polyether triterpenoids. Bioorg. Chem. 2019, 92, 103276. [Google Scholar] [CrossRef]
- López-Arencibia, A.; Bethencourt-Estrella, C.J.; Freijo, M.B.; Reyes-Batlle, M.; Sifaoui, I.; Nicolás-Hernández, D.S.; McNaughton-Smith, G.; Lorenzo-Morales, J.; Abad-Grillo, T.; Piñero, J.E. New phenalenone analogues with improved activity against Leishmania species. Biomed. Pharmacother. 2020, 132, 110814. [Google Scholar] [CrossRef] [PubMed]
- López-Arencibia, A.; Reyes-Batlle, M.; Freijo, M.B.; Sifaoui, I.; Bethencourt-Estrella, C.J.; Rizo-Liendo, A.; Chiboub, O.; McNaughton-Smith, G.; Lorenzo-Morales, J.; Abad-Grillo, T.; et al. In vitro activity of 1H-phenalen-1-one derivatives against Leishmania spp. and evidence of programmed cell death. Parasites Vectors 2019, 12, 1–10. [Google Scholar] [CrossRef]
- Bethencourt-Estrella, C.; Delgado-Hernández, S.; López-Arencibia, A.; Nicolás-Hernández, D.S.; Sifaoui, I.; Tejedor, D.; García-Tellado, F.; Lorenzo-Morales, J.; Piñero, J. Acrylonitrile Derivatives against Trypanosoma cruzi: In Vitro Activity and Programmed Cell Death Study. Pharmaceuticals 2021, 14, 552. [Google Scholar] [CrossRef] [PubMed]
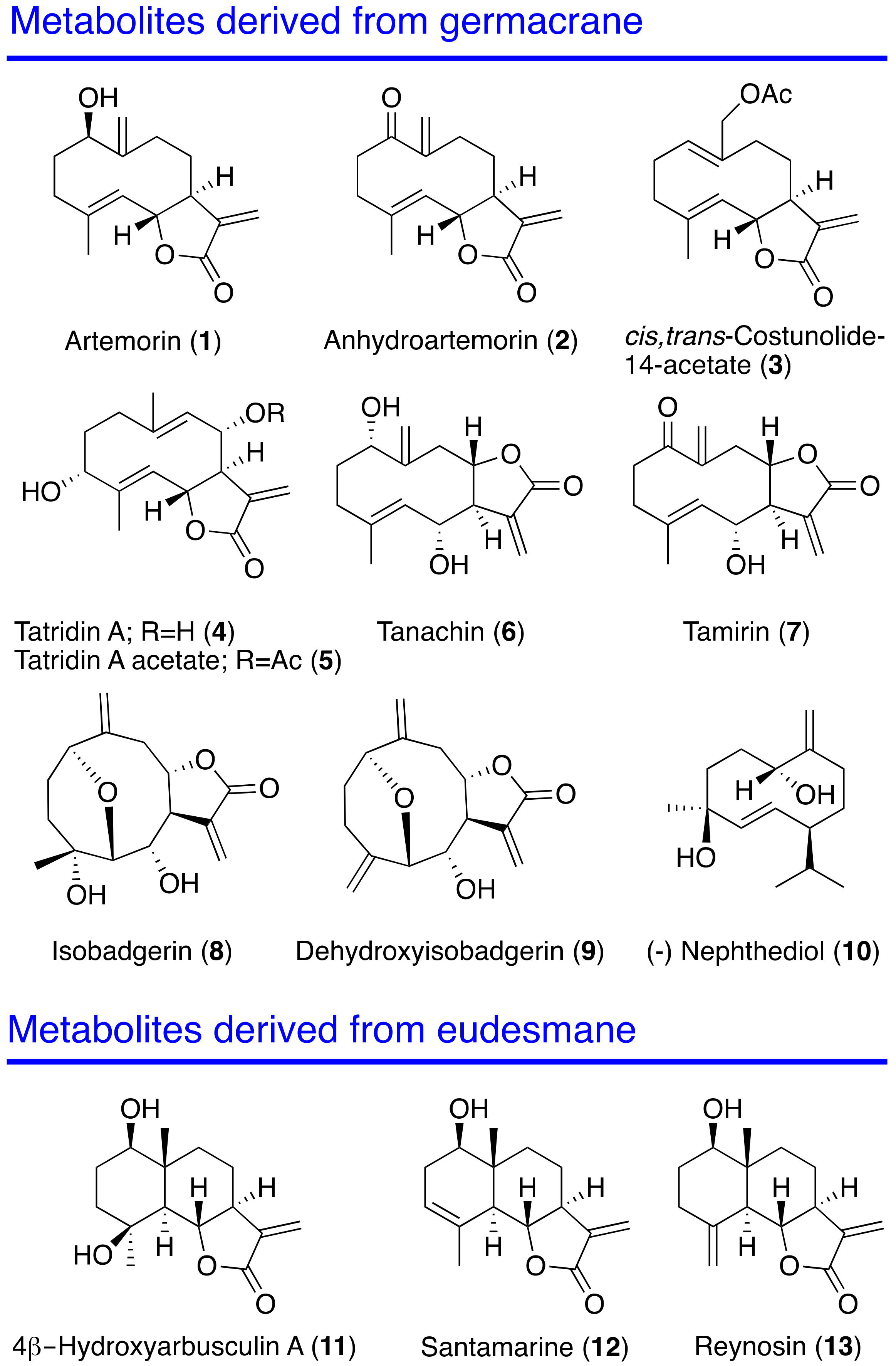
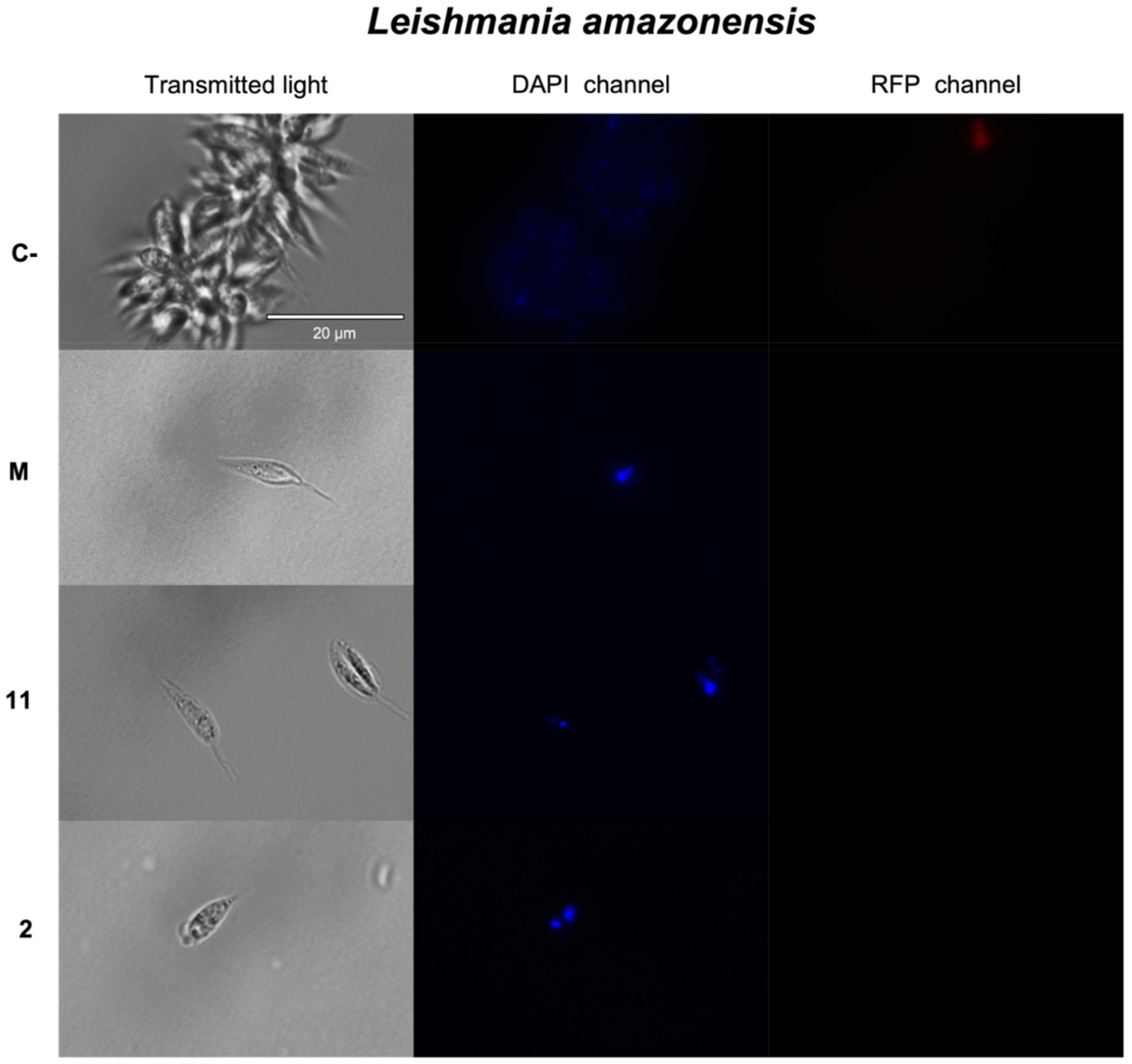
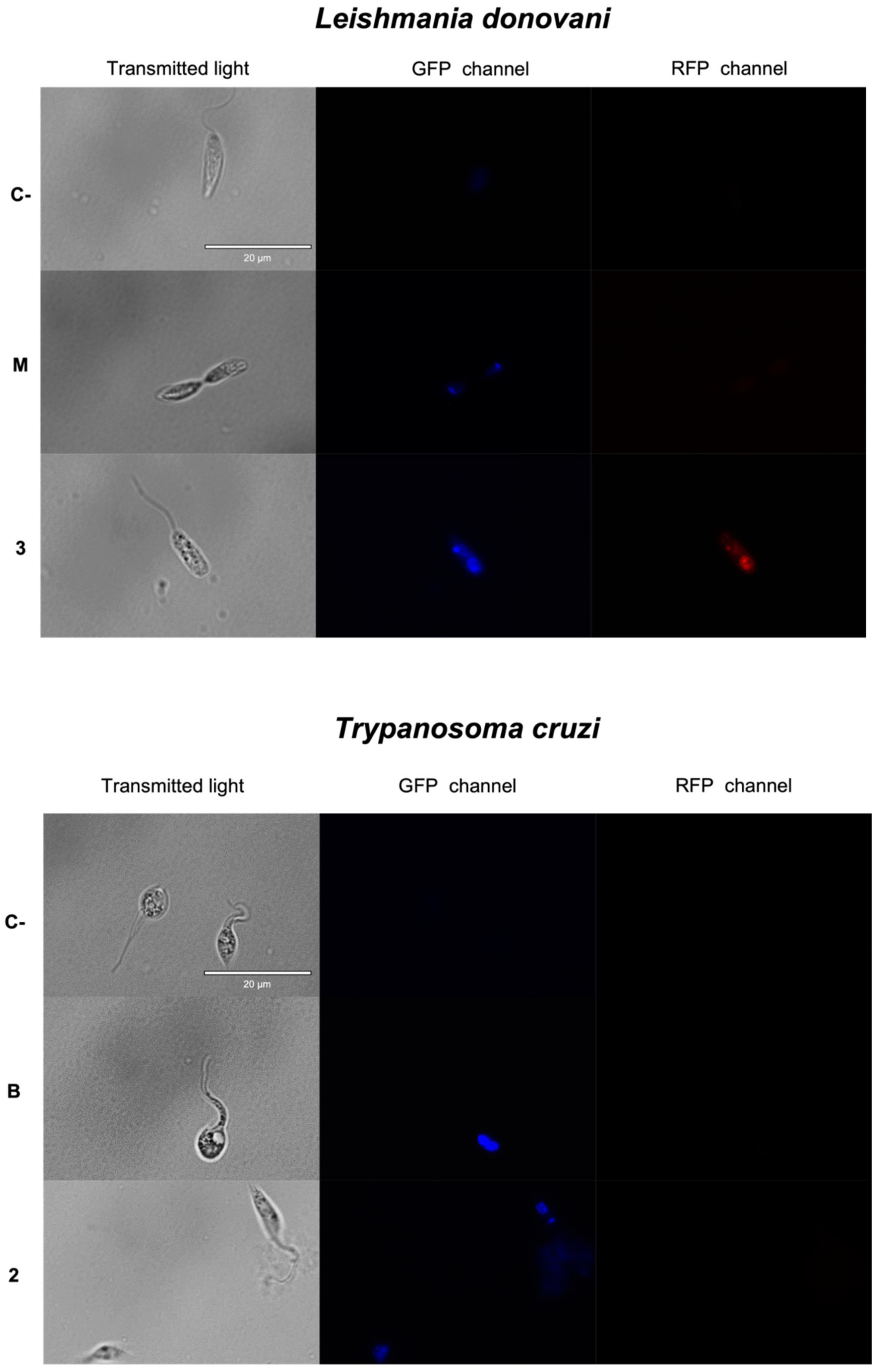
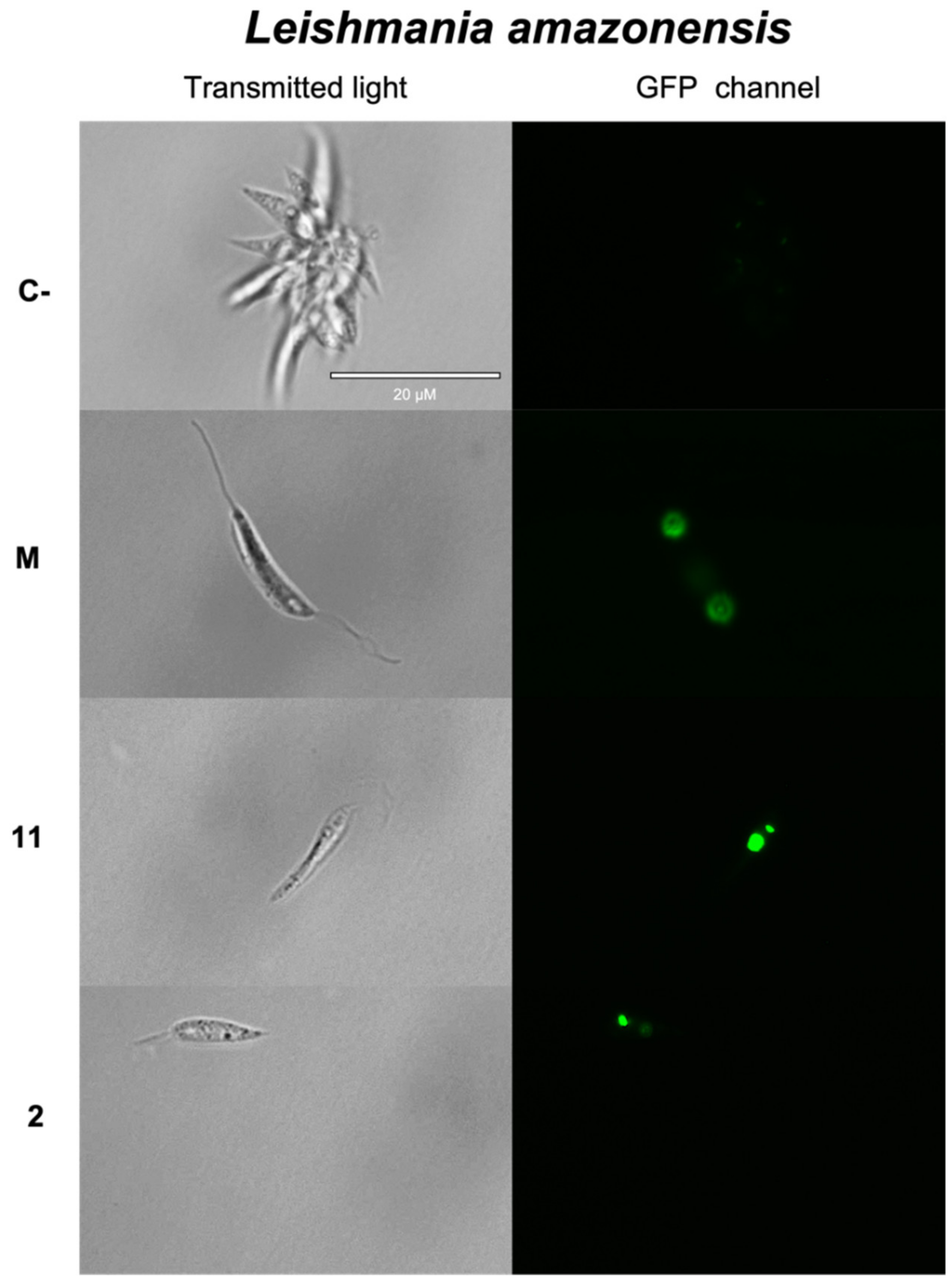
| Compounds | Promastigotes L. donovani IC50 (μM) | Promastigotes L. amazonensis IC50 (μM) | Amastigotes L. amazonensis IC50 (μM) | Epimastigotes T. cruzi IC50 (μM) | Macrophages J744A.1 CC50 (μM) |
|---|---|---|---|---|---|
| Artemorin (1) | >201.35 | 40.87 ± 2.82 | 40.13 ± 3.44 | 80.98 ± 11.96 | >402.71 |
| Anhydroartemorin (2) | >101.50 | 25.56 ± 5.88 | 7.44 ± 0.45 | 13.01 ± 4.95 | 179.01 ± 2.27 |
