Chinese Herbal Medicine for the Treatment of Depression: Effects on the Neuroendocrine-Immune Network
Abstract
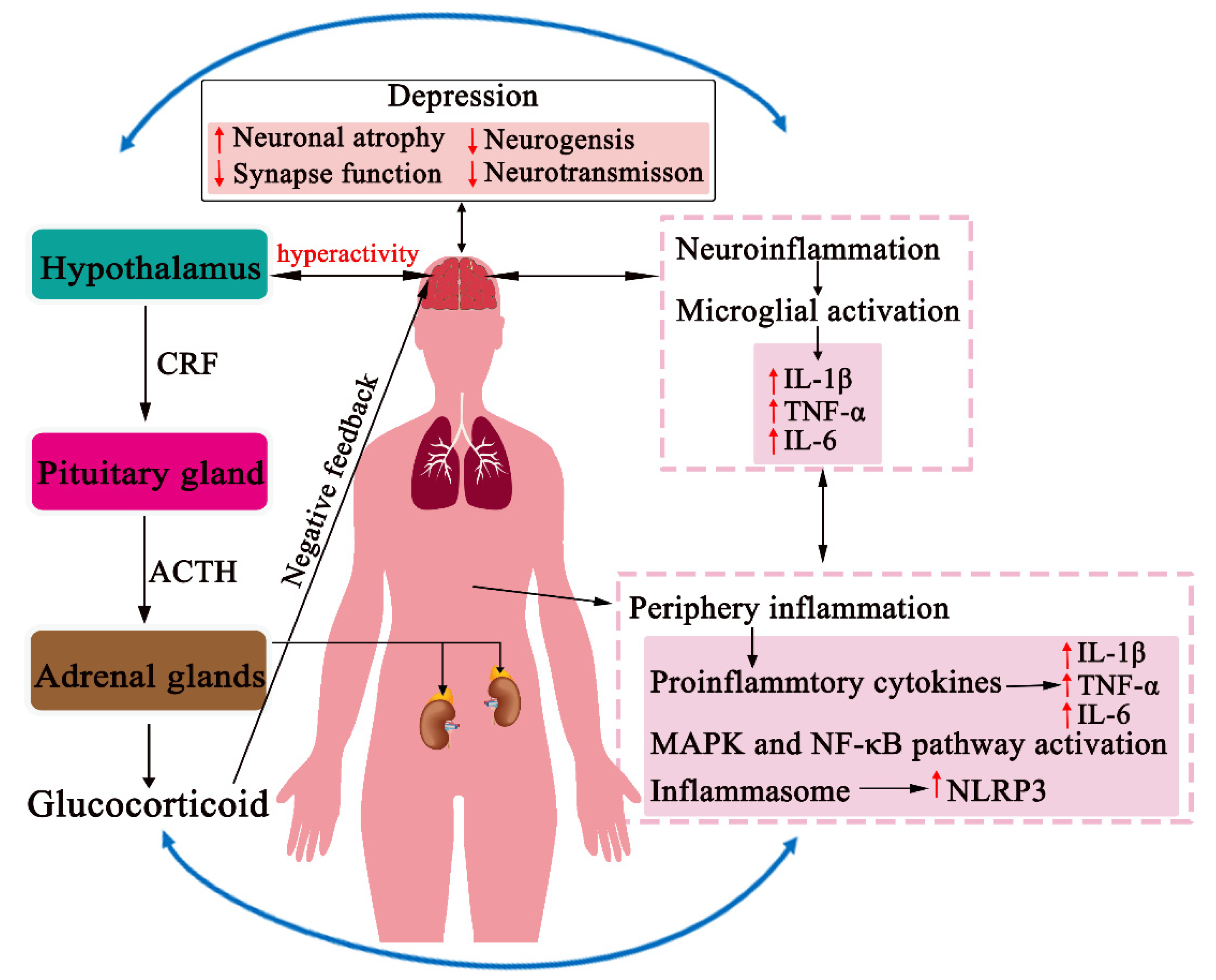
1. Introduction
2. Inflammation in the Pathogenesis of Depression
3. CHM Regulation of the Neuroimmune System
3.1. Proinflammatory Cytokines and Cytokine Receptors
3.2. Proinflammatory Signaling Pathway
3.3. Inflammasome
| Herb | Herbal Constituent | Animal Model | Behavioral Test | Administration Dose/Time/Route of Treatment | Effects on Mediators of Inflammation | Effects on Hormones of the HPA Axis | Reference |
|---|---|---|---|---|---|---|---|
| Fallopia multiflora (Thunb.) Harald. | 2, 3, 5, 4′-Tetrahydroxystilbene-2-O-β-D-glucoside | LPS-induced depressive mice | TPT, FST, SPT | 30, 60 mg/kg, 7 days, i.p. | ↓ IL-6, TNF-α, IL-1β in hippocampus and PFC | ND | [84] |
| Acanthopanax sessiliflorus (Rupr. et Maxim.) Seem. | Chiisanoside | LPS-induced depressive mice | TST, FST | 2.5, 5.0 mg/kg, 7 days, i.p. | ↓ IL-6, TNF-α in serum ↓ NF-κB in hippocampus | ND | [85] |
| Polygala tenuifolia Willd. | Senegenin | CUMS mice | TST, FST, SPT | 4, 8 mg/kg, 21 days, i.g. | ↓ NF-κB/NLRP3 signal pathway in hippocampus | ND | [86] |
| Gastrodia elata Bl. | Gastrodin | CUS rats | SPT, FST, Morris water test | 50, 100, 200 mg/kg, 14 days, i.p. | ↓ NF-κB and IL-1β expression in hippocampus | ND | [87] |
| Cinnamomum cassia Presl | Trans-Cinnamaldehyde | CUMS rats | Sucrose consumptions, FST | 10 mg/kg, 3 weeks, p.o. | ↓ IL-1β, IL-18, TNF-α in serum ↓ NF-κB/NLRP3 in PFC and hippocampus | ND | [28] |
| Lonicera japonica Thunb. | Lonicera japonicapolysaccharide | CUMS mice | OFT, EPM, TST, FST | 30, 100 mg/kg, 21 days, i.g. | ↓ NLRP3, IL-1β, caspase-1 in hippocampus | ND | [88] |
| Andrographis paniculata (Burm. f.) Nees | Andrographolide | CUMS mice | FST, SPT, TST, Y maze | 2.5, 5 mg/kg, 14 days, p.o. | ↓ IL-1β, IL-6, TNF-α, NF-κB signaling, NLRP3 in PFC | ND | [89] |
| Houpoea officinalis (Rehder and E. H. Wilson) N. H. Xia and C. Y. Wu | Honokiol | LPS-induced depressive mice | FST, TST, | 2.5, 5 10 mg/kg, 11 days, p.o. | ↓ NF-κB activation in hippocampus ↓ IL-1β, TNF-α, IFN-γ in serum | ND | [90] |
| Gardenia jasminoides Ellis /Crocus sativus L. | Crocin | LPS-induced depressive mice | SPT, FST, TST, OFT | 20, 40 mg/kg, 7 days, i.p. | ↓ TNF-α, IL-1β, IL-18 in BV-2 microglial cells and hippocampus ↓ NF-κB and NLRP3 in hippocampus | ND | [91] |
| Perilla frutescens (Linn.) Britt. | Perilla aldehyde | CUMS rats | SPT, FST, OFT | 20, 40 mg/kg, 3 weeks, i.g. | ↓ TNF-α, IL-1β in hippocampus ↓ NLRP3 in hippocampus | ND | [92] |
| LPS-induced depressive mice | TST, FST | 60, 120 mg/kg, 7 days, i.g. | ↓ TNF-α, IL-6 in serum and PFC | ND | [93] | ||
| Essential oil of Perilla frutescens | CUMS mice | OFT, TST, FST, SPT | 3, 6, 9 mg/kg, 3 weeks, g.i. | ↓ TNF-α, IL-6, IL-1 in plasma | ND | [94] | |
| Polygonum aviculare L. | Polygonum aviculare L. extract | Restraint-stressed mice | FST, SPT, OFT | 100, 200 mg/kg, 15 days, p.o. | ↓ TNF-α, IL-6, IL-1β in the brain | ND | [95] |
| Hemerocallis fulva (L.) L. | Ethanol extracts | LPS-induced depressive mice | SPT | 180 mg/kg, 7 days, p.o. | ↓ NF-κB signaling pathway in PFC | ND | [96] |
| Angelica sinensis (Oliv.) Diels | Ferulic Acid | CUMS mice | SPT | 20, 40, 80 mg/kg, 4 weeks, p.o. | ↓ TNF-α, IL-6, IL-1β, microglial activation, NF-κB and NLRP3 in PFC | ND | [97] |
| Paeonialactiflora Pall | Paeoniflorin | IFN-α-induced depressive mice | SPT, OFT, TST, FST | 10, 20, 40 mg/kg, 4 weeks, i.g. | ↓ TNF-α, IL-6, IL-1β, IL-9, IL-10, IL-12, MCP-1 in serum, mPFC, vHi and amygdala | ND | [98] |
| Xiaobuxin-Tang | Total flavonoid extract | LPS-induced depressive mice | TST, FST | 25, 50, 100 mg/kg, 1 h, i.p. | ↓ TNF-α, IL-1β in the barin | ND | [99] |
| Ginkgo biloba L. | EGb761 | LPS-induced depressive mice | FST, TST, SPT | 50, 100, 150 mg/kg, 10 days, p.o. | ↓ IL-6, TNF-α, IL-1β, IL-17A in hippocampus ↑ IL-10 in hippocampus | ND | [100] |
| Pueraria lobate (Willd.) Ohwi | Puerarin | CUS rats | SPT, NSFT, FST | 30, 60, 120 mg/kg, 20 days, i.g. | ND | ↓ CRH, CORT, ACTH in serum | [101] |
| Tribulus terrestris Linnaeus | Tribulus Terrestris Saponins | CMS rats | OFT, SPT | 0.375, 0.75, 2.25 g/kg, 4 weeks, i.g. | ND | ↓ CRH, CORT in serum | [102] |
| Rehmannia glutinosa (Gaert.) Libosch. ex Fisch. et Mey. | Ethanol extracts | CUMS rats | SPT | 150, 300, 600 mg/kg, 3 weeks, p.o. | ND | ↓ CORT in serum | [103] |
| Panax ginseng C.A. Meyer | Ginseng total saponins | LPS-induced depressive mice/RAW264.7 cells; CUMS rats | FST, TST, SPT | 200 mg/kg, 7 days, i.g.; 12.5, 25, 50 mg/kg, 6 weeks, i.g. | ↓ IL-1β, IL-6, TNF-α, IDO mRNA in hippocampus | ↓ CORT in serum ↑ GR mRNA in hippocampus | [104,105] |
| Ginsenoside Rg1 | CSDS mice; CUMS rats | Social interaction test, SPT, FST, TST | 20, 40 mg/kg, 34 days, i.g.; 5, 10, 20 mg/kg, 28 days, i.g. | ↓ IL-6, TNF-α, IL-1β, microglial activation, p-NF-κB in hippocampus | ↓ CORT level in serum ↑ GR protein in PFC and hippocampus | [72,106] | |
| Ginsenoside Rg3 | LPS-induced depressive mice; CUS rats | TPT, FST, EPMT, NSFT, OFT | 20, 40 mg/kg, 3 days, i.g.; 10, 20, 40 mg/kg, 14 days, i.g. | ↓ IL-6, TNF-α in plasma ↓ IL-6, IL-1β, IDO, microglial activation, NF-κB pathway in brain | ↓ CRH, CORT, ACTH in serum | [107,108] | |
| Salvia miltiorrhiza Bunge | Salvianolic acid B | CMS mice | SPT, FST, TST | 20 mg/kg, 3 weeks, i.p. | ↓ IL-1β, TNF-α in hippocampus and cortex ↑ IL-10, TGF-β in hippocampus and cortex | ↓ CORT in plasma | [109] |
| Aquilaria spp. | Agarwood Essential Oil | Restraint stress-induced mice | TST, FST | 10, 20, 40 mg/kg, 10 days, i.p. | ↓ IL-1β, IL-1α, IL-6 in serum | ↓ CRF, CRF receptor in cortex ↓ CORT, ACTH in serum | [110] |
| Chaihu-Shugan-San | Saikosaponin A | CUMS rats | SPT, NPFT, FST | 25, 50 or 100 mg/kg, 4 weeks, p.o. | ↓ IL-1β, IL-6, TNF-α in hippocampus | ↓ CRH in hypothalamus ↓ GR mRNA in hippocampus | [53] |
| Rhodiola rosea L. | Salidroside | OBX rats | TST, FST, SPT | 20, 40 mg/kg, 2 weeks, p.o.; 20, 40 mg/kg, 2 weeks, i.g. | ↓ TNF-α, IL-1β in hippocampus ↓ IL-1β, IL-6, TNF-α, NF-κB activation in PFC | ↑ GR in hippocampus ↓ CRH in hypothalamus | [111,112] |
| Epimedium brevicornu Maxim. | Icariin | CMS rats; CSD mice | SPT, FST, social avoidance evaluations | 20, 40 mg/kg, 35 days, p.o.; 25, 50 mg/kg, 28 days, i.g. | ↓ IL-1β, TNF-α, NF-κB signaling pathway, NLRP3/caspase-1/IL-1β axis activation in hippocampus | ↓ CORT, IL-6 in serum ↑ GR in livers | [83,113] |
