Antiangiogenic Drugs in NASH: Evidence of a Possible New Therapeutic Approach
Abstract
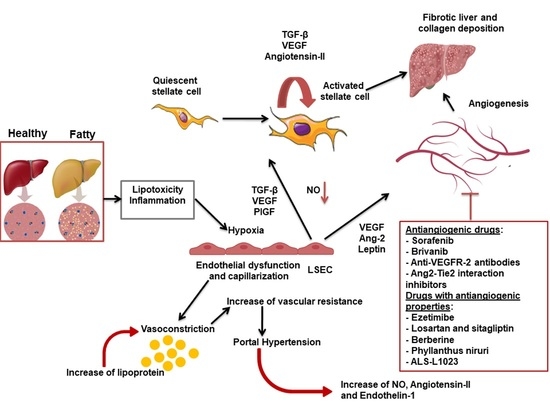
1. Introduction
2. Antiangiogenic Drugs and NASH
2.1. Sorafenib
2.2. Brivanib
2.3. Anti-VEGFR-2 Antibody
2.4. Ang2–Tie2 Interaction Inhibitors: L1-10
3. Drugs with Antiangiogenic Activities and NASH
3.1. Ezetimibe
3.2. Losartan and Sitagliptin
4. Phytotherapeutic Compounds with Antiangiogenic Properties and NASH
4.1. Berberine
4.2. Phyllanthus Niruri
4.3. ALS-L1023
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Min-Debartolo, J.; Schlerman, F.; Akare, S.; Wang, J.; McMahon, J.; Zhan, Y.; Syed, J.; He, W.; Zhang, B.; Martinez, R.V. Thrombospondin-I is a critical modulator in non-alcoholic steatohepatitis (NASH). PLoS ONE 2019, 14, e0226854. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Bellentani, S.; Marino, M. Epidemiology and natural history of non-alcoholic fatty liver disease (NAFLD). Ann. Hepatol. 2009, 8, S4–S8. [Google Scholar] [CrossRef]
- McCullough, A.J. Pathophysiology of nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2006, 40 (Suppl. 1), S17–S29. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Tilg, H.; Effenberger, M. From NAFLD to MAFLD: When pathophysiology succeeds. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, M. Current Options and Future Directions for NAFLD and NASH Treatment. Int. J. Mol. Sci. 2021, 22, 7571. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Weiskirchen, R. Non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH)-related liver fibrosis: Mechanisms, treatment and prevention. Ann. Transl. Med. 2021, 9, 729. [Google Scholar] [CrossRef] [PubMed]
- Natale, G.; Bocci, G.; Lenzi, P. Looking for the Word “Angiogenesis” in the History of Health Sciences: From Ancient Times to the First Decades of the Twentieth Century. World J. Surg. 2017, 41, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Natale, G.; Bocci, G. Discovery and development of the cardiovascular system with a focus on angiogenesis: A historical over-view. Ital. J. Anat. Embryol. 2019, 124, 247–270. [Google Scholar] [CrossRef]
- Coulon, S.; Heindryckx, F.; Geerts, A.; Van Steenkiste, C.; Colle, I.; Van Vlierberghe, H. Angiogenesis in chronic liver disease and its complications. Liver Int. 2011, 31, 146–162. [Google Scholar] [CrossRef]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Harris, W.P.; et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 4317–4345. [Google Scholar] [CrossRef]
- van der Graaff, D.; Kwanten, W.; Francque, S. The potential role of vascular alterations and subsequent impaired liver blood flow and hepatic hypoxia in the pathophysiology of non-alcoholic steatohepatitis. Med. Hypotheses 2019, 122, 188–197. [Google Scholar] [CrossRef]
- Poisson, J.; Lemoinne, S.; Boulanger, C.M.; Durand, F.; Moreau, R.; Valla, D.; Rautou, P.-E. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J. Hepatol. 2016, 66, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Semela, D.; Bruix, J.; Colle, I.; Pinzani, M.; Bosch, J. Angiogenesis in liver disease. J. Hepatol. 2009, 50, 604–620. [Google Scholar] [CrossRef]
- Novo, E.; Cannito, S.; Paternostro, C.; Bocca, C.; Miglietta, A.; Parola, M. Cellular and molecular mechanisms in liver fibrogenesis. Arch. Biochem. Biophys. 2014, 548, 20–37. [Google Scholar] [CrossRef]
- Ramirez-Pedraza, M.; Fernández, M. Interplay Between Macrophages and Angiogenesis: A Double-Edged Sword in Liver Disease. Front. Immunol. 2019, 10, 2882. [Google Scholar] [CrossRef] [PubMed]
- Jahn, D.; Kircher, S.; Hermanns, H.M.; Geier, A. Animal models of NAFLD from a hepatologist’s point of view. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Plaza, A.; Naranjo, V.; Blonda, A.M.; Cano, V.; González-Martín, C.; Gil-Ortega, M.; Ruiz-Gayo, M.; Merino, B. Inflammatory stress and altered angiogenesis evoked by very high-fat diets in mouse liver. Endocrinol. Diabetes Nutr. 2019, 66, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Kitade, M.; Yoshiji, H.; Kojima, H.; Ikenaka, Y.; Noguchi, R.; Kaji, K.; Yoshii, J.; Yanase, K.; Namisaki, T.; Asada, K.; et al. Leptin-mediated neovascularization is a prerequisite for progression of nonalcoholic steatohepatitis in rats. Hepatology 2006, 44, 983–991. [Google Scholar] [CrossRef]
