Fungal Enzyme l-Lysine α-Oxidase Affects the Amino Acid Metabolism in the Brain and Decreases the Polyamine Level
Abstract
1. Introduction
2. Results
2.1. LO Pharmacokinetics: Enzyme Stays in the Brain Much Longer than in the Bloodstream
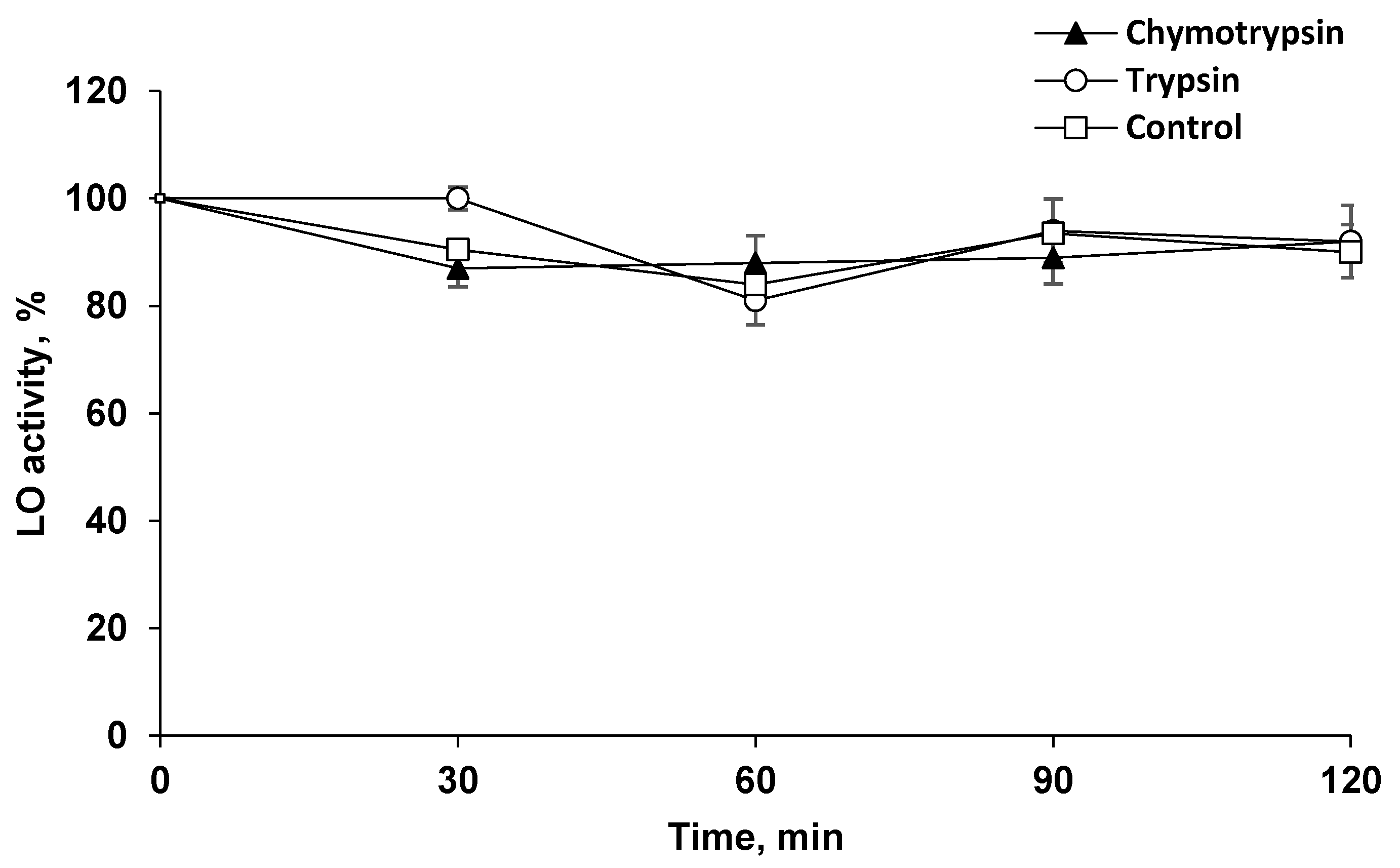
2.2. LO Showed Resistance to Proteolysis
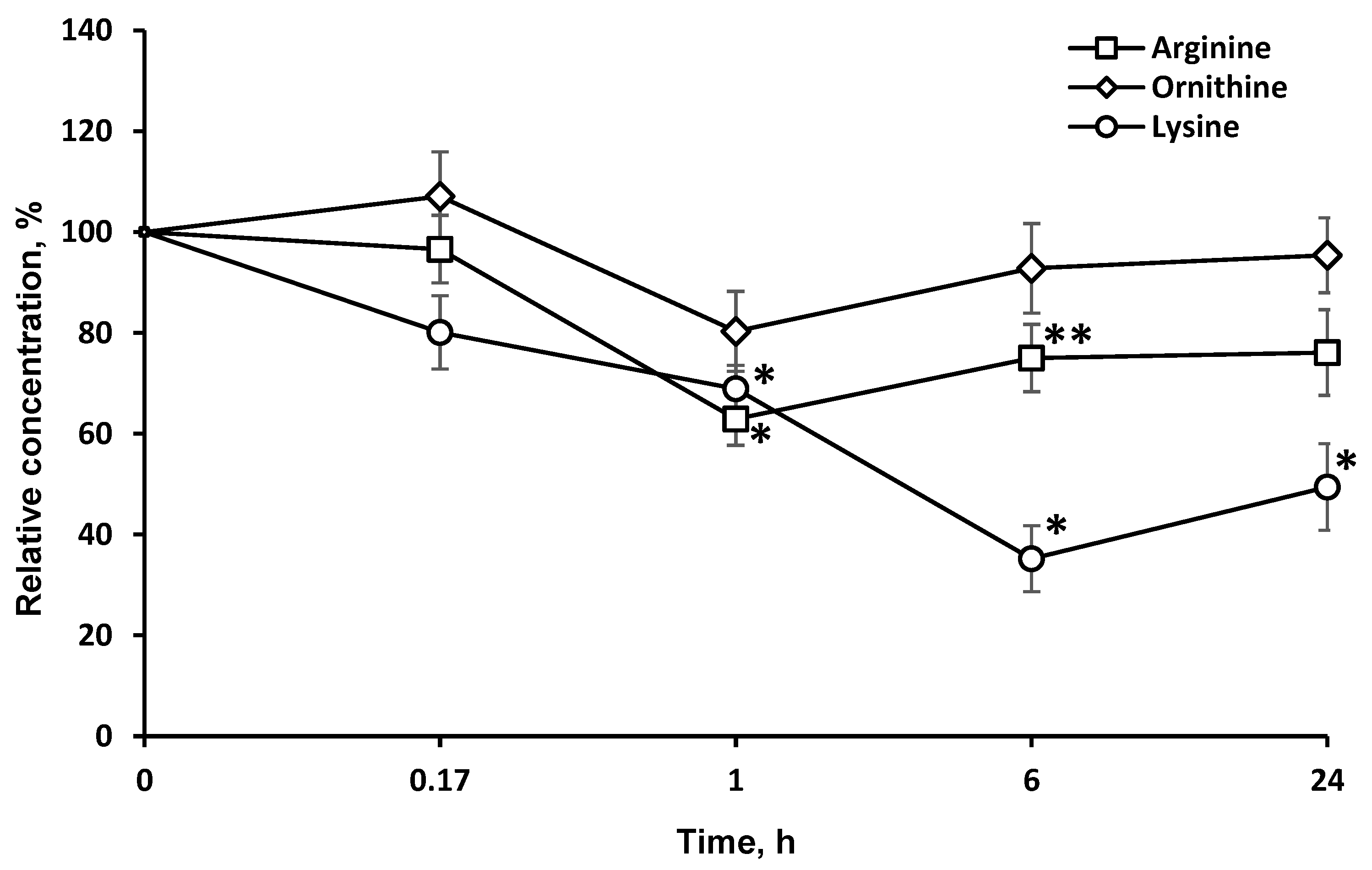
2.3. Concentrations of Target Amino Acids Are Decreased in the Brain
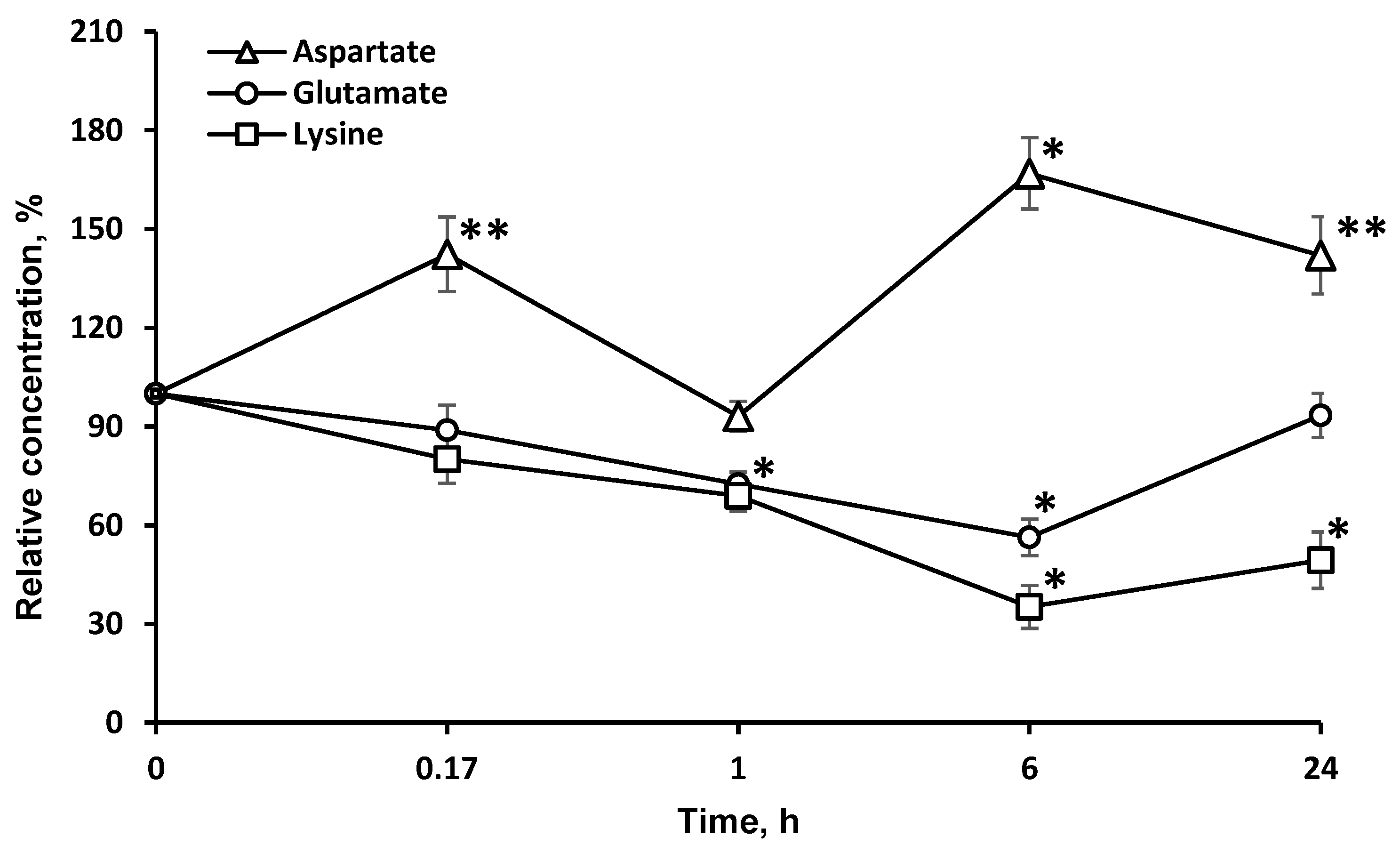
2.4. Concentrations of Amino Acids, Which Are Directly Associated with the Tricarboxylic Acid Cycle (l-asp and l-glu), Are Significantly Changed in the Brain
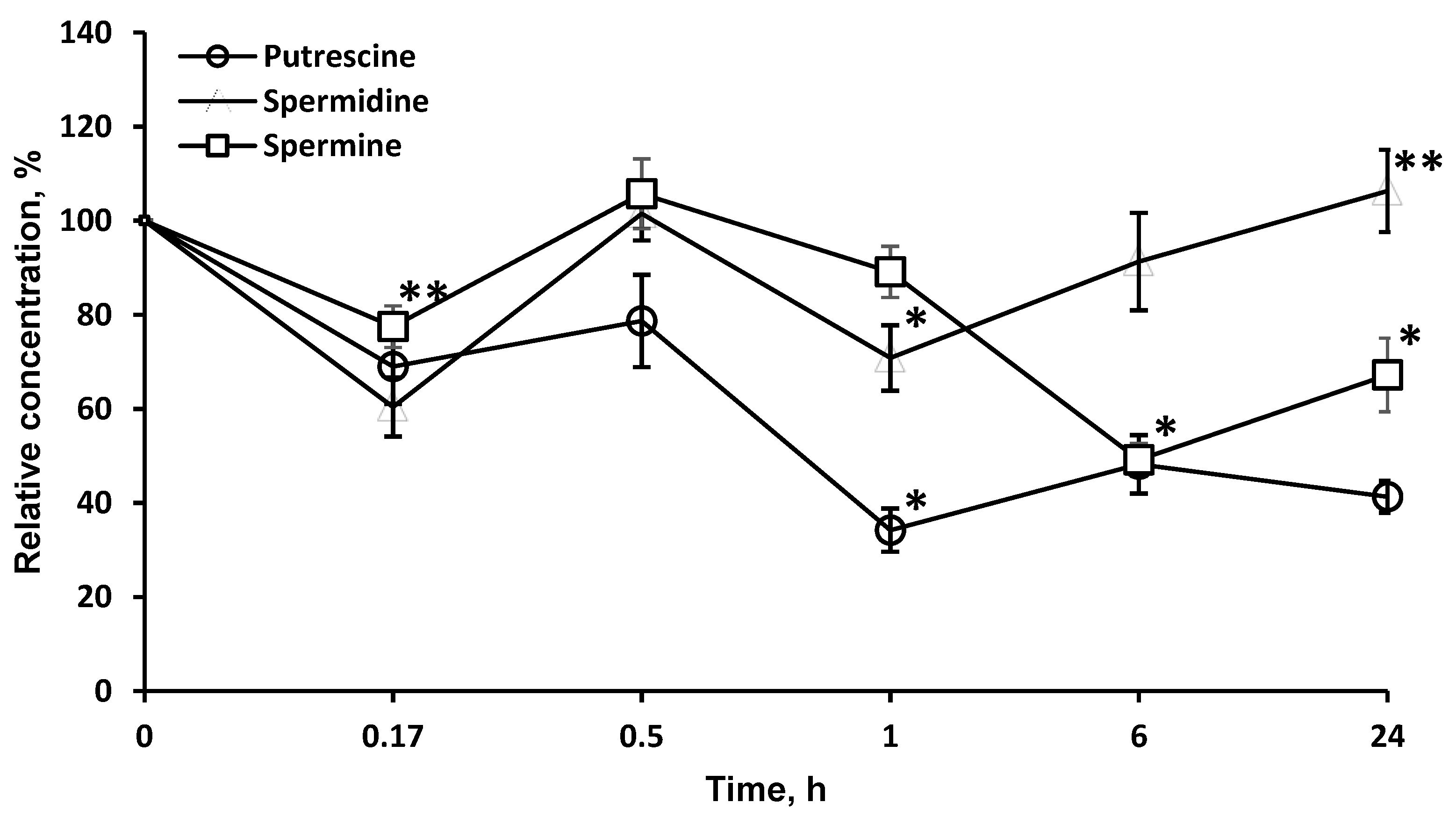
2.5. LO Caused a Significant and Long-Term Decrease of the Polyamines Concentrations
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. l-Lysine α-Oxidase
4.4. Blood Sampling
4.5. Primary Antibodies
4.6. LO Determination in Blood Plasma by Enzyme Immunoassay
4.7. LO Enzymatic Activity
4.8. Protein
4.9. Polyamines (PA)
4.10. Resistance of LO to Proteolysis
4.11. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations:
| LO | l-lysine α-oxidase |
| AA | amino acid |
| PA | polyamines |
| LAAO | l-amino acid oxidase |
| BBB | blood–brain barrier |
| l-lys | l-lysine |
| l-orn | l-ornithine |
| l-arg | l-arginine |
| l-val | valine |
| l-glu | l-glutamic acid |
| l-asp | l-aspartic acid |
References
- Smriga, M.; Torii, K. L-Lysine acts like a partial serotonin receptor 4 antagonist and inhibits serotonin-mediated intestinal pathologies and anxiety in rats. Proc. Natl. Acad. Sci. USA 2003, 100, 15370–15375. [Google Scholar] [CrossRef] [PubMed]
