Inhibition of 11β-HSD1 Ameliorates Cognition and Molecular Detrimental Changes after Chronic Mild Stress in SAMP8 Mice
Abstract
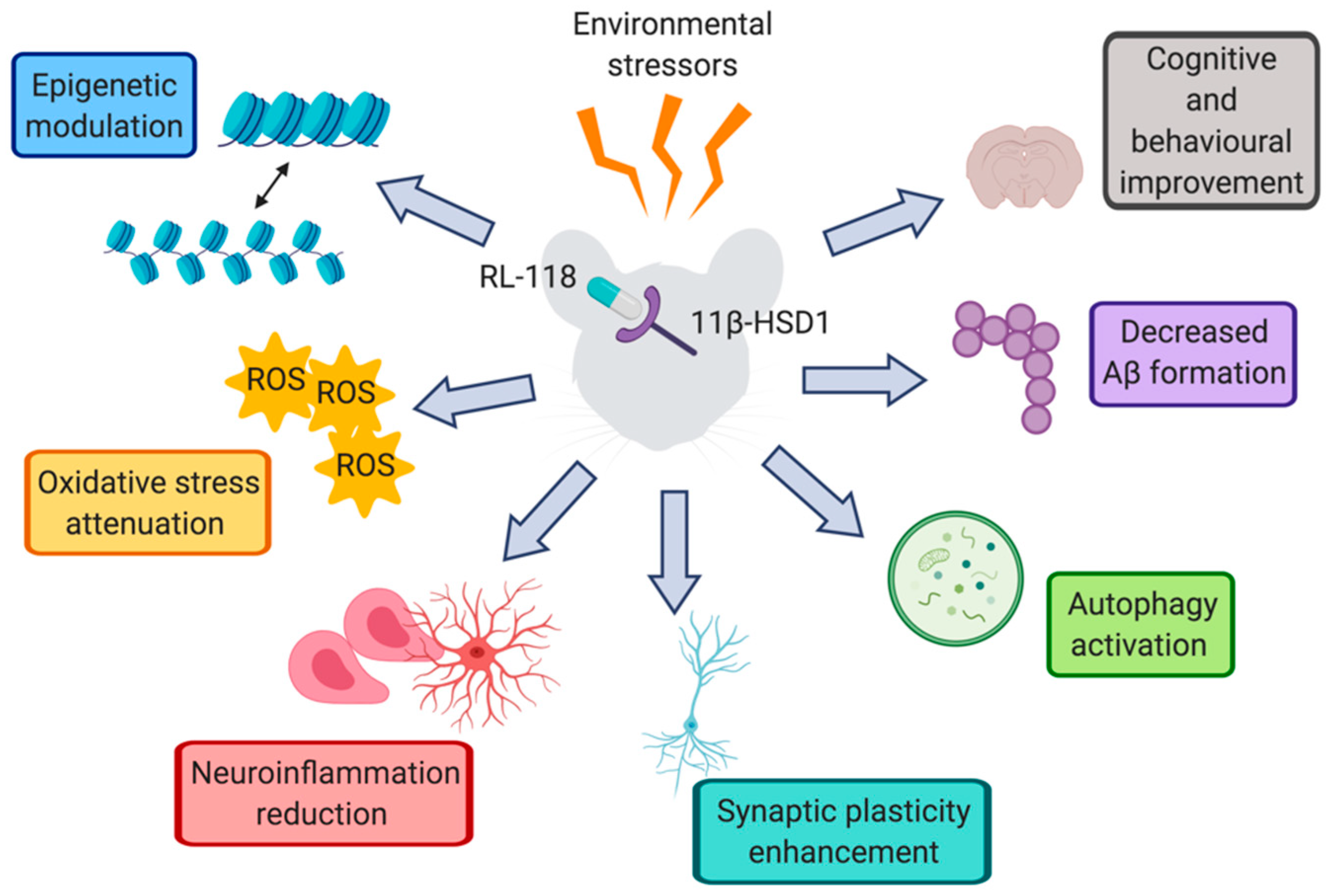
:1. Introduction
2. Results
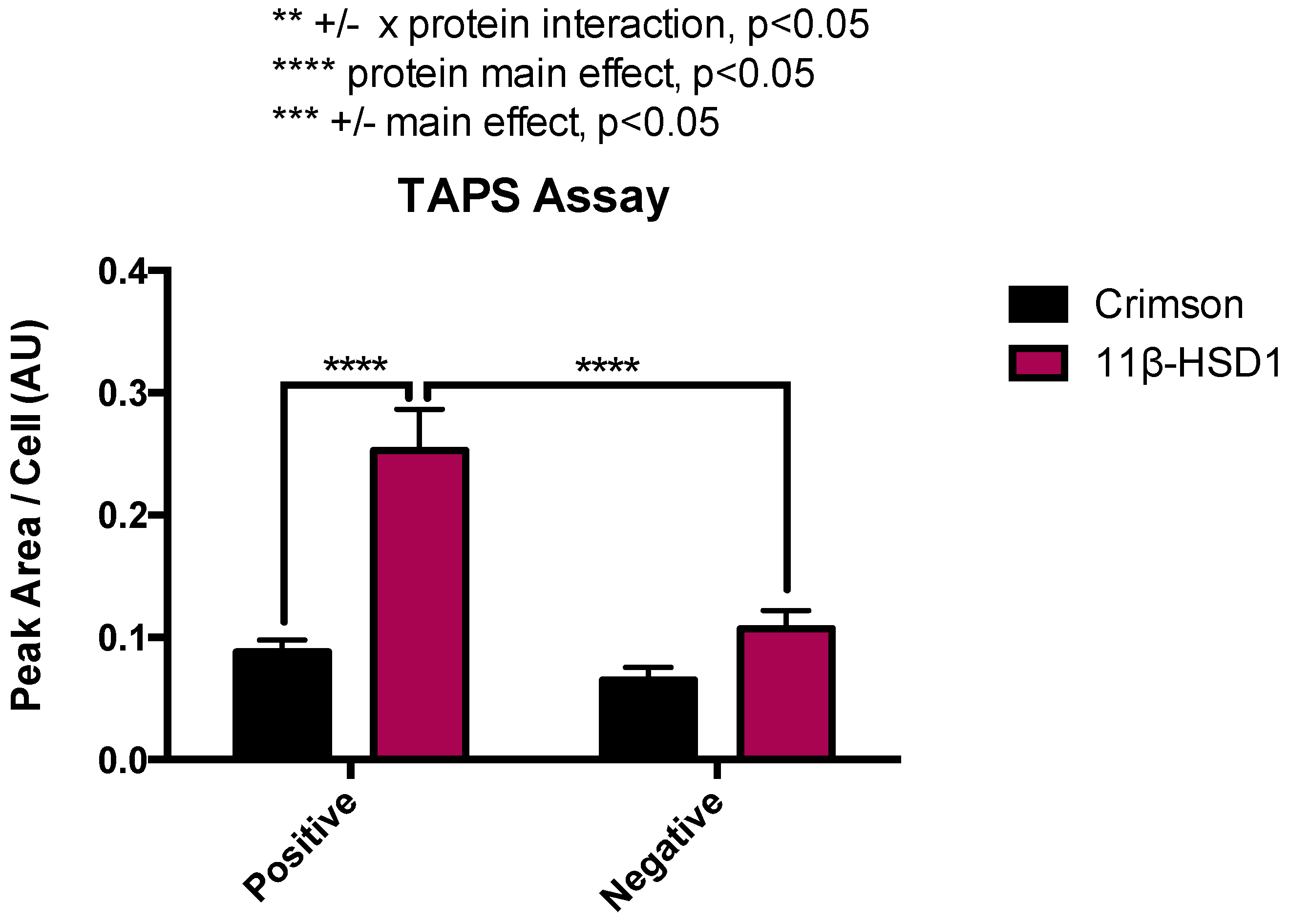
2.1. RL-118 Demonstrates Target Engagement with 11β-HSD1 Enzyme in the TAPS Assay
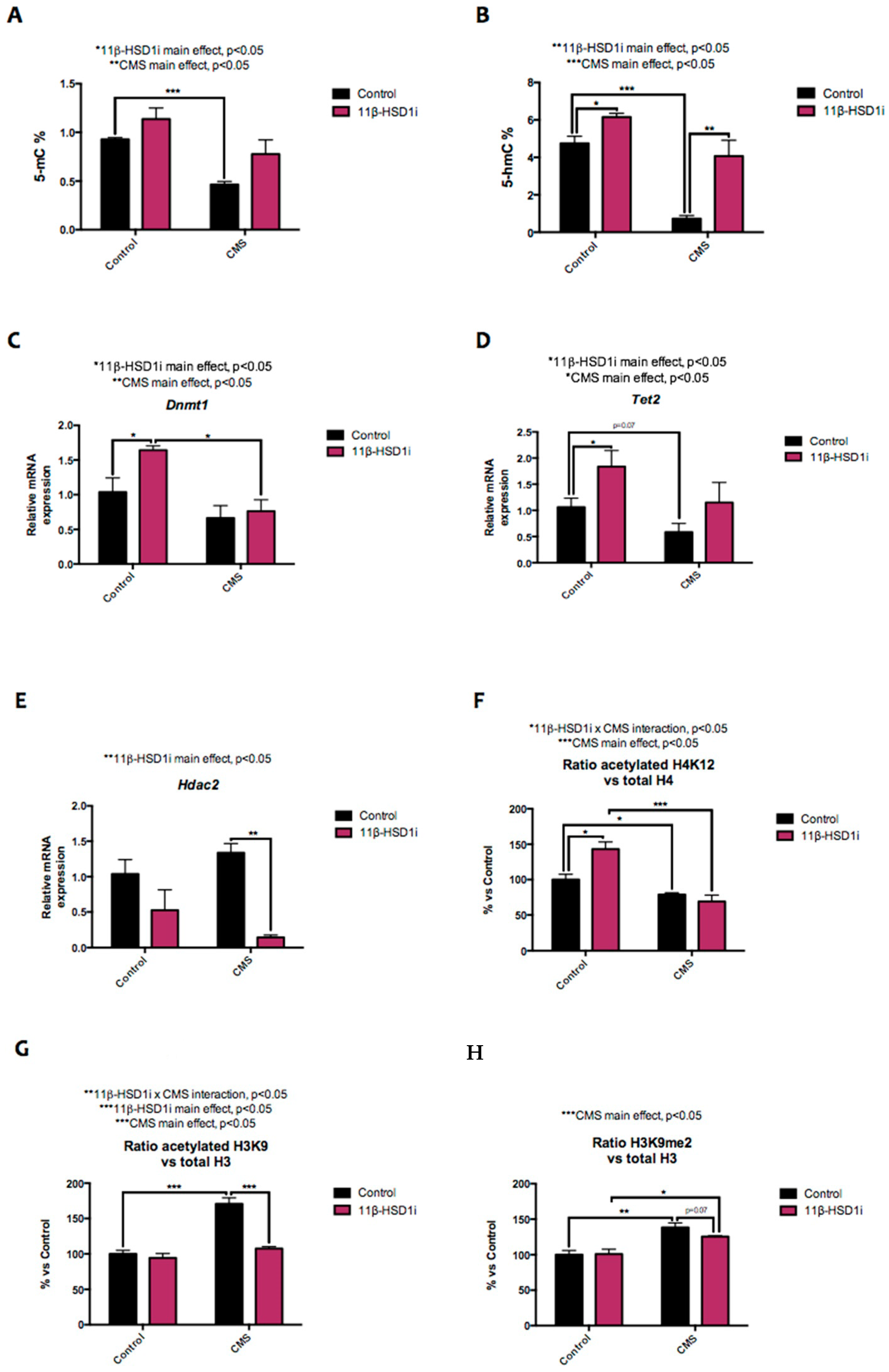
2.2. CMS-Modulated Epigenetic Marks Are Controlled by 11β-HSD1 Inhibition
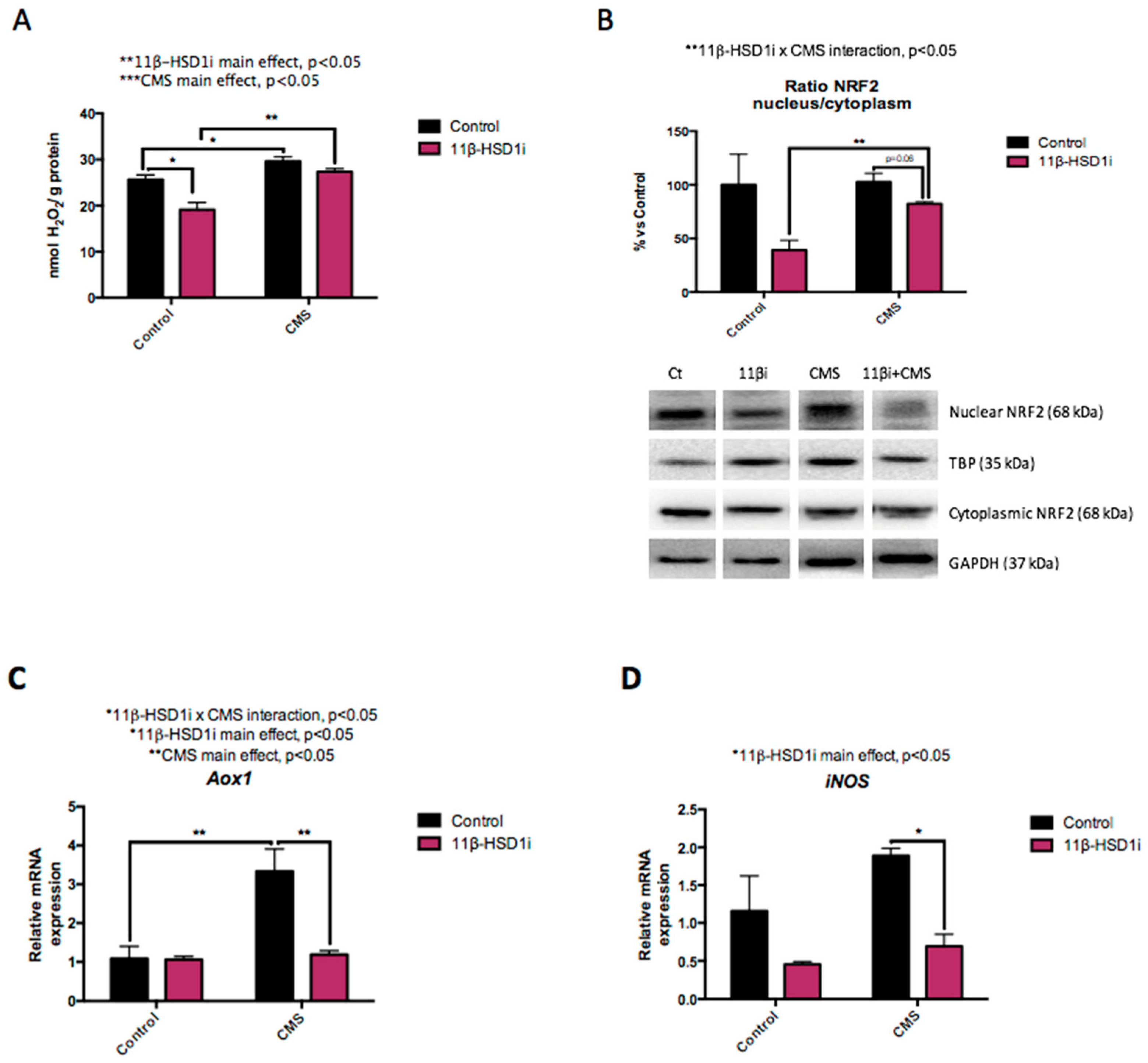
2.3. OS Increase Induced by CMS Was Prevented by RL-118 Treatment
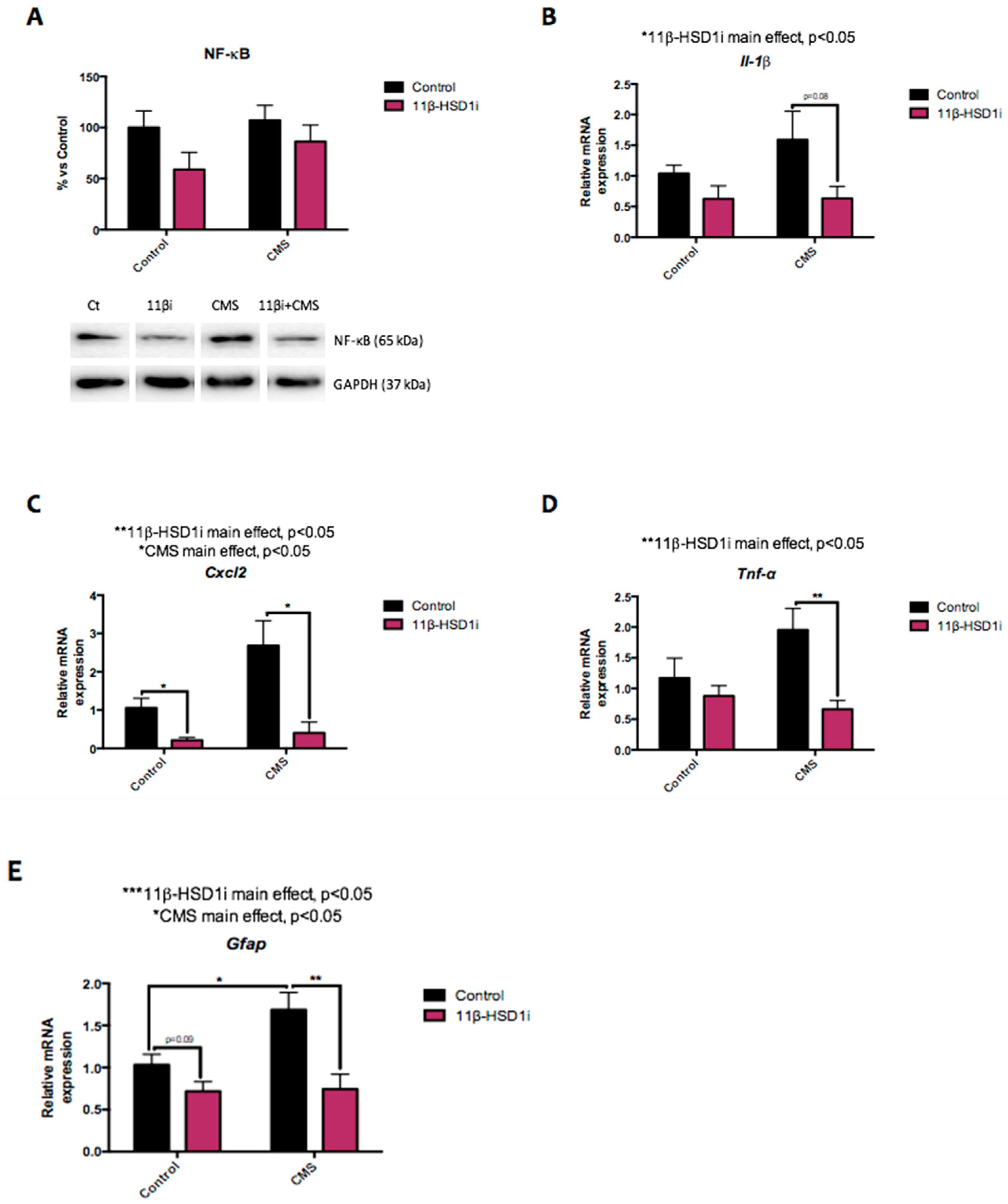
2.4. Pro-Inflammatory Markers Were Reduced after 11β-HSD1 Inhibition
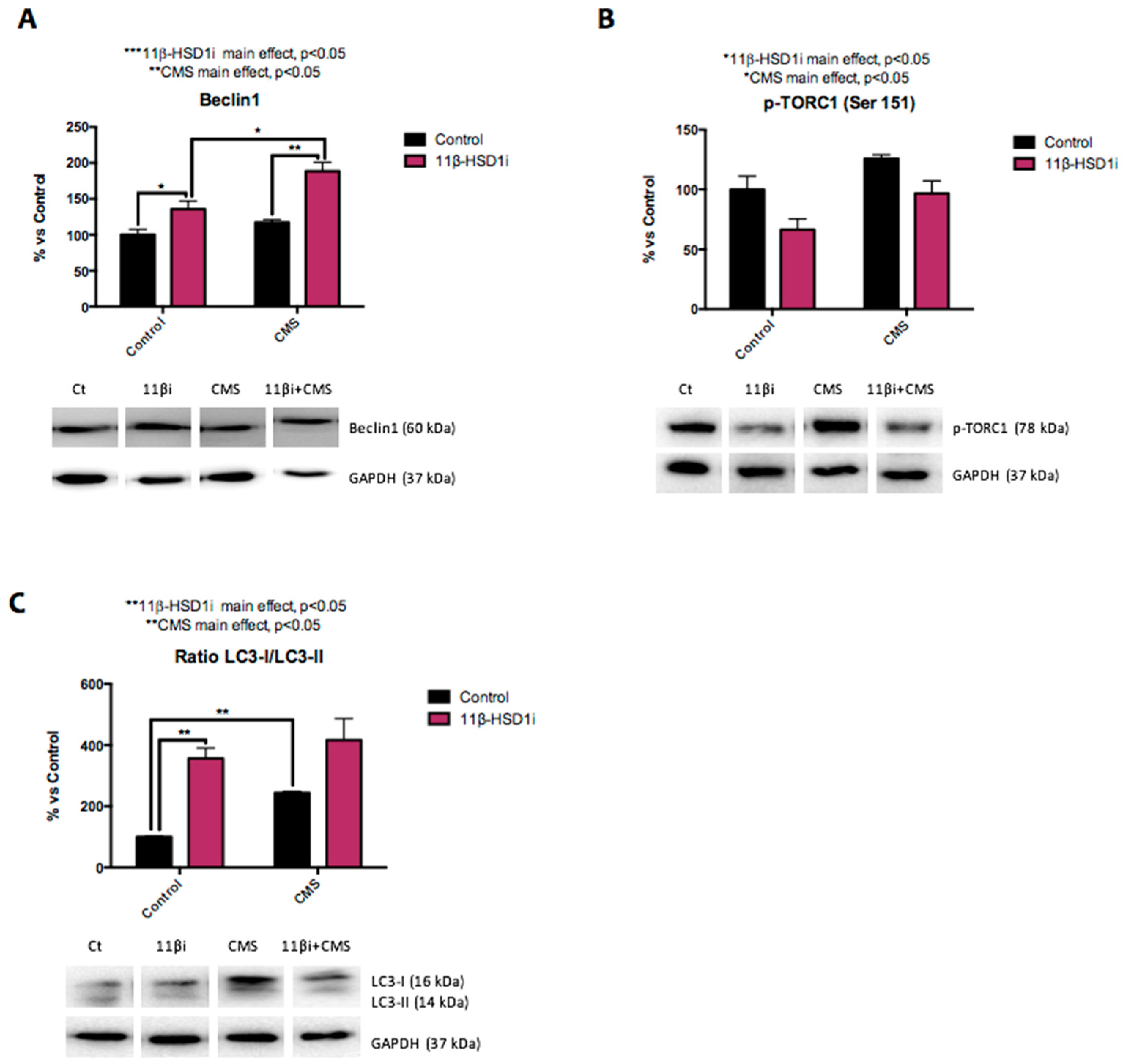
2.5. 11β-HSD1 Inhibition Promoted CMS-Induced Autophagy
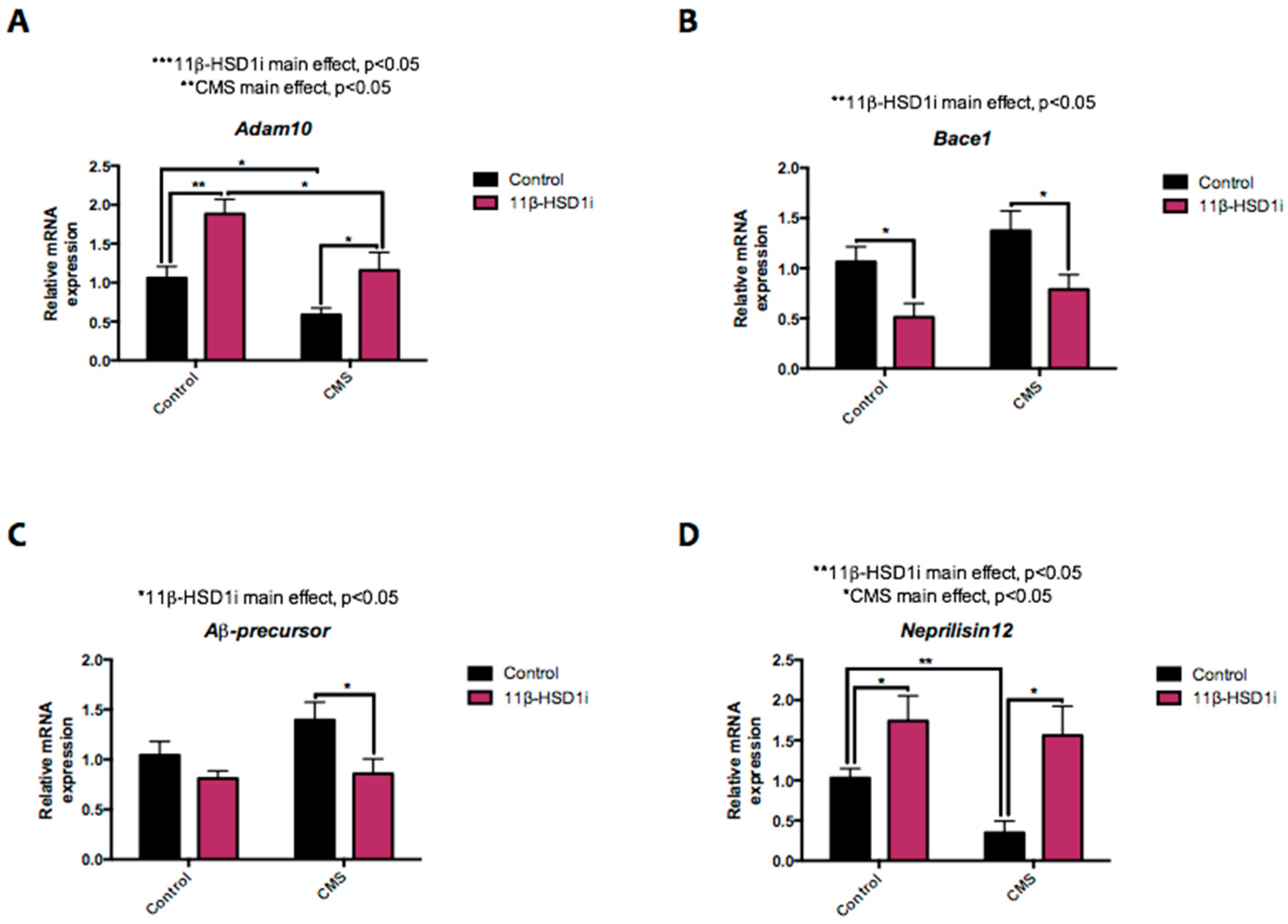
2.6. 11β-HSD1 Inhibition Rescued Mice from the Injurious Effects of CMS on APP Processing
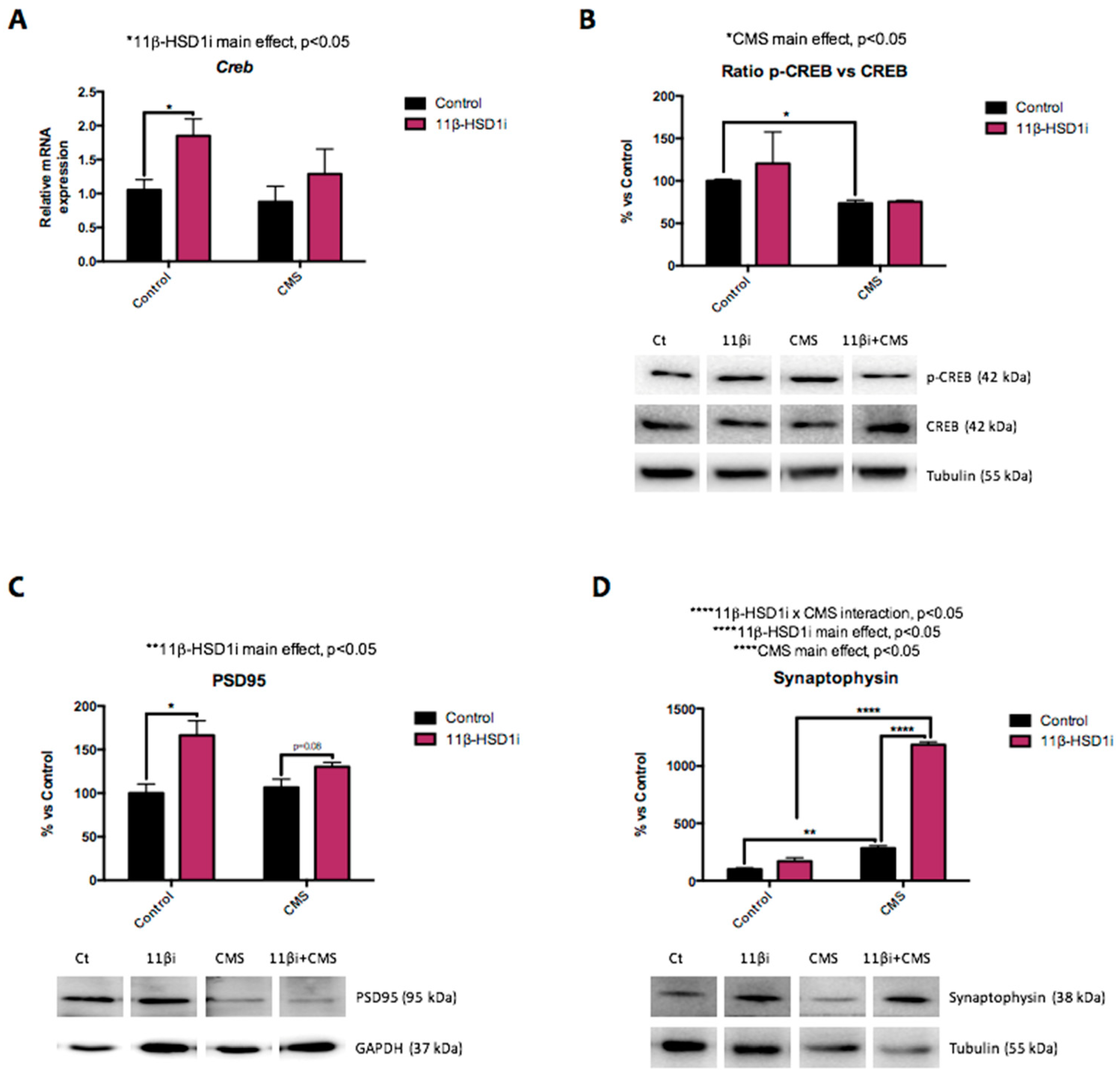
2.7. 11β-HSD1 Inhibition by RL-118 Changed Synaptic Plasticity Markers after CMS
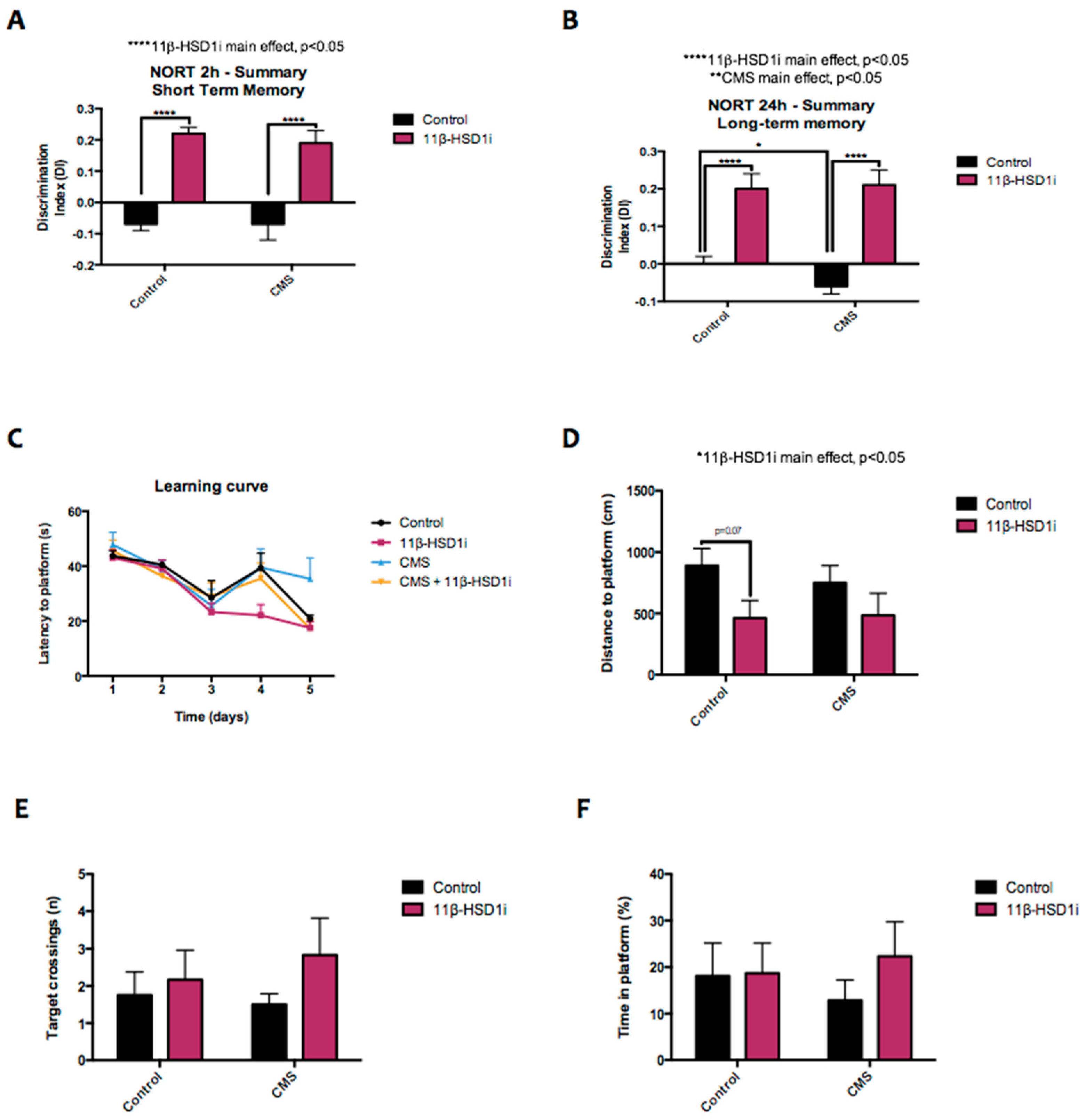
2.8. 11β-HSD1 Inhibition Increased Memory and Learning Abilities
3. Discussion
4. Materials and Methods
4.1. Cloning
4.2. Transient Expression of the Fluorescent Target Protein
4.3. TAPS Assay
4.3.1. Compound Incubation
4.3.2. FACS Sorting
4.3.3. Mass Spectrometry Detection of the Compounds in Cell Lysates
4.4. Animals
4.5. Chronic Mild Stress Treatment
4.6. Behavioral and Cognitive Tests
4.6.1. Novel Object Recognition Test (NORT)
4.6.2. Morris Water Maze (MWM)
4.7. Brain Processing
4.8. Western Blotting
4.9. RNA Extraction and Gene Expression Determination by q-PCR
4.10. Global DNA Methylation and Hydroxymethylation Determination
4.11. Oxidative Stress Determination