| cis,trans-costunolide-14-acetate (3) | 5.41 ± 1.28 | 8.62 ± 1.00 | 24.57 ± 3.64 | 8.88 ± 0.91 | 51.04 ± 7.54 |
| Tatridin A (4) | >94.58 | >94.58 | >94.58 | 32.38 ± 6.55 | |
| Tatridin A acetate (5) | >81.60 | >81.60 | >81.60 | 19.29 ± 0.91 | |
| Tanachin (6) | >94.58 | >94.58 | >94.58 | 99.24 ± 4.43 | |
| Tamirin (7) | 4.13 ± 1.25 | 11.71 ± 2.52 | >69.00 | 23.32 ± 1.27 | |
| Isobadgerin (8) | >89.18 | >89.18 | >89.18 | 89.68 ± 3.92 | |
| Dehydroxyisobadgerin (9) | 34.04 ± 3.32 | 25.54 ± 3.2 | 59.78 ± 9.76 | 49.98 ± 13.08 | |
| Nephthediol (10) | >105.32 | >105.32 | >105.32 | 23.58 ± 0.21 | |
| 4b-Hydroxyarbusculin A (11) | >201.35 | 76.39 ± 5.72 | 15.81 ± 1.01 | >201.35 | >402.71 |
| Santamarine (12) | 12.13 ± 4.67 | >100.67 | 9.74 ± 2.68 | 77.08 ± 13.97 | |
| Reynosin (13) | >201.35 | 40.19 ± 8.78 | 58.23 ± 13.13 | 77.92 ± 13.85 | |
