Simulation of Physicochemical and Pharmacokinetic Properties of Vitamin D3 and Its Natural Derivatives
Abstract
1. Introduction
2. Results
2.1. Physicochemical Properties
2.2. Metabolism and Transport Characteristics
2.3. PK Parameter
2.4. Correlation of Physicochemical and Pharmacokinetic Parameters
3. Discussion
4. Materials and Methods
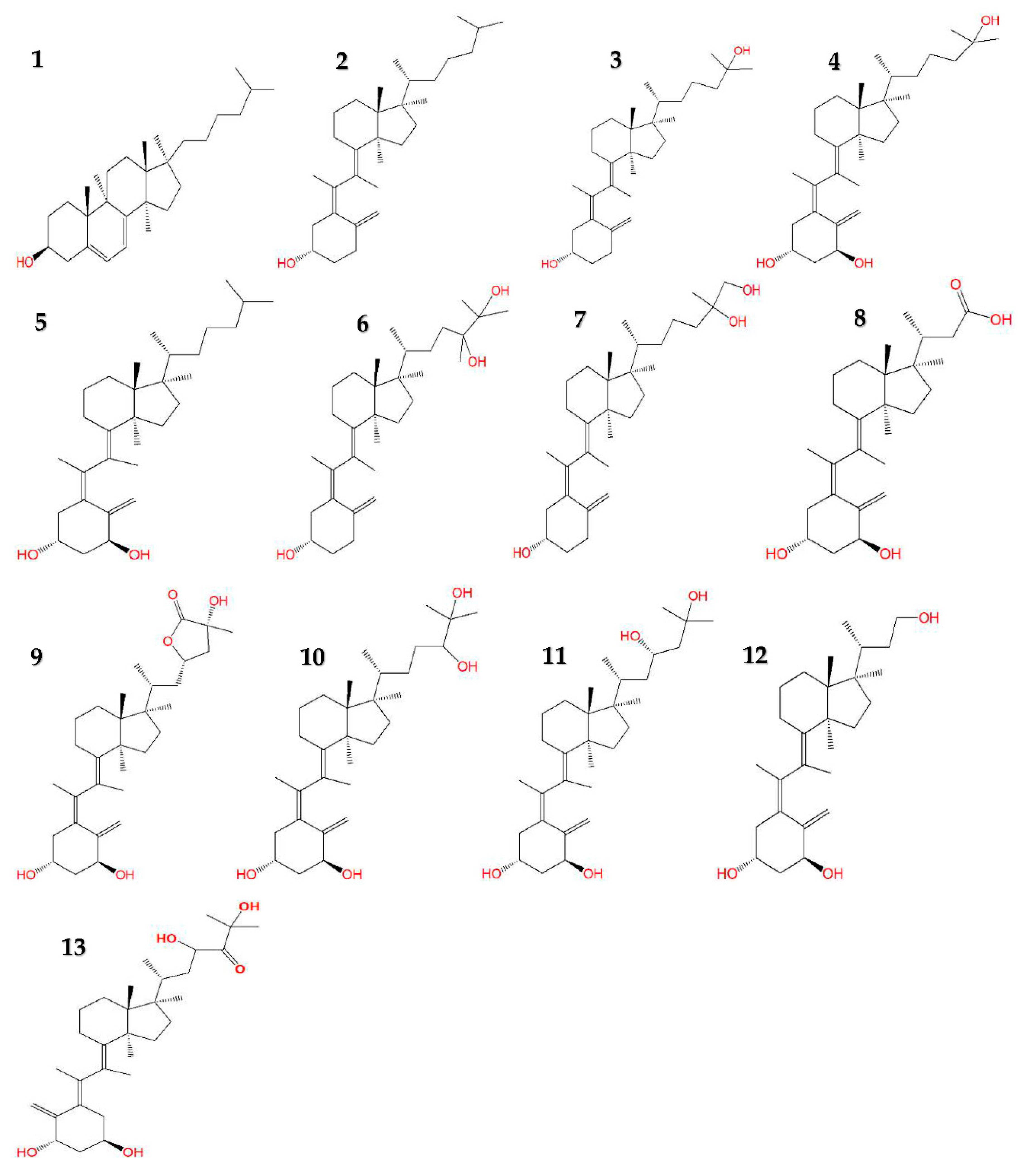
4.1. Vitamin D3 Derivative Structures
4.2. GastroPlusTM
4.3. Estimations of Physicochemical, Biopharmaceutical and Metabolism
4.4. Pharmacokinetic (PK) Analyses
4.5. Correlation Analyses by Microsoft Excel
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Jones, G. Extrarenal vitamin d activation and interactions between vitamin d2, vitamin d3, and vitamin d analogs. Annu. Rev. Nutr. 2013, 33, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Battault, S.; Whiting, S.J.; Peltier, S.L.; Sadrin, S.; Gerber, G.; Maixent, J.M. Vitamin D metabolism, functions and needs: From science to health claims. Eur. J. Nutr. 2013, 52, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Hewison, M.; Gardner, D.G.; Wagner, C.L.; Sergeev, I.N.; Rutten, E.; Pittas, A.G.; Boland, R.; Ferrucci, L.; Bikle, D.D. Vitamin D: Beyond bone. Ann. N. Y. Acad. Sci. 2013, 1287, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, S.; Mikhail, M.; Islam, S.; Aloia, J.F. Vitamin D and Abdominal Aortic Calcification in Older African American Women, the PODA Clinical Trial. Nutrients 2020, 12, 861. [Google Scholar] [CrossRef]
- Kojima, G.; Iliffe, S.; Tanabe, M. Vitamin D supplementation as a potential cause of U-shaped associations between vitamin D levels and negative health outcomes: A decision tree analysis for risk of frailty. BMC Geriatr. 2017, 17, 236. [Google Scholar] [CrossRef]
- Asif, A.; Farooq, N. Vitamin D Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Sigmon, J.R.; Yentzer, B.A.; Feldman, S.R. Calcitriol ointment: A review of a topical vitamin D analog for psoriasis. J. Dermatol. Treat. 2009, 20, 208–212. [Google Scholar] [CrossRef]