| Curcuma longa L. | Curcumin | CUMS rats; CUS rats | SPT, FST, EPM, Shuttle-box testing | 40 mg/kg, 5 weeks, i.p.; 100 mg/kg, 4 weeks, i.g.; 2.5, 5, 10 mg/kg, 21 days, p.o. | ↓ TNF-α, IL-1β, IL-6, NF-κB in mPFC ↓ TNF-α, IL-1β, IL-6 mRNA, NLRP3 in hippocampus | ↓ CORT in serum ↑ GR mRNA in serum | [114,115,116] |
| Polygonum cuspidatum Siebold et Zucc. | Resveratrol | Ouabain-induced depressive mice; Hippocampal neuron cells; CUMS rats | OFT, EPM, Barnes maze performance, object recognition, passive avoidance experiments, SPT, FST | 10 mg/kg, 10 weeks, p.o.; 15 mg/kg, 21 days, i.g. | ↓ IL-1β, IL-17A, IL-8, TNF-α in serum and hippocampal neuron cells | ↓ CORT in serum ↓ CRF mRNA in hypothalamus | [117,118] |
| Bupleurum chinense DC. | Saikosaponin D | LPS-induced depressive mice; UCMS rats | SPT, TST, FST, OFT | 1 mg/kg, 7 days, i.g.; 0.75, 1.5 mg/kg, 21 days, i.g. | ↓ microglia activation in hippocampus ↓ IL-6, TNF-α, IL-1β in vivo and vitro ↓ TLR4/NF-κB signaling pathway in hippocampus | ↓ CORT in serum ↑ GR in hippocampus | [52,119] |
| Scutellaria baicalensis Georgi | Baicalin | CUMS mice; CUMS rats; CORT-induced depressive-like mice | SPT, OFT, TST FST | 60 mg/kg, 14 days, i.g.; 20, 40 mg/kg, i.g., 3 weeks; 10, 20 mg/kg, 21 days, i.g. | ↓ IL-1β, TNF-α, IL-6, TLR4 in the hippocampus ↓ GSK3β/NF-κB/NLRP3 signal pathway in hippocampus | ↓ GR mRNA, GRα in hippocampus | [120,121,122] |
| CHM Formula | Plant Name/Ratio in Fixed Combination | Daily Human Dose | Animal Model | Behavioral Test | Administration Dose/Time/Route of Treatment | Effects on Mediators of Inflammation | Effects on Hormones of the HPA Axis | Reference |
|---|---|---|---|---|---|---|---|---|
| Xiaoyao Pills | Bupleurum chinense DC., Osmanthus fragrans var. aurantiacus Makino, Paeonia lactiflora Pall., Smilax glabra Roxb, Atractylodes macrocephala Koidz., Mentha haplocalyx Briq., Zingiber officinale Roscoe and Glycyrrhiza uralensis Fisch.; 3:3:3:3:3:1:2:1.5 | 2 times/day | LPS-induced depressive mice/rats | TST, FST, OFT, NSFT | 0.4836, 0.93, 1.86 g/kg, 14 days, i.g. | ↓ IL-6 in serum and hippocampus ↓ TNF-α in hippocampus and cortex | ND | [123,124,125] |
| Mahuang-Fuzi-Xixin Decoction | Aconitum carmichaeli Pcbx., Ephedra sinica Stapf and Asarum sieboldii Miq.; 3:2:1 | 3 times/day | LPS-induced depressive mice | SPT, OFT, TST, FST | 2.5, 12.5, 25 g/kg, 1 week, p.o. | ↓ IL-1β, NLRP3 in hippocampus | ND | [126] |
| Jieyu Anshen granule | Bupleurum abchasicum Manden., Ziziphus jujuba Mill., Dens Draconis, Polygala tenuifolia Willd., Lilium brownie var. viridulum Baker, Atractylodes macrocephala Koidz., Triticum aestivum L., Angelica sinensis (Oliv.) Diels, Acorus tatarinowii Schott, Pinellia ternate (Thunb.) Makino, Glycyrrhiza uralensis Fisch., Gardenia jasminoides J. Ellis, Arisaema Cum Bile, Curcuma longa L., Smilax glabra Roxb., and Fructus Jujubae.; 4:5:10:4:10:3:10:3:4:3:3:4:4:4:5:3 | 5 g; 2 times/day | PSD rats | OFT, SPT, water maze test | 1, 3 g/kg, 4 weeks, i.g. | ↓ NF-κB signaling in PFC and hippocampus | ND | [127,128] |
| Jiaotai wan | Coptis chinensis Franch. and Cinnamomum cassia.; 10:1 | 1.5–2.5 g/day | LPS-induced depressive mice | TST, FST, SPT, OFT | 4.2, 8.4 g/kg, 7 days, i.g. | ↓ TNF-α, IL-6 in serum ↓ NF-κB signaling in brain | ND | [129,130] |
| Shen-Qi-Jie-Yu Decoction | Astragalus membranaceus (Fisch) Bunge, Curcuma aromatica Salisb, Ziziphus jujuba var spinosa (Bunge) Hu ex HF Chow, Cornus officinalis Sieb et Zucc (Cornaceae), Codonopsis pilosula (Franch) Nannf, Citrus reticulata Blanco, Citrus medica L, and Angelica sinensis (Oliv) Diels.; 10:7.5:7.5:7.5:6:5:5:5 | 1 time/day | Postpartum depressive rat model | OFT, SPT, FST | 1.25 g/mL, 1, 2, 4 weeks, i.g. | ↓ IL-1β and IL-6 in serum ↓ IL-1RI and gp130 in hippocampus | ND | [54] |
| Jieyuanshen Decoction | Bupleurum chinense DC., Scutellaria baicalensis Georgi, Ziziphusjujuba Mill. var. spinosa (Bunge) Hu ex H.F. Chou, Glycyrrhiza uralensis Fisch., Lilium brownie F.E. Brown var. viridulum Baker, and Pinelliaternata (Thunb.) Breit.; 1:1.5:0.5:1:1:3 | 2 times/day | CUS rats | SPT, OFT | 8.2, 16.3, 32.7 g/kg, 28 days, i.g. | ND | ↓ CORT, ACTH, CRH in serum ↑ GR in hippocampus | [131] |
| Zhizihoupo Decoction | Gardenia jasminoides Ellis, Citrus aurantium L., and Magnolia officinalis Rehd. et Wils.; 1:1:7 | 2 times/day | CUMS rats | SPT, FST, OPT | 3.66, 7.32, 14.64 g/kg, 3 weeks, i.g. | ND | ↓ ACTH, CORT in plasma | [132] |
| Shuyu San | Bupleurum chinense DC., Curcuma aromatica Salisb., Mentha canadensis Linnaeus, Gardenia jasminoides Ellis, Smilax glabra Roxb., Polygala tenuifolia Willd., Acorus gramineus Soland., Ziziphus jujuba var. spinosa (Bunge) Hu ex H. F. Chow., and Albizia julibrissin Durazz.; 5:7.5:3:5:5:5:5:7.5:5 | 2 times/day | UCMS rats | TST, FST | 2.5, 7.5, 25 g/kg, 3 weeks, g.p. | ND | ↓ CRH, ACTH, CORT in serum | [133] |
| Chaihu-Shugan-San | Bupleurum chinense DC., Citrus reticulata Blanco, Ligusticum sinense ‘Chuanxiong’, Cyperus rotundus L., Citrus × aurantium Linnaeus, Paeonia lactiflora Pall., and Glycyrrhiza uralensis Fisch.; 4:4:3:3:3:3:1 | 2 times/day | ApoE-/- mice; UMS rats | SPT, OFT, LDET, TST | 3, 9 g/kg, 16 weeks, i.g.; 5.9 g/kg, 2 weeks, i.g. | ↓ TNF-α, IL-1β, IL-6 in plasma and hippocampus | ↓ CRH, ACTH in plasma | [124,134,135,136,137] |
| Kaixin San | Panax ginseng C.A. Meyer, Poria cocos (Schw.) Wolf, Polygala tenuifolia Willd, and Acorus tatarinowii Schott.; 1:1:25:50 or 3:2:2:3 or 1:1:1:2 | 2 times/day | CUMS rats; CUMS rats | SPT | 338, 676 mg/kg, 3 weeks, p.o.; 3, 10 g/kg, 6 weeks, i.g. | ↓ COX-2, IL-2, IL-6, TNF-α in serum and hippocampus ↑ IL-10, IFN-γ in hippocampus and serum | ↓ CRH, ACTH, CORT in serum and organs | [29,138,139,140] |
| Si-Ni San | Citrus aurantium L., Bupleurum chinense DC., Paeonia lactiflora Pall., and Glycyrrhiza uralensis Fisch.; 2:2:3:2 | 2 times/day | Reserpine-induced rats; Mice | FST, SPT, OFT, TST | 0.75, 1.5, 3.0 g/kg, 2 weeks, p.o.; 325, 650, 1300 mg/kg, 60 min, p.o. | ↓ IL-1β, IL-6, TNF-α in serum, liver, and hippocampus ↓ NF-κB in hippocampus | ↓ CORT in serum | [16,17,141] |
| Banxia houpo Decoction | Pinellia ternate (Thunb.) Breit., Smilax glabra Roxb, Houpoea officinalis (Rehder and E. H. Wilson) N. H. Xia and C. Y. Wu, Zingiber officinale Roscoe, and Folium Perillae; 4:4:3:3:2 | 2 times/day | CUMS rats | SPT | 3.29, 6.58 g/kg, 6 weeks, i.g. | ↓ NLRP3 activation in livers, hypothalamus, PFC | ↓ CORT, CRF in serum | [15,142] |
4. CHM Modulation of the HPA Axis
4.1. CRF Antagonists
4.2. Corticotrophin Releasing Factor 1 (CRF1) Receptor Antagonists
4.3. GR Agonists or Antagonists
5. CHM Effects on the Neuroendocrine-Immune Network
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 30 January 2020).