- Coulon, S.; Legry, V.; Heindryckx, F.; Van Steenkiste, C.; Casteleyn, C.; Olievier, K.; Libbrecht, L.; Carmeliet, P.; Jonckx, B.; Stassen, J.-M.; et al. Role of vascular endothelial growth factor in the pathophysiology of nonalcoholic steatohepatitis in two rodent models. Hepatology 2013, 57, 1793–1805. [Google Scholar] [CrossRef]
- Lefere, S.; Van De Velde, F.; Hoorens, A.; Raevens, S.; Van Campenhout, S.; Vandierendonck, A.; Neyt, S.; Vandeghinste, B.; Vanhove, C.; Debbaut, C.; et al. Angiopoietin-2 Promotes Pathological Angiogenesis and Is a Therapeutic Target in Murine Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 1087–1104. [Google Scholar] [CrossRef]
- Povero, D.; Eguchi, A.; Niesman, I.R.; Andronikou, N.; Jeu, X.D.M.D.; Mulya, A.; Berk, M.; Lazic, M.; Thapaliya, S.; Parola, M.; et al. Lipid-Induced Toxicity Stimulates Hepatocytes to Release Angiogenic Microparticles That Require Vanin-1 for Uptake by Endothelial Cells. Sci. Signal. 2013, 6, ra88. [Google Scholar] [CrossRef]
- Lei, L.; Ei Mourabit, H.; Housset, C.; Cadoret, A.; Lemoinne, S. Role of Angiogenesis in the Pathogenesis of NAFLD. J. Clin. Med. 2021, 10, 1338. [Google Scholar] [CrossRef]
- Khazaei, M.; Tahergorabi, Z. Leptin and its cardiovascular effects: Focus on angiogenesis. Adv. Biomed. Res. 2015, 4, 79. [Google Scholar] [CrossRef]
- Manjunathan, R.; Devarajan, N.; Ragunathan, M. Possible Mechanism of Human Recombinant Leptin-Induced VEGF A Synthesis via PI3K/Akt/mTOR/S6 Kinase Signaling Pathway while Inducing Angiogenesis: An Analysis Using Chicken Chorioallantoic Membrane Model. J. Vasc. Res. 2021, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lefere, S.; Devisscher, L.; Geerts, A. Angiogenesis in the progression of non-alcoholic fatty liver disease. Acta Gastroenterol. Belg. 2020, 83, 301–307. [Google Scholar] [PubMed]
- Lemoinne, S.; Cadoret, A.; Rautou, P.; El Mourabit, H.; Ratziu, V.; Corpechot, C.; Rey, C.; Bosselut, N.; Barbu, V.; Wendum, D.; et al. Portal myofibroblasts promote vascular remodeling underlying cirrhosis formation through the release of microparticles. Hepatology 2015, 61, 1041–1055. [Google Scholar] [CrossRef] [PubMed]
- Parmar, D.; Apte, M. Angiopoietin inhibitors: A review on targeting tumor angiogenesis. Eur. J. Pharmacol. 2021, 899, 174021. [Google Scholar] [CrossRef]
- Ferolla, S.M.; Silva, L.C.; Ferrari, M.D.L.A.; Da Cunha, A.S.; Martins, F.D.S.; Couto, C.A.; Ferrari, T.C.A. Dietary approach in the treatment of nonalcoholic fatty liver disease. World J. Hepatol. 2015, 7, 2522–2534. [Google Scholar] [CrossRef] [PubMed]
- Eaton, C.B.; Gramling, R.; Parker, D.R.; Roberts, M.B.; Lu, B.; Ridker, P.M. Prospective association of vascular endothelial growth factor-A (VEGF-A) with coronary heart disease mortality in Southeastern New England. Atherosclerosis 2008, 200, 221–227. [Google Scholar] [CrossRef]
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011, 34, 274–285. [Google Scholar] [CrossRef]
- Bonis, P.A.; Friedman, S.L.; Kaplan, M.M. Is Liver Fibrosis Reversible? N. Engl. J. Med. 2001, 344, 452–454. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, A. Understanding the mechanism of hepatic fibrosis and potential therapeutic approaches. Saudi J. Gastroenterol. 2012, 18, 155–167. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.M.; Hilgard, P.; Gane, E.; Blanc, J.-F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Eisen, T.; Stadler, W.M.; Szczylik, C.; Oudard, S.; Siebels, M.; Negrier, S.; Chevreau, C.; Solska, E.; Desai, A.A.; et al. Sorafenib in Advanced Clear-Cell Renal-Cell Carcinoma. N. Engl. J. Med. 2007, 356, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A.; Plosker, G.L. Sorafenib: A review of its use in patients with radioactive iodine-refractory, metastatic differentiated thyroid carcinoma. Target. Oncol. 2015, 10, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cao, Y.; Chen, C.; Zhang, X.; McNabola, A.; Wilkie, D.; Wilhelm, S.; Lynch, M.; Carter, C. Sorafenib Blocks the RAF/MEK/ERK Pathway, Inhibits Tumor Angiogenesis, and Induces Tumor Cell Apoptosis in Hepatocellular Carcinoma Model PLC/PRF/5. Cancer Res. 2006, 66, 11851–11858. [Google Scholar] [CrossRef]
