(Radio)Theranostic Patient Management in Oncology Exemplified by Neuroendocrine Neoplasms, Prostate Cancer, and Breast Cancer
Abstract
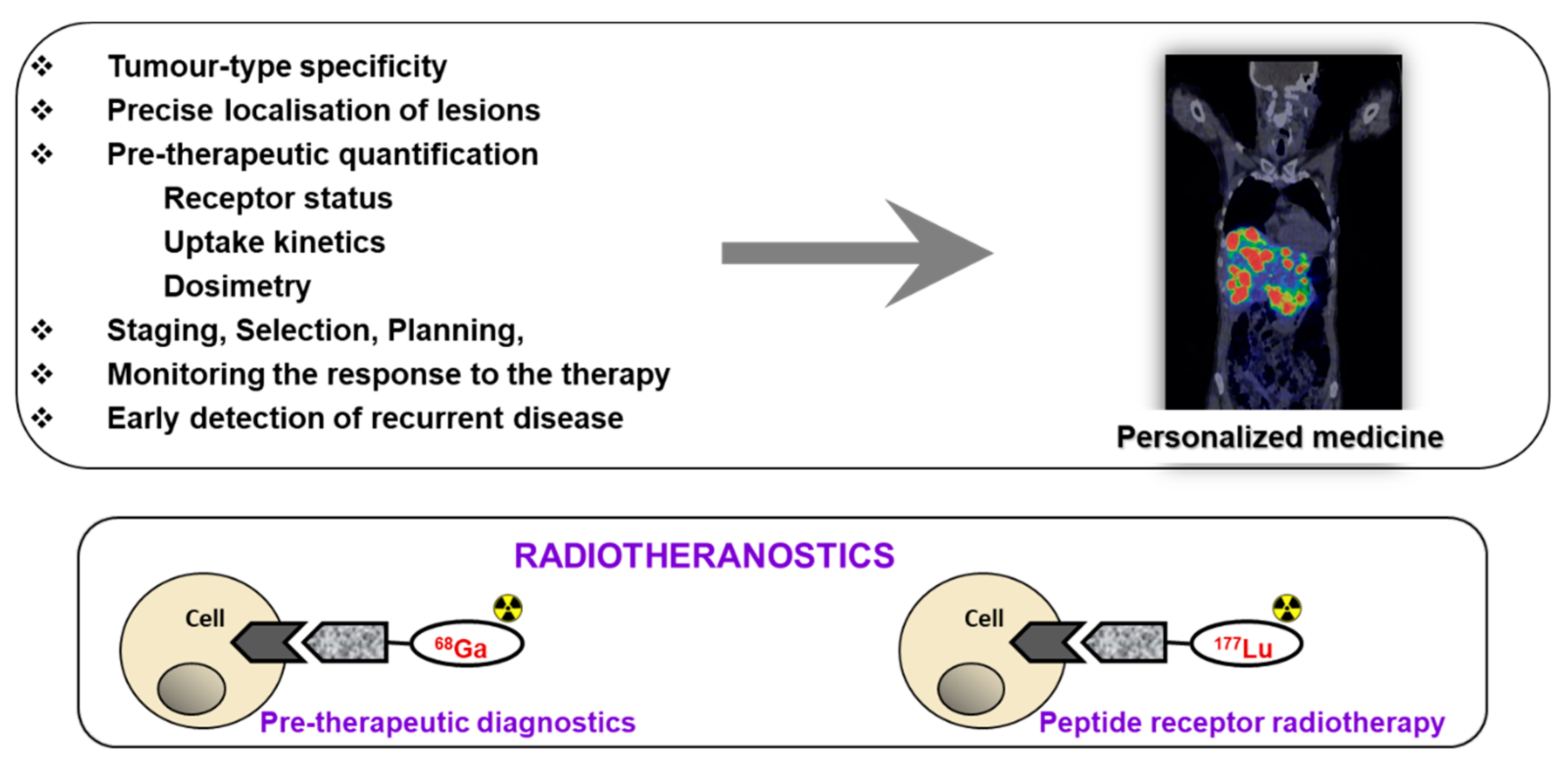
1. Introduction
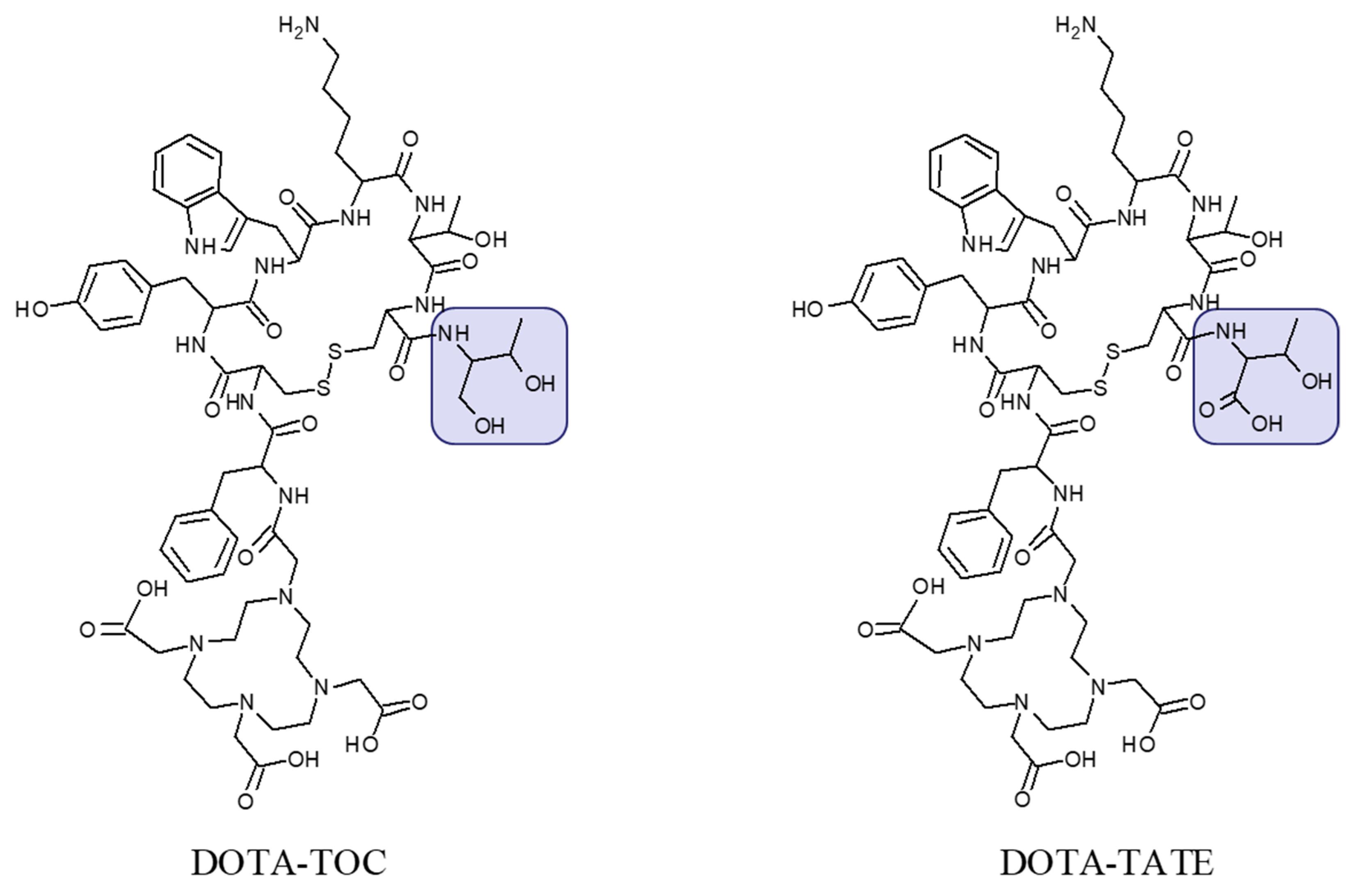
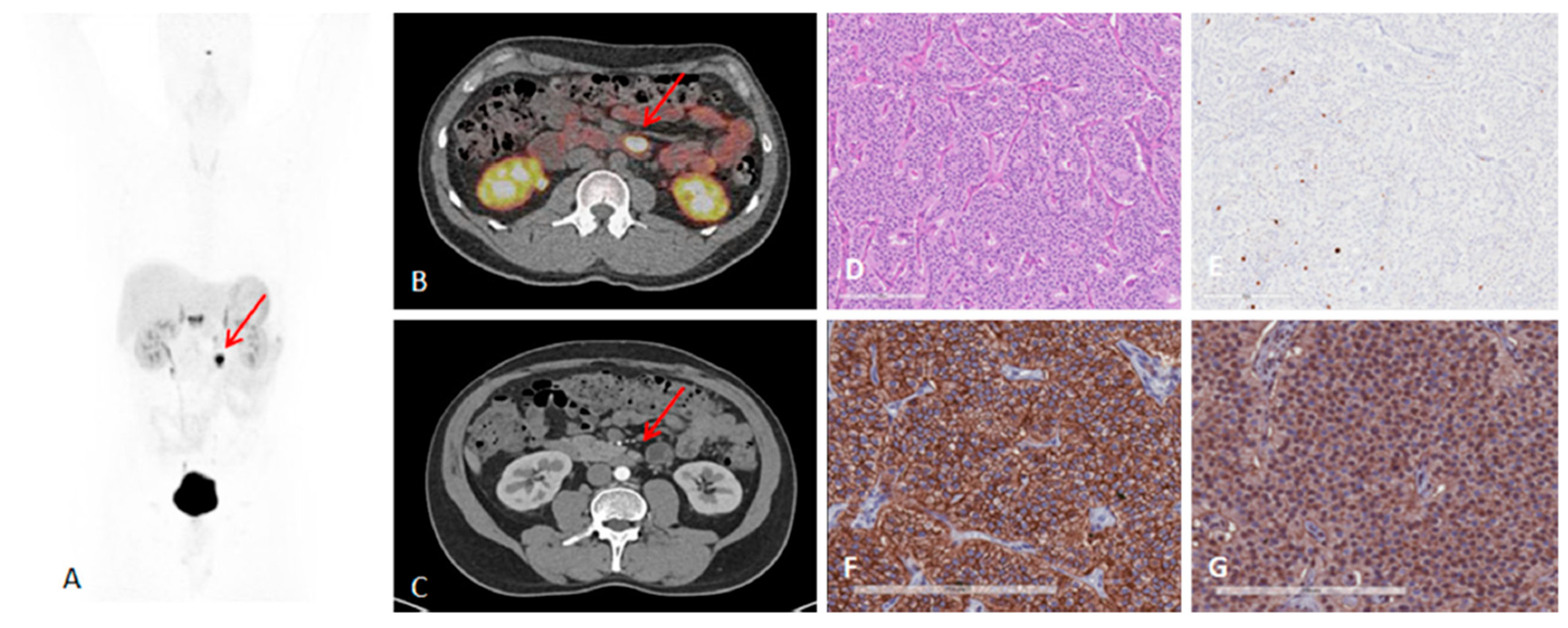
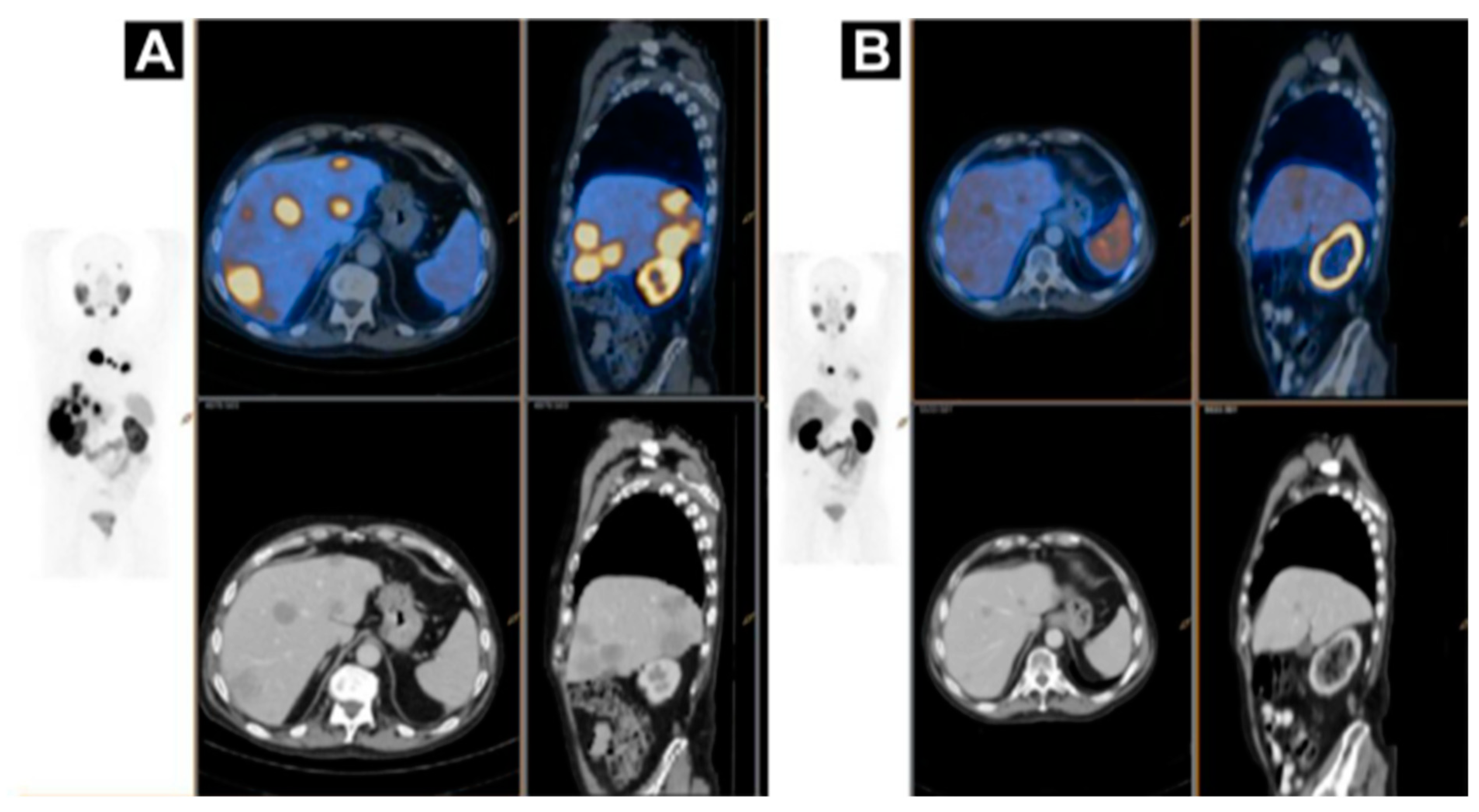
2. Targeting SSTR on Neuroendocrine Neoplasms
Impact of SST Radiopharmaceuticals on Patient Treatment Management
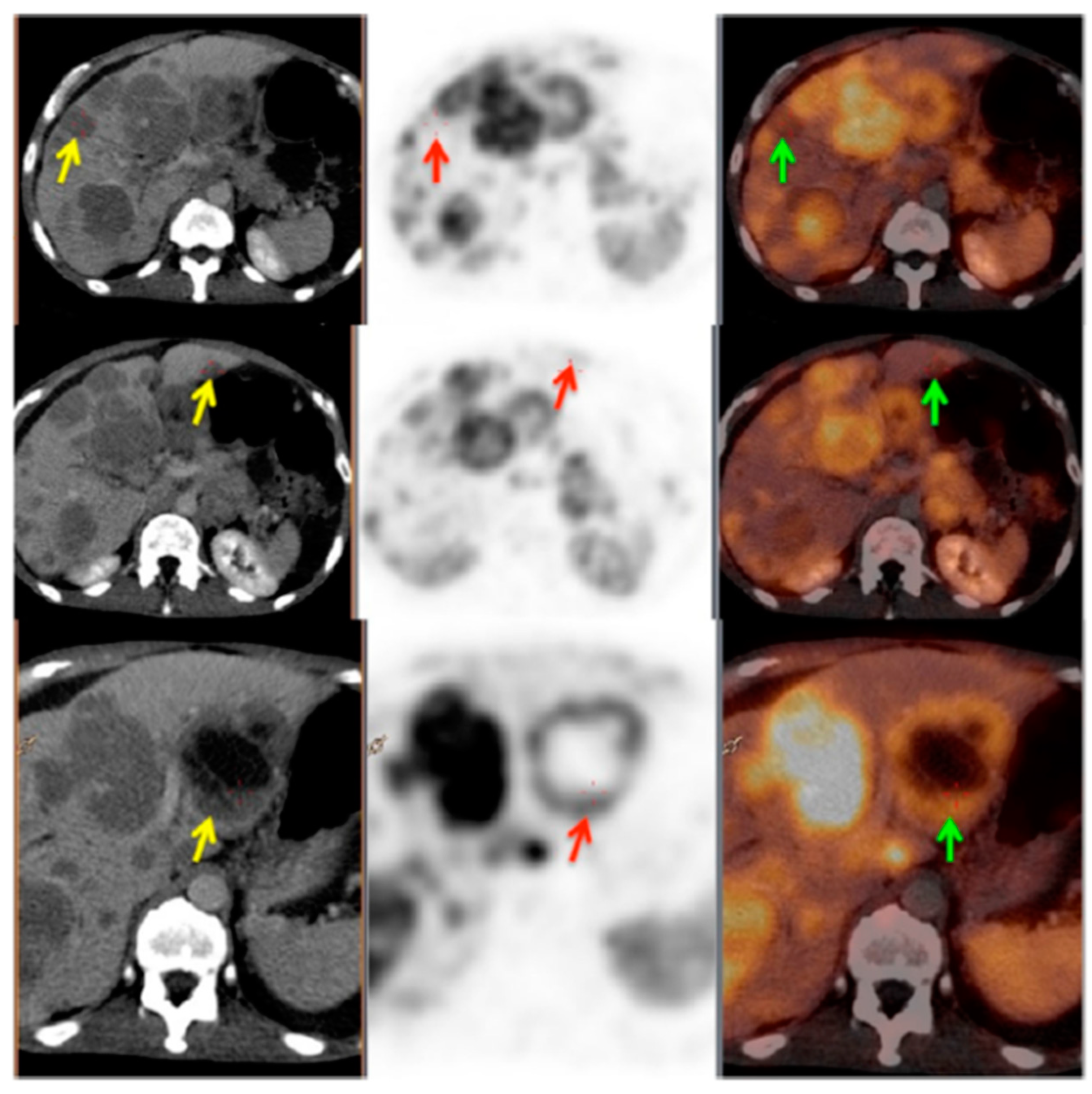
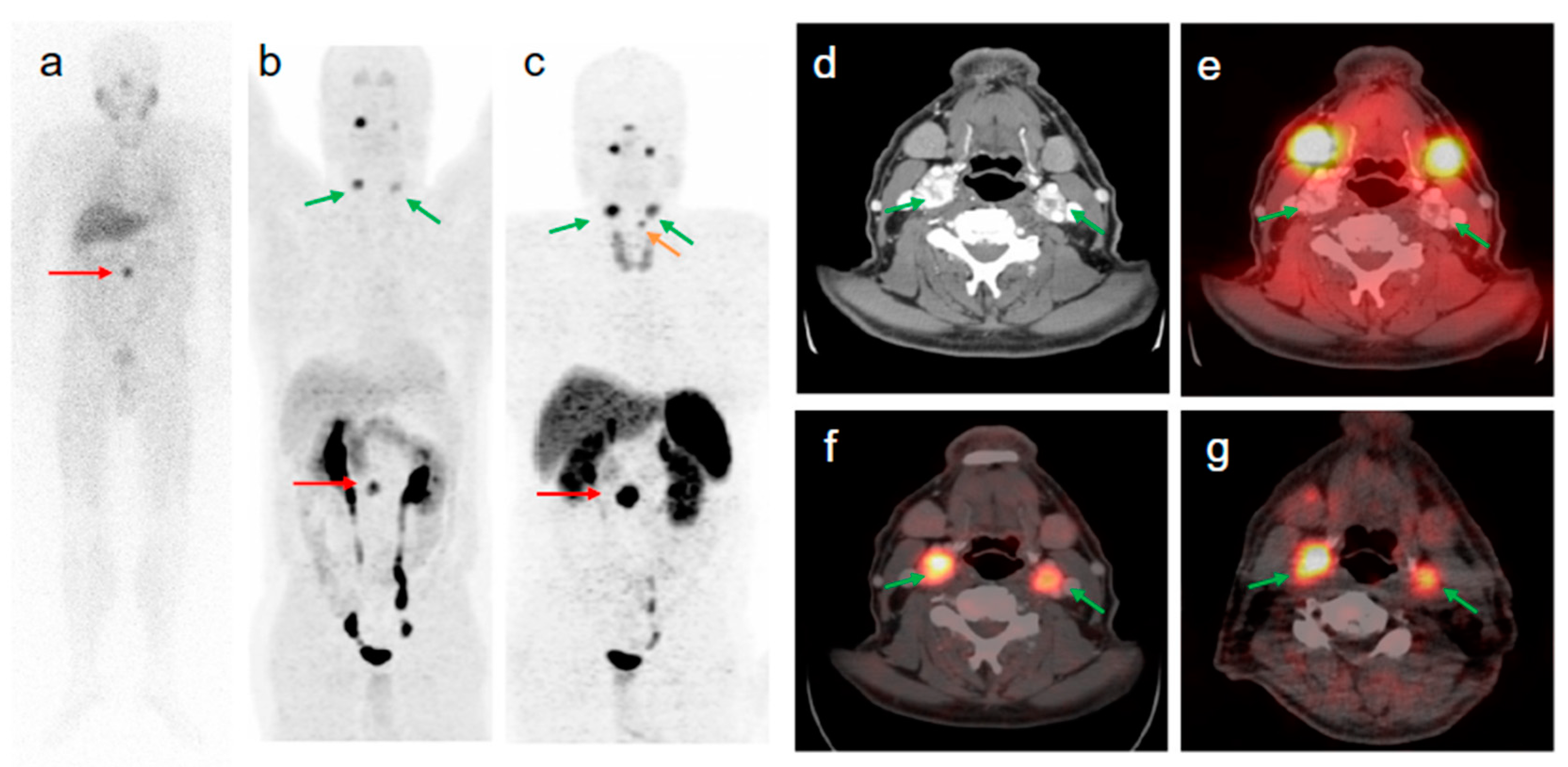
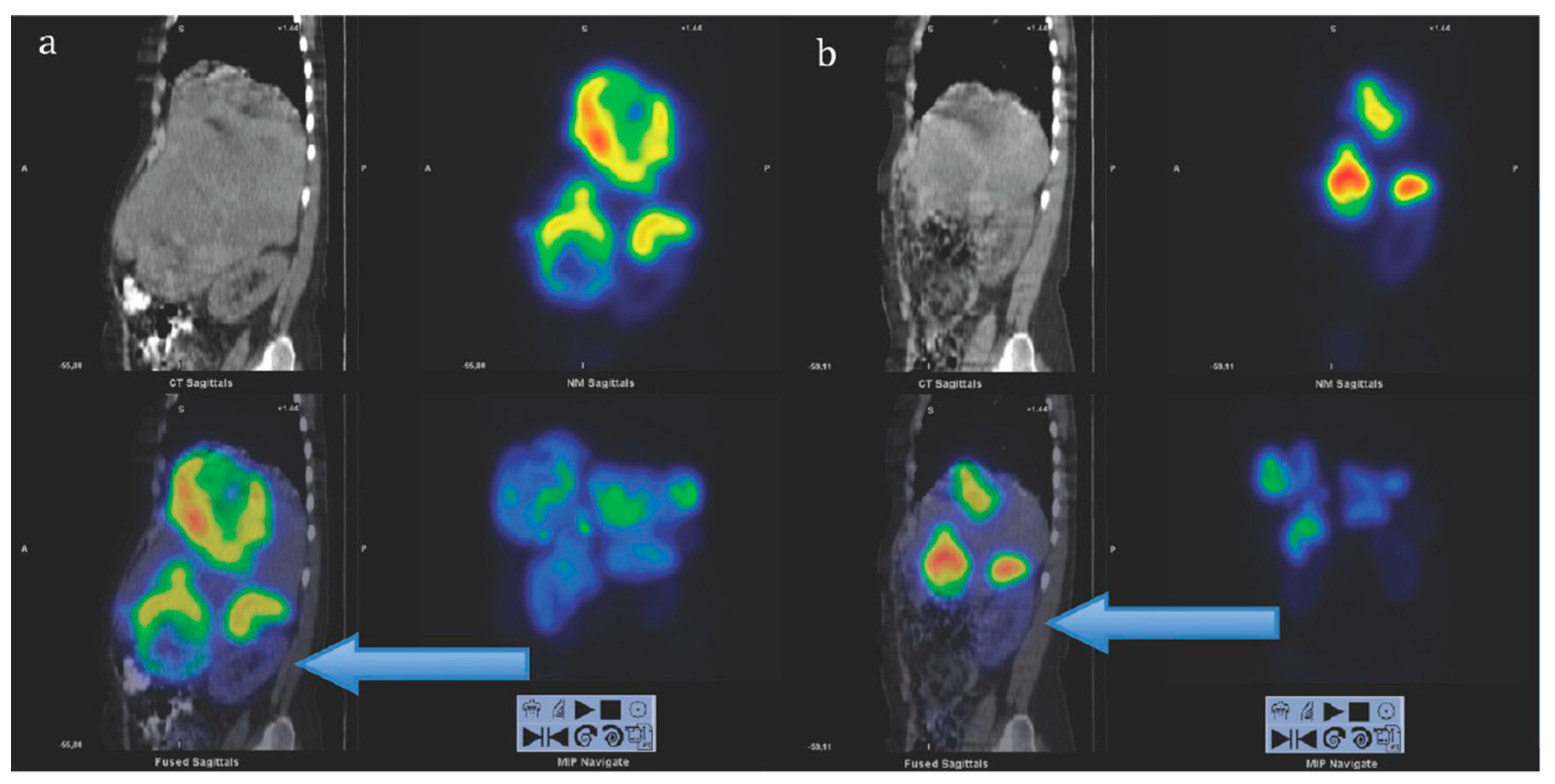
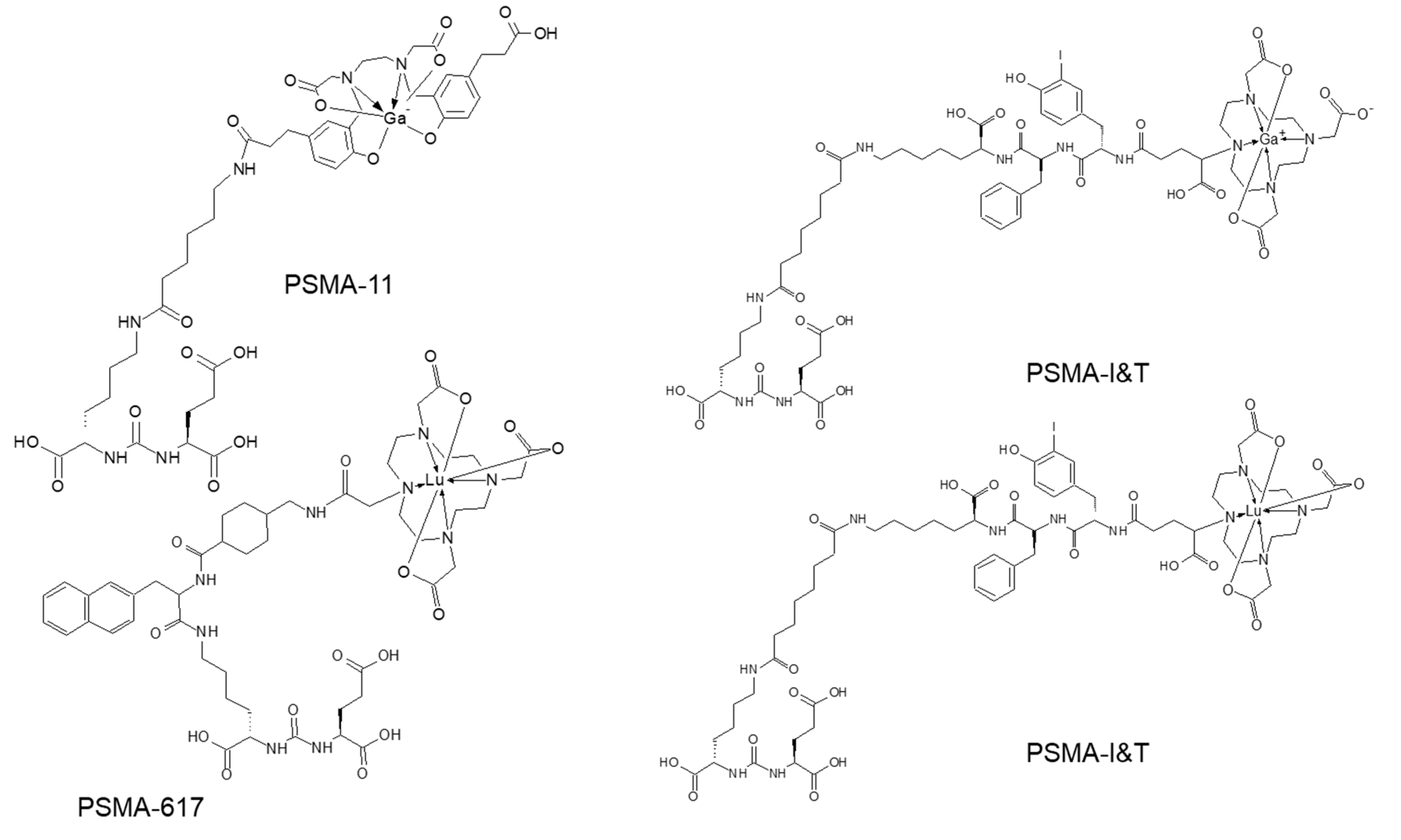
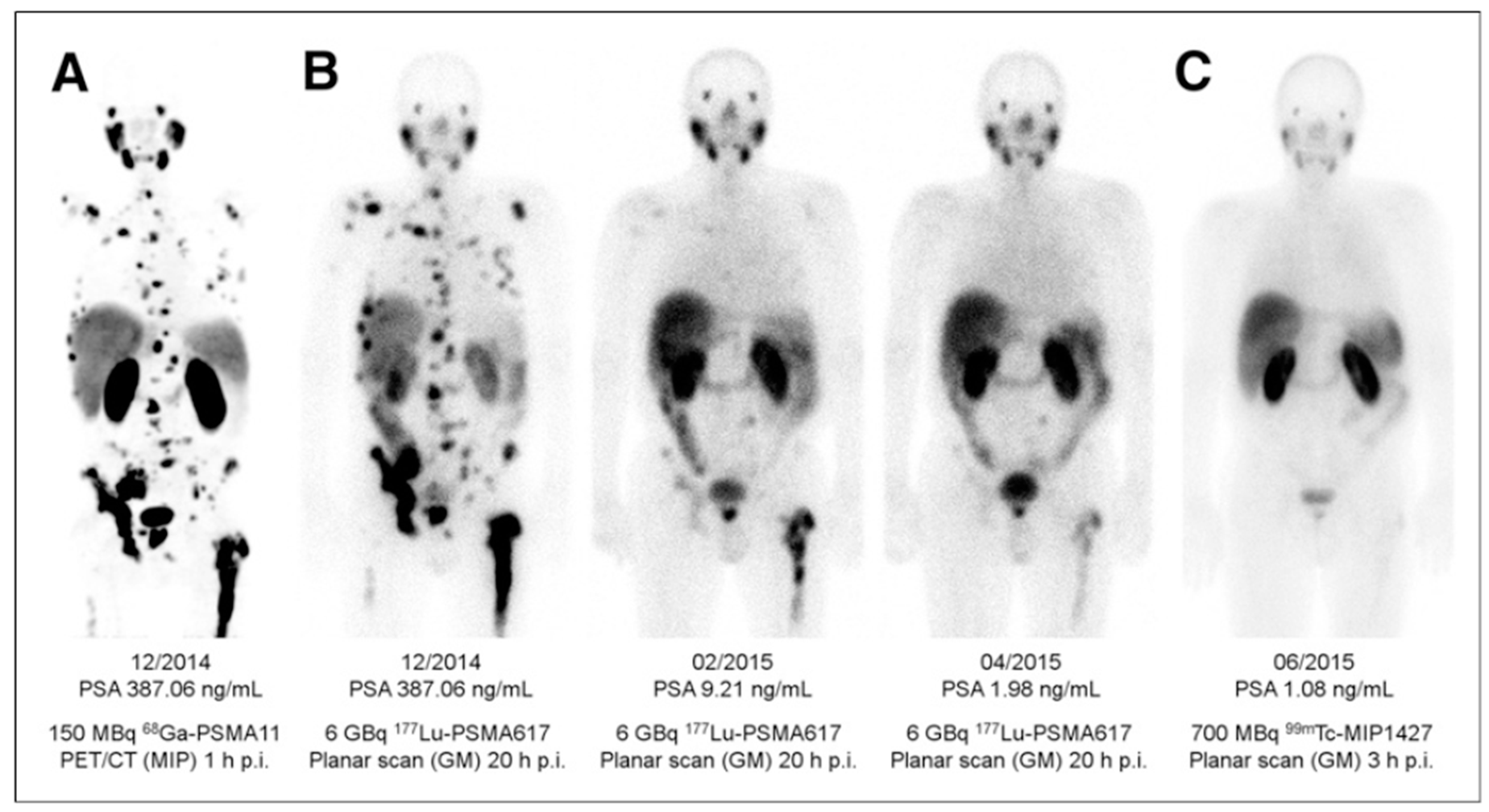
3. Targeting PSMA on Prostate Cancer
Impact of PSMA-Targeted Radiopharmaceuticals on Patient Treatment Management
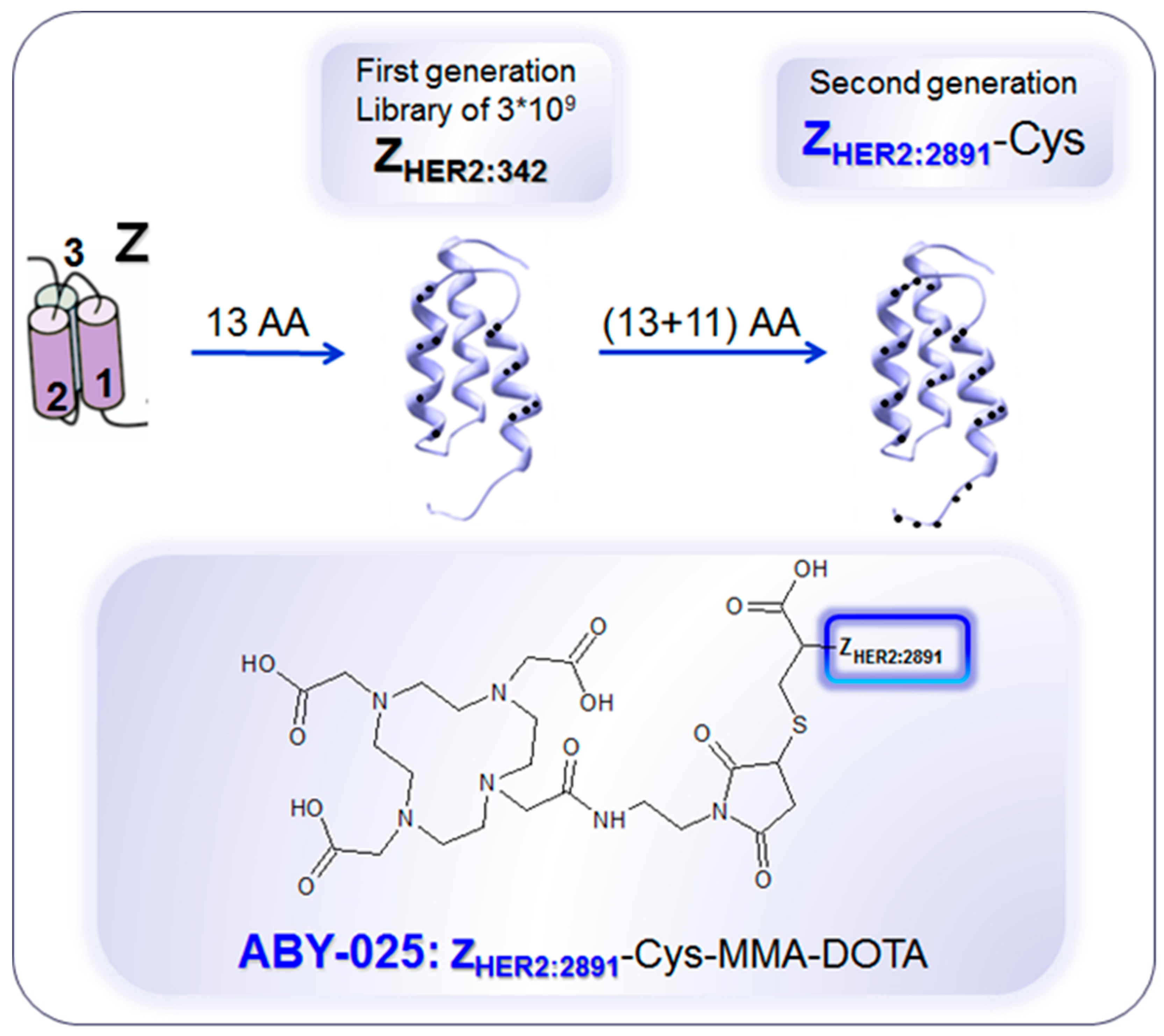
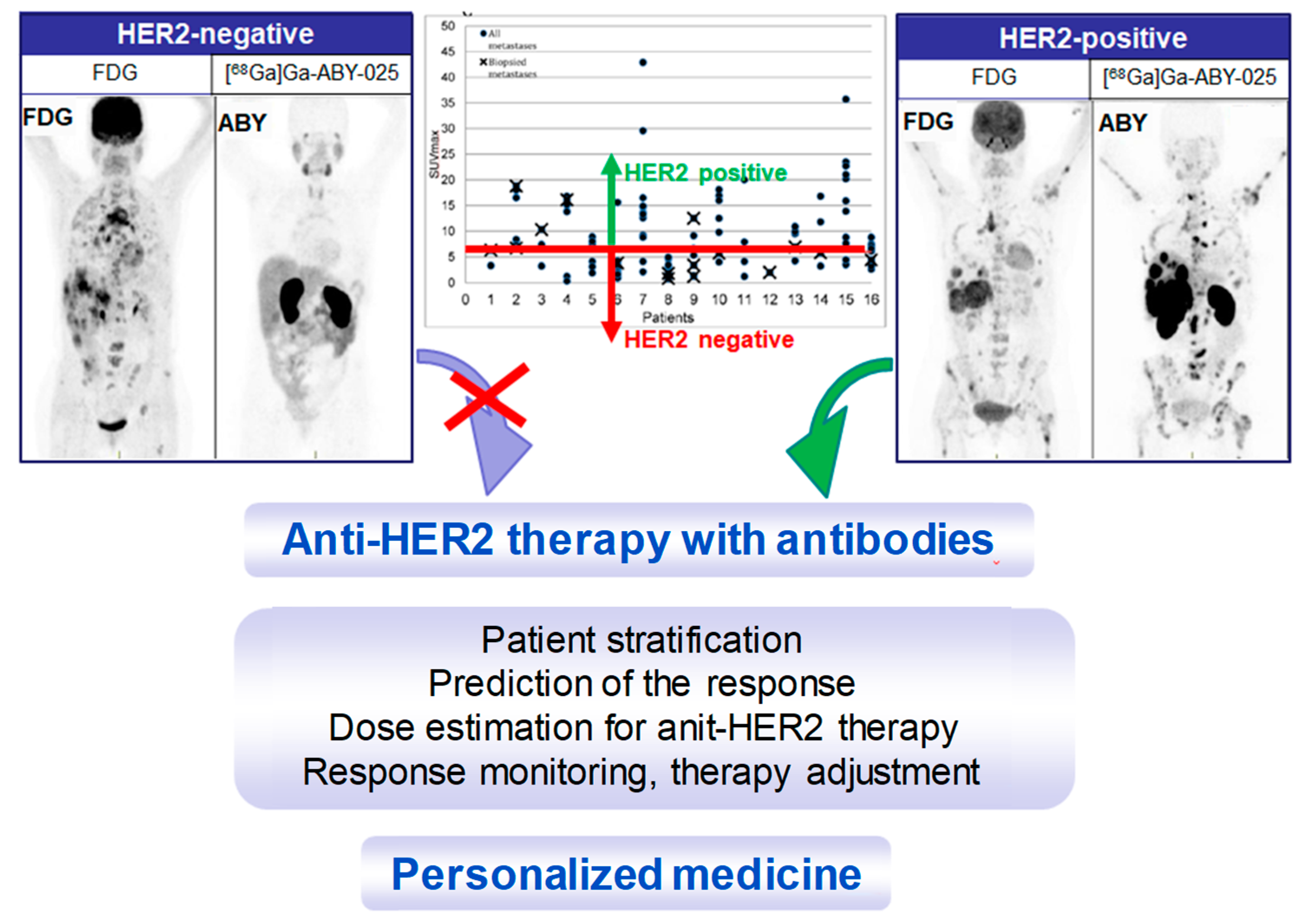
4. Targeting HER2 on Breast Cancer
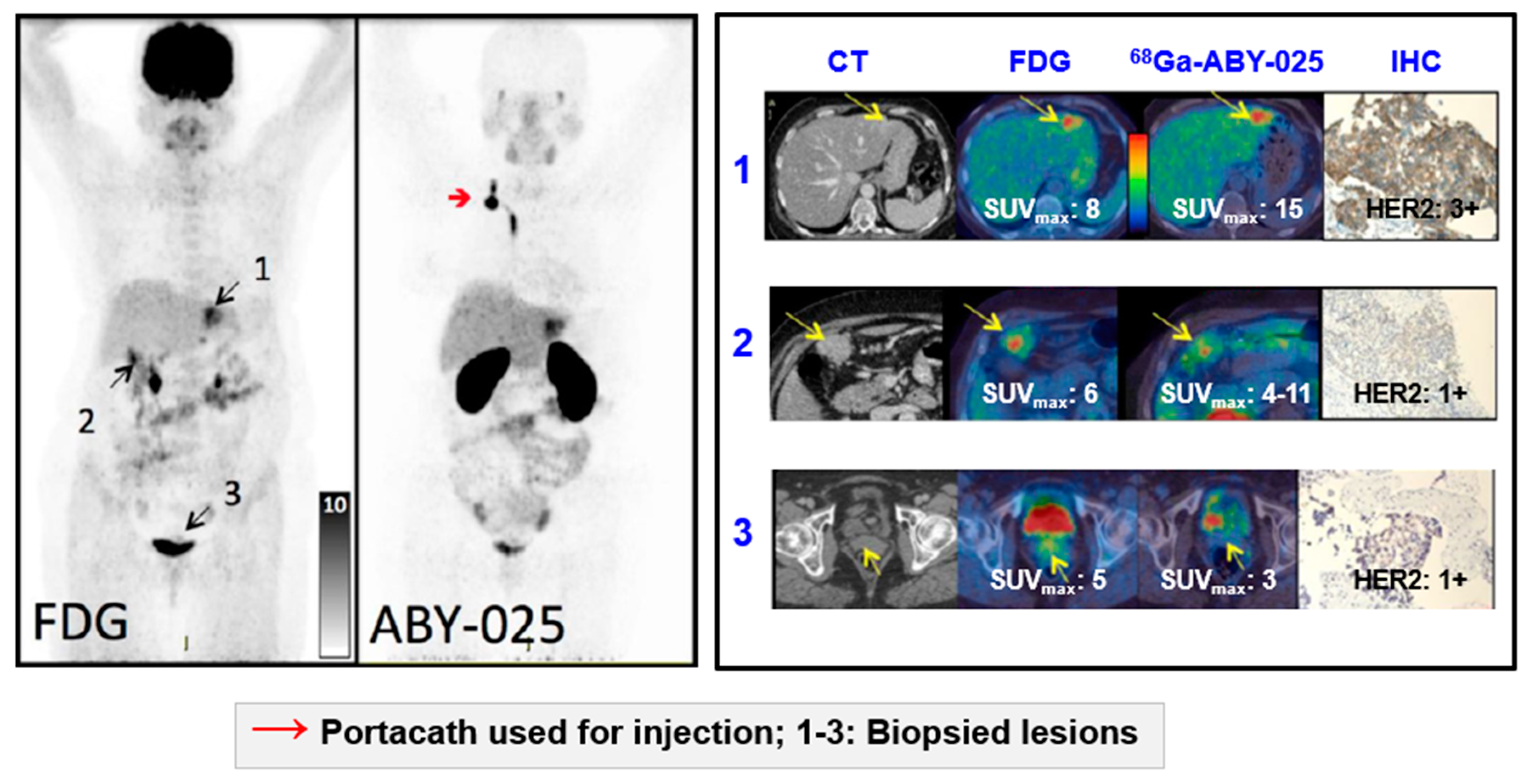
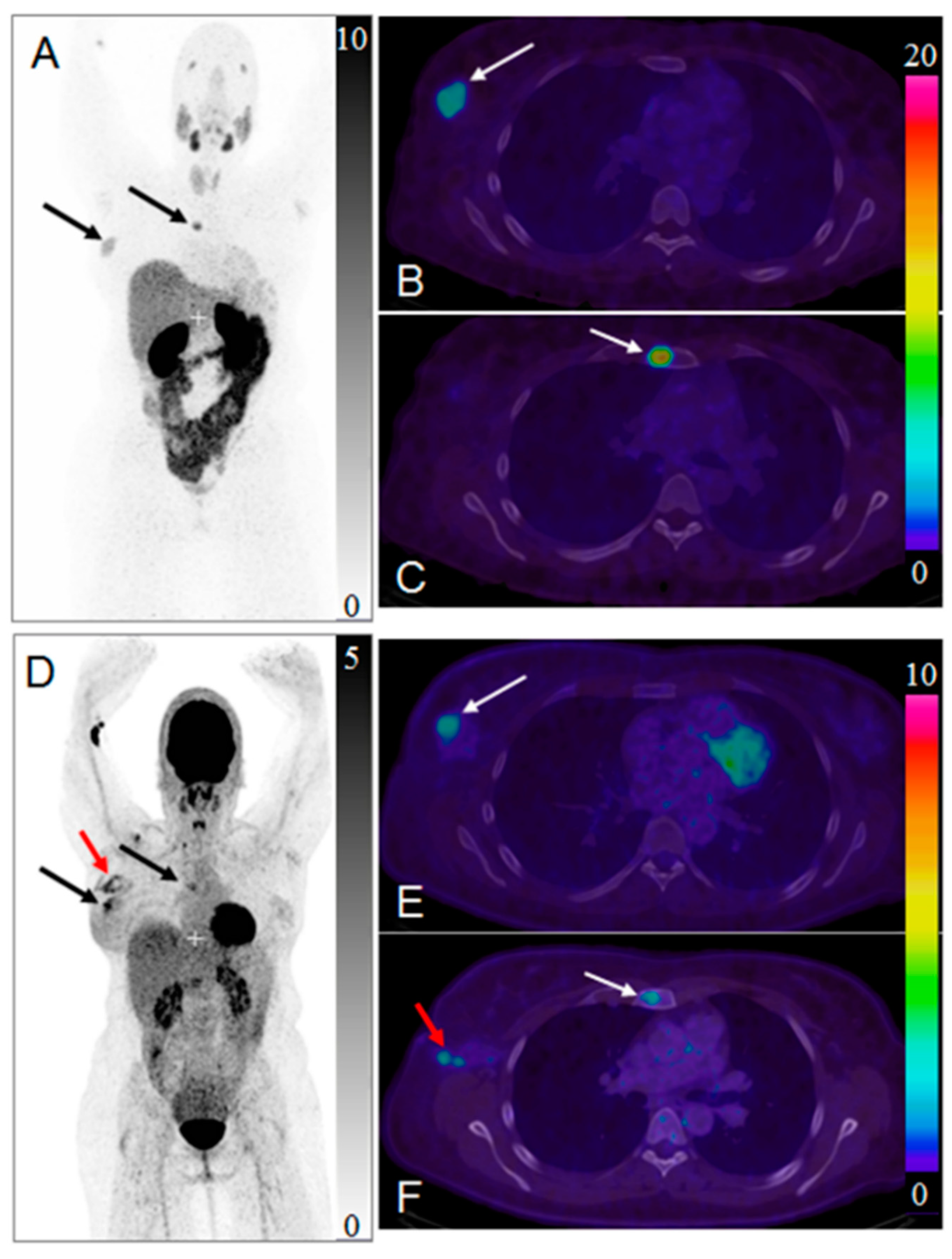
Impact of [68Ga]Ga-ABY-025 PET-CT on Patient Treatment Management
5. Conclusions
Funding
Conflicts of Interest
References
- Velikyan, I. Radionuclides for imaging and therapy in oncology. In Cancer Theranostics; Chen, X., Wong, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 285–325. [Google Scholar]
- Velikyan, I.; Sundin, A.; Eriksson, B.; Lundqvist, H.; Sorensen, J.; Bergstrom, M.; Langstrom, B. In vivo binding of [68ga]-Dotatoc to somatostatin receptors in neuroendocrine tumours--Impact of peptide mass. Nucl. Med. Biol. 2010, 37, 265–275. [Google Scholar] [CrossRef]
- Sorensen, J.; Velikyan, I.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Sandberg, D.; Nilsson, G.; Olofsson, H.; Sandstrom, M.; Lubberink, M.; et al. Measuring her2-Expression in metastatic breast cancer using 68ga-aby025 pet/ct. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, S226. [Google Scholar]
- Velikyan, I.; Wennborg, A.; Feldwisch, J.; Orlova, A.; Tolmachev, V.; Lubberink, M.; Sandstrom, M.; Lindman, H.; Carlsson, J.; Sorensen, J. Gmp compliant preparation of a 68gallium-Labeled affibody analogue for breast cancer patient examination: First-In-Man. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, S228–S229. [Google Scholar]
- Sörensen, J.; Velikyan, I.; Sandberg, D.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Orlova, A.; Sandström, M.; Lubberink, M.; Olofsson, H.; et al. Measuring her2-Receptor expression in metastatic breast cancer using [68ga]aby-025 affibody pet/ct. Theranostics 2016, 6, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Zidan, J.; Dashkovsky, I.; Stayerman, C.; Basher, W.; Cozacov, C.; Hadary, A. Comparison of her-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br. J. Cancer 2005, 93, 552–556. [Google Scholar] [CrossRef]
- Werner, R.A.; Thackeray, J.T.; Pomper, M.G.; Bengel, F.M.; Gorin, M.A.; Derlin, T.; Rowe, S.P. Recent updates on molecular imaging reporting and data systems (mi-rads) for theranostic radiotracers-Navigating pitfalls of sstr- and psma-Targeted pet/ct. J. Clin. Med. 2019, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I. 68ga-Based radiopharmaceuticals: Production and application relationship. Molecules 2015, 20, 12913–12943. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the united states. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Pencharz, D.; Gnanasegaran, G.; Navalkissoor, S. Theranostics in neuroendocrine tumours: Somatostatin receptor imaging and therapy. Br. J. Radiol. 2018, 91, 20180108. [Google Scholar] [CrossRef]
- Singh, S.; Poon, R.; Wong, R.; Metser, U. 68ga pet imaging in patients with neuroendocrine tumors: A systematic review and meta-Analysis. Clin. Nucl. Med. 2018, 43, 802–810. [Google Scholar] [CrossRef]
- Sorbye, H.; Kong, G.; Grozinsky-Glasberg, S. Prrt in high-Grade gastroenteropancreatic neuroendocrine neoplasms (who g3). Endocr. Relat. Cancer 2019. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Kunz, P.; Hobday, T.; Hendifar, A.; et al. Health-Related quality of life in patients with progressive midgut neuroendocrine tumors treated with (177)lu-Dotatate in the phase iii netter-1 trial. J. Clin. Oncol. 2018, 36, 2578–2584. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 trial of (177)lu-Dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, S.M.; Millo, C.; Neychev, V.; Aufforth, R.; Keutgen, X.; Glanville, J.; Alimchandani, M.; Nilubol, N.; Herscovitch, P.; Quezado, M.; et al. Feasibility of radio-Guided surgery with (6)(8)gallium-Dotatate in patients with gastro-Entero-Pancreatic neuroendocrine tumors. Ann. Surg. Oncol. 2015, 22 (Suppl. 3), S676–682. [Google Scholar] [CrossRef]
- El Lakis, M.; Gianakou, A.; Nockel, P.; Wiseman, D.; Tirosh, A.; Quezado, M.A.; Patel, D.; Nilubol, N.; Pacak, K.; Sadowski, S.M.; et al. Radioguided surgery with gallium 68 dotatate for patients with neuroendocrine tumors. JAMA Surg. 2019, 154, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Cherk, M.H.; Kong, G.; Hicks, R.J.; Hofman, M.S. Changes in biodistribution on (68)ga-Dota-Octreotate pet/ct after long acting somatostatin analogue therapy in neuroendocrine tumour patients may result in pseudoprogression. Cancer Imaging 2018, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I. Positron emitting [68ga]ga-Based imaging agents: Chemistry and diversity. Med. Chem. 2011, 7, 338–372. [Google Scholar] [CrossRef]
- Sabet, A.; Nagarajah, J.; Dogan, A.S.; Biersack, H.J.; Sabet, A.; Guhlke, S.; Ezziddin, S. Does prrt with standard activities of 177luoctreotate really achieve relevant somatostatin receptor saturation in target tumor lesions?: Insights from intra-therapeutic receptor imaging in patients with metastatic gastroenteropancreatic neuroendocrine tumors. EJNMMI Res. 2013, 3, 1–6. [Google Scholar]
- Sundin, A.; Arnold, R.; Baudin, E.; Cwikla, J.B.; Eriksson, B.; Fanti, S.; Fazio, N.; Giammarile, F.; Hicks, R.J.; Kjaer, A.; et al. Enets consensus guidelines for the standards of care in neuroendocrine tumors: Radiological, nuclear medicine & hybrid imaging. Neuroendocrinology 2017, 105, 212–244. [Google Scholar]
- Hope, T.A.; Bergsland, E.K.; Bozkurt, M.F.; Graham, M.; Heaney, A.P.; Herrmann, K.; Howe, J.R.; Kulke, M.H.; Kunz, P.L.; Mailman, J.; et al. Appropriate use criteria for somatostatin receptor pet imaging in neuroendocrine tumors. J. Nucl. Med. 2018, 59, 66–74. [Google Scholar] [CrossRef]
- Sollini, M.; Farioli, D.; Froio, A.; Chella, A.; Asti, M.; Boni, R.; Grassi, E.; Roncali, M.; Versari, A.; Erba, P.A. Brief report on the use of radiolabeled somatostatin analogs for the diagnosis and treatment of metastatic small-Cell lung cancer patients. J. Thorac. Oncol. 2013, 8, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, V.; Nanni, C.; Fanti, S. The use of gallium-68 labeled sstrs in pet/ct imaging. PET Clin. 2014, 9, 323–329. [Google Scholar] [CrossRef]
