Zebrafish as a Model System to Study the Mechanism of Cutaneous Wound Healing and Drug Discovery: Advantages and Challenges
Abstract
:1. Introduction
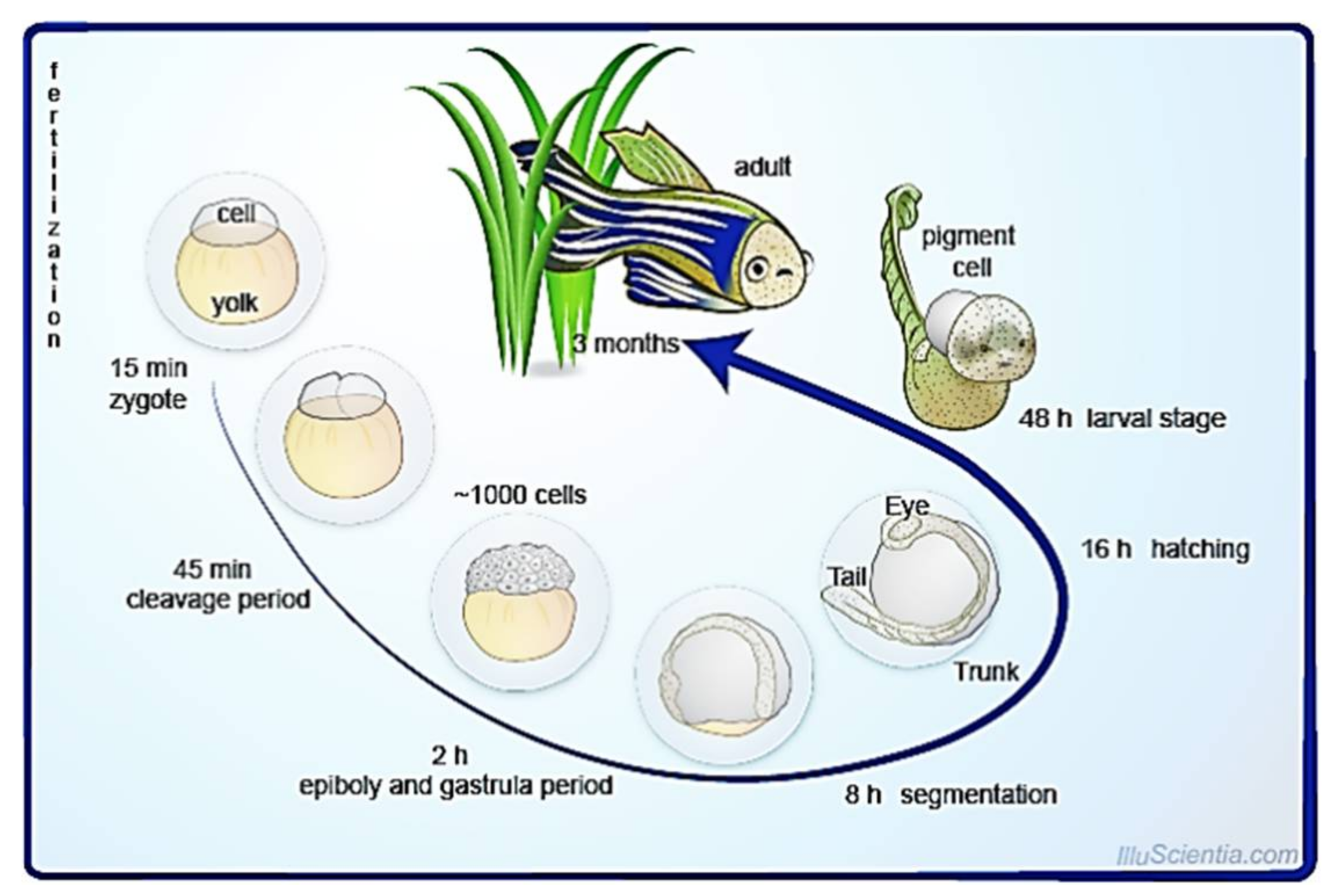
2. Biology of Zebrafish
2.1. Skin Structure of Zebrafish
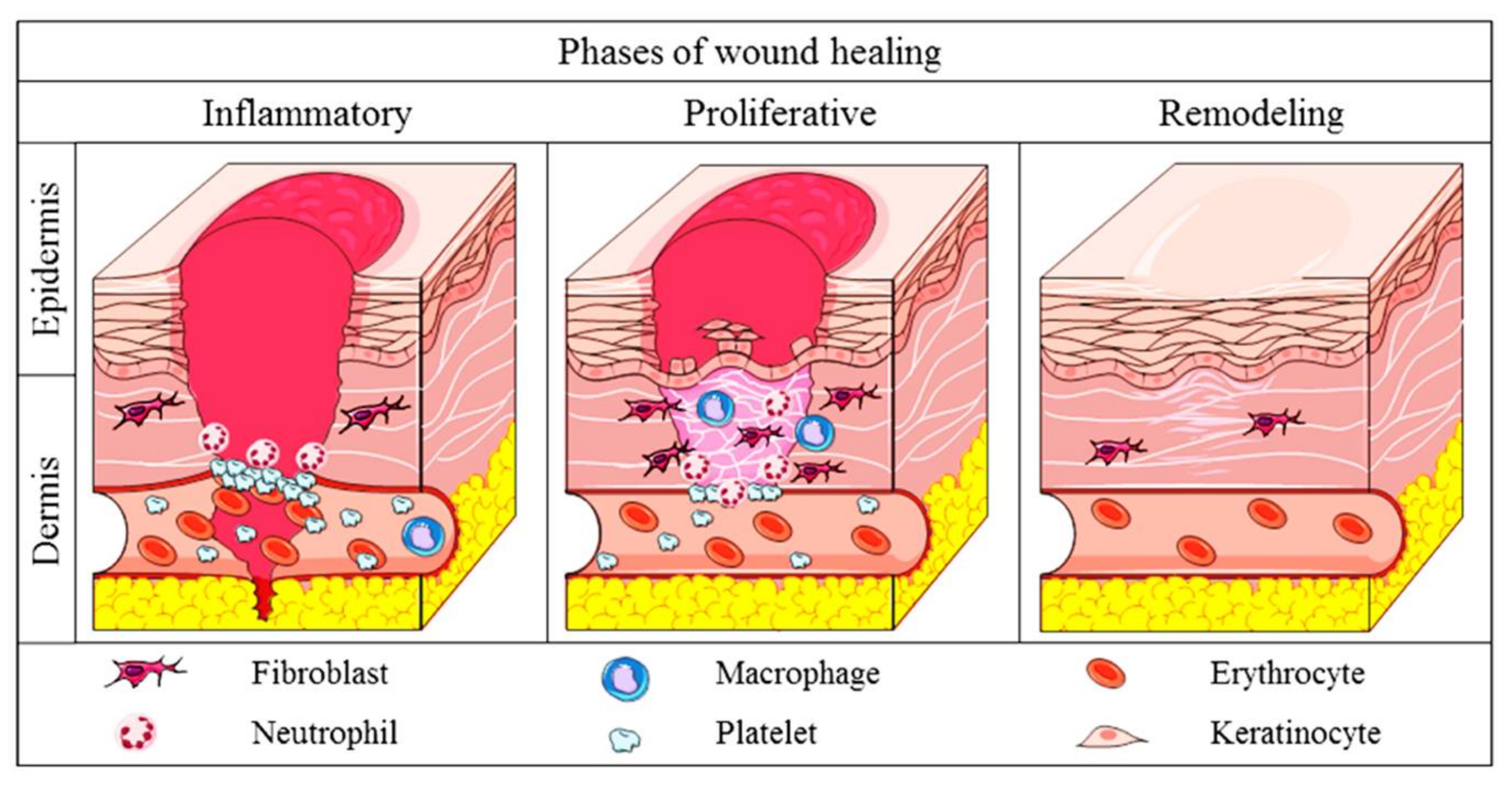
2.2. Physiology of Cutaneous Wound Healing in Mammals
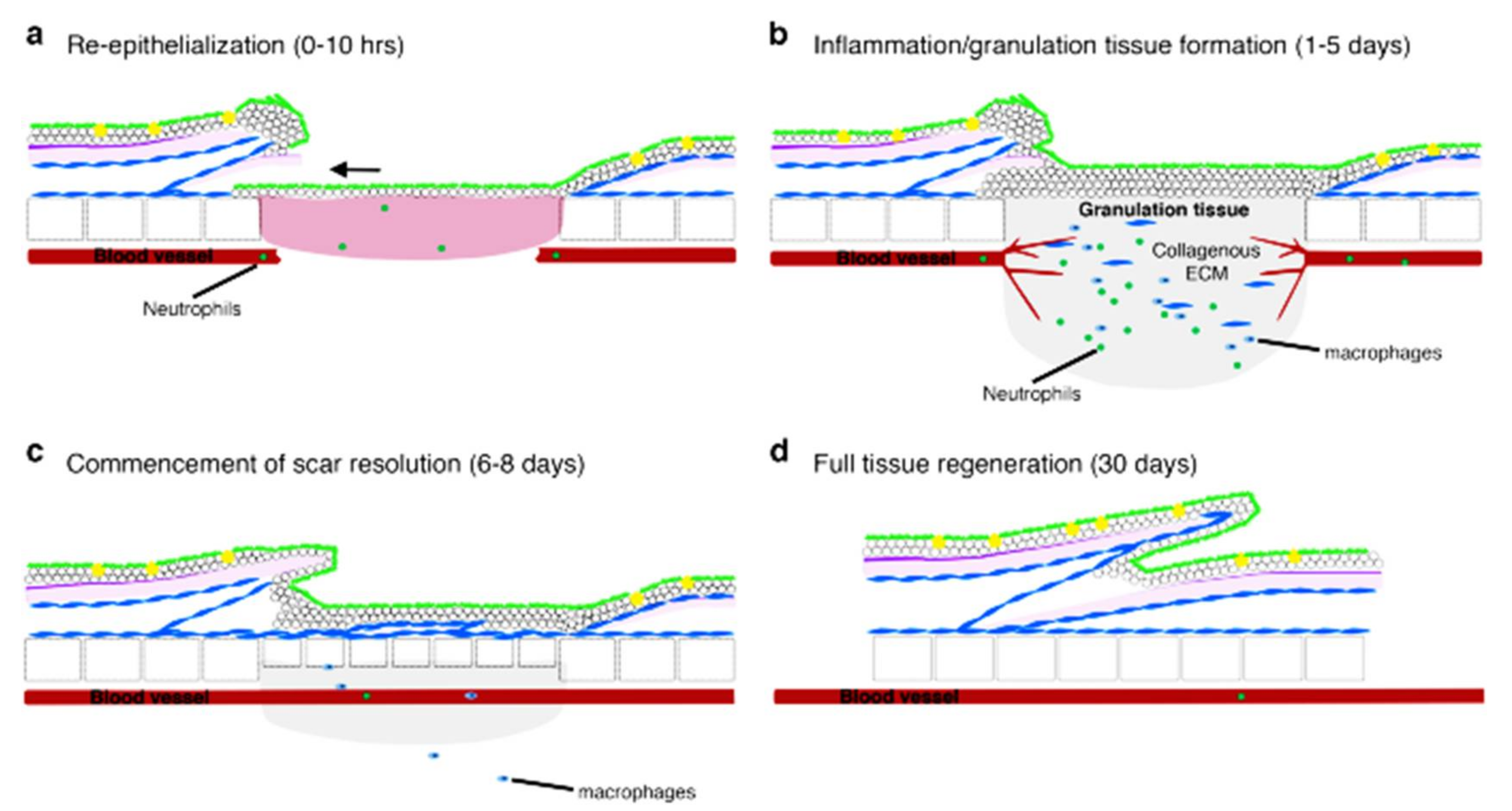
2.3. Comparison of Wound Healing: Mammals and Zebrafish
3. Zebrafish as a Model to Study the Mechanism of Cutaneous Wound Healing
Signaling Mechanism in Zebrafish Model for Wound Healing
4. Zebrafish as a Model for Drug Discovery in Cutaneous Wound Healing
4.1. Natural Compounds
4.2. Nanoparticles
4.3. Formulated Drugs
5. Advantages of Using Zebrafish as a Cutaneous Model in Wound Healing
5.1. External, Transparent, and Rapid Development
5.2. Large Number of Offspring and Ease of Breeding
5.3. Short Reproductive Cycle
5.4. The Ease of Maintenance of a Large Number of Species
5.5. Genetics of Zebrafish
5.6. Manipulation of Genome Activity
5.7. Ease of Drug Introduction
5.8. Test Samples for Molecular Biological Analysis
6. Challenges in Handling Zebrafish as a Wound-Healing Model
7. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naomi, R.; Fauzi, M.B. Cellulose/collagen dressings for diabetic foot ulcer: A review. Pharmaceutics 2020, 12, 881. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Deng, Z.; Xu, W.; Li, Y.; Xu, C.; Chen, H.; Shen, J. A novel dual-response chemosensor for bioimaging of Exogenous/Endogenous hypochlorite and hydrazine in living cells, Pseudomonas aeruginosa and zebrafish. Sens. Actuators B Chem. 2020, 321, 128450. [Google Scholar] [CrossRef]
- Arunachalam, M.; Raja, M.; Vijayakumar, C.; Malaiammal, P.; Mayden, R.L. Natural history of zebrafish (Danio rerio) in India. Zebrafish 2013, 10, 1–14. [Google Scholar] [CrossRef]
- Chen, H.; Ding, F.; Zhou, Z.; He, X.; Shen, J. FRET-based sensor for visualizing pH variation with colorimetric/ratiometric strategy and application for bioimaging in living cells, bacteria and zebrafish. Analyst 2020, 145, 4283–4294. [Google Scholar] [CrossRef] [PubMed]
- Veldman, M.B.; Lin, S. Zebrafish as a developmental model organism for pediatric research. Pediatr. Res. 2008, 64, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Beffagna, G. Zebrafish as a smart model to understand regeneration after heart injury: How fish could help humans. Front. Cardiovasc. Med. 2019, 6, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Ding, F.; Sun, X.; Zheng, Y.; Xu, W.; Ye, L.; Chen, H.; Shen, J. Renovated multifunctional colorimetric/fluorometric sensor for simultaneous detection, imaging of pH variance and antimicrobial therapies. Sens. Actuators B Chem. 2021, 332, 129496. [Google Scholar] [CrossRef]
