Synthesis of Imidazole-Based Medicinal Molecules Utilizing the van Leusen Imidazole Synthesis
Abstract
1. Introduction
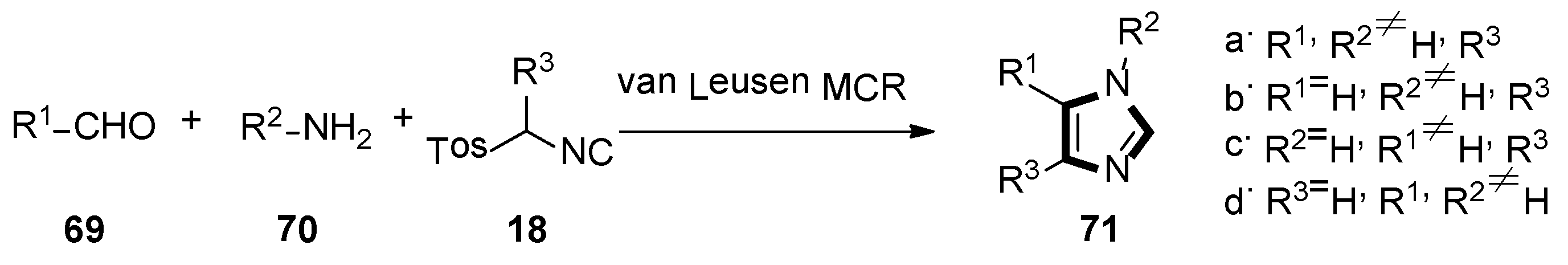
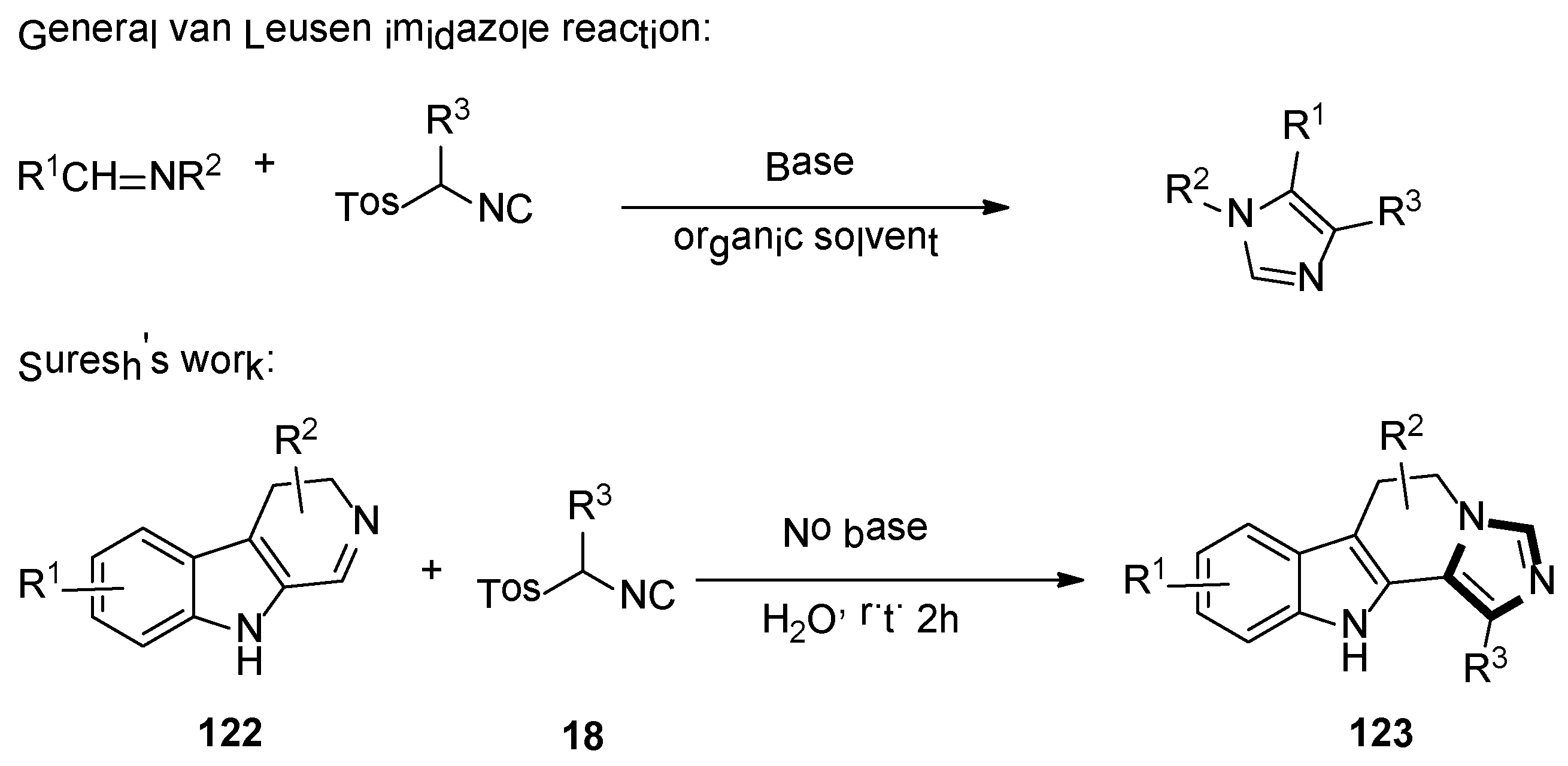
2. General van Leusen Imidazole Synthesis
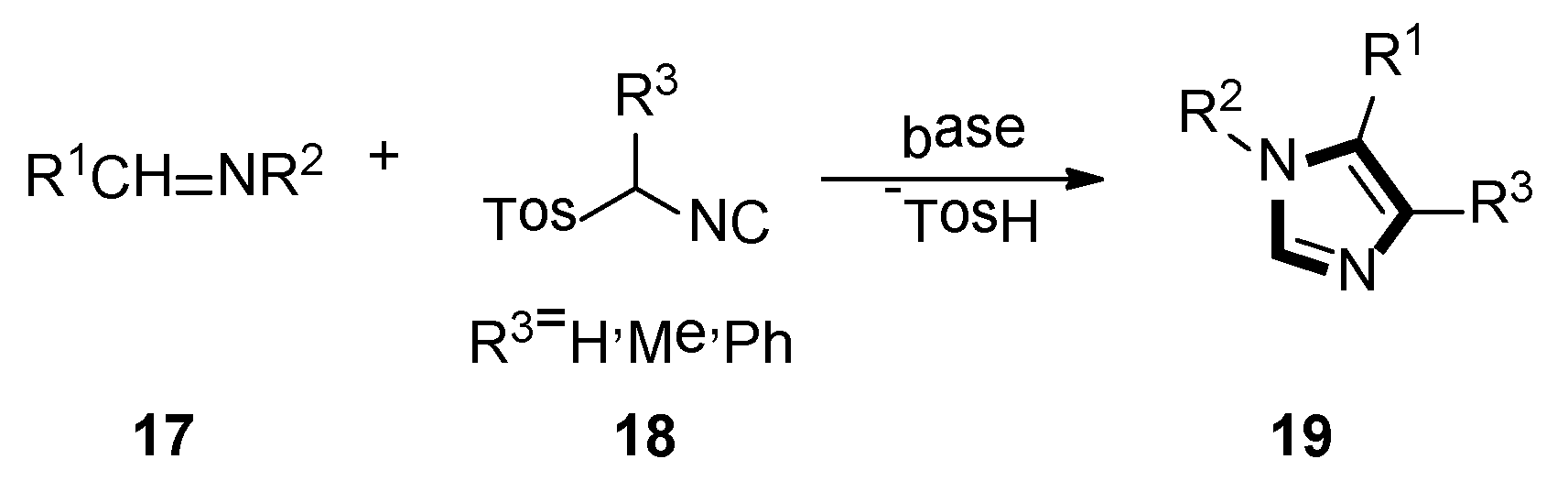
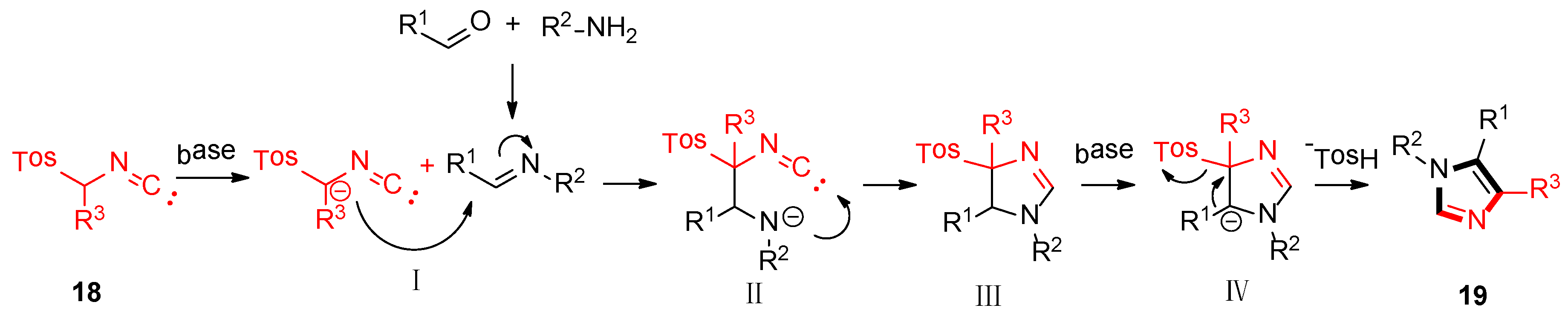
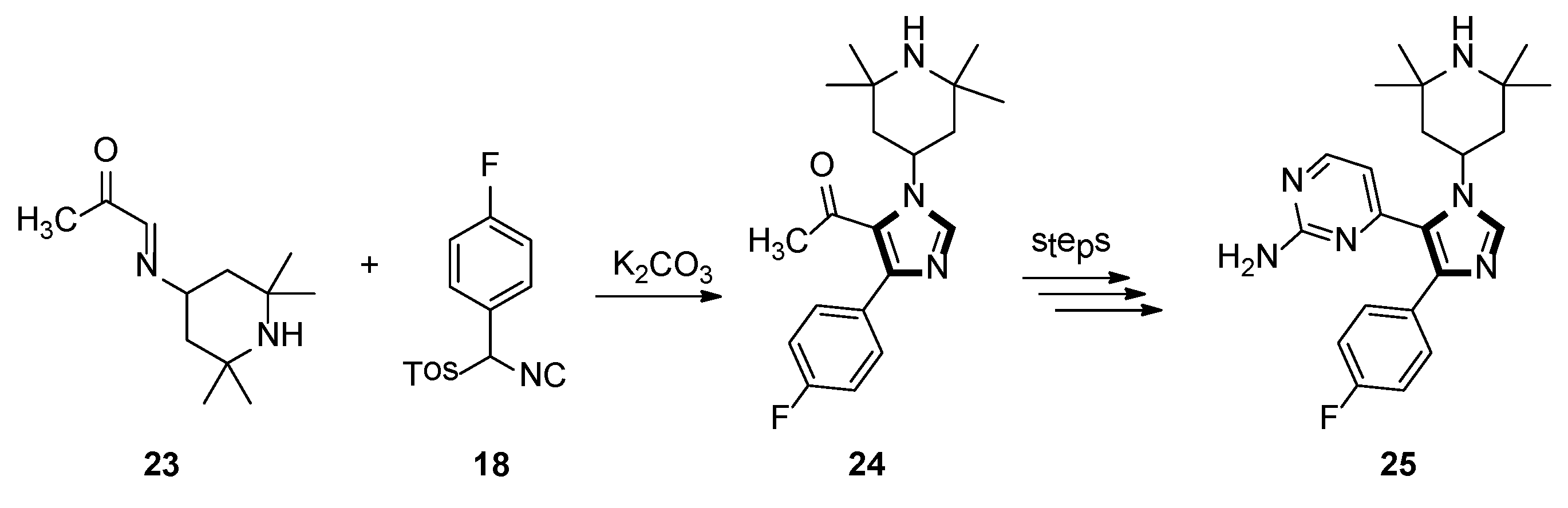
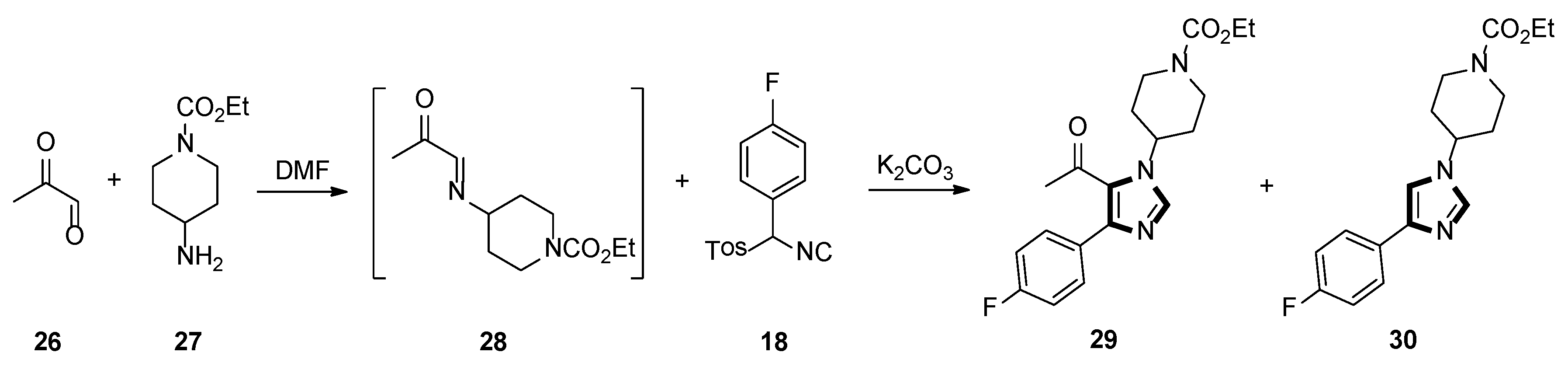
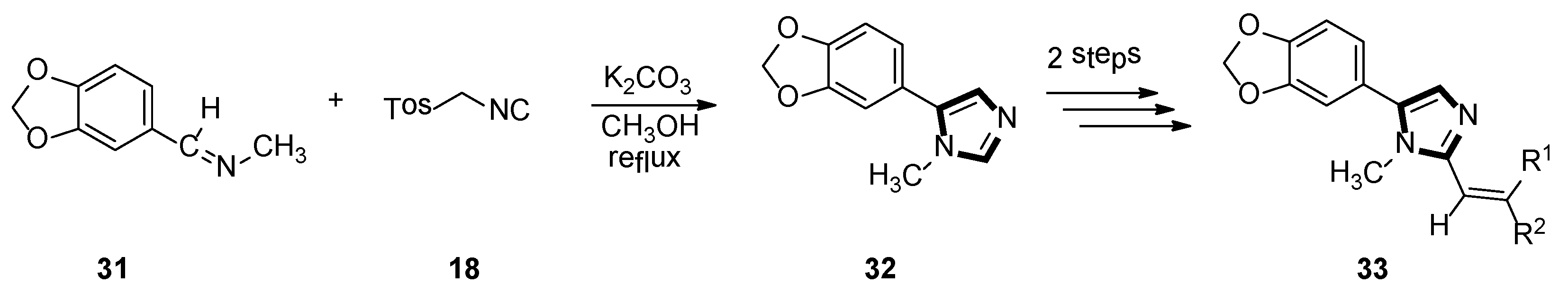
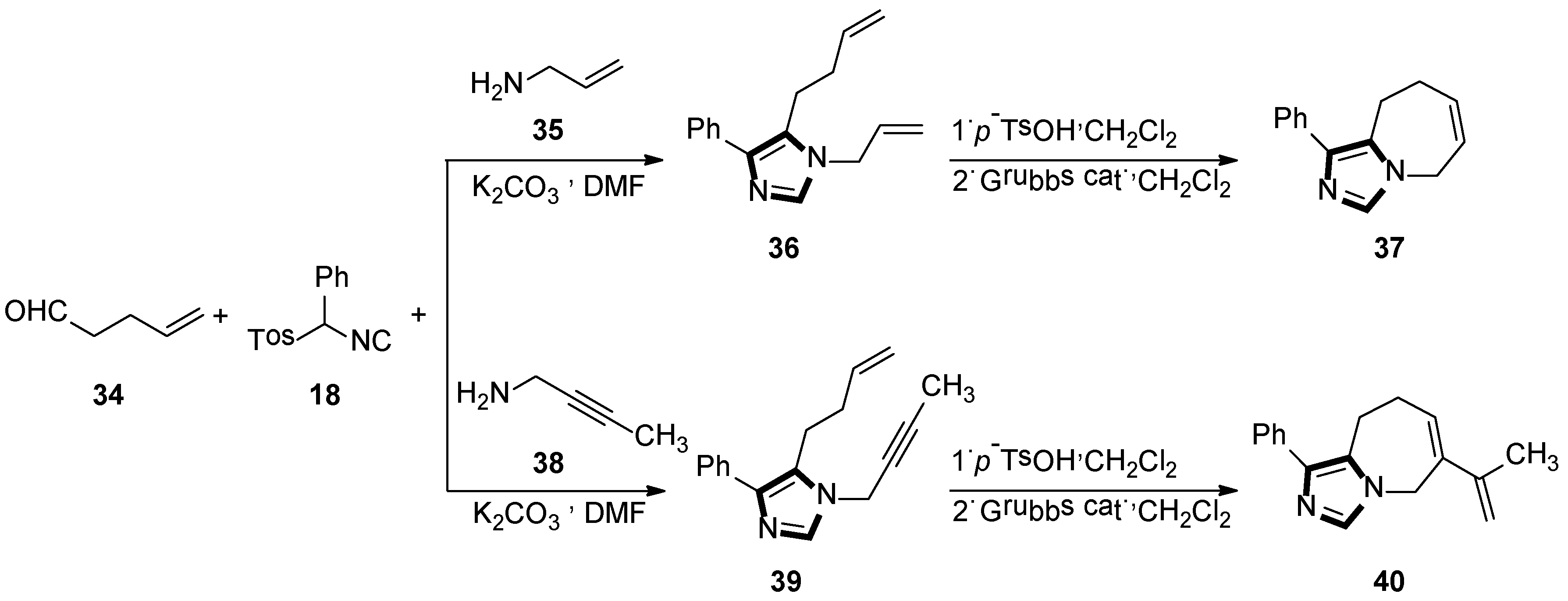
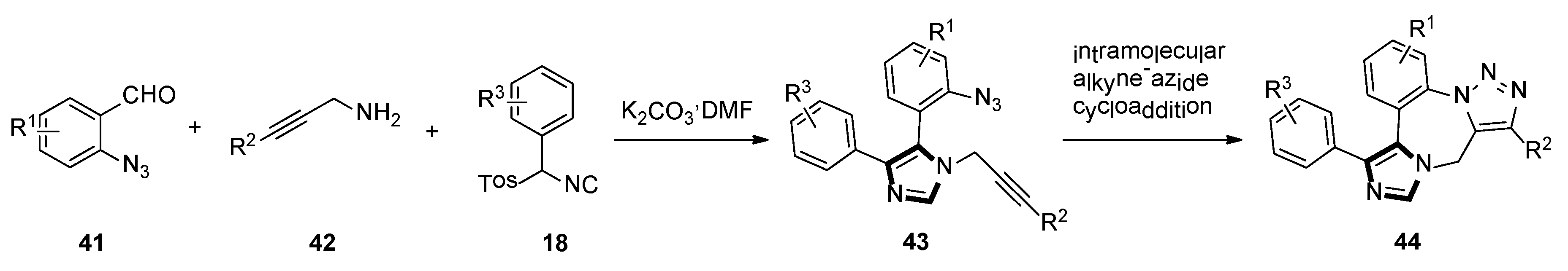
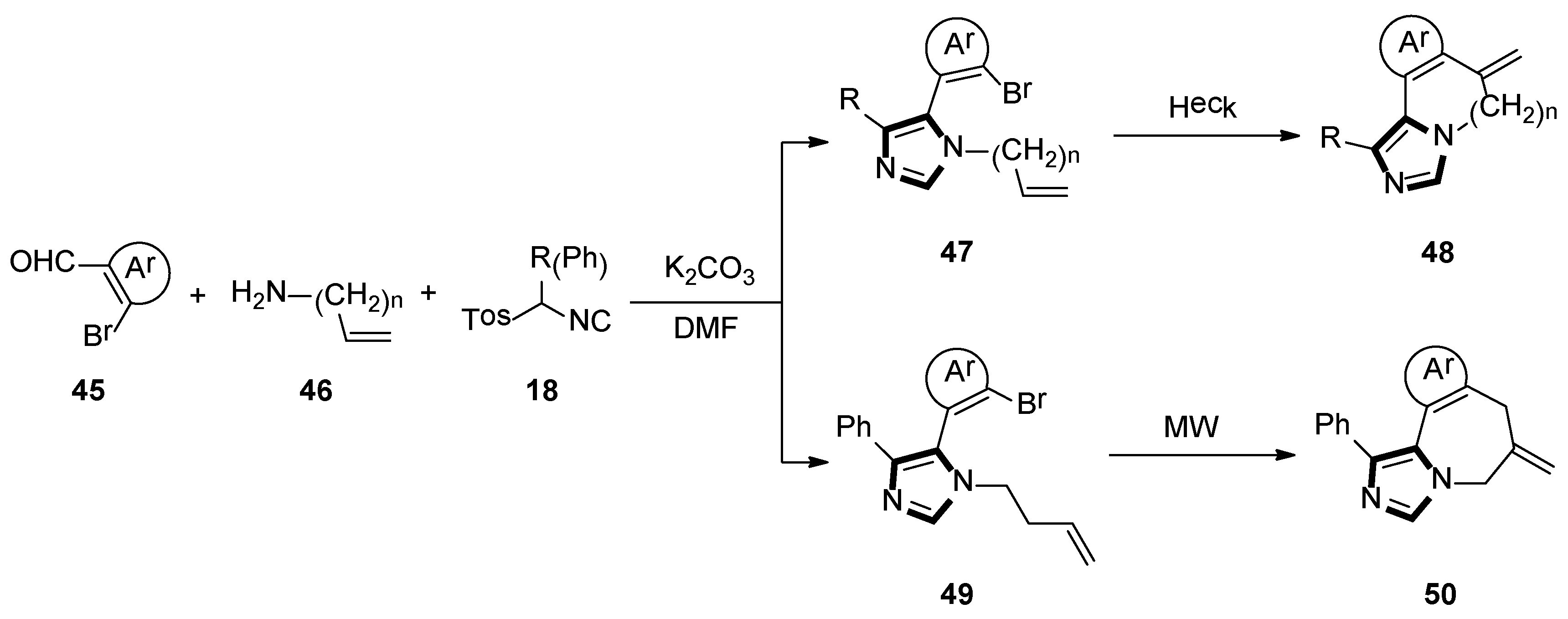
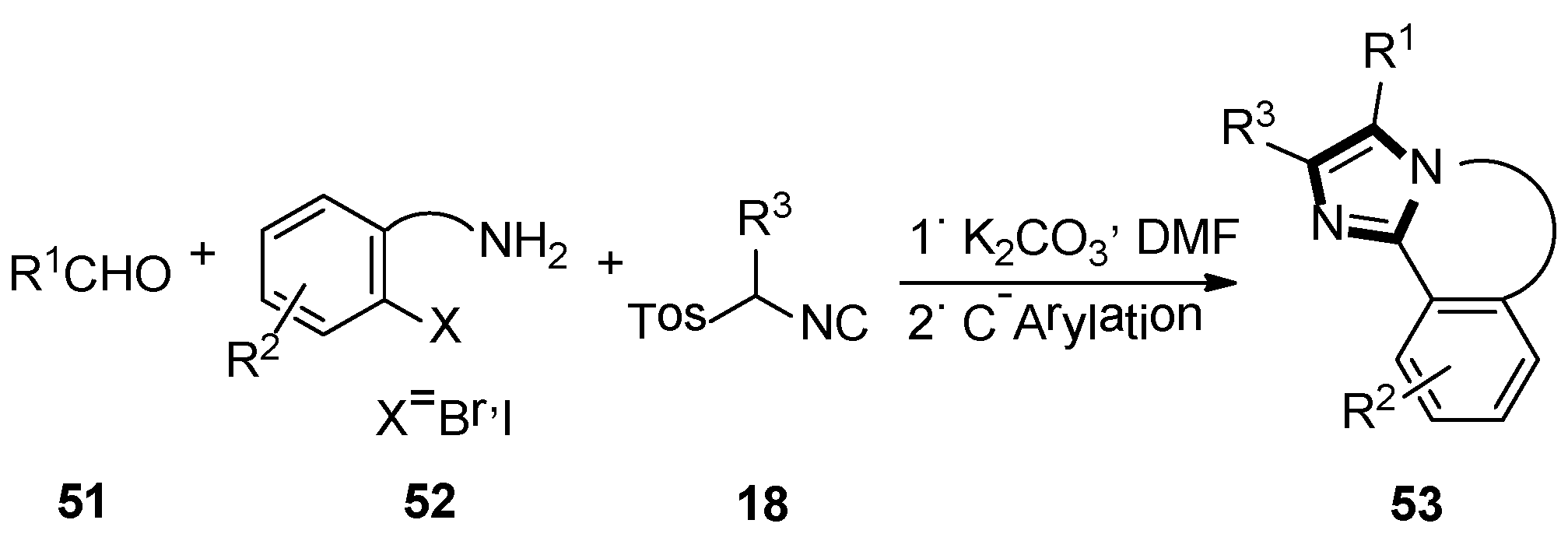
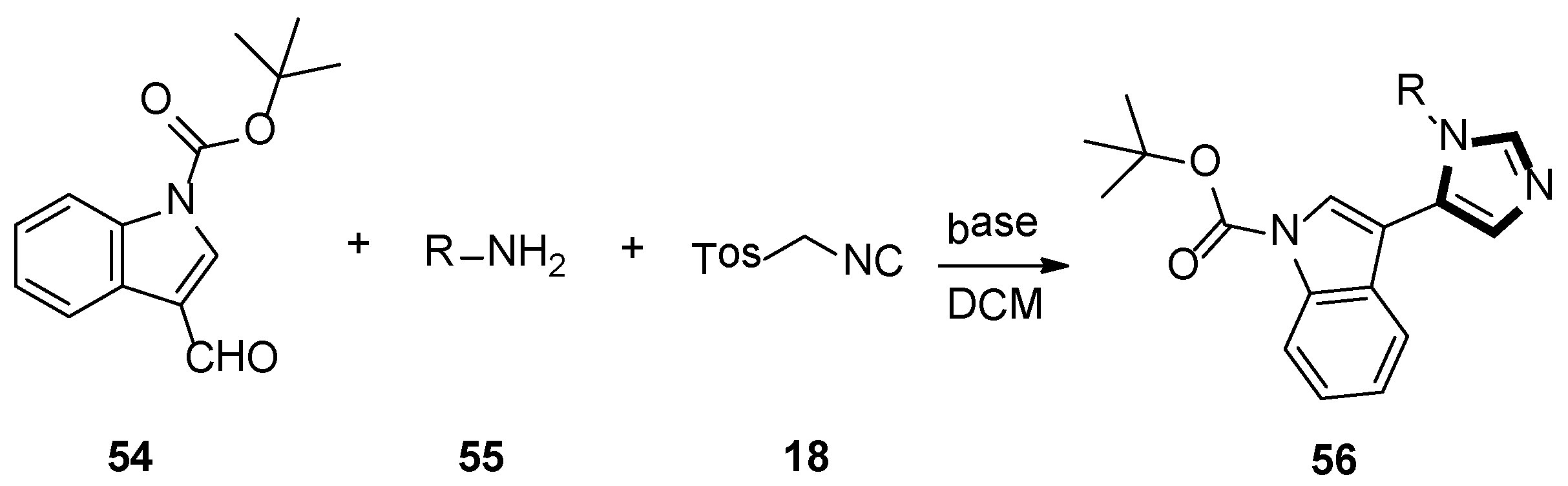
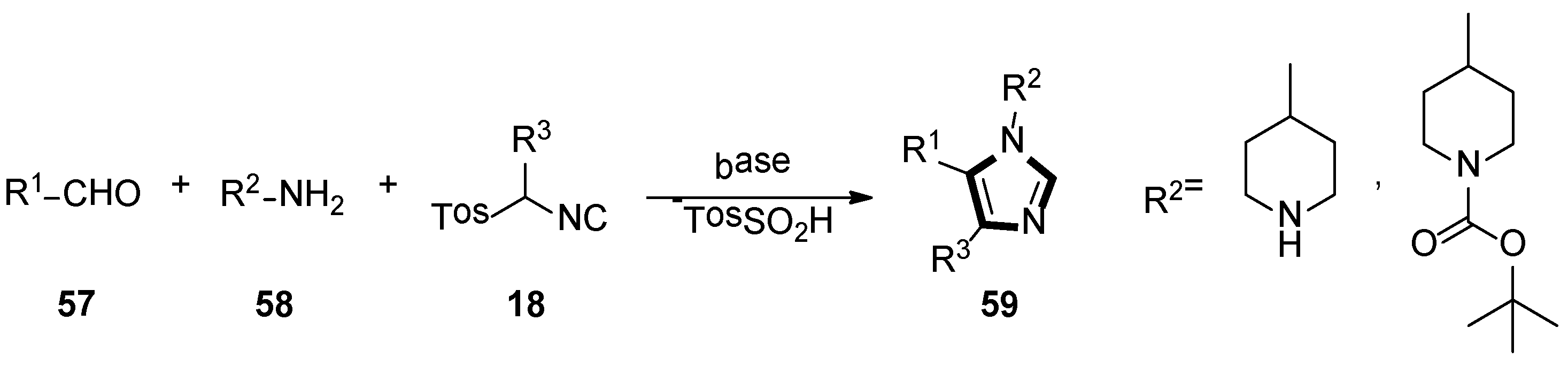
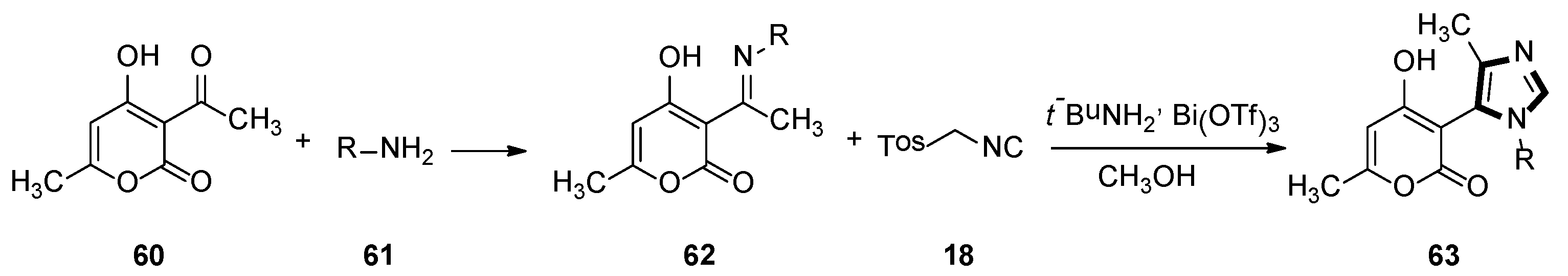
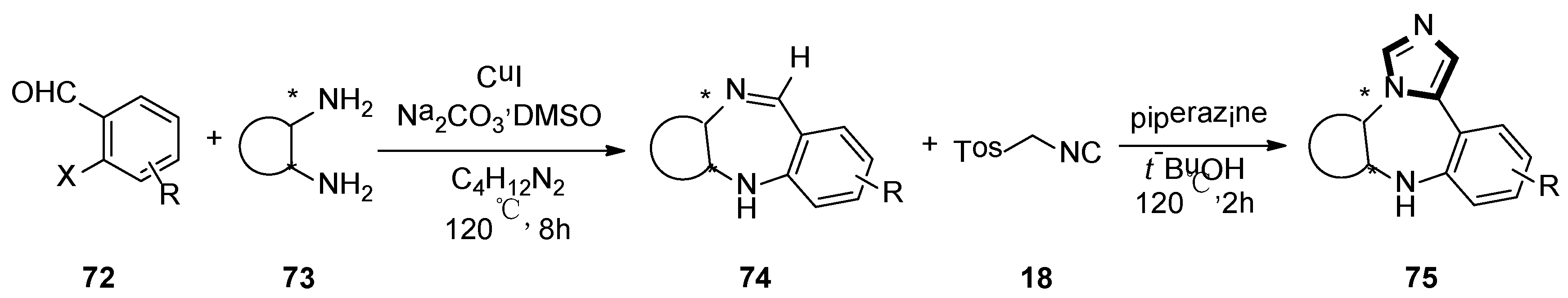
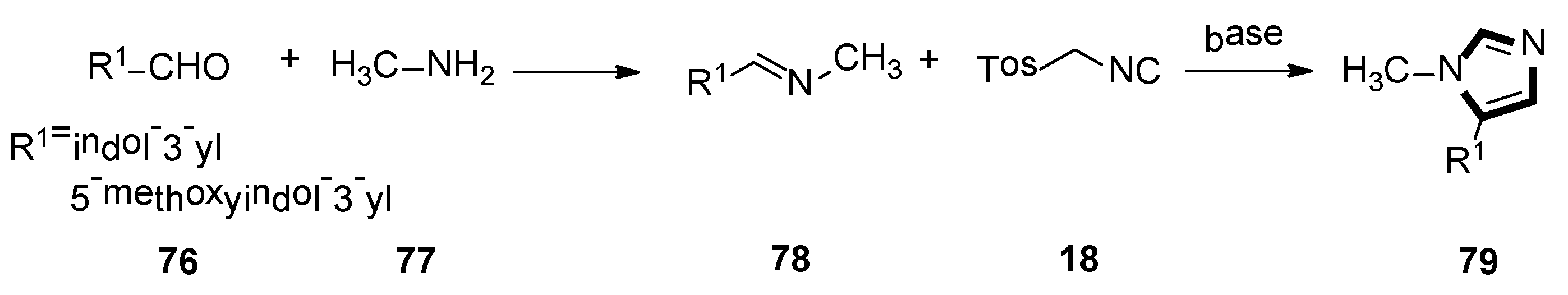
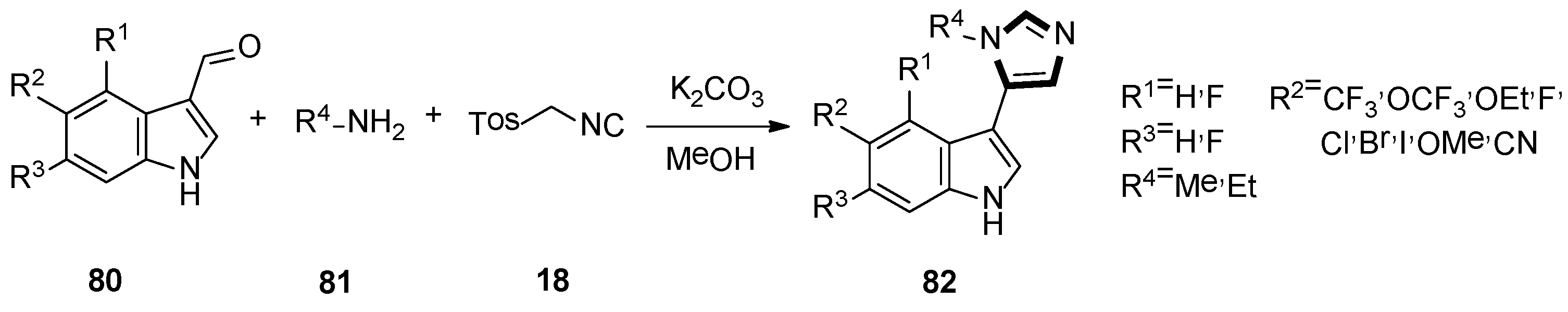
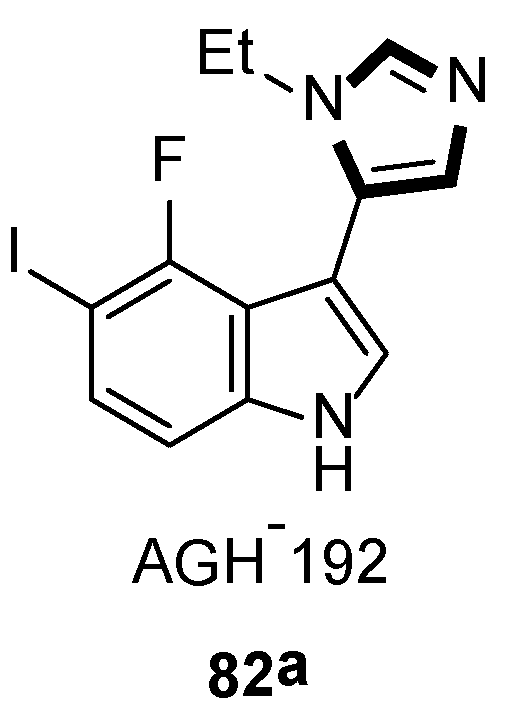
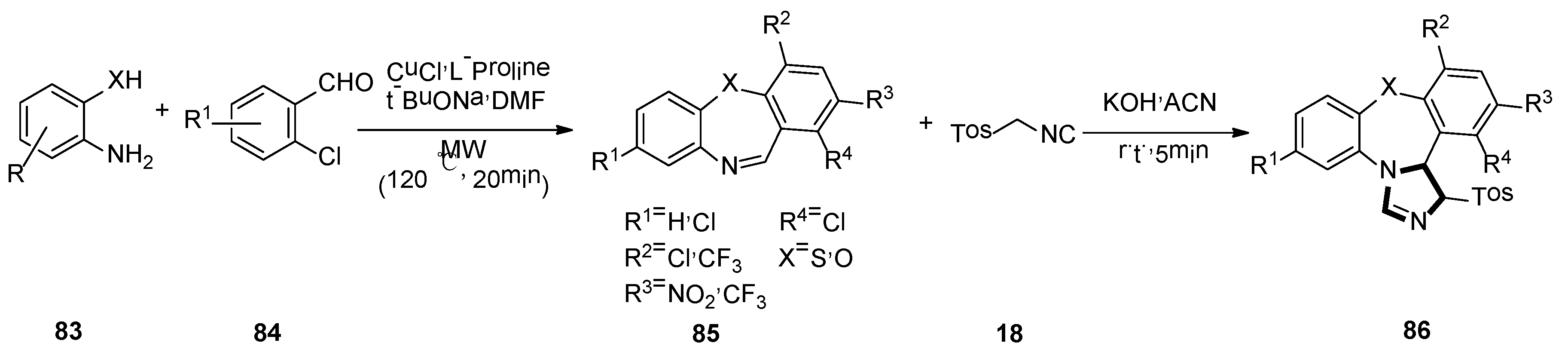
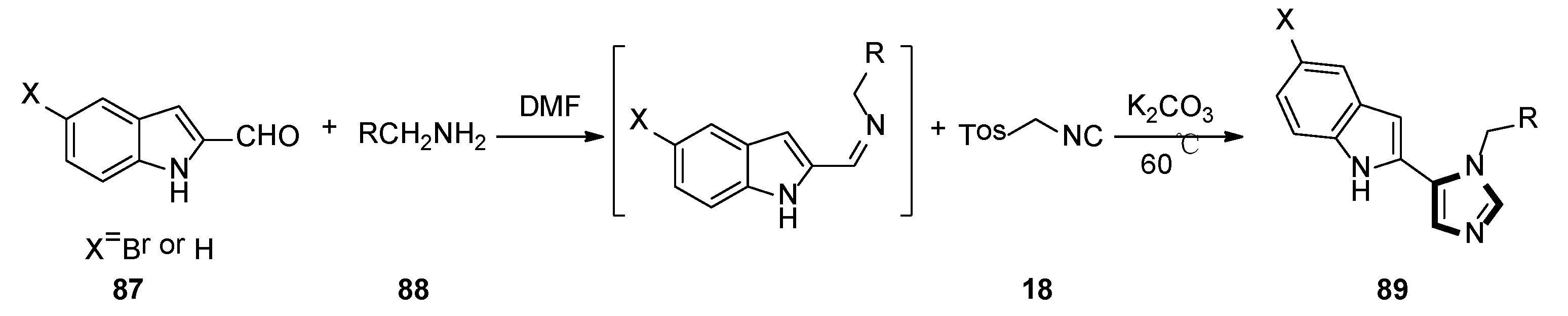
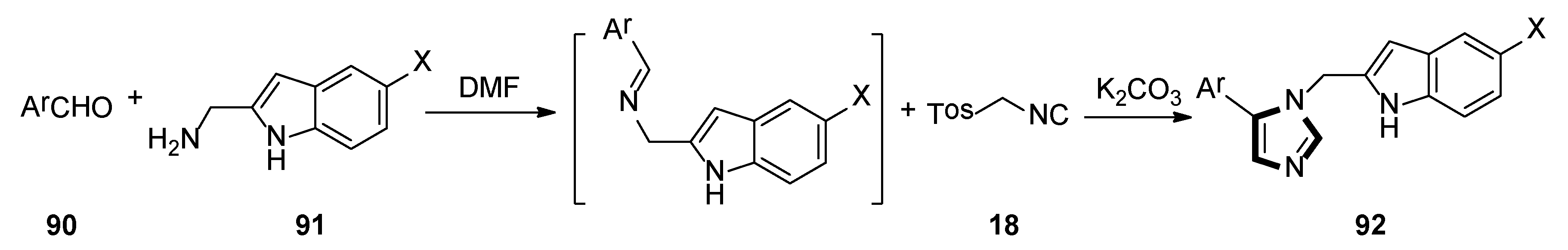
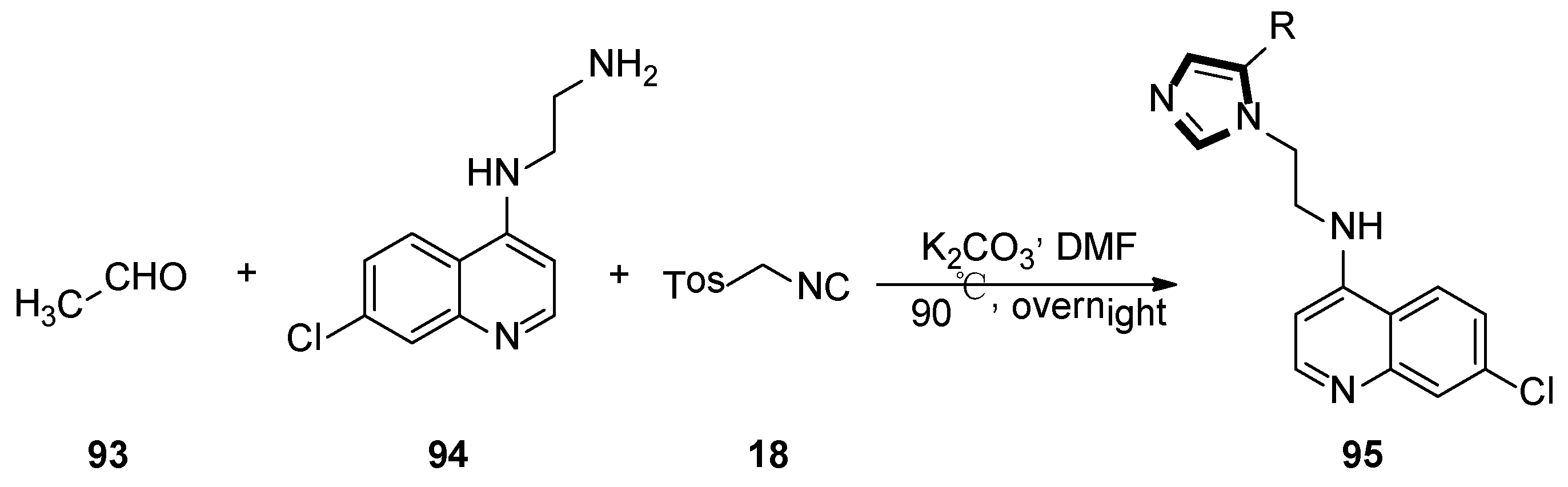
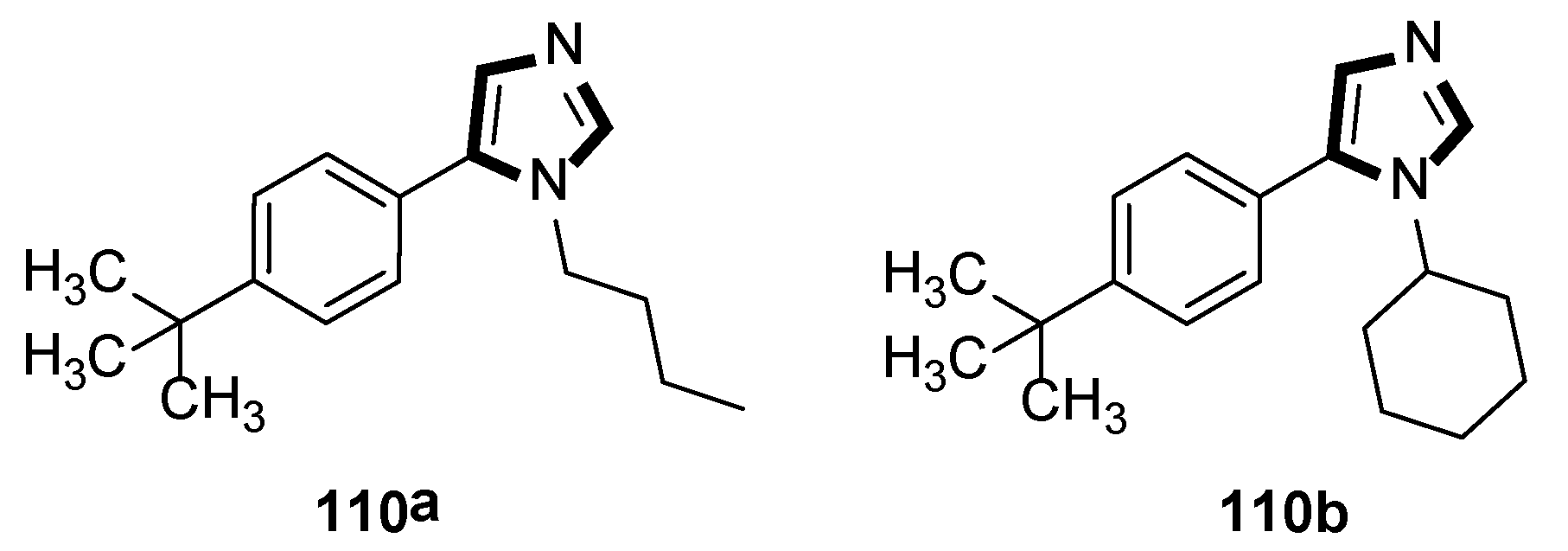
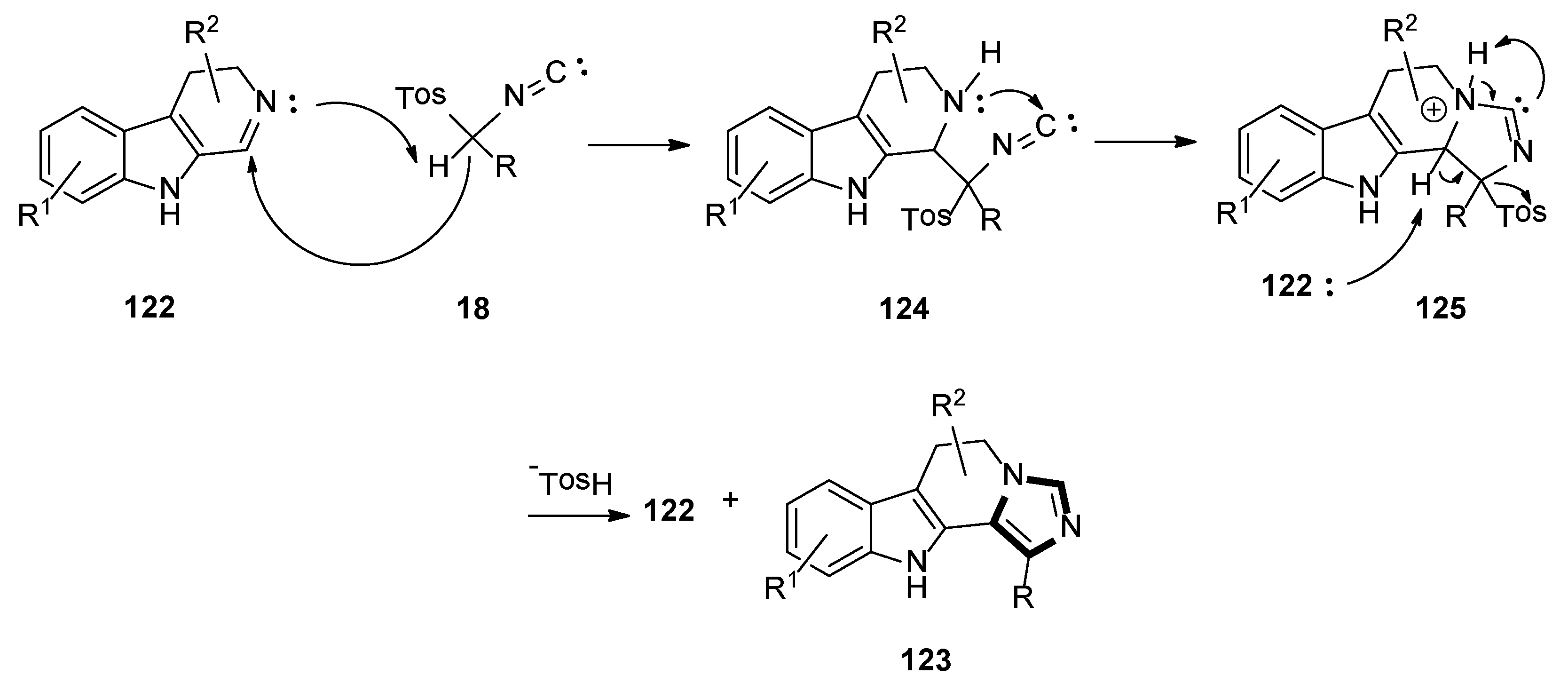
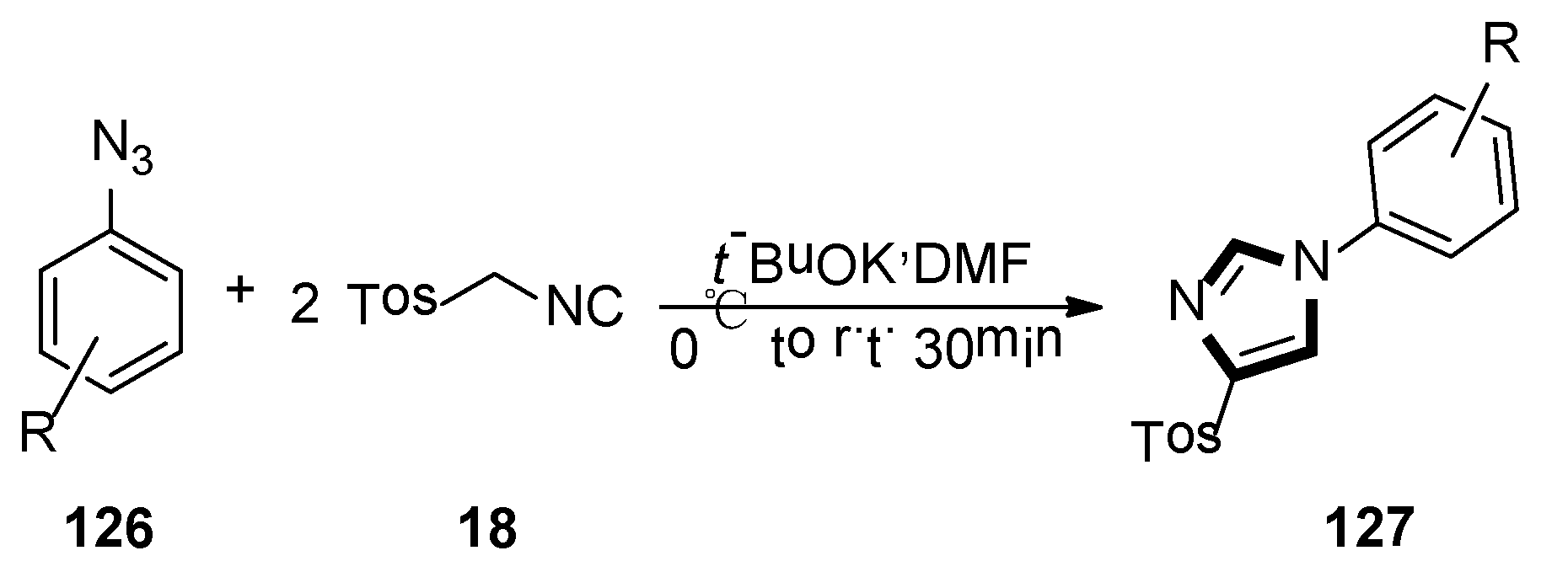
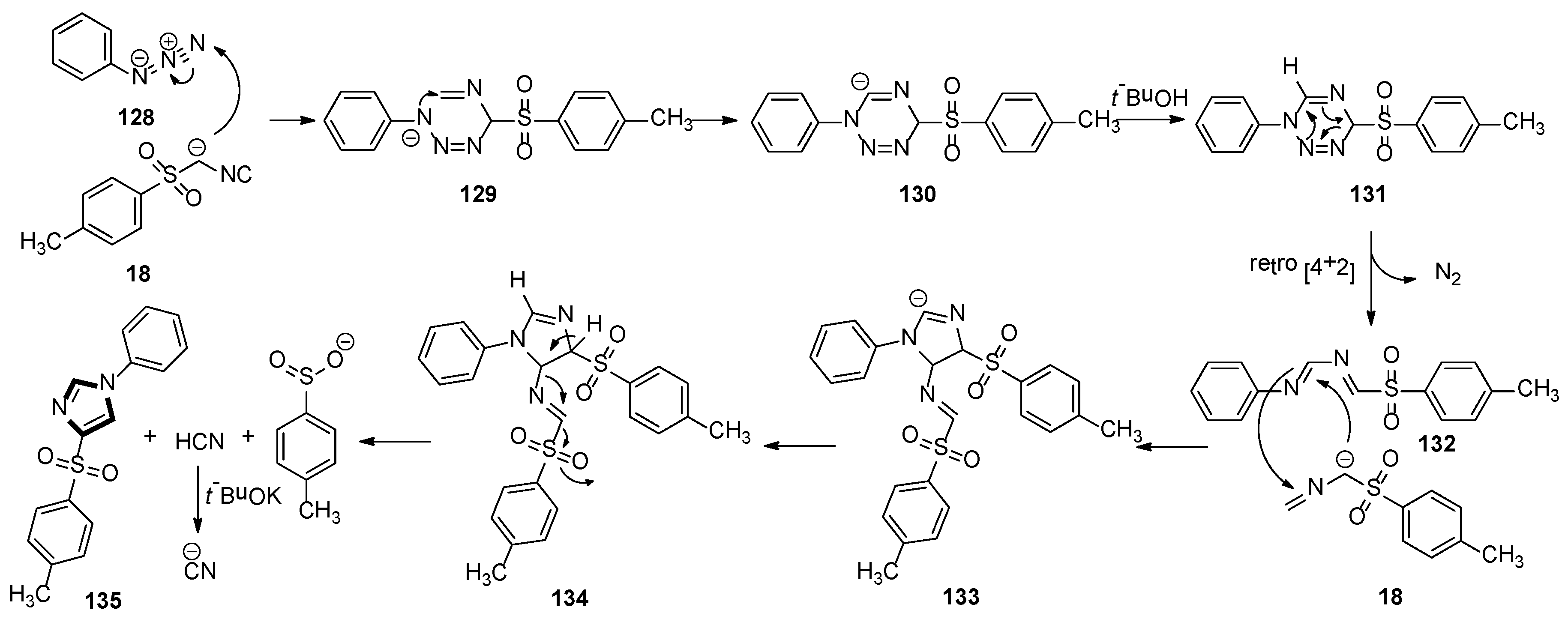
3. Developments of the van Leusen Imidazole Synthesis
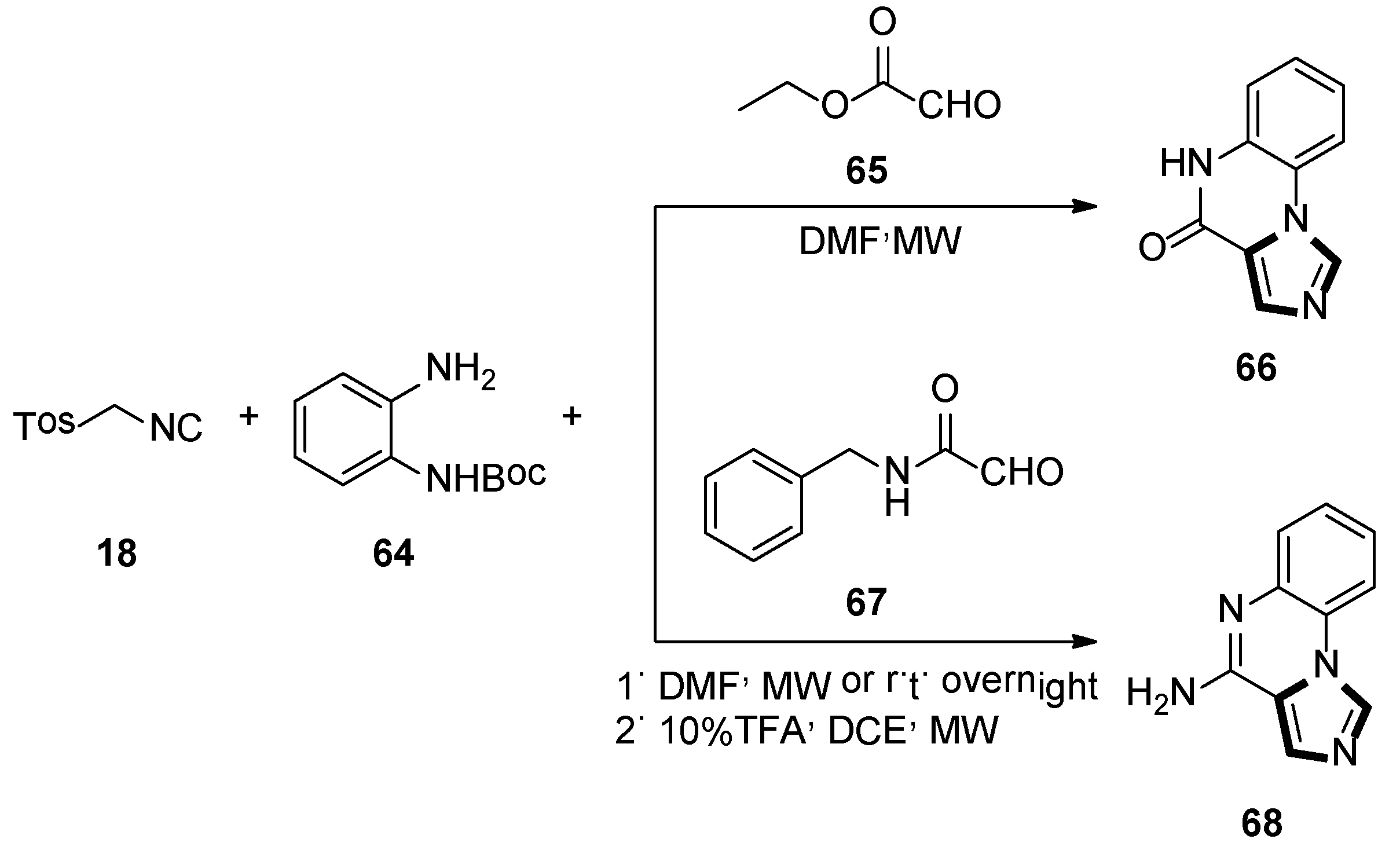
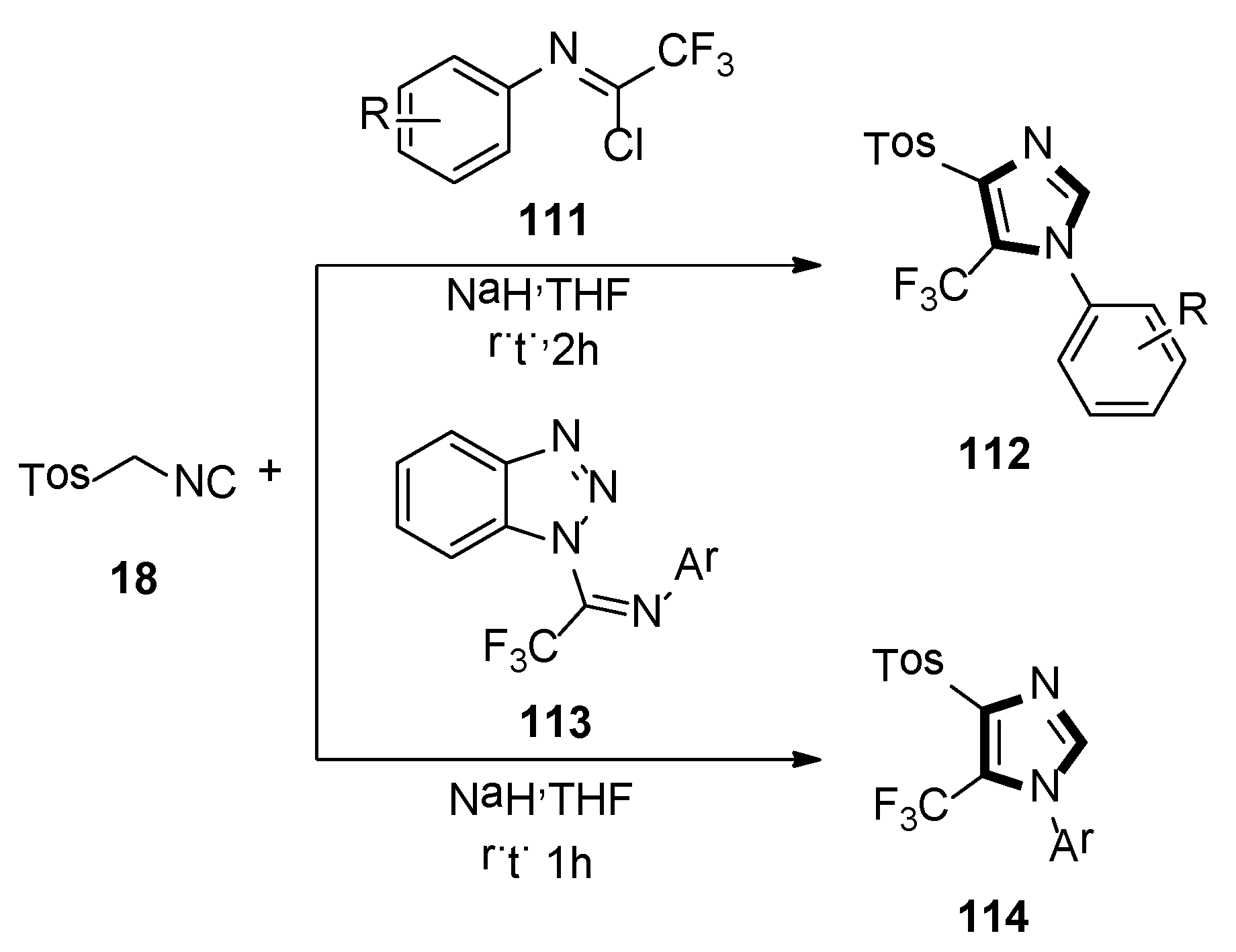
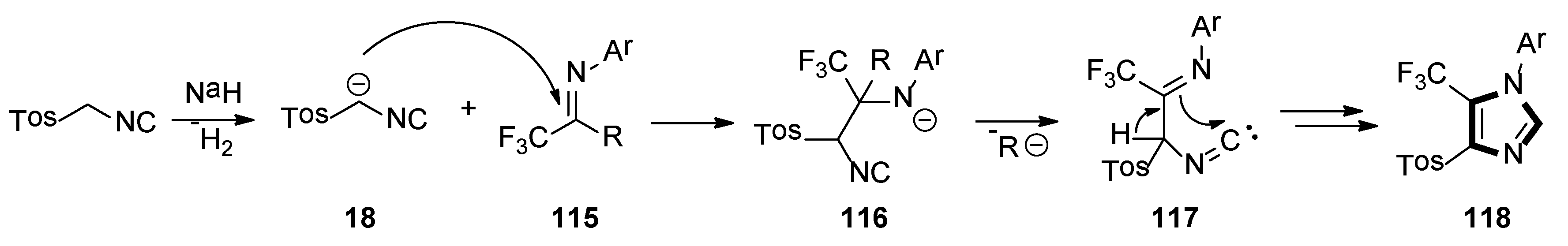
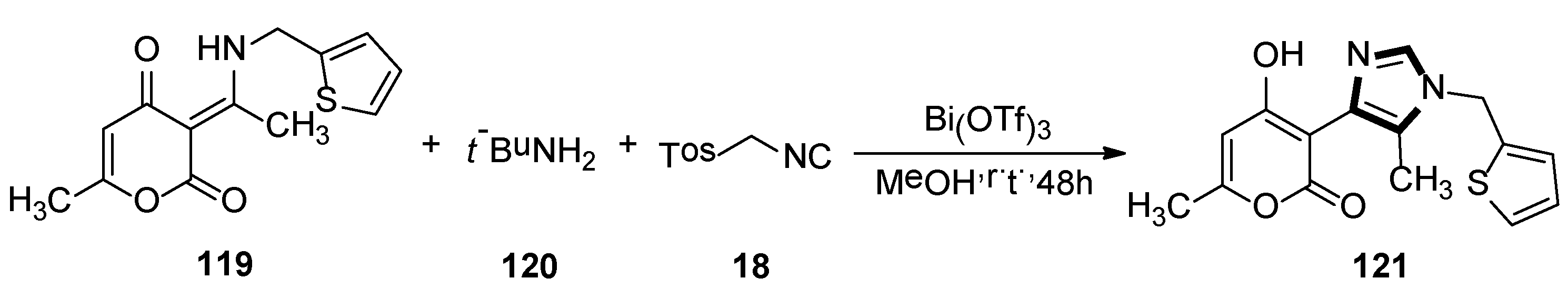
4. Other van Leusen Imidazole Synthesis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mishra, R.; Ganguly, S. Imidazole as an anti-epileptic: An overview. Med. Chem. Res. 2012, 21, 3929–3939. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, X.M.; Damu, G.L.; Geng, R.X.; Zhou, C.H. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 2014, 34, 340–437. [Google Scholar] [CrossRef]
- Gaba, M.; Mohan, C. Development of drugs based on imidazole and benzimidazole bioactive heterocycles: Recent advances and future directions. Med. Chem. Res. 2015, 25, 173–210. [Google Scholar] [CrossRef]