- Smriga, M.; Ando, T.; Akutsu, M.; Furukawa, Y.; Miwa, K.; Morinaga, Y. Oral treatment with L-lysine and L-arginine reduces anxiety and basal cortisol levels in healthy humans. Biomed. Res. 2007, 28, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Duparc, C.; André, C.; Ménard, J.; Godouet-Getti, B.; Wils, J.; Cailleux, A.F.; Moreau-Grange, L.; Louiset, E.; Lefebvre, H. L-Lysine acts as a serotonin type 4 receptor antagonist to counteract in vitro and in vivo the stimulatory effect of serotonergic agents on aldosterone secretion in man. Horm. Metab. Res. 2017, 49, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Jafarnejad, A.; Bathaie, S.Z.; Nakhjavani, M.; Hassan, M.Z.; Banasadegh, S. The improvement effect of L-Lys as a chemical chaperone on STZ-induced diabetic rats, protein structure and function. Diabetes Metab. Res. Rev. 2008, 24, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Sauer, S.W.; Opp, S.; Hoffmann, G.F.; Koeller, D.M.; Okun, J.G.; Kölker, S. Therapeutic modulation of cerebral L-lysine metabolism in a mouse model for glutaric aciduria type I. Brain 2011, 134, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Posset, R.; Opp, S.; Struys, E.A.; Völkl, A.; Mohr, H.; Hoffmann, G.F.; Kölker, S.; Sauer, S.W.; Okun, J.G. Understanding cerebral L-lysine metabolism: The role of L-pipecolate metabolism in Gcdh-deficient mice as a model for glutaric aciduria type I. J. Inherit. Metab. Dis. 2015, 38, 265–272. [Google Scholar] [CrossRef]
- Plecko, B.; Hikel, C.; Korenke, G.C.; Schmitt, B.; Baumgartner, M.; Baumeister, F.; Jakobs, C.; Struys, E.; Erwa, W.; Stöckler-Ipsiroglu, S. Pipecolic acid as a diagnostic marker of pyridoxine-dependent epilepsy. Neuropediatrics 2005, 36, 200–205. [Google Scholar] [CrossRef]
