Strategies for the Synthesis of 19-nor-Vitamin D Analogs
Abstract
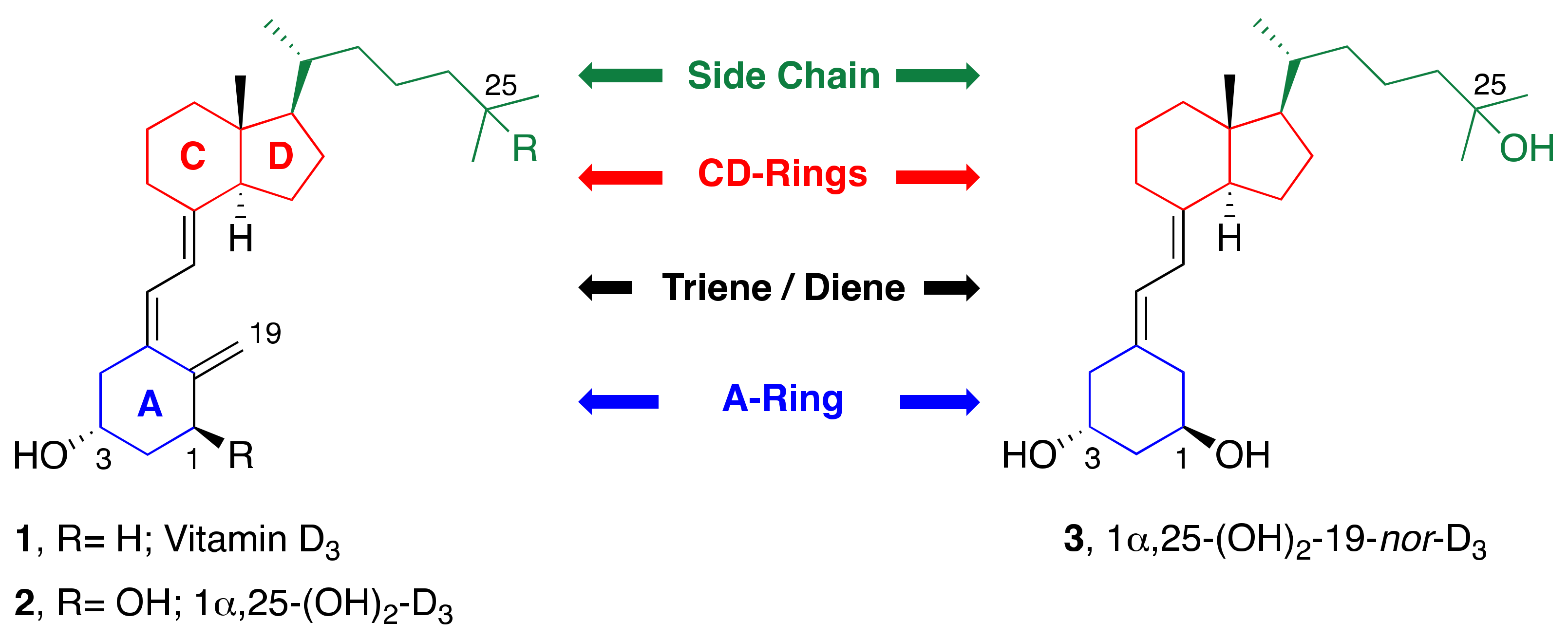
1. Introduction
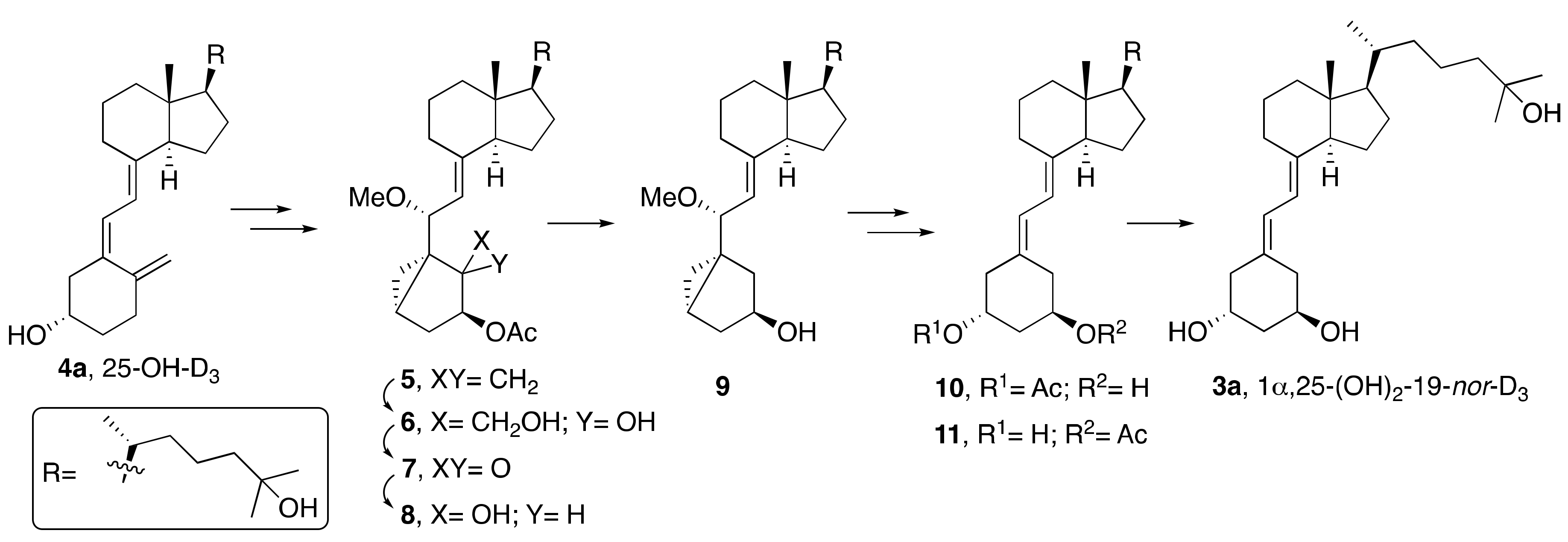
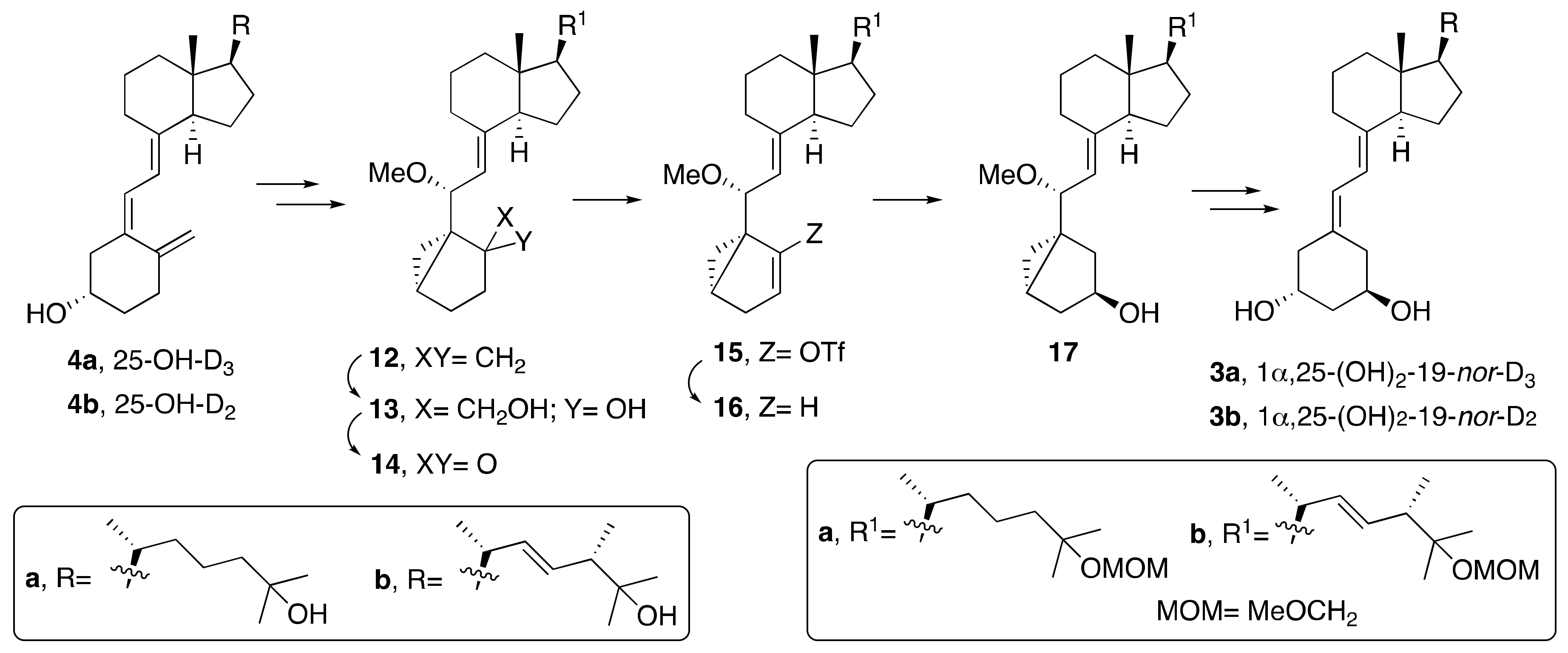
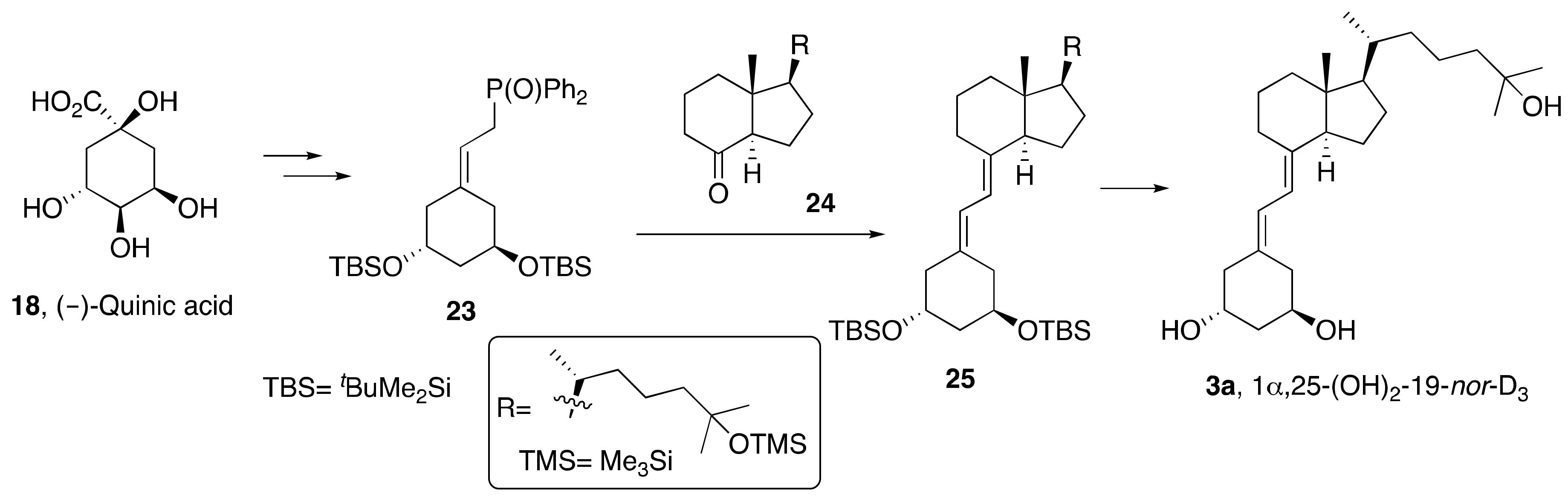
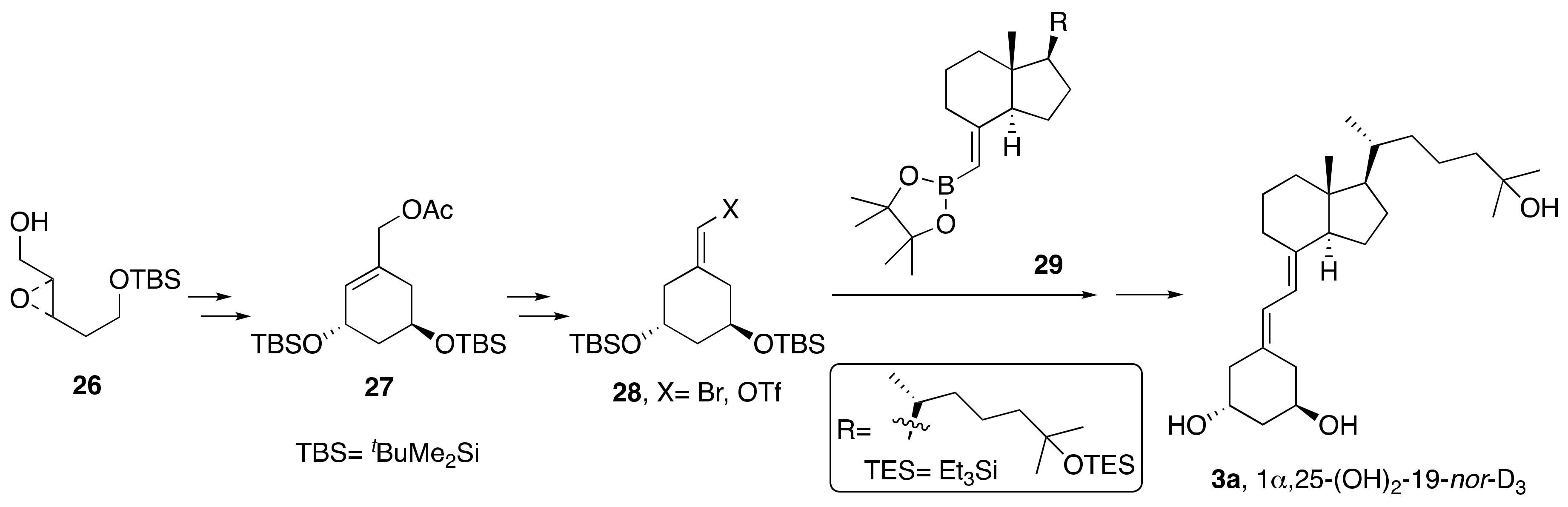
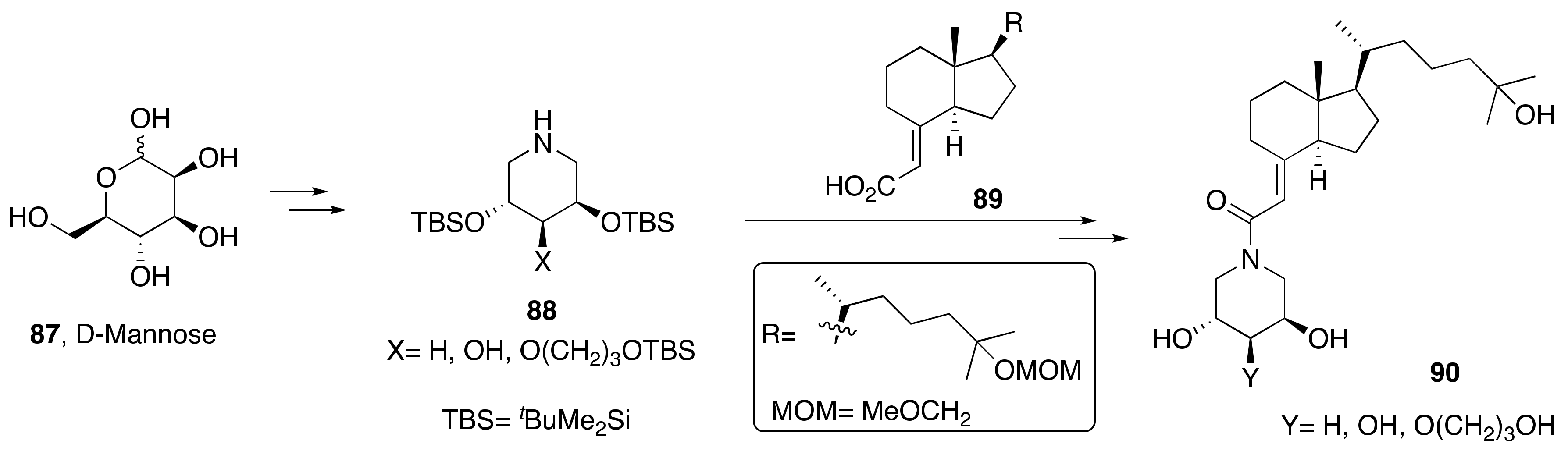
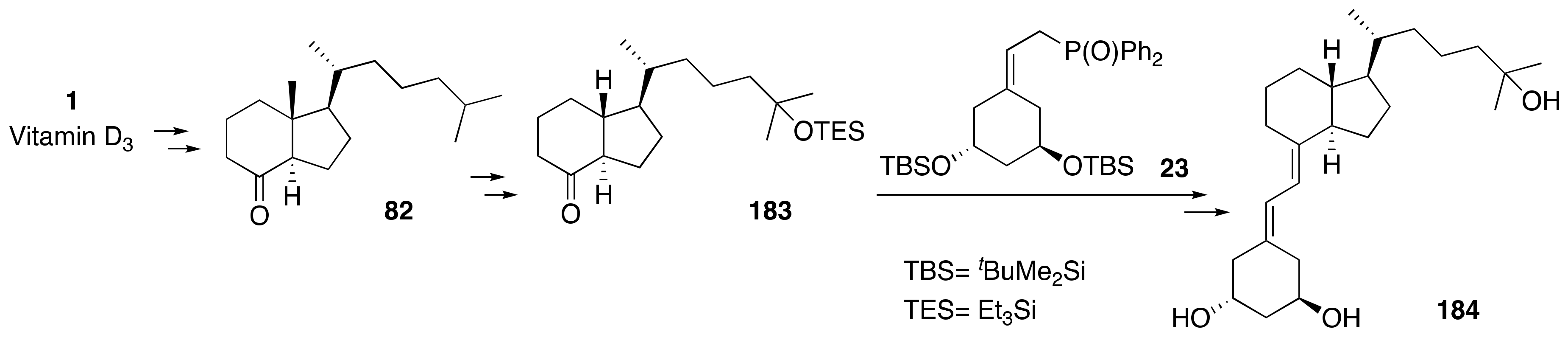
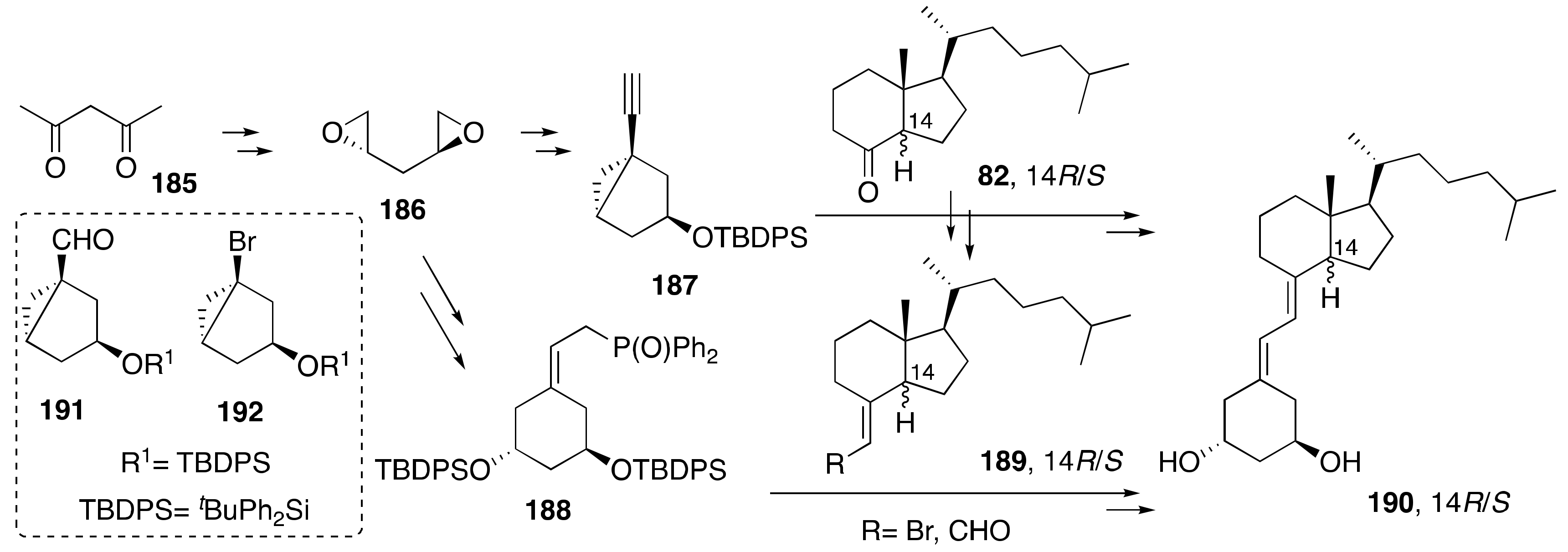
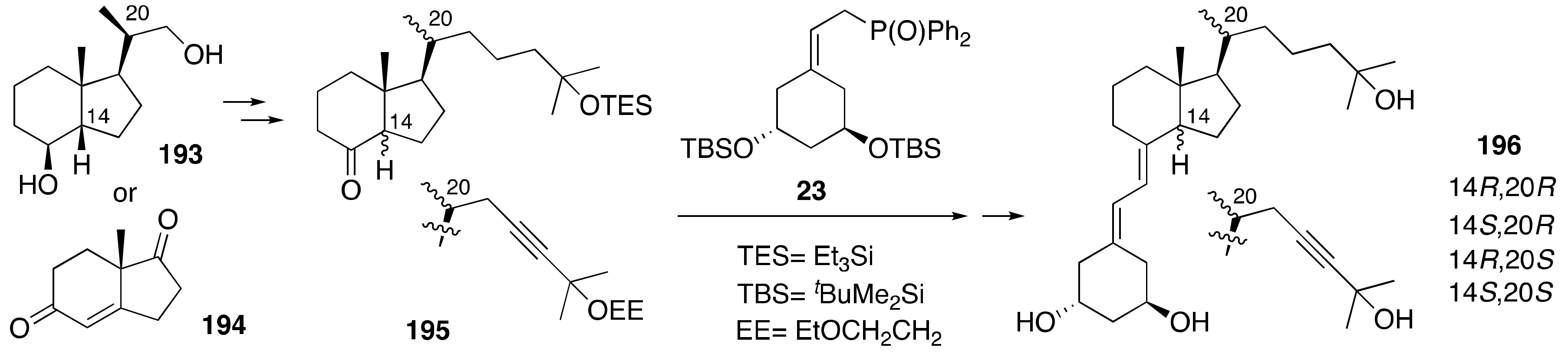
2. Synthesis of 1α,25-(OH)2-19-nor-Vitamin D
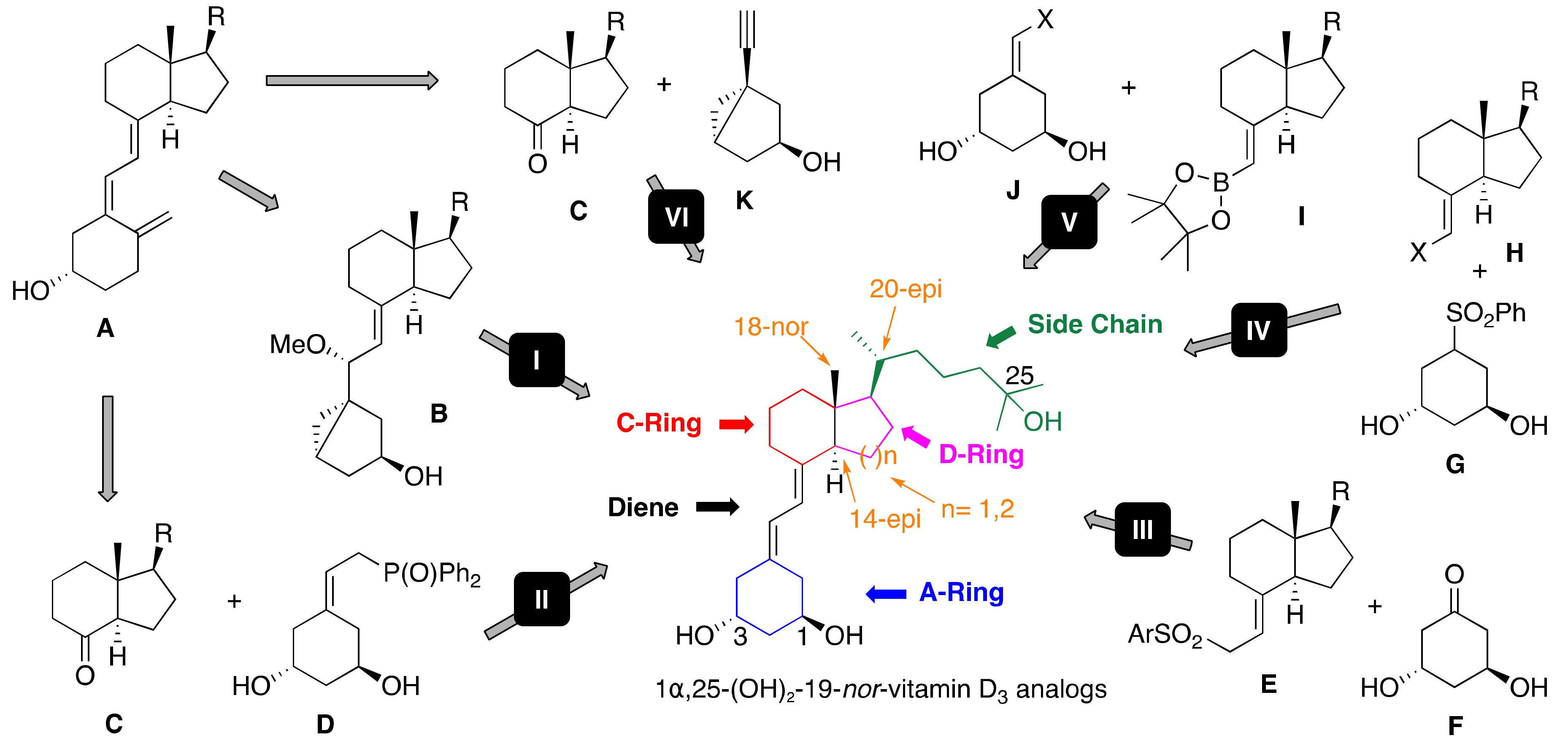
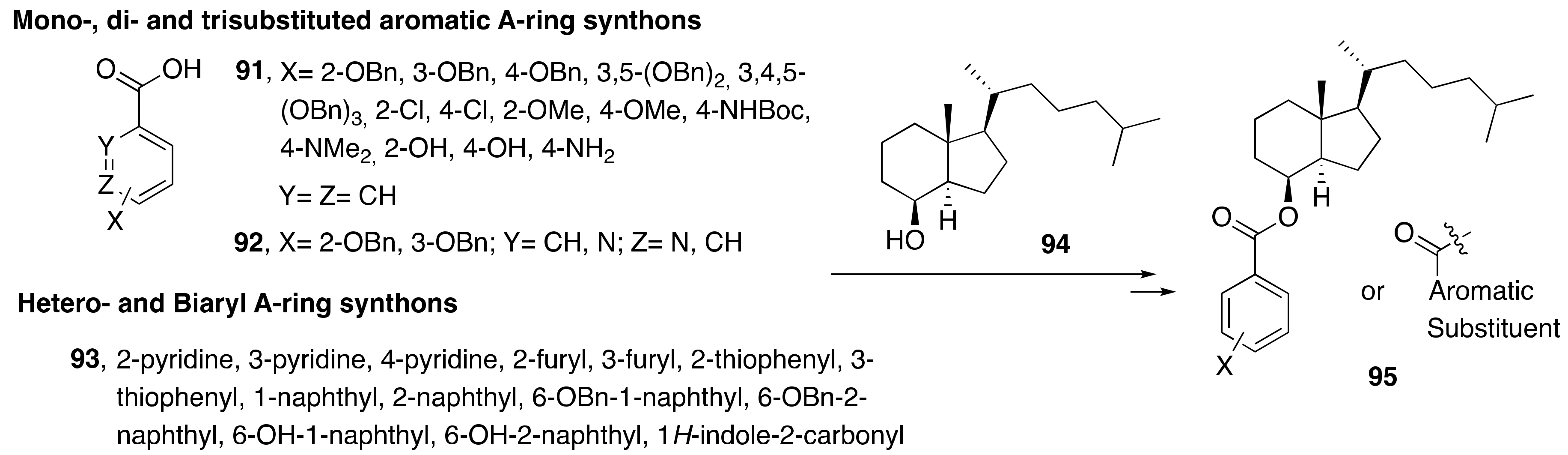
3. Synthesis of A-Ring-Modified 1α,25-(OH)2-19-nor-Vitamin D
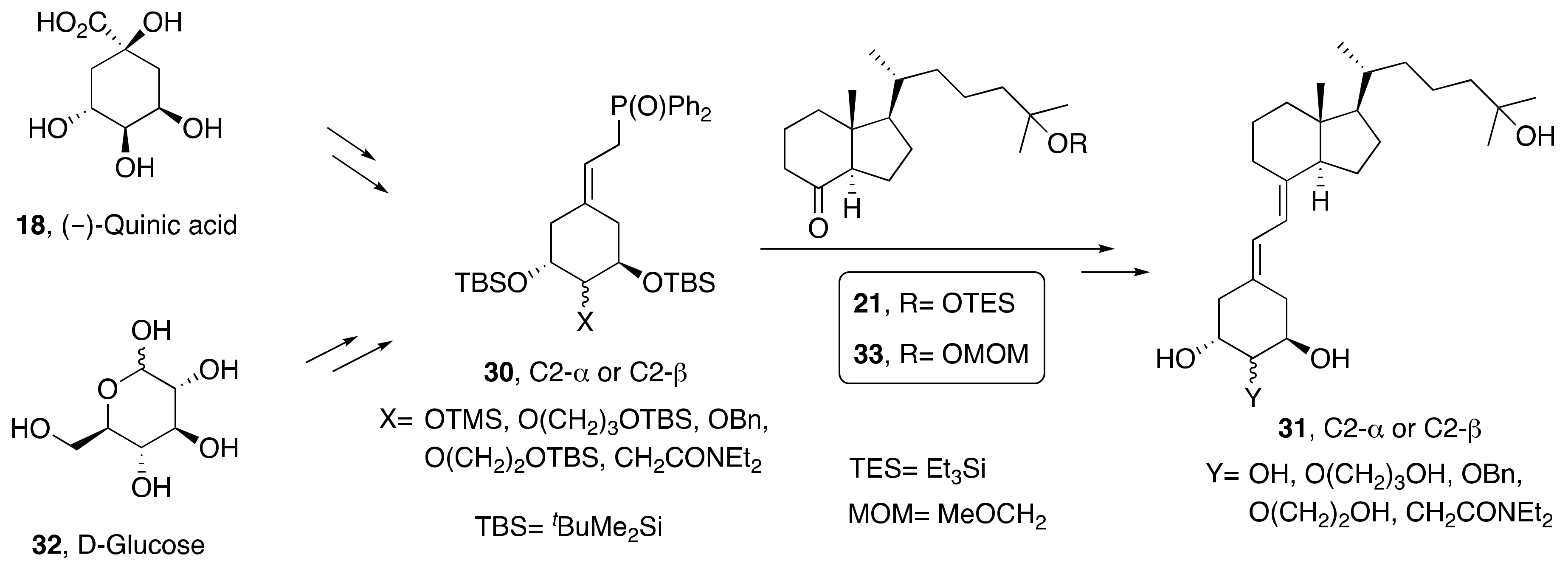
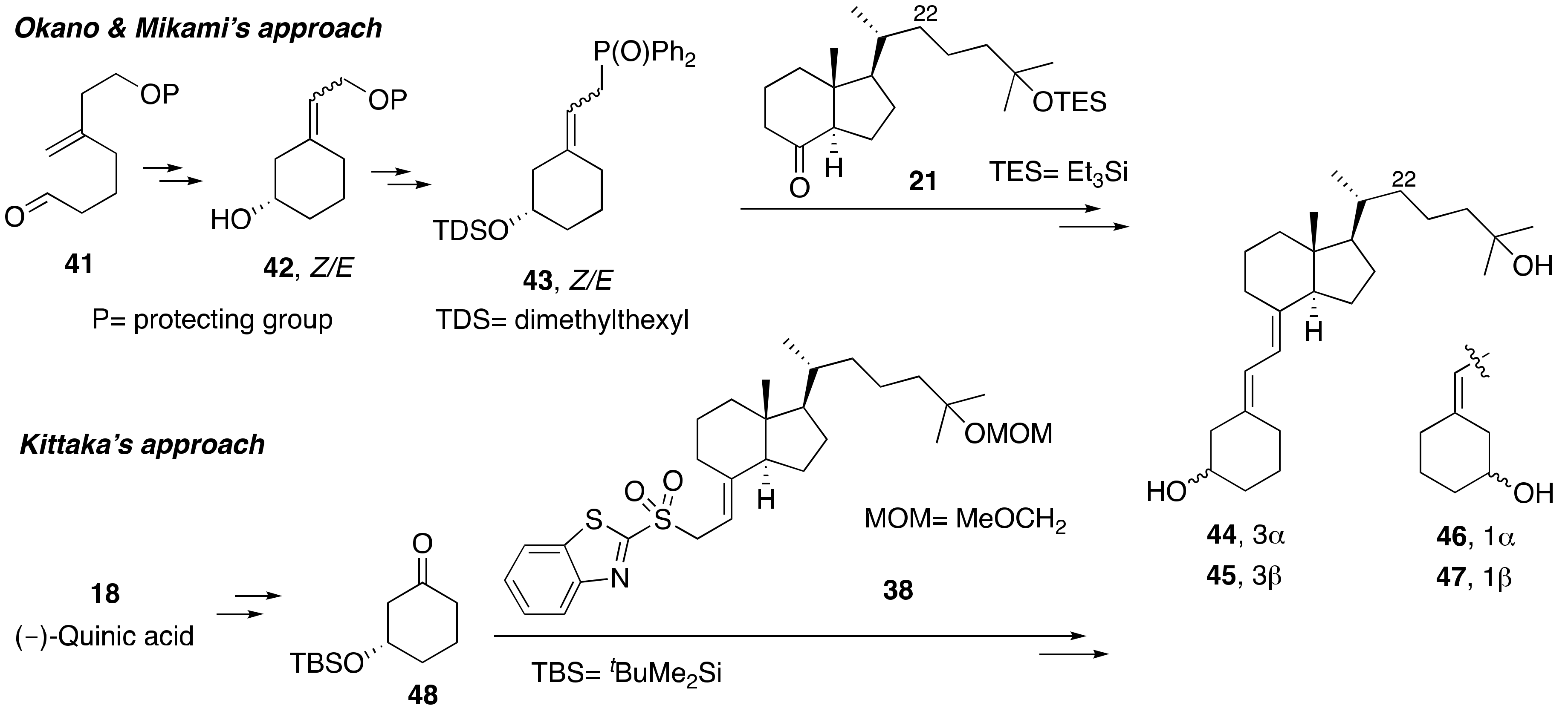
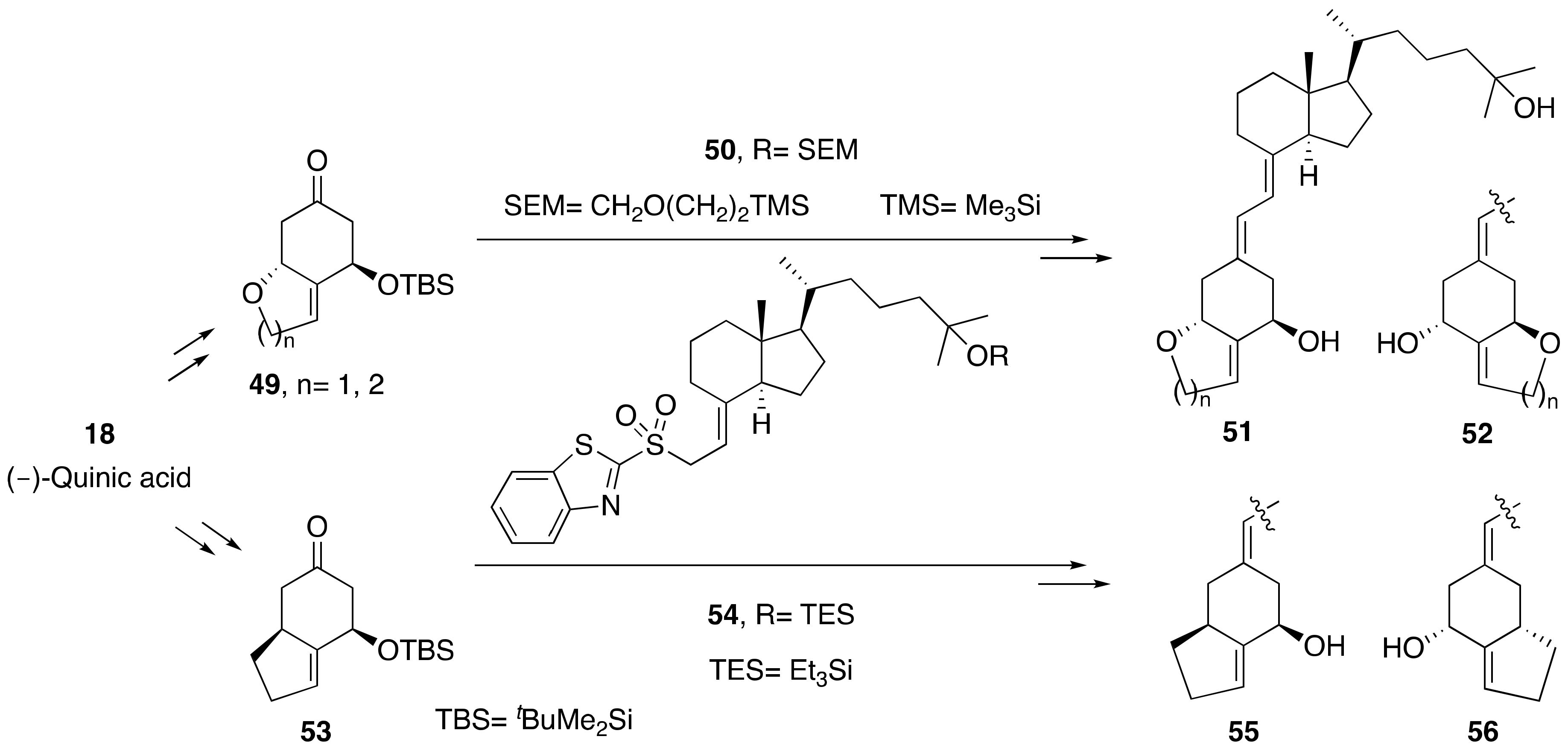
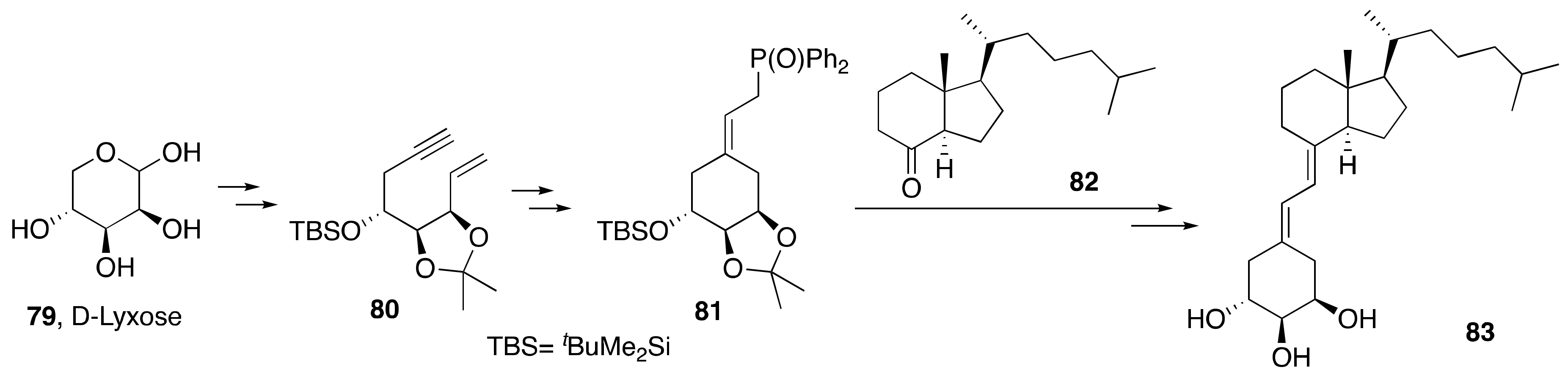
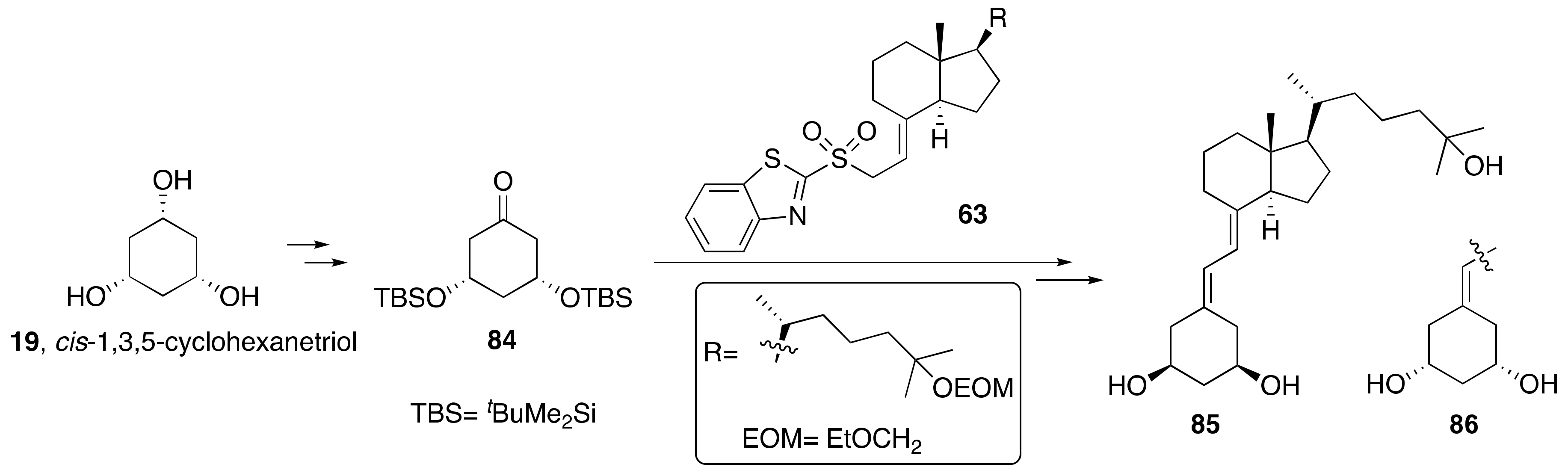
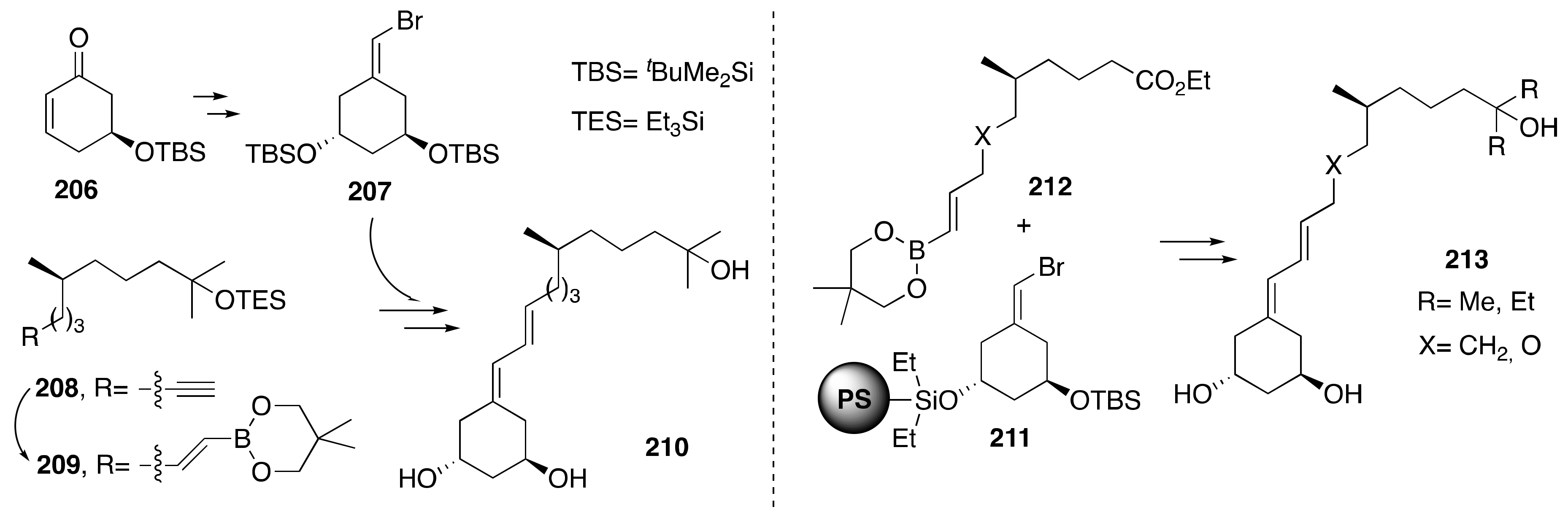
3.1. Synthesis of A-Ring- and Diene-Modified Analogs
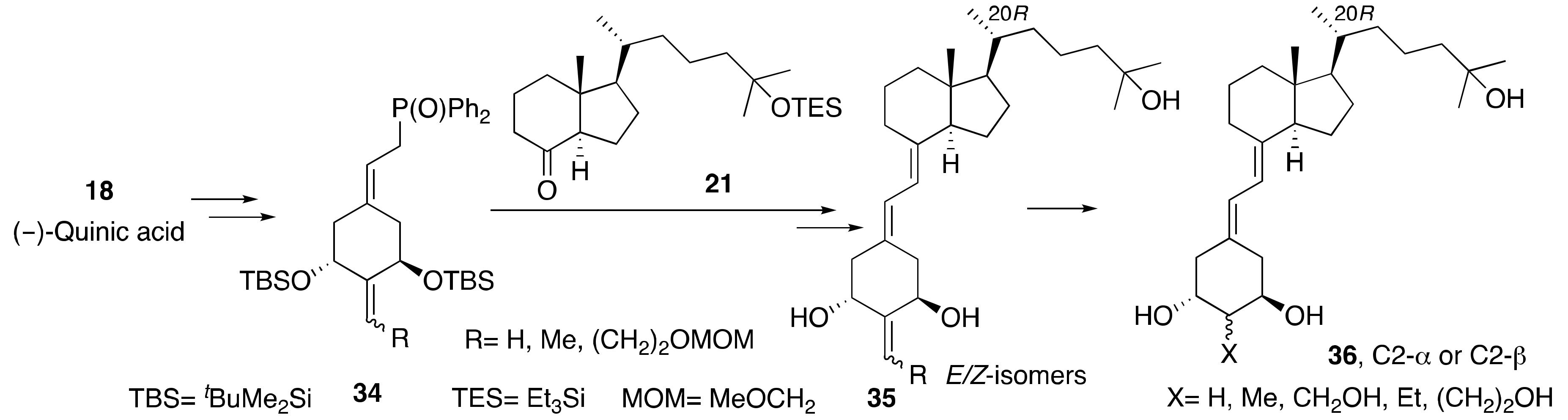
3.2. Synthesis of A-Ring-, D-Ring-, and Side-Chain-Modified Analogs
3.3. Synthesis of A-Ring-, CD-Ring-, and Side-Chain-Modified Analogs
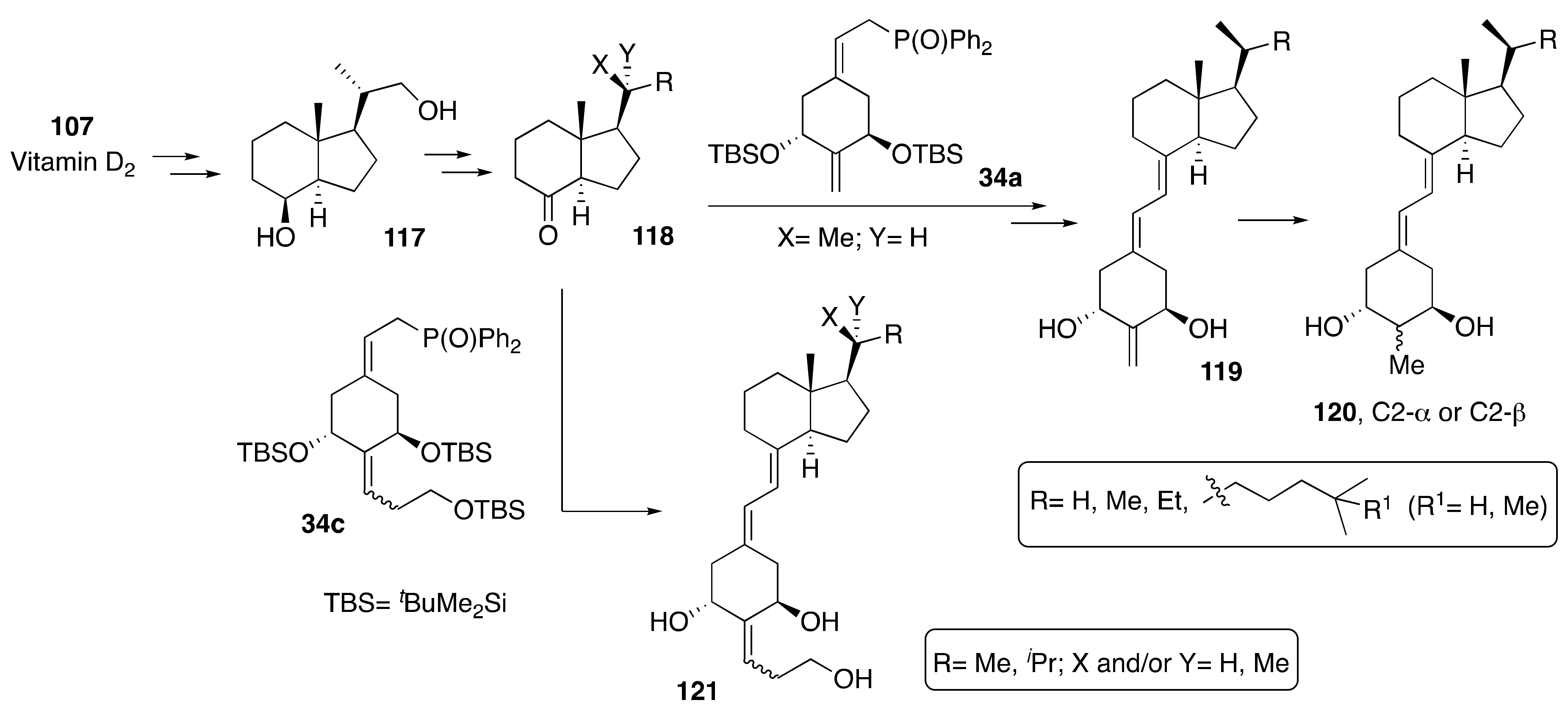
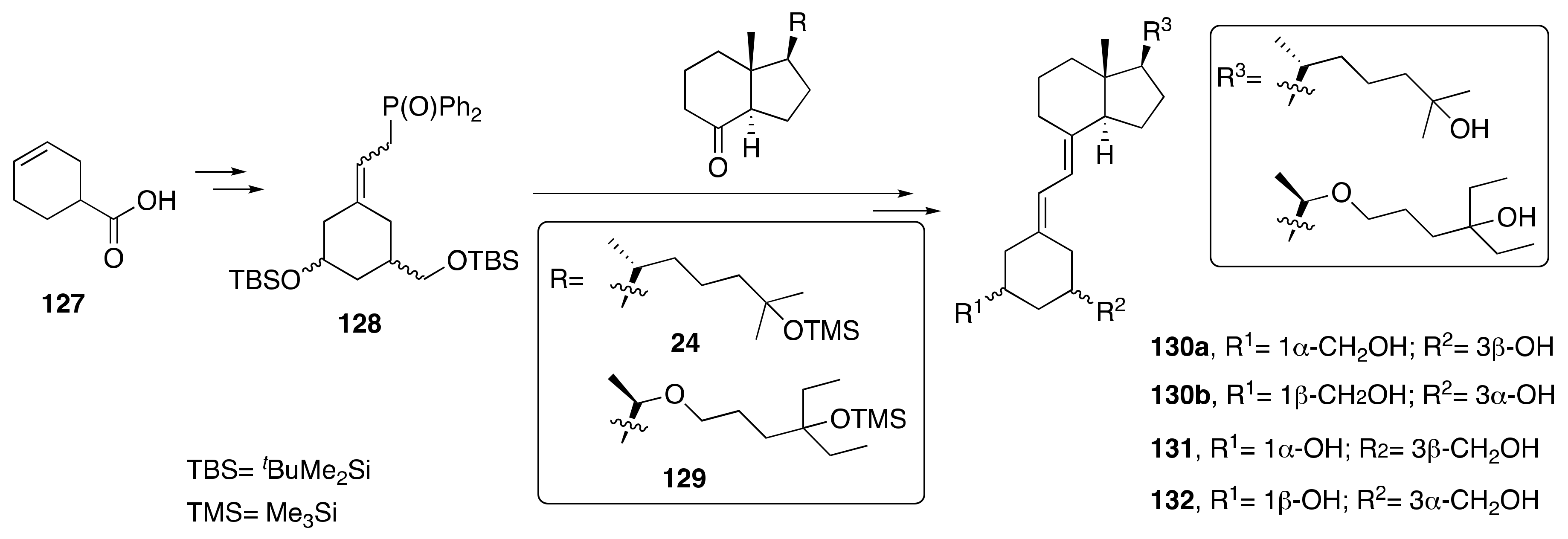
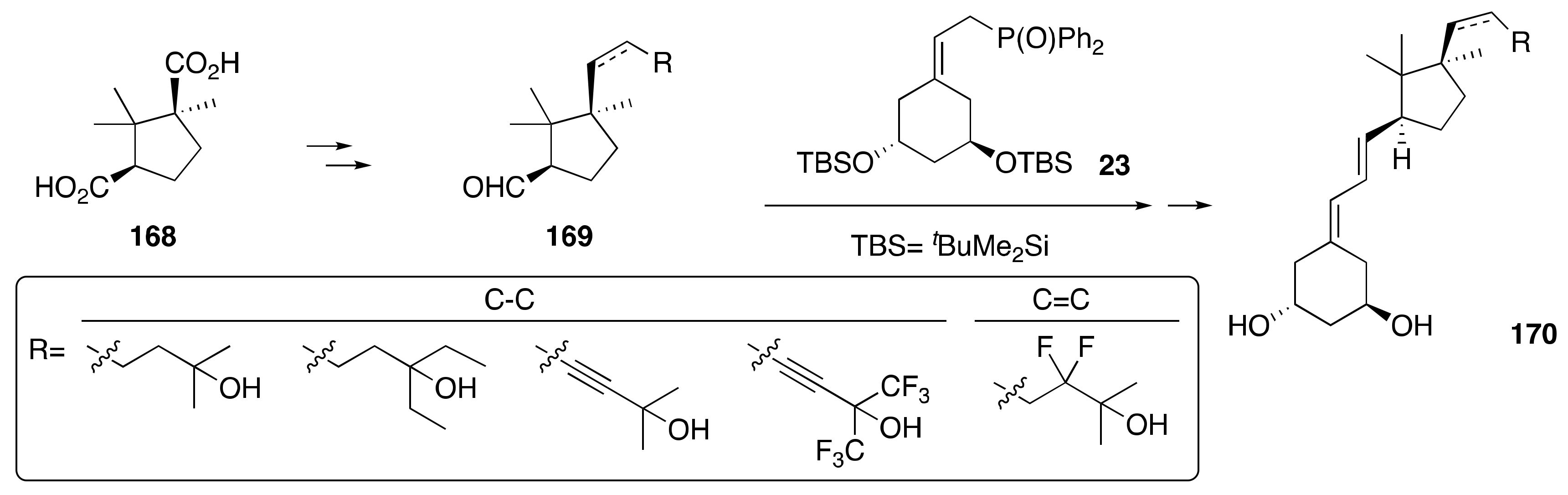
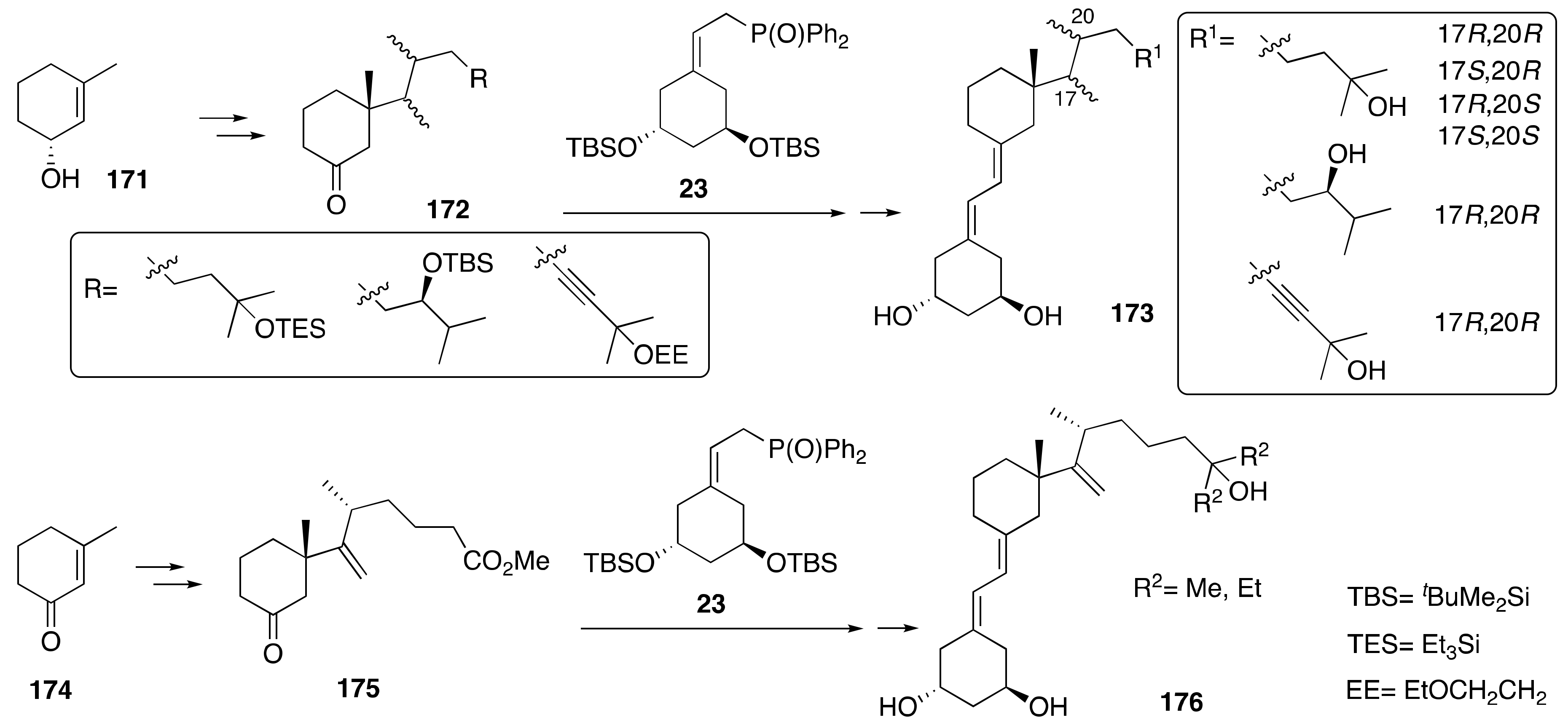
3.4. Synthesis of A-Ring- and Side-Chain-Modified Analogs
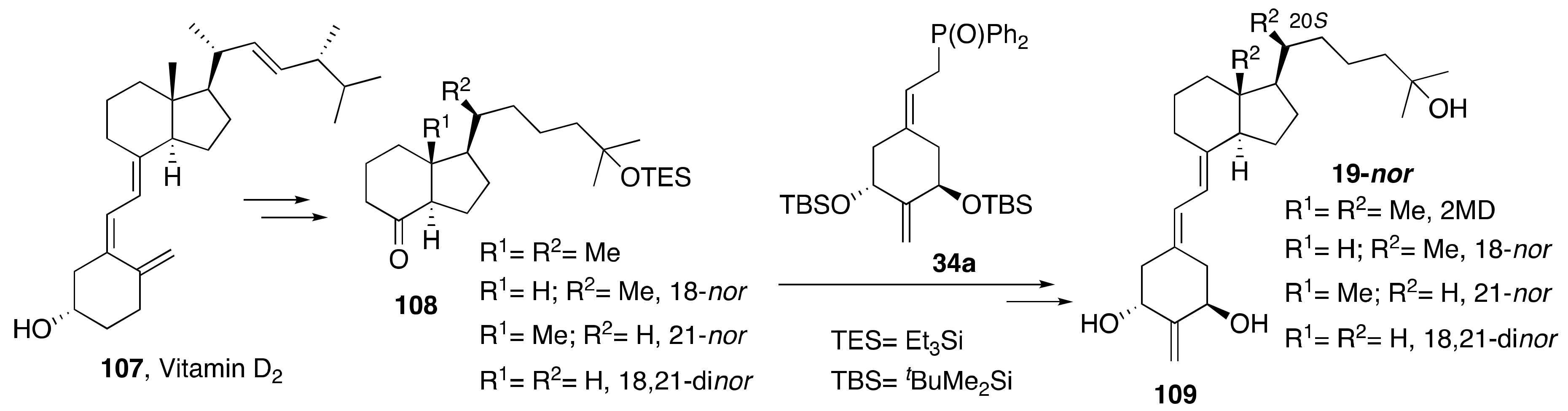
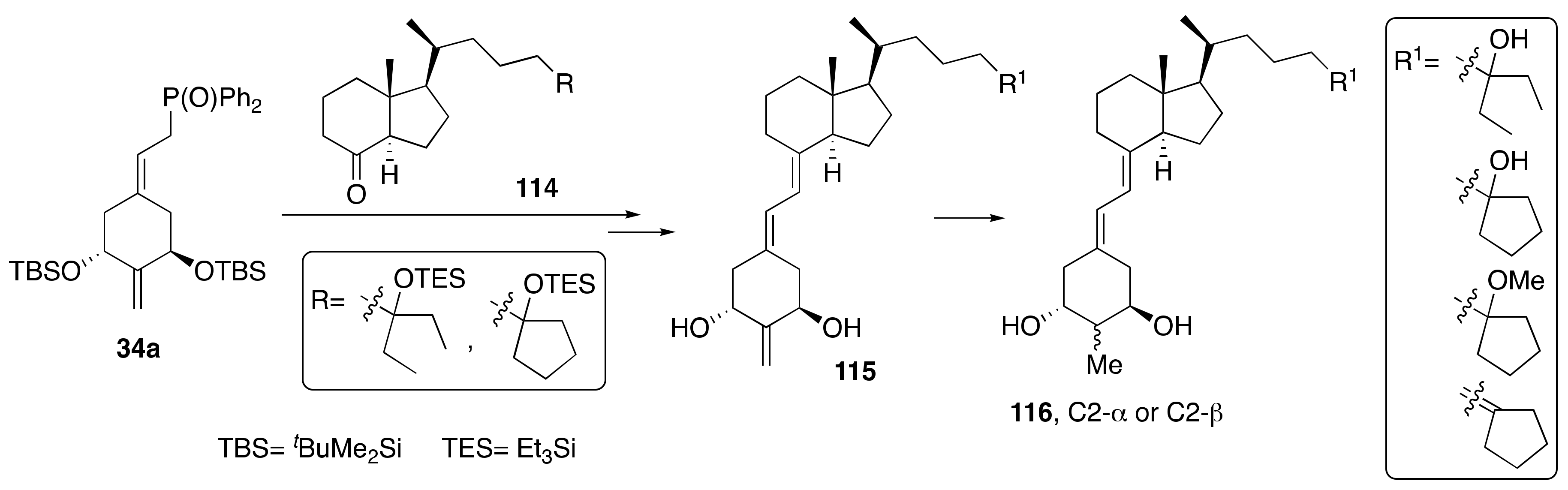
4. Synthesis of Diene-Modified 1α,25-(OH)2-19-nor-Vitamin D
5. Synthesis of CD-Ring-Modified 1α,25-(OH)2-19-nor-Vitamin D
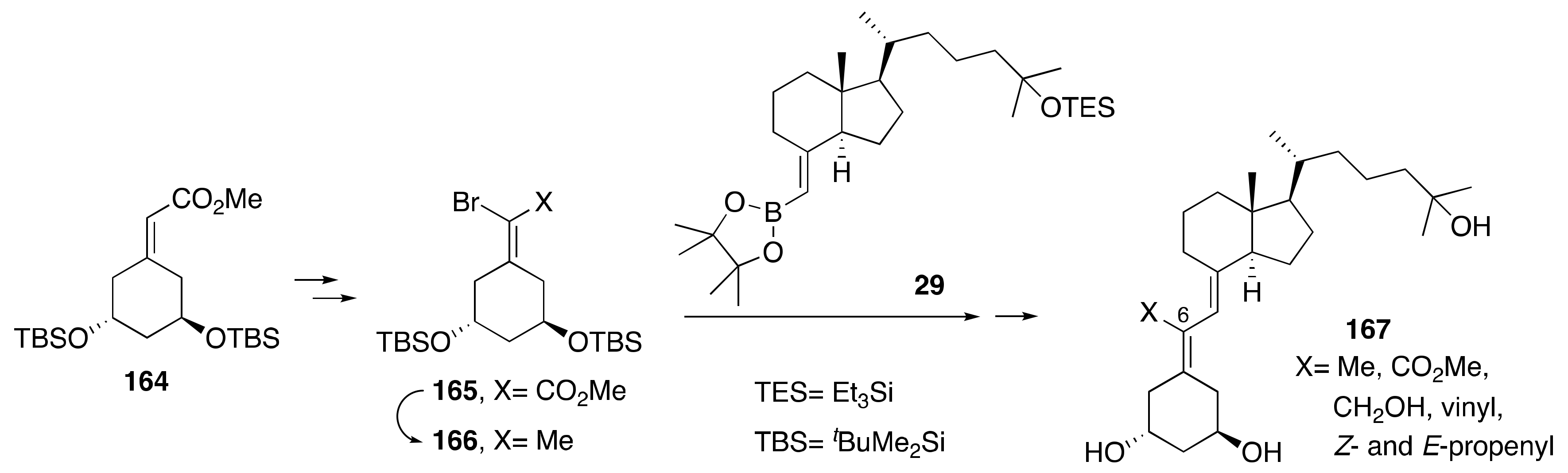
5.1. Synthesis of C-Ring- and Side-Chain-Modified Analogs
5.2. Synthesis of D-Ring- and Side-Chain-Modified Analogs
5.3. Synthesis of CD-Ring-Modified Analogs
5.4. Synthesis of CD-Ring- and Side-Chain-Modified Analogs
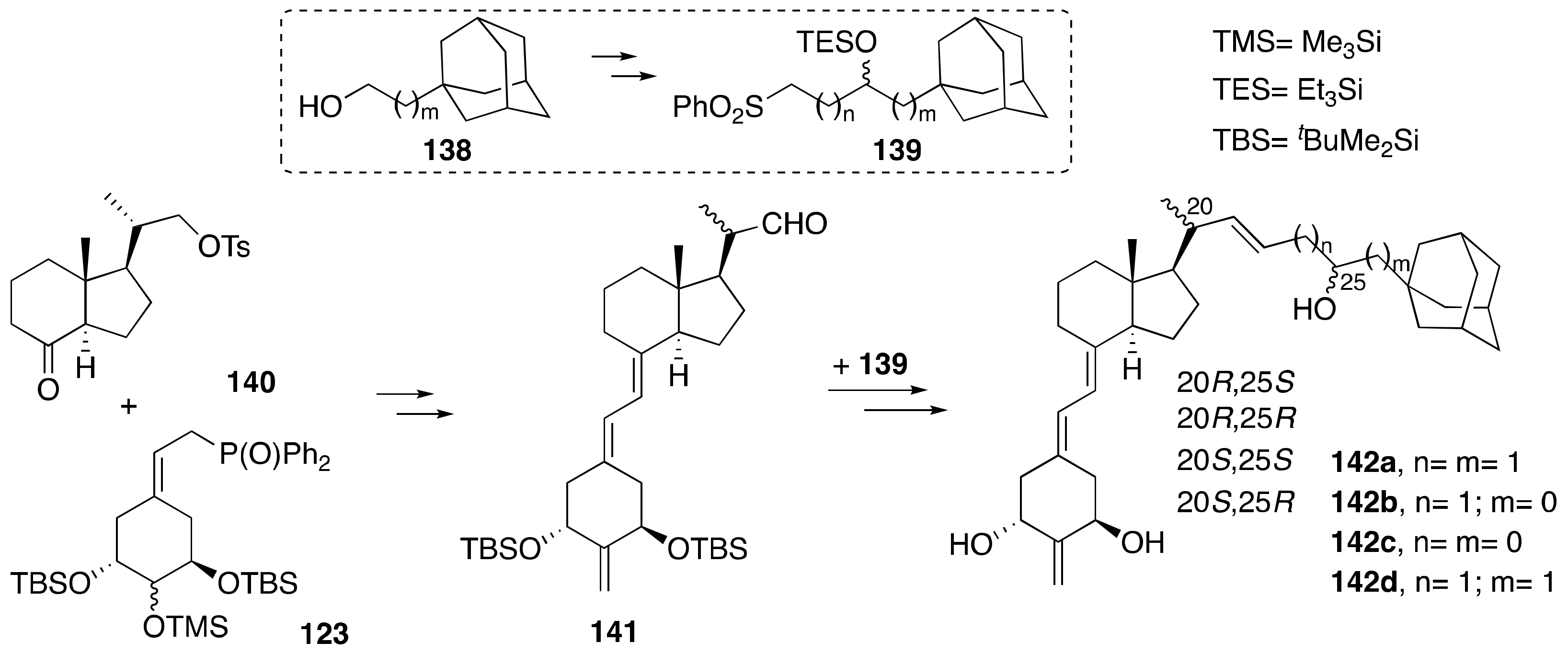
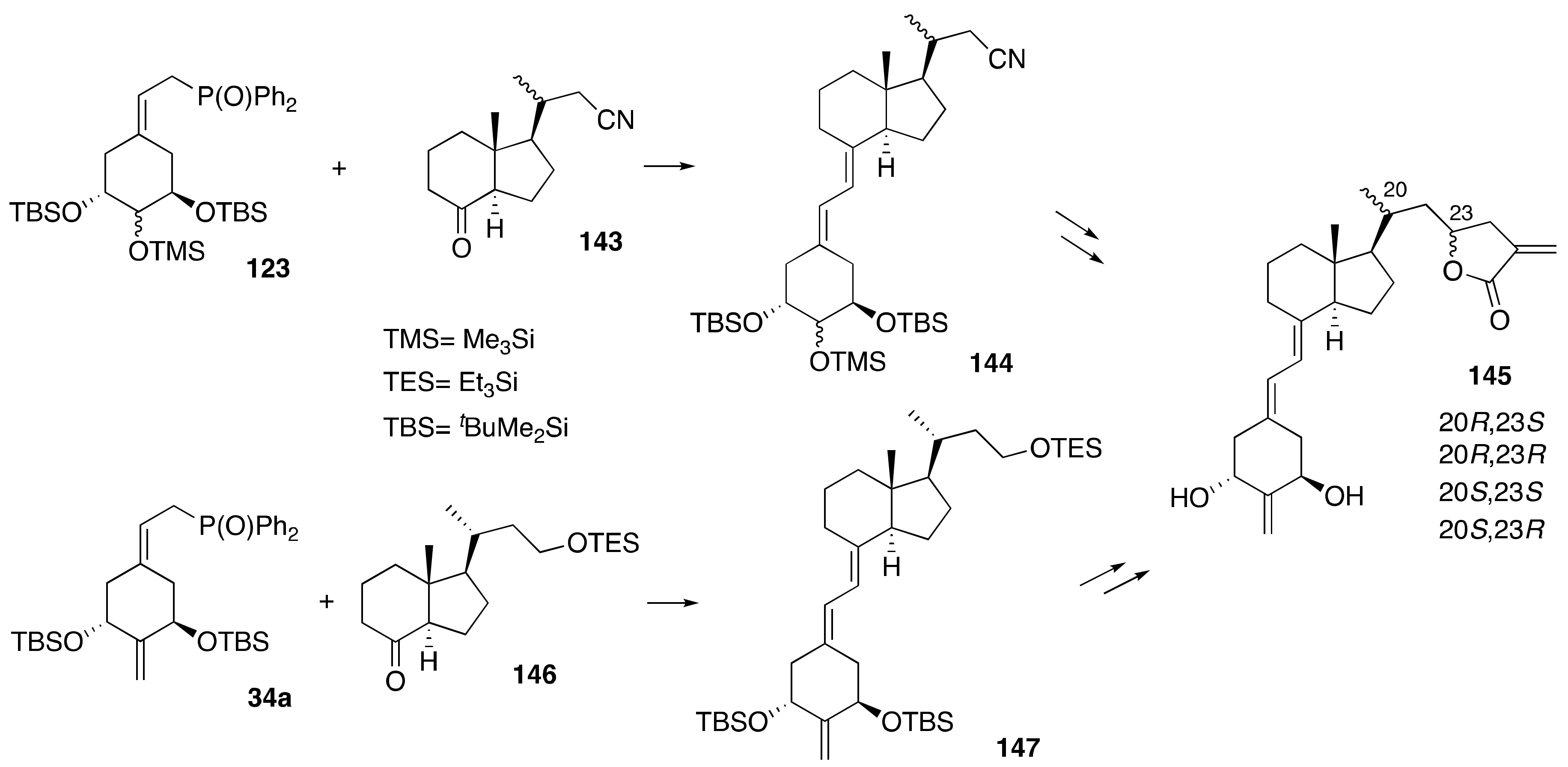
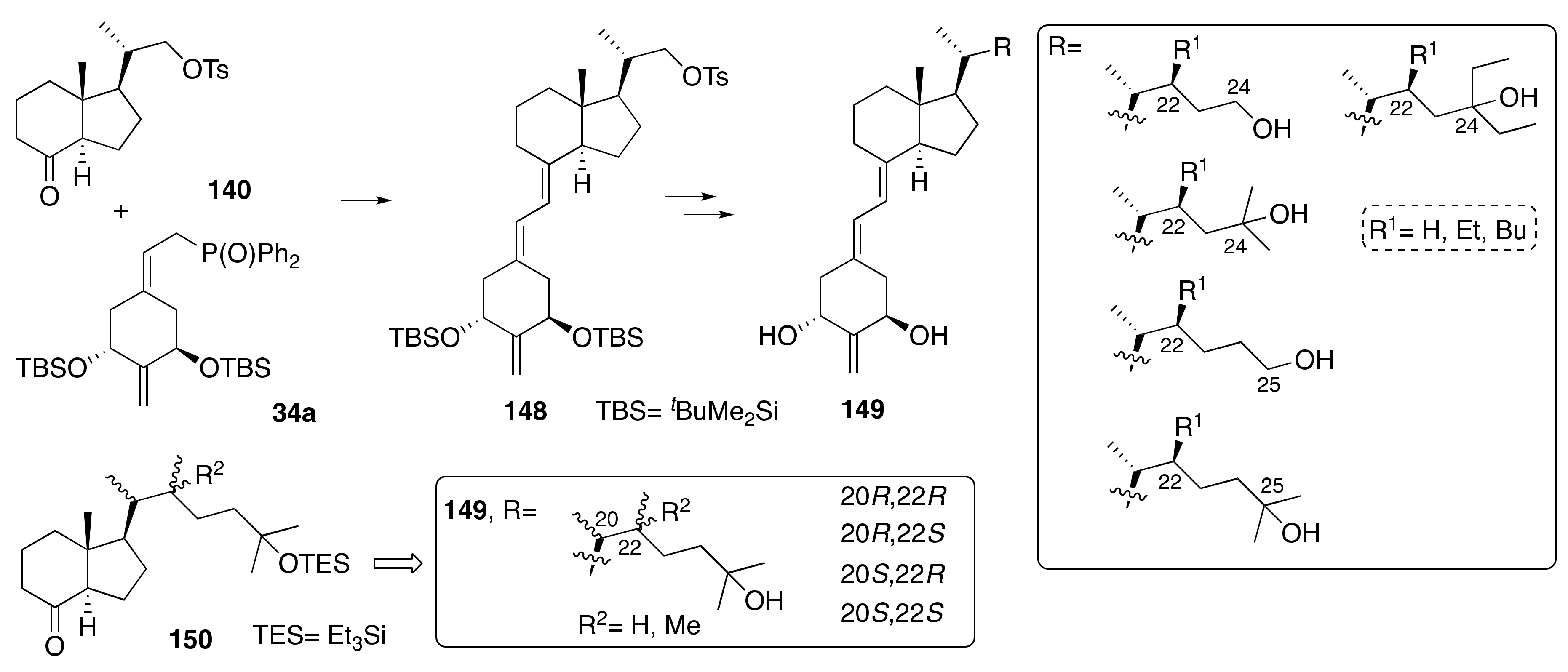
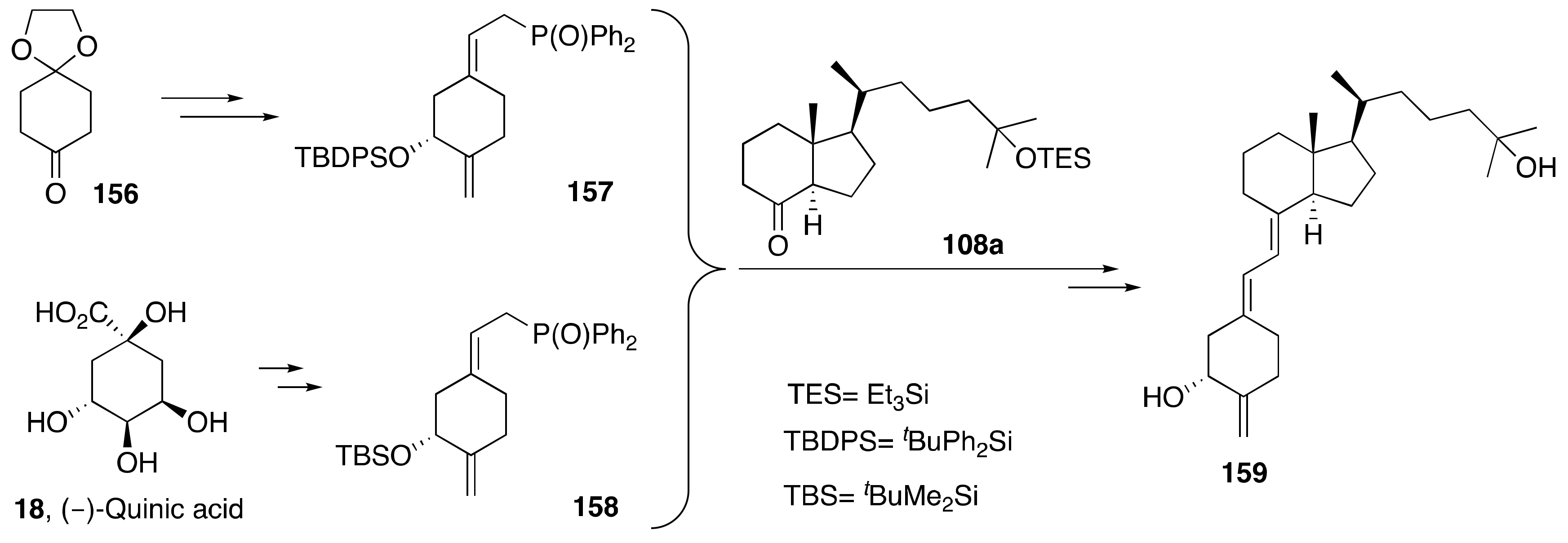
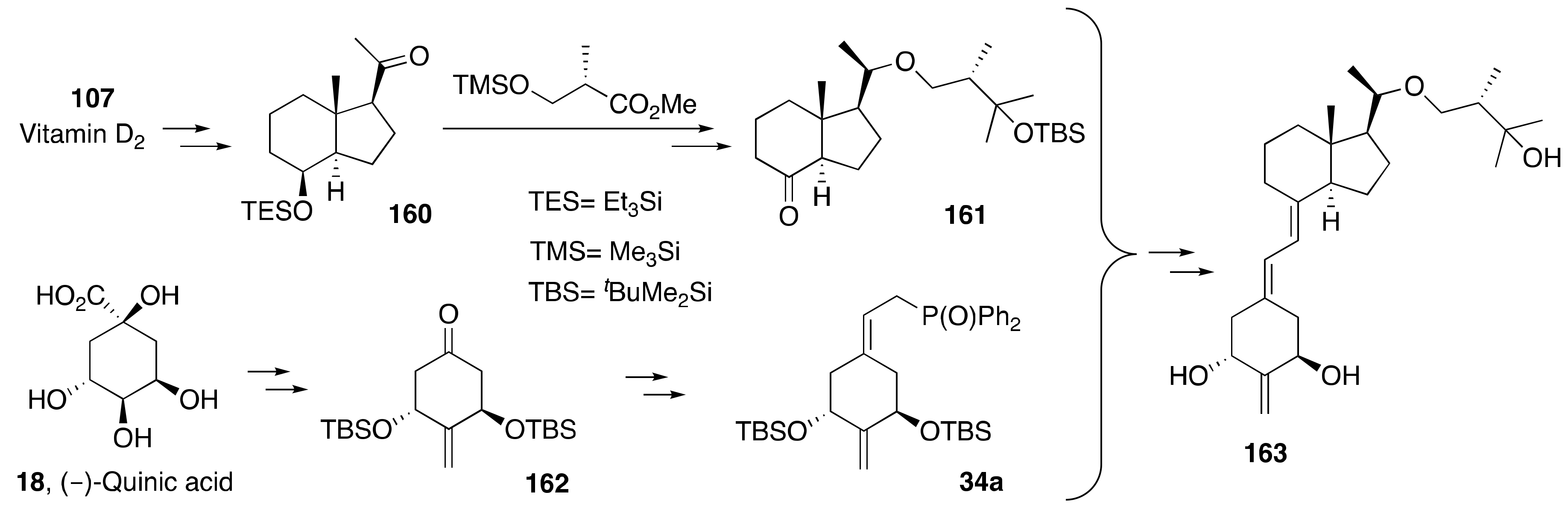
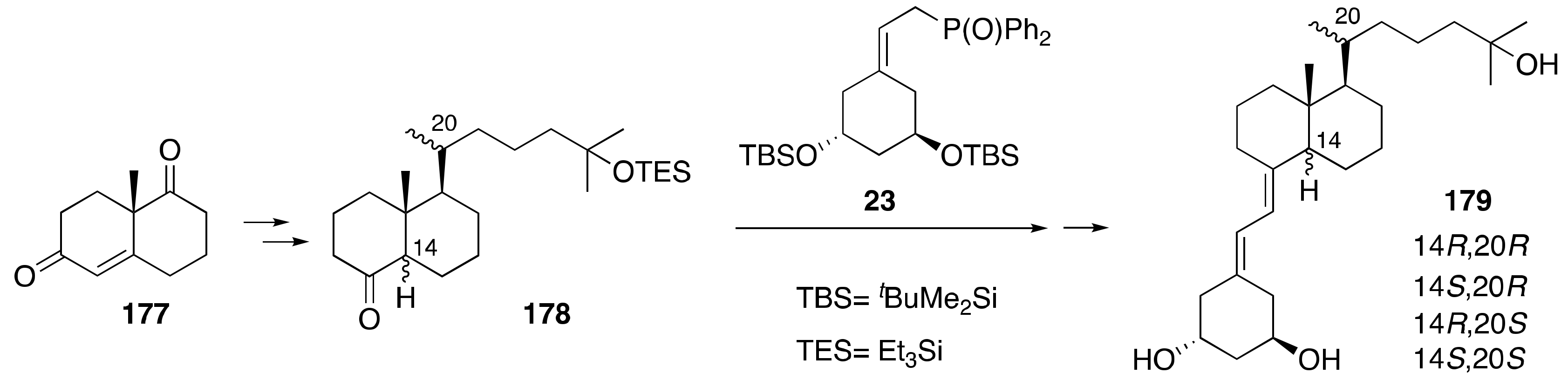
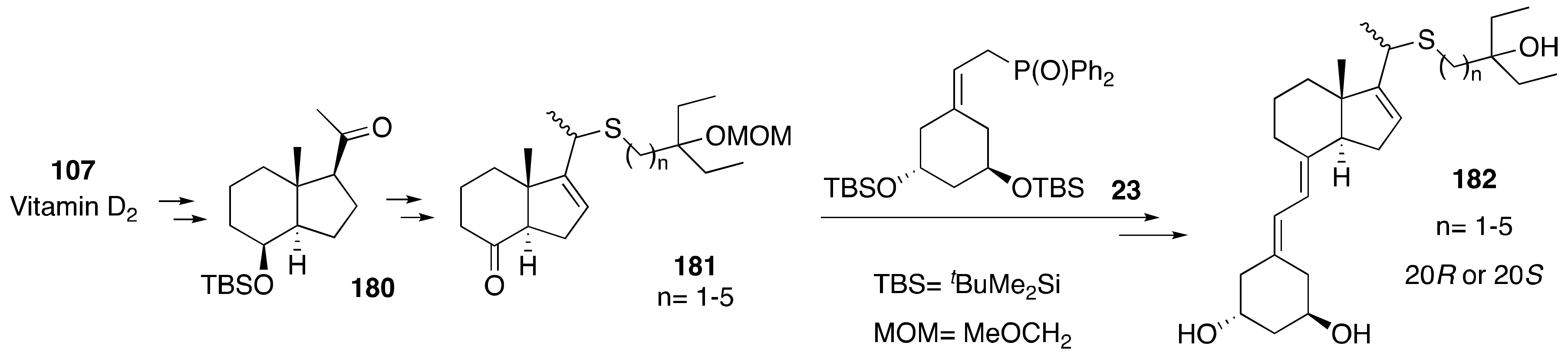
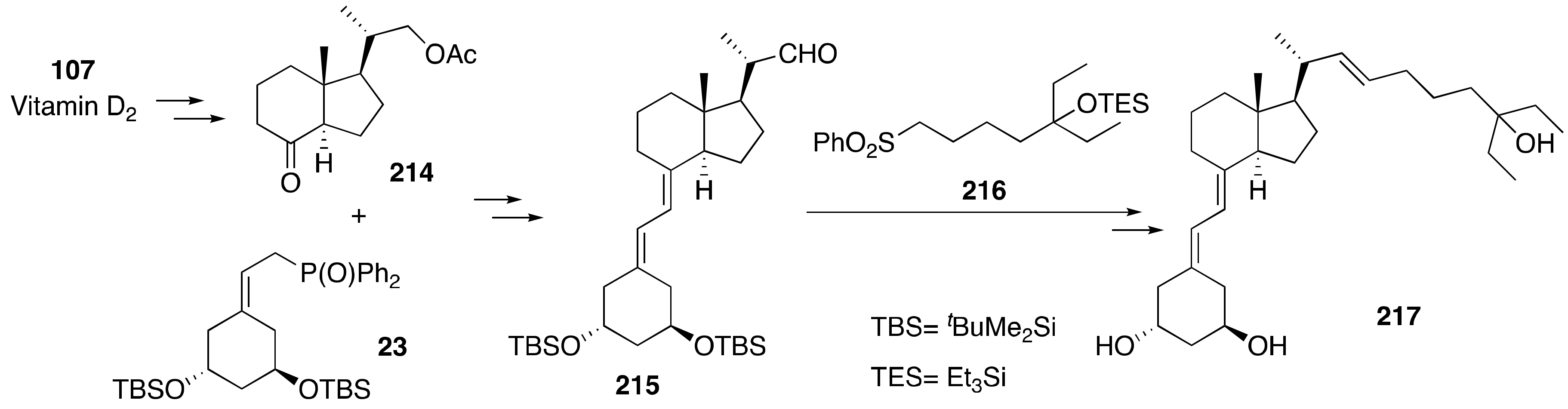
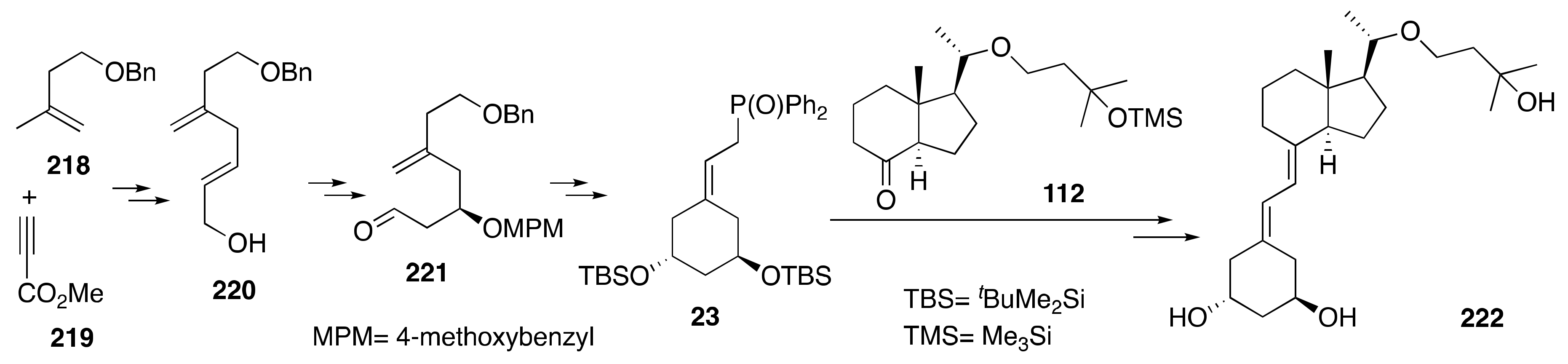
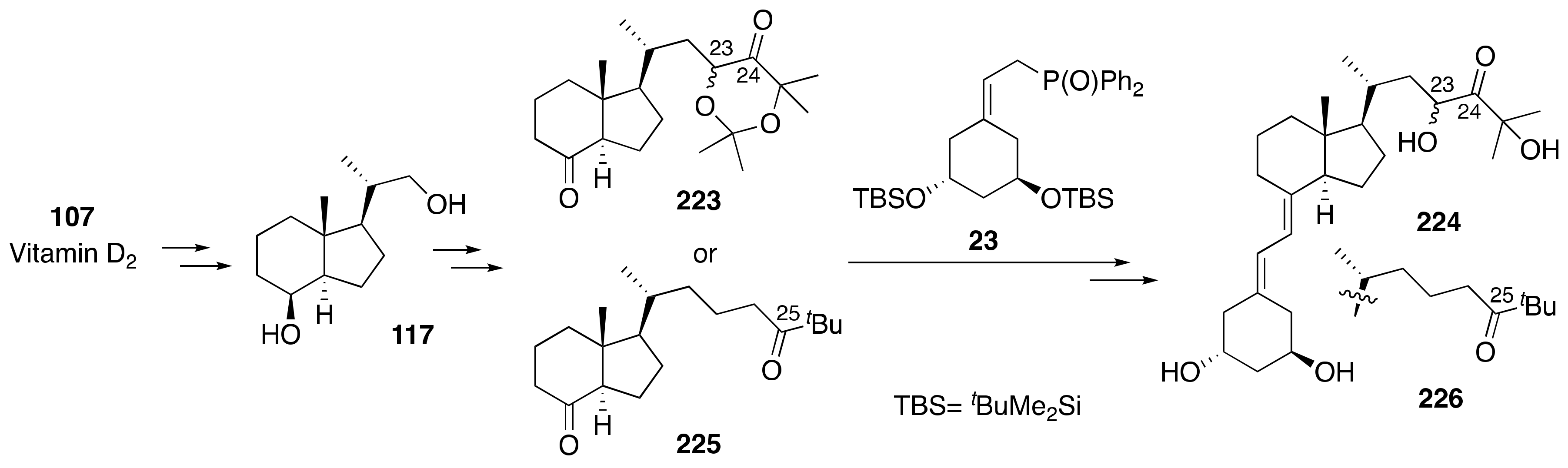
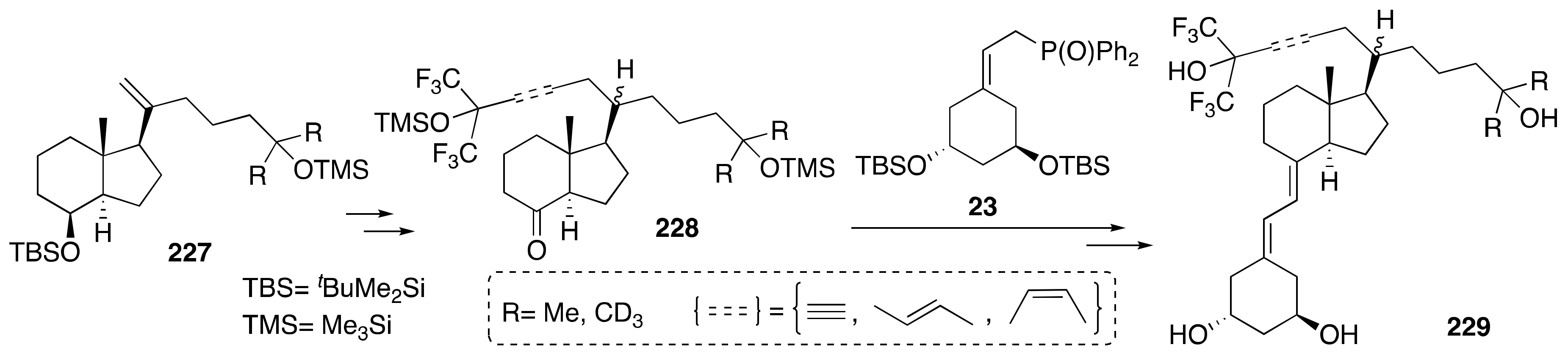
6. Synthesis of Side-Chain-Modified 1α,25-(OH)2-19-nor-Vitamin D
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol. Renal Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Holick, M.F. Vitamin D and prostate cancer prevention and treatment. Trends Endocrinol. Metab. 2003, 14, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Leyssens, C.; Verlinden, L.; Verstuyf, A. Antineoplastic effects of 1,25(OH)2D3 and its analogs in breast, prostate and colorectal cancer. Endocr. Relat. Cancer 2013, 20, R31–R47. [Google Scholar] [CrossRef]
- Fernández, S.; Hernández-Martín, A.; González-García, T.; Ferrero, M. Synthesis of 6-s-cis and 6-s-trans A-Ring Modified Vitamin D Analogues. Curr. Top. Med. Chem. 2014, 14, 2424–2445. [Google Scholar] [CrossRef] [PubMed]