- Fan, Y.L.; Jin, X.H.; Huang, Z.P.; Yu, H.F.; Zeng, Z.G.; Gao, T.; Feng, L.S. Recent advances of imidazole-containing derivatives as anti-tubercular agents. Eur. J. Med. Chem. 2018, 150, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Daraji, D.G.; Prajapati, N.P.; Patel, H.D. Synthesis and applications of 2-substituted imidazole and its derivatives: A review. J. Heterocycl. Chem. 2019, 56, 2299–2317. [Google Scholar] [CrossRef]
- Khabnadideh, S.; Rezaci, Z.; Khalafi, N.A.; Motazedian, M.H.; Eskandari, M. Synthesis of metronidazole derivatives as antigiardiasis agents. DARU 2007, 15, 17–20. [Google Scholar]
- Satyanarayana, V.; Sivakumar, A. An efficient and novel one-pot synthesis of 2,4,5-triaryl-1H-imidazoles catalyzed by UO2(NO3)2·6H2O under heterogeneous conditions. Chem. Pap. 2011, 65, 519–526. [Google Scholar] [CrossRef]
- Van den, B.H. Biochemical effects of miconazole on fungi—I Effects on the uptake and/or utilization of purines, pyrimidines, nucleosides, amino acids and glucose by Candida albicans. Biochem. Pharmacol. 1974, 23, 887–899. [Google Scholar]
- Carrilo-Muñoz, A.J.; Tur, C.; Torres, J.; Seymour, A.C. In-Vitro antifungal activity of sertaconazole, bifonazole, ketoconazole, and miconazole against yeasts of the Candida genus. J. Antimicrob. Chemother. 1996, 37, 815–819. [Google Scholar] [CrossRef]
- Che, H.; Tuyen, T.N.; Kim, H.P.; Park, H. 1,5-Diarylimidazoles with strong inhibitory activity against COX-2 catalyzed PGE2 production from LPS-induced RAW 264.7 cells. Bioorg. Med. Chem. Lett. 2010, 20, 4035–4037. [Google Scholar] [CrossRef]
- Forster, L.; Ludwig, J.; Kaptur, M.; Bovens, S.; Elfringhoff, A.S.; Holtfrerich, A.; Lehr, M. 1-Indol-1-yl-propan-2-ones and related heterocyclic compounds as dual inhibitors of cytosolic phospholipase A2α and fatty acid amide hydrolase. Bioorg. Med. Chem. 2010, 18, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.S.; Nichols, C.; Bird, L.E.; Fujiwara, T.; Sugimoto, H.; Stuart, D.I.; Stammers, D.K. Binding of the second generation non-nucleoside inhibitor S-1153 to HIV-1 reverse transcriptase involves extensive main chain hydrogen bonding. J. Biol. Chem. 2000, 275, 14316–14320. [Google Scholar] [CrossRef]
- Zhan, P.; Liu, X.; Zhu, J.; Fang, Z.; Li, Z.; Pannecouque, C.; Clercq, E.D. Synthesis and biological evaluation of imidazole thioacetanilides as novel non-nucleoside HIV-1 reverse transcriptase inhibitors. Bioorg. Med. Chem. 2009, 17, 5775–5781. [Google Scholar] [CrossRef] [PubMed]