- Pothula, S.; Kato, T.; Liu, R.J.; Wu, M.; Gerhard, D.; Shinohara, R.; Sliby, A.N.; Chowdhury, G.M.; Behar, K.L.; Sanacora, G.; et al. Cell-Type specific modulation of NMDA receptors triggers antidepressant actions. Mol. Psychiatry 2020. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.Y.; Qin, X.Y.; Yuan, M.M.; Lu, G.J.; Cheng, Y. 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside Reverses Stress-Induced Depression via Inflammatory and Oxidative Stress Pathways. Oxid. Med. Cell. Longev. 2018, 2018, 9501427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Cheng, Y.C. Challenge and Prospect of Traditional Chinese Medicine in Depression Treatment. Front. Neurosci. 2019, 13, 190. [Google Scholar] [CrossRef] [PubMed]
- Fogaca, M.V.; Duman, R.S. Cortical GABAergic Dysfunction in Stress and Depression: New Insights for Therapeutic Interventions. Front. Cell. Neurosci. 2019, 13, 87. [Google Scholar] [CrossRef]
- Kim, Y.K.; Na, K.S.; Myint, A.M.; Leonard, B.E. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 277–284. [Google Scholar] [CrossRef]
- Ignacio, Z.M.; da Silva, R.S.; Plissari, M.E.; Quevedo, J.; Reus, G.Z. Physical Exercise and Neuroinflammation in Major Depressive Disorder. Mol. Neurobiol. 2019, 56, 8323–8335. [Google Scholar] [CrossRef]
- Zunszain, P.A.; Anacker, C.; Cattaneo, A.; Carvalho, L.A.; Pariante, C.M. Glucocorticoids, cytokines and brain abnormalities in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 722–729. [Google Scholar] [CrossRef]
- Jiang, Y.Z.; Peng, T.M.; Gaur, U.; Silva, M.; Little, P.; Chen, Z.; Qiu, W.; Zhang, Y.D.; Zheng, W.H. Role of Corticotropin Releasing Factor in the Neuroimmune Mechanisms of Depression: Examination of Current Pharmaceutical and Herbal Therapies. Front. Cell. Neurosci. 2019, 13, 290. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Liang, Y.; Yang, Y.; Liu, Z.; Yao, K.; Chen, Z.; Zhai, S. Chinese Herbal Medicine for the Treatment of Depression: Applications, Efficacies and Mechanisms. Curr. Pharm. Des. 2017, 23, 5180–5190. [Google Scholar] [CrossRef]
- Feng, D.D.; Tang, T.; Lin, X.P.; Yang, Z.Y.; Yang, S.; Xia, Z.A.; Wang, Y.; Zheng, P.; Wang, Y.; Zhang, C.H. Nine traditional Chinese herbal formulas for the treatment of depression: An ethnopharmacology, phytochemistry, and pharmacology review. Neuropsychiatr. Dis. Treat. 2016, 12, 2387–2402. [Google Scholar] [CrossRef]
- Li, C.; Huang, J.; Cheng, Y.C.; Zhang, Y.W. Traditional Chinese Medicine in Depression Treatment: From Molecules to Systems. Front. Pharmacol. 2020, 11, 586. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Guo, X. Neurobiology of Chinese Herbal Medicine on Major Depressive Disorder. Int. Rev. Neurobiol. 2017, 135, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Yeung, W.F.; Chung, K.F.; Ng, K.Y.; Yu, Y.M.; Zhang, S.P.; Ng, B.F.; Ziea, E.T. Prescription of Chinese Herbal Medicine in Pattern-Based Traditional Chinese Medicine Treatment for Depression: A Systematic Review. Evid. Based Complement. Altern. Med. 2015, 2015, 160189. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.K.; Zheng, Y.J.; Zhang, Y.X.; Liu, J.H.; Jiao, R.Q.; Pan, Y.; Kong, L.D. Banxia-Houpu decoction restores glucose intolerance in CUMS rats through improvement of insulin signaling and suppression of NLRP3 inflammasome activation in liver and brain. J. Ethnopharmacol. 2017, 209, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.T.; Li, J.; Liu, B.B.; Li, C.F. Screening of the antidepressant-like effect of the traditional Chinese medicinal formula Si-Ni-San and their possible mechanism of action in mice. Pharmacogn. Res. 2013, 5, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Chen, T.; Dong, H.; Zhu, L.; Ju, W. Si-Ni-San Prevents Reserpine-Induced Depression by Inhibiting Inflammation and Regulating CYP450 Enzymatic Activity. Front. Pharmacol. 2019, 10, 1518. [Google Scholar] [CrossRef]
- Smith, R.S. The macrophage theory of depression. Med. Hypotheses 1991, 35, 298–306. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Franklin, T.; Iwata, M.; Duman, R.S. Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci. 2016, 17, 497–511. [Google Scholar] [CrossRef]
- Kohler, O.; Krogh, J.; Mors, O.; Benros, M.E. Inflammation in Depression and the Potential for Anti-Inflammatory Treatment. Curr. Neuropharmacol. 2016, 14, 732–742. [Google Scholar] [CrossRef]
- Zhang, J.C.; Yao, W.; Hashimoto, K. Brain-Derived Neurotrophic Factor (BDNF)-TrkB Signaling in Inflammation-Related Depression and Potential Therapeutic Targets. Curr. Neuropharmacol. 2016, 14, 721–731. [Google Scholar] [CrossRef]
- Halaris, A. Inflammation and depression but where does the inflammation come from? Curr. Opin. Psychiatry 2019, 32, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Li, G.; Xu, G.; Liu, J.; Wan, X.; Zhang, J.; Xie, S.; Cheng, J.; Gao, S. Inflammatory cytokines derived from peripheral blood contribute to the modified electroconvulsive therapy-induced cognitive deficits in major depressive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.; Tam, W.W.; Zhang, M.W.; Ho, C.S.; Husain, S.F.; McIntyre, R.S.; Ho, R.C. IL-1β, IL-6, TNF-α and CRP in Elderly Patients with Depression or Alzheimer’s disease: Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 12050. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, C.; Swain, M.G. Immune-to-Brain Communication Pathways in Inflammation-Associated Sickness and Depression. Curr. Top. Behav. Neurosci. 2017, 31, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.C.; Su, Y.P.; Su, K.P.; Chen, P.C. Recurrence of depressive disorders after interferon-induced depression. Transl. Psychiatry 2017, 7, e1026. [Google Scholar] [CrossRef]
- Zhao, Y.; Shang, P.; Wang, M.; Xie, M.; Liu, J. Neuroprotective Effects of Fluoxetine Against Chronic Stress-Induced Neural Inflammation and Apoptosis: Involvement of the p38 Activity. Front. Physiol. 2020, 11, 351. [Google Scholar] [CrossRef]
- Wang, M.; Yan, S.; Zhou, Y.; Xie, P. trans-Cinnamaldehyde Reverses Depressive-Like Behaviors in Chronic Unpredictable Mild Stress Rats by Inhibiting NF-κB/NLRP3 Inflammasome Pathway. Evid. Based Complement. Altern. Med. 2020, 2020, 4572185. [Google Scholar] [CrossRef]
- Dong, X.Z.; Wang, D.X.; Lu, Y.P.; Yuan, S.; Liu, P.; Hu, Y. Antidepressant effects of Kai-Xin-San in fluoxetine-resistant depression rats. Braz. J. Med. Biol. Res. 2017, 50, e6161. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Lawson, M.A.; Kelley, K.W. Inflammation-Associated depression: From serotonin to kynurenine. Psychoneuroendocrinology 2011, 36, 426–436. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, Z.; Shen, F.; Xie, P.; Wang, J.; Zhu, A.S.; Zhu, G. Ginsenoside Rg1 Prevents PTSD-Like Behaviors in Mice Through Promoting Synaptic Proteins, Reducing Kir4.1 and TNF-α in the Hippocampus. Mol. Neurobiol. 2020, 1–14. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Turkheimer, F.E.; Althubaity, N.; Schubert, J.; Nettis, M.A.; Cousins, O.; Dima, D.; Mondelli, V.; Bullmore, E.T.; Pariante, C.; Veronese, M. Increased serum peripheral C-reactive protein is associated with reduced brain barriers permeability of TSPO radioligands in healthy volunteers and depressed patients: Implications for inflammation and depression. Brain Behav. Immun. 2020, 91, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, J.D.; Cha, D.S.; Mansur, R.B.; McIntyre, R.S. Inflamed moods: A review of the interactions between inflammation and mood disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 53, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.L.; Chen, J.G.; Wang, F. Microglia: A Central Player in Depression. Curr. Med. Sci. 2020, 40, 391–400. [Google Scholar] [CrossRef]
- Torres-Platas, S.G.; Cruceanu, C.; Chen, G.G.; Turecki, G.; Mechawar, N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav. Immun. 2014, 42, 50–59. [Google Scholar] [CrossRef]
- Setiawan, E.; Wilson, A.A.; Mizrahi, R.; Rusjan, P.M.; Miler, L.; Rajkowska, G.; Suridjan, I.; Kennedy, J.L.; Rekkas, P.V.; Houle, S.; et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 2015, 72, 268–275. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; You, Z. Switching of the Microglial Activation Phenotype Is a Possible Treatment for Depression Disorder. Front. Cell. Neurosci. 2018, 12, 306. [Google Scholar] [CrossRef]
- Brown, G.C. The endotoxin hypothesis of neurodegeneration. J. Neuroinflamm. 2019, 16, 180. [Google Scholar] [CrossRef]
- Bollinger, J.L.; Wohleb, E.S. The formative role of microglia in stress-induced synaptic deficits and associated behavioral consequences. Neurosci. Lett. 2019, 711, 134369. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Terwilliger, R.; Duman, C.H.; Duman, R.S. Stress-Induced Neuronal Colony Stimulating Factor 1 Provokes Microglia-Mediated Neuronal Remodeling and Depressive-Like Behavior. Biol. Psychiatry 2018, 83, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Shen, C.Y.; Jiang, J.G. Antidepressant active ingredients from herbs and nutraceuticals used in TCM: Pharmacological mechanisms and prospects for drug discovery. Pharmacol. Res. 2019, 150, 104520. [Google Scholar] [CrossRef]
- Guan, F.; Lam, W.; Hu, R.; Kim, Y.K.; Han, H.; Cheng, Y.C. Majority of Chinese Medicine Herb Category “Qing Re Yao” Have Multiple Mechanisms of Anti-inflammatory Activity. Sci. Rep. 2018, 8, 7416. [Google Scholar] [CrossRef]