- Glebocka, A.; Chiellini, G. A-ring analogs of 1,25-dihydroxyvitamin D3. Arch. Biochem. Biophys. 2012, 523, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Agoston, E.S.; Hatcher, M.A.; Kensler, T.W.; Posner, G.H. Vitamin D analogs as anticarcinogenic agents. Anti Cancer Agents Med. Chem. 2006, 6, 53–71. [Google Scholar] [CrossRef]
- Saito, N.; Honzawa, S.; Kittaka, A. Recent results on A-ring modification of 1α,25-dihydroxyvitamin D3: Design and synthesis of VDR-agonists and antagonists with high biological activity. Curr. Top. Med. Chem. 2006, 6, 1273–1288. [Google Scholar] [CrossRef]
- Binderup, L.; Binderup, E.; Godtfredsen, W.O.; Kissmeyer, A.M. Development of new vitamin D analogs. In Vitamin D, 2nd ed.; Feldman, D., Pike, J.W., Glorieux., F.H., Eds.; Academic Press: London, UK, 2005; pp. 1489–1510. [Google Scholar] [CrossRef]
- Peleg, S.; Posner, G.H. Vitamin D analogs as modulators of vitamin D receptor action. Curr. Topics Med. Chem. 2003, 3, 1555–1572. [Google Scholar] [CrossRef]
- Zhu, G.-D.; Okamura, W.H. Synthesis of Vitamin D (Calciferol). Chem. Rev. 1995, 95, 1877–1952. [Google Scholar] [CrossRef]
- Lythgoe, B. Synthetic approaches to vitamin D and its relatives. Chem. Soc. Rev. 1980, 9, 449–475. [Google Scholar] [CrossRef]
- Perlman, K.L.; Sicinski, R.R.; Schnoes, H.K.; DeLuca, H.F. 1α,25-Dihydroxy-19-nor-vitamin D3, a novel vitamin D-related compound with potential therapeutic activity. Tetrahedron Lett. 1990, 31, 1823–1824. [Google Scholar] [CrossRef]
- Toyoda, A.; Nagai, H.; Yamada, T.; Moriguchi, Y.; Abe, J.; Tsuchida, T.; Nagasawa, K. Novel synthesis of 1 α,25-dihydroxy-19-norvitamin D from 25-hydroxyvitamin D. Tetrahedron 2009, 65, 10002–10008. [Google Scholar] [CrossRef]
- Huang, P.-Q.; Sabbe, K.; Pottie, M.; Vandewalle, M.; ,P. A novel synthesis of 19-nor 1 α,25-Dihydroxyvitamin D3 and related analogues. Tetrahedron Lett. 1995, 36, 8299–8302. [Google Scholar] [CrossRef]
- Perlman, K.L.; Swenson, R.E.; Paaren, H.E.; Schnoes, H.K.; DeLuca, H.F. Novel synthesis of 19-nor-vitamin D compounds. Tetrahedron Lett. 1991, 32, 7663–7666. [Google Scholar] [CrossRef]
- Nagai, Y.; Tanami, T.; Abe, J.; Nagai, H.; Hamamizu, T.; Kominato, K.; Iida, K.; Nagasawa, K. Synthesis of 19-Nor-Vitamin D A-Ring Synthons via Ring-Closing Olefin Metathesis. Asian J. Org. Chem. 2014, 3, 994–999. [Google Scholar] [CrossRef]
- Sicinski, R.R.; Perlman, K.L.; DeLuca, H.F. Synthesis and Biological Activity of 2-Hydroxy and 2-Alkoxy Analogs of l α,25-Dihydroxy-19-norvitaminD3. J. Med. Chem. 1994, 37, 3730–3738. [Google Scholar] [CrossRef]
- Shimizu, M.; Iwasaki, Y.; Shibamoto, Y.; Sato, M.; DeLuca, H.F.; Yamada, S. Novel Synthesis of 2-Substituted 19-Norvitamin D A-Ring Phosphine Oxide from D-Glucose as a Building Block. Bioorg. Med. Chem. Lett. 2003, 13, 809–812. [Google Scholar] [CrossRef]
- Sicinski, R.R.; Prahl, J.M.; Smith, C.M.; DeLuca, H.F. New 1 α,25-Dihydroxy-19-norvitamin D3 Compounds of High Biological Activity: Synthesis and Biological Evaluation of 2-Hydroxymethyl, 2-Methyl, and 2-Methylene Analogues. J. Med. Chem. 1998, 41, 4662–4674. [Google Scholar] [CrossRef]