- Valdez, C.A.; Tripp, J.C.; Miyamoto, Y.; Kalisiak, J.; Hruz, P.; Andersen, Y.S.; Brown, S.E.; Kangas, K.; Arzu, L.V.; Davids, B.J.; et al. Synthesis and electrochemistry of 2-ethenyl and 2-ethanyl derivatives of 5-nitroimidazole and antimicrobial activity against Giardia lamblia. J. Med. Chem. 2009, 52, 4038–4053. [Google Scholar] [CrossRef]
- Kapoor, V.K.; Chadha, R.; Venisetty, P.K.; Prasanth, S. Medicinal significance of nitroimidazoles-Some recent advances. J. Sci. Ind. Res. 2003, 62, 659–665. [Google Scholar]
- Josephy, P.D.; Palcic, B.; Skarsgard, L.D. In Vitro metabolism of misonidazole. Br. J. Cancer 1981, 43, 443–450. [Google Scholar] [CrossRef]
- Pectasides, D.; Yianniotis, H.; Alevizakos, N.; Bafaloukos, D.; Barbounis, V.; Varthalitis, J.; Dimitriadis, M.; Athanassiou, A. Treatment of metastatic malignant melanoma with dacarbazine, vindesine and cisplatin. Br. J. Cancer 1989, 60, 627–629. [Google Scholar] [CrossRef]
- Kitbunnadaj, R.; Zuiderveld, O.P.; Christophe, B.; Hulscher, S.; Menge, W.M.; Gelens, E.; Snip, E.; Bakker, R.A.; Celanire, S.; Gillard, M.; et al. Identification of 4-(1H-imidazol-4(5)-ylmethyl)pyridine (immethridine) as a novel, potent, and highly selective histamine H3 receptor agonist. J. Med. Chem. 2004, 47, 2414–2417. [Google Scholar] [CrossRef]
- Motawaj, M.; Arrang, J.M. Ciproxifan, a histamine H3-receptor antagonist/inverse agonist, modulates methamphetamine-induced sensitization in mice. Eur. J. Neurosci. 2011, 33, 1197–1204. [Google Scholar] [CrossRef]
- Hille, U.E.; Zimmer, C.; Vock, C.A.; Hartmann, R.W. First selective CYP11B1 inhibitors for the treatment of cortisol-dependent diseases. ACS Med. Chem. Lett. 2011, 2, 2–6. [Google Scholar] [CrossRef]
- Salerno, L.; Modica, M.N.; Romeo, G.; Pittala, V.; Siracusa, M.A.; Amato, M.E.; Acquaviva, R.; Di Giacomo, C.; Sorrenti, V. Novel inhibitors of nitric oxide synthase with antioxidant properties. Eur. J. Med. Chem. 2012, 49, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Van Leusen, A.M.; Wildeman, J.; Oldenziel, O.H. Base-induced cycloaddition of sulfonylmethyl isocyanides to C,N double bonds. Synthesis of 1,5-disubstituted and 1,4,5-trisubstituted imidazoles from aldimines and imidoyl chlorides. J. Org. Chem. 1977, 42, 1153–1159. [Google Scholar] [CrossRef]