- Houten, S.M.; te Brinke, H.; Denis, S.; Ruiter, J.P.N.; Knegt, A.C.; de Klerk, J.B.C.; Augoustides-Savvopoulou, P.; Häberle, J.; Baumgartner, M.R.; Coşkun, T.; et al. Genetic basis of hyperlysinemia. Orphanet J. Rare Dis. 2013, 8, 1–8. [Google Scholar] [CrossRef]
- Severyanova, L.A.; Lazarenko, V.A.; Plotnikov, D.V.; Dolgintsev, M.E.; Kriukov, A.A. L-Lysine as the Molecule Influencing Selective Brain Activity in Pain-Induced Behavior of Rats. Int. J. Mol. Sci. 2019, 20, 1899. [Google Scholar] [CrossRef]
- Hallen, A.; Jamie, J.F.; Cooper, A.J. Lysine metabolism in mammalian brain: An update on the importance of recent discoveries. Amino Acids 2013, 45, 1249–1272. [Google Scholar] [CrossRef]
- Gupta, R.; Agarwal, A.; Agarwal, S.; Mohan, P.; Husain, M.; Alger, J.; Pandey, C.; Datta, D. Reversal of acute human brain ischemic injury by lysine induced therapeutic angiogenesis: Preliminary results of a pilot study. Internet J. Neurol. 2004, 4, 1–8. [Google Scholar]
- Pokrovsky, V.S.; Treshalina, H.M.; Lukasheva, E.V.; Sedakova, L.A.; Medentzev, A.G.; Arinbasarova, A.Y.; Berezov, T.T. Enzymatic properties and anticancer activity of L-lysine α-oxidase from Trichoderma cf. aureoviride Rifai BKMF-4268D. Anti-Cancer Drugs 2013, 24, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Lukasheva, E.V.; Ribakova, Y.S.; Fedorova, T.N.; Makletsova, M.G.; Arinbasarova, A.Y.; Medentzev, A.G.; Berezov, T.T. The effect of L-lysine alpha-oxidase from Trichoderma cf. aureoviride Rifai VKM F-4268D on the rat pheochromocytoma PC12 cell line. Biochem. Suppl. Ser. B Biomed. Chem. 2014, 8, 130–133. [Google Scholar] [CrossRef]
