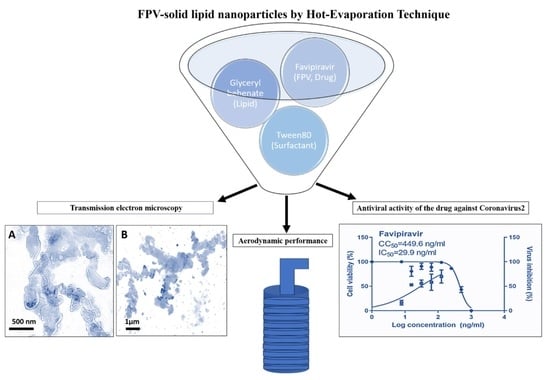
Physicochemical Characteristics and In Vitro Toxicity/Anti-SARS-CoV-2 Activity of Favipiravir Solid Lipid Nanoparticles (SLNs)
Abstract
:1. Introduction
2. Results and Discussion
2.1. FPV-SLN Formulation Physical-Chemical Characterization
2.1.1. Characterization of FPV-SLNs
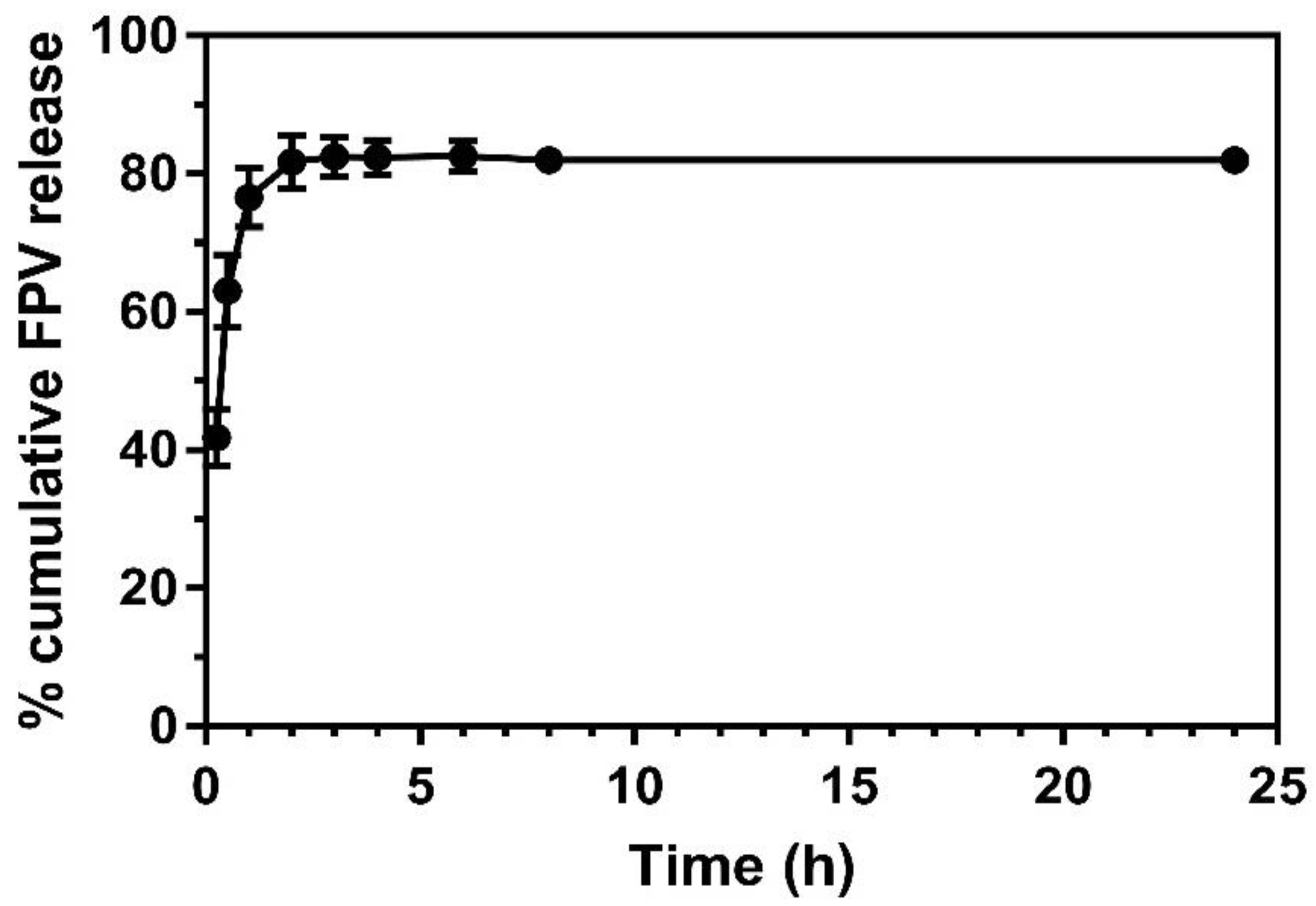
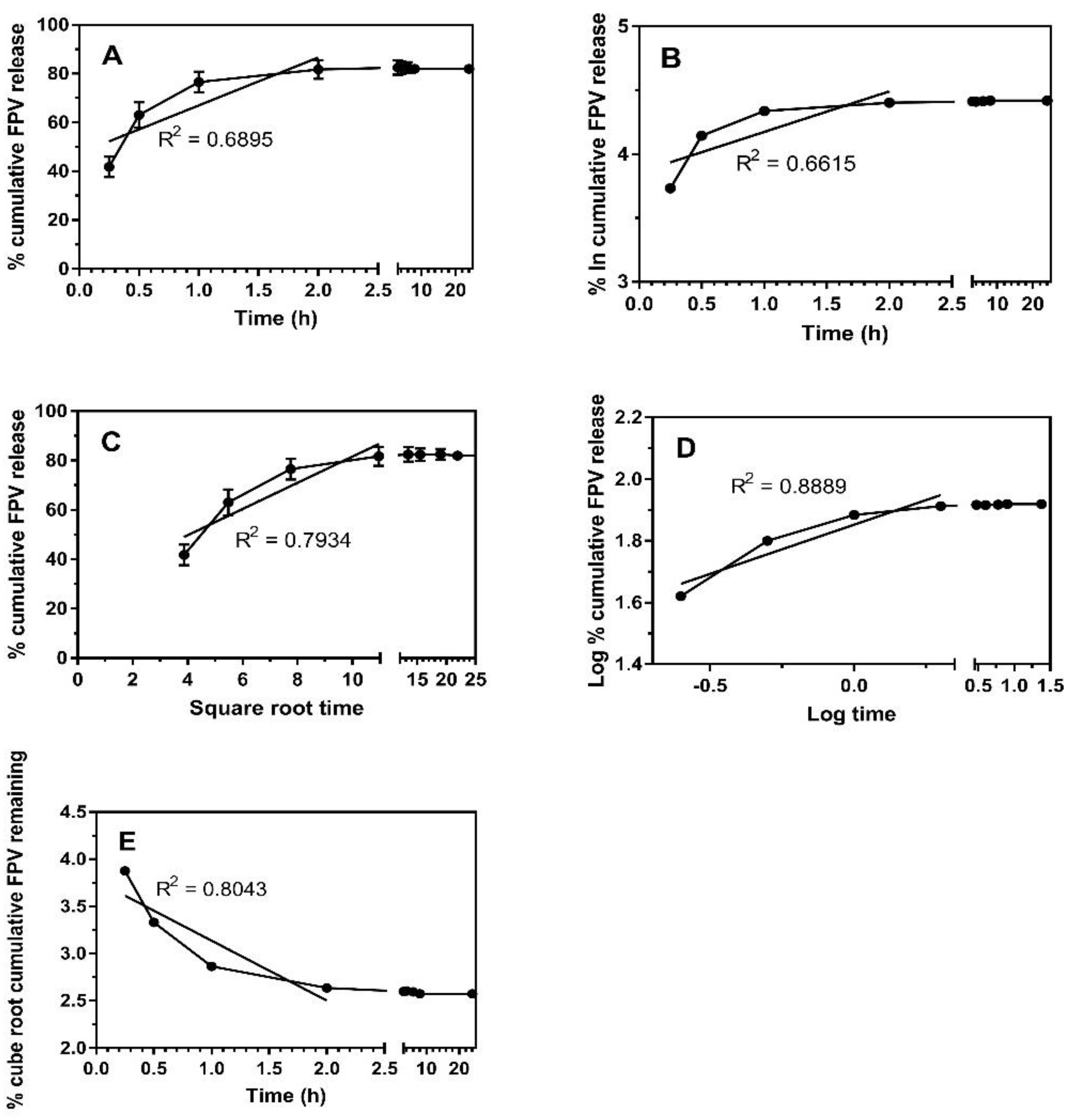
2.1.2. FPV-SLNs’ Drug Release Profile
