Abstract
The relevance of oxidative stress in the pathogenesis of several diseases (including inflammatory disorders) has traditionally led to the search for new sources of antioxidant compounds. In this work, we report the selection of fractions with high antioxidant action from B. tetraphylla (BT) leaf extracts. In vitro methods (DPPH and ABTS assays; determination of phenolic and flavonoid contents) were used to select products derived from B. tetraphylla with high antioxidant action. Then, the samples with the highest potentials were evaluated in a model of injury based on the inoculation of a lethal dose of heat-inactivated Escherichia coli in Tenebrio molitor larvae. Due to its higher antioxidant properties, the methanolic extract (BTME) was chosen to be fractionated using Sephadex LH-20 column-based chromatography. Two fractions from BTME (BTFC and BTFD) were the most active fractions. Pre-treatment with these fractions protected larvae of T. molitor from the stress induced by inoculation of heat-inactivated E. coli. Similarly, BTFC and BTFD increased the lifespan of larvae infected with a lethal dose of enteroaggregative E. coli 042. NMR data indicated the presence of aliphatic compounds (terpenes, fatty acids, carbohydrates) and aromatic compounds (phenolic compounds). These findings suggested that products derived from B. tetraphylla leaves are promising candidates for the development of antioxidant and anti-infective agents able to treat oxidative-related dysfunctions.
1. Introduction
A substantial amount of evidence has indicated the key role of free radicals and reactive oxygen species (ROS) in the etiology of degenerative pathologies associated with aging (Parkinson’s and Alzheimer’s diseases), cancer, cardiovascular diseases, and diabetes [1,2,3,4,5,6,7]. Free radicals are highly reactive molecules characterized by having unpaired electrons in the last valence layer, thus becoming potent oxidizing agents [8,9]. These entities are produced as a result of normal cellular metabolism and play an important role in cell function and signaling; however, they can also damage important macromolecules (DNA, proteins, and lipids), thereby impairing cellular functions and leading to cell death [2,3,8].
Due to the reactivity of free radicals, organisms have developed an efficient antioxidant defense system formed by enzymes (such as superoxide dismutase and catalase) and proteins (glutathione reductase, thioredoxin) [8,9]. However, in many situations, this system cannot cope with the overproduction of reactive species, generating a so-called oxidative stress state, which is related to the clinical manifestations described above [1]. An alternative way of combatting the damage caused by free radicals is the use of exogenous substances collectively called antioxidants [4,8,10].
The antioxidants (natural or synthetic) can act through different mechanisms in the organism, such as: (i) direct neutralization of free radicals; (ii) expression of molecules from the host antioxidant defense systems; (iii) inhibition of oxidant enzymes (leading to reduction of free radicals’ generation/propagation) [10,11,12,13]. These compounds can also be used in the food industry to maintain the physical-chemical quality of fruits, meat, and other foods [14,15,16]. Due to the side effects of synthetic antioxidants, those from natural sources are preferred; this context leads to a constant search for plant-derived compounds with this property [4,10,11,12,14,15,16,17,18].
In addition, antioxidant compounds have been related to the therapeutic properties of the species considered medicinal. In fact, substances with antioxidant actions have been detected in different products derived from plants (juices, teas, extracts, infusions) used in the treatment and prevention of diseases [10,11,12,14,17]. However, the antioxidant potential of some medicinal plants is still unexploited; and the neotropical species called Buchenavia tetraphylla (Aubl.) RA Howard (synonymy Buchenavia capitata; Combretoideae family) is a good example. This plant is distributed from Cuba (Central America) to southeastern Brazil (South America). B. tetraphylla is popularly known as “tanimbuca” in Brazil, where it has ethnomedicinal importance for communities in the northeast region [19]. It is also known to have a broad spectrum of antimicrobial activity, inhibiting bacteria, fungi, and virus [20,21,22]. Buchenavianine and two derivatives (O-demethylbuchenavianine, N,O-bis-(demethyl)buchenavianine) have been isolated from B. tetraphylla. These compounds are classified as flavoalkaloids with a piperidine moiety at carbon 8 [20,23].
The antioxidant potential of plant products has been traditionally characterized by in vitro methods; however, the biological relevance of these tests has been contested by several works [10,24,25]. In general, in vitro methods have been limited to sample prospection and compound isolation. Thus, the need to employ cell-based methods and in vivo models for a better understanding of the pharmacological action of a candidate as an antioxidant agent is evident [10]. In this work, in vitro methods were used to select products derived from B. tetraphylla with antioxidant action. Then, the samples with the highest potentials were evaluated in alternative models of stress based on Tenebrio molitor larvae inoculated with Escherichia coli.
2. Results
2.1. Comparison of the Antioxidant Activity of Extracts Obtained from B. tetraphylla Leaves
Initially, the phenolic and flavonoid contents were compared in different extracts of B. tetraphylla (BTHE: hexane extract; BTCE: chloroform extract; BTEE: ethyl acetate extract; BTME: methanolic extract) (Table 1). Among the extracts, BTME presented higher concentrations of both classes of compounds with values of 123.03 ± 1.51 mg of gallic acid equivalent (GAE) per mg of dry extract (mg GAE/mg) and 108.90 ± 0.07 mg of quercetin equivalent (QE) per mg of dry extract (mg QE/mg) (p < 0.05). A Pearson coefficient of 0.71 was found between the phenolic and flavonoid contents, indicating a strong correlation.

Table 1.
Comparative analysis of total phenolic compounds, flavonoid content, and DPPH radical scavenging of the crude extracts from the leaves of Buchenavia tetraphylla.
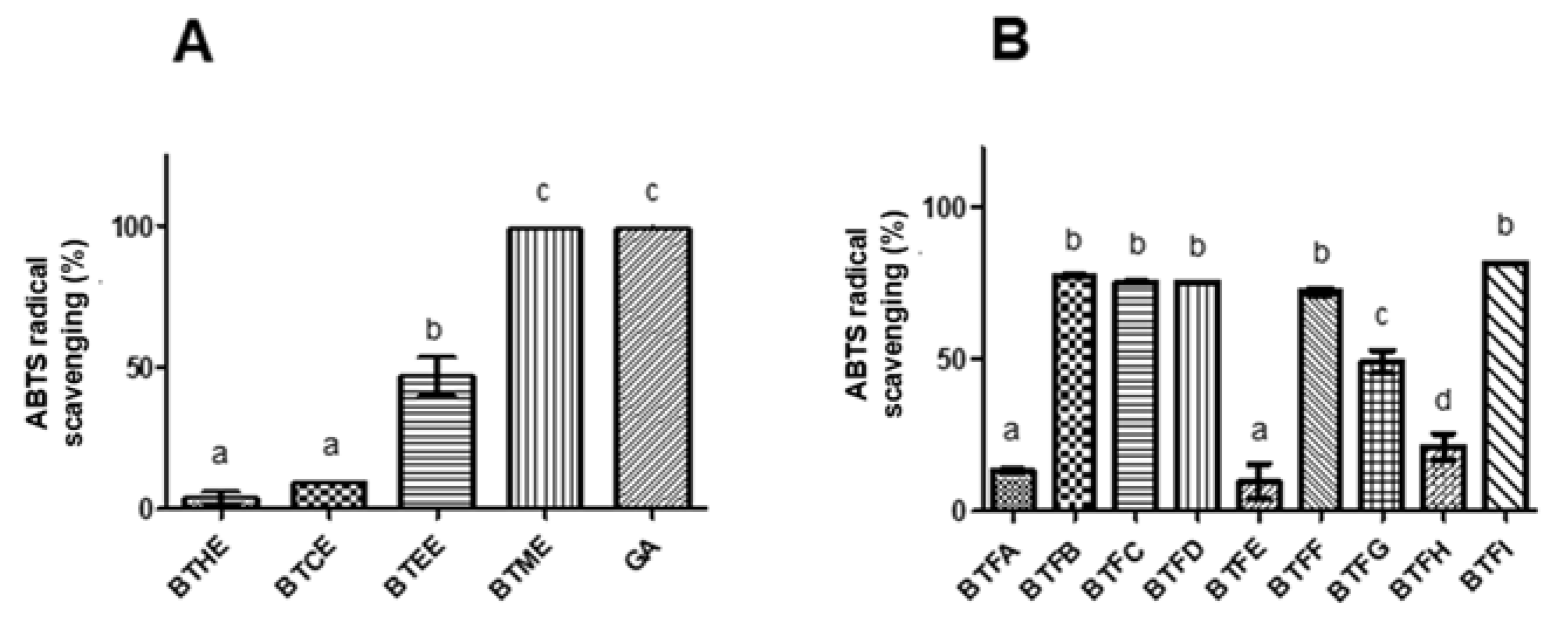
The antioxidant potential of the extracts and fractions was evaluated using the DPPH (2,2-diphenyl-1-picrylhydrazyl radical) and ABTS ((2,2-azino-bis (3-ethylbenzo-thiazoline-6-sulfonic acid) radical) methods. In the DPPH assay, the highest in vitro antioxidant activity was observed for BTME with an EC50 (half maximal effective concentration) of 79.04 μg/mL (Table 1) and higher scavenging action in almost all tested concentrations in relation to other extracts (p < 0.001). The EC50 found for Trolox was 44.10 μg/mL (positive control). Similarly, in the ABTS assay, a greater scavenging action was observed for BTME (approximately 100%; p < 0.05), followed by BTEE (approximately 50%) (Figure 1A). Strong correlations were also found between the levels of phenolic compounds and the scavenger actions towards DPPH (0.96) and ABTS radicals (0.89). The flavonoid contents were moderately correlated with DPPH scavenging (0.68) and strongly correlated with ABTS scavenging (0.95). The results for both antioxidant assays were strongly related (0.85).