- Wan, P.T.; Garnett, M.J.; Roe, M.; Lee, S.; Niculescu-Duvaz, D.; Good, V.M.; Project, C.G.; Jones, C.; Marshall, C.J.; Springer, C.J.; et al. Mechanism of Activation of the RAF-ERK Signaling Pathway by Oncogenic Mutations of B-RAF. Cell 2004, 116, 855–867. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 Exhibits Broad Spectrum Oral Antitumor Activity and Targets the RAF/MEK/ERK Pathway and Receptor Tyrosine Kinases Involved in Tumor Progression and Angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef]
- Deng, Y.-R.; Ma, H.-D.; Tsuneyama, K.; Yang, W.; Wang, Y.-H.; Lu, F.-T.; Liu, C.-H.; Liu, P.; He, X.-S.; Diehl, A.M.; et al. STAT3-mediated attenuation of CCl4-induced mouse liver fibrosis by the protein kinase inhibitor sorafenib. J. Autoimmun. 2013, 46, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Chou, H.; Fiel, M.I.; Friedman, S.L. Antifibrotic Activity of Sorafenib in Experimental Hepatic Fibrosis: Refinement of Inhibitory Targets, Dosing, and Window of Efficacy In Vivo. Dig. Dis. Sci. 2013, 58, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Stefano, J.; Pereira, I.V.A.; Torres, M.; Bida, P.; Coelho, A.; Xerfan, M.; Cogliati, B.; Barbeiro, D.; Mazo, D.; Kubrusly, M.; et al. Sorafenib prevents liver fibrosis in a non-alcoholic steatohepatitis (NASH) rodent model. Braz. J. Med. Biol. Res. 2015, 48, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, J.; Zhang, D.; Zhang, J.; Ma, J.; Jiang, H. New insights into the antifibrotic effects of sorafenib on hepatic stellate cells and liver fibrosis. J. Hepatol. 2010, 53, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Hennenberg, M.; Trebicka, J.; Kohistani, Z.; Stark, C.; Nischalke, H.D.; Krämer, B.; Körner, C.; Klein, S.; Granzow, M.; Fischer, H.-P.; et al. Hepatic and HSC-specific sorafenib effects in rats with established secondary biliary cirrhosis. Lab. Investig. 2011, 91, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Mejias, M.; Garcia-Pras, E.; Tiani, C.; Miquel, R.; Bosch, J.; Fernandez, M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology 2009, 49, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-Y.; Liu, R.-S.; Lee, P.-C.; Yeh, Y.-C.; Huang, Y.-T.; Lee, W.-P.; Lee, K.-C.; Hsieh, Y.-C.; Lee, F.-Y.; Tan, T.-W.; et al. Anti-VEGFR agents ameliorate hepatic venous dysregulation/microcirculatory dysfunction, splanchnic venous pooling and ascites of NASH-cirrhotic rat. Liver Int. 2014, 34, 521–534. [Google Scholar] [CrossRef]
- Jian, C.; Fu, J.; Cheng, X.; Shen, L.-J.; Ji, Y.-X.; Wang, X.; Pan, S.; Tian, H.; Tian, S.; Liao, R.; et al. Low-Dose Sorafenib Acts as a Mitochondrial Uncoupler and Ameliorates Nonalcoholic Steatohepatitis. Cell Metab. 2020, 31, 892–908.e11. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef]
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.-R.; Feldstein, A.F.; Zein, N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010, 51, 1972–1978. [Google Scholar] [CrossRef]
- White, D.L.; Kanwal, F.; El–Serag, H.B. Association between Nonalcoholic Fatty Liver Disease and Risk for Hepatocellular Cancer, Based on Systematic Review. Clin. Gastroenterol. Hepatol. 2012, 10, 1342–1359.e2. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; Khedr, E.G.; El-Bahrawy, H.A.; El-Fattah, E.E.A. Sorafenib effect on liver neoplastic changes in rats: More than a kinase inhibitor. Clin. Exp. Med. 2016, 17, 185–191. [Google Scholar] [CrossRef]
- Qu, K.; Huang, Z.; Lin, T.; Liu, S.; Chang, H.; Yan, Z.; Zhang, H.; Liu, C. New insight into the anti-liver fibrosis effect of mul-titargeted tyrosine kinase inhibitors: From molecular target to clinical trials. Front. Pharmacol. 2016, 6, 300. [Google Scholar] [CrossRef] [PubMed]
- Raoul, J.L.; Gilabert, M.; Adhoute, X.; Edeline, J. An in-depth review of chemical angiogenesis inhibitors for treating hepato-cellular carcinoma. Expert Opin. Pharmacother. 2017, 18, 1467–1476. [Google Scholar] [CrossRef]