- Maffione, A.M.; Karunanithi, S.; Kumar, R.; Rubello, D.; Alavi, A. Nuclear medicine procedures in the diagnosis of net: A historical perspective. PET Clin. 2014, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.; Schwartz, L. Imaging of neuroendocrine tumors. Semin. Oncol. 2013, 40, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Balogova, S.; Talbot, J.N.; Nataf, V.; Michaud, L.; Huchet, V.; Kerrou, K.; Montravers, F. 18f-fluorodihydroxyphenylalanine vs other radiopharmaceuticals for imaging neuroendocrine tumours according to their type. Eur. J. Nucl. Med. Mol. Imaging 2013, 1–24. [Google Scholar] [CrossRef]
- Kroiss, A.; Putzer, D.; Frech, A.; Decristoforo, C.; Uprimny, C.; Gasser, R.W.; Shulkin, B.L.; Url, C.; Widmann, G.; Prommegger, R.; et al. A retrospective comparison between 68ga-Dota-Toc pet/ct and 18f-Dopa pet/ct in patients with extra-Adrenal paraganglioma. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1800–1808. [Google Scholar] [CrossRef]
- Yilmaz, S.; Ocak, M.; Asa, S.; Gülsen, F.; Halac, M.; Kabasakal, L. Appearance of intracranial meningioma in FDG and 68ga-DOTATOC PET/CT. Rev. Esp. Med. Nucl. Imagen Mol. 2013, 32, 60–61. [Google Scholar]
- Castaldi, P.; Treglia, G.; Rufini, V. Multifocal head and neck paraganglioma evaluated with different pet tracers: Comparison between fluorine-18-Fluorodeoxyglucose and gallium-68-Somatostatin receptor pet/ct. Nucl. Med. Mol. Imaging 2013, 47, 218–219. [Google Scholar] [CrossRef][Green Version]
- Epstude, M.; Tornquist, K.; Riklin, C.; di Lenardo, F.; Winterhalder, R.; Hug, U.; Strobel, K. Comparison of (18)f-fdg pet/ct and (68)ga-Dotatate pet/ct imaging in metastasized merkel cell carcinoma. Clin. Nucl. Med. 2013, 38, 283–284. [Google Scholar] [CrossRef]
- Venkitaraman, B.; Karunanithi, S.; Kumar, A.; Khilnani, G.C.; Kumar, R. Role of 68ga-Dotatoc pet/ct in initial evaluation of patients with suspected bronchopulmonary carcinoid. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 856–864. [Google Scholar] [CrossRef]
- Rufini, V.; Treglia, G.; Castaldi, P.; Perotti, G.; Giordano, A. Comparison of metaiodobenzylguanidine scintigraphy with positron emission tomography in the diagnostic work-up of pheochromocytoma and paraganglioma: A systematic review. Q. J. Nucl. Med. Mol. Imaging 2013, 57, 122–133. [Google Scholar] [PubMed]
- Damle, N.A.; Kumar, R.; Tripathi, M.; Bal, C. Positive (68)ga-Dotanoc pet/ct with negative (131)i-Metaiodobenzylguanidine scan in a case of glomus jugulare. Indian J. Endocrinol. Metab. 2013, 17, 765–767. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Thakar, A.; Suman, K.C.S.; Dhull, V.S.; Singh, H.; Naswa, N.; Reddy, R.M.; Karunanithi, S.; Kumar, R.; Malhotra, A.; et al. 68ga-Dotanoc pet/ct for baseline evaluation of patients with head and neck paraganglioma. J. Nucl. Med. 2013, 54, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Charron, M. Contemporary approach to diagnosis and treatment of neuroblastoma. Q. J. Nucl. Med. Mol. Imaging 2013, 57, 40–52. [Google Scholar] [PubMed]
- Treglia, G.; Inzani, F.; Campanini, N.; Rindi, G.; Agnes, S.; Giordano, A.; Rufini, V. A case of insulinoma detected by 68ga-Dotanoc pet/ct and missed by 18f-Dihydroxyphenylalanine pet/ct. Clin. Nucl. Med. 2013, 38, e267–e268. [Google Scholar] [CrossRef]
- Breer, S.; Brunkhorst, T.; Beil, F.T.; Peldschus, K.; Heiland, M.; Klutmann, S.; Barvencik, F.; Zustin, J.; Gratz, K.F.; Amling, M. 68ga dota-Tate pet/ct allows tumor localization in patients with tumor-Induced osteomalacia but negative 111in-Octreotide spect/ct. Bone 2014, 64, 222–227. [Google Scholar] [CrossRef]
- Jadhav, S.; Kasaliwal, R.; Lele, V.; Rangarajan, V.; Chandra, P.; Shah, H.; Malhotra, G.; Jagtap, V.S.; Budyal, S.; Lila, A.R.; et al. Functional imaging in primary tumour-Induced osteomalacia: Relative performance of fdg pet/ct vs somatostatin receptor-based functional scans: A series of nine patients. Clin. Endocrinol. 2014, 81, 31–37. [Google Scholar] [CrossRef]
- Gilardi, L.; Colandrea, M.; Fracassi, S.L.; Sansovini, M.; Paganelli, G. 68ga-dota0-tyr3octreotide (dotatoc) positron emission tomography (pet)/ct in five cases of ectopic adrenocorticotropin-Secreting tumours. Clin. Endocrinol. 2014, 81, 152–153. [Google Scholar] [CrossRef]
- Walker, R.C.; Smith, G.T.; Liu, E.; Moore, B.; Clanton, J.; Stabin, M. Measured human dosimetry of 68ga-Dotatate. J. Nucl. Med. 2013, 54, 855–860. [Google Scholar] [CrossRef]
- Toumpanakis, C.; Kim, M.K.; Rinke, A.; Bergestuen, D.S.; Thirlwell, C.; Khan, M.S.; Salazar, R.; Oberg, K. Combination of cross-Sectional and molecular imaging studies in the localization of gastroenteropancreatic neuroendocrine tumors. Neuroendocrinology 2014, 99, 63–74. [Google Scholar] [CrossRef]
- Gabriel, S.; Garrigue, P.; Dahan, L.; Castinetti, F.; Sebag, F.; Baumstark, K.; Archange, C.; Jha, A.; Pacak, K.; Guillet, B.; et al. Prospective evaluation of (68) ga-Dotatate pet/ct in limited disease neuroendocrine tumours and/or elevated serum neuroendocrine biomarkers. Clin. Endocrinol. (Oxf.) 2018, 82, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Kroiss, A.S.; Uprimny, C.; Shulkin, B.L.; Gruber, L.; Frech, A.; Url, C.; Riechelmann, H.; Sprinzl, G.M.; Thome, C.; Treglia, G.; et al. (68)ga-Dotatoc pet/ct in the localization of head and neck paraganglioma compared with (18)f-Dopa pet/ct and (123)i-mibg spect/ct. Nucl. Med. Biol. 2019, 71, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, B.; Manafi-Farid, R.; Eftekhari, M.; Fard-Esfahani, A.; Emami-Ardekani, A.; Geramifar, P.; Akhlaghi, M.; Hashemi Taheri, A.P.; Beiki, D. Diagnostic fficiency of (68)ga-Dotatate pet/ct as ompared to (99m)tc-Octreotide spect/ct andonventional orphologic odalities in euroendocrine umors. Asia Ocean J. Nucl. Med. Biol. 2019, 7, 129–140. [Google Scholar] [PubMed]
- Han, S.; Suh, C.H.; Woo, S.; Kim, Y.J.; Lee, J.J. Performance of (68)ga-Dota-Conjugated somatostatin receptor-Targeting peptide pet in detection of pheochromocytoma and paraganglioma: A systematic review and metaanalysis. J. Nucl. Med. 2019, 60, 369–376. [Google Scholar] [CrossRef]
- Naswa, N.; Sharma, P.; Gupta, S.K.; Karunanithi, S.; Reddy, R.M.; Patnecha, M.; Lata, S.; Kumar, R.; Malhotra, A.; Bal, C. Dual tracer functional imaging of gastroenteropancreatic neuroendocrine tumors using 68ga-dota-noc pet-ct and 18f-fdg pet-ct: Competitive or complimentary? Clin. Nucl. Med. 2014, 39, e27–e34. [Google Scholar] [CrossRef]
- Zhao, X.; Xiao, J.; Xing, B.; Wang, R.; Zhu, Z.; Li, F. Comparison of 68ga dotatate to 18f-fdg uptake is useful in the differentiation of residual or recurrent pituitary adenoma from the remaining pituitary tissue after transsphenoidal adenomectomy. Clin. Nucl. Med. 2014, 39, 605–608. [Google Scholar] [CrossRef]
- Lococo, F.; Treglia, G. Which is the best strategy for diagnosing bronchial carcinoid tumours? The role of dual tracer pet/ct scan. Hell. J. Nucl. Med. 2014, 17, 7–9. [Google Scholar]