- Schuster, I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim. Biophys. Acta 2011, 1814, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Rosen, C.J.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; Kovacs, C.S.; et al. IOM committee members respond to Endocrine Society vitamin D guideline. J. Clin. Endocrinol. Metab. 2012, 97, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Ben-Eltriki, M.; Deb, S.; Guns, E.S. Calcitriol in Combination Therapy for Prostate Cancer: Pharmacokinetic and Pharmacodynamic Interactions. J. Cancer 2016, 7, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.E.; Reddy, G.S.; Brown, A.J.; Williard, P.G. Synthesis, stereochemistry, and biological activity of 1alpha,23,25-trihydroxy-24-oxovitamin D3, a major natural metabolite of 1alpha,25-dihydroxyvitamin D3. Biochemistry 1997, 36, 9429–9437. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, S.; Sumitani, K.; Hiura, K.; Kawata, T.; Okawa, M.; Hakeda, Y.; Kumegawa, M. Biological activity assessment of 1 alpha,25-dihydroxyvitamin D3-26,23-lactone and its intermediate metabolites in vivo and in vitro. Endocrinology 1990, 127, 695–701. [Google Scholar] [CrossRef]
- Dukas, L.; Schacht, E.; Mazor, Z.; Stahelin, H.B. Treatment with alfacalcidol in elderly people significantly decreases the high risk of falls associated with a low creatinine clearance of <65 mL/min. Osteoporos. Int. 2005, 16, 198–203. [Google Scholar]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D bioavailability: State of the art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205. [Google Scholar] [CrossRef]
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar]
- Toner, C.D.; Davis, C.D.; Milner, J.A. The vitamin D and cancer conundrum: Aiming at a moving target. J. Am. Diet. Assoc. 2010, 110, 1492–1500. [Google Scholar] [CrossRef]
- Hochberg, Z.; Hochberg, I. Evolutionary Perspective in Rickets and Vitamin D. Front. Endocrinol. 2019, 10, 306. [Google Scholar] [CrossRef]
- Gad, S.C. Preclinical Development Handbook: ADME and Biopharmaceutical Properties; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Shargel, L.; Yu, A.B.C. Applied Biopharmaceutics & Pharmacokinetics, 7th ed.; McGraw-Hill Education: New York, NY, USA, 2016. [Google Scholar]
- Drug Bank. Available online: www.drugbank.ca (accessed on 10 July 2020).