- Ma, B.B.; Peng, Y.X.; Zhao, P.C.; Huang, W. cis and trans Isomers distinguished by imidazole N-alkylation after Debus-Radziszewski reaction starting from 2,7-di-tert-butyl-pyrene-4,5,9,10-tetraone. Tetrahedron 2015, 71, 3195–3202. [Google Scholar] [CrossRef]
- Benincori, T.; Brenna, E.; Sannicolo, F. Studies on Wallach’s imidazole synthesis. J. Chem. Soc. Perkin Trans. 1993, 1, 675–679. [Google Scholar] [CrossRef]
- Tandon, V.K.; Rai, S. p-Toluenesulfonylmethyl isocyanide: A versatile synthon in organic chemistry. Suljiur Rep. 2003, 24, 307–385. [Google Scholar]
- Akritopoulou-Zanze, I. Isocyanide-based multicomponent reactions in drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 324–331. [Google Scholar] [CrossRef]
- Lujan-Montelongo, J.A.; Estevez, A.O.; Fleming, F.F. Alkyl sulfinates: Formal nucleophiles for synthesizing TosMIC analogs. Eur. J. Org. Chem. 2015, 7, 1602–1605. [Google Scholar] [CrossRef]
- Mathiyazhagan, A.D.; Anilkumar, G. Recent advances and applications of p-toluenesulfonylmethyl isocyanide (TosMIC). Org. Biomol. Chem. 2019, 17, 6735–6747. [Google Scholar] [CrossRef]
- Van Leusen, D.; van Leusen, A.M. Synthetic uses of tosylmethyl isocyanide (TosMIC). In Organic Reactions; Overman, L., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; Volume 57, pp. 417–666. [Google Scholar]
- Frankowski, A.; Deredas, D.; Lenouen, D.; Tschamber, T.; Streith, J. On the way to glycoprocessing inhibitors. Synthesis of an imidazo-L-xylo-piperidinose derivative. Helv. Chim. Acta 1995, 78, 1837–1842. [Google Scholar] [CrossRef]
- Sisko, J. A one-pot synthesis of 1-(2,2,6,6-tetramethyl-4-piperidinyl)-4-(4-fluorophenyl)-5-(2-amino-4-pyrimidinyl)-imidazole: A potent inhibitor of P38 MAP kinase. J. Org. Chem. 1998, 63, 4529–4531. [Google Scholar] [CrossRef]