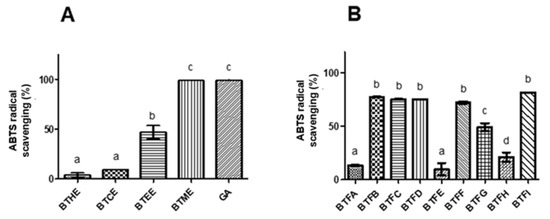
Figure 1.
Comparative evaluation of antioxidant activity by the ABTS assay of the crude extracts (A) and fractions (B) of Buchenavia tetraphylla leaves. BTHE: hexane extract; BTCE: chloroform extract; BTEE: ethyl acetate extract; BTME: methanolic extract; BTFA: Fraction A; BTFB: Fraction B; BTFC: Fraction C; BTFD: Fraction D; BTFE: Fraction E; BTFF: Fraction F; BTFG: Fraction G; BTFH: Fraction H; BTFI: Fraction I. In each graph, values with significant differences (p < 0.05) are indicated by different superscript letters (a, b, c). The results are expressed as the mean ± standard deviation calculated from three independent assays performed in triplicate (n = 3).
2.2. Comparison of Phenolic and Flavonoid Content and Antioxidant Activity of Fractions Obtained from Methanolic Extract of B. tetraphylla Leaves
Since a higher level of antioxidant activity was observed in BTME, it was submitted to fractionation using Sephadex LH-20 column chromatography. A total of 9 non-repetitive fractions were obtained (BTFA to BTFI). Among them, the highest phenolic content was detected in BTFD (168.99 ± 2.22 μg GAE/μg), followed by BTFC (156.02 ± 4.51 μg GAE/μg), BTFG (127.62 ± 19.11 μg GAE/μg), and BTFI (110.15 ± 0.78 μg GAE/μg) (Table 2). Almost the same pattern was observed for the flavonoid content; in this case, BTFC had the highest values (68.26 ± 2.87 μg QE/μg) (p < 0.0001), followed by BTFD (56.01 ± 5.54 μg QE/μg), BTFG (45.27 ± 4.13 μg QE/μg), and BTFI (39.29 ± 2.89 μg QE/μg) (Table 2). A strong correlation (Pearson coefficient of 0.88) was observed among the concentration of phenolic and flavonoid compounds in the fractions obtained from BTME. Following, the antioxidant action of each fraction was investigated, and BTFC showed the highest activity against the DPPH radical (EC50: 50.41 μg/mL), followed by BTFD (EC50: 237.7641 μg/mL), BTFG (EC50: 294.38 μg/mL), and BTFI (EC50: 376.25 μg/mL). On the other hand, the fractions BTFB, BTFC, BTFD, BTFF, and BTFI scavenged approximately 80% of the ABTS radical (p < 0.05), and no statistical differences were observed between them (Figure 1B).

Table 2.
Comparative analysis of total phenolic compounds and flavonoid content and DPPH radical scavenging of the crude extracts from leaves of Buchenavia tetraphylla.
2.3. Evaluation of the Hemolytic Effects of Extracts and Fractions from Buchenavia tetraphylla Leaves
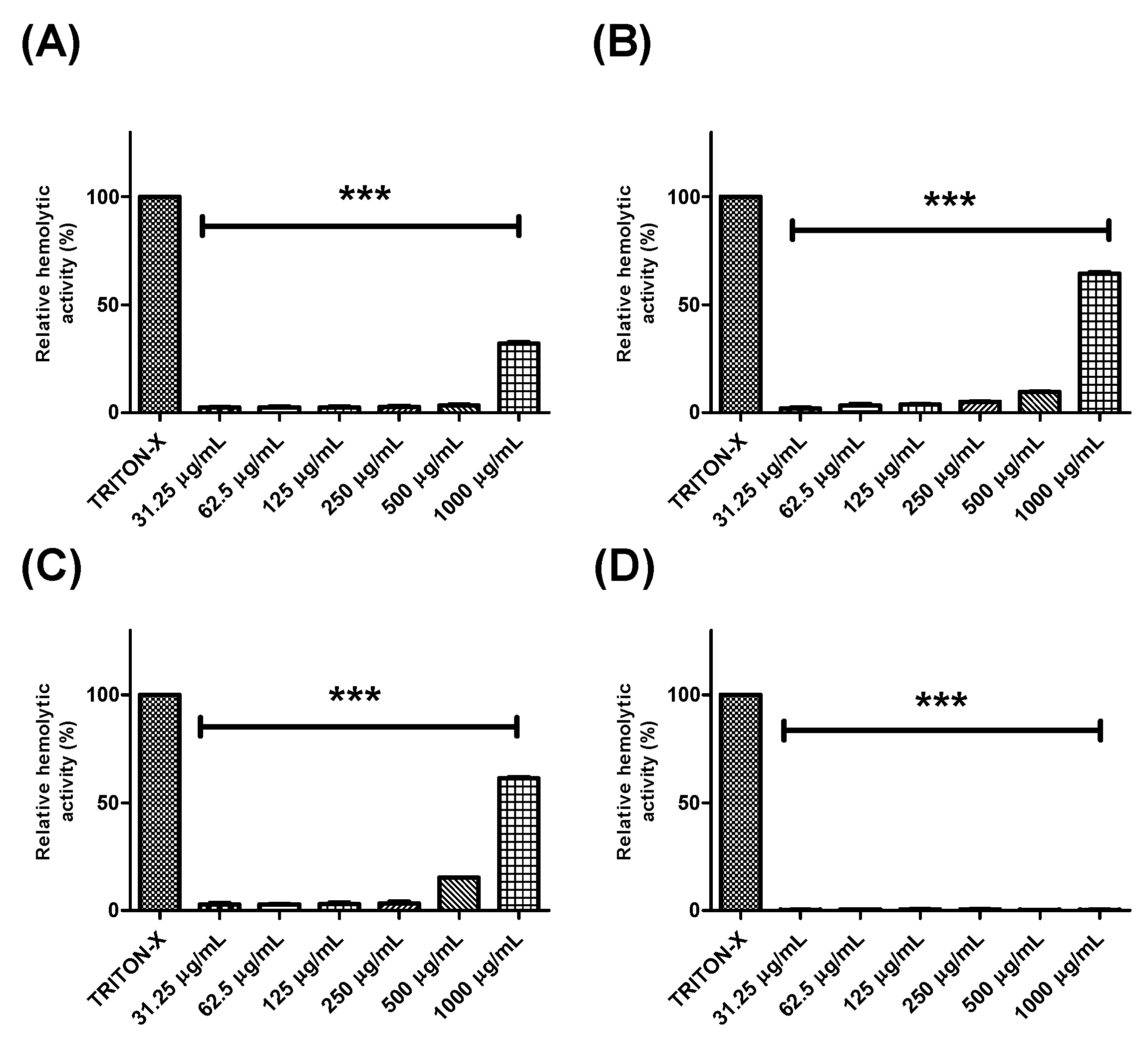
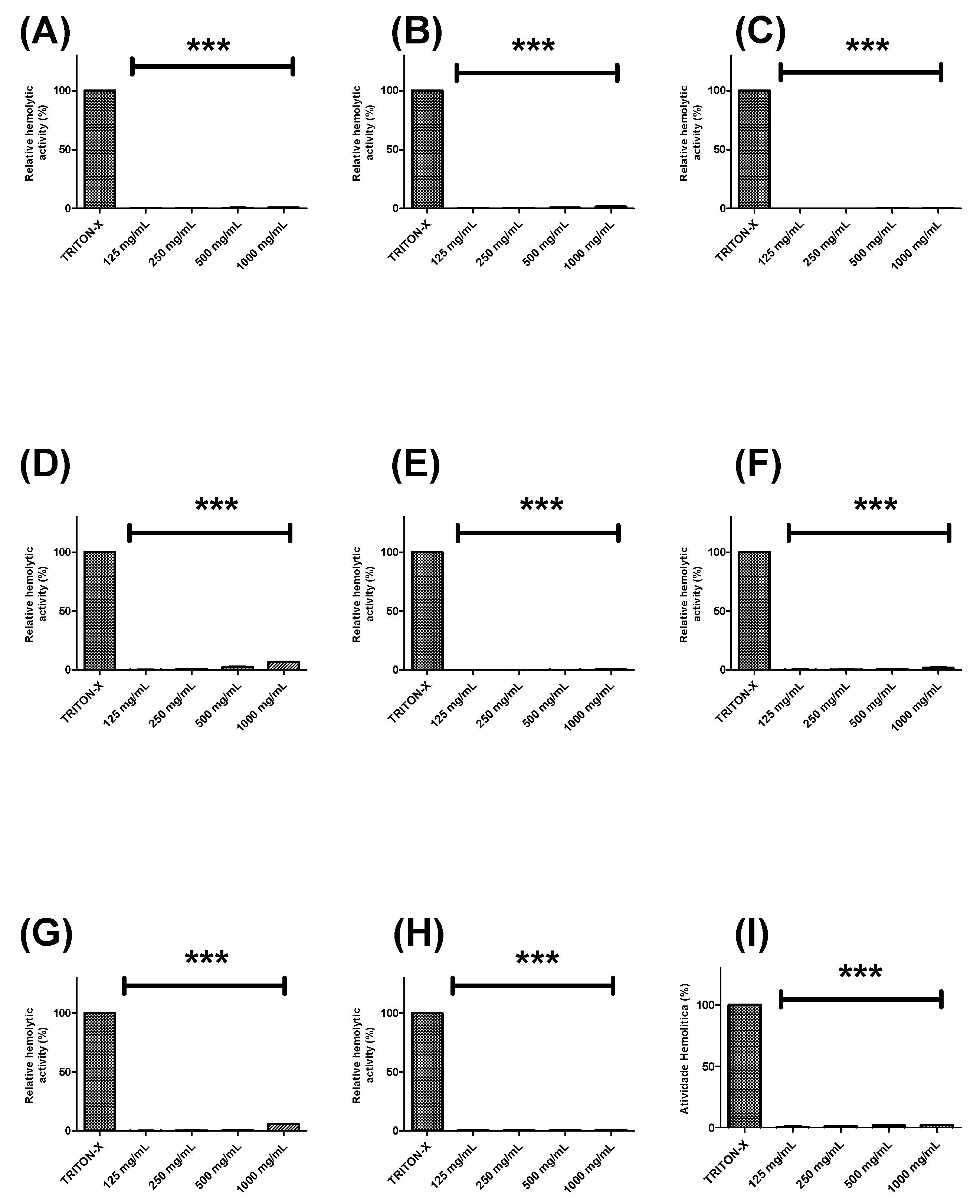