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 10 July 2020).
- SimulationsPlus. GastroPlus Simulation Software for Drug Discovery and Development Manual. 2019. Available online: https://www.simulations-plus.com/ (accessed on 10 July 2020).
- Fraczkiewicz, R.; Lobell, M.; Goller, A.H.; Krenz, U.; Schoenneis, R.; Clark, R.D.; Hillisch, A. Best of both worlds: Combining pharma data and state of the art modeling technology to improve in Silico pKa prediction. J. Chem. Inf. Model. 2015, 55, 389–397. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: Physiology, Molecualr Biology, and Clinical Applications; Humana Press: New York, NY, USA, 2010. [Google Scholar]
- LIPID MAPS® Lipidomics Gateway. Available online: www.lipidmaps.org (accessed on 10 July 2020).
- Human Metabolome Database. Available online: www.hmdb.ca (accessed on 10 July 2020).
- Zimmerman, D.R.; Reinhardt, T.A.; Kremer, R.; Beitz, D.C.; Reddy, G.S.; Horst, R.L. Calcitroic acid is a major catabolic metabolite in the metabolism of 1 alpha-dihydroxyvitamin D(2). Arch. Biochem. Biophys. 2001, 392, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Debs, S.; Pandey, M.; Adomat, H.; Guns, E.S. Cytochrome P450 3A-mediated microsomal biotransformation of 1alpha,25-dihydroxyvitamin D3 in mouse and human liver: Drug-related induction and inhibition of catabolism. Drug Metab. Dispos. 2012, 40, 907–918. [Google Scholar]
- Gupta, R.P.; Hollis, B.W.; Patel, S.B.; Patrick, K.S.; Bell, N.H. CYP3A4 is a human microsomal vitamin D 25-hydroxylase. J. Bone Miner. Res. 2004, 19, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Javle, M.; Lam, G.N.; Henner, W.D.; Wong, A.; Trump, D.L. Pharmacokinetics and tolerability of a single dose of DN-101, a new formulation of calcitriol, in patients with cancer. Clin. Cancer Res. 2005, 11, 7794–7799. [Google Scholar] [CrossRef]
- Jin, S.E.; Park, J.S.; Kim, C.K. Pharmacokinetics of oral calcitriol in healthy human based on the analysis with an enzyme immunoassay. Pharmacol. Res. 2009, 60, 57–60. [Google Scholar] [CrossRef]
- Muindi, J.R.; Johnson, C.S.; Trump, D.L.; Christy, R.; Engler, K.L.; Fakih, M.G. A phase I and pharmacokinetics study of intravenous calcitriol in combination with oral dexamethasone and gefitinib in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2009, 65, 33–40. [Google Scholar] [CrossRef]
- Fassio, A.; Adami, G.; Rossini, M.; Giollo, A.; Caimmi, C.; Bixio, R.; Viapiana, O.; Milleri, S.; Gatti, M.; Gatti, D. Pharmacokinetics of Oral Cholecalciferol in Healthy Subjects with Vitamin D Deficiency: A Randomized Open-Label Study. Nutrients 2020, 12, 1553. [Google Scholar] [CrossRef]