- Zang, L.; Maddison, L.A.; Chen, W. Zebrafish as a model for obesity and diabetes. Front. Cell Dev. Biol. 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arjmand, B.; Tayanloo-Beik, A.; Foroughi Heravani, N.; Alaei, S.; Payab, M.; Alavi-Moghadam, S.; Goodarzi, P.; Gholami, M.; Larijani, B. Zebrafish for personalized regenerative medicine; a more predictive humanized model of endocrine disease. Front. Endocrinol. 2020, 11, 396. [Google Scholar] [CrossRef]
- Antinucci, P.; Hindges, R. A crystal-clear zebrafish for in vivo imaging. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hason, M.; Bartůněk, P. Zebrafish models of cancer—New insights on modeling human cancer in a non-mammalian vertebrate. Genes 2019, 10, 935. [Google Scholar] [CrossRef] [Green Version]
- Zang, L.; Shimada, Y.; Nishimura, N. Development of a novel zebrafish model for type 2 diabetes mellitus. Sci. Rep. 2017, 7, 1461. [Google Scholar] [CrossRef] [Green Version]
- Kanungo, J.; Cuevas, E.; Ali, S.F.; Paule, M.G. Zebrafish model in drug safety assessment. Curr. Pharm. Des. 2014, 20, 5416–5429. [Google Scholar] [CrossRef]
- Rubinstein, A.L. Zebrafish: From disease modeling to drug discovery. Curr. Opin. Drug Discov. Devel. 2003, 6, 218–223. [Google Scholar]
- Bruneel, B.; Mathä, M.; Paesen, R.; Ameloot, M.; Weninger, W.J.; Huysseune, A. Imaging the zebrafish dentition: From traditional approaches to emerging technologies. Zebrafish 2015, 12, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Costa, A.; Shepherd, I.T. Zebrafish development and genetics: Introducing undergraduates to developmental biology and genetics in a large introductory laboratory class. Zebrafish 2009, 6, 177. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.R. Zebrafish: Development of a vertebrate model organism. Curr. Protoc. Essent. Lab. Tech. 2018, 16, e19. [Google Scholar] [CrossRef] [Green Version]
- Pathak, N.H.; Barresi, M.J.F. Zebrafish as a model to understand vertebrate development. In The Zebrafish in Biomedical Research: Biology, Husbandry, Diseases, and Research Applications; Cartner, S.C., Eisen, J.S., Farmer, S.C., Guillemin, K.J., Kent, M.L., Sanders, G.E., Eds.; Academic Press: Birmingham, AL, USA, 2020; pp. 559–591. [Google Scholar]
- Zebrafish Cycle. Available online: https://commons.wikimedia.org/wiki/File:Zebrafish_Cycle.png#filelinks (accessed on 2 August 2021).
- Le Guellec, D.; Morvan-Dubois, G.; Sire, J.Y. Skin development in bony fish with particular emphasis on collagen deposition in the dermis of the zebrafish (Danio rerio). Int. J. Dev. Biol. 2004, 48, 217–231. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Uitto, J. Zebrafish as a model system to study skin biology and pathology. J. Investig. Dermatol. 2014, 134, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Frank, M.; Thisse, C.; Thisse, B.; Uitto, J. Zebrafish: A model system to study heritable skin diseases. J. Investig. Dermatol. 2010, 131, 571. [Google Scholar] [CrossRef] [Green Version]
- Naomi, R.; Ratanavaraporn, J.; Fauzi, M.B. Comprehensive review of hybrid collagen and silk fibroin for cutaneous wound healing. Materials 2020, 13, 3097. [Google Scholar] [CrossRef]