Further, the hemolytic potential of each extract or fraction was evaluated using human erythrocytes (Figure 2 and Figure 3). BTHE, BTCE, and BTEE induced toxic effects when tested at the highest concentrations (500 μg/mL and 1000 μg/mL) (Figure 2A–C). In contrast, it was observed that the BTME and its fractions did not induce significant hemolytic activity, even at the highest tested concentrations (Figure 2D and Figure 3).
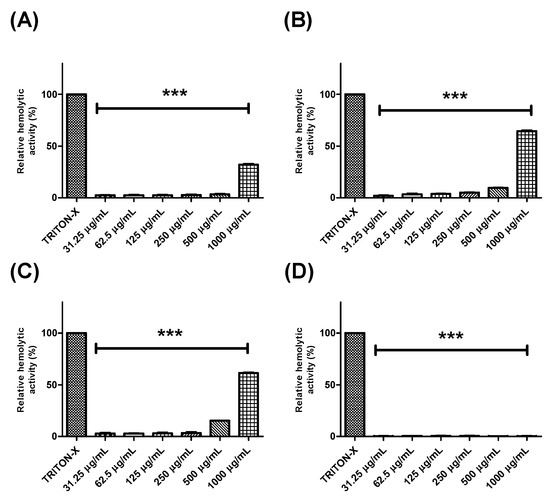
Figure 2.
Hemolytic activity of the crude extracts of Buchenavia tetraphylla leaves. (A) BTHE (hexane extract); (B) BTCE (chloroform extract); (C) BTEE (ethyl acetate extract); (D) BTME (methanolic extract). *** Significant differences in relation to triton-X (p < 0.0001). The results are expressed as the mean ± standard deviation calculated from three independent assays performed in quadruplicate (n = 4).
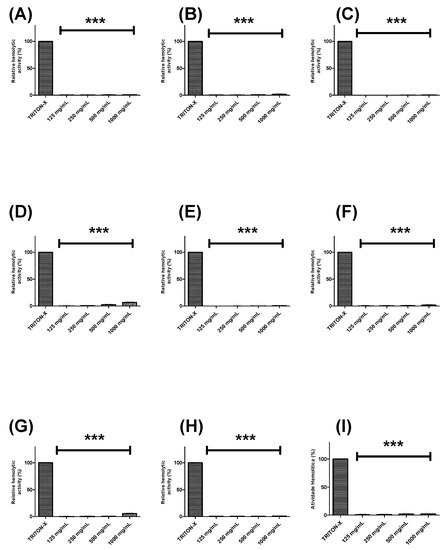
Figure 3.
Hemolytic activity of the fractions obtained from the methanolic extract of Buchenavia tetraphylla leaves. (A) BTFA (Fraction A); (B) BTFB (Fraction B); (C) BTFC (Fraction C); (D) BTFD (Fraction D); (E) BTFE (Fraction E); (F) BTFF (Fraction F); (G) BTFG (Fraction G); (H) BTFH (Fraction H); (I) BTFI (Fraction I). *** Significant differences in relation to triton-X (p < 0.0001). The results are expressed as the mean ± standard deviation calculated from three independent assays performed in quadruplicate (n = 4).
2.4. Effects of Extracts and Fractions from Buchenavia tetraphylla Leaves on the Survival of Tenebrio molitor larvae Submitted to Stress Induced by Heat-Killed Escherichia coli
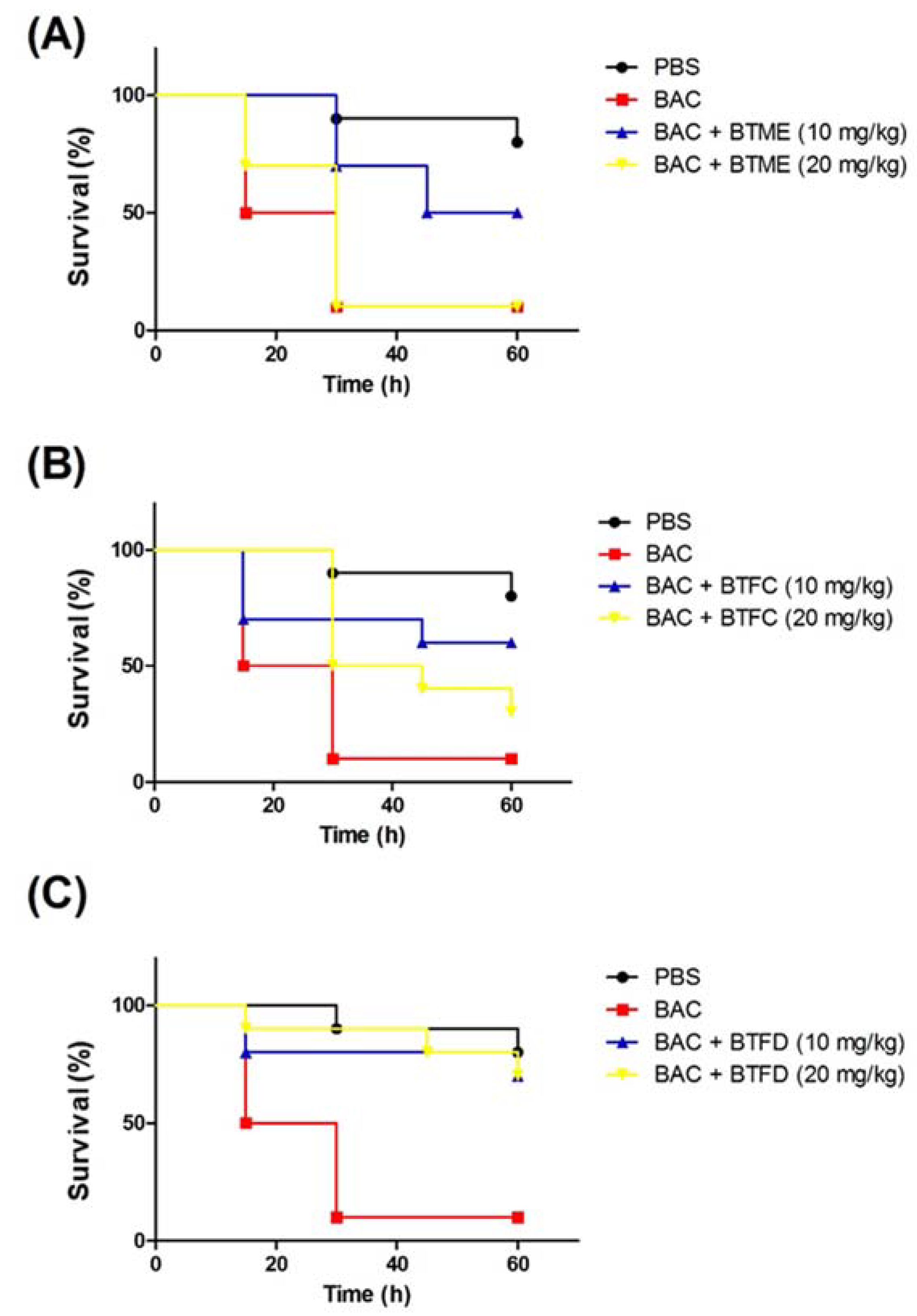
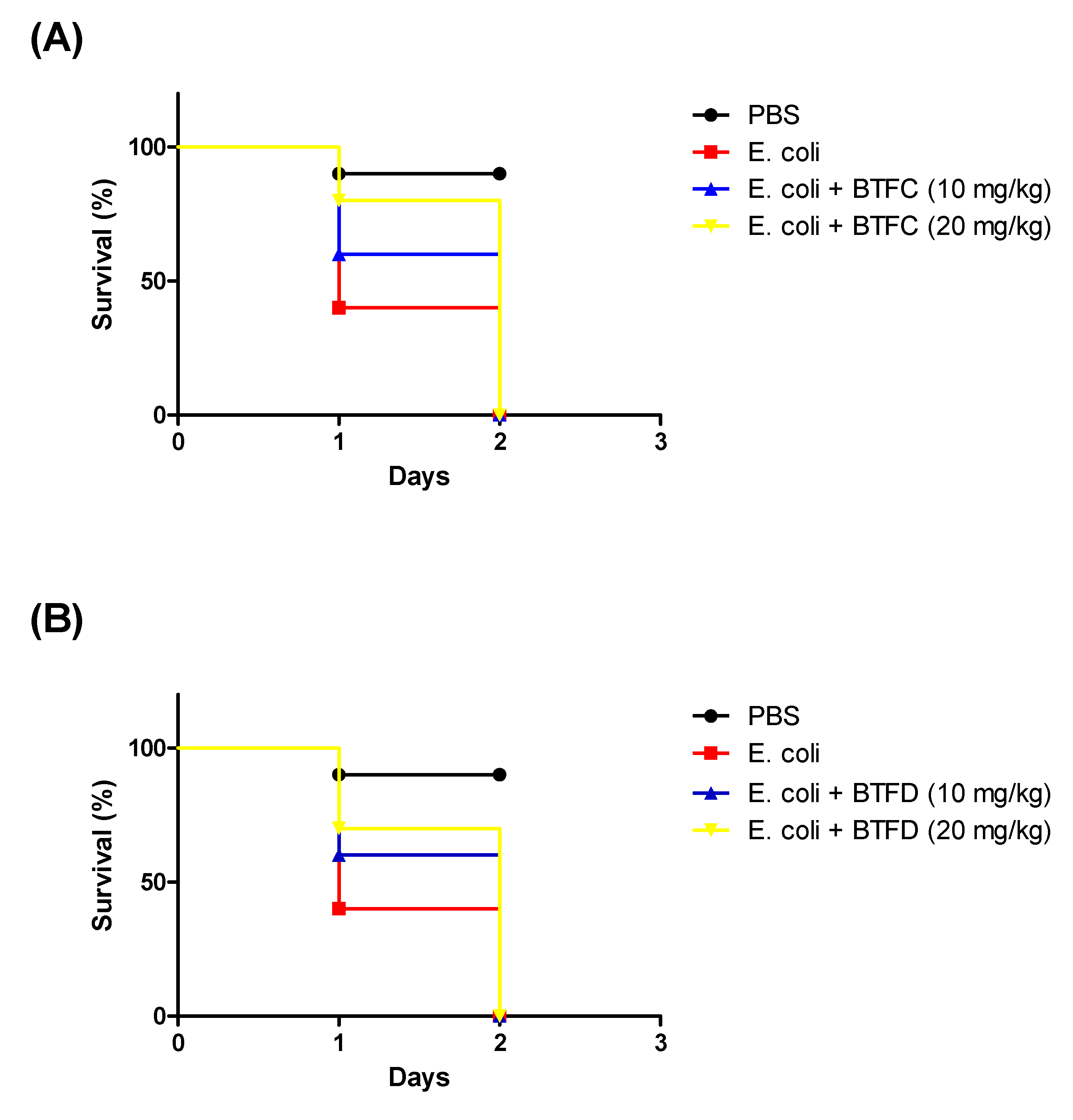
Based on the results presented in the above activities, we decided to evaluate the effects of the methanolic extract and its most active fractions (BTFC and BTFD) in a model of stress induced by heat-killed E. coli OP50 in T. molitor larvae. The heat treatment was used to ensure that larval death was not induced by bacterial growth; it was caused by the components present in the bacteria, such as lipopolysaccharide (LPS). Additionally, we used the nonpathogenic E. coli OP50 strain [26]. In this sense, at this point, we did not evaluate the antimicrobial action of the extract/fractions, but the ability of these to inhibit stress pathways induced by the presence of the bacteria. First, we evaluated the effects of several concentrations (measured at OD600 (optical density at 600 nm)) of heat-killed E. coli OP50 (data not shown). The best results were obtained with the suspension at an OD600 of 0.7; this dose induced the death of 50% of