- Mentaverri, R.; Souberbielle, J.C.; Brami, G.; Daniel, C.; Fardellone, P. Pharmacokinetics of a New Pharmaceutical Form of Vitamin D3 100,000 IU in Soft Capsule. Nutrients 2019, 11, 703. [Google Scholar] [CrossRef]
- Ben-Eltriki, M.; Deb, S.; Adomat, H.; Tomlinson Guns, E.S. Calcitriol and 20(S)-protopanaxadiol synergistically inhibit growth and induce apoptosis in human prostate cancer cells. J. Steroid Biochem. Mol. Biol. 2016, 158, 207–219. [Google Scholar] [CrossRef]
- Thummel, K.E.; Wilkinson, G.R. In vitro and in vivo drug interactions involving human CYP3A. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 389–430. [Google Scholar] [CrossRef]
- Lutz, J.D.; Kirby, B.J.; Wang, L.; Song, Q.; Ling, J.; Massetto, B.; Worth, A.; Kearney, B.P.; Mathias, A. Cytochrome P450 3A Induction Predicts P-glycoprotein Induction; Part 2: Prediction of Decreased Substrate Exposure After Rifabutin or Carbamazepine. Clin. Pharmacol. Ther. 2018, 104, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.A.; Cooley, K.; Skidmore, B.; Fritz, H.; Campbell, T.; Seely, D. Vitamin d: Pharmacokinetics and safety when used in conjunction with the pharmaceutical drugs used in cancer patients: A systematic review. Cancers 2013, 5, 255–280. [Google Scholar] [CrossRef] [PubMed]
| Compound | log P | MW (g/mol) | Solubility (µg/mL) | Diff. Coeff (cm2/s × 10−5) | Peff (cm/s x 10-4) | pKa Microstates |
|---|---|---|---|---|---|---|
| Calcitriol (1,25-dihydroxyvitamin D3) | 5.50 | 416.65 | 0.65 | 0.56 | 3.45 | 13.09 |
| 24R,25-dihydroxyvitamin D3 | 5.17 | 416.65 | 0.65 | 0.56 | 4.08 | 13.04 |
| Calcifediol (25-hydroxyvitamin D3) | 6.67 | 400.65 | 0.11 | 0.56 | 6.41 | 12.98 |
| 25S,26-Dihydroxyvitamin D3 | 5.20 | 416.65 | 0.62 | 0.56 | 4.25 | 13.09 |
| Calcitroic acid (1-hydroxy-23-carboxytetranorvitamin D3) | 3.22 | 374.52 | 110.00 | 0.62 | 3.61 | 4.96 |
| Vitamin D3 (Cholecalciferol) | 8.80 | 384.65 | 0.02 | 0.57 | 7.93 | 13.26 |
| Provitamin D3 (7-dehydrocholesterol) | 9.02 | 384.65 | 0.06 | 0.58 | 8.14 | 13.34 |
| Alfacalcidol (1-hydroxyvitamin D3) | 7.20 | 400.65 | 0.08 | 0.56 | 4.21 | 13.32 |
| (23S,25R)-1,25-dihydroxyvitamin D3-26,23-lactone | 3.36 | 444.62 | 24.30 | 0.57 | 2.56 | 12.91 |
| Calcitetrol (1,24R,25-trihydroxyvitamin D3) | 4.00 | 432.65 | 4.61 | 0.56 | 2.47 | 13.10 |
| 1,23S,25-trihydroxyvitamin D3 | 3.98 | 432.65 | 4.77 | 0.56 | 2.37 | 13.27 |
| Tetranorcholecalciferol (1,23-dihydroxy-24,25,26,27-tetranorvitamin D3) | 3.71 | 360.54 | 6.90 | 0.62 | 3.65 | 13.27 |