- Krupyanko, V.I.; Medentsev, A.G.; Lukasheva, E.V.; Arinbasarova, A.Y. Kinetic characteristics of L-lysine α-oxidase from Trichoderma cf. aureoviride Rifai VKM F-4268D: Substrate specificity and allosteric effects. Biochem. Biophys. Rep. 2017, 9, 9–12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Treshalina, H.M.; Tcherkassova, J.R.; Andronova, N.V.; Lukasheva, E.V.; Babayeva, G.; Klinski, E.Y.; Treshchalin, M.I.; Tsurkan, S.A. Modeling of ex vivo internalization method of water-soluble anticancer drugs in small intestine using chemiluminescence. Sib. J. Oncol. 2019, 18, 75–81. [Google Scholar] [CrossRef]
- Asselin, B.L.; Whitin, J.C.; Coppola, D.J.; Rupp, I.P.; Sallan, S.E.; Cohen, H.J. Comparative pharmacokinetic studies of three asparaginase preparations. J. Clin. Oncol. 1993, 11, 1780–1786. [Google Scholar] [CrossRef]
- Puetter, J.; Flenker, I. The behavior of L-asparaginase and L-asparagine in whole mice after asparaginase-application. Eur. J. Drug Metab. Pharmacokinet. 1976, 1, 149–154. [Google Scholar] [CrossRef]
- Morozova, E.A.; Anufrieva, N.V.; Davydov, D.Z.; Komarova, M.V.; Dyakov, I.N.; Rodionov, A.N.; Demidkina, T.V.; Pokrovsky, V.S. Plasma methionine depletion and pharmacokinetic properties in mice of methionine γ-lyase from Citrobacter freundii, Clostridium tetani and Clostridium sporogenes. Biomed. Pharmacother. 2017, 88, 978–984. [Google Scholar] [CrossRef]
- Van Der Meer, L.T.; Terry, S.Y.; van Ingen Schenau, D.S.; Andree, K.C.; Franssen, G.M.; Roeleveld, D.M.; Metselaar, J.M.; Reinheckel, T.; Hoogerbrugge, P.M.; Boerman, O.C.; et al. In vivo imaging of antileukemic drug asparaginase reveals a rapid macrophage-mediated clearance from the bone marrow. J. Nucl. Med. 2017, 58, 214–220. [Google Scholar] [CrossRef]