the larvae after 15 h and of 90% after 30 h. These larvae presented typical myelination points related to stress induction in this organism. The pre-treatment with BTME at 10 mg/kg was able to inhibit animal death, with survival rates of 100%, 70%, and 50% after 15 h, 30 h, and 60 h of infection, respectively. The concentration of 20 mg/kg showed no significant protective action (Figure 4A).
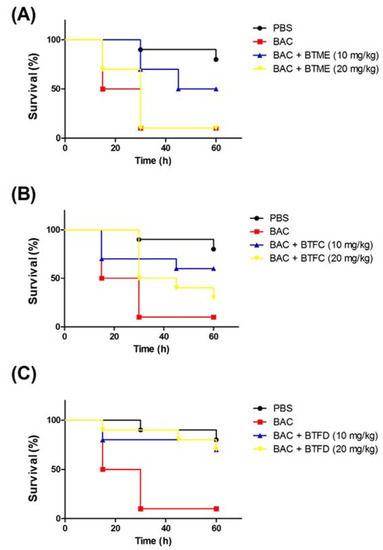
Figure 4.
Effects of methanolic extract of Buchenavia tetraphylla leaves (BTME) and its fractions (BTFC and BTFD) on the survival of Tenebrio molitor larvae challenged with heat-killed Escherichia coli OP50. (A) Effects of BTME on the survival of T. molitor larvae challenged with heat-killed E. coli OP50; (B) effects of BTFC on the survival of T. molitor larvae challenged with heat-killed E. coli OP50; (C) effects of BTFD on the survival of T. molitor larvae challenged with heat-killed E. coli OP50. The larvae (n = 10/group) were pre-treated with each sample (at 10 mg/kg or 20 mg/kg) 12 h prior to inoculation of heat-killed bacteria. Larvae treated with phosphate-saline buffer (PBS) or E. coli OP50 (BAC) were used as negative or positive controls, respectively. In this set of assays, larvae survival was recorded each 12 h. The experiment was repeated three times.
The results obtained with BTFD were even more significant. The group treated with 20 mg/kg exhibited viability rates of 90% and 80% after 45 h and 60 h, respectively. Similar results were obtained with BTFD at 10 mg/kg, where viability remained at 80% after 45 h and 70% after 60 h (Figure 4C). Regarding BTFC, the best protective action was observed for the dose of 10 mg/kg. In this case, survival rates remained at 70% up to 45 h and ended at 60% (60 h). In the case of the 20 mg/kg dose, after 15 h, 80% of the larvae remained viable, and this rate progressively decreased to 50% (30 h), 40% (45 h), and reached 30% after 60 h (Figure 4B).
2.5. Effects of Fractions from Buchenavia tetraphylla Leaves in the Lifespan of Tenebrio molitor larvae Infected by Enteroaggregative Escherichia coli
We also evaluated the efficacy of BTFC and BTFD in a model of infection provoked by Enteroaggregative E. coli 042. The larvae were treated with each fraction 2 h after the infection. E. coli 042 killed most of the larvae in less than 24 h (median survival of one day). In contrast, the group treated with B. tetraphylla had median survivals of two days (Figure 5). It is important to highlight that these fractions did not have antimicrobial activity towards E. coli.