| 1,23S,25-trihydroxy-24-oxo-vitamin D3 | 3.00 | 446.63 | 62.80 | 0.56 | 1.82 | 12.88 |
| Vitamin D3 Derivatives | BBB Penetration | Predicated CYP fm |
|---|---|---|
| Calcitriol (1,25-dihydroxyvitamin D3) | High | 3A4 = 100% |
| 24R,25-dihydroxyvitamin D3 | High | 3A4 = 100% |
| Calcifediol (25-hydroxyvitamin D3) | High | 3A4 = 100% |
| 25S,26-Dihydroxyvitamin D3 | High | N/A |
| Calcitroic acid (1-hydroxy-23-carboxytetranorvitamin D3) | High | 2C9 = 100% |
| Vitamin D3 (Cholecalciferol) | High | 2C19 = 24.76%; 3A4 = 75.24% |
| Provitamin D3 (7-dehydrocholesterol) | High | 2C9 = 16.09%; 2C19 = 17.71%; 3A4 = 66.21% |
| Alfacalcidol (1-hydroxyvitamin D3) | High | 3A4 = 100% |
| (23S,25R)-1,25-dihydroxyvitamin D3-26,23-lactone | High | 3A4 = 100% |
| Calcitetrol (1,24R,25-trihydroxyvitamin D3) | Low | 3A4 = 100% |
| 1,23S,25-trihydroxyvitamin D3 | Low | 3A4 = 100% |
| Tetranorcholecalciferol (1,23-dihydroxy-24,25,26,27-tetranorvitamin D3) | High | N/A |
| 1,23S,25-trihydroxy-24-oxo-vitamin D3 | Low | 3A4 = 100% |
| Compound | Fa% | F% | Cmax (ng/mL) | CmaxLiver (ng/mL) | Tmax (h) | AUC0-∞ (ng-h/mL) | AUC0-24 (ng-h/mL) | T1/2 (h) | CL (L/h) |
|---|---|---|---|---|---|---|---|---|---|
| Calcitriol (1,25-dihydroxyvitamin D3) | 8.62 | 5.44 | 9.86 | 13.97 | 5.20 | 402.48 | 176.25 | 2.43 | 27.58 |
| 24R,25-dihydroxyvitamin D3 | 8.57 | 6.76 | 16.86 | 20.47 | 9.76 | 924.70 | 340.49 | 4.30 | 15.90 |
| Calcifediol (25-hydroxyvitamin D3) | 2.24 | 1.78 | 5.83 | 7.61 | 4.80 | 187.72 | 96.43 | 4.98 | 15.20 |
| 25S,26-Dihydroxyvitamin D3 | 8.31 | 8.31 | 83.16 | 85.98 | 24.00 | 1179.60 | 1179.60 | N/A | N/A |
| Calcitroic acid (1-hydroxy-23-carboxytetranorvitamin D3) | 99.95 | 94.76 | 3040.00 | 3480.90 | 1.92 | 36318.00 | 32571.00 | 6.88 | 2.61 |
| Vitamin D3 (Cholecalciferol) | 0.24 | 0.20 | 0.58 | 0.67 | 15.28 | 56.42 | 11.83 | 7.57 | 11.15 |
| Provitamin D3 (7-dehydrocholesterol) | 1.64 | 1.42 | 0.59 | 7.40 | 5.12 | 196.05 | 102.34 | 7.98 | 10.21 |
| Alfacalcidol (1-hydroxyvitamin D3) | 2.03 | 1.61 | 6.00 | 7.91 | 4.64 | 152.43 | 89.59 | 4.85 | 15.21 |
| (23S,25R)-1,25-dihydroxyvitamin D3-26,23-lactone | 90.15 | 43.39 | 105.35 | 165.31 | 4.32 | 1147.60 | 1146.60 | 1.45 | 37.81 |
| Calcitetrol (1,24R,25-trihydroxyvitamin D3) | 37.95 | 22.58 | 37.26 | 53.49 | 5.36 | 1312.10 | 673.27 | 2.00 | 30.62 |
| 1,23S,25-trihydroxyvitamin D3 | 38.45 | 13.26 | 15.92 | 27.24 | 4.16 | 475.23 | 253.95 | 1.21 | 49.54 |
| Tetranorcholecalciferol (1,23-dihydroxy-24,25,26,27-tetranorvitamin D3) | 58.99 | 58.99 | 667.36 | 683.08 | 24.00 | 9466.20 | 9466.20 | N/A | N/A |