- Huang, R.Q.; Ke, W.L.; Qu, Y.H.; Zhu, J.H.; Pei, Y.Y.; Jiang, C. Characterization of lactoferrin receptor in brain endothelial capillary cells and mouse brain. J. Biomed. Sci. 2007, 14, 121–128. [Google Scholar] [CrossRef]
- Fillebeen, C.; Descamps, L.; Dehouck, M.P.; Fenart, L.; Benaissa, M.; Spik, G.; Cecchelli, R.; Pierce, A. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. J. Biol. Chem. 1999, 274, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Maeda, J.; Higuchi, M.; Inoue, K.; Akita, H.; Harashima, H.; Suhara, T. Pharmacokinetics and brain uptake of lactoferrin in rats. Life Sci. 2006, 78, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ke, W.; Liu, Y.; Jiang, C.; Pei, Y. The use of lactoferrin as a ligand for targeting the polyamidoamine-based gene delivery system to the brain. Biomaterials 2008, 29, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Tschöp, M.; Robinson, S.M.; Heiman, M.L. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J. Pharmacol. Exp. Ther. 2002, 302, 822–827. [Google Scholar] [CrossRef]
- Pena, I.A.; Marques, L.A.; Laranjeira, Â.B.; Yunes, J.A.; Eberlin, M.N.; MacKenzie, A.; Arruda, P. Mouse lysine catabolism to aminoadipate occurs primarily through the saccharopine pathway; implications for pyridoxine dependent epilepsy (PDE). Biochim. Biophys. Acta Bba Mol. Basis Dis. 2017, 1863, 21–128. [Google Scholar] [CrossRef]
- Chang, Y.F. Pipecolic acid pathway: The major lysine metabolic route in the rat brain. Biochem. Biophys. Res. Commun. 1976, 69, 174–180. [Google Scholar] [CrossRef]