| Miltefosine | 3.32 ± 0.27 | 6.48 ± 0.24 | 3.12 ± 0.30 | 72.19 ± 3.06 | |
| Benznidazole | 6.94 ± 1.94 | >1536 |
| Compounds | Promastigotes L. donovani SI | Promastigotes L. amazonensis SI | Amastigotes L. amazonensis SI | Epimastigotes T. cruzi SI |
|---|---|---|---|---|
| Artemorin (1) | ND | >9.85 | >10.04 | >4.97 |
| Anhydroartemorin (2) | <1.76 | 7.00 | 24.06 | 13.76 |
| cis,trans-costunolide-14-acetate (3) | 9.43 | 5.92 | 2.08 | 5.75 |
| Tatridin A (4) | <0.34 | <0.34 | ND | <0.34 |
| Tatridin A acetate (5) | <0.24 | <0.24 | ND | <0.24 |
| Tanachin (6) | <1.05 | <1.05 | ND | <1.05 |
| Tamirin (7) | 5.65 | 1.99 | ND | <0.34 |
| Isobadgerin (8) | <1.01 | <1.01 | ND | <1.01 |
| Dehydroxyisobadgerin (9) | 1.47 | 1.96 | ND | 0.84 |
| Nephthediol (10) | <0.22 | <0.22 | ND | <0.22 |
| 4b-Hydroxyarbusculin A (11) | ND | >5.27 | >25.47 | ND |