- Lococo, F.; Cesario, A.; Paci, M.; Filice, A.; Versari, A.; Rapicetta, C.; Ricchetti, T.; Sgarbi, G.; Alifano, M.; Cavazza, A.; et al. Pet/ct assessment of neuroendocrine tumors of the lung with special emphasis on bronchial carcinoids. Tumor Biol. 2014, 35, 8369–8377. [Google Scholar] [CrossRef]
- Treglia, G.; Giovanella, L. Could 68ga-Somatostatin analogues replace other pet tracers in evaluating extra-Adrenal paragangliomas? Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1797–1799. [Google Scholar] [CrossRef]
- Hindie, E. The netpet score: Combining fdg and somatostatin receptor imaging for optimal management of patients with metastatic well-Differentiated neuroendocrine tumors. Theranostics 2017, 7, 1159–1163. [Google Scholar] [CrossRef]
- Ashwathanarayana, A.G.; Biswal, C.K.; Sood, A.; Parihar, A.S.; Kapoor, R.; Mittal, B.R. Imaging-Guided use of combined (177)lu-Dotatate and capecitabine therapy in metastatic mediastinal paraganglioma. J. Nucl. Med. Technol. 2017, 45, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Waseem, N.; Aparici, C.M.; Kunz, P.L. Evaluating the role of theranostics in grade 3 neuroendocrine neoplasms. J. Nucl. Med. 2019, 60, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kulkarni, H.R.; Singh, A.; Niepsch, K.; Muller, D.; Baum, R.P. Peptide receptor radionuclide therapy in grade 3 neuroendocrine neoplasms: Safety and survival analysis in 69 patients. J. Nucl. Med. 2019, 60, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Schraml, C.; Schwenzer, N.F.; Sperling, O.; Aschoff, P.; Lichy, M.P.; Uller, M.; Brendle, C.; Werner, M.K.; Claussen, C.D.; Pfannenberg, C. Staging of neuroendocrine tumours: Comparison of [68ga]dotatoc multiphase pet/ct and whole-Body mri. Cancer Imaging 2013, 13, 63–72. [Google Scholar] [CrossRef]
- Graf, R.; Nyuyki, F.; Steffen, I.G.; Michel, R.; Fahdt, D.; Wust, P.; Brenner, W.; Budach, V.; Wurm, R.; Plotkin, M. Contribution of 68ga-Dotatoc pet/ct to target volume delineation of skull base meningiomas treated with stereotactic radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 68–73. [Google Scholar] [CrossRef]
- Klinaki, I.; Al-Nahhas, A.; Soneji, N.; Win, Z. 68ga dota-Tate pet/ct uptake in spinal lesions and mri correlation on a patient with neuroendocrine tumor: Potential pitfalls. Clin. Nucl. Med. 2013, 38, e449–e453. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Ba-Ssalamah, A.; Weber, M.; Mitterhauser, M.; Eidherr, H.; Wadsak, W.; Raderer, M.; Trattnig, S.; Herneth, A.; Karanikas, G. Gadoxetate-Enhanced versus diffusion-Weighted mri for fused ga-68-Dotanoc pet/mri in patients with neuroendocrine tumours of the upper abdomen. Eur. Radiol. 2013, 23, 1978–1985. [Google Scholar] [CrossRef]
- Schmid-Tannwald, C.; Schmid-Tannwald, C.M.; Morelli, J.N.; Neumann, R.; Haug, A.R.; Jansen, N.; Nikolaou, K.; Schramm, N.; Reiser, M.F.; Rist, C. Comparison of abdominal mri with diffusion-Weighted imaging to 68ga-Dotatate pet/ct in detection of neuroendocrine tumors of the pancreas. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 897–907. [Google Scholar] [CrossRef]
- Wulfert, S.; Kratochwil, C.; Choyke, P.L.; Afshar-Oromieh, A.; Mier, W.; Kauczor, H.U.; Schenk, J.P.; Haberkorn, U.; Giesel, F.L. Multimodal imaging for early functional response assessment of 90y-/177lu-dotatoc peptide receptor targeted radiotherapy with dw-mri and 68ga-Dotatoc-pet/ct. Mol. Imaging Biol. 2014, 16, 586–594. [Google Scholar] [CrossRef]
- Gaertner, F.C.; Beer, A.J.; Souvatzoglou, M.; Eiber, M.; Furst, S.; Ziegler, S.I.; Brohl, F.; Schwaiger, M.; Scheidhauer, K. Evaluation of feasibility and image quality of 68ga-Dotatoc positron emission tomography/magnetic resonance in comparison with positron emission tomography/computed tomography in patients with neuroendocrine tumors. Invest. Radiol. 2013, 48, 263–272. [Google Scholar] [CrossRef]
- Wiesmüller, M.; Quick, H.H.; Navalpakkam, B.; Lell, M.M.; Uder, M.; Ritt, P.; Schmidt, D.; Beck, M.; Kuwert, T.; Von Gall, C.C. Comparison of lesion detection and quantitation of tracer uptake between pet from a simultaneously acquiring whole-Body pet/mr hybrid scanner and pet from pet/ct. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Beiderwellen, K.J.; Poeppel, T.D.; Hartung-Knemeyer, V.; Buchbender, C.; Kuehl, H.; Bockisch, A.; Lauenstein, T.C. Simultaneous 68ga-dotatoc pet/mri in patients with gastroenteropancreatic neuroendocrine tumors: Initial results. Invest. Radiol. 2013, 48, 273–279. [Google Scholar] [CrossRef]
- Al-Nabhani, K.Z.; Syed, R.; Michopoulou, S.; Alkalbani, J.; Afaq, A.; Panagiotidis, E.; O′Meara, C.; Groves, A.; Ell, P.; Bomanji, J. Qualitative and quantitative comparison of pet/ct and pet/mr imaging in clinical practice. J. Nucl. Med. 2014, 55, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Thorwarth, D.; Müller, A.C.; Pfannenberg, C.; Beyer, T. Combined PET/MR imaging using 68Ga-DOTATOC for radiotherapy treatment planning in meningioma patients. In Theranostics, Gallium-68, and Other Radionuclides; Recent Results in Cancer Research; Springer: Berlin/Heidelberg, Germany, 2013; Volume 194, pp. 425–439. [Google Scholar]
- Afaq, A.; Syed, R.; Bomanji, J. Pet/mri: A new technology in the field of molecular imaging. Br. Med. Bull. 2013, 108, 159–171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuyumcu, S.; Özkan, Z.G.; Sanli, Y.; Yilmaz, E.; Mudun, A.; Adalet, I.; Unal, S. Physiological and tumoral uptake of 68ga-Dotatate: Standardized uptake values and challenges in interpretation. Ann. Nucl. Med. 2013, 27, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Todorović-Tirnanić, M.V.; Gajić, M.M.; Obradović, V.B.; Baum, R.P. Gallium-68 dotatoc pet/ct in vivo characterization of somatostatin receptor expression in the prostate. Cancer Biother. Radiopharm. 2014, 29, 108–115. [Google Scholar] [CrossRef]
- Reindl, O.; Loidl, A.; Franz, B.; Hofer, J.F.; Pichler, R. Pitfall in follow-Up imaging of pancreatic neuroendocrine tumor by somatostatin receptor pet. Neuroendocrinol. Lett. 2013, 34, 273–274. [Google Scholar]
- Treglia, G.; Giovanella, L.; Muoio, B.; Caldarella, C. Splenosis mimicking relapse of a neuroendocrine tumor at gallium-68-Dotatoc pet/ct. Nucl. Med. Mol. Imaging 2014, 48, 163–165. [Google Scholar] [CrossRef]
- Kulkarni, H.R.; Prasad, V.; Kaemmerer, D.; Hommann, M.; Baum, R.P. High uptake of (68)ga-Dotatoc in spleen as compared to splenosis: Measurement by pet/ct. Recent Results Cancer Res. 2013, 194, 373–378. [Google Scholar]
- Calissendorff, J.; Sundin, A.; Falhammar, H. 68Ga-DOTA-TOC-PET/CT detects heart metastases from ileal neuroendocrine tumors. Endocrine 2013, 47, 169–176. [Google Scholar] [CrossRef]