- Chang, Y.F. Lysine metabolism in the human and the monkey: Demonstration of pipecolic acid formation in the brain and other organs. Neurochem. Res. 1982, 7, 577–588. [Google Scholar] [CrossRef]
- Ko, K.C.; Wang, B.; Tai, P.C.; Derby, C.D. Identification of potent bactericidal compounds produced by escapin, an L-amino acid oxidase in the ink of the sea hare Aplysia californica. Antimicrob. Agents Chemother. 2008, 52, 4455–4462. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.; Wang, M.; Chang, Y.; Zhang, F.; Ban, Z.; Tang, R.; Gan, Q.; Wu, S.; Guo, Y.; et al. The lysine catabolite saccharopine impairs development by disrupting mitochondrial homeostasis. J. Cell Biol. 2019, 218, 580–597. [Google Scholar] [CrossRef]
- Treshalina, H.M.; Lukasheva, E.V.; Sedakova, L.A.; Firsova, G.A.; Guerassimova, G.K.; Gogichaeva, N.V.; Berezov, T.T. Anticancer enzyme L-lysine α-oxidase. Appl. Biochem. Biotechnol. 2000, 88, 267–273. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Sayed, N.; Ditsworth, D.; Thompson, C.B. Brick by brick: Metabolism and tumor cell growth. Curr. Opin. Genet. Dev. 2008, 18, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Komatsuzaki, S.; Ediga, R.D.; Okun, J.G.; Kölker, S.; Sauer, S.W. Impairment of astrocytic glutaminolysis in glutaric aciduria type I. J. Inherit. Metab. Dis. 2018, 41, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Makletsova, M.G.; Syatkin, S.P.; Poleshchuk, V.V.; Urazgildeeva, G.R.; Chigaleychik, L.A.; Sungrapova, C.Y.; Illarioshkin, S.N. Polyamines in Parkinson’s Disease: Their Role in Oxidative Stress Induction and Protein Aggregation. J. Neurol. Res. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. The functional role of polyamines in eukaryotic cells. Int. J. Biochem. Cell Biol. 2019, 107, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Gamble, L.D.; Purgato, S.; Murray, J.; Xiao, L.; Denise, M.T.; Hanssen, K.M.; Giorgi, F.M.; Carter, D.R.; Gifford, A.J.; Valli, E.; et al. Inhibition of polyamine synthesis and uptake reduces tumor progression and prolongs survival in mouse models of neuroblastoma. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Kay, J.E.; Lindsay, V.J. Control of ornithine decarboxylase activity in stimulated human lymphocytes by putrescine and spermidine. Biochem. J. 1973, 132, 791–796. [Google Scholar] [CrossRef]
- Hayashi, S.I.; Murakami, Y.; Matsufuji, S. Ornithine decarboxylase antizyme: A novel type of regulatory protein. Trends Biochem. Sci. 1996, 21, 27–30. [Google Scholar] [CrossRef]
- Coffino, P. Regulation of cellular polyamines by antizyme. Nat. Rev. Mol. Cell Biol. 2001, 2, 188–194. [Google Scholar] [CrossRef]
- Skatchkov, S.N.; Antonov, S.M.; Eaton, M.J. Glia and glial polyamines. Role in brain function in health and disease. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2016, 10, 73–98. [Google Scholar] [CrossRef]
- Benedikt, J.; Inyushin, M.; Kucheryavykh, Y.V.; Rivera, Y.; Kucheryavykh, L.Y.; Nichols, C.G.; Eaton, M.J.; Skatchkov, S.N. Intracellular polyamines enhance astrocytic coupling. Neuroreport 2012, 23, 1021. [Google Scholar] [CrossRef]
- Arinbasarova, A.Y.; Ashin, V.V.; Makrushin, K.V.; Medentsev, A.G.; Lukasheva, E.V.; Berezov, T.T. Isolation and properties of L-lysine-α-oxidase from the fungus Trichoderma cf. aureoviride RIFAI VKM F-4268D. Microbiology 2012, 81, 549–554. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sethi, R.; Chava, S.R.; Bashir, S.; Castro, M.E. An improved high performance liquid chromatographic method for identification and quantization of polyamines as benzoylated derivatives. Am. J. Anal. Chem. 2011, 2, 456. [Google Scholar] [CrossRef]
