Effects of Red and White Ginseng Preparations on Electrical Activity of the Brain in Elderly Subjects: A Randomized, Double-Blind, Placebo-Controlled, Three-Armed Cross-Over Study
Abstract
1. Introduction
2. Results
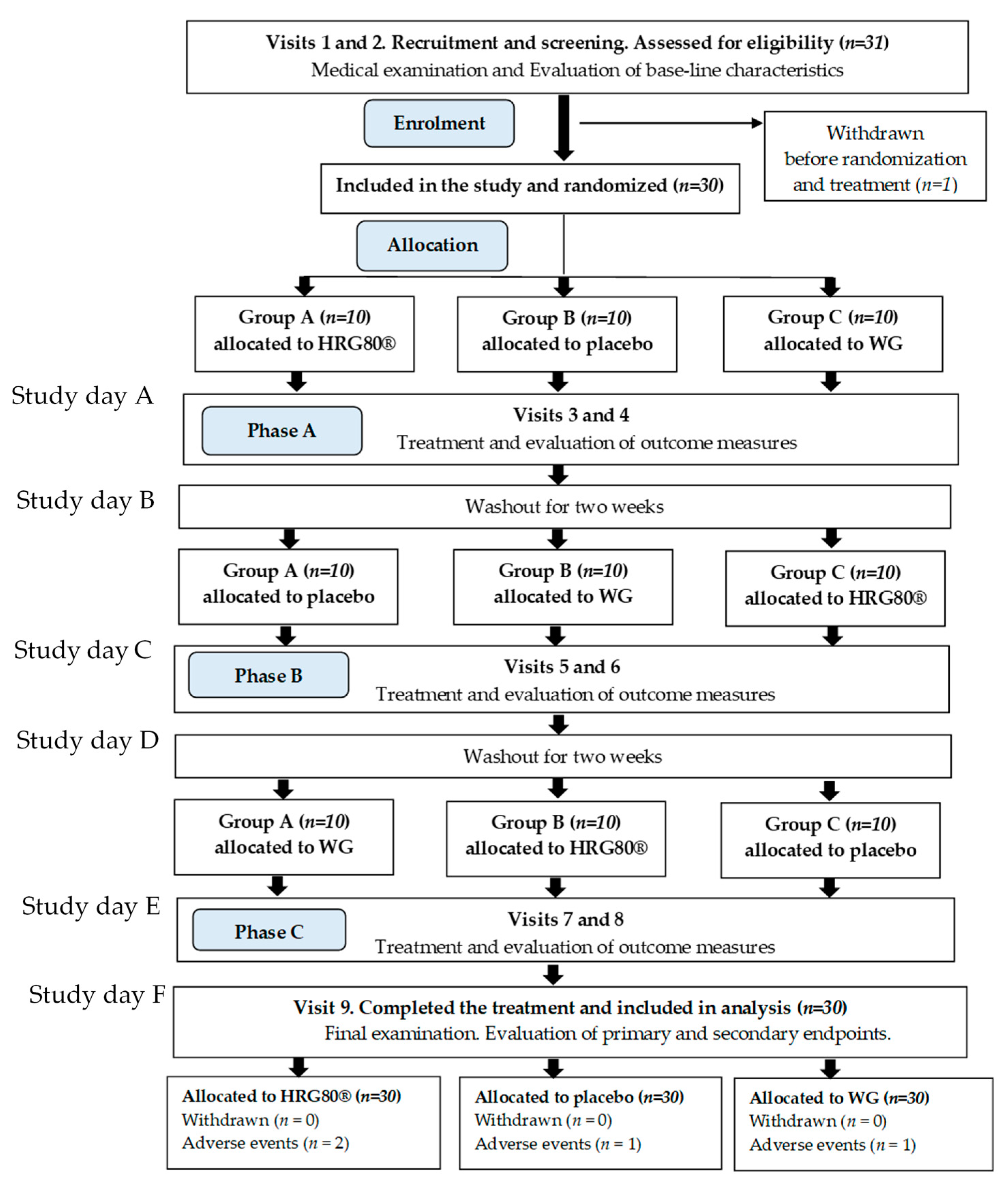
2.1. Baseline Data of Study Participants, Their Disposition and Treatment Compliance
2.2. Efficacy of Treatment
2.2.1. Efficacy Outcome Measures and Endpoints
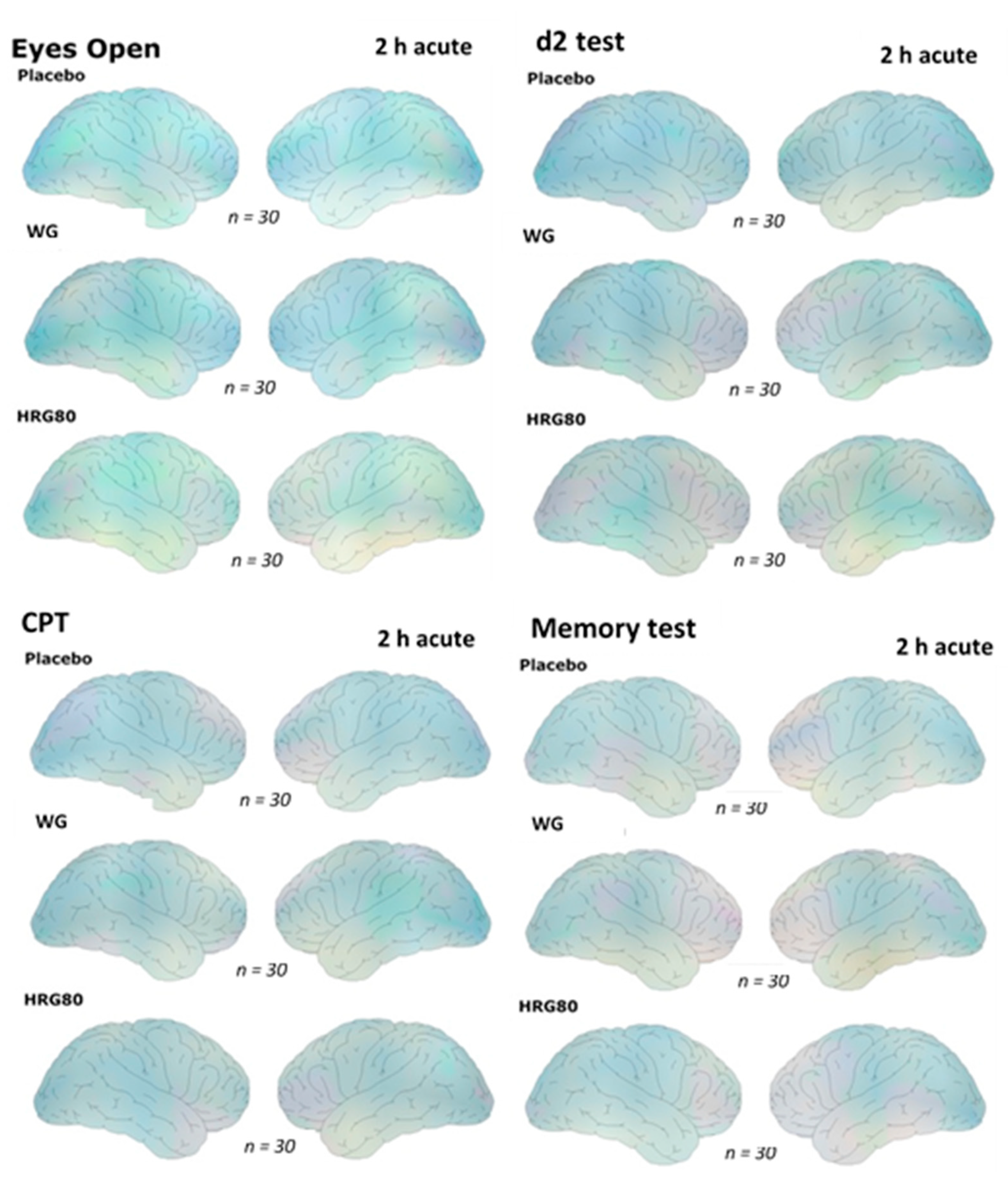
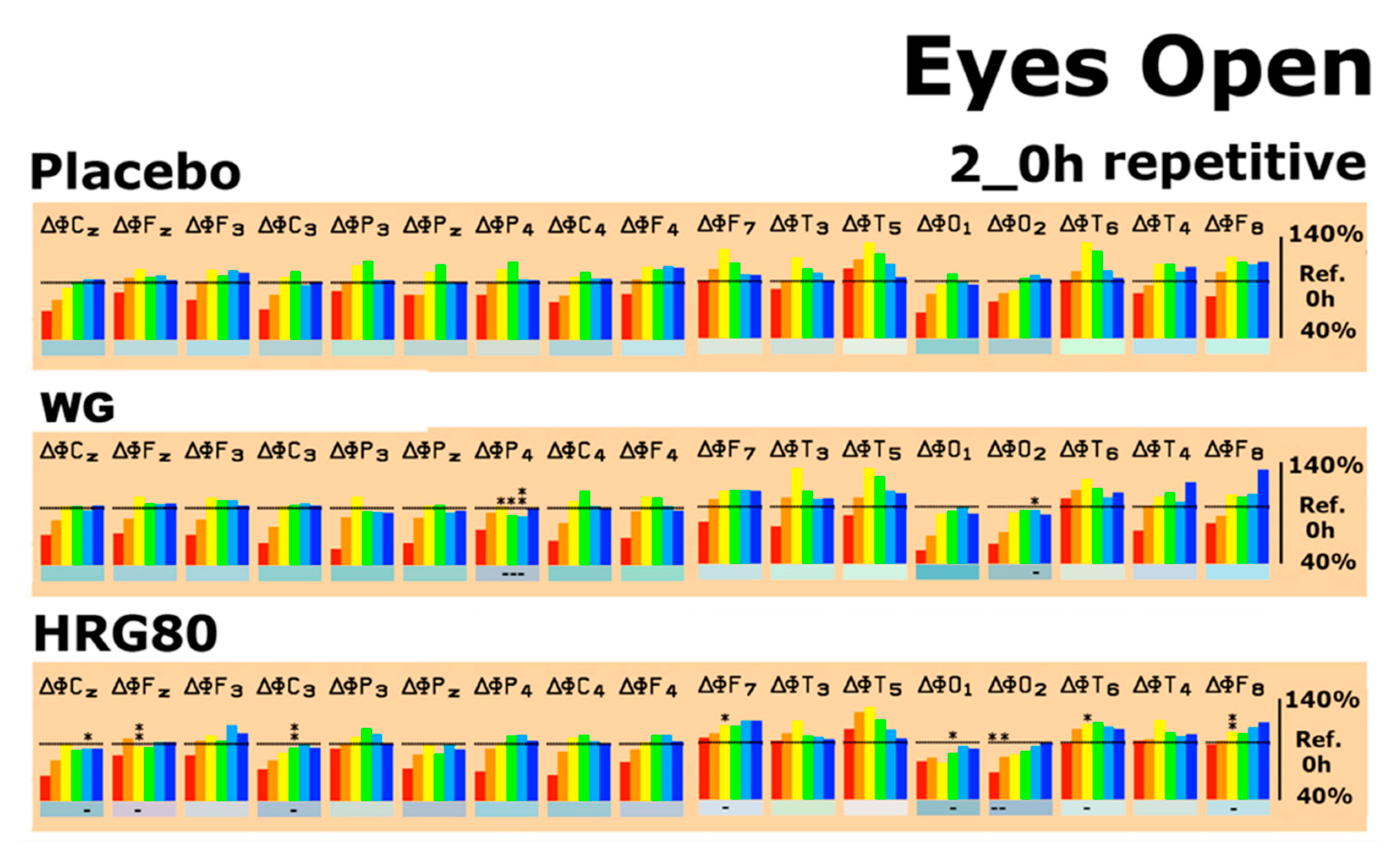
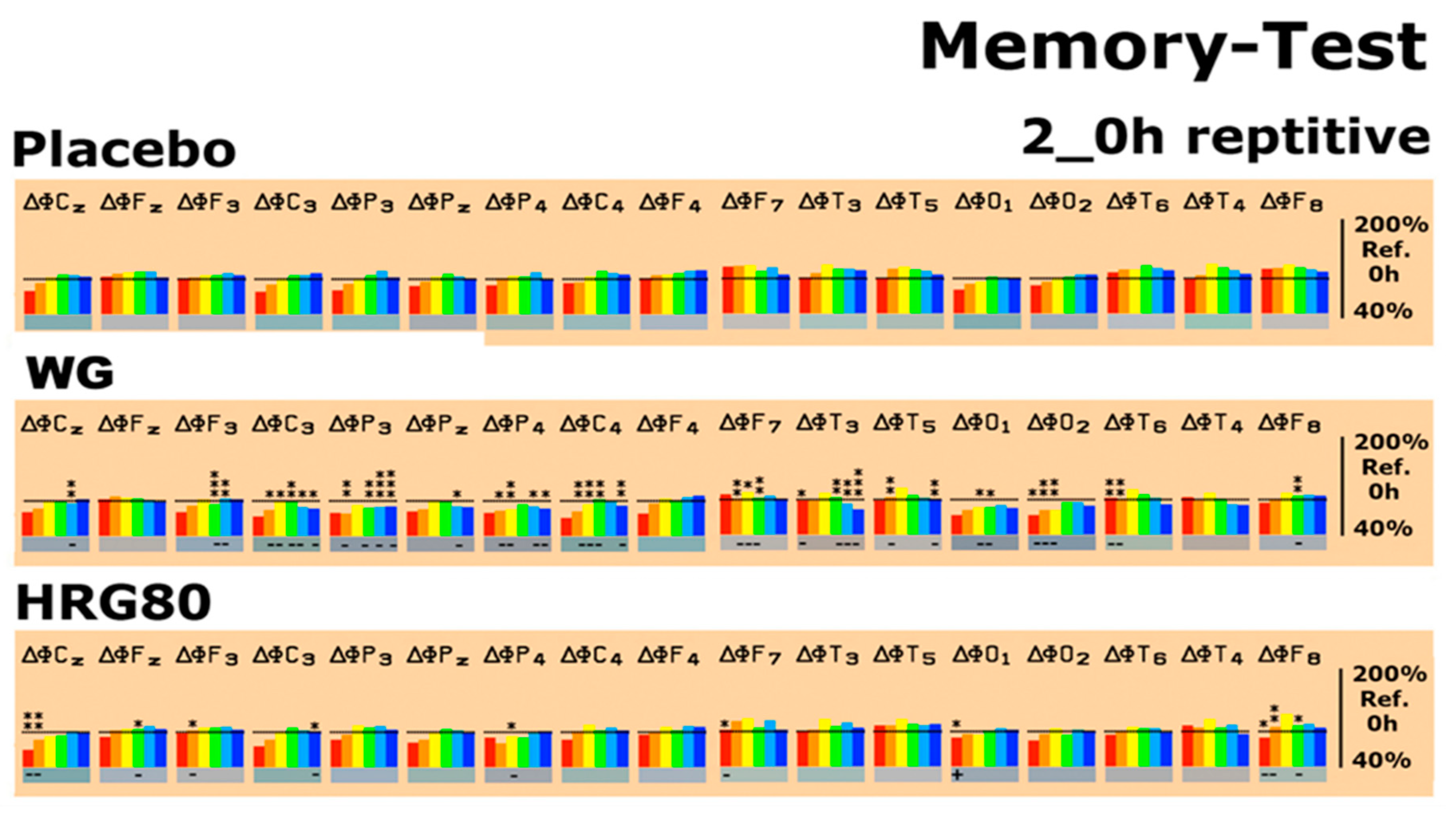
2.2.2. Quantitative EEG Results after Acute and Repetitive Dosing
2.2.3. Efficacy of Red and White Ginseng Documented by Discriminant Analysis
2.3. Safety Evaluation
3. Discussion
4. Materials and Methods
4.1. Participant Eligibility and Study Population
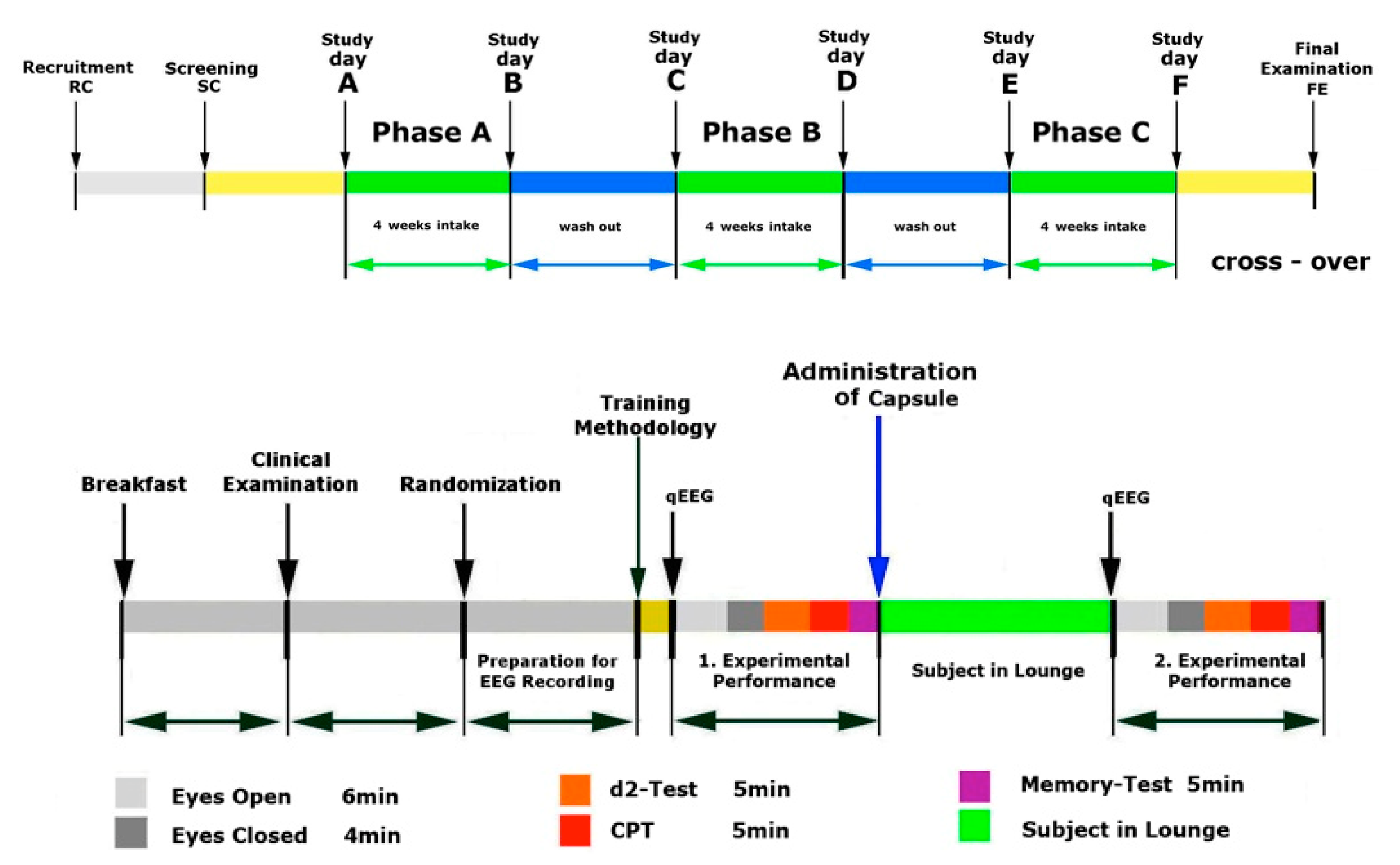
4.2. Study Design
4.3. Intervention and Comparator
Randomization, Blinding, Allocation Concealment, and Evaluation of Compliance
4.4. Study Procedures and Follow-up
4.4.1. Phase A, Screening and Training
4.4.2. Phase A, Treatment and Assessment
4.4.3. Phase B, Treatment and Assessment
4.4.4. Phase C, Treatment and Assessment
4.5. Efficacy and Safety Evaluation
Efficacy Outcome
4.6. Safety Outcomes
4.7. Sample Size Considerations
4.8. Statistical Analysis
5. Conclusions
- Red ginseng has an effect on the CNS in humans
- Red ginseng has an effect on the CNS in elderly subjects with mild cognitive impairments
- Cultivated in standard conditions, red ginseng has an effect on the CNS of elderly subjects with mild cognitive impairments
- Hydroponically cultivated in standard conditions, red ginseng has an effect on the CNS in elderly subjects with mild cognitive impairments