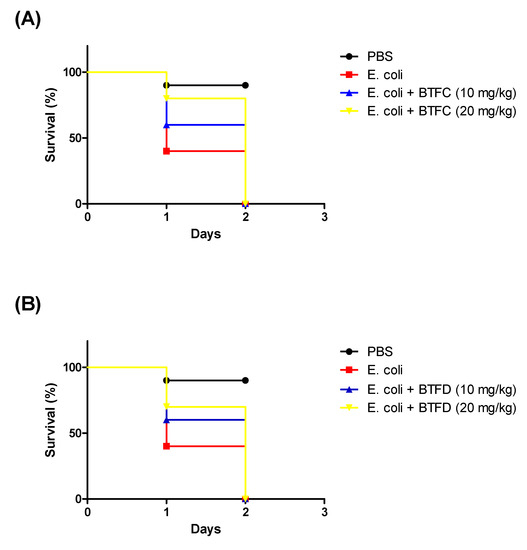
Figure 5.
Effects of fractions from methanolic extract of Buchenavia tetraphylla leaves (BTFC and BTFD) on the survival of Tenebrio molitor larvae challenged with Escherichia coli 042. (A) Effects of BTFC on the survival of Tenebrio molitor larvae challenged with E. coli 042. (B) Effects of BTFD on the survival of Tenebrio molitor larvae challenged with E. coli 042. The larvae (n = 10/group) received a lethal dose of EAEC 042 and after 2 h were treated with fraction BTFC and BTFD (at 10 mg/kg or 20 mg/kg). Larvae treated with phosphate-saline buffer (PBS) or EAEC 042 were used as negative or positive controls, respectively. In this set of assays, larvae survival was recorded each 24 h. The experiment was repeated three times.
2.6. Nuclear Magnetic Resonance Analysis of Buchenavia tetraphylla Leaves
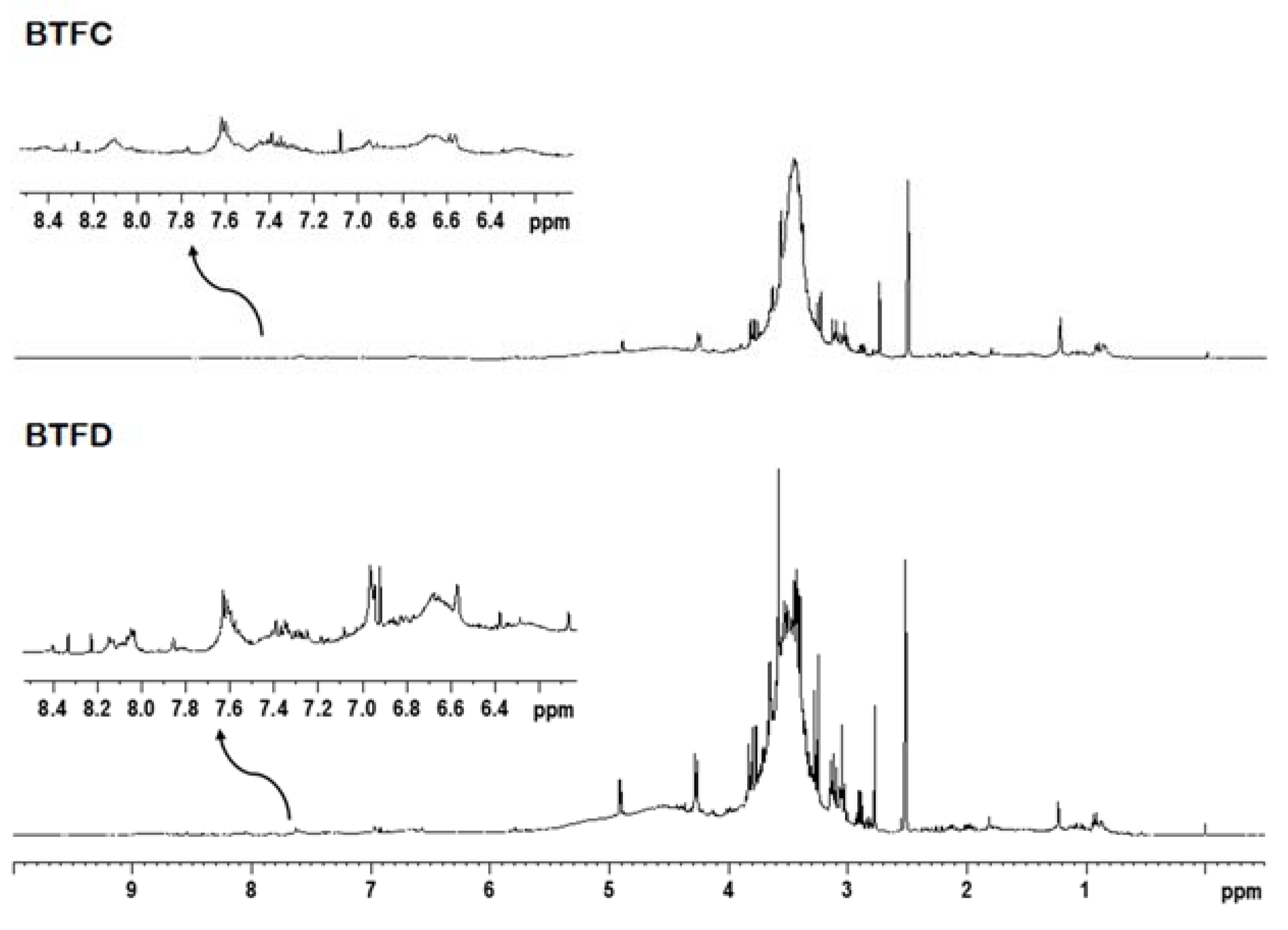
The 1H NMR analysis and two-dimensional experiments revealed similar profiles for BTFC and BTFD. The intense overlapping made difficult the identification of metabolites; however, it was possible to suggest the presence of some classes of compounds. The fractions showed signals in spectral regions related to aliphatic and aromatic compounds (Figure 6). The signals at δ 0.50–5.90 ppm attributed to an aliphatic compound (such as terpenoids, fatty acids, carbohydrates) were more expressive in comparison to the signals at δ 6.00–8.50 ppm related to aromatic compounds (such as phenolic compounds). This was observed mainly in the BTFD fraction.
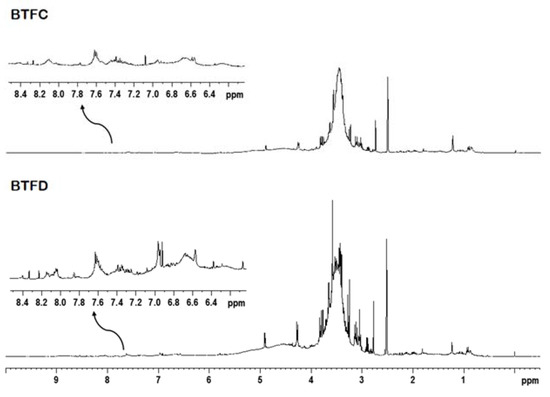
Figure 6.
Representative 1H NMR spectrum of the active fractions (BTFC and BTFD) obtained from the methanolic extract of Buchenavia tetraphylla leaves.
3. Discussion
The role of oxidative stress in the development of severe clinical conditions has promoted the search for new antioxidant agents from natural products [1,2,10,24,25]. Herein, we report the selection of high antioxidant fractions from B. tetraphylla leaf extracts using in vitro and in vivo approaches. Products derived from this plant have been shown as potential candidates for the development of new antimicrobial agents [20,21,22]; however, their antioxidant actions have not been properly addressed. The in vitro results obtained in this study showed that the methanolic extract (BTME) had a higher antioxidant activity, and this effect was correlated with its higher phenolic and flavonoid content. Among the fractions obtained from this extract, the best potentials were found for BTFC and BTFD (these fractions also exhibited greater levels of phenolic compounds). Furthermore, these agents were not toxic towards human erythrocytes.
These samples were selected to be evaluated in a model of stress induced by heat-killed E. coli in T. molitor larvae. This insect has been used as a model organism in studies of microbial pathogenesis and drug development (antimicrobial, antivirulence, and immunomodulator agents) [27,28,29]. Several factors have supported the use of this animal. First, T. molitor is susceptible to pathogens such as Candida albicans, E. coli, and Staphylococcus aureus, which are able to persist within the infected insect, and cause changes in tissues, hemolymph, and phagocytes [30,31,32]. Second, the immune system of this insect has some known signaling pathways, such as the Toll pathway, the prophenoloxidase cascade, and the autophagy pathway [33,34,35,36].
The antioxidant defense system of T. molitor is composed of several antioxidant and detoxifying enzymes such as superoxide dismutases, peroxidases, catalases, as well as tyrosinase, acetylcholinesterase, carboxylesterase, and glutathione S-transferase [37,38,39]. Previous studies have shown that during infection, the T. molitor larvae overproduce reactive species in response to the pathogen presence, leading to increased activity of several antioxidants and detoxifying enzymes that are correlated with larvae death [37,38,40].