- Morgan, D.M.L. Determination of Polyamines as Their Benzoylated Derivatives by HPLC. In Polyamine Protocols; Morgan, D.M.L., Ed.; Humana Press: Totowa, NJ, USA, 1998; Volume 79, pp. 111–118. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukasheva, E.V.; Makletsova, M.G.; Lukashev, A.N.; Babayeva, G.; Arinbasarova, A.Y.; Medentsev, A.G. Fungal Enzyme l-Lysine α-Oxidase Affects the Amino Acid Metabolism in the Brain and Decreases the Polyamine Level. Pharmaceuticals 2020, 13, 398. https://doi.org/10.3390/ph13110398
Lukasheva EV, Makletsova MG, Lukashev AN, Babayeva G, Arinbasarova AY, Medentsev AG. Fungal Enzyme l-Lysine α-Oxidase Affects the Amino Acid Metabolism in the Brain and Decreases the Polyamine Level. Pharmaceuticals. 2020; 13(11):398. https://doi.org/10.3390/ph13110398
Chicago/Turabian StyleLukasheva, Elena V., Marina G. Makletsova, Alexander N. Lukashev, Gulalek Babayeva, Anna Yu. Arinbasarova, and Alexander G. Medentsev. 2020. "Fungal Enzyme l-Lysine α-Oxidase Affects the Amino Acid Metabolism in the Brain and Decreases the Polyamine Level" Pharmaceuticals 13, no. 11: 398. https://doi.org/10.3390/ph13110398
APA StyleLukasheva, E. V., Makletsova, M. G., Lukashev, A. N., Babayeva, G., Arinbasarova, A. Y., & Medentsev, A. G. (2020). Fungal Enzyme l-Lysine α-Oxidase Affects the Amino Acid Metabolism in the Brain and Decreases the Polyamine Level. Pharmaceuticals, 13(11), 398. https://doi.org/10.3390/ph13110398