- The overall effects of white and red ginseng on the electrical activity of the brain are different, suggesting different pharmacological activity of the red and white ginseng preparations
- A treatment duration of 4 weeks seems to be sufficient to uncover the action of ginseng on the activity of the human brain
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Adverse Events, Tolerability and Vital Signs
Appendix A.1. Summary of Adverse Events
| Overview of the Adverse Events | |||
|---|---|---|---|
| AE (n = 4) | Study Medication | Diagnosis | Comments |
| Subject no. 007 | Phase A HRG80® | Scaphoid fracture | The subject fell on 31 October 2019 around midday in front of his door and did not take any painkillers. An X-ray was conducted on 1 November 2019 and a scaphoid fracture was diagnosed. He took 600 mg of the painkiller ibuprofen twice a day until 13 November 2019. Hand surgery was carried out on 13 November 2019 in the morning under general anesthesia and he was released at 2:00 p.m. He took 600 mg of ibuprofen once a day until 18 November 2019. |
| Subject no. 007 | Phase C Placebo | Urinary incontinence | Prostate biopsy under anesthesia on 6 March 2020 at 10:00 a.m. because of urinary incontinence. Discharge from hospital on same day (6 March 2020 at 2.00 pm). Intake of painkillers from 6 March 2020 to 7 March 2020. |
| Unconsciousness | The sudden occurrence of unconsciousness on 7 March 2020 at 3:00 p.m. (duration: 2 min). Examination on 9 March 2020 without pathological findings. | ||
| Subject no. 013 | Phase C HRG80® | Back pain | Subject had back pain on 11 March 2020 and took four painkillers. She felt better during the examination on 12 March 2020. |