- Sisko, J.; Kassick, A.J.; Mellinger, R.; Filan, J.J.; Allen, A.; Olsen, M.A. An investigation of imidazole and oxazole syntheses using aryl-substituted TosMIC reagents. J. Org. Chem. 2000, 65, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Vanelle, P.; Meuche, J.; Maldonado, J.; Crozet, M.P.; Delmas, F.; Timon-David, P. Functional derivatives of 5-benzo[1,3]-5-yl-1-methyl-1H-imidazole-2-carbaldehyde and evaluation of leishmanicidal activity. Eur. J. Med. Chem. 2000, 35, 157–162. [Google Scholar] [CrossRef]
- Gracias, V.; Gasiecki, A.F.; Djuric, S.W. Synthesis of fused bicyclic imidazoles by sequential van Leusen/ring-closing metathesis reactions. Org. Lett. 2005, 7, 3183–3186. [Google Scholar] [CrossRef] [PubMed]
- Gracias, V.; Gasiecki, A.F.; Djuric, S.W. Synthesis of fused imidazo azepine derivatives by sequential van Leusen/enyne metathesis reactions. Tetrahedron Lett. 2005, 46, 9049–9052. [Google Scholar] [CrossRef]
- Gracias, V.; Darczak, D.; Gasiecki, A.F.; Djuric, S.W. Synthesis of fused triazolo-imidazole derivatives by sequential van Leusen/alkyne–azide cycloaddition reactions. Tetrahedron Lett. 2005, 46, 9053–9056. [Google Scholar] [CrossRef]
- Beebe, X.; Gracias, V.; Djuric, S.W. Synthesis of fused imidazo-pyridine and -azepine derivatives by sequential van Leusen/Heck reactions. Tetrahedron Lett. 2006, 47, 3225–3228. [Google Scholar] [CrossRef]
- Gracias, V.; Gasiecki, A.F.; Pagano, T.G.; Djuric, S.W. Synthesis of fused imidazole rings by sequential van Leusen/C–H bond activation. Tetrahedron Lett. 2006, 47, 8873–8876. [Google Scholar] [CrossRef]
- Beck, B.; Leppert, C.A.; Mueller, B.K.; Dömling, A. Discovery of pyrroloimidazoles as agents stimulating neurite outgrowth. QSAR Comb. Sci. 2006, 25, 527–535. [Google Scholar] [CrossRef]
- Domling, A.; Beck, B.; Herdtweck, E.; Antuch, W.; Oefner, C.; Yehia, N.; Gracia-Marques, A. Parallel synthesis of arrays of 1,4,5-trisubstituted 1-(4-piperidyl)-imidazoles by IMCR: A novel class of aspartyl protease inhibitors. Arkivoc 2007, 2007, 99–109. [Google Scholar]
- Fodili, M.; Nedjar-Kolli, B.; Garrigues, B.; Lherbet, C.; Hoffmann, P. Synthesis of imidazoles from ketimines using tosylmethyl isocyanide (TosMIC) catalyzed by bismuth triflate. Lett. Org. Chem. 2009, 6, 354–358. [Google Scholar] [CrossRef]
- De Moliner, F.; Hulme, C. A van Leusen deprotection-cyclization strategy as a fast entry into two imidazoquinoxaline families. Tetrahedron Lett. 2012, 53, 5787–5790. [Google Scholar] [CrossRef] [PubMed]