- Mapelli, P.; Tam, H.H.; Sharma, R.; Aboagye, E.O.; Al-Nahhas, A. Frequency and significance of physiological versus pathological uptake of 68ga-dotatate in the pancreas: Validation with morphological imaging. Nucl. Med. Commun. 2014, 35, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Brogsitter, C.; Hofmockel, T.; Kotzerke, J. (68)ga dotatate uptake in vertebral hemangioma. Clin. Nucl. Med. 2014, 39, 462–463. [Google Scholar] [CrossRef]
- Sandström, M.; Velikyan, I.; Garske-Román, U.; Sörensen, J.; Eriksson, B.; Granberg, D.; Lundqvist, H.; Sundin, A.; Lubberink, M. Comparative biodistribution and radiation dosimetry of 68ga-Dotatoc and 68ga-Dotatate in patients with neuroendocrine tumors. J. Nucl. Med. 2013, 54, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Versari, A.; Sollini, M.; Frasoldati, A.; Fraternali, A.; Filice, A.; Froio, A.; Asti, M.; Fioroni, F.; Cremonini, N.; Putzer, D.; et al. Differentiated thyroid cancer: A new perspective with radiolabeled somatostatin analogues for imaging and treatment of patients. Thyroid 2014, 24, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Taïeb, D.; Varoquaux, A.; Chen, C.C.; Pacak, K. Current and future trends in the anatomical and functional imaging of head and neck paragangliomas. Semin. Nucl. Med. 2013, 43, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Blaickner, M.; Baum, R.P. Relevance of pet for pretherapeutic prediction of doses in peptide receptor radionuclide therapy. PET Clin. 2014, 9, 99–112. [Google Scholar] [CrossRef]
- Kulkarni, H.R.; Baum, R.P. Patient selection for personalized peptide receptor radionuclide therapy using ga-68 somatostatin receptor pet/ct. PET Clin. 2014, 9, 83–90. [Google Scholar] [CrossRef]
- Slavikova, K.; Montravers, F.; Treglia, G.; Kunikowska, J.; Kaliska, L.; Vereb, M.; Talbot, J.N.; Balogova, S. What is currently the best radiopharmaceutical for the hybrid pet/ct detection of recurrent medullary thyroid carcinoma? Curr. Radiopharm. 2013, 6, 96–105. [Google Scholar]
- Treglia, G.; Castaldi, P.; Villani, M.F.; Perotti, G.; Filice, A.; Ambrosini, V.; Cremonini, N.; Versari, A.; Fanti, S.; Giordano, A.; et al. Comparison of different positron emission tomography tracers in patients with recurrent medullary thyroid carcinoma: Our experience and a review of the literature. In Recent Results in Cancer Research; Springer: Berlin/Heidelberg, Germany, 2013; Volume 194, pp. 385–393. [Google Scholar]
- Haug, A.R.; Auernhammer, C.J.; Wangler, B.; Schmidt, G.P.; Uebleis, C.; Goke, B.; Cumming, P.; Bartenstein, P.; Tiling, R.; Hacker, M. 68ga-Dotatate pet/ct for the early prediction of response to somatostatin receptor-Mediated radionuclide therapy in patients with well-Differentiated neuroendocrine tumors. J. Nucl. Med. 2010, 51, 1349–1356. [Google Scholar] [CrossRef]
- Sainz-Esteban, A.; Prasad, V.; Schuchardt, C.; Zachert, C.; Carril, J.M.; Baum, R.P. Comparison of sequential planar 177lu-Dota-Tate dosimetry scans with 68ga-Dota-Tate pet/ct images in patients with metastasized neuroendocrine tumours undergoing peptide receptor radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 501–511. [Google Scholar] [CrossRef]
- Kratochwil, C.; Stefanova, M.; Mavriopoulou, E.; Holland-Letz, T.; Dimitrakopoulou-Strauss, A.; Afshar-Oromieh, A.; Mier, W.; Haberkorn, U.; Giesel, F.L. Suv of [68ga]dotatoc-pet/ct predicts response probability of prrt in neuroendocrine tumors. Mol. Imaging Biol. 2015, 17, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Kulkarni, H.R.; Singh, A.; Kaemmerer, D.; Mueller, D.; Prasad, V.; Hommann, M.; Robiller, F.C.; Niepsch, K.; Franz, H.; et al. Results and adverse events of personalized peptide receptor radionuclide therapy with (90)yttrium and (177)lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget 2018, 9, 16932–16950. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.R.; Cindea-Drimus, R.; Auernhammer, C.J.; Reincke, M.; Beuschlein, F.; Wangler, B.; Uebleis, C.; Schmidt, G.P.; Spitzweg, C.; Bartenstein, P.; et al. Neuroendocrine tumor recurrence: Diagnosis with 68ga-Dotatate pet/ct. Radiology 2014, 270, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Menda, Y.; Ponto, L.L.; Schultz, M.K.; Zamba, G.K.; Watkins, G.L.; Bushnell, D.L.; Madsen, M.T.; Sunderland, J.J.; Graham, M.M.; O′Dorisio, T.M.; et al. Repeatability of gallium-68 dotatoc positron emission tomographic imaging in neuroendocrine tumors. Pancreas 2013, 42, 937–943. [Google Scholar] [CrossRef]
- Kulkarni, H.R.; Baum, R.P. Theranostics with ga-68 somatostatin receptor pet/ct: Monitoring response to peptide receptor radionuclide therapy. PET Clin. 2014, 9, 91–97. [Google Scholar] [CrossRef]
- Giesel, F.L.; Stefanova, M.; Schwartz, L.H.; Afshar-Oromieh, A.; Eisenhut, M.; Haberkorn, U.; Kratochwil, C. Impact of peptide receptor radionuclide therapy on the 68ga-Dotatoc-pet/ct uptake in normal tissue. Q. J. Nucl. Med. Mol. Imaging 2013, 57, 171–176. [Google Scholar]
- Combs, S.E.; Welzel, T.; Habermehl, D.; Rieken, S.; Dittmar, J.O.; Kessel, K.; Jäkel, O.; Haberkorn, U.; Debus, J. Prospective evaluation of early treatment outcome in patients with meningiomas treated with particle therapy based on target volume definition with mri and 68ga-dotatoc-pet. Acta Oncol. 2013, 52, 514–520. [Google Scholar] [CrossRef]
- Jois, B.; Asopa, R.; Basu, S. Somatostatin receptor imaging in non-131i-Avid metastatic differentiated thyroid carcinoma for determining the feasibility of peptide receptor radionuclide therapy with 177lu-Dotatate: Low fraction of patients suitable for peptide receptor radionuclide therapy and evidence of chromogranin a level-Positive neuroendocrine differentiation. Clin. Nucl. Med. 2014, 39, 505–510. [Google Scholar]
- Oksuz, M.O.; Winter, L.; Pfannenberg, C.; Reischl, G.; Mussig, K.; Bares, R.; Dittmann, H. Peptide receptor radionuclide therapy of neuroendocrine tumors with (90)y-Dotatoc: Is treatment response predictable by pre-Therapeutic uptake of (68)ga-Dotatoc? Diagn. Interv. Imaging 2014, 95, 289–300. [Google Scholar] [CrossRef]
- Kroiss, A.; Putzer, D.; Decristoforo, C.; Uprimny, C.; Warwitz, B.; Nilica, B.; Gabriel, M.; Kendler, D.; Waitz, D.; Widmann, G.; et al. 68ga-dota-toc uptake in neuroendocrine tumour and healthy tissue: Differentiation of physiological uptake and pathological processes in pet/ct. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 514–523. [Google Scholar] [CrossRef]