The ability of T. molitor to produce reactive species in response to deleterious stimulus makes this insect a potential model for the study of antioxidant substances. Despite these advantages, no study has exploited T. molitor larvae for the screening of plant-derived antioxidant compounds. The protective action of the agent is evidenced by the increased survival of the treated larvae compared to untreated larvae. This approach using larvae could bring more information than those traditionally used for antioxidant prospecting, based on the chemical interaction of compounds and without biological relevance [10,24].
Since T. molitor is a multicellular organism, this approach also presents advantages over those that use cells as some insights into the toxicity of the antioxidant agent can also be assessed. Furthermore, the use of this insect offers some advantages in relation to Caenorhabditis elegans, the invertebrate organism traditionally used for in vivo antioxidant evaluation [41,42], such as ease of handling and direct inoculation of the compound agent in the larvae. In our in vivo model of stress induced by heat-killed E. coli (OP50 strain), BTFD induced higher protective effects than BTME and BTFC. However, in our assays using the live EAEC strain, the fractions had similar results (both increasing the larvae median survival in one day). It is important to highlight that BTFC and BTFD did no display antimicrobial activity in vitro (data not shown), suggesting that their protective effects are related to the antioxidant properties. In this sense, we showed the efficacy of two fractions rich in antioxidants to reduce the deleterious effects of E. coli-induced injury in T. molitor larvae.
Previous works reported the predominant presence of flavonoids and alkaloids in B. tetraphylla and other species from the same genus [20,21,43,44]. In this present research, the most bioactive fractions (BTFC and BTFD) showed a similar chemical composition with the presence of aliphatic (terpenes, fatty acids, carbohydrates) and aromatic compounds (phenolic compounds). In general, the pharmacological potentials of some terpenes are associated with their antioxidant action [45,46]. These studies were reviewed by Gonzalez-Burgos and Gomez-Serranillos [45], who highlighted some structural features involved in the antioxidant action of each type of terpene.
Among the classes of compounds detected, phenolic compounds are the most usually related to antioxidant activity. The high antioxidant abilities of phenolic compounds are related to their phenolic hydroxyl groups that can donate hydrogen atom or transfer electrons, resulting in the scavenging of harmful free radicals (such as hydroxyl radicals). The aromatic groups present in the phenolic acids can also delocalize the unpaired electron [47,48]. According to Dai and Mumper [47], the flavonoids are able to perform electron transfer (mainly due to the ortho-dihydroxy structure on the B ring) and electron delocalization (the 2,3-double bond with a 4-oxo function in the C ring, which relocates from the B ring). The authors also highlighted the essential role of 3- and 5-hydroxyl groups with the 4-oxo function in A and C rings and 3-hydroxyl groups.
4. Material and Methods
4.1. Collection and Extract Preparation
Leaves of B. tetraphylla were collected in November 2013, in Catimbau National Park (Pernambuco, Brazil). The samples were processed according to the taxonomic techniques, identified, and deposited in the Herbarium of Agronomic Institute of Pernambuco (voucher: IPA 80349). The leaves of B. tetraphylla were subjected to drying at room temperature and then pulverized using a Macsalab mill (Model 200 LAB). This material was stored in a closed, dark container until used.
For extraction, 25 g of the powder were mixed with 100 mL of the first solvent (hexane; for BTHE) on a rotary shaker table (125 rpm) at 25 °C. After 72 h, the sample was filtered, and the extracted liquid was dried in a rotary evaporator (45 rpm) at 50 °C. The remaining leaf residue was further extracted with 100 mL of chloroform (BTCE), and the above procedure was repeated completely, subsequently performed with ethyl acetate (BTEE), and finally, with methanol (BTME).
4.2. Fractionation of the Methanolic Extract
The methanolic extract (2 g) was fractionated by chromatography using a column (80 cm × 2.5 cm) incorporated with Sephadex LH-20 (GE Healthcare®, Chicago, IL, USA; 60 cm high). The systems of eluents were based on the combination of methanol and ethyl acetate (as shown below). A total of 120 fractions (7 mL each) were obtained and analyzed by fluorescent black light (25W; 127V; Empalux®, Curitiba, Brazil) and thin layer chromatography (POLYGRAM® SIL G60/UV254; 20 × 20 cm; 0.20 mm; Macherey-Nagel®, Düren, Germany). After these procedures, fractions with similar phytochemical profiles were gathered, resulting in 9 different fractions: BTFA (Fractions 1–4), BTFB (Fractions 5–11), BTFC (Fractions 12–18), BTFD (Fractions 19–26), BTFE (Fractions 27–36), BTFF (Fractions 37–54), BTFG (Fractions 55–61), BTFH (Fractions 62–69), and BTFI (Fractions 70–120). The elution systems (methanol:ethyl acetate; v/v) for obtaining each fraction were: 7:3 for BTFA; 6:4 for BTFB, BTFC, BTFD, BTFE, BTFF; 5:5 for BTFG; 6:4 for BTFH and BTFI.
4.3. Total Phenolic Content
Obtaining the total phenolic compounds in crude extracts and fractions was performed using the Folin-Ciocalteu reagent [49]. Samples of each extract/fraction (200 μL at 1000 μg/mL) were mixed with 1.0 mL of Folin-Ciocalteu reagent, and 800 μL of 20% sodium carbonate were added after 3 min. The mixture was incubated at room temperature, protected from light, and allowed to stand for 2 h. The absorbance of the mixture was measured at 765 nm (GeneQuant 1300, GE Healthcare). The total phenolic content was calculated in mg of gallic acid equivalent (GAE) per mg of dry extract using a calibration curve obtained with gallic acid (y = 0.0043x + 0.0153; R2 = 0.9932). The results were expressed as mean ± standard deviation calculated from three independent assays performed in triplicate (n = 3).
4.4. Flavonoid Content
For flavonoid content, 100 μL (at 1000 μg/mL) of each sample were mixed with 100 μL of the reagent solution (2 g of aluminum chloride diluted in 2% ethanol solution). The mixture was incubated at room temperature and protected from light, and after 60 min, the absorbance was measured at 420 nm [50]. The content of flavonoids was calculated in mg of quercetin equivalent (QE) per mg of dry extract using a calibration curve constructed with standard quercetin solution (y = 0.004x + 0.0121; R2 = 0.993). The results are expressed as the mean ± standard deviation calculated from three independent assays performed in triplicate (n = 3).
4.5. DPPH Assay
An aliquot of 250 μL of 1 mM DPPH solution (2,2-diphenyl-1-picrylhydrazyl; Sigma-Aldrich) was added to 40 μL of different sample concentrations (31.25–1000 μg/mL) and homogenized. After 30 min, the absorbance was measured at 517 nm [51]. Trolox was used as the control compound. The DPPH sequestering activity was calculated using the formula below. The results were expressed as the mean ± standard deviation calculated from three independent assays performed in triplicate (n = 3).
where: Ac = absorbance control; As = sample absorbance
DPPH scavenging (%) = (Ac − As)/Ac × 100
4.6. ABTS Assay
The ABTS (2,2-azino-bis (3-ethylbenzo-thiazoline-6-sulfonic acid)) radical cation was prepared 16 h prior to the assay by mixing 5 mL of the stock solution (7 mM) with 88 µL of the 140 mM potassium persulfate solution. Aliquots (20 μL) of each extract/fraction and 2 mL of the ABTS radical were mixed, and the absorbance of the solutions was monitored at 734 nm after 6, 15, 30, 45, 60, and 120 min, respectively [52]. Gallic acid was used as the positive control. The ABTS scavenging was calculated using the formula below. The results were expressed as the mean ± standard deviation calculated from three independent assays performed in triplicate (n = 3).
where Ac (control absorbance) and Aa (sample absorbance).
ABTS scavenging (%) = (Ac − Aa)/Ac × 100
4.7. Hemolytic Activity
Blood (5–10 mL) was obtained from healthy, non-smoker volunteers by venipuncture, after signing a free informed consent form. Human erythrocytes from citrated blood were immediately isolated by centrifugation at 1500 rpm for 10 min. After removal of the plasma, the erythrocytes were washed three times with phosphate-buffered saline (PBS; pH 7.4), and then, a suspension of 1% erythrocytes was prepared as the same buffer. Following, an aliquot of 1.1 mL of erythrocyte suspension was mixed with 0.4 mL of each extract/fraction (concentration range: 125 to 1000 μg/mL). The negative control and positive control received 0.4 mL of PBS and Triton X, respectively. After 60 min of incubation at room temperature, the cells were centrifuged, and the supernatant was used to measure the absorbance of hemoglobin released at 540 nm [21]. The hemolytic activity was expressed in relation to the action of Triton X-100 and calculated using the formula below. The results were expressed as the mean ± standard deviation calculated from three independent assays performed in quadruplicate (n = 4).
where: Aa is sample absorbance; Ab is the absorbance of the negative control; and Ac is the absorbance of the positive control.