2.1.3. Fourier Transform Infrared Spectroscopy (FTIR)
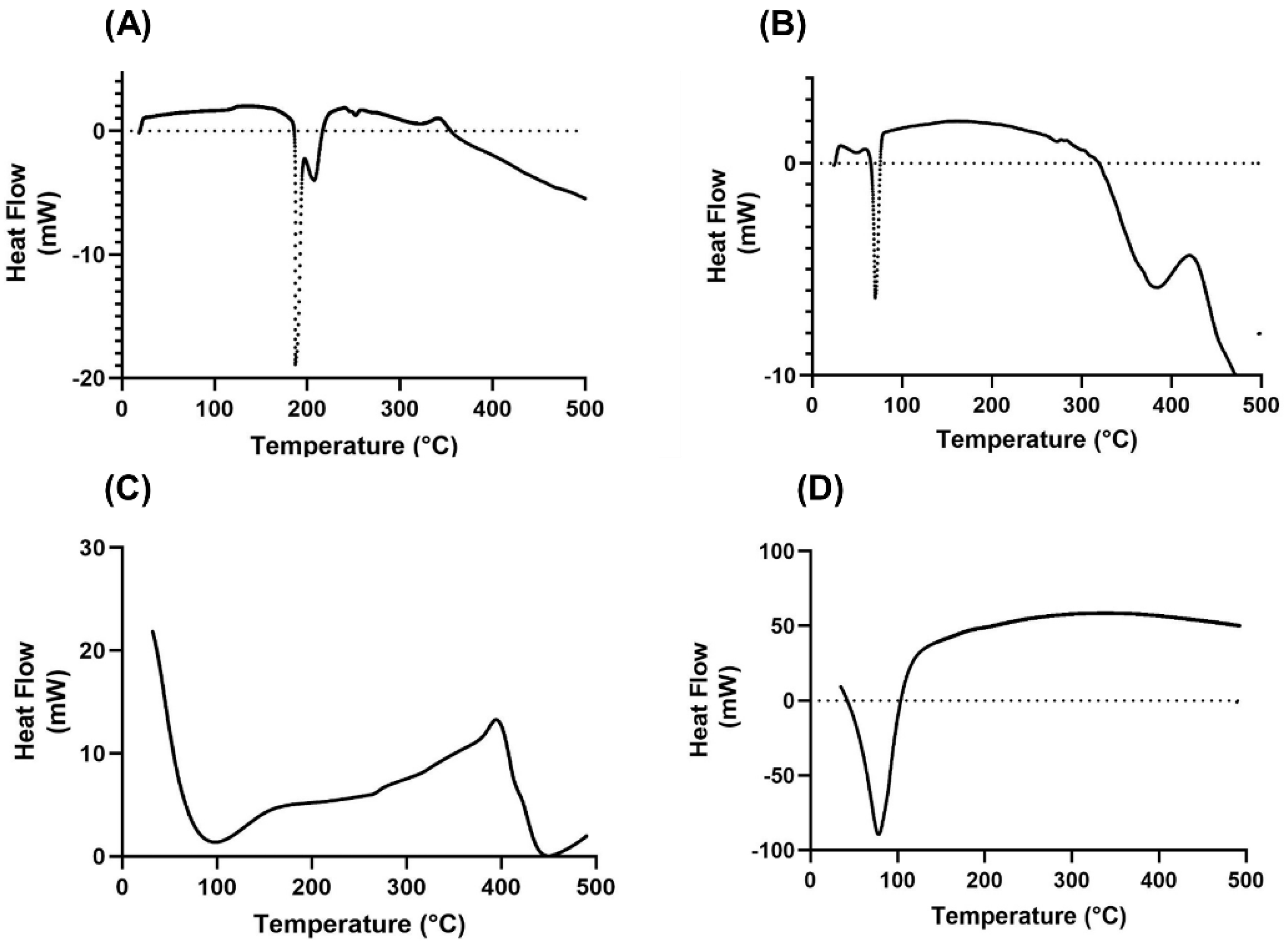
2.1.4. Thermal Properties Analysis
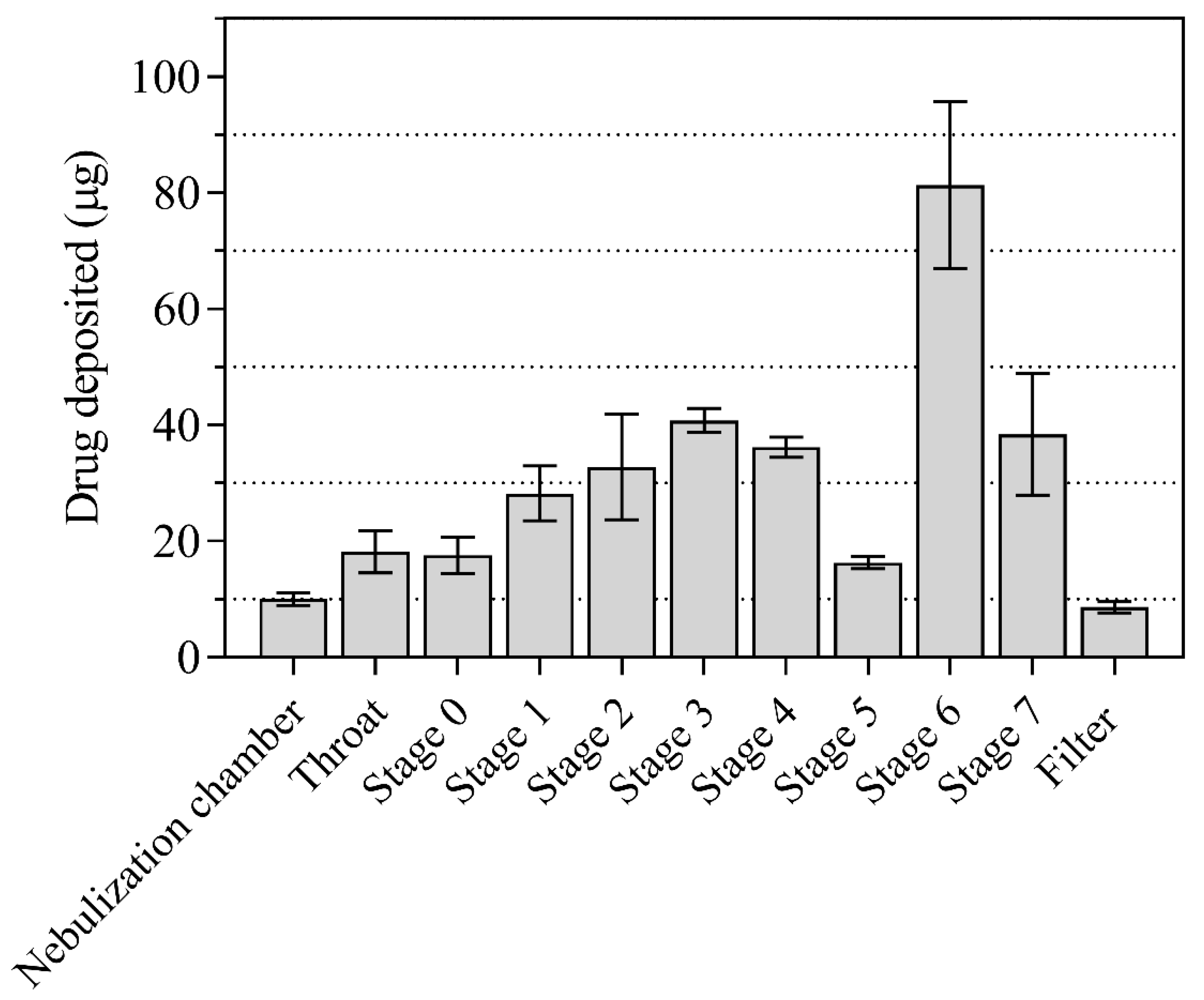
2.1.5. In Vitro Aerodynamic Evaluation of Nebulized FPV-SLN Formulation
2.2. In Vitro Bio-Characterization
Cytotoxicity and FPV-SLNs Anti-Viral Activity
3. Materials and Methods
3.1. Materials
3.2. Formulation of the Favipiravir Solid Lipid Nanoparticles Formulation (FPV-SLNs)
3.3. Particle Characterization of FPV-SLN Formulations
3.3.1. Zeta Potential, Particle Size, and Polydispersity Index Analysis
3.3.2. Measurement of Encapsulation Efficiency
3.3.3. Transmission Electron Microscopy (TEM)
3.3.4. In Vitro FPV Release from the Nanoparticles
3.3.5. Fourier Transform Infrared Spectroscopy (FTIR)
3.3.6. Thermal Properties Analysis
3.3.7. High-Performance Liquid Chromatography (HPLC)
3.3.8. In Vitro Aerosol Performance of Nebulized FPV-SLN Using ACI
3.4. In Vitro Bio-Characterization
3.4.1. The FPV-SLNs Cytotoxicity Assay on Vero-E6 Cells
3.4.2. Inhibitory Concentration 50 (IC50) Determination
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO—World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report-61. 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ (accessed on 22 March 2020).