4.12. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catania, C.; Sotiropoulous, I.; Silva, R.; Onofri, C.; Breen, K.C.; Sousa, N.; Almeida, O.F.X. The amyloidogenic potential and behavioral correlates of stress. Mol. Psychiatry 2009, 14, 95–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sotiropoulos, I.; Cerqueira, J.; Catania, C.; Takashima, A.; Sousa, N.; Almeida, O.F.X. Stress and glucocorticoid footprints in the brain—The path from depression to Alzheimer’s disease. Neurosci. Biobehav. Rev. 2008, 32, 1161–1173. [Google Scholar] [CrossRef]
- Pazirandeh, A.; Xue, Y.; Prestegaard, T.; Jondal, M.; Okret, S. Effects of altered glucocorticoid sensitivity in the T cell lineage on thymocyte and T cell homeostasis. FASEB J. 2002, 16, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Sandi, C. Stress and cognition. WIREs Cogn. Sci. 2013, 4, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Dhabhar, F.S. The Short-Term Stress Response—Mother Nature’s Mechanism for Enhancing Protection and Performance under Conditions of Threat, Challenge, and Opportunity. Front. Neuroendocrinol. 2018, 49, 175–192. [Google Scholar] [CrossRef]
- Tatomir, A.; Micu, C.; Crivii, C. The impact of stress and glucocorticoids on memory. Clujul Med. 2014, 87, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yuan, J.; Pang, J.; Ma, J.; Han, B.; Geng, Y.; Shen, L.; Wang, H.; Ma, Q.; Wang, Y.; et al. Effects of Chronic Stress on Cognition in Male SAMP8 Mice. Cell Physiol. Biochem. 2016, 39, 1078–1086. [Google Scholar] [CrossRef]
- Vyas, S.; Rodrigues, A.J.; Silva, J.M.; Tronche, F.; Almeida, O.F.X.; Sousa, N.; Sotiropoulous, I. Chronic Stress and Glucocorticoids: From Neuronal Plasticity to Neurodegeneration. Neural Plast. 2016, 2016, 6391686. [Google Scholar] [CrossRef] [Green Version]