- Sicinski, R.R.; Rotkiewicz, P.; Kolinski, A.; Sicinska, W.; Prahl, J.M.; Smith, C.M.; DeLuca, H.F. 2-Ethyl and 2-Ethylidene Analogues of 1 α,25-Dihydroxy-19-norvitamin D3: Synthesis, Conformational Analysis, Biological Activities, and Docking to the Modeled rVDR Ligand Binding Domain. J. Med. Chem. 2002, 45, 3366–3380. [Google Scholar] [CrossRef]
- Glebocka, A.; Sicinski, R.R.; Plum, L.A.; Clagett-Dame, M.; DeLuca, H.F. New 2-Alkylidene 1 α,25-Dihydroxy-19-norvitamin D3 Analogues of High Intestinal Activity: Synthesis and Biological Evaluation of 2-(3′-Alkoxypropylidene) and 2-(3′-Hydroxypropylidene) Derivatives. J. Med. Chem. 2006, 49, 2909–2920. [Google Scholar] [CrossRef]
- Ono, K.; Yoshida, A.; Saito, N.; Fujishima, T.; Honzawa, S.; Suhara, Y.; Kishimoto, S.; Sugiura, T.; Waku, K.; Takayama, H.; et al. Efficient Synthesis of 2-Modified 1 α,25-Dihydroxy-19-norvitamin D3 with Julia Olefination: High Potency in Induction of Differentiation on HL-60 Cells. J. Org. Chem. 2003, 68, 7407–7415. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.A.; Kittaka, A. Novel 2-Alkyl-1 α,25-dihydroxy-19-norvitamin D3: Efficient Synthesis with Julia Olefination, Evaluation of Biological Activity and Development of New Analyzing System for Co-Activator Recruitment. Anticancer Res. 2006, 26, 2621–2631. [Google Scholar]
- Okano, T.; Nakagawa, K.; Kubodera, N.; Ozono, K.; Isaka, A.; Osawa, A.; Terada, M.; Mikami, K. Catalytic asymmetric syntheses and biological activities of singly dehydroxylated 19-nor-1 α,25-dihydroxyvitamin D3 A-ring analogs in cancer cell differentiation and apoptosis. Chem. Biol. 2000, 7, 173–184. [Google Scholar] [CrossRef]
- Arai, M.A.; Tsutsumi, R.; Hara, H.; Chen, T.C.; Sakaki, T.; Urushino, N.; Inouye, K.; Kittaka, A. Synthesis of 25-hydroxy-19-norvitamin D3 analogs and their antiproliferative activities on prostate cells. Heterocycles 2005, 66, 469–479. [Google Scholar] [CrossRef]
- Sicinski, R.R.; Glebocka, A.; Plum, L.A.; DeLuca, H.F. Design, Synthesis, and Biological Evaluation of a 1 α,25-Dihydroxy-19-norvitamin D3 Analogue with a Frozen A-Ring Conformation. J. Med. Chem. 2007, 50, 6154–6164. [Google Scholar] [CrossRef] [PubMed]
- Glebocka, A.; Sokolowska, K.; Sicinski, R.R.; Plum, L.A.; DeLuca, H.F. New 1 α,25-Dihydroxy-19-norvitamin D3 Compounds Constrained in a Single A-Ring Conformation: Synthesis of the Analogues by Ring-Closing Metathesis Route and Their Biological Evaluation. J. Med. Chem. 2009, 52, 3496–3504. [Google Scholar] [CrossRef] [PubMed]
- Glebocka, A.; Sicinski, R.R.; Plum, L.A.; DeLuca, H.F. New 1 α,25-dihydroxy-19-norvitamin D3 analogs with frozen A-Ring conformation. J. Steroid Biochem. Mol. Biol. 2010, 121, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Abella, L.; Fernández, S.; Verstuyf, A.; Verlinden, L.; Gotor, V.; Ferrero, M. Synthesis, Conformational Analysis, and Biological Evaluation of 19-nor-Vitamin D3 Analogues with A-Ring Modifications. J. Med. Chem. 2009, 52, 6158–6162. [Google Scholar] [CrossRef]