- Richardson, P.; Griffin, I.; Tucker, C.; Smith, D.; Oechsle, O.; Phelan, A.; Rawling, M.; Savory, E.; Stebbing, J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020, 395, e30–e31. [Google Scholar] [CrossRef] [Green Version]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.; Di Napoli, R. Features, evaluation and treatment of coronavirus (COVID-19). StatPearls 2021. [Google Scholar]
- Jiang, F.; Deng, L.; Zhang, L.; Cai, Y.; Cheung, C.W.; Xia, Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). J. Gen. Intern. Med. 2020, 35, 1545–1549. [Google Scholar] [CrossRef] [Green Version]
- Sahakijpijarn, S.; Moon, C.; Warnken, Z.N.; Maier, E.Y.; DeVore, J.E.; Christensen, D.J.; Koleng, J.J.; Williams, R.O. In Vivo pharmacokinetic study of remdesivir dry powder for inhalation in hamsters. Int. J. Pharm. X 2021, 3, 100073. [Google Scholar] [CrossRef]
- Lin, H.X.J.; Cho, S.; Aravamudan, V.M.; Sanda, H.Y.; Palraj, R.; Molton, J.S.; Venkatachalam, I. Remdesivir in coronavirus disease 2019 (COVID-19) treatment: A review of evidence. Infection 2021, 49, 401–410. [Google Scholar] [CrossRef]
- Ucar, B.; Acar, T.; Arayici, P.P.; Derman, S. A nanotechnological approach in the current therapy of COVID-19: Model drug oseltamivir-phosphate loaded PLGA nanoparticles targeted with spike protein binder peptide of SARS-CoV-2. Nanotechnology 2021, 32, 485601. [Google Scholar] [CrossRef]
- Chagla, Z. The BNT162b2 (BioNTech/Pfizer) vaccine had 95% efficacy against COVID-19 ≥7 days after the 2nd dose. Ann. Intern. Med. 2021, 174, JC15. [Google Scholar] [CrossRef]
- WHO. Background Document on the AZD1222 Vaccine Against COVID-19 Developed by Oxford University and AstraZeneca: Background Document to the WHO Interim Recommendations for Use of the AZD1222 (ChAdOx1-S [Recombinant]) Vaccine Against COVID19 Developed by Oxford University and AstraZeneca; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- WHO. Interim Recommendations for use of the Pfizer–BioNTech COVID-19 Vaccine, BNT162b2, Under Emergency use Listing: Interim guidance; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Eedara, B.; Alabsi, W.; Encinas-Basurto, D.; Polt, R.; Ledford, J.; Mansour, H. Inhalation delivery for the treatment and prevention of COVID-19 infection. Pharmaceutis 2021, 13, 1077. [Google Scholar] [CrossRef]
- Vartak, R.; Patil, S.M.; Saraswat, A.; Patki, M.; Kunda, N.K.; Patel, K. Aerosolized nanoliposomal carrier of remdesivir: An effective alternative for COVID-19 treatment in vitro. Nanomedicine 2021, 16, 1187–1202. [Google Scholar] [CrossRef] [PubMed]
- Dhanani, J.; Fraser, J.F.; Chan, H.-K.; Rello, J.; Cohen, J.; Roberts, J.A. Fundamentals of aerosol therapy in critical care. Crit. Care 2016, 20, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patton, J.S.; Platz, R.M. (D) Routes of delivery: Case studies: (2) Pulmonary delivery of peptides and proteins for systemic action. Adv. Drug Deliv. Rev. 1992, 8, 179–196. [Google Scholar] [CrossRef]
- Pilcer, G.; Amighi, K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef]
- Johnson, K.A. Preparation of peptide and protein powders for inhalation. Adv. Drug Deliv. Rev. 1997, 26, 3–15. [Google Scholar] [CrossRef]
- Kim, C.S.; Duncan, B.; Creran, B.; Rotello, V.M. Triggered nanoparticles as therapeutics. Nano Today 2013, 8, 439–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, C.-S. The Fundamentals of Aerosol Dynamics; World Scientific: Singapore, 1996. [Google Scholar]
- Mitchell, J.P.; Berlinski, A.; Canisius, S.; Cipolla, D.; Dolovich, M.B.; Gonda, I.; Hochhaus, G.; Kadrichu, N.; Lyapustina, S.; Mansour, H.M.; et al. Urgent appeal from international society for aerosols in medicine (ISAM) during COVID-19: Clinical decision makers and governmental agencies should consider the inhaled route of administration: A statement from the ISAM regulatory and standardization issues networking group. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 235–238. [Google Scholar] [CrossRef]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 588–599. [Google Scholar] [CrossRef]