| Subject no. 018 | Phase B/ WG | Higher blood pressure | Subject no. 018 had high blood pressure and went to the doctor on 7 January 2020. She received a prescription for 5 mg bisoprolol. She took the bisoprolol from 7 January 2020 to 17 January 2020 (phase B, taking Treatment A) and as required during the washout phase (four weeks, from 17 January 2020 to 11 February 2020). She did not take bisoprolol during the last phase (phase 3, Treatment B). |
Appendix A.2. Tolerability
| Tolerability | ||||||
|---|---|---|---|---|---|---|
| Placebo | HRG80® | WG | ||||
| Acute (n) | Repetitive (n) | Acute (n) | Repetitive (n) | Acute (n) | Repetitive (n) | |
| Very Good | 30 | 30 | 30 | 30 | 30 | 30 |
| Good | 0 | 0 | 0 | 0 | 0 | 0 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 |
| Poor | 0 | 0 | 0 | 0 | 0 | 0 |
Appendix A.3. Vital Signs and Physical Findings
| Blood Pressure (mmHg) and Pulse/Heart Rate (bpm) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute | Repetitive | |||||||||||
| 0 h Baseline | 2 h after Intake | 0 h Baseline | 2 h after Intake | |||||||||
| 1st Record | 2nd Record | 1st Record | 2nd Record | |||||||||
| SBP | DBP | Pulse | SBP | DBP | Pulse | SBP | DBP | Pulse | SBP | DBP | Pulse | |
| (mmHg) | (mmHg) | (bpm) | (mmHg) | (mmHg) | (bpm) | (mmHg) | (mmHg) | (bpm) | (mmHg) | (mmHg) | (bpm) | |
| n = 30 | n = 30 | n = 30 | n = 30 | n = 30 | n = 30 | n = 30 | n = 30 | n = 30 | n = 30 | n = 30 | n = 30 | |
| Screening | 139.50 (±21.11) | 84.33 (±15.06) | 68.17 (±8.07) | |||||||||
| Placebo | 133.20 (±20.52) | 80.43 (±10.88) | 67.83 (±7.86) | 134.80 (±18.42) | 80.57 (±10.88) | 63.80 (±10.70) | 133.83 (±18.29) | 81.57 (±10.80) | 69.00 (±9.09) | 139.13 (±19.66) | 81.60 (±10.36) | 62.83 (±9.17) p < 0.01 |
| HRG80® | 139.30 (±20.37) | 82.53 (±13.46) | 68.63 (±12.04) | 138.50 (±23.28) | 81.30 (±11.43) | 64.80 (±10.35) | 135.70 (±19.30) | 82.43 (±9.47) | 67.83 (±7.43) | 136.87 (±21.24) | 81.70 (±12.33) | 62.43 (±8.29) p < 0.01 |