- Sikervar, V.; Fleet, J.C.; Fuchs, P.L. A general approach to the synthesis of enantiopure 19-nor-Vitamin D3 and its C-2 phosphate analogs prepared from cyclohexadienyl sulfone. Chem. Commun. 2012, 48, 9077–9079. [Google Scholar] [CrossRef]
- Ibe, K.; Aoki, H.; Takagi, H.; Ken-mochi, K.; Hasegawa, Y.; Hayashi, N.; Okamoto, S. Preparation of 2-hydroxy A-ring precursors for synthesis of vitamin D3 analogues from lyxose. Tetrahedron Lett. 2015, 56, 2315–2318. [Google Scholar] [CrossRef]
- González-García, T.; Verstuyf, A.; Verlinden, L.; Fernández, S.; Ferrero, M. Enzymatic Desymmetrization of 19-nor-Vitamin D3 A-Ring Synthon Precursor: Synthesis, Structure Elucidation, and Biological Activity of 1 α,25-Dihydroxy-3-epi-19-nor-vitamin D3 and 1 α,25-Dihydroxy-19-nor-vitamin D3. Adv. Synth. Catal. 2018, 360, 2762–2772. [Google Scholar] [CrossRef]
- Suhara, Y.; Kittaka, A.; Ono, K.; Kurihara, M.; Fujishima, T.; Yoshida, A.; Takayama, H. Design and Efficient Synthesis of New STable 1 α,25-Dihydroxy-19-norvitamin D3 Analogues Containing Amide Bond. Bioorg. Med. Chem. Lett. 2002, 12, 3533–3536. [Google Scholar] [CrossRef]
- DeBerardinis, A.M.; Madden, D.J.; Banerjee, U.; Sail, V.; Raccuia, D.S.; De Carlo, D.; Lemieux, S.M.; Meares, A.; Hadden, M.K. Structure-Activity Relationships for Vitamin D3-Based Aromatic A-Ring Analogues as Hedgehog Pathway Inhibitors. J. Med. Chem. 2014, 57, 3724–3736. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Miyamoto, Y.; Takaku, H.; Matsuo, M.; Nakabayashi, M.; Masuno, H.; Udagawa, N.; DeLuca, H.F.; Ikura, T.; Ito, N. 2-Substituted-16-ene-22-thia-1 α,25-dihydroxy-26,27-dimethyl-19-norvitamin D3 analogs: Synthesis, biological evaluation, and crystal structure. Bioorg. Med. Chem. Lett. 2008, 16, 6949–6964. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Y.; Tian, H.; De Clercq, P.; Vandewalle, M.; Berthier, M.; Pellegrino, G.; Maillos, P.; Pascal, J.-C. A Practical Synthesis of 14-epi-19-nor-1 α,25-Dihydroxyvitamin D3 Analogues and Their A-ring Epimers. Eur. J. Org. Chem. 2001, 2001, 3779–3788. [Google Scholar] [CrossRef]
- Barycki, R.; Sicinski, R.R.; Plum, L.A.; Grzywacz, P.; Clagett-Dame, M.; DeLuca, H.F. Removal of the 20-methyl group from 2-methylene-19-nor-(20S)-1 α,25-dihydroxyvitamin D3 (2MD) selectively eliminates bone calcium mobilization activity. Bioorg. Med. Chem. 2009, 17, 7658–7669. [Google Scholar] [CrossRef]
- Mikami, K.; Koizumi, Y.; Osawa, A.; Terada, M.; Takayama, H.; Nakagawa, K.; Okano, T. BINOL-Ti-Catalyzed Carbonyl-Ene Cyclization by Tuning the 6-Br-Ligand for the Synthesis of 2-Methyl-19-nor-22-oxa Vitamin D Analogue with Significant Differentiation Activity. Synlett 1999, 12, 1899–1902. [Google Scholar] [CrossRef]
- Mikami, K.; Ohba, S.; Ohmura, H.; Kubodera, N.; Nakagawa, K.; Okano, T. Asymmetric Catalytic Ene-Cyclization Approach to 2-Fluoro-19-Nor-1,25-Dihydroxyvitamin D3 A-Ring Analog with Significant Transactivation Activity. Chirality 2001, 13, 366–371. [Google Scholar] [CrossRef]