- Sotiropoulos, I.; Catania, C.; Pinto, L.G.; Silva, R.; Pollerberg, G.E.; Takashima, A.; Sousa, N.; Almeida, O.F.X. Stress acts cumulatively to precipitate Alzheimer’s disease-like tau pathology and cognitive deficits. J. Neurosci. 2011, 31, 7840–7847. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.R.; Spencer-Segal, J.L. Glucocorticoids and the Brain after Critical Illness. Endocrinology 2021, 162, bqaa242. [Google Scholar] [CrossRef]
- Harman, M.F.; Martín, M.G. Epigenetic mechanisms related to cognitive decline during aging. J. Neurosci. Res. 2020, 98, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Puigoriol-Illamola, D.; Martínez-Damas, M.; Griñán-Ferré, C.; Pallàs, M. Chronic Mild Stress Modified Epigenetic Mechanisms Leading to Accelerated Senescence and Impaired Cognitive Performance in Mice. Int. J. Mol. Sci. 2020, 21, 1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, V.K.; Singh, T.G.; Mehta, V. Stressed mitochondria: A target to intrude alzheimer’s disease. Mitochondrion 2021, 59, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Bisht, K.; Sharma, K.; Tremblay, M.È. Chronic stress as a risk factor for Alzheimer’s disease: Roles of microglia-mediated synaptic remodeling, inflammation, and oxidative stress. Neurobiol. Stress 2018, 9, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Puigoriol-Illamola, D.; Leiva, R.; Vázquez-Carrera, M.; Vázquez, S.; Griñán-Ferré, C.; Pallàs, M. 11β-HSD1 Inhibition Rescues SAMP8 Cognitive Impairment Induced by Metabolic Stress. Mol. Neurobiol. 2020, 57, 551–565. [Google Scholar] [CrossRef]
- Irwin, M.R.; Miller, A.H. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav. Immun. 2007, 21, 374–383. [Google Scholar] [CrossRef]
- Pace, T.W.; Hu, F.; Miller, A.H. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav. Immun. 2007, 21, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Bonet-Costa, V.; Pomatto, L.C.-D.; Davies, K.J.A. The Proteasome and Oxidative Stress in Alzheimer’s Disease. Antioxid. Redox Signal. 2016, 25, 886–901. [Google Scholar] [CrossRef]