| WG | 141.77 (±20.09) | 82.83 (±10.56) | 67.57 (±8.01) | 138.57 (±19.12) | 80.03 (±9.81) | 60.67 (±8.53) p < 0.002 | 136.77 (±19.07) | 83.23 (±11.36) | 67.57 (±10.05) | 133.77 (±21.60) | 78.93 (±12.05) | 61.97 (±10.13) p < 0.036) |
| Final Examination | 143.43 (±20.71) | 83.07 (±11.32) | 62.87 (±10.31) | |||||||||
Appendix B. CONSORT 2010 Checklist
| Section/Topic | Item No | Checklist Item | Reported on Page No |
| Title and abstract | |||
| 1a | Identification as a randomized trial in the title | 1 | |
| 1b | Structured summary of trial design, methods, results, and conclusions (for specific guidance see CONSORT for abstracts) | 1 | |
| Introduction | |||
| Background and objectives | 2a | Scientific background and explanation of rationale | 2 |
| 2b | Specific objectives or hypotheses | 2 | |
| Methods | |||
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio | 2, 11, Figure 1 and Figure 6 Table 1 |
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons | n/a | |
| Participants | 4a | Eligibility criteria for participants | 10 |
| 4b | Settings and locations where the data were collected | 10 | |
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | 12 |
| Outcomes | 6a | Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed | 13,14 Figure 2 |
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | n/a | |
| Sample size | 7a | How sample size was determined | 14 |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | n/a | |
| Randomization: | |||
| Sequence generation | 8a | Method used to generate the random allocation sequence | 12 |
| 8b | Type of randomization; details of any restriction (such as blocking and block size) | 12 | |