- Fallarini, S.; Massarotti, A.; Gesu, A.; Giovarruscio, S.; Zabetta, G.C.; Bergo, R.; Giannelli, B.; Brunco, A.; Lombardi, G.; Sorba, G.; et al. In silico-driven multicomponent synthesis of 4,5-and 1,5-disubstituted imidazoles as indoleamine 2,3-dioxygenase inhibitors. Med. Chem. Comm. 2016, 7, 409–419. [Google Scholar] [CrossRef]
- Murugesh, V.; Harish, B.; Adiseshu, M.; Nanubolu, J.B.; Suresh, S. Tandem copper-catalyzed N-arylation-condensation and van Leusen reaction: Synthesis of 1,4-benzodiazepines and imidazobenzodiazepines (ImBDs). Adv. Synthesis Catal. 2016, 358, 1309–1321. [Google Scholar] [CrossRef]
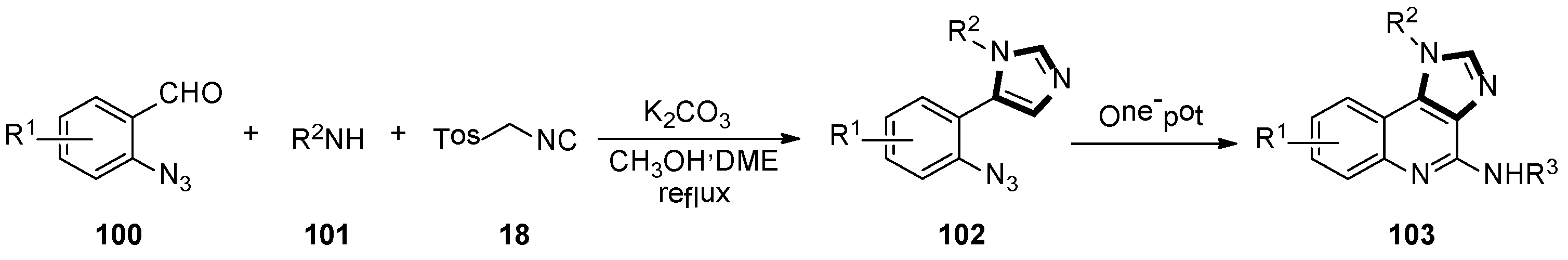
- Hogendorf, A.S.; Hogendorf, A.; Kurczab, R.; Satala, G.; Lenda, T.; Walczak, M.; Latacz, G.; Handzlik, J.; Kiec-Kononowicz, K.; Wieronska, J.M.; et al. Low-basicity 5-HT7 receptor agonists synthesized using the van Leusen multicomponent protocol. Sci. Rep. 2017, 7, 1444. [Google Scholar] [CrossRef] [PubMed]
- Hogendorf, A.S.; Hogendorf, A.; Popiolek-Barczyk, K.; Ciechanowska, A.; Mika, J.; Satala, G.; Walczak, M.; Latacz, G.; Handzlik, J.; Kiec-Kononowicz, K.; et al. Fluorinated indole-imidazole conjugates: Selective orally bioavailable 5-HT7 receptor low-basicity agonists, potential neuropathic painkillers. Eur. J. Med. Chem. 2019, 170, 261–275. [Google Scholar] [CrossRef]
- Saha, D.; Kaur, T.; Sharma, A. Facile construction of imidazo-benzothia-/oxazepines via quick and efficient van Leusen protocol. Asian J. Org. Chem. 2017, 6, 527–533. [Google Scholar] [CrossRef]
- Brant, M.G.; Goodwin-Tindall, J.; Stover, K.R.; Stafford, P.M.; Wu, F.; Meek, A.R.; Schiavini, P.; Wohnig, S.; Weaver, D.F. Identification of potent indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors based on a phenylimidazole scaffold. ACS Med. Chem. Lett. 2018, 9, 131–136. [Google Scholar] [CrossRef]
- Kondaparla, S.; Manhas, A.; Dola, V.R.; Srivastava, K.; Puri, S.K.; Katti, S.B. Design, synthesis and antiplasmodial activity of novel imidazole derivatives based on 7-chloro-4-aminoquinoline. Bioorg. Chem. 2018, 80, 204–211. [Google Scholar] [CrossRef]
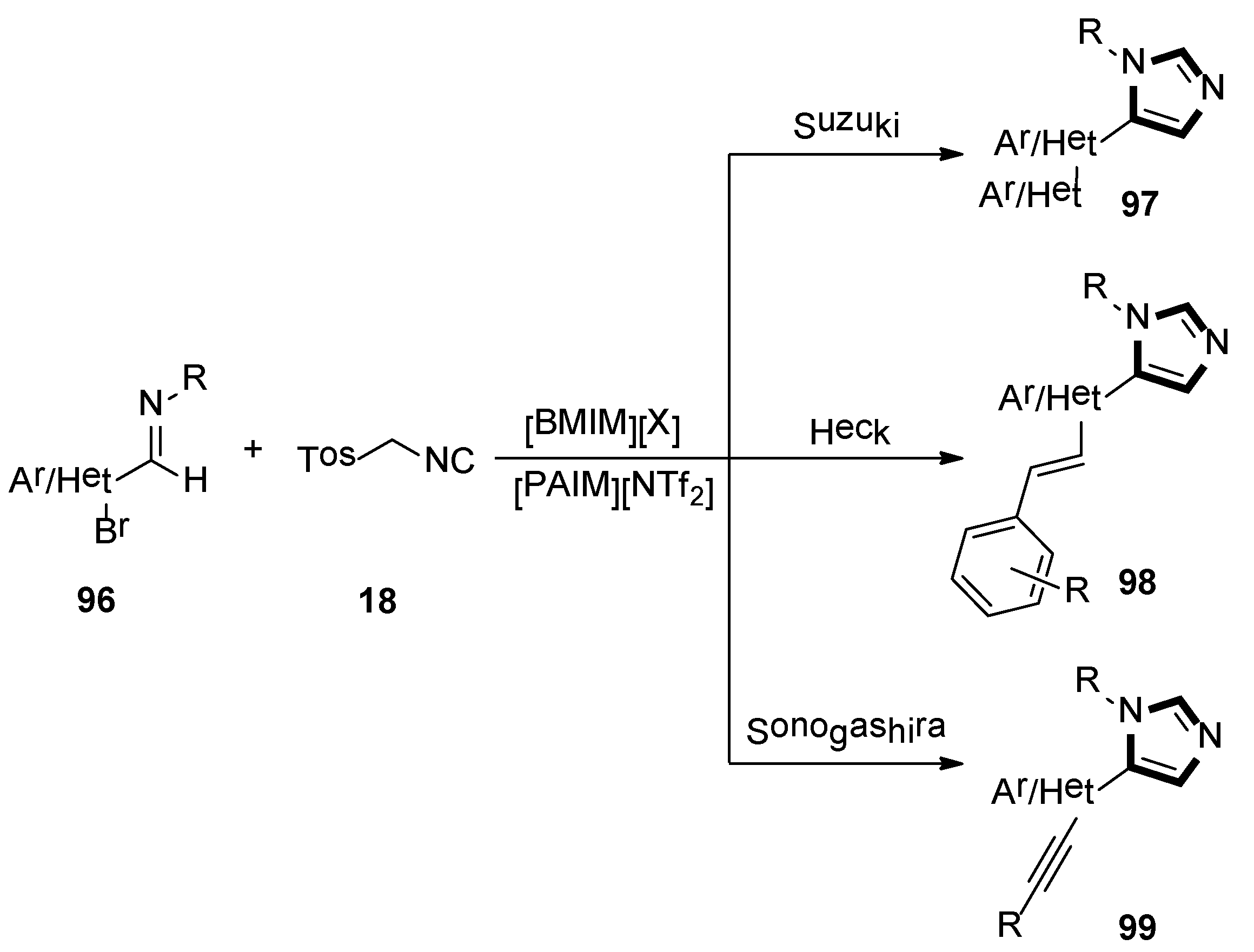