- Dean, R. Hess; FAARC. Nebulizers: Principles and performance. Respir. Care 2000, 45, 609. [Google Scholar]
- Alhaddad, B.; Smith, F.J.; Robertson, T.; Watman, G.; Taylor, K.M.G. Patients’ practices and experiences of using nebuliser therapy in the management of COPD at home. BMJ Open Respir. Res. 2015, 2, e000076. [Google Scholar] [CrossRef] [Green Version]
- Tchesnokov, E.P.; Feng, J.Y.; Porter, D.P.; Götte, M. Mechanism of Inhibition of Ebola virus RNA-dependent RNA polymerase by remdesivir. Viruses 2019, 11, 326. [Google Scholar] [CrossRef] [Green Version]
- Furuta, Y.; Gowen, B.B.; Takahashi, K.; Shiraki, K.; Smee, D.F.; Barnard, D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir. Res. 2013, 100, 446–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) In Vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Shiraki, K.; Daikoku, T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol. Ther. 2020, 209, 107512. [Google Scholar] [CrossRef]
- Joshi, S.; Parkar, J.; Ansari, A.; Vora, A.; Talwar, D.; Tiwaskar, M.; Patil, S.; Barkate, H. Role of favipiravir in the treatment of COVID-19. Int. J. Infect. Dis. 2021, 102, 501–508. [Google Scholar] [CrossRef]
- Jin, Z.; Smith, L.K.; Rajwanshi, V.K.; Kim, B.; Deval, J. The ambiguous base-pairing and high substrate efficiency of T-705 (Favipiravir) ribofuranosyl 5′-triphosphate towards influenza a virus polymerase. PLoS ONE 2013, 8, e68347. [Google Scholar] [CrossRef]
- Oestereich, L.; Lüdtke, A.; Wurr, S.; Rieger, T.; Munoz-Fontela, C.; Günther, S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antivir. Res. 2014, 105, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, G.; Madou, M.J.; Kalra, S.; Chopra, V.; Ghosh, D.; Martinez-Chapa, S.O. Nanotechnology for COVID-19: Therapeutics and vaccine research. ACS Nano 2020, 14, 7760–7782. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Ratho, R.K.; Panda, J.J. Respiratory delivery of favipiravir-tocilizumab combination through mucoadhesive protein-lipidic nanovesicles: Prospective therapeutics against COVID-19. VirusDisease 2021, 32, 131–136. [Google Scholar] [CrossRef]
- Elkodous, M.A.; Olojede, S.O.; Morsi, M.; El-Sayyad, G.S. Nanomaterial-based drug delivery systems as promising carriers for patients with COVID-19. RSC Adv. 2021, 11, 26463–26480. [Google Scholar] [CrossRef]
- Chun, H.; Yeom, M.; Kim, H.-O.; Lim, J.-W.; Na, W.; Park, G.; Park, C.; Kang, A.; Yun, D.; Kim, J.; et al. Efficient antiviral co-delivery using polymersomes by controlling the surface density of cell-targeting groups for influenza A virus treatment. Polym. Chem. 2018, 9, 2116–2123. [Google Scholar] [CrossRef]
- DeLong, R.K.; Risor, A.; Kanomata, M.; Laymon, A.; Jones, B.; Zimmerman, S.D.; Williams, J.; Witkowski, C.; Warner, M.; Ruff, M.; et al. Characterization of biomolecular nanoconjugates by high-throughput delivery and spectroscopic difference. Nanomedicine 2012, 7, 1851–1862. [Google Scholar] [CrossRef] [Green Version]
- Mehanna, M.M.; Mohyeldin, S.; Elgindy, N.A. Respirable nanocarriers as a promising strategy for antitubercular drug delivery. J. Control. Release 2014, 187, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-H.; Bebawy, M.; Loo, C.-Y.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Fabrication of curcumin micellar nanoparticles with enhanced anti-cancer activity. J. Biomed. Nanotechnol. 2015, 11, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Tulbah, A.; Pisano, E.; Scalia, S.; Young, P.M.; Traini, D.; Ong, H.X. Inhaled simvastatin nanoparticles for inflammatory lung disease. Nanomedicine 2017, 12, 2471–2485. [Google Scholar] [CrossRef] [PubMed]
- Sou, K.; Inenaga, S.; Takeoka, S.; Tsuchida, E. Loading of curcumin into macrophages using lipid-based nanoparticles. Int. J. Pharm. 2008, 352, 287–293. [Google Scholar] [CrossRef]