- Ruf, J.; Schiefer, J.; Kropf, S.; Furth, C.; Ulrich, G.; Kosiek, O.; Denecke, T.; Pavel, M.; Pascher, A.; Wiedenmann, B.; et al. Quantification in ga-68-dota(0)-phe(1)-tyr(3)-Octreotide positron emission tomography/computed tomography: Can we be impartial about partial volume effects? Neuroendocrinology 2013, 97, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Kim, Y.I. Prognostic value of maximum standardized uptake value in 68ga-Somatostatin receptor positron emission tomography for neuroendocrine tumors: A systematic review and meta-Analysis. Clin. Nucl. Med. 2019, 44, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Ito, T.; Jensen, R.T. Imaging of pancreatic neuroendocrine tumors: Recent advances, current status, and controversies. Expert Rev. Anticancer Ther. 2018, 18, 837–860. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, A.; Kebebew, E. The utility of (68)ga-Dotatate positron-Emission tomography/computed tomography in the diagnosis, management, follow-Up and prognosis of neuroendocrine tumors. Future Oncol. 2018, 14, 111–122. [Google Scholar] [CrossRef]
- Yu, J.; Li, N.; Li, J.; Lu, M.; Leal, J.P.; Tan, H.; Su, H.; Fan, Y.; Zhang, Y.; Zhao, W.; et al. The correlation between [(68)ga]dotatate pet/ct and cell proliferation in patients with gep-nens. Mol. Imaging Biol. 2019, 21, 984–990. [Google Scholar] [CrossRef]
- Chan, H.; Moseley, C.; Zhang, L.; Bergsland, E.K.; Pampaloni, M.H.; Van Loon, K.; Hope, T.A. Correlation of dotatoc uptake and pathologic grade in neuroendocrine tumors. Pancreas 2019, 48, 948–952. [Google Scholar] [CrossRef]
- Velikyan, I.; Sundin, A.; Sörensen, J.; Lubberink, M.; Sandström, M.; Garske-Román, U.; Lundqvist, H.; Granberg, D.; Eriksson, B. Quantitative and qualitative intrapatient comparison of 68ga-Dotatoc and 68ga-Dotatate: Net uptake rate for accurate quantification. J. Nucl. Med. 2014, 55, 204–210. [Google Scholar] [CrossRef]
- Ilan, E.; Velikyan, I.; Sandstrom, M.; Sundin, A.; Lubberink, M. Tumor-To-Blood ratio for assessment of somatostatin receptor density in neuroendocrine tumors using (68)ga-Dotatoc and (68)ga-Dotatate. J. Nucl. Med. 2019, 61, 217–221. [Google Scholar] [CrossRef]
- Ohnona, J.; Nataf, V.; Gauthe, M.; Balogova, S.; Belissant Benesty, O.; Zhang-Yin, J.; Talbot, J.N.; Montravers, F. Prognostic value of functional tumor burden on 68ga-Dotatoc pet/ct in patients with pancreatic neuro-Endocrine tumors. Neoplasma 2019, 66, 140–148. [Google Scholar] [CrossRef]
- Toriihara, A.; Baratto, L.; Nobashi, T.; Park, S.; Hatami, N.; Davidzon, G.; Kunz, P.L.; Iagaru, A. Prognostic value of somatostatin receptor expressing tumor volume calculated from (68)ga-dotatate pet/ct in patients with well-Differentiated neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2244–2251. [Google Scholar] [CrossRef]
- Graf, J.; Pape, U.F.; Jann, H.; Denecke, T.; Arsenic, R.; Brenner, W.; Pavel, M.; Prasad, V. Prognostic significance of somatostatin receptor heterogeneity in progressive neuroendocrine tumor treated with lu-177 dotatoc or lu-177 dotatate. Eur. J. Nucl. Med. Mol. Imaging 2019. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Ilhan, H.; Lehner, S.; Papp, L.; Zsoter, N.; Schatka, I.; Muegge, D.O.; Javadi, M.S.; Higuchi, T.; Buck, A.K.; et al. Pre-Therapy somatostatin receptor-Based heterogeneity predicts overall survival in pancreatic neuroendocrine tumor patients undergoing peptide receptor radionuclide therapy. Mol. Imaging Biol. 2019, 21, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Coura-Filho, G.B.; Hoff, A.; Duarte, P.S.; Buchpiguel, C.A.; Josefsson, A.; Hobbs, R.F.; Sgouros, G.; Sapienza, M.T. 68ga-Dotatate pet: Temporal variation of maximum standardized uptake value in normal tissues and neuroendocrine tumours. Nucl. Med. Commun. 2019, 40, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I. Continued rapid growth in ga applications: Update 2013 to june 2014. J. Labelled. Comp. Radiopharm. 2015, 99–121. [Google Scholar] [CrossRef] [PubMed]
- Paquet, M.; Gauthe, M.; Zhang Yin, J.; Nataf, V.; Belissant, O.; Orcel, P.; Roux, C.; Talbot, J.N.; Montravers, F. Diagnostic performance and impact on patient management of (68)ga-dota-toc pet/ct for detecting osteomalacia-Associated tumours. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Barrio, M.; Czernin, J.; Fanti, S.; Ambrosini, V.; Binse, I.; Du, L.; Eiber, M.; Herrmann, K.; Fendler, W.P. The impact of somatostatin receptor-Directed pet/ct on the management of patients with neuroendocrine tumor: A systematic review and meta-Analysis. J. Nucl. Med. 2017, 58, 756–761. [Google Scholar] [CrossRef]
- Herrmann, K.; Czernin, J.; Wolin, E.M.; Gupta, P.; Barrio, M.; Gutierrez, A.; Schiepers, C.; Mosessian, S.; Phelps, M.E.; Allen-Auerbach, M.S. Impact of 68ga-Dotatate pet/ct on the management of neuroendocrine tumors: The referring physician’s perspective. J. Nucl. Med. 2015, 56, 70–75. [Google Scholar] [CrossRef]
- Calais, J.; Czernin, J.; Eiber, M.; Fendler, W.P.; Gartmann, J.; Heaney, A.P.; Hendifar, A.E.; Pisegna, J.R.; Hecht, J.R.; Wolin, E.M.; et al. Most of the intended management changes after (68)ga-Dotatate pet/ct are implemented. J. Nucl. Med. 2017, 58, 1793–1796. [Google Scholar] [CrossRef]
- Crown, A.; Rocha, F.G.; Raghu, P.; Lin, B.; Funk, G.; Alseidi, A.; Hubka, M.; Rosales, J.; Lee, M.; Kennecke, H. Impact of initial imaging with gallium-68 dotatate pet/ct on diagnosis and management of patients with neuroendocrine tumors. J. Surg. Oncol. 2019, 121, 480–485. [Google Scholar] [CrossRef]
- Skoura, E.; Priftakis, D.; Novruzov, F.; Caplin, M.E.; Gnanasegaran, G.; Navalkissoor, S.; Bomanji, J. The impact of ga-68 dotatate pet/ct imaging on management of patients with paragangliomas. Nucl. Med. Commun. 2019, 41, 169–174. [Google Scholar] [CrossRef]
- Kong, G.; Hicks, R.J. Peptide receptor radiotherapy: Current approaches and future directions. Curr. Treat. Options Oncol. 2019, 20, 77. [Google Scholar] [CrossRef] [PubMed]
- Garske-Roman, U.; Sandstrom, M.; Fross Baron, K.; Lundin, L.; Hellman, P.; Welin, S.; Johansson, S.; Khan, T.; Lundqvist, H.; Eriksson, B.; et al. Prospective observational study of (177)lu-Dota-Octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (nets): Feasibility and impact of a dosimetry-Guided study protocol on outcome and toxicity. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 970–988. [Google Scholar] [CrossRef] [PubMed]
- Garske, U.; Sandstrom, M.; Johansson, S.; Granberg, D.; Lundqvist, H.; Lubberink, M.; Sundin, A.; Eriksson, B. Lessons on tumour response: Imaging during therapy with (177)lu-Dota-Octreotate. A case report on a patient with a large volume of poorly differentiated neuroendocrine carcinoma. Theranostics 2012, 2, 459–471. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mease, R.C.; Foss, C.A.; Pomper, M.G. Pet imaging in prostate cancer: Focus on prostate-specific membrane antigen. Curr. Top. Med. Chem. 2013, 13, 951–962. [Google Scholar] [CrossRef]
- Kopka, K.; Benesova, M.; Barinka, C.; Haberkorn, U.; Babich, J. Glu-Ureido-Based inhibitors of prostate-Specific membrane antigen: Lessons learned during the development of a novel class of low-Molecular-Weight theranostic radiotracers. J. Nucl. Med. 2017, 58, 17S–26S. [Google Scholar] [CrossRef]