- Lu, Z.B.; Ou, J.Y.; Cao, H.H.; Liu, J.S.; Yu, L.Z. Heat-Clearing Chinese Medicines in Lipopolysaccharide-Induced Inflammation. Chin. J. Integr. Med. 2020, 26, 552–559. [Google Scholar] [CrossRef]
- Panossian, A.; Brendler, T. The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections. Pharmaceuticals 2020, 13, 236. [Google Scholar] [CrossRef] [PubMed]
- Adzic, M.; Brkic, Z.; Mitic, M.; Francija, E.; Jovicic, M.J.; Radulovic, J.; Maric, N.P. Therapeutic Strategies for Treatment of Inflammation-related Depression. Curr. Neuropharmacol. 2018, 16, 176–209. [Google Scholar] [CrossRef] [PubMed]
- Young, J.J.; Bruno, D.; Pomara, N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J. Affect. Disord. 2014, 169, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.D.; Li, H.; Wan, F.; Su, X.Y.; Lu, Y.; Chen, D.F.; Zhang, Y.Y. Polysaccharides extracted from the roots of Bupleurum chinense DC modulates macrophage functions. Chin. J. Nat. Med. 2017, 15, 889–898. [Google Scholar] [CrossRef]
- Shiu, L.Y.; Huang, H.H.; Chen, C.Y.; Cheng, H.Y.; Chen, C.I.; Kuo, S.M. Reparative and toxicity-reducing effects of liposome-encapsulated saikosaponin in mice with liver fibrosis. Biosci. Rep. 2020, 40, BSR20201219. [Google Scholar] [CrossRef]
- Zhang, B.Z.; Guo, X.T.; Chen, J.W.; Zhao, Y.; Cong, X.; Jiang, Z.L.; Cao, R.F.; Cui, K.; Gao, S.S.; Tian, W.R. Saikosaponin-D attenuates heat stress-induced oxidative damage in LLC-PK1 cells by increasing the expression of anti-oxidant enzymes and HSP72. Am. J. Chin. Med. 2014, 42, 1261–1277. [Google Scholar] [CrossRef]
- Su, J.; Pan, Y.W.; Wang, S.Q.; Li, X.Z.; Huang, F.; Ma, S.P. Saikosaponin-d attenuated lipopolysaccharide-induced depressive-like behaviors via inhibiting microglia activation and neuroinflammation. Int. Immunopharmacol. 2020, 80, 106181. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Chen, S.J.; Liang, W.N.; Wang, M.; Li, C.F.; Wang, S.S.; Dong, S.Q.; Yi, L.T.; Li, C.D. Saikosaponin A attenuates perimenopausal depression-like symptoms by chronic unpredictable mild stress. Neurosci. Lett. 2018, 662, 283–289. [Google Scholar] [CrossRef]
- Li, J.; Zhao, R.; Li, X.; Sun, W.; Qu, M.; Tang, Q.; Yang, X.; Zhang, S. Shen-Qi-Jie-Yu-Fang exerts effects on a rat model of postpartum depression by regulating inflammatory cytokines and CD4+CD25+ regulatory T cells. Neuropsychiatr. Dis. Treat. 2016, 12, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Su, W.J.; Zhang, Y.; Chen, Y.; Gong, H.; Lian, Y.J.; Peng, W.; Liu, Y.Z.; Wang, Y.X.; You, Z.L.; Feng, S.J.; et al. NLRP3 gene knockout blocks NF-κB and MAPK signaling pathway in CUMS-induced depression mouse model. Behav. Brain Res. 2017, 322, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.F.; Zhuang, Q.S.; Shen, L. Genetic overlap between type 2 diabetes and major depressive disorder identified by bioinformatics analysis. Oncotarget 2016, 7, 17410–17414. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, W.; Zhou, X.; Zhuang, F.; Chen, Q.; Li, M.; Ma, T.; Gu, S. Antidepressant-Like effects of alarin produced by activation of TrkB receptor signaling pathways in chronic stress mice. Behav. Brain Res. 2015, 280, 128–140. [Google Scholar] [CrossRef]
- Masson, J.; Emerit, M.B.; Hamon, M.; Darmon, M. Serotonergic signaling: Multiple effectors and pleiotropic effects. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 685–713. [Google Scholar] [CrossRef]
- Duric, V.; Banasr, M.; Licznerski, P.; Schmidt, H.D.; Stockmeier, C.A.; Simen, A.A.; Newton, S.S.; Duman, R.S. A negative regulator of MAP kinase causes depressive behavior. Nat. Med. 2010, 16, 1328–1332. [Google Scholar] [CrossRef]
- Welcome, M.O.; Mastorakis, N.E. Stress-Induced blood brain barrier disruption: Molecular mechanisms and signaling pathways. Pharmacol. Res. 2020, 157, 104769. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.J.; Cole, S.W.; Bower, J.E.; Irwin, M.R.; Taylor, S.E.; Arevalo, J.; Fuligni, A. Depressive symptoms and immune transcriptional profiles in late adolescents. Brain Behav. Immun. 2019, 80, 163–169. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, H.L.; Cai, L.L.; Yan, M.; Dong, S.J.; Mao, B. Tanreqing Injection Attenuates Lipopolysaccharide-Induced Airway Inflammation through MAPK/NF-κB Signaling Pathways in Rats Model. Evid. Based Complement. Altern. Med. 2016, 2016, 5292346. [Google Scholar] [CrossRef] [PubMed]
- Shih, R.H.; Wang, C.Y.; Yang, C.M. NF-κB Signaling Pathways in Neurological Inflammation: A Mini Review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Pedrajas, R.; Ramirez-Lamelas, D.T.; Muriach, B.; Sanchez-Villarejo, M.V.; Almansa, I.; Vidal-Gil, L.; Romero, F.J.; Barcia, J.M.; Muriach, M. Cocaine promotes oxidative stress and microglial-macrophage activation in rat cerebellum. Front. Cell. Neurosci. 2015, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Kaltschmidt, B.; Kaltschmidt, C. NF-κB in the nervous system. Cold Spring Harb. Perspect. Biol. 2009, 1, a001271. [Google Scholar] [CrossRef] [PubMed]
- Caviedes, A.; Lafourcade, C.; Soto, C.; Wyneken, U. BDNF/NF-κB Signaling in the Neurobiology of Depression. Curr. Pharm. Des. 2017, 23, 3154–3163. [Google Scholar] [CrossRef]
- Pradere, J.P.; Hernandez, C.; Koppe, C.; Friedman, R.A.; Luedde, T.; Schwabe, R.F. Negative regulation of NF-κB p65 activity by serine 536 phosphorylation. Sci. Signal. 2016, 9, ra85. [Google Scholar] [CrossRef]
- Koo, J.W.; Russo, S.J.; Ferguson, D.; Nestler, E.J.; Duman, R.S. Nuclear factor-κB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl. Acad. Sci. USA 2010, 107, 2669–2674. [Google Scholar] [CrossRef]
- Bottcher, M.; Muller-Fielitz, H.; Sundaram, S.M.; Gallet, S.; Neve, V.; Shionoya, K.; Zager, A.; Quan, N.; Liu, X.; Schmidt-Ullrich, R.; et al. NF-κB signaling in tanycytes mediates inflammation-induced anorexia. Mol. Metab. 2020, 39, 101022. [Google Scholar] [CrossRef]
- Jin, Y.; Cui, R.; Zhao, L.; Fan, J.; Li, B. Mechanisms of Panax ginseng action as an antidepressant. Cell Prolif. 2019, 52, e12696. [Google Scholar] [CrossRef]
- Lou, T.; Huang, Q.; Su, H.; Zhao, D.; Li, X. Targeting Sirtuin 1 signaling pathway by ginsenosides. J. Ethnopharmacol. 2020, 268, 113657. [Google Scholar] [CrossRef]
- Jiang, N.; Lv, J.; Wang, H.; Huang, H.; Wang, Q.; Lu, C.; Zeng, G.; Liu, X.M. Ginsenoside Rg1 ameliorates chronic social defeat stress-induced depressive-like behaviors and hippocampal neuroinflammation. Life Sci. 2020, 252, 117669. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Song, Q.; Wang, P.; Li, Y.; Yang, M.; Yu, S.Y. Neuroprotective Effects of Ginsenoside-Rg1 Against Depression-Like Behaviors via Suppressing Glial Activation, Synaptic Deficits, and Neuronal Apoptosis in Rats. Front. Immunol. 2018, 9, 2889. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.F.; Song, X.Y.; Chu, S.F.; Chen, J.; Ji, H.J.; Chen, X.Y.; Yuan, Y.H.; Han, N.; Zhang, J.T.; Chen, N.H. Inhibitory effect of ginsenoside Rg1 on lipopolysaccharide-induced microglial activation in mice. Brain Res. 2011, 1374, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, F.N.; Costa, A.P.; Ghisleni, G.; Diaz, A.P.; Rodrigues, A.L.S.; Peluffo, H.; Kaster, M.P. NLRP3 inflammasome-driven pathways in depression: Clinical and preclinical findings. Brain Behav. Immun. 2017, 64, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Arbore, G.; Kemper, C. A novel “complement-metabolism-inflammasome axis” as a key regulator of immune cell effector function. Eur. J. Immunol. 2016, 46, 1563–1573. [Google Scholar] [CrossRef]
- Walsh, J.G.; Muruve, D.A.; Power, C. Inflammasomes in the CNS. Nat. Rev. Neurosci. 2014, 15, 84–97. [Google Scholar] [CrossRef]
- Shao, B.Z.; Cao, Q.; Liu, C. Targeting NLRP3 Inflammasome in the Treatment of CNS Diseases. Front. Mol. Neurosci. 2018, 11, 320. [Google Scholar] [CrossRef]
- Alcocer-Gómez, E.; de Miguel, M.; Casas-Barquero, N.; Núñez-Vasco, J.; Sánchez-Alcazar, J.A.; Fernández-Rodríguez, A.; Cordero, M.D. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav. Immun. 2014, 36, 111–117. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Liu, Y.Z.; Shen, X.L.; Wu, T.Y.; Zhang, T.; Wang, W.; Wang, Y.X.; Jiang, C.L. NLRP3 Inflammasome Mediates Chronic Mild Stress-Induced Depression in Mice via Neuroinflammation. Int. J. Neuropsychopharmacol. 2015, 18, pyv006. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Peng, Y.L.; Liu, Y.Z.; Wu, T.Y.; Shen, X.L.; Zhou, J.R.; Sun, D.Y.; Huang, A.J.; Wang, X.; et al. Involvement of inflammasome activation in lipopolysaccharide-induced mice depressive-like behaviors. CNS Neurosci. Ther. 2014, 20, 119–124. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, P.; Zhao, Y.; Zhang, L.; Zhang, Z.; Dong, X.; Wu, Z.; Xu, Y.; Chen, Y. trans-Cinnamaldehyde Inhibits Microglial Activation and Improves Neuronal Survival against Neuroinflammation in BV2 Microglial Cells with Lipopolysaccharide Stimulation. Evid. Based Complement. Altern. Med. 2017, 2017, 4730878. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xu, C.; Wu, X.; Liu, F.; Du, Y.; Sun, J.; Tao, J.; Dong, J. Icariin exerts an antidepressant effect in an unpredictable chronic mild stress model of depression in rats and is associated with the regulation of hippocampal neuroinflammation. Neuroscience 2015, 294, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, C.; He, H.; Ding, W. 2, 3, 5, 4′-Tetrahydroxystilbene-2-O-β-D-glucoside prevention of lipopolysaccharide-induced depressive-like behaviors in mice involves neuroinflammation and oxido-nitrosative stress inhibition. Behav. Pharmacol. 2017, 28, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Liu, X.; Liu, J.; Zhao, Y.; Li, H.; Cai, E.; Li, P.; Gao, Y. Study on antidepressant activity of chiisanoside in mice. Int. Immunopharmacol. 2018, 57, 33–42. [Google Scholar] [CrossRef]