- Chou, T.; Finn, R.S. Brivanib: A review of development. Future Oncol. 2012, 8, 1083–1090. [Google Scholar] [CrossRef]
- Nakamura, I.; Zakharia, K.; Banini, B.A.; Mikhail, D.S.; Kim, T.H.; Yang, J.D.; Moser, C.D.; Shaleh, H.M.; Thornburgh, S.R.; Walters, I.; et al. Brivanib Attenuates Hepatic Fibrosis In Vivo and Stellate Cell Activation In Vitro by Inhibition of FGF, VEGF and PDGF Signaling. PLoS ONE 2014, 9, e92273. [Google Scholar] [CrossRef]
- Kiss, E.A.; Saharinen, P. Anti-angiogenic Targets: Angiopoietin and Angiopoietin-Receptors. In Tumor Angiogenesis; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–24. [Google Scholar] [CrossRef]
- Mueller, S.B.; Kontos, C.D. Tie1: An orphan receptor provides context for angiopoietin-2/Tie2 signaling. J. Clin. Investig. 2016, 126, 3188–3191. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, E.A.; Lampinen, A.; Giri, H.; Anisimov, A.; Kim, M.; Allen, B.; Fang, S.; D’Amico, G.; Sipilä, T.J.; Lohela, M.; et al. Tie1 controls angiopoietin function in vascular remodeling and inflammation. J. Clin. Investig. 2016, 126, 3495–3510. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Allen, B.; Korhonen, E.A.; Nitschké, M.; Yang, H.W.; Baluk, P.; Saharinen, P.; Alitalo, K.; Daly, C.; Thurston, G.; et al. Opposing actions of angiopoietin-2 on Tie2 signaling and FOXO1 activation. J. Clin. Investig. 2016, 126, 3511–3525. [Google Scholar] [CrossRef]
- Takakura, K.; Koido, S.; Fujii, M.; Hashiguchi, T.; Shibazaki, Y.; Yoneyama, H.; Katagi, H.; Kajihara, M.; Misawa, T.; Homma, S.; et al. Characterization of non-alcoholic steatohepatitis-derived hepatocellular carcinoma as a human stratification model in mice. Anticancer Res. 2014, 34, 4849–4855. [Google Scholar]
- Oliner, J.; Min, H.; Leal, J.; Yu, D.; Rao, S.; You, E.; Tang, X.; Kim, H.; Meyer, S.; Han, S.J.; et al. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell 2004, 6, 507–516. [Google Scholar] [CrossRef]
- Suzuki, R.; Yamamoto, H.; Ngan, C.Y.; Ohtsuka, M.; Kitani, K.; Uemura, M.; Nishimura, J.; Takemasa, I.; Mizushima, T.; Sekimoto, M.; et al. Inhibition of angiopoietin 2 attenuates lumen formation of tumour-associated vessels in vivo. Int. J. Oncol. 2013, 43, 1447–1455. [Google Scholar] [CrossRef][Green Version]
- Marth, C.; Vergote, I.; Scambia, G.; Oberaigner, W.; Clamp, A.; Berger, R.; Kurzeder, C.; Colombo, N.; Vuylsteke, P.; Lorusso, D.; et al. ENGOT-ov-6/TRINOVA-2: Randomised, double-blind, phase 3 study of pegylated liposomal doxorubicin plus trebananib or placebo in women with recurrent partially platinum-sensitive or resistant ovarian cancer. Eur. J. Cancer 2017, 70, 111–121. [Google Scholar] [CrossRef]
- Monk, B.J.; Poveda, A.; Vergote, I.; Raspagliesi, F.; Fujiwara, K.; Bae, D.-S.; Oaknin, A.; Ray-Coquard, I.; Provencher, D.M.; Karlan, B.Y.; et al. Anti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1): A randomised, multicentre, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014, 15, 799–808. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Blanc, J.; Miles, S.; Ganten, T.; Trojan, J.; Cebon, J.; Liem, A.K.; Lipton, L.; Gupta, C.; Wu, B.; et al. Phase II Study of First-Line Trebananib Plus Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Oncologist 2017, 22, 780-e65. [Google Scholar] [CrossRef] [PubMed]
- Pelton, K.; Coticchia, C.M.; Curatolo, A.S.; Schaffner, C.P.; Zurakowski, D.; Solomon, K.R.; Moses, M.A. Hypercholesterolemia Induces Angiogenesis and Accelerates Growth of Breast Tumors in Vivo. Am. J. Pathol. 2014, 184, 2099–2110. [Google Scholar] [CrossRef]
- Solomon, K.R.; Pelton, K.; Boucher, K.; Joo, J.; Tully, C.; Zurakowski, D.; Schaffner, C.P.; Kim, J.; Freeman, M.R. Ezetimibe Is an Inhibitor of Tumor Angiogenesis. Am. J. Pathol. 2009, 174, 1017–1026. [Google Scholar] [CrossRef]
- Ehling, J.; Bartneck, M.; Wei, X.; Gremse, F.; Fech, V.; Möckel, D.; Baeck, C.; Hittatiya, K.; Eulberg, D.; Luedde, T.; et al. CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut 2014, 63, 1960–1971. [Google Scholar] [CrossRef] [PubMed]