- Nakagawa, K.; Okano, T.; Ozono, K.; Kato, S.; Kubodera, N.; Ohba, S.; Itoh, Y.; Mikami, K. Catalytic asymmetric synthesis and anticancer effects of the novel non-calcemic analog of vitamin D, 2 α-fluoro-19-nor-22-oxa-1 α,25-dihydroxyvitamin D3 in metastatic lung carcinoma. J. Fluorine Chem. 2007, 128, 654–667. [Google Scholar] [CrossRef]
- Sicinski, R.R.; Prah, J.M.; Smith, C.M.; DeLuca, H.F. New highly calcemic 1 α,25-dihydroxy-19-norvitamin D3 compounds with modified side chain: 26,27-dihomo- and 26,27-dimethylene analogs in 20S-series. Steroids 2002, 67, 247–256. [Google Scholar] [CrossRef]
- Plum, L.A.; Prahl, J.M.; Ma, X.; Sicinski, R.R.; Gowlugari, S.; Clagett-Dame, M.; DeLuca, H.F. Biologically active noncalcemic analogs of 1 α,25-dihydroxyvitamin D with an abbreviated side chain containing no hydroxyl. Proc. Natl. Acad. Sci. USA 2004, 101, 6900–6904. [Google Scholar] [CrossRef] [PubMed]
- Grzywacz, P.; Plum, L.A.; Sicinski, R.R.; Clagett-Dame, M.; DeLuca, H.F. Methyl substitution of the 25-hydroxy group on 2-methylene-19-nor-1 α,25-dihydroxyvitamin D3 (2MD) reduces potency but allows bone selectivity. Arch. Biochem. Biophys. 2007, 460, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Glebocka, A.; Sicinski, R.R.; Plum, L.A.; DeLuca, H.F. Synthesis and Biological Activity of 2-(3′-Hydroxypropylidene)-1α-hydroxy-19-norvitamin D Analogues with Shortened Alkyl Side Chains. J. Med. Chem. 2011, 54, 6832–6842. [Google Scholar] [CrossRef]
- Shimizu, M.; Miyamoto, Y.; Kobayashi, E.; Shimazaki, M.; Yamamoto, K.; Reischl, W.; Yamada, S. Synthesis and biological activities of new 1 α,25-dihydroxy-19-norvitamin D3 analogs with modifications in both the A-ring and the side chain. Bioorg. Med. Chem. 2006, 14, 4277–4294. [Google Scholar] [CrossRef]
- Shimazaki, M.; Miyamoto, Y.; Yamamoto, K.; Yamada, S.; Takami, M.; Shinki, T.; Udagawa, N.; Shimizu, M. Analogs of 1 α,25-dihydroxyvitamin D3 with high potency in induction of osteoclastogenesis and prevention of dendritic cell differentiation: Synthesis and biological evaluation of 2-substituted 19-norvitamin D analogs. Bioorg. Med. Chem. 2006, 14, 4645–4656. [Google Scholar] [CrossRef]
- Kobayashi, E.; Shimazaki, M.; Miyamoto, Y.; Masuno, H.; Yamamoto, K.; DeLuca, H.F.; Yamada, S.; Shimizu, M. Structure-activity relationships of 19-norvitamin D analogs having a fluoroethylidene group at the C-2 position. Bioorg. Med. Chem. 2007, 15, 1475–1482. [Google Scholar] [CrossRef]
- Hatcher, M.A.; Peleg, S.; Dolan, P.; Kensler, T.W.; Sarjeanta, A.; Posner, G.H. A-ring hydroxymethyl 19-nor analogs of the natural hormone 1 α,25-dihydroxyvitamin D3: Synthesis and preliminary biological evaluation. Bioorg. Med. Chem. 2005, 13, 3964–3976. [Google Scholar] [CrossRef]
- Shimizu, M.; Iwasaki, Y.; Shimazaki, M.; Amano, Y.; Yamamoto, K.; Reischl, W.; Yamada, S. New derivatives of 1 α,25-dihydroxy-19-norvitamin D3 with two substituents at C-2: Synthesis and biological activity. Bioorg. Med. Chem. Lett. 2005, 15, 1451–1455. [Google Scholar] [CrossRef]