- Eder, M.; Eisenhut, M.; Babich, J.; Haberkorn, U. Psma as a target for radiolabelled small molecules. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 819–823. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschik, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. Pet imaging with a [68ga]gallium-labelled psma ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495. [Google Scholar] [CrossRef]
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68ga-psma-11 pet accuracy in localizing recurrent prostate cancer: A prospective single-arm clinical trial. JAMA Oncol 2019, 5, 856–863. [Google Scholar] [CrossRef]
- Bouchelouche, K.; Choyke, P.L. Prostate-specific membrane antigen positron emission tomography in prostate cancer: A step toward personalized medicine. Curr. Opin. Oncol. 2016, 28, 216–221. [Google Scholar] [CrossRef]
- Bouchelouche, K.; Choyke, P.L. Advances in prostate-specific membrane antigen pet of prostate cancer. Curr. Opin. Oncol. 2018, 30, 189–196. [Google Scholar] [CrossRef]
- Alipour, R.; Azad, A.; Hofman, M.S. Guiding management of therapy in prostate cancer: Time to switch from conventional imaging to psma pet? Ther. Adv. Med. Oncol. 2019, 11, 1758835919876828. [Google Scholar] [CrossRef] [PubMed]
- Eissa, A.; Elsherbiny, A.; Coelho, R.F.; Rassweiler, J.; Davis, J.W.; Porpiglia, F.; Patel, V.R.; Prandini, N.; Micali, S.; Sighinolfi, M.C.; et al. The role of 68ga-psma pet/ct scan in biochemical recurrence after primary treatment for prostate cancer: A systematic review of the literature. Minerva Urol. Nefrol. 2018, 70, 462–478. [Google Scholar] [CrossRef] [PubMed]
- Lenzo, N.P.; Meyrick, D.; Turner, J.H. Review of gallium-68 psma pet/ct imaging in the management of prostate cancer. Diagnostics (Basel) 2018, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Maurer, T.; Weirich, G.; Schottelius, M.; Weineisen, M.; Frisch, B.; Okur, A.; Kubler, H.; Thalgott, M.; Navab, N.; Schwaiger, M.; et al. Prostate-Specific membrane antigen-radioguided surgery for metastatic lymph nodes in prostate cancer. Eur. Urol. 2015, 68, 530–534. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 prostate-Specific membrane antigen positron emission tomography in advanced prostate cancer-Updated diagnostic utility, sensitivity, specificity, and distribution of prostate-Specific membrane antigen-Avid lesions: A systematic review and meta-Analysis. Eur. Urol. 2019. [Google Scholar] [CrossRef]
- Yaxley, J.W.; Raveenthiran, S.; Nouhaud, F.X.; Samaratunga, H.; Yaxley, W.J.; Coughlin, G.; Yaxley, A.J.; Gianduzzo, T.; Kua, B.; McEwan, L.; et al. Risk of metastatic disease on (68) gallium-Prostate-Specific membrane antigen positron emission tomography/computed tomography scan for primary staging of 1253 men at the diagnosis of prostate cancer. BJU Int. 2019, 124, 401–407. [Google Scholar] [CrossRef] [PubMed]
- De Visschere, P.J.L.; Standaert, C.; Futterer, J.J.; Villeirs, G.M.; Panebianco, V.; Walz, J.; Maurer, T.; Hadaschik, B.A.; Lecouvet, F.E.; Giannarini, G.; et al. A systematic review on the role of imaging in early recurrent prostate cancer. Eur. Urol. Oncol. 2019, 2, 47–76. [Google Scholar] [CrossRef]
- Barber, T.W.; Singh, A.; Kulkarni, H.R.; Niepsch, K.; Billah, B.; Baum, R.P. Clinical outcomes of (177)lu-Psma radioligand therapy in earlier and later phases of metastatic castration-resistant prostate cancer grouped by previous taxane chemotherapy. J. Nucl. Med. 2019, 60, 955–962. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benešová, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. Psma-Targeted radionuclide therapy of metastatic castration-Resistant prostate cancer with 177lu-Labeled psma-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef]
- Habl, G.; Sauter, K.; Schiller, K.; Dewes, S.; Maurer, T.; Eiber, M.; Combs, S.E. (68) ga-psma-pet for radiation treatment planning in prostate cancer recurrences after surgery: Individualized medicine or new standard in salvage treatment. Prostate 2017, 77, 920–927. [Google Scholar] [CrossRef]
- Woythal, N.; Arsenic, R.; Kempkensteffen, C.; Miller, K.; Janssen, J.-C.; Huang, K.; Makowski, M.R.; Brenner, W.; Prasad, V. Immunohistochemical validation of psma expression measured by 68ga-psma pet/ct in primary prostate cancer. J. Nucl. Med. 2018, 59, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Kabasakal, L.; Demirci, E.; Ocak, M.; Akyel, R.; Nematyazar, J.; Aygun, A.; Halac, M.; Talat, Z.; Araman, A. Evaluation of psma pet/ct imaging using a 68ga-hbed-cc ligand in patients with prostate cancer and the value of early pelvic imaging. Nucl. Med. Commun. 2015, 36, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Woo, S.; Kim, Y.J.; Suh, C.H. Impact of (68)ga-psma pet on the management of patients with prostate cancer: A systematic review and meta-Analysis. Eur. Urol. 2018, 74, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.; Amit, U.; Saad, A.; Hahiashvili, M.; Goshen, E.; Portnoy, O.; Berger, R.; Goldstein, A.; Sadetsky, I.; Weizman, N.; et al. Gallium-68 prostate-specific membrane antigen pet-ct and the clinical management of prostate cancer. Nucl. Med. Commun. 2019, 40, 913–919. [Google Scholar] [CrossRef]
- Ekmekcioglu, O.; Busstra, M.; Klass, N.D.; Verzijlbergen, F. Bridging the imaging gap: Psma pet/ct has a high impact on treatment planning in prostate cancer patients with biochemical recurrence-a narrative review of the literature. J. Nucl. Med. 2019, 60, 1394–1398. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, D.A.; Garcia Schuler, H.I.; Muehlematter, U.J.; Eberli, D.; Muller, J.; Muller, A.; Gablinger, R.; Kranzbuhler, H.; Omlin, A.; Kaufmann, P.A.; et al. Impact of (68)ga-psma-11 pet staging on clinical decision-Making in patients with intermediate or high-Risk prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 47, 652–664. [Google Scholar] [CrossRef]
- Schmidt-Hegemann, N.S.; Eze, C.; Li, M.; Rogowski, P.; Schaefer, C.; Stief, C.; Buchner, A.; Zamboglou, C.; Fendler, W.P.; Ganswindt, U.; et al. Impact of (68)ga-psma pet/ct on the radiotherapeutic approach to prostate cancer in comparison to ct: A retrospective analysis. J. Nucl. Med. 2019, 60, 963–970. [Google Scholar] [CrossRef]
- Rousseau, C.; Le Thiec, M.; Ferrer, L.; Rusu, D.; Rauscher, A.; Maucherat, B.; Frindel, M.; Baumgartner, P.; Fleury, V.; Denis, A.; et al. Preliminary results of a (68) ga-psma pet/ct prospective study in prostate cancer patients with occult recurrence: Diagnostic performance and impact on therapeutic decision-Making. Prostate 2019, 79, 1514–1522. [Google Scholar] [CrossRef]
- Hoffmann, M.A.; Wieler, H.J.; Baues, C.; Kuntz, N.J.; Richardsen, I.; Schreckenberger, M. The impact of 68ga-psma pet/ct and pet/mri on the management of prostate cancer. Urology 2019, 130, 1–12. [Google Scholar] [CrossRef]