- Wallace, H.A.; Zito, P.M. Wound Healing Phases; StatPearls: Bethesda, MD, USA, 2019. [Google Scholar]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin acute wound healing: A comprehensive review. Int. J. Inflam. 2019, 2019, 1–15. [Google Scholar] [CrossRef]
- Gushiken, L.F.S.; Beserra, F.P.; Bastos, J.K.; Jackson, C.J.; Pellizzon, C.H. Cutaneous wound healing: An update from physiopathology to current therapies. Life 2021, 11, 665. [Google Scholar] [CrossRef]
- Rosowski, E.E. Determining macrophage versus neutrophil contributions to innate immunity using larval zebrafish. Dis. Model. Mech. 2020, 13, dmm041889. [Google Scholar] [CrossRef] [Green Version]
- Richardson, R.J. Parallels between vertebrate cardiac and cutaneous wound healing and regeneration. NPJ Regen. Med. 2018, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Rousselle, P.; Montmasson, M.; Garnier, C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2019, 75, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.A.; Power, D.M. Skin and scale regeneration after mechanical damage in a teleost. Mol. Immunol. 2018, 95, 73–82. [Google Scholar] [CrossRef] [PubMed]
- LeBert, D.C.; Huttenlocher, A. Inflammation and wound repair. Semin. Immunol. 2014, 26, 320. [Google Scholar] [CrossRef]
- Richardson, R.; Hammerschmidt, M. The role of Rho kinase (Rock) in re-epithelialization of adult zebrafish skin wounds. Small GTPases 2016, 9, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.; Slanchev, K.; Kraus, C.; Knyphausen, P.; Eming, S.; Hammerschmidt, M. Adult zebrafish as a model system for cutaneous wound-healing research. J. Invest Dermatol. 2013, 133, 1655–1665. [Google Scholar] [CrossRef] [Green Version]
- Nikaido, M.; Subkhankulova, T.; Uroshlev, L.A.; Kasianov, A.J.; Sosa, K.C.; Bavister, G.; Yang, X.; Rodrigues, F.S.L.M.; Carney, T.J.; Schwetlick, H.; et al. Zebrafish pigment cells develop directly from persistent highly multipotent progenitors. bioRxiv 2021. [Google Scholar] [CrossRef]
- Lisse, T.S.; King, B.L.; Rieger, S. Comparative transcriptomic profiling of hydrogen peroxide signaling networks in zebrafish and human keratinocytes: Implications toward conservation, migration and wound healing. Sci. Rep. 2016, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Seo, S.B.; Dananjaya, S.H.S.; Nikapitiya, C.; Park, B.K.; Gooneratne, R.; Kim, T.-Y.; Lee, J.; Kim, C.-H.; De Zoysa, M. Silver nanoparticles enhance wound healing in zebrafish (Danio rerio). Fish Shellfish Immunol. 2017, 68, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Caraguel, F.; Bessonov, N.; Demongeot, J.; Dhouailly, D.; Volpert, V. Wound healing and scale modelling in zebrafish. Acta Biotheor. 2016, 64, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.Y.; Liu, Y.; Shan, L.T.; Xu, Y.Q.; Liang, J.; Lai, Y.H.; Hsiao, C.D. Evaluation of collagen mixture on promoting skin wound healing in zebrafish caused by acetic acid administration. Biochem. Biophys. Res. Commun. 