- Igarashi, M.; Yoshimoto, N.; Yamamoto, K.; Shimizu, M.; Ishizawa, M.; Makishima, M.; DeLuca, H.F.; Yamada, S. Identification of a highly potent vitamin D receptor antagonist: (25S)-26-Adamantyl-25-hydroxy-2-methylene-22,23-didehydro-19,27-dinor-20-epi-vitamin D3 (ADMI3). Arch. Biochem. Biophys. 2007, 460, 240–253. [Google Scholar] [CrossRef]
- Nakabayashi, M.; Yamada, S.; Yoshimoto, N.; Tanaka, T.; Igarashi, M.; Ikura, T.; Ito, N.; Makishima, M.; Tokiwa, H.; DeLuca, H.F.; et al. Crystal Structures of Rat Vitamin D Receptor Bound to Adamantyl Vitamin D Analogs: Structural Basis for Vitamin D Receptor Antagonism and Partial Agonism. J. Med. Chem. 2008, 51, 5320–5329. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, N.; Inaba, Y.; Yamada, S.; Makishima, M.; Shimizu, M.; Yamamoto, K. 2-Methylene 19-nor-25-dehydro-1α-hydroxyvitamin D3 26,23-lactones: Synthesis, biological activities and molecular basis of passive antagonism. Bioorg. Med. Chem. 2008, 16, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Chiellini, G.; Grzywacz, P.; Plum, L.A.; Barycki, R.; Clagett-Dame, M.; DeLuca, H.F. Synthesis and biological properties of 2-methylene-19-nor-25-dehydro-1α-hydroxyvitamin D3-26,23-lactones—Weak agonists. Bioorg. Med. Chem. 2008, 16, 8563–8573. [Google Scholar] [CrossRef][Green Version]
- Sakamaki, Y.; Inaba, Y.; Yoshimoto, N.; Yamamoto, K. Potent Antagonist for the Vitamin D Receptor: Vitamin D Analogues with Simple Side Chain Structure. J. Med. Chem. 2010, 53, 5813–5826. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, N.; Sakamaki, Y.; Haeta, M.; Kato, A.; Inaba, Y.; Itoh, T.; Nakabayashi, M.; Ito, N.; Yamamoto, K. Butyl Pocket Formation in the Vitamin D Receptor Strongly Affects the Agonistic or Antagonistic Behavior of Ligands. J. Med. Chem. 2012, 55, 4373–4381. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.; Sicinski, R.R.; Grzywacz, P.; Thoden, J.B.; Plum, L.A.; Clagett-Dame, M.; DeLuca, H.F. A 20S Combined with a 22R Configuration Markedly Increases both in Vivo and in Vitro Biological Activity of 1α,25-Dihydroxy-22-methyl-2-methylene-19-norvitamin D3. J. Med. Chem. 2012, 55, 4352–4366. [Google Scholar] [CrossRef]
- Grzywacz, P.; Chiellini, G.; Plum, L.A.; Clagett-Dame, M.; DeLuca, H.F. Removal of the 26-Methyl Group from 19-nor-1 α,25-Dihydroxyvitamin D3 Markedly Reduces in Vivo Calcemic Activity without Altering in Vitro VDR Binding, HL-60 Cell Differentiation, and Transcription. J. Med. Chem. 2010, 53, 8642–8649. [Google Scholar] [CrossRef]
- Grzywacz, P.; Plum, L.A.; Clagett-Dame, M.; DeLuca, H.F. 26- and 27-Methyl groups of 2-substituted, 19-nor-1 α,25-dihydroxylated vitamin D compounds are essential for calcium mobilization in vivo. Bioorg. Chem. 2013, 47, 9–16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sibilska, I.; Barycka, K.M.; Sicinski, R.R.; Plum, L.A.; DeLuca, H.F. 1-Desoxy analog of 2MD: Synthesis and biological activity of (20S)-25-hydroxy-2-methylene-19-norvitamin D3. J. Steroid Biochem. Mol. Biol. 2010, 121, 51–55. [Google Scholar] [CrossRef]
