3-Pentylcatechol, a Non-Allergenic Urushiol Derivative, Displays Anti-Helicobacter pylori Activity In Vivo
Abstract
1. Introduction
2. Results
2.1. Confirmation of Infection and Associated Gastric Disorders after H. pylori Inoculation
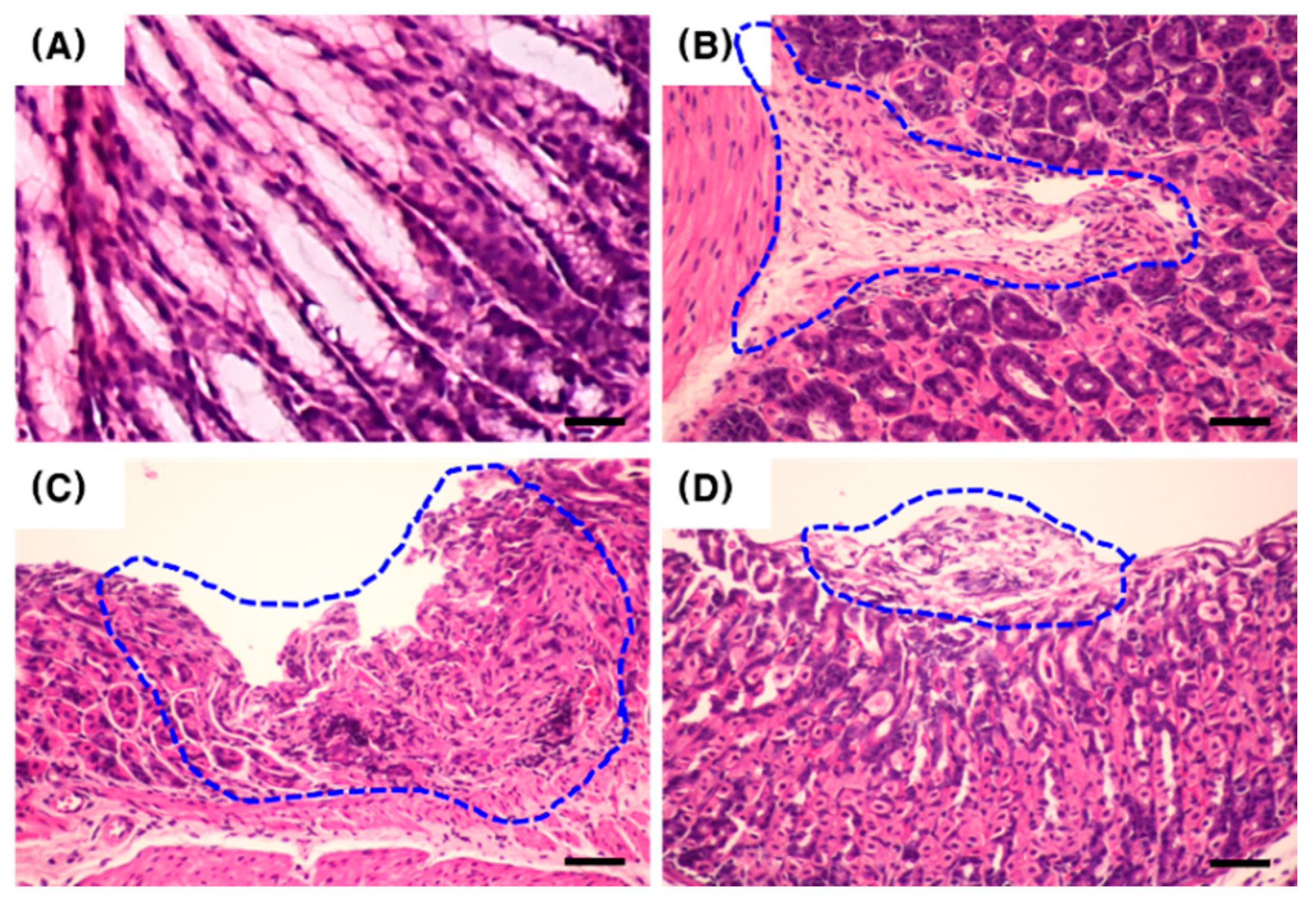
2.2. Effect of PC on the Gastric Tissue Histology of H. pylori-Infected Mice
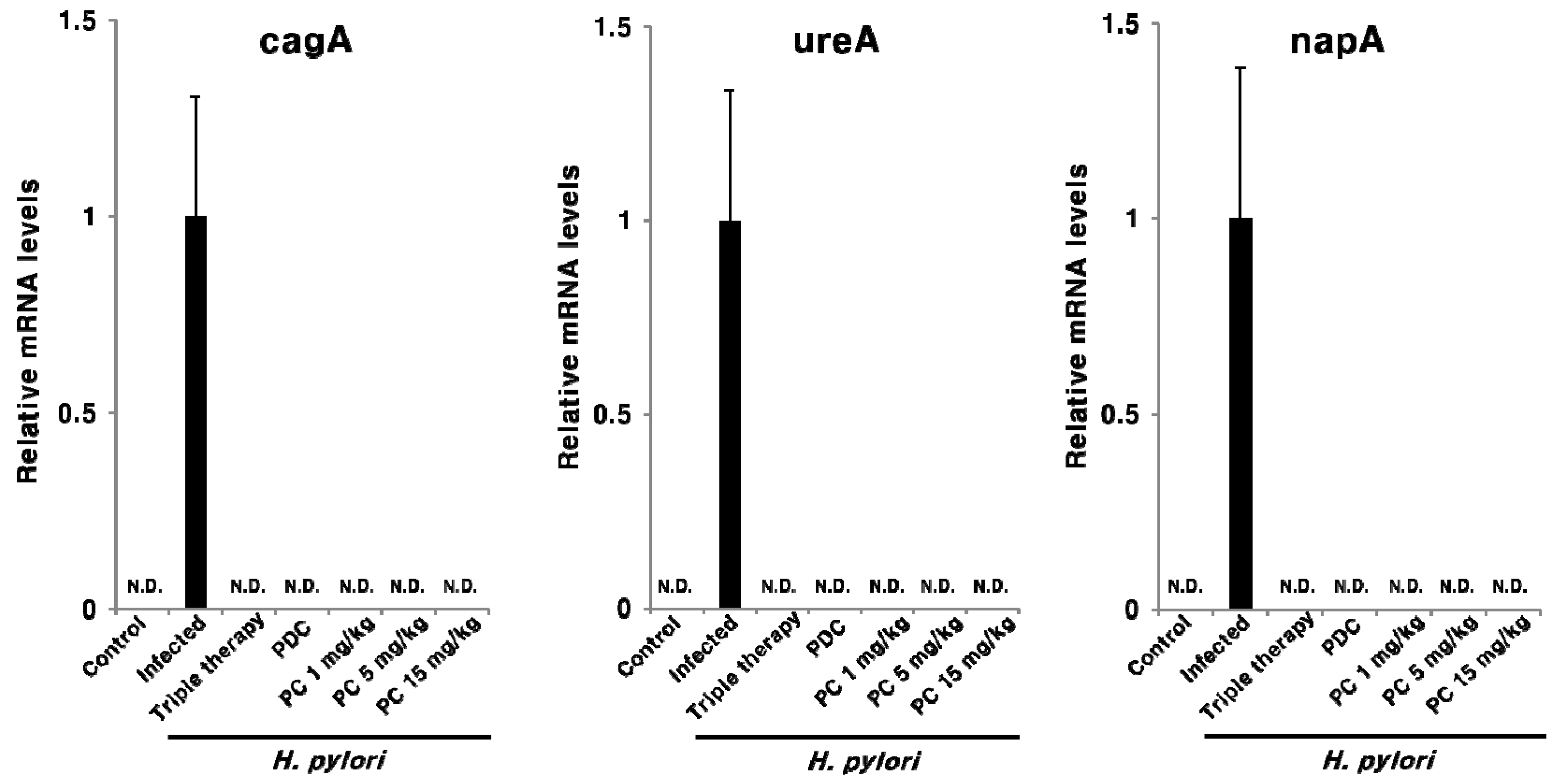
2.3. Effect of PC on H. pylori Eradication and Cytokine Expression
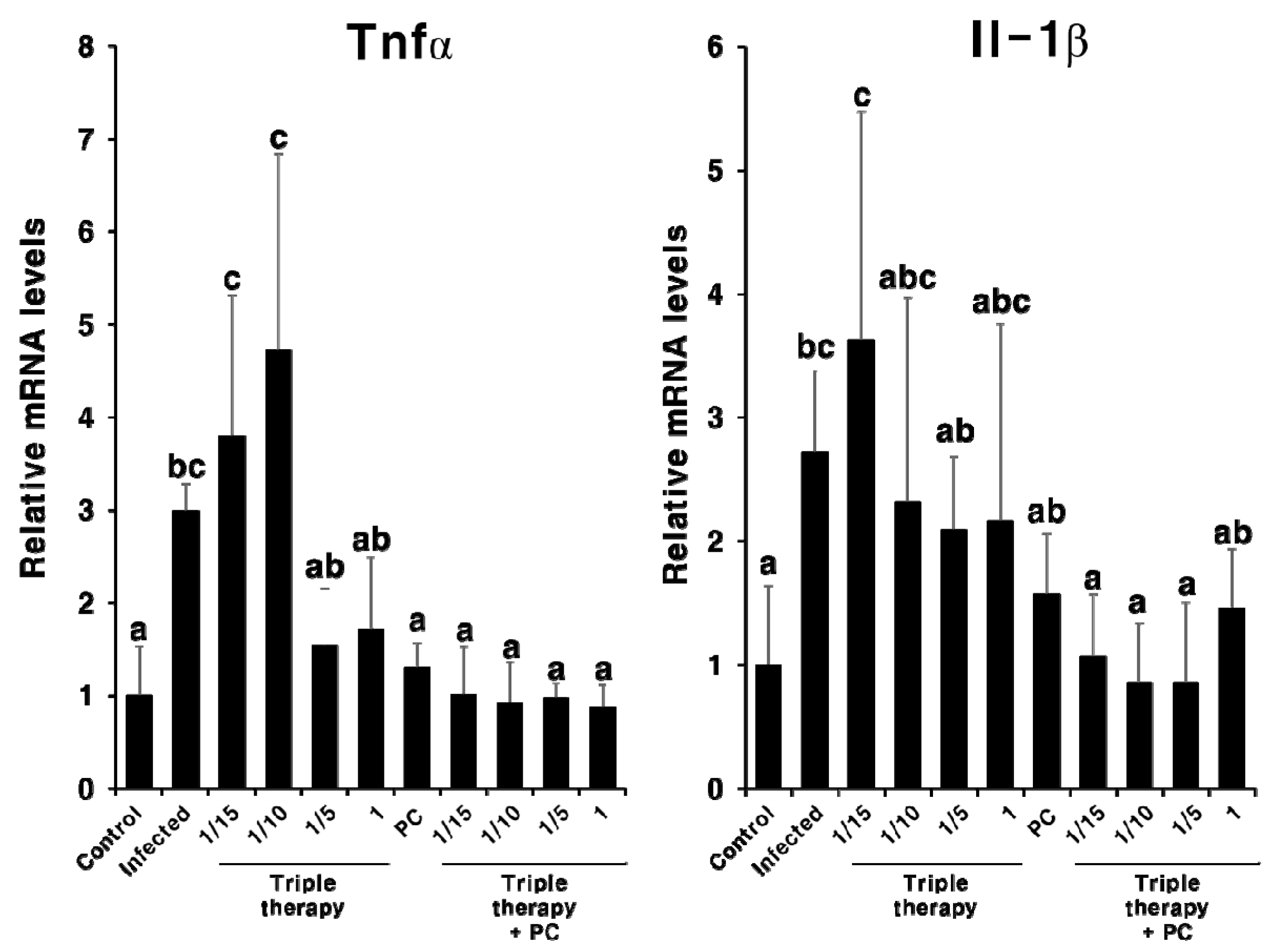
2.4. Synergistic Effect of PC in Combination with Triple Therapy
2.5. Hepatotoxicity of PC
3. Discussion
4. Materials and Methods
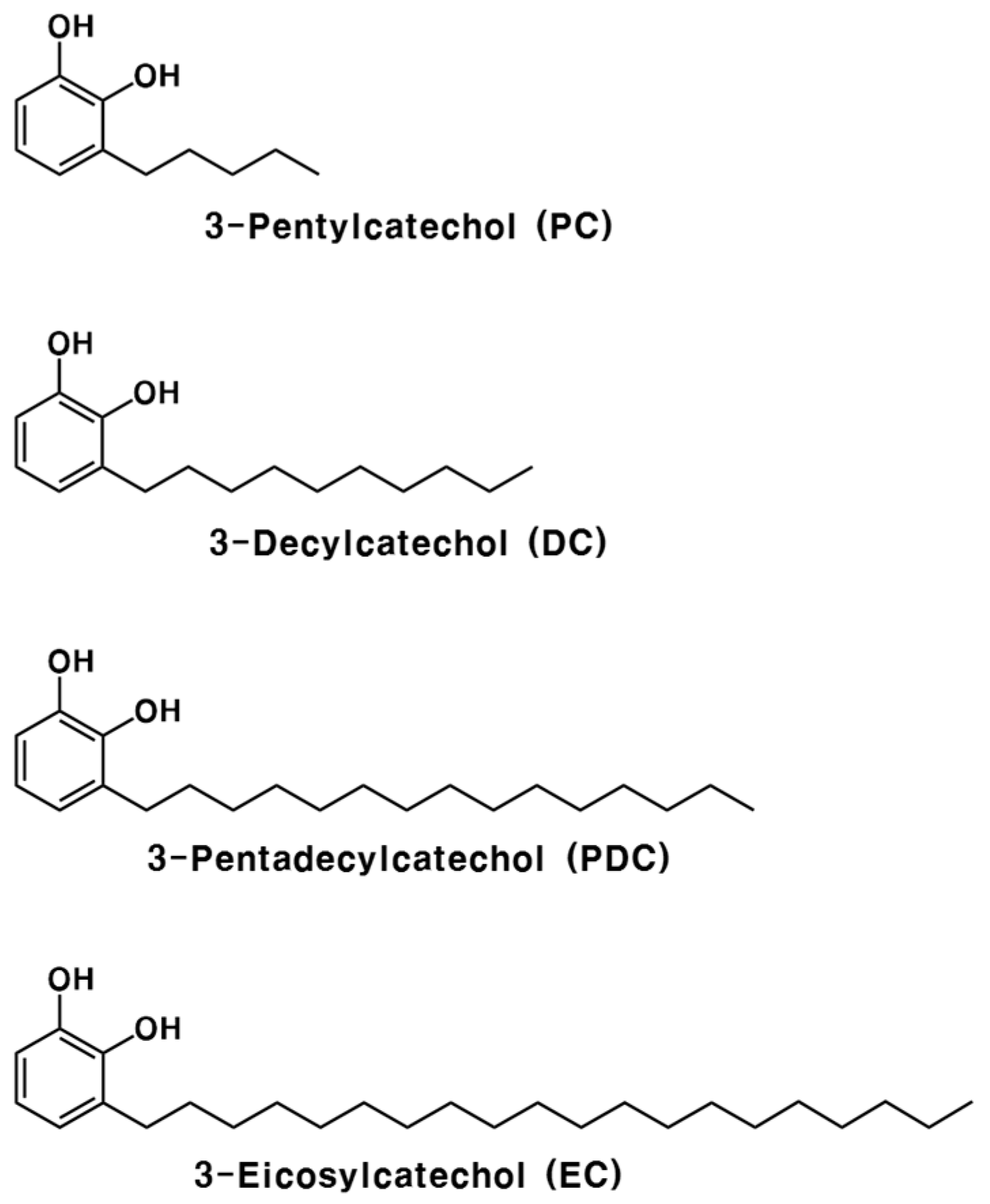
4.1. Chemicals