2018, 505, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S.; Pichu, S.; Pankajam, T.; Dharanibalan, K.; Djonov, V.; Chatterjee, S. Nitric oxide regulates intussusceptive-like angiogenesis in wound repair in chicken embryo and transgenic zebrafish models. Nitric Oxide Biol. Chem. 2018, 82, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Noishiki, C.; Yuge, S.; Ando, K.; Wakayama, Y.; Mochizuki, N.; Ogawa, R.; Fukuhara, S. Live imaging of angiogenesis during cutaneous wound healing in adult zebrafish. Angiogenes 2019, 22, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Wu, J.Q.; Fan, R.Y.; He, Z.H.; Li, C.Y.; He, M.F. Isoliquiritin promote angiogenesis by recruiting macrophages to improve the healing of zebrafish wounds. Fish Shellfish Immunol. 2020, 100, 238–245. [Google Scholar] [CrossRef]
- Edirisinghe, S.L.; Rajapaksha, D.C.; Nikapitiya, C.; Oh, C.; Lee, K.A.; Kang, D.H.; De Zoysa, M. Spirulina maxima derived marine pectin promotes the in vitro and in vivo regeneration and wound healing in zebrafish. Fish Shellfish Immunol. 2020, 107, 414–425. [Google Scholar] [CrossRef]
- Comte, A.; Roux, J.; Robinson-Rechavi, M. Molecular signaling in zebrafish development and the vertebrate phylotypic period. Evol. Dev. 2010, 12, 156. [Google Scholar] [CrossRef] [Green Version]
- Hameedaldeen, A.; Liu, J.; Batres, A.; Graves, G.S.; Graves, D.T. FOXO1, TGF-β eegulation and wound healing. Int. J. Mol. Sci. 2014, 15, 16257–16269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, D.Y.; Hutcheon, A.E.K.; Zieske, J.D.; Guo, X. Epidermal growth factor stimulates transforming growth factor-beta receptor type ii expression in corneal epithelial cells. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purba, E.R.; Saita, E.; Maruyama, I.N. Activation of the EGF receptor by ligand binding and oncogenic mutations: The “rotation model”. Cells 2017, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, R.; Metzger, M.; Knyphausen, P.; Ramezani, T.; Slanchev, K.; Kraus, C.; Schmelzer, E.; Hammerschmidt, M. Re-epithelialization of cutaneous wounds in adult zebrafish combines mechanisms of wound closure in embryonic and adult mammals. Development 2016, 143, 2077–2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.L.; Tsai, Y.C.; Korivi, M.; Chang, C.T.; Hseu, Y.C. Lucidone Promotes the cutaneous wound healing process via activation of the PI3K/AKT, Wnt/β-catenin and NF-κB signaling pathways. Biochim. Biophys. Acta-Mol. Cell Res. 2017, 1864, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Chávez, M.N.; Aedo, G.; Fierro, F.A.; Allende, M.L.; Egaña, J.T. Zebrafish as an emerging model organism to study angiogenesis in development and regeneration. Front. Physiol. 2016, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of zebrafish in drug discovery toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Meijer, A.H.; Schaaf, M.J.M. Modeling inflammation in zebrafish for the development of anti-inflammatory drugs. Front. Cell Dev. Biol. 2020, 8, 1819. [Google Scholar] [CrossRef]