- Chen, B.; Kawai, M.; Wu-Wong, J.R. Synthesis of VS-105: A novel and potent vitamin D receptor agonist with reduced hypercalcemic effects. Bioorg. Med. Chem. Lett. 2013, 23, 5949–5952. [Google Scholar] [CrossRef]
- Sokolowska, K.; Sicinski, R.R.; Mouriño, A.; Plum, L.A.; DeLuca, H.F. Synthesis and biological evaluation of novel 6-substituted analogs of 1 α,25-dihydroxy-19-norvitamin D3. J. Steroid Biochem. Mol. Biol. 2013, 136, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sabbe, K.; De Clercq, P.; Vandewalle, M.; Bouillon, R.; Verstuyf, A. Vitamin D3: Synthesis of seco C-9,11,21-trisnor-17-Methyl-1 α,25-dihydroxyvitamin D3 Analogues. Bioorg. Med. Chem. Lett. 2002, 12, 1629–1632. [Google Scholar] [CrossRef]
- Zhu, G.-D.; Chen, Y.; Zhou, X.; Vandewalle, M.; De Clercq, P.J. Synthesis of CD-ring modified l α,25-dihydroxy Vitamin D Analogues: C-ring Analogues. Bioorg. Med. Chem. Lett. 1996, 6, 1703–1708. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, G.-D.; Van Haver, D.; Vandewalle, M.; De Clercq, P.J.; Verstuyf, A.; Bouillon, R. Synthesis, Biological Activity, and Conformational Analysis of Four seco-D-15,19-bisnor-1 α,25-Dihydroxyvitamin D Analogues, Diastereomeric at C17 and C20. J. Med. Chem. 1999, 42, 3539–3556. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; De Clercq, P.; Vandewalle, M. Synthesis of New Vitamin D3 Analogues with a Decalin-type CD-Ring. Tetrahedron Lett. 1996, 37, 9361–9364. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Gao, L.-J.; Murad, I.; Verstuyf, A.; Verlinden, L.; Verboven, C.; Bouillon, R.; Viterbo, D.; Milanesio, M.; Van Haver, D.; et al. Synthesis, biological activity, and conformational analysis of CD-ring modified trans-decalin 1 α,25-dihydroxyvitamin D analogs. Org. Biomol. Chem. 2003, 1, 257–267. [Google Scholar] [CrossRef]
- Takaku, H.; Miyamoto, Y.; Asami, S.; Shimazaki, M.; Yamada, S.; Yamamoto, K.; Udagawa, N.; DeLuca, H.F.; Shimizu, M. Synthesis and structure-activity relationships of 16-ene-22-thia-1 α,25-dihydroxy-26, 27-dimethyl-19-norvitamin D3 analogs having side chains of different sizes. Bioorg. Med. Chem. 2008, 16, 1796–1815. [Google Scholar] [CrossRef]
- Sicinski, R.R.; Perlman, K.L.; Prahl, J.; Smith, C.; DeLuca, H.F. Synthesis and Biological Activity of 1 α,25-Dihydroxy-18-norvitamin D3 and 1 α,25-Dihydroxy-18,19-dinorvitamin D3. J. Med. Chem. 1996, 39, 4497–4506. [Google Scholar] [CrossRef]
- Zhou, S.-Z.; Anne, S.; Vandewalle, M. A Practical Synthesis of A-ring Precursors for 19-Nor-l α,25-dihydroxyvitamin D3 Analogues. Tetrahedron Lett. 1996, 37, 7637–7640. [Google Scholar] [CrossRef]
- Yong, W.; Vandewalle, M. A New A-ring Precursor for 19-Nor-l α,25-dihydroxyvitamin D3 Analogues. Synlett 1996, 9, 911–912. [Google Scholar] [CrossRef]
- Van Gool, M.; Zhao, X.; Sabbe, K.; Vandewalle, M. Synthesis of 14,20-Bis-epi-1α,25-dihydroxy-19-norvitamin D3 and Analogues. Eur. J. Org. Chem. 1999, 1999, 2241–2248. [Google Scholar] [CrossRef]
- Hilpert, H.; Wirz, B. Novel versatile approach to an enantiopure 19-nor, des-C,D vitamin D3 derivative. Tetrahedron 2001, 57, 681–694. [Google Scholar] [CrossRef]