- Ishikawaa, K.; Mochidaa, S.; Mashibaa, S.; Inaoa, M.; Matsuia, A.; Ikedab, H.; Ohnoa, A.; Shibuyac, M.; Fujiwara, K. Expressions of Vascular Endothelial Growth Factor in Nonparenchymal as Well as Parenchymal Cells in Rat Liver after Necrosis. Biochem. Biophys. Res. Commun. 1999, 254, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Ohnishi, H.; Morimoto, N.; Minami, S.; Ishioka, M.; Watanabe, S.; Tsukui, M.; Takaoka, Y.; Nomoto, H.; Isoda, N.; et al. Ezetimibe suppresses development of liver tumors by inhibiting angiogenesis in mice fed a high-fat diet. Cancer Sci. 2019, 110, 771–783. [Google Scholar] [CrossRef]
- Horie, Y.; Suzuki, A.; Kataoka, E.; Sasaki, T.; Hamada, K.; Sasaki, J.; Mizuno, K.; Hasegawa, G.; Kishimoto, H.; Iizuka, M.; et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J. Clin. Investig. 2004, 113, 1774–1783. [Google Scholar] [CrossRef]
- Yoshiji, H.; Fukui, H. Renin-angiotensin system and progression of chronic liver diseases. J. Gastroenterol. 2006, 41, 1020–1022. [Google Scholar] [CrossRef]
- Yoshiji, H.; Kuriyama, S.; Yoshii, J.; Ikenaka, Y.; Noguchi, R.; Nakatani, T.; Tsujinoue, H.; Fukui, H. Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology 2001, 34, 745–750. [Google Scholar] [CrossRef]
- Yoshiji, H.; Noguchi, R.; Namisaki, T.; Moriya, K.; Kitade, M.; Aihara, Y.; Douhara, A.; Kawaratani, H.; Nishimura, N.; Fukui, H. Combination of sorafenib and angiotensin-II receptor blocker attenuates preneoplastic lesion development in a non-diabetic rat model of steatohepatitis. J. Gastroenterol. 2014, 49, 1421–1429. [Google Scholar] [CrossRef]
- Yoshiji, H.; Noguchi, R.; Ikenaka, Y.; Kaji, K.; Aihara, Y.; Fukui, H. Impact of renin-angiotensin system in hepatocellular carcinoma. Curr. Cancer Drug Targets 2011, 11, 431–441. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, T.; Zhao, J.; Guo, C.; Yao, J.; Gao, P.; Dong, J.; Liao, L. Effects and Safety of Sitagliptin in Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 2020, 52, 517–526. [Google Scholar] [CrossRef]
- Kajiyama, H.; Kikkawa, F.; Maeda, O.; Suzuki, T.; Ino, K.; Mizutani, S. Increased Expression of Dipeptidyl Peptidase IV in Human Mesothelial Cells by Malignant Ascites from Ovarian Carcinoma Patients. Oncology 2002, 63, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Okura, Y.; Namisaki, T.; Moriya, K.; Kitade, M.; Takeda, K.; Kaji, K.; Noguchi, R.; Nishimura, N.; Seki, K.; Kawaratani, H.; et al. Combination treatment of dipeptidyl peptidase IV inhibitor (sitagliptin) and angiotensin-II type 1 receptor blocker (losartan) suppresses progression in a non-diabetic rat model of steatohepatitis. Hepatol. Res. 2017, 47, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Huang, W.-Y.; Lai, C.-H.; Hsu, Y.-M.; Yao, Y.-H.; Chen, T.-Y.; Wu, J.-Y.; Peng, S.-F.; Lin, Y.-H. Development of novel nanoparticles shelled with heparin for berberine delivery to treat Helicobacter pylori. Acta Biomater. 2011, 7, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xun, K.; Wang, Y.; Chen, X. A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anti-Cancer Drugs 2009, 20, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Hesari, A.; Ghasemi, F.; Cicero, A.F.G.; Mohajeri, M.; Rezaei, O.; Gheibi-Hayat, S.M.; Sahebkar, A. Berberine: A potential adjunct for the treatment of gastrointestinal cancers? J. Cell. Biochem. 2018, 119, 9655–9663. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Shao, D.; Zhao, Y.; Zhang, F.; Zheng, X.; Tan, Y.; He, K.; Li, J.; Chen, L. Berberine Reverses Hypoxia-induced Chemoresistance in Breast Cancer through the Inhibition of AMPK- HIF-1α. Int. J. Biol. Sci. 2017, 13, 794–803. [Google Scholar] [CrossRef]
- Xu, J.; Wu, W.; Zhang, H.; Yang, L. Berberine alleviates amyloid β25-35-induced inflammatory response in human neuroblastoma cells by inhibiting proinflammatory factors. Exp. Ther. Med. 2018, 16, 4865–4872. [Google Scholar] [CrossRef]
- Hamsa, T.; Kuttan, G. Antiangiogenic activity of berberine is mediated through the downregulation of hypoxia-inducible factor-1, VEGF, and proinflammatory mediators. Drug Chem. Toxicol. 2012, 35, 57–70. [Google Scholar] [CrossRef]