4.2. H. pylori Strain and Culture Conditions
4.3. Animals and Infection
4.4. PC Treatment after H. pylori Infection
4.5. Histological Examination
4.6. RNA Analysis
4.7. Determination of Plasma Glutamate Oxaloacetate Transaminase (GOT) and Glutamate Pyruvate Transaminase (GPT) Levels
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kusters, J.G.; van Vliet, A.H.M.; Kuipers, E.J. Pathogenesis of Helicobacter Pylori Infection. Clin. Microbiol. Rev. 2006, 19, 449–490. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.J.; Warren, J.R. Unidentified Curved Bacilli in the Stomach of Patients with Gastritis and Peptic Ulceration. Lancet 1984, 1, 1311–1315. [Google Scholar] [CrossRef]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter Pylori Infection and the Development of Gastric Cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, C.S. Helicobacter Pylori Gastritis, Peptic Ulcer, and Gastric Cancer: Clinical and Molecular Aspects. Clin. Infect. Dis. 1997, 25, 1017–1019. [Google Scholar] [CrossRef] [PubMed]
- Parsonnet, J.; Hansen, S.; Rodriguez, L.; Gelb, A.B.; Warnke, R.A.; Jellum, E.; Orentreich, N.; Vogelman, J.H.; Friedman, G.D. Helicobacter Pylori Infection and Gastric Lymphoma. N. Engl. J. Med. 1994, 330, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Shin, H.