- He, M.; Halima, M.; Xie, Y.; Schaaf, M.J.M.; Meijer, A.H.; Wang, M. Ginsenoside Rg1 acts as a selective glucocorticoid receptor agonist with anti-inflammatory action without affecting tissue regeneration in zebrafish larvae. Cells 2020, 9, 1107. [Google Scholar] [CrossRef]
- Naomi, R.; Ridzuan, P.M.; Bahari, H. Current insights into collagen type, I. Polymers 2021, 13, 2642. [Google Scholar] [CrossRef]
- De Oliveira, S.; Rosowski, E.E.; Huttenlocher, A. Neutrophil migration in infection and wound repair: Going forward in reverse. Nat. Rev. Immunol. 2016, 16, 378–391. [Google Scholar] [CrossRef] [Green Version]
- Sung, W.-N.; Kwok, H.-H.; Rhee, M.-H.; Yue, P.Y.-K.; Wong, R.N.-S. Korean red ginseng extract induces angiogenesis through activation of glucocorticoid receptor. J. Ginseng Res. 2017, 41, 486. [Google Scholar] [CrossRef]
- Raghupathy, S.; Vaidyanathan, L.; Sivaswamy, L.T.S. Adult zebrafish model of wound inflammation to study wound healing potency of curcuma longa extracts. Annu. Res. Rev. Biol. 2017, 18, 1–8. [Google Scholar] [CrossRef]
- Ansari, S.; Jilani, S.; Abbasi, H.; Hashimi, A.; Ahmed, Y.; Khatoon, R.; Rifas, A.L. Curcuma longa: A treasure of medicinal properties. Cell Orthocellular Med. Pharm. Assoc. 2020, 10, 9.1–9.7. [Google Scholar]
- Salehi, B.; Rodrigues, C.F.; Peron, G.; Dall’Acqua, S.; Sharifi-Rad, J.; Azmi, L.; Shukla, I.; Baghel, U.S.; Mishra, A.P.; Elissawy, A.M.; et al. Curcumin nanoformulations for antimicrobial and wound healing purposes. Phyther. Res. 2021, 35, 2487–2499. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, M.; Kang, M.C.; Lee, H.H.L.; Cho, C.H.; Choi, I.; Park, Y.; Lee, S.H. Antioxidant effects of turmeric leaf extract against hydrogen peroxide-induced oxidative stress in vitro in vero cells and in vivo in zebrafish. Antioxidants 2021, 10, 112. [Google Scholar] [CrossRef]
- Chylińska-Wrzos, P.; Lis-Sochocka, M.; Jodłowska-Jędrych, B. Use of propolis in difficult to heal diabetic wounds. Short review. Pol. J. Public Health 2017, 127, 173–175. [Google Scholar] [CrossRef] [Green Version]
- Wibowo, I.; Utami, N.; Anggraeni, T.; Barlian, A.; Putra, R.E.; Indriani, A.D.; Masadah, R.; Ekawardhani, S. Propolis can improve caudal fin regeneration in zebrafish (Danio rerio) induced by the combined administration of Alloxan and glucose. Zebrafish 2021, 18, 274–281. [Google Scholar] [CrossRef]
- Pensado-López, A.; Fernández-Rey, J.; Reimunde, P.; Crecente-Campo, J.; Sánchez, L.; Andón, F.T. Zebrafish models for the safety and therapeutic testing of nanoparticles with a focus on macrophages. Nanomaterials 2021, 11, 1784. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nachtergael, A.; Nguyen, T.M.; Cornet, V.; Duez, P.; Muller, M.; Huong, D.T.L.; Kestemont, P. Anti-inflammatory properties of the ethanol extract from Clerodendrum cyrtophyllum Turcz based on in vitro and in vivo studies. J. Ethnopharmacol. 2020, 23, 112739. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Le, H.D.; Kim, T.N.T.; The, H.P.; Nguyen, T.M.; Cornet, V.; Lambert, J.; Kestemont, P. Anti–Inflammatory and antioxidant properties of the ethanol extract of Clerodendrum cyrtophyllum Turcz in copper sulfate-induced inflammation in zebrafish. Antioxidants 2020, 9, 192. [Google Scholar] [CrossRef] [Green Version]