- Schiavone, S.; Jaquet, V.; Trabace, L.; Krause, K.-H. Severe Life Stress and Oxidative Stress in the Brain: From Animal Models to Human Pathology. Antioxid. Redox Signal. 2013, 18, 1475–1490. [Google Scholar] [CrossRef] [Green Version]
- Pittenger, C.; Duman, R.S. Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology 2008, 33, 88–109. [Google Scholar] [CrossRef]
- Sandi, C. Stress, cognitive impairment and cell adhesion molecules. Nat. Rev. Neurosci. 2004, 5, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Leiva, R.; Ferré, C.G.; Seira, C.; Valverde, E.; McBride, A.; Binnie, M.; Pérez, B.; Luque, F.J.; Pallàs, M.; Bidon-Chanal, A.; et al. Design, synthesis and in vivo study of novel pyrrolidine-based 11β-HSD1 inhibitors for age-related cognitive dysfunction. Eur. J. Med. Chem. 2017, 139, 412–428. [Google Scholar] [CrossRef] [Green Version]
- Illamola, D.P.; Griñán-Ferré, C.; Vasilopoulou, F.; Leiva, R.; Vázquez, S.; Pallàs, M. 11β-HSD1 Inhibition by RL-118 Promotes Autophagy and Correlates with Reduced Oxidative Stress and Inflammation, Enhancing Cognitive Performance in SAMP8 Mouse Model. Mol. Neurobiol. 2018, 55, 8904–8915. [Google Scholar] [CrossRef] [PubMed]
- Weger, M.; Sandi, C. High anxiety trait: A vulnerable phenotype for stress-induced depression. Neurosci. Biobehav. Rev. 2018, 87, 27–37. [Google Scholar] [CrossRef]
- Mohler, E.G.; Browman, K.E.; Roderwald, V.A.; Cronin, E.A.; Markosyan, S.; Bitner, R.S.; Strakhova, M.I.; Drescher, K.U.; Hornberger, W.; Rohde, J.J.; et al. Acute Inhibition of 11β-Hydroxysteroid Dehydrogenase Type-1 Improves Memory in Rodent Models of Cognition. J. Neurosci. 2011, 31, 5406–5413. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.C.; Carter, R.N.; Noble, J.; Chitnis, S.; Dutia, A.; Paterson, J.M.; Mullins, J.J.; Seckl, J.R.; Yau, J.L.W. 11β-Hydroxysteroid dehydrogenase type 1 expression is increased in the aged mouse hippocampus and parietal cortex and causes memory impairments. J. Neurosci. 2010, 30, 6916–6920. [Google Scholar] [CrossRef]
- Yau, J.L.; McNair, K.M.; Noble, J.; Brownstein, D.; Hibberd, C.; Morton, N.; Mullins, J.J.; Morris, R.G.; Cobb, S.; Seckl, J.R. Enhanced hippocampal long-term potentiation and spatial learning in aged 11beta-hydroxysteroid dehydrogenase type 1 knock-out mice. J. Neurosci. 2007, 27, 10487–10496. [Google Scholar] [CrossRef] [Green Version]
- Yau, J.L.; Wheelan, N.; Noble, J.; Walker, B.R.; Webster, S.; Kenyon, C.J.; Ludwig, M.; Seckl, J.R. Intrahippocampal glucocorticoids generated by 11β-HSD1 affect memory in aged mice. Neurobiol. Aging 2015, 36, 334–343. [Google Scholar] [CrossRef] [Green Version]
- Webster, S.P.; McBride, A.; Binnie, M.; Sooy, K.; Seckl, J.R.; Andrew, R.; Pallin, T.D.; Hunt, H.J.; Perrior, T.R.; Ruffles, V.S.; et al. Selection and early clinical evaluation of the brain-penetrant 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) inhibitor UE2343 (XanamemTM). Br. J. Pharmacol. 2017, 174, 396–408. [Google Scholar] [CrossRef]
- Wilson, K.; Webster, S.P.; Iredale, J.P.; Zheng, X.; Homer, N.Z.; Pham, N.T.; Auer, M.; Mole, D.J. Detecting drug-target binding in cells using fluorescence-activated cell sorting coupled with mass spectrometry analysis. Methods Appl. Fluoresc. 2017, 6, 015002. [Google Scholar] [CrossRef]
- Fink, G. Stress: Concepts, Definition and History. Neurosci. Biobehav. Psychol. 2017, 549–555. [Google Scholar] [CrossRef]