- Barbaud, M.; Frindel, M.; Ferrer, L.; Le Thiec, M.; Rusu, D.; Rauscher, A.; Maucherat, B.; Baumgartner, P.; Fleury, V.; Colombie, M.; et al. 68ga-psma-11 pet-ct study in prostate cancer patients with biochemical recurrence and non-Contributive 18f-choline pet-ct: Impact on therapeutic decision-Making and biomarker changes. Prostate 2019, 79, 454–461. [Google Scholar] [CrossRef]
- Farolfi, A.; Ceci, F.; Castellucci, P.; Graziani, T.; Siepe, G.; Lambertini, A.; Schiavina, R.; Lodi, F.; Morganti, A.G.; Fanti, S. (68)ga-psma-11 pet/ct in prostate cancer patients with biochemical recurrence after radical prostatectomy and psa <0.5 ng/ml. Efficacy and impact on treatment strategy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 11–19. [Google Scholar] [PubMed]
- Roach, P.J.; Francis, R.; Emmett, L.; Hsiao, E.; Kneebone, A.; Hruby, G.; Eade, T.; Nguyen, Q.A.; Thompson, B.D.; Cusick, T.; et al. The impact of (68)ga-psma pet/ct on management intent in prostate cancer: Results of an australian prospective multicenter study. J. Nucl. Med. 2018, 59, 82–88. [Google Scholar] [CrossRef]
- Emmett, L.; Crumbaker, M.; Ho, B.; Willowson, K.; Eu, P.; Ratnayake, L.; Epstein, R.; Blanksby, A.; Horvath, L.; Guminski, A.; et al. Results of a prospective phase 2 pilot trial of (177)lu-psma-617 therapy for metastatic castration-Resistant prostate cancer including imaging predictors of treatment response and patterns of progression. Clin. Genitourin. Cancer 2019, 17, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Haberkorn, U.; Kopka, K.; Giesel, F.; Kratochwil, C. Future trends in prostate cancer theranostics with psma ligands. Clin. Transl. Imaging 2016, 4, 487–489. [Google Scholar] [CrossRef][Green Version]
- van Leeuwen, P.J.; Donswijk, M.; Nandurkar, R.; Stricker, P.; Ho, B.; Heijmink, S.; Wit, E.M.K.; Tillier, C.; van Muilenkom, E.; Nguyen, Q.; et al. Gallium-68-Prostate-Specific membrane antigen ((68) ga-psma) positron emission tomography (pet)/computed tomography (ct) predicts complete biochemical response from radical prostatectomy and lymph node dissection in intermediate- and high-Risk prostate cancer. BJU Int. 2019, 124, 62–68. [Google Scholar] [PubMed]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [(177)lu]-psma-617 radionuclide treatment in patients with metastatic castration-Resistant prostate cancer (lupsma trial): A single-Centre, single-Arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Kabasakal, L.; AbuQbeitah, M.; Aygun, A.; Yeyin, N.; Ocak, M.; Demirci, E.; Toklu, T. Pre-Therapeutic dosimetry of normal organs and tissues of (177)lu-psma-617 prostate-Specific membrane antigen (psma) inhibitor in patients with castration-Resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1976–1983. [Google Scholar] [CrossRef]
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassmann, M.; Klette, I.; Eiber, M.; et al. 68ga- and 177lu-Labeled psma i&t: Optimization of a psma-Targeted theranostic concept and first proof-Of-Concept human studies. J. Nucl. Med. 2015, 56, 1169–1176. [Google Scholar]
- Heck, M.M.; Tauber, R.; Schwaiger, S.; Retz, M.; D′Alessandria, C.; Maurer, T.; Gafita, A.; Wester, H.J.; Gschwend, J.E.; Weber, W.A.; et al. Treatment outcome, toxicity, and predictive factors for radioligand therapy with (177)lu-psma-i&t in metastatic castration-Resistant prostate cancer. Eur. Urol. 2019, 75, 920–926. [Google Scholar]
- von Eyben, F.E.; Roviello, G.; Kiljunen, T.; Uprimny, C.; Virgolini, I.; Kairemo, K.; Joensuu, T. Third-Line treatment and (177)lu-psma radioligand therapy of metastatic castration-Resistant prostate cancer: A systematic review. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 496–508. [Google Scholar] [CrossRef]
- Virgolini, I.; Decristoforo, C.; Haug, A.; Fanti, S.; Uprimny, C. Current status of theranostics in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 471–495. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Thieme, A.; Allmann, J.; D′Alessandria, C.; Maurer, T.; Retz, M.; Tauber, R.; Heck, M.M.; Wester, H.J.; Tamaki, N.; et al. Radiation dosimetry for (177)lu-psma i&t in metastatic castration-resistant prostate cancer: Absorbed dose in normal organs and tumor lesions. J. Nucl. Med. 2017, 58, 445–450. [Google Scholar] [PubMed]
- Scarpa, L.; Buxbaum, S.; Kendler, D.; Fink, K.; Bektic, J.; Gruber, L.; Decristoforo, C.; Uprimny, C.; Lukas, P.; Horninger, W.; et al. The (68)ga/(177)lu theragnostic concept in psma targeting of castration-Resistant prostate cancer: Correlation of suvmax values and absorbed dose estimates. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Kulkarni, H.R.; Schuchardt, C.; Singh, A.; Wirtz, M.; Wiessalla, S.; Schottelius, M.; Mueller, D.; Klette, I.; Wester, H.J. 177lu-Labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-Resistant prostate cancer: Safety and efficacy. J. Nucl. Med. 2016, 57, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Delgado Bolton, R.C.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. Eanm procedure guidelines for radionuclide therapy with (177)lu-Labelled psma-Ligands ((177)lu-psma-rlt). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Derks, Y.H.W.; Lowik, D.; Sedelaar, J.P.M.; Gotthardt, M.; Boerman, O.C.; Rijpkema, M.; Lutje, S.; Heskamp, S. Psma-Targeting agents for radio- and fluorescence-Guided prostate cancer surgery. Theranostics 2019, 9, 6824–6839. [Google Scholar] [CrossRef]
- Maurer, T.; Gschwend, J.E.; Eiber, M. Prostate-Specific membrane antigen-guided salvage lymph node dissection in recurrent prostate cancer: A novel technology to detect lymph node metastases. Curr. Opin. Urol. 2018, 28, 191–196. [Google Scholar] [CrossRef]
- Cortes, J.; Fumoleau, P.; Bianchi, G.V.; Petrella, T.M.; Gelmon, K.; Pivot, X.; Verma, S.; Albanell, J.; Conte, P.; Lluch, A.; et al. Pertuzumab monotherapy after trastuzumab-Based treatment and subsequent reintroduction of trastuzumab: Activity and tolerability in patients with advanced human epidermal growth factor receptor 2-Positive breast cancer. J. Clin. Oncol. 2012, 30, 1594–1600. [Google Scholar] [CrossRef]
- Houssami, N.; Macaskill, P.; Balleine, R.L.; Bilous, M.; Pegram, M.D. Her2 discordance between primary breast cancer and its paired metastasis: Tumor biology or test artefact? Insights through meta-Analysis. Breast Cancer Res. Treat. 2011, 129, 659–674. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Dieras, V.; Guardino, E.; et al. Trastuzumab emtansine for her2-Positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. The erbb/her family of protein-Tyrosine kinases and cancer. Pharmacol. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef] [PubMed]