- Anand, P.; Nair, H.B.B.; Sung, B.; Kunnumakkara, A.B.; Yadav, V.R.; Tekmal, R.R.; Aggarwal, B.B. Retracted: Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity In Vitro and superior bioavailability in vivo. Biochem. Pharmacol. 2010, 79, 330–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashidi, H.; Ellis, M.J.; Cartmell, S.H.; Chaudhuri, J.B. Simvastatin release from poly (lactide-co-glycolide) membrane scaffolds. Polymers 2010, 2, 709–718. [Google Scholar] [CrossRef] [Green Version]
- Assaf, K.; Duek, E.A.D.R.; Oliveira, N.M. Efficacy of a combination of simvastatin and poly (DL-lactic-co-glycolic acid) in stimulating the regeneration of bone defects. Mater. Res. 2012, 16, 215–220. [Google Scholar] [CrossRef]
- Azarmi, S.; Roa, W.H.; Löbenberg, R. Targeted delivery of nanoparticles for the treatment of lung diseases. Adv. Drug Deliv. Rev. 2008, 60, 863–875. [Google Scholar] [CrossRef]
- Ong, H.X.; Traini, D.; Cipolla, D.; Gonda, I.; Bebawy, M.; Agus, H.; Young, P.M. Liposomal nanoparticles control the uptake of ciprofloxacin across respiratory epithelia. Pharm. Res. 2012, 29, 3335–3346. [Google Scholar] [CrossRef]
- Tulbah, A.; Pisano, E.; Landh, E.; Scalia, S.; Young, P.; Traini, D.; Ong, H.X. Simvastatin nanoparticles reduce inflammation in LPS-stimulated alveolar macrophages. J. Pharm. Sci. 2019, 108, 3890–3897. [Google Scholar] [CrossRef]
- Lee, W.-H.; Loo, C.-Y.; Ong, H.-X.; Traini, D.; Young, P.M.; Rohanizadeh, R.; Wing-Hin, L.; Ching-Yee, L.; Hui-Xin, O.; Daniela, T.; et al. Synthesis and characterization of inhalable flavonoid nanoparticle for lung cancer cell targeting. J. Biomed. Nanotechnol. 2016, 12, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Mangal, S.; Gao, W.; Li, T.; Zhou, Q.T. Pulmonary delivery of nanoparticle chemotherapy for the treatment of lung cancers: Challenges and opportunities. Acta Pharmacol. Sin. 2017, 38, 782–797. [Google Scholar] [CrossRef]
- Lee, W.-H.; Loo, J.C.Y.; Traini, D.; Young, P. Inhalation of nanoparticle-based drug for lung cancer treatment: Advantages and challenges. Asian J. Pharm. Sci. 2015, 10, 481–489. [Google Scholar] [CrossRef]
- Huang, Z.-R.; Hua, S.-C.; Yang, Y.-L.; Fang, J.-Y. Development and evaluation of lipid nanoparticles for camptothecin delivery: A comparison of solid lipid nanoparticles, nanostructured lipid carriers, and lipid emulsion. Acta Pharmacol. Sin. 2008, 29, 1094–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.A.; Abdulbaqi, I.M.; Assi, R.A.; Murugaiyah, V.; Darwis, Y. Lyophilized hybrid nanostructured lipid carriers to enhance the cellular uptake of verapamil: Statistical optimization and In Vitro evaluation. Nanoscale Res. Lett. 2018, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.M.; Vaughan-Jones, R. Assessment of the potential uses of liposomes for lymphoscintigraphy and lymphatic drug delivery failure of 99 m-technetium marker to represent intact liposomes in lymph nodes. Biochim. Biophys. Acta Gen. Subj. 1984, 801, 76–86. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, Y.; Cai, J.; Duan, Y.; Wang, R.; Zhang, H.; Ruan, Q.; Li, J.; Zhao, L.; Ping, Y.; et al. Pathological changes in the lungs and lymphatic organs of 12 COVID-19 autopsy cases. Natl. Sci. Rev. 2020, 7, 1868–1878. [Google Scholar] [CrossRef]
- Videira, M.A.; Botelho, M.F.; Santos, A.C.; Gouveia, L.; De Lima, J.P.; Almeida, A. Lymphatic uptake of pulmonary delivered radiolabelled solid lipid nanoparticles. J. Drug Target. 2002, 10, 607–613. [Google Scholar] [CrossRef]
- Yamali, K.C.; Gudipati, M.; Nadendla, R.R. Design, characterization and In-Vitro evaluation of favipiravir orodispersible films. J. Pharm. Res. Int. 2021, 49–60. [Google Scholar] [CrossRef]
- Aburahma, M.H.; Badr-Eldin, S.M. Compritol 888 ATO: A multifunctional lipid excipient in drug delivery systems and nanopharmaceuticals. Expert Opin. Drug Deliv. 2014, 11, 1865–1883. [Google Scholar] [CrossRef]
- Ren, W.; Tian, G.; Jian, S.; Gu, Z.; Zhou, L.; Yan, L.; Yin, W.; Zhao, Y. Tween coated NaYF4:Yb,Er/NaYF4 core/shell upconversion nanoparticles for bioimaging and drug delivery. RSC Adv. 2012, 2, 7037–7041. [Google Scholar] [CrossRef]