Hemolytic activity (%) = [(Aa − Ab) × 100]/(Ac − Ab)
4.8. Toxicity Model Using Heat-Killed E. coli
Larvae of T. molitor (~100 mg) were randomly allocated into groups (n = 10). After anesthesia and disinfection, 10 μL of the most active samples (methanolic extract or fractions C and D; at 10 mg/kg or 20 mg/kg) were injected in the ventral membrane between the second and third abdominal segments (tail to the head) [29]. One hour after the sample inoculation, the larvae received 10 μL of heat-killed E. coli OP50 (optical density at 600 nm: 0.7). The viability of the larvae was evaluated after 15, 30, 45, and 60 h (by evaluation the lack of movement after mechanical stimulus). Larvae inoculated with the microorganism and treated with PBS were used as the negative control; while larvae that received two doses of PBS were the positive control. The experiment was performed in three independent assays.
4.9. Infection Model Using Enteroaggregative E. coli
Larvae (n = 10) were prepared as described above and infected with 10 μL of enteroaggregative E. coli (EAEC) 042 (optical density at 600 nm: 0.1). After two hours, each animal received 10 μL of BTFC or BTFD (at 10 mg/kg or 20 mg/kg). Larvae inoculated with E. coli 042 and treated with PBS were used as the negative control; while larvae that received two doses of PBS were the positive control. The experiment was repeated three independent assays.
4.10. Nuclear Magnetic Resonance Analysis
The chemical characterization of the most active fractions (BTFC and BTFD) was performed by nuclear magnetic resonance (NMR) analysis. 1D and 2D NMR data were acquired at 298 K in DMSO-d6 on a Bruker AVANCE III 400 NMR spectrometer operating at 9.4 T, observing 1H and 13C at 400 and 100 MHz, respectively. The NMR spectrometer was equipped with a 5 mm direct detection probe (BBO) with a z-gradient. One-bond (1H-13C HSQC) and long-range (1H-13C HMBC) NMR correlation experiments were optimized for average coupling constant 1J(C,H) and LRJ(C,H) of 140 and 8 Hz, respectively. All 1H and 13C NMR chemical shifts (δ) were given in ppm related to the TMS signal at 0.00 as an internal reference and the coupling constants (J) in Hz.
4.11. Statistical Analysis
The results were expressed as the mean ± standard deviation (SD). Statistical significance was determined by one-way ANOVA or two-way ANOVA followed by Tukey and Bonferroni tests. A p-value < 0.05 was considered statistically significant. Determination of EC50 (half maximal effective concentration) was performed by linear regression. Correlations were assessed using the Pearson coefficient. The larvae survival assays were analyzed using the Kaplan–Meier method to calculate survival fractions, and the log-rank test was used to compare survival curves.
5. Conclusions
In this study, the use of in vitro antioxidant assays allowed the selection of fractions from a methanolic extract with a high activity and low toxicity. The fractions (BTFC and BTFD) were able to extend the lifespan of T. molitor larvae submitted to stress induced by heat-killed E. coli significantly. The therapeutic treatment with these fractions had also positive effects on the infection induced by the pathogenic strain E. coli 042 (EAEC). 1H NMR data indicated the presence of aliphatic (terpenes, fatty acids, carbohydrates) and aromatic compounds (phenolic compounds). These findings suggested that products derived from B. tetraphylla leaves are a promising candidate for the development of antioxidant agents able to treat the oxidative-related dysfunctions.
Author Contributions
T.F.S., M.V.d.S., M.T.d.S.C., and L.C.N.d.S. conceived of the study and performed the study design. T.F.S., V.L.d.M.L., N.H.d.S., J.R.G.d.S.A., M.V.d.S., M.T.d.S.C., and L.C.N.d.S. provided the reagents and equipment for all assays. J.R.N.C.F., M.M.L.B.F., N.M.d.S., and A.P.S.d.S. prepared the extracts and performed the in vivo and in vitro antioxidant assays. A.C.B.d.S., A.Z., and A.G.A. performed the anti-infective assays with T. molitor. J.R.G.d.S.A. and L.M.D. performed the chemical characterization of the extracts. All authors interpreted and discussed the results. T.F.S., J.R.G.d.S.A., L.M.D., A.C.B.d.S., M.T.d.S.C., and L.C.N.d.S. drafted and revised the manuscript. All authors approved the final version of this manuscript.
Funding
This work was funded by Fundação de Amparo à Ciência e Tecnologia de Pernambuco (FACEPE) Fundação de Amparo à Pesquisa e Desenvolvimento Científico do Maranhão (FAPEMA; BEPP-02241/18), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Acknowledgments
The authors thank the support given by the Laboratory of Natural Products and the Laboratory of Molecular Biology, Department of Biochemistry, of the Federal University of Pernambuco (UFPE;Universidade Federal de Oer).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, M.J.; Salazar, L.; Grijalva, M. Oxidative Stress Modulation and ROS-Mediated Toxicity in Cancer: A review on in vitro models for plant-derived compounds. Oxid. Med. Cell. Longev. 2017, 2017, 4586068. [Google Scholar] [CrossRef] [PubMed]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef] [PubMed]
- De Andrade Teles, R.B.; Diniz, T.C.; Costa Pinto, T.C.; de Oliveira Junior, R.G.; Gama, E.S.M.; de Lavor, E.M.; Fernandes, A.W.C.; de Oliveira, A.P.; de Almeida Ribeiro, F.P.R.; da Silva, A.A.M.; et al. Flavonoids as therapeutic agents in alzheimer’s and parkinson’s diseases: A systematic review of preclinical evidences. Oxid. Med. Cell. Longev. 2018, 2018, 7043213. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H. Mitochondrial dysfunction and oxidative stress in asthma: Implications for mitochondria-targeted antioxidant therapeutics. Pharmaceuticals 2011, 4, 429–456. [Google Scholar] [CrossRef] [PubMed]
- Sillar, J.R.; Germon, Z.P.; DeIuliis, G.N.; Dun, M.D. The role of reactive oxygen species in acute myeloid leukaemia. Int. J. Mol. Sci. 2019, 20, 6003. [Google Scholar] [CrossRef]
- Teixeira, J.P.; de Castro, A.A.; Soares, F.V.; da Cunha, E.F.F.; Ramalho, T.C. Future therapeutic perspectives into the alzheimer’s disease targeting the oxidative stress hypothesis. Molecules 2019, 24, 4410. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Nascimento da Silva, L.C.; Bezerra Filho, C.M.; Paula, R.A.; Silva, E.S.C.S.; Oliveira de Souza, L.I.; Silva, M.V.; Correia, M.T.; Figueiredo, R.C. In vitro cell-based assays for evaluation of antioxidant potential of plant-derived products. Free Radic. Res. 2016, 50, 801–812. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.C.; Alves, N.M.; de Castro, M.C.; Higino, T.M.; da Cunha, C.R.; Pereira, V.R.; da Paz, N.V.; Coelho, L.C.; Correia, M.T.; de Figueiredo, R.C. pCramoll and rCramoll as new preventive agents against the oxidative dysfunction induced by hydrogen peroxide. Oxid. Med. Cell. Longev. 2015, 2015, 520872. [Google Scholar] [PubMed]
- Cacciatore, I.; Fornasari, E.; Baldassarre, L.; Cornacchia, C.; Fulle, S.; Di Filippo, E.S.; Pietrangelo, T.; Pinnen, F. A potent (R)-alpha-bis-lipoyl derivative containing 8-hydroxyquinoline scaffold: Synthesis and biological evaluation of its neuroprotective capabilities in sh-sy5y human neuroblastoma cells. Pharmaceuticals 2013, 6, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Marranzano, M.; Rosa, R.L.; Malaguarnera, M.; Palmeri, R.; Tessitori, M.; Barbera, A.C. Polyphenols: Plant sources and food industry applications. Curr. Pharm. Des. 2018, 24, 4125–4130. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.S.; Santos, M.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; da Silva Lannes, S.C.; da Silva, M.V. Natural antioxidants used in meat products: A brief review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, S.C.; Moldao-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- da Silva, L.C.; da Silva, C.A., Jr.; de Souza, R.M.; Jose Macedo, A.; da Silva, M.V.; dos Santos Correia, M.T. Comparative analysis of the antioxidant and DNA protection capacities of Anadenanthera colubrina, Libidibia ferrea and Pityrocarpa moniliformis fruits. Food Chem. Toxicol. 2011, 49, 2222–2228. [Google Scholar] [CrossRef]