- Yasukawa, K.; Okuno, T.; Yokomizo, T. Eicosanoids in skin wound healing. Int. J. Mol. Sci. 2020, 21, 8435. [Google Scholar] [CrossRef]
- Bieren, J.E. Eicosanoids in tissue repair. Immunol. Cell Biol. 2019, 97, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [Green Version]
- Khansa, I.; Schoenbrunner, A.R.; Kraft, C.T.; Janis, J.E. Silver in wound care—Friend or foe?: A comprehensive review. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2390. [Google Scholar] [CrossRef]
- Kurahashi, T.; Fujii, J. Roles of antioxidative enzymes in wound healing. J. Dev. Biol. 2015, 3, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, H.; Silverton, S.; Debolt, K.; Shapiro, I. Superoxide dismutase and catalase activities in the growth cartilage: Relationship between oxidoreductase activity and chondrocyte maturation. J. Bone Miner. Res. 1991, 6, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Gao, Y.; Wang, F.; Wang, Y.; Cao, M.; Zhang, W.; Liang, Y.; Song, M.; Jiang, G. Toxicity of silver nanoparticles on wound healing: A case study of zebrafish fin regeneration model. Sci. Total Environ. 2020, 717, 137178. [Google Scholar] [CrossRef] [PubMed]
- Uthaman, A.; Lal, H.M.; Thomas, S. Fundamentals of silver nanoparticles and their toxicological aspects. In Polymer Nanocomposites Based on Silver Nanoparticles: Synthesis, Characterization and Applications; Li, T., Maria, H.J., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–24. ISBN 978-3-030-44259-0. [Google Scholar]
- Rajapaksha, D.C.; Edirisinghe, S.L.; Nikapitiya, C.; Dananjaya, S.; Kwun, H.J.; Kim, C.H.; Oh, C.; Kang, D.H.; De Zoysa, M. Spirulina maxima derived pectin nanoparticles enhance the immunomodulation, stress tolerance, and wound healing in zebrafish. Mar. Drugs 2020, 18, 556. [Google Scholar] [CrossRef] [PubMed]
- Sharif, F.; Steenbergen, P.J.; Metz, J.R.; Champagne, D.L. Long-lasting effects of dexamethasone on immune cells and wound healing in the zebrafish. Wound Repair Regen. 2015, 23, 855–865. [Google Scholar] [CrossRef]
- Charafeddine, R.A.; Nosanchuk, J.D.; Sharp, D.J. Targeting microtubules for wound repair. Adv. Wound Care 2016, 5, 444–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilgus, T.A.; Roy, S.; McDaniel, J.C. Neutrophils and wound repair: Positive actions and negative reactions. Adv. Wound Care 2013, 2, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckman, S. Analyzing Wound Healing in Zebrafish Embryos. Available online: https://www.biotek.com/resources/application-notes/analyzing-wound-healing-in-zebrafish-embryos/ (accessed on 14 August 2021).