- Fetahu, I.S.; Ma, D.; Rabidou, K.; Argueta, C.; Smith, M.; Liu, H.; Wu, F.; Shi, Y.G. Epigenetic signatures of methylated DNA cytosine in Alzheimer’s disease. Sci. Adv. 2019, 5, eaaw2880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherwani, S.I.; Khan, H.A. Role of 5-hydroxymethylcytosine in neurodegeneration. Gene 2015, 570, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, J.; Chen, R.; Wang, L.; Li, B.; Cheng, H.; Duan, X.; Zhu, H.; Wei, W.; Li, J.; et al. Dissecting the precise role of H3K9 methylation in crosstalk with DNA maintenance methylation in mammals. Nat. Commun. 2016, 7, 12464. [Google Scholar] [CrossRef] [Green Version]
- Turner, B.M. Histone acetylation and an epigenetic code. BioEssays 2000, 22, 836–845. [Google Scholar] [CrossRef]
- Watson, J.D.; Baker, T.A.; Gann, A.; Levine, M.; Losik, R. Molecular Biology of the Gene, 7th ed.; Pearson/CSH Press: Boston, MA, USA, 2014. [Google Scholar]
- Kadmiel, M.; Cidlowski, J.A. Glucocorticoid receptor signalling in health and disease. Trends Pharmacol. Sci. 2013, 34, 518–530. [Google Scholar] [CrossRef] [Green Version]
- Picard, M.; McEwen, B.S.; Epel, E.S.; Sandi, C. An energetic view of stress: Focus on mitochondria. Front. Neuroendocr. 2018, 49, 72–85. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Corpas, R.; Puigoriol-Illamola, D.; Palomera-Ávalos, V.; Sanfeliu, C.; Pallàs, M. Understanding Epigenetics in the Neurodegeneration of Alzheimer’s Disease: SAMP8 Mouse Model. J. Alzheimer’s Dis. 2018, 62, 943–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.A.; Johnson, D.A.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Vargas, M.R.; Chen, P.-C. The Nrf2-ARE Pathway: An Indicator and Modulator of Oxidative Stress in Neurodegeneration. Ann. N. Y. Acad. Sci. 2008, 1147, 61–69. [Google Scholar] [CrossRef]