- Teles Fujishima, M.A.; Silva, N.; Ramos, R.D.S.; Batista Ferreira, E.F.; Santos, K.; Silva, C.; Silva, J.O.D.; Campos Rosa, J.M.; Santos, C. An Antioxidant Potential, Quantum-Chemical and Molecular Docking Study of the Major Chemical Constituents Present in the Leaves of Curatella americana Linn. Pharmaceuticals 2018, 11, 72. [Google Scholar] [CrossRef]
- Agra, M.F.; Baracho, G.S.; Nurit, K.; Basilio, I.J.; Coelho, V.P. Medicinal and poisonous diversity of the flora of “Cariri Paraibano”, Brazil. J. Ethnopharmacol. 2007, 111, 383–395. [Google Scholar] [CrossRef]
- Beutler, J.A.; Cardellina, J.H., 2nd; McMahon, J.B.; Boyd, M.R.; Cragg, G.M. Anti-HIV and cytotoxic alkaloids from Buchenavia capitata. J. Nat. Prod. 1992, 55, 207–213. [Google Scholar] [CrossRef]
- De Oliveira, Y.L.; Nascimento da Silva, L.C.; da Silva, A.G.; Macedo, A.J.; de Araujo, J.M.; Correia, M.T.; da Silva, M.V. Antimicrobial activity and phytochemical screening of Buchenavia tetraphylla (Aubl.) R. A. Howard (Combretaceae: Combretoideae). Sci. World J. 2012, 2012, 849302. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti Filho, J.R.; Silva, T.F.; Nobre, W.Q.; Oliveira de Souza, L.I.; Silva, E.S.F.C.S.; Figueiredo, R.C.; de Gusmao, N.B.; Silva, M.V.; Nascimento da Silva, L.C.; Correia, M.T. Antimicrobial activity of Buchenavia tetraphylla against Candida albicans strains isolated from vaginal secretions. Pharm. Biol. 2017, 55, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Blair, L.M.; Calvert, M.B.; Sperry, J. Flavoalkaloids-Isolation, Biological Activity, and Total Synthesis. Alkaloids Chem. Biol. 2017, 77, 85–115. [Google Scholar] [PubMed]
- Becker, K.; Schroecksnadel, S.; Gostner, J.; Zaknun, C.; Schennach, H.; Uberall, F.; Fuchs, D. Comparison of in vitro tests for antioxidant and immunomodulatory capacities of compounds. Phytomedicine 2014, 21, 164–171. [Google Scholar] [CrossRef]
- Pellegrini, N.; Vitaglione, P.; Granato, D.; Fogliano, V. Twenty-five years of total antioxidant capacity measurement of foods and biological fluids: Merits and limitations. J. Sci. Food Agric. 2018. [Google Scholar] [CrossRef]
- Yun, E.J.; Lee, S.H.; Kim, S.; Kim, S.H.; Kim, K.H. Global profiling of metabolic response of Caenorhabditis elegans against Escherichia coli O157: H7. Process. Biochem. 2017, 53, 36–43. [Google Scholar] [CrossRef]
- Canteri de Souza, P.; Custodio Caloni, C.; Wilson, D.; Sergio Almeida, R. An Invertebrate Host to Study Fungal Infections, Mycotoxins and Antifungal Drugs: Tenebrio molitor. J. Fungi 2018, 4, 125. [Google Scholar] [CrossRef]
- Guo, Z.; Pfohl, K.; Karlovsky, P.; Dehne, H.W.; Altincicek, B. Dissemination of Fusarium proliferatum by mealworm beetle Tenebrio molitor. PLoS ONE 2018, 13, e0204602. [Google Scholar] [CrossRef]
- Czarniewska, E.; Urbanski, A.; Chowanski, S.; Kuczer, M. The long-term immunological effects of alloferon and its analogues in the mealworm Tenebrio molitor. Insect Sci. 2018, 25, 429–438. [Google Scholar] [CrossRef]
- McGonigle, J.E.; Purves, J.; Rolff, J. Intracellular survival of Staphylococcus aureus during persistent infection in the insect Tenebrio molitor. Dev. Comp. Immunol. 2016, 59, 34–38. [Google Scholar] [CrossRef]
- Seong, J.H.; Jo, Y.H.; Seo, G.W.; Park, S.; Park, K.B.; Cho, J.H.; Ko, H.J.; Kim, C.E.; Patnaik, B.B.; Jun, S.A.; et al. Molecular Cloning and Effects of Tm14-3-3zeta-Silencing on Larval Survivability Against, E. coli and C. albicans in Tenebrio molitor. Genes 2018, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- De Souza, P.C.; Morey, A.T.; Castanheira, G.M.; Bocate, K.P.; Panagio, L.A.; Ito, F.A.; Furlaneto, M.C.; Yamada-Ogatta, S.F.; Costa, I.N.; Mora-Montes, H.M.; et al. Tenebrio molitor (Coleoptera: Tenebrionidae) as an alternative host to study fungal infections. J. Microbiol. Methods 2015, 118, 182–186. [Google Scholar] [CrossRef]
- Johnston, P.R.; Makarova, O.; Rolff, J. Inducible defenses stay up late: Temporal patterns of immune gene expression in Tenebrio molitor. G3 2013, 4, 947–955. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, S.K.; So, Y.I.; Park, H.Y.; Li, X.H.; Yeom, D.H.; Lee, M.N.; Lee, B.L.; Lee, J.H. Protease IV, a quorum sensing-dependent protease of Pseudomonas aeruginosa modulates insect innate immunity. Mol. Microbiol. 2014, 94, 1298–1314. [Google Scholar] [CrossRef] [PubMed]
- Tindwa, H.; Jo, Y.H.; Patnaik, B.B.; Lee, Y.S.; Kang, S.S.; Han, Y.S. Molecular cloning and characterization of autophagy-related gene TmATG8 in Listeria-invaded hemocytes of Tenebrio molitor. Dev. Comp. Immunol. 2015, 51, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.T.; Lee, M.R.; Lee, S.J.; Kim, S.; Nai, Y.S.; Kim, J.S. Tenebrio molitor Gram-negative-binding protein 3 (TmGNBP3) is essential for inducing downstream antifungal Tenecin 1 gene expression against infection with Beauveria bassiana JEF-007. Insect Sci. 2018, 25, 969–977. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Ze, S.Z.; Stanley, D.W.; Yang, B. Parasitization by Scleroderma guani influences expression of superoxide dismutase genes in Tenebrio molitor. Arch. Insect Biochem. Physiol. 2014, 87, 40–52. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Lewis, E.E.; Tarasco, E. Activity changes of antioxidant and detoxifying enzymes in Tenebrio molitor (Coleoptera: Tenebrionidae) larvae infected by the entomopathogenic nematode Heterorhabditis beicherriana (Rhabditida: Heterorhabditidae). Parasitol. Res. 2016, 115, 4485–4494. [Google Scholar] [CrossRef]
- Gulevsky, A.K.; Relina, L.I.; Grishchenkova, Y.A. Variations of the antioxidant system during development of the cold-tolerant beetle, Tenebrio molitor. Cryo Lett. 2006, 27, 283–290. [Google Scholar]
- Medina-Gomez, H.; Farriols, M.; Santos, F.; Gonzalez-Hernandez, A.; Torres-Guzman, J.C.; Lanz, H.; Contreras-Garduno, J. Pathogen-produced catalase affects immune priming: A potential pathogen strategy. Microb. Pathog. 2018, 125, 93–95. [Google Scholar] [CrossRef]
- Peixoto, H.; Roxo, M.; Silva, E.; Valente, K.; Braun, M.; Wang, X.; Wink, M. Bark Extract of the Amazonian Tree Endopleura uchi (Humiriaceae) Extends Lifespan and Enhances Stress Resistance in Caenorhabditis elegans. Molecules 2019, 24, 915. [Google Scholar] [CrossRef] [PubMed]
- Rohn, I.; Raschke, S.; Aschner, M.; Tuck, S.; Kuehnelt, D.; Kipp, A.; Schwerdtle, T.; Bornhorst, J. Treatment of Caenorhabditis elegans with small selenium species enhances antioxidant defense systems. Mol. Nutr. Food Res. 2019, 63, e1801304. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, G.R.; Brighenti, F.L.; Delbem, A.C.; Delbem, A.C.; Khouri, S.; Gontijo, A.V.; Pascoal, A.C.; Salvador, M.J.; Koga-Ito, C.Y. Antifungal activity of extracts and isolated compounds from Buchenavia tomentosa on Candida albicans and non-albicans. Future Microbiol. 2015, 10, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, G.R.; Gontijo, A.V.L.; Salvador, M.J.; Tanaka, M.H.; Brighenti, F.L.; Delbem, A.C.B.; Delbem, A.C.B.; Koga-Ito, C.Y. Effects of acetone fraction from Buchenavia tomentosa aqueous extract and gallic acid on Candida albicans biofilms and virulence factors. Front. Microbiol. 2018, 9, 647. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, E.; Gomez-Serranillos, M.P. Terpene compounds in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Castaneda-Arriaga, R.; Perez-Gonzalez, A.; Reina, M.; Alvarez-Idaboy, J.R.; Galano, A. Comprehensive investigation of the antioxidant and pro-oxidant effects of phenolic compounds: A double-edged sword in the context of oxidative stress? J. Phys. Chem. B 2018, 122, 6198–6214. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apicult. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).