| Santamarine (12) | 6.35 | <0.77 | ND | 7.91 |
| Reynosin (13) | <0.39 | 1.94 | ND | 1.34 |
| Miltefosine | 21.74 | 11.14 | 23.17 | ND |
| Benznidazole | ND | ND | ND | 221.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bethencourt-Estrella, C.J.; Nocchi, N.; López-Arencibia, A.; San Nicolás-Hernández, D.; Souto, M.L.; Suárez-Gómez, B.; Díaz-Marrero, A.R.; Fernández, J.J.; Lorenzo-Morales, J.; Piñero, J.E. Antikinetoplastid Activity of Sesquiterpenes Isolated from the Zoanthid Palythoa aff. clavata. Pharmaceuticals 2021, 14, 1095. https://doi.org/10.3390/ph14111095
Bethencourt-Estrella CJ, Nocchi N, López-Arencibia A, San Nicolás-Hernández D, Souto ML, Suárez-Gómez B, Díaz-Marrero AR, Fernández JJ, Lorenzo-Morales J, Piñero JE. Antikinetoplastid Activity of Sesquiterpenes Isolated from the Zoanthid Palythoa aff. clavata. Pharmaceuticals. 2021; 14(11):1095. https://doi.org/10.3390/ph14111095
Chicago/Turabian StyleBethencourt-Estrella, Carlos J., Nathalia Nocchi, Atteneri López-Arencibia, Desirée San Nicolás-Hernández, María L. Souto, Blanca Suárez-Gómez, Ana R. Díaz-Marrero, José J. Fernández, Jacob Lorenzo-Morales, and José E. Piñero. 2021. "Antikinetoplastid Activity of Sesquiterpenes Isolated from the Zoanthid Palythoa aff. clavata" Pharmaceuticals 14, no. 11: 1095. https://doi.org/10.3390/ph14111095
APA StyleBethencourt-Estrella, C. J., Nocchi, N., López-Arencibia, A., San Nicolás-Hernández, D., Souto, M. L., Suárez-Gómez, B., Díaz-Marrero, A. R., Fernández, J. J., Lorenzo-Morales, J., & Piñero, J. E. (2021). Antikinetoplastid Activity of Sesquiterpenes Isolated from the Zoanthid Palythoa aff. clavata. Pharmaceuticals, 14(11), 1095. https://doi.org/10.3390/ph14111095