- Citri, A.; Yarden, Y. Egf-erbb signalling: Towards the systems level. Nat. Rev. Mol. Cell Biol. 2006, 7, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Natali, P.G.; Nicotra, M.R.; Bigotti, A.; Venturo, I.; Slamon, D.J.; Fendly, B.M.; Ullrich, A. Expression of the p185 encoded by her2 oncogene in normal and transformed human tissues. Int. J. Cancer 1990, 45, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against her2 for metastatic breast cancer that overexpresses her2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Nahta, R.; Esteva, F.J. Trastuzumab: Triumphs and tribulations. Oncogene 2007, 26, 3637–3643. [Google Scholar] [CrossRef]
- Molina, R.; Barak, V.; van Dalen, A.; Duffy, M.J.; Einarsson, R.; Gion, M.; Goike, H.; Lamerz, R.; Nap, M.; Soletormos, G.; et al. Tumor markers in breast cancer-European group on tumor markers recommendations. Tumour. Biol. 2005, 26, 281–293. [Google Scholar] [CrossRef]
- Viani, G.A.; Afonso, S.L.; Stefano, E.J.; De Fendi, L.I.; Soares, F.V. Adjuvant trastuzumab in the treatment of her-2-Positive early breast cancer: A meta-Analysis of published randomized trials. BMC Cancer 2007, 7, 153. [Google Scholar] [CrossRef]
- Karlsson, E.; Sandelin, K.; Appelgren, J.; Zhou, W.; Jirstrom, K.; Bergh, J.; Warnberg, F. Clonal alteration of breast cancer receptors between primary ductal carcinoma in situ (dcis) and corresponding local events. Eur. J. Cancer 2014, 50, 517–524. [Google Scholar] [CrossRef]
- Lindstrom, L.S.; Karlsson, E.; Wilking, U.M.; Johansson, U.; Hartman, J.; Lidbrink, E.K.; Hatschek, T.; Skoog, L.; Bergh, J. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J. Clin. Oncol. 2012, 30, 2601–2608. [Google Scholar] [CrossRef]
- Corcoran, E.B.; Hanson, R.N. Imaging egfr and her2 by pet and spect: A review. Med. Res. Rev. 2014, 34, 596–643. [Google Scholar] [CrossRef]
- Perik, P.J.; Lub-De Hooge, M.N.; Gietema, J.A.; van der Graaf, W.T.; de Korte, M.A.; Jonkman, S.; Kosterink, J.G.; van Veldhuisen, D.J.; Sleijfer, D.T.; Jager, P.L.; et al. Indium-111-Labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-Positive metastatic breast cancer. J. Clin. Oncol. 2006, 24, 2276–2282. [Google Scholar] [CrossRef] [PubMed]
- Dijkers, E.C.; Oude Munnink, T.H.; Kosterink, J.G.; Brouwers, A.H.; Jager, P.L.; de Jong, J.R.; van Dongen, G.A.; Schroder, C.P.; Lub-de Hooge, M.N.; de Vries, E.G. Biodistribution of 89zr-Trastuzumab and pet imaging of her2-Positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 2010, 87, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Beylergil, V.; Morris, P.G.; Smith-Jones, P.M.; Modi, S.; Solit, D.; Hudis, C.A.; Lu, Y.; O′Donoghue, J.; Lyashchenko, S.K.; Carrasquillo, J.A.; et al. Pilot study of 68ga-dota-f(ab’)2-Trastuzumab in patients with breast cancer. Nucl. Med. Commun. 2013, 34, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, B.; Moks, T.; Jansson, B.; Abrahmsen, L.; Elmblad, A.; Holmgren, E.; Henrichson, C.; Jones, T.A.; Uhlen, M. A synthetic igg-binding domain based on staphylococcal protein a. Protein Eng. 1987, 1, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Nord, K.; Nilsson, J.; Nilsson, B.; Uhlen, M.; Nygren, P.A. A combinatorial library of an alpha-Helical bacterial receptor domain. Protein Eng. 1995, 8, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Nord, K.; Gunneriusson, E.; Ringdahl, J.; Stahl, S.; Uhlen, M.; Nygren, P.A. Binding proteins selected from combinatorial libraries of an alpha-Helical bacterial receptor domain. Nat. Biotechnol. 1997, 15, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Feldwisch, J.; Tolmachev, V.; Lendel, C.; Herne, N.; Sjoberg, A.; Larsson, B.; Rosik, D.; Lindqvist, E.; Fant, G.; Hoiden-Guthenberg, I.; et al. Design of an optimized scaffold for affibody molecules. J. Mol. Biol. 2010, 398, 232–247. [Google Scholar] [CrossRef]
- Ahlgren, S.; Orlova, A.; Wallberg, H.; Hansson, M.; Sandstrom, M.; Lewsley, R.; Wennborg, A.; Abrahmsen, L.; Tolmachev, V.; Feldwisch, J. Targeting of her2-Expressing tumors using 111in-aby-025, a second-Generation affibody molecule with a fundamentally reengineered scaffold. J. Nucl. Med. 2010, 51, 1131–1138. [Google Scholar] [CrossRef]
- Eigenbrot, C.; Ultsch, M.; Dubnovitsky, A.; Abrahmsen, L.; Hard, T. Structural basis for high-Affinity her2 receptor binding by an engineered protein. Proc. Natl. Acad. Sci. USA 2010, 107, 15039–15044. [Google Scholar] [CrossRef]
- Velikyan, I.; Wennborg, A.; Feldwisch, J.; Lindman, H.; Carlsson, J.; Sorensen, J. Good manufacturing practice production of [(68)ga]ga-aby-025 for her2 specific breast cancer imaging. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 135–153. [Google Scholar]
- Velikyan, I.; Schweighofer, P.; Feldwisch, J.; Seemann, J.; Frejd, F.Y.; Lindman, H.; Sorensen, J. Diagnostic her2-binding radiopharmaceutical, ga-68 ga-aby-025, for routine clinical use in breast cancer patients. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 12–23. [Google Scholar] [PubMed]
- Sorensen, J.; Sandberg, D.; Sandstrom, M.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Astrom, G.; Lubberink, M.; Garske-Roman, U.; Carlsson, J.; et al. First-In-Human molecular imaging of her2 expression in breast cancer metastases using the 111in-aby-025 affibody molecule. J. Nucl. Med. 2014, 55, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, D.; Tolmachev, V.; Velikyan, I.; Olofsson, H.; Wennborg, A.; Feldwisch, J.; Carlsson, J.; Lindman, H.; Sorensen, J. Intra-Image referencing for simplified assessment of her2-Expression in breast cancer metastases using the affibody molecule aby-025 with pet and spect. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Sandstrom, M.; Lindskog, K.; Velikyan, I.; Wennborg, A.; Feldwisch, J.; Sandberg, D.; Tolmachev, V.; Orlova, A.; Sorensen, J.; Carlsson, J.; et al. Biodistribution and radiation dosimetry of the anti-her2 affibody molecule 68ga-aby-025 in breast cancer patients. J. Nucl. Med. 2016, 57, 867–871. [Google Scholar] [CrossRef]
- Schrijver, W.; Suijkerbuijk, K.P.M.; van Gils, C.H.; van der Wall, E.; Moelans, C.B.; van Diest, P.J. Receptor conversion in distant breast cancer metastases: A systematic review and meta-Analysis. J. Natl. Cancer Inst. 2018, 110, 568–580. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velikyan, I. (Radio)Theranostic Patient Management in Oncology Exemplified by Neuroendocrine Neoplasms, Prostate Cancer, and Breast Cancer. Pharmaceuticals 2020, 13, 39. https://doi.org/10.3390/ph13030039
Velikyan I. (Radio)Theranostic Patient Management in Oncology Exemplified by Neuroendocrine Neoplasms, Prostate Cancer, and Breast Cancer. Pharmaceuticals. 2020; 13(3):39. https://doi.org/10.3390/ph13030039
Chicago/Turabian StyleVelikyan, Irina. 2020. "(Radio)Theranostic Patient Management in Oncology Exemplified by Neuroendocrine Neoplasms, Prostate Cancer, and Breast Cancer" Pharmaceuticals 13, no. 3: 39. https://doi.org/10.3390/ph13030039
APA StyleVelikyan, I. (2020). (Radio)Theranostic Patient Management in Oncology Exemplified by Neuroendocrine Neoplasms, Prostate Cancer, and Breast Cancer. Pharmaceuticals, 13(3), 39. https://doi.org/10.3390/ph13030039