- Savanur, H.M.; Kalkhambkar, R.G.; Laali, K.K. Libraries of C-5 substituted imidazoles and oxazoles by sequential van Leusen (vL)-Suzuki, vL-Heck and vL-Sonogashira in imidazolium-ILs with piperidine-appended-IL as base. Eur. J. Org. Chem. 2018, 38, 5285–5288. [Google Scholar] [CrossRef]
- Guan, Z.R.; Liu, Z.M.; Ding, M.W. New efficient synthesis of 1H-imidazo-[4,5-c]quinolines by a sequential van Leusen/Staudinger/aza-Wittig/carbodiimide-mediated cyclization. Tetrahedron 2018, 74, 7186–7192. [Google Scholar] [CrossRef]
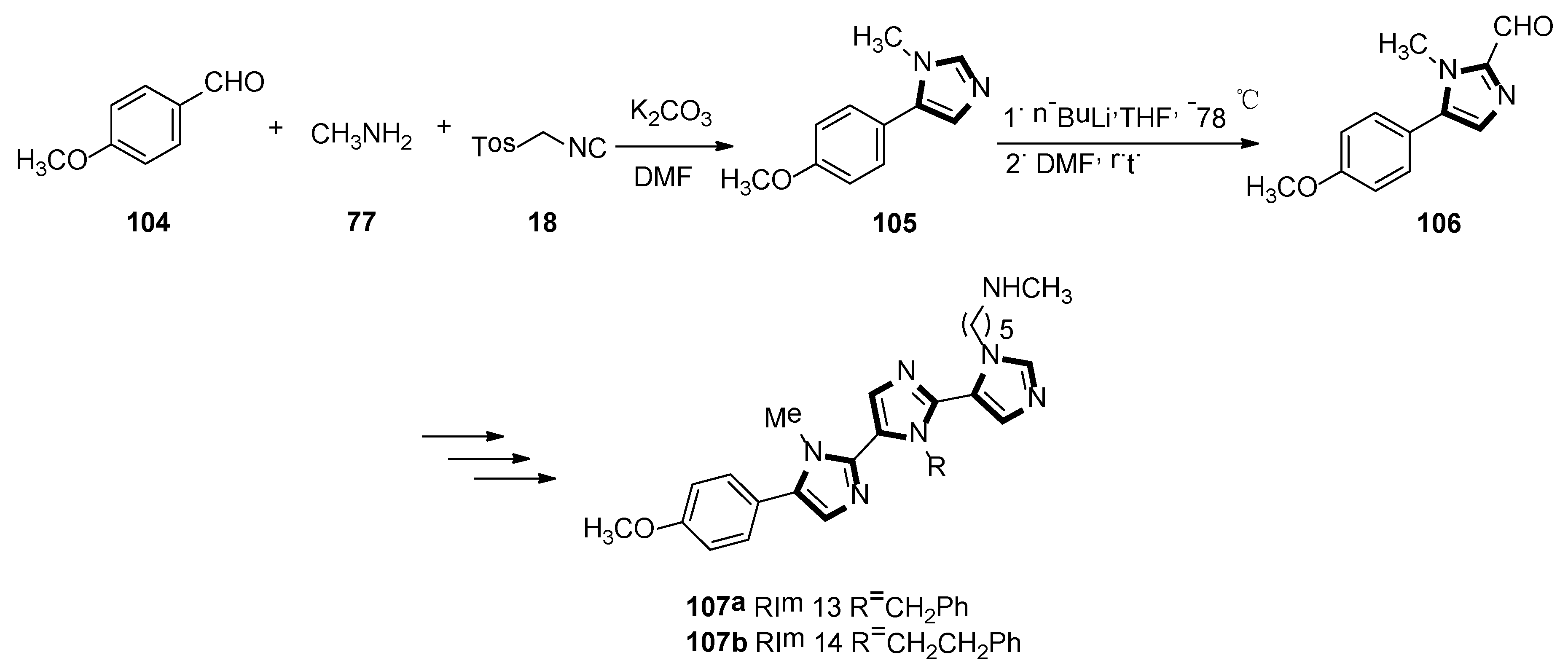
- Lammi, C.; Sgrignani, J.; Arnoldi, A.; Lesma, G.; Spatti, C.; Silvani, A.; Grazioso, G. Computationally driven structure optimization, synthesis, and biological evaluation of imidazole-based proprotein convertase subtilisin/kexin 9 (PCSK9) inhibitors. J. Med. Chem. 2019, 62, 6163–6174. [Google Scholar] [CrossRef] [PubMed]
- Rashamuse, T.J.; Harrison, A.T.; Mosebi, S.; van Vuuren, S.; Coyanis, E.M.; Bode, M.L. Design, synthesis and biological evaluation of imidazole and oxazole fragments as HIV-1 integrase-LEDGF/p75 disruptors and inhibitors of microbial pathogens. Bioorg. Med. Chem. 2020, 28, 115210. [Google Scholar] [CrossRef]
- Bunev, A.S.; Vasiliev, M.A.; Statsyuk, V.E.; Ostapenko, G.I.; Peregudov, A.S. Synthesis of 1-aryl-4-tosyl-5-(trifluoromethyl)-1H-imidazoles. J. Fluor. Chem. 2014, 163, 34–37. [Google Scholar] [CrossRef]
- Bunev, A.S.; Varakina, E.V.; Khochenkov, D.A.; Peregudov, A.S. 1-Imidoyl-1,2,3-benzotriazoles—Novel reagents for the synthesis of 1-aryl-5-trifluoromethylimidazoles. Russ. J. Org. Chem. 2019, 55, 493–497. [Google Scholar] [CrossRef]
- Fodili, M.; Nedjar-Kolli, B.; Vedrenne, M.; Saffon-Merceron, N.; Lherbet, C.; Hoffmann, P. The first example of an unusual rearrangement in the van Leusen imidazole synthesis. Chem. Heterocycl. Compd. 2015, 51, 940–943. [Google Scholar] [CrossRef]
- Satyam, K.; Murugesh, V.; Suresh, S. The base-free van Leusen reaction of cyclic imines on water: Synthesis of N-fused imidazo 6,11-dihydro β-carboline derivatives. Org. Biomol. Chem. 2019, 17, 5234–5238. [Google Scholar] [CrossRef] [PubMed]