- Keenan, S.R.; Currie, P.D. The developmental phases of zebrafish myogenesis. J. Dev. Biol. 2019, 7, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, M.S.; Borrelli, M.R.; Hong, W.X.; Malhotra, S.; Cheung, A.T.M.; Ransom, R.C.; Rennert, R.C.; Morrison, S.D.; Lorenz, H.P.; Longaker, M.T. Embryonic skin development and repair. Organogenesis 2017, 14, 46–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adatto, I.; Lawrence, C.; Thompson, M.; Zon, L.I. A new system for the rapid collection of large numbers of developmentally staged zebrafish embryos. PLoS ONE 2011, 6, 1–7. [Google Scholar] [CrossRef]
- Ribas, L.; Piferrer, F. The zebrafish (Danio rerio) as a model organism, with emphasis on applications for finfish aquaculture research. Rev. Aquac. 2014, 6, 209–240. [Google Scholar] [CrossRef]
- Avdesh, A.; Chen, M.; Martin-Iverson, M.T.; Mondal, A.; Ong, D.; Rainey-Smith, S.; Taddei, K.; Lardelli, M.; Groth, D.M.; Verdile, G.; et al. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: An introduction. J. Vis. Exp. 2012, 4196. [Google Scholar] [CrossRef] [Green Version]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Lardelli, M. Using zebrafish in human disease research: Some advantages, disadvantages and ethical considerations. In Proceedings of the 2008 ANZCCART Conference, Auckland, New Zealand, 29 June–1 July 2008; pp. 23–28. [Google Scholar]
- Deonarine, K.; Panelli, M.C.; Stashower, M.E.; Jin, P.; Smith, K.; Slade, H.B.; Norwood, C.; Wang, E.; Marincola, F.M.; Stroncek, D.F. Gene expression profiling of cutaneous wound healing. J. Transl. Med. 2007, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Fadool, J.M.; Dowling, J.E. Zebrafish: A model system for the study of eye genetics. Prog. Retin. Eye Res. 2008, 27, 110. [Google Scholar] [CrossRef] [Green Version]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef]
- Gut, P.; Reischauer, S.; Stainier, D.Y.R.; Arnaout, R. Little fish, big data: Zebrafish as a model for cardiovascular and metabolic disease. Physiol. Rev. 2016, 97, 889–938. [Google Scholar] [CrossRef]
- Yuan, S.; Sun, Z. Microinjection of mRNA and morpholino antisense oligonucleotides in zebrafish embryos. J. Vis. Exp. 2009, 1113. [Google Scholar] [CrossRef] [Green Version]
- D’Amora, M.; Giordani, S. The utility of zebrafish as a model for screening developmental neurotoxicity. Front. Neurosci. 2018, 12, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Hofsten, J.; Olsson, P.-E. Zebrafish sex determination and differentiation: Involvement of FTZ-F1 genes. Reprod. Biol. Endocrinol. 2005, 3, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engeland, C.G.; Sabzehei, B.; Marucha, P.T. Sex hormones and mucosal wound healing. Brain. Behav. Immun. 2009, 23, 635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruneel, B.; Witten, P.E. Power and challenges of using zebrafish as a model for skeletal tissue imaging. Connect. Tissue Res. 2015, 56, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Champagne, D.L.; Spaink, H.P.; Richardson, M.K. Zebrafish embryos and larvae: A new generation of disease models and drug screens. Birth Defects Res. 2011, 93, 115–133. [Google Scholar] [CrossRef]
| Author | Aim | Strain | Type of Wound | Age | Follow Up Duration | Observation | Signaling Pathway Involved |
|---|---|---|---|---|---|---|---|
| Richardson et al. (2013) [34] | To demonstrate adult zebrafish as a model for cutaneous wounds | Tg(krt4:egfp)gz7, Tg(mpx:GFP)i114, Tg(lyz:;EGFP) nz117, Tg(lyz:dsRED2)nz50, Tg(fli1a:EGFP)y1, Tg(kdrl:HSRAS:mCherry) s896, and Tg(hsp70l:dnfgfr1-EGFP)pd1 | Full thickness | 6–12 months | 4 h–24 days |
| Transgenic inhibition of FGF signaling. |
| Lisse et al. (2016) [36] | To access the effectiveness of H2O2 in epidermal wound healing | Nacre | Epidermal | Not specified | 0.5 h–4 days |
| Activation of EGF, FOXO1, and IKKα pathways. |
| Caraguel et al. (2016) [38] | To develop a differential approach in wound-healing modelling | Danio rerio | Full thickness | Not specified | 2 h–14 days |
| Activation of EGF pathway. |
| Richardson et al. (2016) [48] | To study the underlying mechanism of cutaneous wound closure | TL, edarz3R367W, Tg(actb2:hras-egfp)vu119, Tg(krt4:egfp)gz7, Tg(hsp70l:EGFP), and Tg(hsp70l:dnfgfr1-EGFP)pd1 | Partial and full thickness | 6–12 months | 30 min to 4 days |