| 1,23S,25-trihydroxy-24-oxo-vitamin D3 | 99.30 | 52.44 | 248.32 | 395.74 | 3.60 | 1563.00 | 1562.50 | 1.72 | 33.55 |
| Physicochemical Property | Pharmacokinetics Parameter | R2 | Interpretation |
|---|---|---|---|
| Log P | Fa% | 0.66 | Moderate positive correlation |
| Log P | F% | 0.53 | Moderate positive correlation |
| Log P | Cmax | 0.16 | Weak positive correlation |
| Log P | Tmax | 0.01 | No correlation |
| Log P | AUC0–24 | 0.16 | Weak positive correlation |
| Log P | T1/2 | 0.52 | Moderate positive correlation |
| Log P | CL (L/h) | 0.30 | Weak positive correlation |
| Log P | CmaxLiver (µg/mL) | 0.17 | Weak positive correlation |
| Solubility (µg/mL) | Fa% | 0.65 | Moderate positive correlation |
| Solubility (µg/mL) | F% | 0.75 | Fairly strong positive correlation |
| Solubility (µg/mL) | Cmax | 0.75 | Fairly strong positive correlation |
| Solubility (µg/mL) | Tmax | 0.13 | Weak positive correlation |
| Solubility (µg/mL) | AUC0–24 | 0.69 | Moderate positive correlation |
| Solubility (µg/mL) | T1/2 | 0.01 | No correlation |
| Solubility (µg/mL) | CL (L/h) | 0.04 | No correlation |
| Solubility (µg/mL) | CmaxLiver (µg/mL) | 0.78 | Fairly strong positive correlation |
| Peff | Fa% | 0.41 | Weak positive correlation |
| Peff | F% | 0.23 | Weak positive correlation |
| Peff | Cmax | 0.02 | No correlation |
| Peff | Tmax | 0.03 | No correlation |
| Peff | AUC0–24 | 0.02 | No correlation |
| Peff | T1/2 | 0.74 | Fairly strong positive correlation |
| Peff | CL (L/h) | 0.46 | Weak positive correlation |
| Peff | CmaxLiver (µg/mL) | 0.03 | No correlation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deb, S.; Reeves, A.A.; Lafortune, S. Simulation of Physicochemical and Pharmacokinetic Properties of Vitamin D3 and Its Natural Derivatives. Pharmaceuticals 2020, 13, 160. https://doi.org/10.3390/ph13080160
Deb S, Reeves AA, Lafortune S. Simulation of Physicochemical and Pharmacokinetic Properties of Vitamin D3 and Its Natural Derivatives. Pharmaceuticals. 2020; 13(8):160. https://doi.org/10.3390/ph13080160
Chicago/Turabian StyleDeb, Subrata, Anthony Allen Reeves, and Suki Lafortune. 2020. "Simulation of Physicochemical and Pharmacokinetic Properties of Vitamin D3 and Its Natural Derivatives" Pharmaceuticals 13, no. 8: 160. https://doi.org/10.3390/ph13080160
APA StyleDeb, S., Reeves, A. A., & Lafortune, S. (2020). Simulation of Physicochemical and Pharmacokinetic Properties of Vitamin D3 and Its Natural Derivatives. Pharmaceuticals, 13(8), 160. https://doi.org/10.3390/ph13080160