- Kura, A.U.; Hussein-Al-Ali, S.H.; Hussein, M.Z.; Fakurazi, S. Preparation of Tween 80-Zn/Al-levodopa-layered double hydroxides nanocomposite for drug delivery system. Sci. World J. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goloveshkin, A.; Korlyukov, A.; Vologzhanina, A. Novel polymorph of favipiravir—an antiviral medication. Pharmceutics 2021, 13, 139. [Google Scholar] [CrossRef]
- Souto, E.B.; Mehnert, W.; Müller, R.H. Polymorphic behaviour of Compritol®888 ATO as bulk lipid and as SLN and NLC. J. Microencapsul. 2006, 23, 417–433. [Google Scholar] [CrossRef]
- Yadav, M.; Schiavone, N.; Guzman-Aranguez, A.; Giansanti, F.; Papucci, L.; Perez De Lara, M.J.; Singh, M.; Kaur, I.P. Atorvastatin-loaded solid lipid nanoparticles as eye drops: Proposed treatment option for age-related macular degeneration (AMD). Drug Deliv. Transl. Res. 2020, 10, 919–944, Erratum in 2020, 10, 1531. [Google Scholar] [CrossRef] [PubMed]
- Landh, E.; Moir, L.M.; dos Reis, L.G.; Traini, D.; Young, P.; Ong, H.X. Inhaled rapamycin solid lipid nano particles for the treatment of lymphangioleiomyomatosis. Eur. J. Pharm. Sci. 2020, 142, 105098. [Google Scholar] [CrossRef]
- Boocock, D.J.; Patel, K.R.; Faust, G.E.; Normolle, D.P.; Marczylo, T.H.; Crowell, J.A.; Brenner, D.E.; Booth, T.D.; Gescher, A.; Steward, W.P. Quantitation of trans-resveratrol and detection of its metabolites in human plasma and urine by high performance liquid chromatography. J. Chromatogr. B 2007, 848, 182–187. [Google Scholar] [CrossRef] [Green Version]
- Abdelrahim, M. Aerodynamic characteristics of nebulized terbutaline sulphate using the Andersen cascade impactor compared to the next generation impactor. Pharm. Dev. Technol. 2010, 16, 137–145. [Google Scholar] [CrossRef]
- Pharmacopeia, U. Inhalation and Nasal Drug Products: Aerosols, Sprays, and Powders—Performance Quality, in United States Pharmacopeia, USP 38–NF 33nd Supplement; US Pharmacopeial Convention: Rockville, MD, USA, 2015; pp. 388–413. [Google Scholar]
- Mostafa, A.; Kandeil, A.; Elshaier, Y.A.M.M.; Kutkat, O.; Moatasim, Y.; Rashad, A.A.; Shehata, M.; Gomaa, M.R.; Mahrous, N.; Mahmoud, S.H.; et al. FDA-approved drugs with potent In Vitro antiviral activity against severe acute respiratory syndrome coronavirus 2. Pharmceuticals 2020, 13, 443. [Google Scholar] [CrossRef]



| FPV-SLNs | Blank SLNs | Unprocessed Favipiravir | |
|---|---|---|---|
| Particle Size (nm) | 693.1 ± 40.3 | 389.3 ± 26.5 | 1056.4 ± 181.2 |
| Polydispersity Index | 0.655 ± 0.020 | 0.491 ± 0.0360 | 0.451 ± 0.036 |
| Zeta Potential (mV) | −13.3 ± 0.3 | −14.8 ± 0.8 | −11.1 ± 0.2 |
| R2 of Sample * | Zero-Order | First-Order | Higuchi | Hixson-Crowell | Korsmeyer–Peppas |
|---|---|---|---|---|---|
| FPV-SLNs | 0.6895 | 0.6615 | 0.7934 | 0.8043 | 0.8889 |
| Results | n ± SD |
|---|---|
| Calculated Delivered Dose (µg ± SD) | 322.3 ± 25.6 |
| Fine Particle Dose (µg ± SD) | 193.9 ± 13.1 |
| Fine Particle Fraction (% ± SD) | 60.2 ± 1.7 |
| MMAD (µm ± SD) | 3.0 ± 0.4 |
| GSD | 2.33 ± 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tulbah, A.S.; Lee, W.-H. Physicochemical Characteristics and In Vitro Toxicity/Anti-SARS-CoV-2 Activity of Favipiravir Solid Lipid Nanoparticles (SLNs). Pharmaceuticals 2021, 14, 1059. https://doi.org/10.3390/ph14101059
Tulbah AS, Lee W-H. Physicochemical Characteristics and In Vitro Toxicity/Anti-SARS-CoV-2 Activity of Favipiravir Solid Lipid Nanoparticles (SLNs). Pharmaceuticals. 2021; 14(10):1059. https://doi.org/10.3390/ph14101059
Chicago/Turabian StyleTulbah, Alaa S., and Wing-Hin Lee. 2021. "Physicochemical Characteristics and In Vitro Toxicity/Anti-SARS-CoV-2 Activity of Favipiravir Solid Lipid Nanoparticles (SLNs)" Pharmaceuticals 14, no. 10: 1059. https://doi.org/10.3390/ph14101059
APA StyleTulbah, A. S., & Lee, W.-H. (2021). Physicochemical Characteristics and In Vitro Toxicity/Anti-SARS-CoV-2 Activity of Favipiravir Solid Lipid Nanoparticles (SLNs). Pharmaceuticals, 14(10), 1059. https://doi.org/10.3390/ph14101059