| Allocation concealment mechanism | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | 12 |
| Implementation | 10 | Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions | 12 |
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how | 12 |
| 11b | If relevant, description of the similarity of interventions | 12 | |
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | 14 |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | n/a | |
| Results | |||
| Participant flow (a diagram is strongly recommended) | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analyzed for the primary outcome | Figure 1 |
| 13b | For each group, losses and exclusions after randomization, together with reasons | n/a | |
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | 10, Figure 6 |
| 14b | Why the trial ended or was stopped | n/a | |
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | Table 1 |
| Numbers analyzed | 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | Figure 1 |
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) | 9–13 Figure 2, Figure 3 and Figure 4 Table 2 |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | Figure 2, Figure 3 and Figure 4 Appendix A | |
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory | n/a |
| Harms | 19 | All important harms or unintended effects in each group | 14, Appendix A |
| Discussion | |||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses | 9 |
| Generalizability | 21 | Generalizability (external validity, applicability) of the trial findings | 8,9 |
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence | 8, 9 |
| Other information | |||
| Registration | 23 | Registration number and name of trial registry | 10 |
| Protocol | 24 | Where the full trial protocol can be accessed, if available | 15 |
| Funding | 25 | Sources of funding and other support (such as supply of drugs), role of funders | 15 |
References
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China; National Pharmacopoeia Committee: Beijing, China, 2010. [Google Scholar]
- Panossian, A.G.; Efferth, T.; Shikov, A.N.; Pozharitskaya, O.N.; Kuchta, K.; Mukherjee, P.K.; Banerjee, S.; Heinrich, M.; Wu, W.; Guo, D.A.; et al. Evolution of the adaptogenic concept from traditional use to medical systems: Pharmacology of stress- and aging-related diseases. Med. Res. Rev. 2021, 41, 630–703. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.Y.; He, Y.F.; Li, L.; Meng, H.; Dong, Y.M.; Yi, F.; Xiao, P.G. A preliminary review of studies on adaptogens: Comparison of their bioactivity in TCM with that of ginseng-like herbs used worldwide. Chin. Med. 2018, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Rauf, A. Adaptogenic herb ginseng (Panax) as medical food: Status quo and future prospects. Biomed. Pharmacother. 2017, 85, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Baeg, I.H.; So, S.H. The world ginseng market and the ginseng (Korea). J. Ginseng Res. 2013, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Radad, K.; Gille, G.; Liu, L.; Rausch, W.D. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J. Pharmacol. Sci. 2006, 100, 175–186. [Google Scholar] [CrossRef] [PubMed]
- So, S.H.; Lee, J.W.; Kim, Y.S.; Hyun, S.H.; Han, C.K. Red ginseng monograph. J. Ginseng Res. 2018, 42, 549–561. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO), Radix Ginseng. WHO Monographs on Medicinal Plants Commonly Used in the Newly Independent States (NIS); World Health Organization: Genova, Switzerland, 2010; pp. 141–160.