- Li, H.; Lin, S.; Qin, T.; Li, H.; Ma, Z.; Ma, S. Senegenin exerts anti-depression effect in mice induced by chronic un-predictable mild stress via inhibition of NF-κB regulating NLRP3 signal pathway. Int. Immunopharmacol. 2017, 53, 24–32. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, R.; Qiao, Y.; Xue, F.; Nie, H.; Zhang, Z.; Wang, Y.; Peng, Z.; Tan, Q. Gastrodin ameliorates depression-like behaviors and up-regulates proliferation of hippocampal-derived neural stem cells in rats: Involvement of its anti-inflammatory action. Behav. Brain Res. 2014, 266, 153–160. [Google Scholar] [CrossRef]
- Liu, P.; Bai, X.; Zhang, T.; Zhou, L.; Li, J.; Zhang, L. The protective effect of Lonicera japonica polysaccharide on mice with depression by inhibiting NLRP3 inflammasome. Ann. Transl. Med. 2019, 7, 811. [Google Scholar] [CrossRef]
- Geng, J.; Liu, J.; Yuan, X.; Liu, W.; Guo, W. Andrographolide triggers autophagy-mediated inflammation inhibition and attenuates chronic unpredictable mild stress (CUMS)-induced depressive-like behavior in mice. Toxicol. Appl. Pharmacol. 2019, 379, 114688. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, P.P.; Hu, K.L.; Li, L.N.; Yu, X.; Lu, Y.; Chang, H.S. Antidepressant-Like Effect and Mechanism of Action of Honokiol on the Mouse Lipopolysaccharide (LPS) Depression Model. Molecules 2019, 24, 2035. [Google Scholar] [CrossRef]
- Zhang, L.; Previn, R.; Lu, L.; Liao, R.F.; Jin, Y.; Wang, R.K. Crocin, a natural product attenuates lipopolysaccharide-induced anxiety and depressive-like behaviors through suppressing NF-kB and NLRP3 signaling pathway. Brain Res. Bull. 2018, 142, 352–359. [Google Scholar] [CrossRef]
- Song, Y.; Sun, R.; Ji, Z.; Li, X.; Fu, Q.; Ma, S. Perilla aldehyde attenuates CUMS-induced depressive-like behaviors via regulating TXNIP/TRX/NLRP3 pathway in rats. Life Sci. 2018, 206, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.W.; Wang, S.Y.; Ma, Z.Q.; Li, R.P.; Li, S.S.; Xue, J.S.; Li, W.; Niu, X.X.; Yan, L.; Zhang, X.; et al. Effects of perillaldehyde on alternations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacol. Biochem. Behav. 2014, 116, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.W.; Li, R.P.; Li, M.; Wang, S.Y.; Zhang, X.; Niu, X.X.; Li, W.; Yan, L.; Wang, Y.; Fu, Q.; et al. Antidepressant-Like effect of essential oil of Perilla frutescens in a chronic, unpredictable, mild stress-induced depression model mice. Chin. J. Nat. Med. 2014, 12, 753–759. [Google Scholar] [CrossRef]
- Park, S.H.; Jang, S.; Son, E.; Lee, S.W.; Park, S.D.; Sung, Y.Y.; Kim, H.K. Polygonum aviculare L. extract reduces fatigue by inhibiting neuroinflammation in restraint-stressed mice. Phytomedicine 2018, 42, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Li, C.F.; Chen, X.Q.; Chen, S.M.; Chen, X.M.; Geng, D.; Liu, Q.; Yi, L.T. Evaluation of the toxicological properties and anti-inflammatory mechanism of Hemerocallis citrina in LPS-induced depressive-like mice. Biomed. Pharmacother. 2017, 91, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Shen, J.D.; Xu, L.P.; Li, H.B.; Li, Y.C.; Yi, L.T. Ferulic acid inhibits neuro-inflammation in mice exposed to chronic unpredictable mild stress. Int. Immunopharmacol. 2017, 45, 128–134. [Google Scholar] [CrossRef]
- Li, J.; Huang, S.; Huang, W.; Wang, W.; Wen, G.; Gao, L.; Fu, X.; Wang, M.; Liang, W.; Kwan, H.Y.; et al. Paeoniflorin ameliorates interferon-α-induced neuroinflammation and depressive-like behaviors in mice. Oncotarget 2017, 8, 8264–8282. [Google Scholar] [CrossRef]
- An, L.; Li, J.; Yu, S.T.; Xue, R.; Yu, N.J.; Chen, H.X.; Zhang, L.M.; Zhao, N.; Li, Y.F.; Zhang, Y.Z. Effects of the total flavonoid extract of Xiaobuxin-Tang on depression-like behavior induced by lipopolysaccharide and proinflammatory cytokine levels in mice. J. Ethnopharmacol. 2015, 163, 83–87. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Pan, F. The effects of EGb761 on lipopolysaccharide-induced depressive-like behaviour in C57BL/6J mice. Cent. Eur. J. Immunol. 2015, 40, 11–17. [Google Scholar] [CrossRef]
- Qiu, Z.K.; Zhang, G.H.; Zhong, D.S.; He, J.L.; Liu, X.; Chen, J.S.; Wei, D.N. Puerarin ameliorated the behavioral deficits induced by chronic stress in rats. Sci. Rep. 2017, 7, 6266. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, D.; Hui, S.; Zhang, Y.; Hu, S. Effect of tribulus terrestris saponins on behavior and neuroendocrine in chronic mild stress depression rats. J. Tradit. Chin. Med. 2013, 33, 228–232. [Google Scholar] [CrossRef][Green Version]
- Wang, J.M.; Pei, L.X.; Zhang, Y.Y.; Cheng, Y.X.; Niu, C.L.; Cui, Y.; Feng, W.S.; Wang, G.F. Ethanol extract of Rehmannia glutinosa exerts antidepressant-like effects on a rat chronic unpredictable mild stress model by involving monoamines and BDNF. Metab. Brain Dis. 2018, 33, 885–892. [Google Scholar] [CrossRef]
- Kang, A.; Hao, H.; Zheng, X.; Liang, Y.; Xie, Y.; Xie, T.; Dai, C.; Zhao, Q.; Wu, X.; Xie, L.; et al. Peripheral anti-inflammatory effects explain the ginsenosides paradox between poor brain distribution and anti-depression efficacy. J. Neuroinflamm. 2011, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Luo, Y.; Zhang, R.; Guo, J. Effects of ginsenosides on hypothalamic-pituitary-adrenal function and brain-derived neurotrophic factor in rats exposed to chronic unpredictable mild stress. Zhongguo Zhongyao Zazhi 2011, 36, 1342–1347. [Google Scholar] [PubMed]
- Mou, Z.; Huang, Q.; Chu, S.F.; Zhang, M.J.; Hu, J.F.; Chen, N.H.; Zhang, J.T. Antidepressive effects of ginsenoside Rg1 via regulation of HPA and HPG axis. Biomed. Pharmacother. 2017, 92, 962–971. [Google Scholar] [CrossRef]
- Kang, A.; Xie, T.; Zhu, D.; Shan, J.; Di, L.; Zheng, X. Suppressive Effect of Ginsenoside Rg3 against Lipopolysaccharide-Induced Depression-Like Behavior and Neuroinflammation in Mice. J. Agric. Food Chem. 2017, 65, 6861–6869. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.N.; Chen, L.F.; Su, J.; Liu, Z.L.; Chen, J.; Lin, Q.F.; Mao, W.D.; Shen, D. The anxiolytic-like effects of ginsenoside Rg3 on chronic unpredictable stress in rats. Sci. Rep. 2018, 8, 7741. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Wu, X.H.; Feng, Y.; Xie, X.F.; Fan, Y.H.; Yan, S.; Zhao, Q.Y.; Peng, C.; You, Z.L. Salvianolic acid B ameliorates depressive-like behaviors in chronic mild stress-treated mice: Involvement of the neuroinflammatory pathway. Acta Pharmacol. Sin. 2016, 37, 1141–1153. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Yu, Z.; Wu, C.; Peng, D.; Liu, X.; Liu, Y.; Yang, Y.; Guo, P.; Wei, J. Agarwood Essential Oil Ameliorates Restrain Stress-Induced Anxiety and Depression by Inhibiting HPA Axis Hyperactivity. Int. J. Mol. Sci. 2018, 19, 3468. [Google Scholar] [CrossRef]
- Yang, S.J.; Yu, H.Y.; Kang, D.Y.; Ma, Z.Q.; Qu, R.; Fu, Q.; Ma, S.P. Antidepressant-Like effects of salidroside on olfactory bulbectomy-induced pro-inflammatory cytokine production and hyperactivity of HPA axis in rats. Pharmacol. Biochem. Behav. 2014, 124, 451–457. [Google Scholar] [CrossRef]
- Zhang, X.; Du, Q.; Liu, C.; Yang, Y.; Wang, J.; Duan, S.; Duan, J. Rhodioloside ameliorates depressive behavior via up-regulation of monoaminergic system activity and anti-inflammatory effect in olfactory bulbectomized rats. Int. Immunopharmacol. 2016, 36, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Du, J.; Xu, C.; Le, J.; Xu, Y.; Liu, B.; Dong, J. Icariin attenuates social defeat-induced down-regulation of glucocorticoid receptor in mice. Pharmacol. Biochem. Behav. 2011, 98, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Song, Q.; Wang, P.; Li, Y.; Yang, M.; Liu, B.; Yu, S.Y. Curcumin Protects Against Chronic Stress-induced Dysregulation of Neuroplasticity and Depression-like Behaviors via Suppressing IL-1β Pathway in Rats. Neuroscience 2018, 392, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Guo, Y.J.; Han, W.X.; Yang, M.Q.; Wen, L.P.; Wang, K.Y.; Jiang, P. Curcumin relieves depressive-like behaviors via inhibition of the NLRP3 inflammasome and kynurenine pathway in rats suffering from chronic unpredictable mild stress. Int. Immunopharmacol. 2019, 67, 138–144. [Google Scholar] [CrossRef]
- Xu, Y.; Ku, B.; Tie, L.; Yao, H.; Jiang, W.; Ma, X.; Li, X. Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res. 2006, 1122, 56–64. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; An, J.; Yuan, G.; Hao, X.; Zhang, Y. Resveratrol ameliorates depressive disorder through the NETRIN1-mediated extracellular signal-regulated kinase/cAMP signal transduction pathway. Mol. Med. Rep. 2018, 17, 4611–4618. [Google Scholar] [CrossRef]