- Fujii, M.; Shibazaki, Y.; Wakamatsu, K.; Honda, Y.; Kawauchi, Y.; Suzuki, K.; Arumugam, S.; Watanabe, K.; Ichida, T.; Asakura, H.; et al. A murine model for non-alcoholic steatohepatitis showing evidence of association between diabetes and hepatocellular carcinoma. Med. Mol. Morphol. 2013, 46, 141–152. [Google Scholar] [CrossRef]
- Luo, Y.; Tian, G.; Zhuang, Z.; Chen, J.; You, N.; Zhuo, L.; Liang, B.; Song, Y.; Zang, S.; Liu, J.; et al. Berberine prevents non-alcoholic steatohepatitis-derived hepatocellular carcinoma by inhibiting inflammation and angiogenesis in mice. Am. J. Transl. Res. 2019, 11, 2668–2682. [Google Scholar]
- Wu, D.; Wen, W.; Qi, C.-L.; Zhao, R.-X.; Lü, J.-H.; Zhong, C.-Y.; Chen, Y.-Y. Ameliorative effect of berberine on renal damage in rats with diabetes induced by high-fat diet and streptozotocin. Phytomedicine 2012, 19, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiao, X.; Feng, K.; Wang, T.; Li, W.; Yuan, T.; Sun, X.; Sun, Q.; Xiang, H.; Wang, H. Berberine Moderates Glucose and Lipid Metabolism through Multipathway Mechanism. Evid.-Based Complement. Altern. Med. 2011, 2011, 924851. [Google Scholar] [CrossRef]
- Kaur, N.; Kaur, B.; Sirhindi, G. Phytochemistry and Pharmacology of Phyllanthus niruri L.: A Review. Phyther. Res. 2017, 31, 980–1004. [Google Scholar] [CrossRef] [PubMed]
- Bagalkotkar, G.; Sagineedu, S.R.; Saad, M.S.; Stanslas, J. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: A review. J. Pharm. Pharmacol. 2006, 58, 1559–1570. [Google Scholar] [CrossRef]
- Khanna, A.; Rizvi, F.; Chander, R. Lipid lowering activity of Phyllanthus niruri in hyperlipemic rats. J. Ethnopharmacol. 2002, 82, 19–22. [Google Scholar] [CrossRef]
- Al Zarzour, R.H.; Alshawsh, M.A.; Asif, M.; Al-Mansoub, M.A.; Mohamed, Z.; Ahmad, M.; Majid, A.M.S.A.; Asmawi, M.Z.; Kaur, G.; Al-Dualimi, D.W.; et al. Adipocytokine Regulation and Antiangiogenic Activity Underlie the Molecular Mechanisms of Therapeutic Effects of Phyllanthus niruri against Non-Alcoholic Fatty Liver Disease. Nutrients 2018, 10, 1057. [Google Scholar] [CrossRef] [PubMed]
- Crandall, D.L.; Hausman, G.J.; Kral, J.G. A Review of the Microcirculation of Adipose Tissue: Anatomic, Metabolic, and Angiogenic Perspectives. Microcirculation 1997, 4, 211–232. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.; Lim, J.; Oh, J.; Shin, S.S.; Yoon, M. The Angiogenesis Inhibitor ALS-L1023 from Lemon-Balm Leaves Attenuates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease through Regulating the Visceral Adipose-Tissue Function. Int. J. Mol. Sci. 2017, 18, 846. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-Y.; Park, B.Y.; Lee, H.S.; Park, E.K.; Hahm, J.C.; Lee, J.; Hong, Y.; Choi, S.; Park, D.; Lee, H.; et al. The anti-angiogenic herbal composition Ob-X inhibits adipose tissue growth in obese mice. Int. J. Obes. 2010, 34, 820–830. [Google Scholar] [CrossRef]
- Rupnick, M.A.; Panigrahy, D.; Zhang, C.-Y.; Dallabrida, S.M.; Lowell, B.B.; Langer, R.; Folkman, M.J. Adipose tissue mass can be regulated through the vasculature. Proc. Natl. Acad. Sci. USA 2002, 99, 10730–10735. [Google Scholar] [CrossRef]
- Brakenhielm, E.; Cao, R.; Gao, B.; Angelin, B.; Cannon, B.; Parini, P.; Cao, Y. Angiogenesis Inhibitor, TNP-470, Prevents Diet-Induced and Genetic Obesity in Mice. Circ. Res. 2004, 94, 1579–1588. [Google Scholar] [CrossRef]
- Park, B.Y.; Lee, H.; Woo, S.; Yoon, M.; Kim, J.; Hong, Y.; Lee, H.S.; Park, E.K.; Hahm, J.C.; Kim, J.W.; et al. Reduction of Adipose Tissue Mass by the Angiogenesis Inhibitor ALS-L1023 from Melissa officinalis. PLoS ONE 2015, 10, e0141612. [Google Scholar] [CrossRef]
- Woo, S.; Yoon, M.; Kim, J.; Hong, Y.; Kim, M.-Y.; Shin, S.S.; Yoon, M. The anti-angiogenic herbal extract from Melissa officinalis inhibits adipogenesis in 3T3-L1 adipocytes and suppresses adipocyte hypertrophy in high fat diet-induced obese C57BL/6J mice. J. Ethnopharmacol. 2016, 178, 238–250. [Google Scholar] [CrossRef]