- European Medicines Agency. EMA/HMPC/321232/2012. Assessment Report on Panax Ginseng C.A. Meyer, Radix. Based on Article 16d (1), Article 16f and Article 16h of Directive 2001/83/EC as Amended (Traditional Use); European Medicines Agency: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Blumenthal, M. The ABC Clinical Guide to Herbs; Theime: Austin, TX, USA, 2003; p. 211e25. [Google Scholar]
- Panax ginseng. Monograph. Altern Med. Rev. 2009, 14, 172.e6.
- Blumenthal, M.; Goldberg, A.; Brinkmann, J. Herbal Medicine: Expanded Commission E Monographs; Integrative Medicine Communications: Austin, TX, USA, 2000; p. 170.e7. [Google Scholar]
- European Directorate for the Quality of Medicines & Health Care. Ginseng radix. In European Pharmacopoeia, Monograph 01/2008:1523; European Directorate for the Quality of Medicines & Health Care: Strasburg, France, 2008. [Google Scholar]
- Zhou, Q.L.; Zhu, D.N.; Yang, X.W.; Xu, W.; Wang, Y.P. Development and validation of a UFLC-MS/MS method for simultaneous quantification of sixty-six saponins and their six aglycones: Application to comparative analysis of red ginseng and white ginseng. J. Pharm. Biomed. Anal. 2018, 159, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.Y.; Moon, J.M.; Kim, B.Y.; Lim, S.H.; Lee, G.S.; Yu, H.S.; Cho, S.I. Comparative study of Korean White Ginseng and Korean Red Ginseng on efficacies of OVA-induced asthma model in mice. J. Ginseng Res. 2015, 39, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Han, M.J.; Kim, D.H. Effects of Red and Fermented Ginseng and Ginsenosides on Allergic Disorders. Biomolecules 2020, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.; Shin, W.C.; Lee, Y.S.; Kim, S.; Baek, N.I.; Lee, D.Y. A Comparative Study on Processed Panax ginseng Products Using HR-MAS NMR-Based Metabolomics. Molecules 2020, 25, 1390. [Google Scholar] [CrossRef] [PubMed]
- Mariage, P.A.; Hovhannisyan, A.; Panossian, A.G. Efficacy of Panax ginseng Meyer Herbal Preparation HRG80 in Preventing and Mitigating Stress-Induced Failure of Cognitive Functions in Healthy Subjects: A Pilot, Randomized, Double-Blind, Placebo-Controlled Crossover Trial. Pharmaceuticals 2020, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Lemerond, T.; Panossian, A.G. Panax ginseng Meyer Herbal Preparation HRG80 for Preventing and Mitigating Stress-Induced Failure of Cognitive Functions in Healthy Subjects. J. Altern Complement Integr. Med. 2020, 6, 100. [Google Scholar]
- Dimpfel, W.; Schombert, L.; Panossian, A.G. Panax Ginseng Preparations Enhance Long Term Potentiation in Rat Hippocampal Slices by Glutamatergic NMDA and Kainate Receptor Mediated Transmission. J. Altern Complement Integr. Med. 2020, 6, 106. [Google Scholar]
- Dimpfel, W. Neurophysiological Biomarker of Mild Cognitive Impairment. Adv. Alzheimers Dis. 2014, 3, 64–77. [Google Scholar] [CrossRef]
- Dimpfel, W.; Schombert, L.; Biller, A. Psychophysiological Effects of Sideritis and Bacopa Extract and Three Combinations Thereof—A Quantitative EEG Study in Subjects Suffering from Mild Cognitive Impairment (MCI). Adv. Alzheimers Dis. 2016, 5, 1–22. [Google Scholar] [CrossRef]