- Necardo, C.; Alfano, A.I.; Del Grosso, E.; Pelliccia, S.; Galli, U.; Novellino, E.; Meneghetti, F.; Giustiniano, M.; Tron, G.C. Aryl azides as forgotten electrophiles in the van Leusen reaction: A multicomponent transformation affording 4-Tosyl-1-arylimidazoles. J. Org. Chem. 2019, 84, 16299–16307. [Google Scholar] [CrossRef]
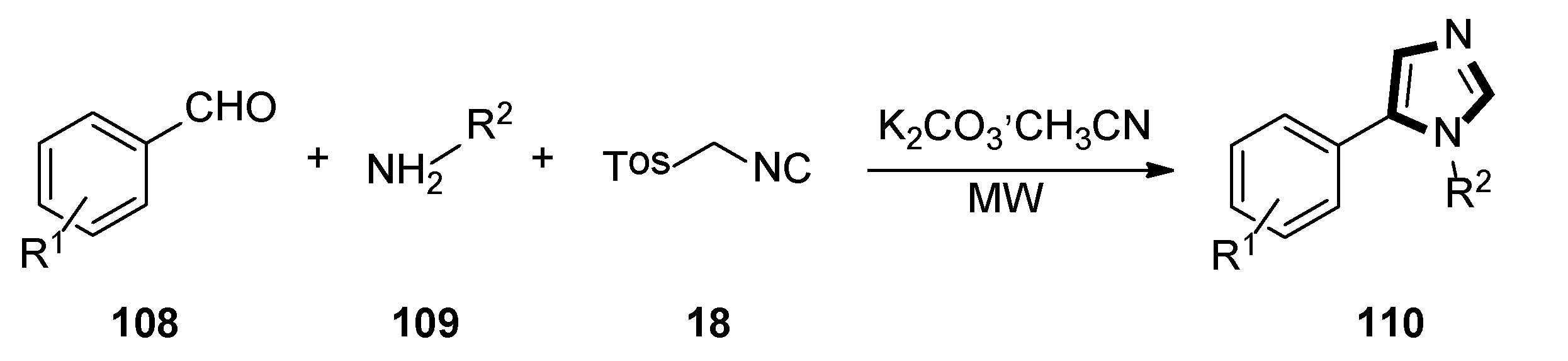
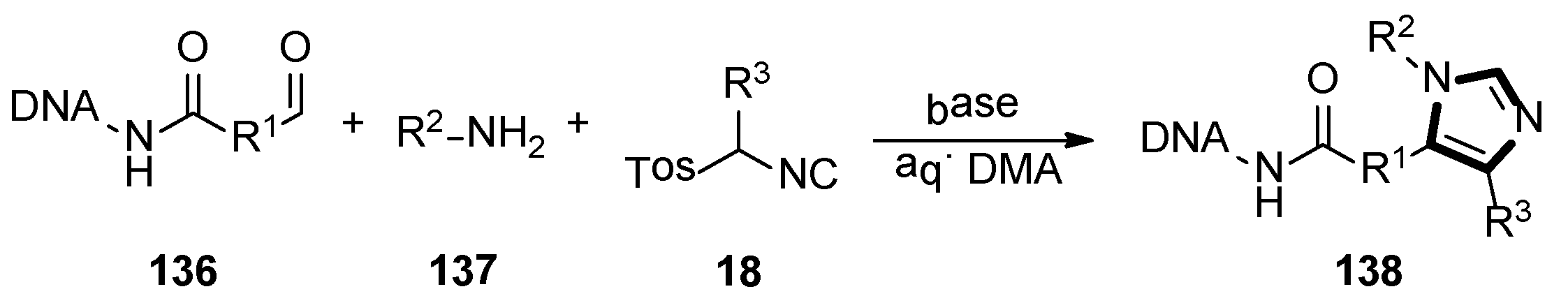
- Geigle, S.N.; Petersen, A.C.; Satz, A.L. Development of DNA-Compatible van Leusen three-component imidazole synthesis. Org. Lett. 2019, 21, 9001–9004. [Google Scholar] [CrossRef]
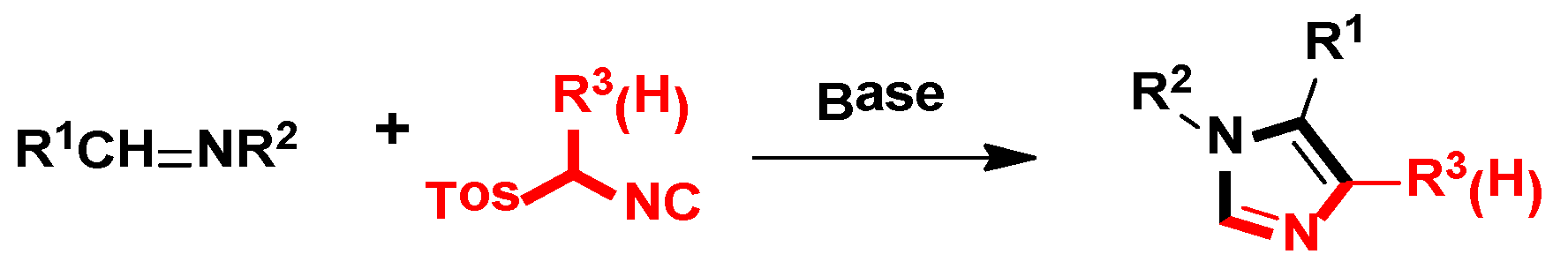
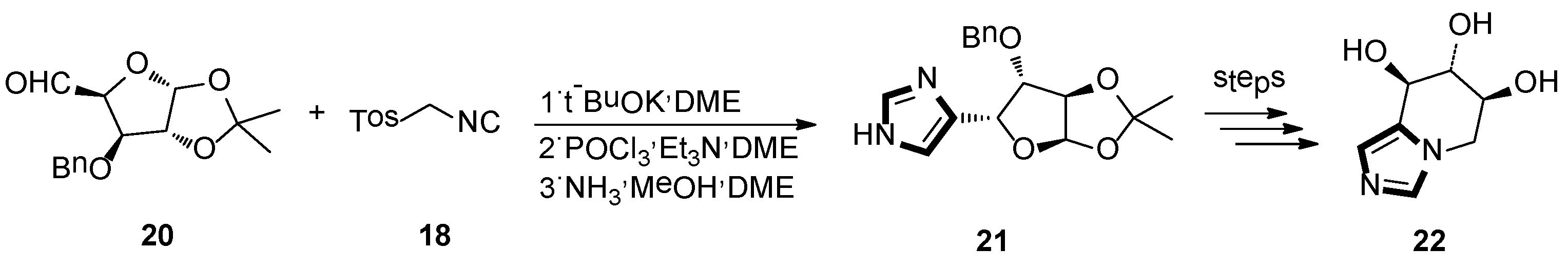

| Pharmacological Activities | Chemical Structures | |
|---|---|---|
| Antibacterial |  | 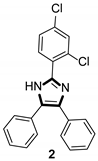 |
| Antifungal | 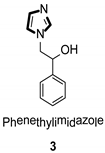 | 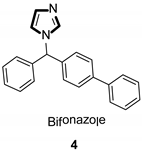 |
| Anti-inflammatory | 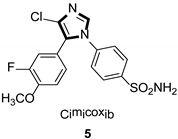 | 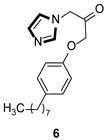 |
| Antiviral |  | 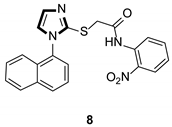 |
| Antiparasitic | 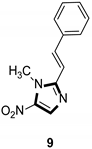 | 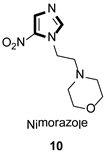 |
| Anticancer | 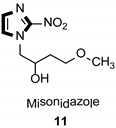 | 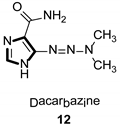 |
| Antihistaminic |  | 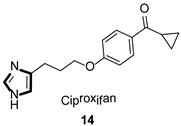 |
| Enzyme inhibitor | 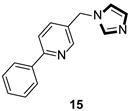 | 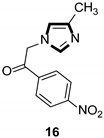 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Ma, Z.; Zhang, D. Synthesis of Imidazole-Based Medicinal Molecules Utilizing the van Leusen Imidazole Synthesis. Pharmaceuticals 2020, 13, 37. https://doi.org/10.3390/ph13030037
Zheng X, Ma Z, Zhang D. Synthesis of Imidazole-Based Medicinal Molecules Utilizing the van Leusen Imidazole Synthesis. Pharmaceuticals. 2020; 13(3):37. https://doi.org/10.3390/ph13030037
Chicago/Turabian StyleZheng, Xunan, Zhengning Ma, and Dawei Zhang. 2020. "Synthesis of Imidazole-Based Medicinal Molecules Utilizing the van Leusen Imidazole Synthesis" Pharmaceuticals 13, no. 3: 37. https://doi.org/10.3390/ph13030037
APA StyleZheng, X., Ma, Z., & Zhang, D. (2020). Synthesis of Imidazole-Based Medicinal Molecules Utilizing the van Leusen Imidazole Synthesis. Pharmaceuticals, 13(3), 37. https://doi.org/10.3390/ph13030037