- Gutierrez, L.S.; Gutierrez, J. Thrombospondin 1 in Metabolic Diseases. Front. Endocrinol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- de Alwis, N.M.W.; Day, C.P. Non-alcoholic fatty liver disease: The mist gradually clears. J. Hepatol. 2008, 48, S104–S112. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.-C.; Ryu, S.; Lee, J.-Y.; Kim, J.-Y.; Wild, S.H.; Byrne, C.D. Effect of exercise on the development of new fatty liver and the resolution of existing fatty liver. J. Hepatol. 2016, 65, 791–797. [Google Scholar] [CrossRef]
- Dehnavi, S.; Kiani, A.; Sadeghi, M.; Biregani, A.F.; Banach, M.; Atkin, S.L.; Jamialahmadi, T.; Sahebkar, A. Targeting AMPK by Statins: A Potential Therapeutic Approach. Drugs 2021, 81, 923–933. [Google Scholar] [CrossRef]
- Imarisio, I.; Paglino, C.; Ganini, C.; Magnani, L.; Caccialanza, R.; Porta, C. The effect of sorafenib treatment on the diabetic status of patients with renal cell or hepatocellular carcinoma. Future Oncol. 2012, 8, 1051–1057. [Google Scholar] [CrossRef]
- Makol, A.; Kanthaje, S.; Dhiman, R.K.; Kalra, N.; Chawla, Y.K.; Chakraborti, A. Association of liver cirrhosis severity with type 2 diabetes mellitus in hepatocellular carcinoma. Exp. Biol. Med. 2018, 243, 323–326. [Google Scholar] [CrossRef]
- Tesori, V.; Piscaglia, A.C.; Samengo, D.; Barba, M.; Bernardini, C.; Scatena, R.; Pontoglio, A.; Castellini, L.; Spelbrink, J.; Maulucci, G.; et al. The multikinase inhibitor Sorafenib enhances glycolysis and synergizes with glycolysis blockade for cancer cell killing. Sci. Rep. 2015, 5, 9149. [Google Scholar] [CrossRef]
- Baffy, G. Origins of Portal Hypertension in Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2018, 63, 563–576. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, Y.; Zhang, X.; Li, L. The vascular endothelial growth factor signaling pathway regulates liver sinusoidal endothelial cells during liver regeneration after partial hepatectomy. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Schoell, A.; Groenland, S.L.; Scherf-Clavel, O.; van Dyk, M.; Huisinga, W.; Michelet, R.; Jaehde, U.; Steeghs, N.; Huitema, A.D.; Kloft, C. Therapeutic drug monitoring of oral targeted antineoplastic drugs. Eur. J. Clin. Pharmacol. 2021, 77, 441–464. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Ding, M.; Li, X.; Zhou, X.; Zhu, Q.; Varela-Ramirez, A.; Yi, C. Comparative evaluation of cardiovascular risks among nine FDA-approved VEGFR-TKIs in patients with solid tumors: A Bayesian network analysis of randomized controlled trials. J. Cancer Res. Clin. Oncol. 2021, 147, 2407–2420. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Qin, S.; Park, J.-W.; Poon, R.T.; Raoul, J.-L.; Philip, P.A.; Hsu, C.-H.; Hu, T.-H.; Heo, J.; Xu, J.; et al. Brivanib Versus Sorafenib As First-Line Therapy in Patients With Unresectable, Advanced Hepatocellular Carcinoma: Results From the Randomized Phase III BRISK-FL Study. J. Clin. Oncol. 2013, 31, 3517–3524. [Google Scholar] [CrossRef] [PubMed]
- Karbownik, A.; Szkutnik-Fiedler, D.; Czyrski, A.; Kostewicz, N.; Kaczmarska, P.; Bekier, M.; Stanisławiak-Rudowicz, J.; Karaźniewicz-Łada, M.; Wolc, A.; Główka, F.; et al. Pharmacokinetic Interaction between Sorafenib and Atorvastatin, and Sorafenib and Metformin in Rats. Pharmaceutics 2020, 12, 600. [Google Scholar] [CrossRef] [PubMed]
- Gomo, C.; Coriat, R.; Faivre, L.; Mir, O.; Ropert, S.; Billemont, B.; Dauphin, A.; Tod, M.; Goldwasser, F.; Blanchet, B. Pharmacokinetic interaction involving sorafenib and the calcium-channel blocker felodipine in a patient with hepatocellular carcinoma. Investig. New Drugs 2011, 29, 1511–1514. [Google Scholar] [CrossRef]
| Antiangiogenic Treatment | Animal Model | Results | Reference |
|---|---|---|---|
| Sorafenib 40 mg/kg, 20 mg/kg, 5 mg/kg, and 1 mg/kg, orally | Male Sprague–Dawley and Wistar rats | Decreased liver fibrosis, reduced HSC proliferation, downregulation of cyclin D1 and cyclin-dependent kinase 4 and inhibition of the ERK and Akt phosphorylation. | Wang et al., 2010 |
| Sorafenib 4 mg/kg intragastrically once a day for four weeks | C57BL/6 (B6) mice | Attenuation of CCl4-induced chronic liver injury and fibrosis. | Deng et al., 2013 |