- Yang, X.H.; Song, S.Q.; Xu, Y. Resveratrol ameliorates chronic unpredictable mild stress-induced depression-like behavior: Involvement of the HPA axis, inflammatory markers, BDNF, and Wnt/β-catenin pathway in rats. Neuropsychiatr. Dis. Treat. 2017, 13, 2727–2736. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhao, Y.H.; Zeng, M.J.; Fang, F.; Li, M.; Qin, T.T.; Ye, L.Y.; Li, H.W.; Qu, R.; Ma, S.P. Saikosaponin D relieves unpredictable chronic mild stress induced depressive-like behavior in rats: Involvement of HPA axis and hippocampal neurogenesis. Psychopharmacology 2017, 234, 3385–3394. [Google Scholar] [CrossRef]
- Guo, L.T.; Wang, S.Q.; Su, J.; Xu, L.X.; Ji, Z.Y.; Zhang, R.Y.; Zhao, Q.W.; Ma, Z.Q.; Deng, X.Y.; Ma, S.P. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J. Neuroinflamm. 2019, 16, 95. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Zeng, M.J.; Zhou, L.P.; Li, Y.Q.; Zhao, F.; Shang, Z.Y.; Deng, X.Y.; Ma, Z.Q.; Fu, Q.; Ma, S.P.; et al. Baicalin exerts neuroprotective effects via inhibiting activation of GSK3β/NF-κB/NLRP3 signal pathway in a rat model of depression. Int. Immunopharmacol. 2018, 64, 175–182. [Google Scholar] [CrossRef]
- Li, Y.C.; Wang, L.L.; Pei, Y.Y.; Shen, J.D.; Li, H.B.; Wang, B.Y.; Bai, M. Baicalin decreases SGK1 expression in the hippocampus and reverses depressive-like behaviors induced by corticosterone. Neuroscience 2015, 311, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Luo, J.; Fang, Y.; Liu, X.; Rao, Z.; Liu, R.; Zeng, N. Xiaoyao Pills Prevent Lipopolysaccharide-Induced Depression by Inhibiting Inflammation and Protecting Nerves. Front. Pharmacol. 2019, 10, 1324. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shergis, J.L.; Di, Y.M.; Zhang, A.L.; Lu, C.; Guo, X.; Fang, Z.; Xue, C.C.; Li, Y. Managing Depression with Bupleurum chinense Herbal Formula: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Altern. Complement. Med. 2020, 26, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, G.; Han, T.; Li, J.; Huang, H.L. Network Meta-Analysis of Chinese Patent Medicines in Treatment of Liver Stagnation and Spleen Deficiency of Depression. Zhongguo Zhongyao Zazhi 2019, 44, 5217–5224. [Google Scholar] [CrossRef]
- Jing, W.; Song, S.; Sun, H.; Chen, Y.; Zhao, Q.; Zhang, Y.; Dai, G.; Ju, W. Mahuang-Fuzi-Xixin Decoction Reverses Depression-Like Behavior in LPS-Induced Mice by Regulating NLRP3 Inflammasome and Neurogenesis. Neural Plast. 2019, 2019, 1571392. [Google Scholar] [CrossRef]
- Du, Y.; Ruan, J.; Zhang, L.; Fu, F. Jieyu Anshen Granule, a Chinese Herbal Formulation, Exerts Effects on Poststroke Depression in Rats. Evid. Based Complement. Altern. Med. 2020, 2020, 7469068. [Google Scholar] [CrossRef]
- Guo, F.; Wang, L.; Yang, Y.; Li, L. Clinical observation on treating post-stroke depression with Anshen Jieyu Wan. Clin. J. Chin. Med. 2018, 10. [Google Scholar] [CrossRef]
- Zhe, Q.; Sulei, W.; Weiwei, T.; Hongyan, L.; Jianwei, W. Effects of Jiaotaiwan on depressive-like behavior in mice after lipopolysaccharide administration. Metab. Brain Dis. 2017, 32, 415–426. [Google Scholar] [CrossRef]
- Li, Y. Clinical observation on application of Jiao Tai Pill on Shenque (CV 8) in treating insomnia. J. Acupunct. Tuina Sci. 2010, 8, 35–37. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, X.; Yang, Y.; Zhao, H.; Wang, Y.; Yao, X.; Wang, L.; Chang, J.; Zou, H. Jieyuanshen Decoction Exerts Antidepressant Effects on Depressive Rat Model Via Regulating Hpa Axis and the Level of Amino Acids Neurotransmitter. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 33–46. [Google Scholar] [CrossRef][Green Version]
- Xing, H.; Zhang, K.; Zhang, R.; Shi, H.; Bi, K.; Chen, X. Antidepressant-Like effect of the water extract of the fixed combination of Gardenia jasminoides, Citrus aurantium and Magnolia officinalis in a rat model of chronic unpredictable mild stress. Phytomedicine 2015, 22, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, M.; Wang, F.; Sun, Z.; Quanzhi, H.; Geng, M.; Chen, H.; Duan, D. Antidepressant-Like effects of shuyusan in rats exposed to chronic stress: Effects on hypothalamic-pituitary-adrenal function. Evid. Based Complement. Altern. Med. 2012, 2012, 940846. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, L.; Yu, A.L.; Wang, Z.L.; Chen, K.; Zheng, W.; Zhou, J.J.; Xie, Q.; Yan, H.B.; Ren, P.; Huang, X. Chaihu-Shugan-San and absorbed meranzin hydrate induce anti-atherosclerosis and behavioral improvements in high-fat diet ApoE-/- mice via anti-inflammatory and BDNF-TrkB pathway. Biomed. Pharmacother. 2019, 115, 108893. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, C.H.; Wang, S.E.; Qiu, J.; Hu, S.Y.; Xiao, G.L. Effects of Chaihu Shugan San on behavior and plasma levels of corticotropin releasing hormone and adrenocorticotropic hormone of rats with chronic mild unpredicted stress depression. Zhong Xi Yi Jie He Xue Bao 2009, 7, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, R.; Huang, X. Meta-Analysis of the clinical effectiveness of traditional Chinese medicine formula Chaihu-Shugan-San in depression. J. Ethnopharmacol. 2012, 141, 571–577. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, X.; Zhang, J.; Chen, Y. Treatment of depression with Chai Hu Shu Gan San: A systematic review and meta-analysis of 42 randomized controlled trials. BMC Complement. Altern. Med. 2018, 18, 66. [Google Scholar] [CrossRef]
- Cao, C.; Liu, M.; Qu, S.; Huang, R.; Qi, M.; Zhu, Z.; Zheng, J.; Chen, Z.; Wang, Z.; Han, Z.; et al. Chinese medicine formula Kai-Xin-San ameliorates depression-like behaviours in chronic unpredictable mild stressed mice by regulating gut microbiota-inflammation-stress system. J. Ethnopharmacol. 2020, 261, 113055. [Google Scholar] [CrossRef]
- Bao, Z.; Zhao, G.; Sun, W.; Chen, B. Clinical Curative Effects of Kaixin Powder on Depression with Mild or Moderate Degree. Chin. Arch. Tradit. Chin. Med. 2011, 29. [Google Scholar] [CrossRef]
- Cao, C.; Xiao, J.; Liu, M.; Ge, Z.; Huang, R.; Qi, M.; Zhu, H.; Zhu, Y.; Duan, J.A. Active components, derived from Kai-xin-san, a herbal formula, increase the expressions of neurotrophic factor NGF and BDNF on mouse astrocyte primary cultures via cAMP-dependent signaling pathway. J. Ethnopharmacol. 2018, 224, 554–562. [Google Scholar] [CrossRef]
- Zhou, J.; Cai, H.; Duan, Y.; Pei, K.; Fan, K.L.; Xu, Y.Y.; Zhao, J.Y.; Liu, J. Research progress on antidepressant effects of Sini San based on three progressive levels of “single herb, herb-pair, and complicated Chinese herbal formula”. Zhongguo Zhongyao Zazhi 2018, 43, 46–51. [Google Scholar] [CrossRef]
- Bo, P.; Chen, Q.M.; Zhu, H.H.; Zhang, X.D.; Xu, H.R.; Zhang, Y.; Cao, Y.J. Clinical observations on 46 cases of globus hystericus treated with modified Banxia Houpu decoction. J. Tradit. Chin. Med. 2010, 30, 103–107. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Juruena, M.F. Early-Life stress and HPA axis trigger recurrent adulthood depression. Epilepsy Behav. 2014, 38, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Juruena, M.F.; Agustini, B.; Cleare, A.J.; Young, A.H. A translational approach to clinical practice via stress-responsive glucocorticoid receptor signaling. Stem Cell Investig. 2017, 4, 13. [Google Scholar] [CrossRef][Green Version]
- Pandey, G.N.; Rizavi, H.S.; Bhaumik, R.; Ren, X. Increased protein and mRNA expression of corticotropin-releasing factor (CRF), decreased CRF receptors and CRF binding protein in specific postmortem brain areas of teenage suicide subjects. Psychoneuroendocrinology 2019, 106, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Horchar, M.J.; Wohleb, E.S. Glucocorticoid receptor antagonism prevents microglia-mediated neuronal remodeling and behavioral despair following chronic unpredictable stress. Brain Behav. Immun. 2019, 81, 329–340. [Google Scholar] [CrossRef]
- Pariante, C.M.; Lightman, S.L. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef]
- Gillespie, C.F.; Nemeroff, C.B. Hypercortisolemia and depression. Psychosom. Med. 2005, 67, S26–S28. [Google Scholar] [CrossRef]
- D’Alessio, L.; Mesarosova, L.; Anink, J.J.; Kochen, S.; Solis, P.; Oddo, S.; Konopka, H.; Lyer, A.M.; Mühlebner, A.; Lucassen, P.J.; et al. Reduced expression of the glucocorticoid receptor in the hippocampus of patients with drug-resistant temporal lobe epilepsy and comorbid depression. Epilepsia 2020, 61, 1595–1605. [Google Scholar] [CrossRef]
- Waters, R.P.; Rivalan, M.; Bangasser, D.A.; Deussing, J.M.; Ising, M.; Wood, S.K.; Holsboer, F.; Summers, C.H. Evidence for the role of corticotropin-releasing factor in major depressive disorder. Neurosci. Biobehav. Rev. 2015, 58, 63–78. [Google Scholar] [CrossRef]
- Fahmy, H.; Kuppast, B.; Ismail, M.T. Structure and Function of Small Non-Peptide CRF Antagonists and Their Potential Clinical Use. Curr. Mol. Pharmacol. 2017, 10, 270–281. [Google Scholar] [CrossRef]
- Bhutada, P.; Mundhada, Y.; Bansod, K.; Ubgade, A.; Quazi, M.; Umathe, S.; Mundhada, D. Reversal by quercetin of corticotrophin releasing factor induced anxiety- and depression-like effect in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Kawai, Y.; Terao, J. Suppressive effect of quercetin on acute stress-induced hypothalamic-pituitary-adrenal axis response in Wistar rats. J. Nutr. Biochem. 2010, 21, 374–380. [Google Scholar] [CrossRef]