- Hanazawa, T.; Wada, T.; Masuda, T.; Okamoto, S.; Sato, F. Novel Synthetic Approach to 19-nor-1 α,25-Dihydroxyvitamin D3 and Its Derivatives by Suzuki-Miyaura Coupling in Solution and on Solid Support. Org. Lett. 2001, 3, 3975–3977. [Google Scholar] [CrossRef] [PubMed]
- Perlman, K.L.; DeLuca, H.F. 1 α-Hydroxy-19-Nor-Vitamin D C-22 Aldehyde. A Valuable Intermediate in the Synthesis of Side Chain Modified 1 α,25-Dihydroxy-19-Nor-Vitamin D3. Tetrahedron Lett. 1992, 33, 2937–2940. [Google Scholar] [CrossRef]
- Mikami, K.; Osawa, A.; Isaka, A.; Sawa, E.; Shimizu, M.; Terada, M.; Kubodera, N.; Nakagawa, K.; Tsugawa, N.; Okano, T. “Symmetry” in the Synthesis of the A-Ring of a Vitamin D Hybrid Analogue with Significant Transactivation Activity: A Combinatorial Sequence of Regioselective Propiolate-Ene, Catalytic Enantioselective Epoxidation and Carbonyl-Ene Cyclization Reactions. Tetrahedron Lett. 1998, 39, 3359–3362. [Google Scholar] [CrossRef]
- Lee, N.E.; Williard, P.G.; Brown, A.J.; Campbell, M.J.; Koeffler, H.P.; Peleg, S.; Rao, D.S.; Reddy, G.S. Synthesis and biological activities of the two C(23) epimers of 1 α,23,25-trihydroxy-24-oxo-19-nor-vitamin D3: Novel analogs of 1 α,23(S),25-trihydroxy-24-oxo-vitamin D3, a natural metabolite of 1 α,25-dihydroxyvitamin D3. Steroids 2000, 65, 252–265. [Google Scholar] [CrossRef]
- Posner, G.H.; Kim, H.J.; Kahraman, M.; Jeon, H.B.; Suh, B.C.; Li, H.; Dolan, P.; Kensler, T.W. Highly antiproliferative, low-calcemic, side-chain ketone analogs of the hormone 1 α,25-dihydroxyvitamin D3. Bioorg. Med. Chem. 2005, 13, 5569–5580. [Google Scholar] [CrossRef]
- Maehr, H.; Uskokovic, M.R. Formal Desymmetrization of the Diastereotopic Chains in Gemini Calcitriol Derivatives with Two Different Side Chains at C-20. Eur. J. Org. Chem. 2004, 2004, 1703–1713. [Google Scholar] [CrossRef]
- Maehr, H.; Lee, H.J.; Perry, B.; Suh, N.; Uskokovic, M.R. Calcitriol Derivatives with Two Different Side Chains at C-20. V. Potent Inhibitors of Mammary Carcinogenesis and Inducers of Leukemia Differentiation. J. Med. Chem. 2009, 52, 5505–5519. [Google Scholar] [CrossRef]
- Pietraszek, A.; Malinska, M.; Chodynski, M.; Krupa, M.; Krajewski, K.; Cmoch, P.; Wozniak, K.; Kutner, A. Synthesis and crystallographic study of 1,25-dihydroxyergocalciferol analogs. Steroids 2013, 78, 1003–1014. [Google Scholar] [CrossRef]












© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, S.; Ferrero, M. Strategies for the Synthesis of 19-nor-Vitamin D Analogs. Pharmaceuticals 2020, 13, 159. https://doi.org/10.3390/ph13080159
Fernández S, Ferrero M. Strategies for the Synthesis of 19-nor-Vitamin D Analogs. Pharmaceuticals. 2020; 13(8):159. https://doi.org/10.3390/ph13080159
Chicago/Turabian StyleFernández, Susana, and Miguel Ferrero. 2020. "Strategies for the Synthesis of 19-nor-Vitamin D Analogs" Pharmaceuticals 13, no. 8: 159. https://doi.org/10.3390/ph13080159
APA StyleFernández, S., & Ferrero, M. (2020). Strategies for the Synthesis of 19-nor-Vitamin D Analogs. Pharmaceuticals, 13(8), 159. https://doi.org/10.3390/ph13080159