| Sorafenib 1.25, 5 or 7 mg/kg/day orally | Male Sprague–Dawley rats | Significant inhibition of liver fibrosis when administered concurrently with TAA. No significant effect on fibrosis when administered after established cirrhosis. | Hong et al., 2013 |
| Sorafenib 2.5 mg/kg/day, orally | Adult Sprague–Dawley rats | Restoration of mitochondrial function and reduction of collagen deposition in a NASH model. Upregulation of PGC1a and MMP9; reduction of TIMP1, TIMP2 mRNA, and IL-6, IL-10 protein. | Stefano et al., 2015 |
| Sorafenib 10, 15, and 30 mg/kg/ every 2 days | Male C57BL/6J mice | Significant reduction of HCC incidence and size in a model of NASH. Suppression of the pathological features of NASH, including hepatic steatosis, inflammation, and fibrosis. | Jian et al., 2020 |
| Sorafenib 10 mg/kg/day orally for 2 weeks | Male albino rats | Prevention of neoplastic changes in the liver with a decrease in size of hepatocellular foci. | El-Ashmawy et al., 2017 |
| Anti-VEGFR-2 (40 mg/kg i.p.) and Anti-PlGF (25mg/kg i.p.) antibodies | Ten-week-old C57BL/6 and homozygous db/db female mice | Prevention of NASH progression by decreasing steatosis and inflammation (anti-VEGFR-2). No effect of anti-PlGF on liver histology. Improvement of the liver vasculature by anti-VEGFR-2. | Coulon et al., 2013 |
| Brivanib (3 mg/kg/day), sorafenib (5 mg/kg/day), orally | Male Wistar rats | Significant decrease in plasma VEGF, FGF, PDGF, hepatic TNFα, IL-1b, IL-6, IL-17; decrease in hepatic leucocytes recruitment, microvascular density and hydroxyproline content; increased hepatic blood flow in NASH-cirrhotic rats. | Yang et al., 2014 |
| Ezetimibe 50 mg/kg orally | PtenΔhep mice (C57BL/6 background) | Blockade of the development of HCC by inhibiting cholesterol-mediated angiogenesis in PtenΔhep mice with hypercholesterolemia. Conversely, no inhibition of angiogenesis in PtenΔhep mice fed with the standard diet | Miura et al., 2019 |
| Berberine 250 mg/kg/day orally | C57BL/6J mice | Suppression of genes related to lipogenesis, inflammation, fibrosis, and angiogenesis. | Luo et al., 2019 |
| L1-10 4 mg/kg i.p. three-times weekly | C57BL/6 mice | Reduction of liver inflammation, balloon, and fibrosis in MCD-fed mice; reduction of angiogenic signaling in cultured endothelial cells. | Lefere et al., 2019 |
| 50% ME of Phyllanthus niruri (1000 mg/kg orally). | Sprague-Dawley rats | Attenuation of NAFLD with a preventive effect on fibrosis accompanied by the inhibition of VEGF production. | Al Zarzour et al., 2018 |
| ALS-L1023 (0.8%, w/w; orally) | C57BL/6J mice | Suppression of steatosis, infiltration of inflammatory cells, and accumulation of collagen in livers. Fewer CD68-positive macrophage numbers and lower expression of inflammatory cytokines. | Kim et al., 2017 |
| Sitagliptin 150 mg/kg/day, losartan 30 mg/kg/day orally, alone and in combination | Fischer 344 rats | Combined treatment suppressed hepatic fibrogenesis and carcinogenesis, with the suppression of HSC activation, neovascularization, and oxidative stress. | Okura et al., 2017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlandi, P.; Solini, A.; Banchi, M.; Brunetto, M.R.; Cioni, D.; Ghiadoni, L.; Bocci, G. Antiangiogenic Drugs in NASH: Evidence of a Possible New Therapeutic Approach. Pharmaceuticals 2021, 14, 995. https://doi.org/10.3390/ph14100995
Orlandi P, Solini A, Banchi M, Brunetto MR, Cioni D, Ghiadoni L, Bocci G. Antiangiogenic Drugs in NASH: Evidence of a Possible New Therapeutic Approach. Pharmaceuticals. 2021; 14(10):995. https://doi.org/10.3390/ph14100995
Chicago/Turabian StyleOrlandi, Paola, Anna Solini, Marta Banchi, Maurizia Rossana Brunetto, Dania Cioni, Lorenzo Ghiadoni, and Guido Bocci. 2021. "Antiangiogenic Drugs in NASH: Evidence of a Possible New Therapeutic Approach" Pharmaceuticals 14, no. 10: 995. https://doi.org/10.3390/ph14100995
APA StyleOrlandi, P., Solini, A., Banchi, M., Brunetto, M. R., Cioni, D., Ghiadoni, L., & Bocci, G. (2021). Antiangiogenic Drugs in NASH: Evidence of a Possible New Therapeutic Approach. Pharmaceuticals, 14(10), 995. https://doi.org/10.3390/ph14100995