- Ketchesin, K.D.; Stinnett, G.S.; Seasholtz, A.F. Corticotropin-Releasing hormone-binding protein and stress: From invertebrates to humans. Stress 2017, 20, 449–464. [Google Scholar] [CrossRef]
- Nielsen, D.M. Corticotropin-Releasing factor type-1 receptor antagonists: The next class of antidepressants? Life Sci. 2006, 78, 909–919. [Google Scholar] [CrossRef]
- Henckens, M.J.; Deussing, J.M.; Chen, A. Region-Specific roles of the corticotropin-releasing factor-urocortin system in stress. Nat. Rev. Neurosci. 2016, 17, 636–651. [Google Scholar] [CrossRef]
- Im, E. Multi-Facets of Corticotropin-Releasing Factor in Modulating Inflammation and Angiogenesis. J. Neurogastroenterol. Motil. 2015, 21, 25–32. [Google Scholar] [CrossRef]
- Dedic, N.; Chen, A.; Deussing, J.M. The CRF Family of Neuropeptides and their Receptors—Mediators of the Central Stress Response. Curr. Mol. Pharmacol. 2018, 11, 4–31. [Google Scholar] [CrossRef]
- Paez-Pereda, M.; Hausch, F.; Holsboer, F. Corticotropin releasing factor receptor antagonists for major depressive disorder. Expert Opin. Investig. Drugs 2011, 20, 519–535. [Google Scholar] [CrossRef]
- Menke, A. Is the HPA Axis as Target for Depression Outdated, or Is There a New Hope? Front. Psychiatry 2019, 10, 101. [Google Scholar] [CrossRef]
- Jutkiewicz, E.M.; Wood, S.K.; Houshyar, H.; Hsin, L.W.; Rice, K.C.; Woods, J.H. The effects of CRF antagonists, antalarmin, CP154,526, LWH234, and R121919, in the forced swim test and on swim-induced increases in adrenocorticotropin in rats. Psychopharmacology 2005, 180, 215–223. [Google Scholar] [CrossRef]
- Simmen, U.; Bobirnac, I.; Ullmer, C.; Lübbert, H.; Berger Büter, K.; Schaffner, W.; Schoeffter, P. Antagonist effect of pseudohypericin at CRF1 receptors. Eur. J. Pharmacol. 2003, 458, 251–256. [Google Scholar] [CrossRef]
- Thiagarajah, A.S.; Eades, L.E.; Thomas, P.R.; Guymer, E.K.; Morand, E.F.; Clarke, D.M.; Leech, M. GILZ: Glitzing up our understanding of the glucocorticoid receptor in psychopathology. Brain Res. 2014, 1574, 60–69. [Google Scholar] [CrossRef]
- Leistner, C.; Menke, A. How to measure glucocorticoid receptor’s sensitivity in patients with stress-related psychiatric disorders. Psychoneuroendocrinology 2018, 91, 235–260. [Google Scholar] [CrossRef]
- Moraitis, A.G.; Block, T.; Nguyen, D.; Belanoff, J.K. The role of glucocorticoid receptors in metabolic syndrome and psychiatric illness. J. Steroid Biochem. Mol. Biol. 2017, 165, 114–120. [Google Scholar] [CrossRef]
- Sarubin, N.; Hilbert, S.; Naumann, F.; Zill, P.; Wimmer, A.M.; Nothdurfter, C.; Rupprecht, R.; Baghai, T.C.; Bühner, M.; Schüle, C. The sex-dependent role of the glucocorticoid receptor in depression: Variations in the NR3C1 gene are associated with major depressive disorder in women but not in men. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 123–133. [Google Scholar] [CrossRef]
- Pandey, G.N.; Rizavi, H.S.; Ren, X.; Dwivedi, Y.; Palkovits, M. Region-Specific alterations in glucocorticoid receptor expression in the postmortem brain of teenage suicide victims. Psychoneuroendocrinology 2013, 38, 2628–2639. [Google Scholar] [CrossRef]
- Guidotti, G.; Calabrese, F.; Anacker, C.; Racagni, G.; Pariante, C.M.; Riva, M.A. Glucocorticoid receptor and FKBP5 expression is altered following exposure to chronic stress: Modulation by antidepressant treatment. Neuropsychopharmacology 2013, 38, 616–627. [Google Scholar] [CrossRef]
- Van Rossum, E.F.; Binder, E.B.; Majer, M.; Koper, J.W.; Ising, M.; Modell, S.; Salyakina, D.; Lamberts, S.W.; Holsboer, F. Polymorphisms of the glucocorticoid receptor gene and major depression. Biol. Psychiatry 2006, 59, 681–688. [Google Scholar] [CrossRef]
- Block, T.S.; Kushner, H.; Kalin, N.; Nelson, C.; Belanoff, J.; Schatzberg, A. Combined Analysis of Mifepristone for Psychotic Depression: Plasma Levels Associated with Clinical Response. Biol. Psychiatry 2018, 84, 46–54. [Google Scholar] [CrossRef]
- Soria, V.; Gonzalez-Rodriguez, A.; Huerta-Ramos, E.; Usall, J.; Cobo, J.; Bioque, M.; Barbero, J.D.; García-Rizo, C.; Tost, M.; Monreal, J.A.; et al. Targeting hypothalamic-pituitary-adrenal axis hormones and sex steroids for improving cognition in major mood disorders and schizophrenia: A systematic review and narrative synthesis. Psychoneuroendocrinology 2018, 93, 8–19. [Google Scholar] [CrossRef]
- Wang, W.; Liu, L.; Yang, X.; Gao, H.; Tang, Q.K.; Yin, L.Y.; Yin, X.Y.; Hao, J.R.; Geng, D.Q.; Gao, C. Ketamine improved depressive-like behaviors via hippocampal glucocorticoid receptor in chronic stress induced-susceptible mice. Behav. Brain Res. 2019, 364, 75–84. [Google Scholar] [CrossRef]
- Garde, D. Corcept Tanks as Depression Drug Comes Up Short In Phase III. Available online: https://www.fiercebiotech.com/biotech/corcept-tanks-as-depression-drug-comes-up-short-phase-iii (accessed on 5 May 2014).
- Clark, R.D. Glucocorticoid receptor antagonists. Curr. Top. Med. Chem. 2008, 8, 813–838. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, M.; Guo, M.; Zhong, X.; Hu, Y. Huang Qin Hua Shi decoction for high-temperature- and high-humidity-induced cognitive-behavioral disorder in rats is associated with deactivation of the hypothalamic-pituitary-adrenal axis. J. Int. Med. Res. 2019, 47, 5752–5766. [Google Scholar] [CrossRef]
- Horowitz, M.A.; Cattaneo, A.; Cattane, N.; Lopizzo, N.; Tojo, L.; Bakunina, N.; Musaelyan, K.; Borsini, A.; Zunszain, P.A.; Pariante, C.M. Glucocorticoids prime the inflammatory response of human hippocampal cells through up-regulation of inflammatory pathways. Brain Behav. Immun. 2020, 87, 777–794. [Google Scholar] [CrossRef]
- Bottasso, E. Toward the Existence of a Sympathetic Neuroplasticity Adaptive Mechanism Influencing the Immune Response. A Hypothetical View-Part II. Front. Endocrinol. 2019, 10, 633. [Google Scholar] [CrossRef]
- Dai, S.; Mo, Y.; Wang, Y.; Xiang, B.; Liao, Q.; Zhou, M.; Li, X.; Li, Y.; Xiong, W.; Li, G.; et al. Chronic Stress Promotes Cancer Development. Front. Oncol. 2020, 10, 1492. [Google Scholar] [CrossRef]
- Chrousos, G.P.; Gold, P.W. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. J. Am. Med. Assoc. 1992, 267, 1244–1252. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Bellavance, M.A.; Rivest, S. The HPA—Immune Axis and the Immunomodulatory Actions of Glucocorticoids in the Brain. Front. Immunol. 2014, 5, 136. [Google Scholar] [CrossRef]
- Dantzer, R. Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol. Rev. 2018, 98, 477–504. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.; Holden, N.S. Separating transrepression and transactivation: A distressing divorce for the glucocorticoid receptor? Mol. Pharmacol. 2007, 72, 799–809. [Google Scholar] [CrossRef] [PubMed]
- De Bosscher, K.; Vanden Berghe, W.; Haegeman, G. The interplay between the glucocorticoid receptor and nuclear factor-κB or activator protein-1: Molecular mechanisms for gene repression. Endocr. Rev. 2003, 24, 488–522. [Google Scholar] [CrossRef]
- Bekhbat, M.; Rowson, S.A.; Neigh, G.N. Checks and balances: The glucocorticoid receptor and NFκB in good times and bad. Front. Neuroendocrinol. 2017, 46, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, W.; Pan, Y.; Kuang, X.; Qi, X.; Sun, H. Chronic blockade of glucocorticoid receptors by RU486 enhances lipopolysaccharide-induced depressive-like behaviour and cytokine production in rats. Brain Behav. Immun. 2011, 25, 706–714. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; Hage, W.E.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Li, K.D.; Yan, K.; Wang, Q.S.; Tian, J.S.; Xu, D.; Zhang, W.Y.; Cui, Y.L. Antidepressant-Like effects of dietary gardenia blue pigment derived from genipin and tyrosine. Food Funct. 2019, 10, 4533–4545. [Google Scholar] [CrossRef]
- Dunn, A.J. Effects of cytokines and infections on brain neurochemistry. Clin. Neurosci. Res. 2006, 6, 52–68. [Google Scholar] [CrossRef]
- Chen, H.; Shi, H.; Liu, Y.; Ren, X.; He, S.; Chang, X.; Yin, Y. Activation of corticotropin-releasing factor receptor 1 aggravates dextran sodium sulphate-induced colitis in mice by promoting M1 macrophage polarization. Mol. Med. Rep. 2018, 17, 234–242. [Google Scholar] [CrossRef]
- Knapp, D.J.; Whitman, B.A.; Wills, T.A.; Angel, R.A.; Overstreet, D.H.; Criswell, H.E.; Ming, Z.; Breese, G.R. Cytokine involvement in stress may depend on corticotrophin releasing factor to sensitize ethanol withdrawal anxiety. Brain Behav. Immun. 2011, 25, S146–S154. [Google Scholar] [CrossRef]
- Chatoo, M.; Li, Y.; Ma, Z.; Coote, J.; Du, J.; Chen, X. Involvement of Corticotropin-Releasing Factor and Receptors in Immune Cells in Irritable Bowel Syndrome. Front. Endocrinol. 2018, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G.; Weber, M.D.; Watkins, L.R.; Maier, S.F. Stress sounds the alarmin: The role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming. Brain Behav. Immun. 2015, 48, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Carbonell, D.; Ye, F.; Ramanath, N.; Dobrowolski, C.; Karn, J. The Glucocorticoid Receptor Is a Critical Regulator of HIV Latency in Human Microglial Cells. J. Neuroimmune Pharmacol. 2019, 14, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ji, P.; Dow, K.E. Corticotropin-Releasing hormone induces proliferation and TNF-α release in cultured rat microglia via MAP kinase signalling pathways. J. Neurochem. 2003, 84, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.J.; Espinosa-Oliva, A.M.; Carrillo-Jimenez, A.; Oliva-Martin, M.J.; Garcia-Revilla, J.; Garcia-Quintanilla, A.; Pablos, R.M.; Venero, J.L. Relevance of chronic stress and the two faces of microglia in Parkinson’s disease. Front. Cell. Neurosci. 2015, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.A.; Hayakawa, K.; Monji, A.; Kanba, S. Missing and Possible Link between Neuroendocrine Factors, Neuropsychiatric Disorders, and Microglia. Front. Integr. Neurosci. 2013, 7, 53. [Google Scholar] [CrossRef]