- Maeda, K.; Ohno, T.; Igarashi, S.; Yoshimura, T.; Yamashiro, K.; Sakai, M. Aldehyde oxidase 1 gene is regulated by Nrf2 pathway. Gene 2012, 505, 374–378. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Jia, Z.; Zhu, H. Regulation of Nrf2 Signaling. React. Oxyg. Species 2019, 8, 312–322. [Google Scholar] [CrossRef]
- Arias-Salvatierra, D.; Silbergeld, E.K.; Acosta-Saavedra, L.C.; Calderon-Aranda, E.S. Role of nitric oxide produced by iNOS through NF-kB pathway in migration of cerebellar granule neurons induced by Lipopolysaccharide. Cell Signal. 2011, 23, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Ki, W.C.; Lee, E.K.; Chung, K.W.; Chung, S.; Lee, B.; Seo, A.Y.; Chung, J.H.; Jung, Y.S.; Im, E.; et al. Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept. Aging Dis. 2019, 10, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, H.; Csernansky, J.G. Effects of stress and stress hormones on amyloid-beta protein and plaque deposition. J. Alzheimers Dis. 2009, 18, 459–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouanes, S.; Popp, J. High Cortisol and the Risk of Dementia and Alzheimer’s Disease: A Review of the Literature. Front. Aging Neurosci. 2019, 11, 43. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, H.; Sun, Q.; Yao, H.; Keegan, A.P.; Mullan, M.; Wilson, J.; Lista, S.; Leyhe, T.; Laske, C.; et al. Increased Plasma Beta-Secretase 1 May Predict Conversion to Alzheimer’s Disease Dementia in Individuals with Mild Cognitive Impairment. Biol. Psychiatry 2018, 83, 447–455. [Google Scholar] [CrossRef]
- Steffke, E.E.; Kirca, D.; Mazei-Robison, M.S.; Robison, A.J. Serum- and glucocorticoid-inducible kinase 1 activity reduces dendritic spines in dorsal hippocampus. Neurosci. Lett. 2020, 725, 134909. [Google Scholar] [CrossRef]