- Dimpfel, W.; Schombert, L.; Keplinger-Dimpfel, I.K.; Panossian, A. Effects of an Adaptogenic Extract on Electrical Activity of the Brain in Elderly Subjects with Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled, Two-Armed Cross-Over Study. Pharmaceuticals 2020, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Schober, F.; Schellenberg, R.; Dimpfel, W. Reflection of Mental Exercise in the Dynamic Quantitative Topographical EEG. Neuropsychobiolgy 1995, 31, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Dimpfel, W.; Hofmann, H.C.; Prohaska, A.; Schober, F.; Schellenberg, R. Source density analysis of functional topographical EEG: Monitoring of cognitive drug action. Eur. J. Med. Res. 1996, 1, 283–290. [Google Scholar] [PubMed]


| Demographic Characteristics | n | Mean | SD |
|---|---|---|---|
| Age | |||
| Total | 30 | 65.63 | 3.68 |
| Male | 17 | 66.47 | 3.71 |
| Female | 13 | 64.54 | 3.48 |
| Body height (cm) | |||
| Total | 30 | 1.74 | 0.10 |
| Male | 17 | 1.80 | 0.06 |
| Female | 13 | 1.65 | 0.06 |
| Weight (kg) | |||
| Total | 30 | 79.80 | 14.49 |
| Male | 17 | 87.12 | 7.87 |
| Female | 13 | 70.23 | 15.81 |
| Body Mass Index (BMI) (kg/cm2) | |||
| Total | 30 | 26.32 | 3.92 |
| Male | 17 | 26.85 | 2.44 |
| Female | 13 | 25.63 | 5.31 |
| Condition | Ginseng | δ | θ | α1 | α2 | β1 | β2 |
|---|---|---|---|---|---|---|---|
| Mediator | Ach | NE | 5-HT | DA | Glu | GABA | |
| Relaxation | HRG80® | ↓Fz, F7, F8, T6 | ↓C3, O1 | ||||
| WG | P4 | P4 | ↓P4, O2 | ||||
| D2 test | HRG80® | ↓F4 | ↓F4, F7, F8 | ↓F4, F8, T5 | ↓F8, Pz | ||
| WG | ↓C3, O1 | T3 | |||||
| CPT | HRG80® | ↑F3, ↓F8 | ↓F8, O2 | ↓T5 | ↓Cz, P4, O2 | ||
| WG | ↓P4 | ↓F7, Pz, O2 | ↓T5, T6, C3, C4 | ↓F7, T5, P4, | ↓F3, P3, P4, O1, O2 | ↓F3, F7, T3, C3, O1, O2 | |
| Memory test | HRG80® | ↓F7, F8, Cz, O1 | ↓F3, F8, Cz, | ↓P4 | ↓Fz, F8 | ↓C3 | |
| WG | ↓T3, T6, O2 | ↓F7, T5, T6, C3, C4, P3, P4 | ↓F7, C3, C4, P4, O2 | ↓F3, F7, F8, T3, C3, P3, C4 | ↓F3, T3, P3, | ↓T3, T5, C3, C4, P3 |
| Challenges during qEEG Recordings on Study Days A, B, C, D, E and F at Baseline (0 h) and 120 min (2 h) after Intake of Two Capsules of HRG80®, WG, or the Placebo | |
|---|---|
| Eyes open (Eo) | 6 min |
| Eyes closed (Ec) | 4 min |
| Concentration test (d2 test) | 5 min |
| Memory test (ME test) | 5 min |
| Calculation performance test (CPT) | 5 min |
| Total time excluding instructing of subjects | 25 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimpfel, W.; Mariage, P.-A.; Panossian, A.G. Effects of Red and White Ginseng Preparations on Electrical Activity of the Brain in Elderly Subjects: A Randomized, Double-Blind, Placebo-Controlled, Three-Armed Cross-Over Study. Pharmaceuticals 2021, 14, 182. https://doi.org/10.3390/ph14030182
Dimpfel W, Mariage P-A, Panossian AG. Effects of Red and White Ginseng Preparations on Electrical Activity of the Brain in Elderly Subjects: A Randomized, Double-Blind, Placebo-Controlled, Three-Armed Cross-Over Study. Pharmaceuticals. 2021; 14(3):182. https://doi.org/10.3390/ph14030182
Chicago/Turabian StyleDimpfel, Wilfried, Pierre-Antoine Mariage, and Alexander G. Panossian. 2021. "Effects of Red and White Ginseng Preparations on Electrical Activity of the Brain in Elderly Subjects: A Randomized, Double-Blind, Placebo-Controlled, Three-Armed Cross-Over Study" Pharmaceuticals 14, no. 3: 182. https://doi.org/10.3390/ph14030182
APA StyleDimpfel, W., Mariage, P.-A., & Panossian, A. G. (2021). Effects of Red and White Ginseng Preparations on Electrical Activity of the Brain in Elderly Subjects: A Randomized, Double-Blind, Placebo-Controlled, Three-Armed Cross-Over Study. Pharmaceuticals, 14(3), 182. https://doi.org/10.3390/ph14030182