- Raison, C.L.; Borisov, A.S.; Woolwine, B.J.; Massung, B.; Vogt, G.; Miller, A.H. Interferon-α effects on diurnal hypothalamic-pituitary-adrenal axis activity: Relationship with proinflammatory cytokines and behavior. Mol. Psychiatry 2010, 15, 535–547. [Google Scholar] [CrossRef]
- Panossian, A.; Seo, E.J.; Efferth, T. Novel molecular mechanisms for the adaptogenic effects of herbal extracts on isolated brain cells using systems biology. Phytomedicine 2018, 50, 257–284. [Google Scholar] [CrossRef]
- Panossian, A.G.; Efferth, T.; Shikov, A.N.; Pozharitskaya, O.N.; Kuchta, K.; Mukherjee, P.K.; Banerjee, S.; Heinrich, M.; Wu, W.; Guo, D.A.; et al. Evolution of the adaptogenic concept from traditional use to medical systems: Pharmacology of stress- and aging-related diseases. Med. Res. Rev. 2021, 41, 630–703. [Google Scholar] [CrossRef]
- Amsterdam, J.D.; Panossian, A.G. Rhodiola rosea L. as a putative botanical antidepressant. Phytomedicine 2016, 23, 770–783. [Google Scholar] [CrossRef]
- Fu, H.; Xu, Z.; Zhang, X.L.; Zheng, G.Q. Kaixinsan, a Well-Known Chinese Herbal Prescription, for Alzheimer’s Disease and Depression: A Preclinical Systematic Review. Front. Neurosci. 2019, 13, 1421. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, X.; Zhang, T.; Chen, C.; Dong, X.; Can, Y.; Liu, P. Behavioral and Biochemical Effects of KXS on Postmyocardial Infarction Depression. Front. Pharmacol. 2020, 11, 561817. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Sun, L.; Liu, X.; Peng, B.; Wang, Q.; Jia, W.; Chen, Y.; Pan, A.; Xiao, P. Preventive action of Kai Xin San aqueous extract on depressive-like symptoms and cognition deficit induced by chronic mild stress. Exp. Biol. Med. 2009, 234, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Wang, P.; Fan, C.; Zhang, P.; Shen, J.; Yu, S.Y. Ginsenoside-Rg1 Rescues Stress-Induced Depression-Like Behaviors via Suppression of Oxidative Stress and Neural Inflammation in Rats. Oxid. Med. Cell. Longev. 2020, 2020, 2325391. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Ji, G.E. The effect of fermented red ginseng on depression is mediated by lipids. Nutr. Neurosci. 2014, 17, 7–15. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Park, J.S.; Lee, E.J.; Lee, S.Y.; Kim, D.H.; Kang, J.L.; Kim, H.S. Anti-Inflammatory mechanism of ginseng saponin metabolite Rh3 in lipopolysaccharide-stimulated microglia: Critical role of 5′-adenosine monophosphate-activated protein kinase signaling pathway. J. Agric. Food Chem. 2015, 63, 3472–3480. [Google Scholar] [CrossRef]
- Ke, L.; Guo, W.; Xu, J.; Zhang, G.; Wang, W.; Huang, W. Ginsenoside Rb1 attenuates activated microglia-induced neuronal damage. Neural Regen. Res. 2014, 9, 252–259. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Wang, Y.; Mu, P.; Chen, C.; Huang, P.; Liu, D. Ginsenoside Rb1 attenuates intestinal ischemia/reperfusion-induced inflammation and oxidative stress via activation of the PI3K/Akt/Nrf2 signaling pathway. Mol. Med. Rep. 2019, 19, 3633–3641. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, H.; Zheng, M.; Xu, W.; Yang, Y.; Shi, F. Ginsenoside Rg3 suppresses the NLRP3 inflammasome activation through inhibition of its assembly. FASEB J. 2020, 34, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Cho, S.H.; Kim, J.E.; Lee, C.; Lee, J.H.; Baek, M.C.; Song, G.Y.; Bae, J.S. Suppressive Effects of Ginsenoside Rh1 on HMGB1-Mediated Septic Responses. Am. J. Chin. Med. 2019, 47, 119–133. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, H.Y.; Choi, Y.J.; Cho, S.H. Antidepressant effects of ginsenoside Rf on behavioral change in the glial degeneration model of depression by reversing glial loss. J. Ginseng Res. 2020, 44, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, L.; Chen, J.; Liu, C.; Li, S.; Hua, M.; Qu, D.; Shao, Z.; Sun, Y. Ginsenoside Rk1 alleviates LPS-induced depression-like behavior in mice by promoting BDNF and suppressing the neuroinflammatory response. Biochem. Biophys. Res. Commun. 2020, 530, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Wang, X.B.; Xue, R.R.; Gao, X.X.; Li, W. Ginsenoside Rg1 attenuates chronic unpredictable mild stress-induced depressive-like effect via regulating NF-κB/NLRP3 pathway in rats. Neuroreport 2019, 30, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, M.; Liu, P.; Guo, D.H.; Wei, R.B.; Rahman, K. Possible mechanism of the antidepressant effect of 3,6′-disinapoyl sucrose from Polygala tenuifolia Willd. J. Pharm. Pharmacol. 2011, 63, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, P.; Guo, D.H.; Rahman, K.; Wang, D.X.; Xie, T.T. Antidepressant effects of the extract YZ-50 from Polygala tenuifolia in chronic mild stress treated rats and its possible mechanisms. Pharm. Biol. 2010, 48, 794–800. [Google Scholar] [CrossRef]
- Liu, P.; Hu, Y.; Guo, D.H.; Wang, D.X.; Tu, H.H.; Ma, L.; Xie, T.T.; Kong, L.Y. Potential antidepressant properties of Radix Polygalae (Yuan Zhi). Phytomedicine 2010, 17, 794–799. [Google Scholar] [CrossRef]
- Lee, S.R.; Lee, S.; Moon, E.; Park, H.J.; Park, H.B.; Kim, K.H. Bioactivity-Guided isolation of anti-inflammatory triterpenoids from the sclerotia of Poria cocos using LPS-stimulated Raw264.7 cells. Bioorganic Chem. 2017, 70, 94–99. [Google Scholar] [CrossRef]
- Chang, W.; Teng, J. β-asarone prevents Aβ25-35-induced inflammatory responses and autophagy in SH-SY5Y cells: Down expression Beclin-1, LC3B and up expression Bcl-2. Int. J. Clin. Exp. Med. 2015, 8, 20658–20663. [Google Scholar]
- Chen, C.; Hu, Y.; Dong, X.Z.; Zhou, X.J.; Mu, L.H.; Liu, P. Proteomic Analysis of the Antidepressant Effects of Shen-Zhi-Ling in Depressed Patients: Identification of Proteins Associated with Platelet Activation and Lipid Metabolism. Cell. Mol. Neurobiol. 2018, 38, 1123–1135. [Google Scholar] [CrossRef]
- Dong, X.Z.; Wang, D.X.; Zhang, T.Y.; Liu, X.; Liu, P.; Hu, Y. Identification of protein targets for the antidepressant effects of Kai-Xin-San in Chinese medicine using isobaric tags for relative and absolute quantitation. Neural Regen. Res. 2020, 15, 302–310. [Google Scholar] [CrossRef]
- Zhao, S.; Iyengar, R. Systems pharmacology: Network analysis to identify multiscale mechanisms of drug action. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.I.; Lim, S.W.; Myung, W.; Kim, D.K.; Lee, S.Y. Differentially expressed genes related to major depressive disorder and antidepressant response: Genome-Wide gene expression analysis. Exp. Mol. Med. 2018, 50, 92. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Hamm, R.; Wikman, G.; Efferth, T. Mechanism of action of Rhodiola, salidroside, tyrosol and triandrin in isolated neuroglial cells: An interactive pathway analysis of the downstream effects using RNA microarray data. Phytomedicine 2014, 21, 1325–1348. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Seo, E.J.; Wikman, G.; Efferth, T. Synergy assessment of fixed combinations of Herba Andrographidis and Radix Eleutherococci extracts by transcriptome-wide microarray profiling. Phytomedicine 2015, 22, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Seo, E.-J.; Efferth, T. Synergy assessments of plant extracts used in the treatment of stress and aging-related disorders. Synergy 2018, 7, 39–48. [Google Scholar] [CrossRef]
- Panossian, A.; Hamm, R.; Kadioglu, O.; Wikman, G.; Efferth, T. Synergy and Antagonism of Active Constituents of ADAPT-232 on Transcriptional Level of Metabolic Regulation of Isolated Neuroglial Cells. Front. Neurosci. 2013, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lu, S.; Zhang, C.; Zhu, L.; Li, Y.; Bai, M.; Xu, E. Quantitative proteomic analysis of the liver reveals antidepressant potential protein targets of Sinisan in a mouse CUMS model of depression. Biomed. Pharmacother. 2020, 130, 110565. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Huang, B.; Zhang, Y.-W. Chinese Herbal Medicine for the Treatment of Depression: Effects on the Neuroendocrine-Immune Network. Pharmaceuticals 2021, 14, 65. https://doi.org/10.3390/ph14010065
Li C, Huang B, Zhang Y-W. Chinese Herbal Medicine for the Treatment of Depression: Effects on the Neuroendocrine-Immune Network. Pharmaceuticals. 2021; 14(1):65. https://doi.org/10.3390/ph14010065
Chicago/Turabian StyleLi, Chan, Bishan Huang, and Yuan-Wei Zhang. 2021. "Chinese Herbal Medicine for the Treatment of Depression: Effects on the Neuroendocrine-Immune Network" Pharmaceuticals 14, no. 1: 65. https://doi.org/10.3390/ph14010065
APA StyleLi, C., Huang, B., & Zhang, Y.-W. (2021). Chinese Herbal Medicine for the Treatment of Depression: Effects on the Neuroendocrine-Immune Network. Pharmaceuticals, 14(1), 65. https://doi.org/10.3390/ph14010065