| Regulation of TGFβ/integrin- and Rock/JNK pathway. |
| Richardson et al. (2016) [33] | To study the role of Rho kinase (Rock) in cutaneous wound healing | Adult transgenic zebrafish | Partial and full thickness | 6–12 months | 1 h to 15 days |
| Activation of Rock pathway for the rapid re-epithelialization process. |
| Seo et al. (2017) [37] | To study the efficacy of silver nanoparticles (AgNP) for wound healing | Wild-type Danio rerio | Epidermal | 4 months | 2–20 days |
| Not specified. |
| Xiong et al. (2018) [39] | To study the effectiveness of a collagen mixture in wound healing | Wild-type AB strain | Full thickness | Not specified | 2–5 days |
| Inhibition of TNF-mediated leukocyte chemotaxis. |
| Vimalraj et al. (2018) [40] | To analyze the role of nitric oxide in wound healing | Adult Tie2-GFP transgenic Zebrafish | Full thickness | 8–10 months | 48 h–14 days |
| Upregulation of the Wnt/β-catenin pathway. |
| Noishiki et al. (2019) [41] | To demonstrate the angiogenesis mechanism during cutaneous wound healing | Tg(kdrl:eGFP)s843, Tg(gata1:DsRed)sd2, and Tg(fli1a:mCherry)ncv501 | Partial thickness | Not specified | 2 days–2 months |
| Activation of VEGF signaling pathway. |
| Liu et al. (2020) [42] | To investigate the role of isoliquiritin in angiogenesis during wound healing | Tg(fli-1:EGFP), and Tg(mpeg:mCherry) | Full thickness | 6 months | Day 1–the 15th day |
| Downregulation of VEGFR tyrosine kinase inhibitor II pathway. |
| Edirisinghe et al. (2020) [43] | To scrutinize the ability of Spirulina maxima in wound healing | Wild-type AB | Full thickness | 4 months | Day 1–day 10 |
| Upregulation of the Wnt/β-catenin pathway. |
| Agent | Model | Treatment Mode | Findings | Reference |
|---|---|---|---|---|
| Natural products | ||||
| Panax ginseng | Tail fin amputation in zebrafish larva | Incubation medium |
| [53] |
| Panax ginseng | Zebrafish embryos | Incubation medium |
| [56] |
| Curcuma longa | Caudal fin transection in adult zebrafish | Topical application |
| [57] |
| Ethanol extract Propolis (Trigona laeviceps)(EEP) | Caudal fin amputation in hyperglycemia model (induced by alloxan and glucose) in adult zebrafish | Water immersion |
| [62] |
| Nanoparticles | ||||
| Silver nanoparticles | Caudal fin regeneration model in adult 3-month-old zebrafish | Water immersion |
| [72] |
| Silver nanoparticles | Laser-induced wound injury, posterior to the gill area in adult zebrafish | Water immersion and direct skin application |
| [37] |
| Spirulina maxima-derived pectin nanoparticles (SmPNPs) | Laser-induced wound at the left flank in adult zebrafish | Direct skin application |
| [74] |
| Spirulina maxima-derived marine pectin (Smp) | Fin regeneration model in zebrafish larvae; laser-induced wound in adult zebrafish | Topical application |
| [43] |
| Formulated drug | ||||
| Nocadazole | Caudal fin amputation in zebrafish embryos | Incubation medium |
| [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naomi, R.; Bahari, H.; Yazid, M.D.; Embong, H.; Othman, F. Zebrafish as a Model System to Study the Mechanism of Cutaneous Wound Healing and Drug Discovery: Advantages and Challenges. Pharmaceuticals 2021, 14, 1058. https://doi.org/10.3390/ph14101058
Naomi R, Bahari H, Yazid MD, Embong H, Othman F. Zebrafish as a Model System to Study the Mechanism of Cutaneous Wound Healing and Drug Discovery: Advantages and Challenges. Pharmaceuticals. 2021; 14(10):1058. https://doi.org/10.3390/ph14101058
Chicago/Turabian StyleNaomi, Ruth, Hasnah Bahari, Muhammad Dain Yazid, Hashim Embong, and Fezah Othman. 2021. "Zebrafish as a Model System to Study the Mechanism of Cutaneous Wound Healing and Drug Discovery: Advantages and Challenges" Pharmaceuticals 14, no. 10: 1058. https://doi.org/10.3390/ph14101058
APA StyleNaomi, R., Bahari, H., Yazid, M. D., Embong, H., & Othman, F. (2021). Zebrafish as a Model System to Study the Mechanism of Cutaneous Wound Healing and Drug Discovery: Advantages and Challenges. Pharmaceuticals, 14(10), 1058. https://doi.org/10.3390/ph14101058