- Mango, D.; Saidi, A.; Cisale, G.Y.; Feligioni, M.; Corbo, M.; Nisticò, R. Targeting Synaptic Plasticity in Experimental Models of Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 778. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferré, C.G.; Illamola, D.P.; Palomera-Avalos, V.; Pérez-Cáceres, D.; Companys-Alemany, J.; Camins, A.; Ortuño-Sahagún, D.; Rodrigo, M.T.; Pallàs, M. Environmental Enrichment Modified Epigenetic Mechanisms in SAMP8 Mouse Hippocampus by Reducing Oxidative Stress and Inflammaging and Achieving Neuroprotection. Front. Aging Neurosci. 2016, 8, 241. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puigoriol-Illamola, D.; Companys-Alemany, J.; McGuire, K.; Homer, N.Z.M.; Leiva, R.; Vázquez, S.; Mole, D.J.; Griñán-Ferré, C.; Pallàs, M. Inhibition of 11β-HSD1 Ameliorates Cognition and Molecular Detrimental Changes after Chronic Mild Stress in SAMP8 Mice. Pharmaceuticals 2021, 14, 1040. https://doi.org/10.3390/ph14101040
Puigoriol-Illamola D, Companys-Alemany J, McGuire K, Homer NZM, Leiva R, Vázquez S, Mole DJ, Griñán-Ferré C, Pallàs M. Inhibition of 11β-HSD1 Ameliorates Cognition and Molecular Detrimental Changes after Chronic Mild Stress in SAMP8 Mice. Pharmaceuticals. 2021; 14(10):1040. https://doi.org/10.3390/ph14101040
Chicago/Turabian StylePuigoriol-Illamola, Dolors, Júlia Companys-Alemany, Kris McGuire, Natalie Z. M. Homer, Rosana Leiva, Santiago Vázquez, Damian J. Mole, Christian Griñán-Ferré, and Mercè Pallàs. 2021. "Inhibition of 11β-HSD1 Ameliorates Cognition and Molecular Detrimental Changes after Chronic Mild Stress in SAMP8 Mice" Pharmaceuticals 14, no. 10: 1040. https://doi.org/10.3390/ph14101040
APA StylePuigoriol-Illamola, D., Companys-Alemany, J., McGuire, K., Homer, N. Z. M., Leiva, R., Vázquez, S., Mole, D. J., Griñán-Ferré, C., & Pallàs, M. (2021). Inhibition of 11β-HSD1 Ameliorates Cognition and Molecular Detrimental Changes after Chronic Mild Stress in SAMP8 Mice. Pharmaceuticals, 14(10), 1040. https://doi.org/10.3390/ph14101040