-R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of Worldwide Burden of Cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Leontiadis, G.I.; Sharma, V.K.; Howden, C.W. Non-Gastrointestinal Tract Associations of Helicobacter Pylori Infection. Arch. Intern. Med. 1999, 159, 925–940. [Google Scholar] [CrossRef]
- Franceschi, F.; Tortora, A.; Gasbarrini, G.; Gasbarrini, A. Helicobacter Pylori and Extragastric Diseases. Helicobacter 2014, 19 (Suppl. 1), 52–58. [Google Scholar] [CrossRef]
- Chey, W.D.; Wong, B.C.Y. American College of Gastroenterology Guideline on the Management of Helicobacter Pylori Infection. Am. J. Gastroenterol. 2007, 102, 1808–1825. [Google Scholar] [CrossRef]
- Delchier, J.C.; Malfertheiner, P.; Thieroff-Ekerdt, R. Use of a Combination Formulation of Bismuth, Metronidazole and Tetracycline with Omeprazole as a Rescue Therapy for Eradication of Helicobacter Pylori. Aliment. Pharmacol. Ther. 2014, 40, 171–177. [Google Scholar] [CrossRef]
- Chuah, S.-K.; Tsay, F.-W.; Hsu, P.-I.; Wu, D.-C. A New Look at Anti-Helicobacter Pylori Therapy. World J. Gastroenterol. 2011, 17, 3971–3975. [Google Scholar] [CrossRef] [PubMed]
- Vakil, N.; Vaira, D. Treatment for H. Pylori Infection: New Challenges with Antimicrobial Resistance. J. Clin. Gastroenterol. 2013, 47, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Gong, E.J.; Yun, S.-C.; Jung, H.-Y.; Lim, H.; Choi, K.-S.; Ahn, J.Y.; Lee, J.H.; Kim, D.H.; Choi, K.D.; Song, H.J.; et al. Meta-Analysis of First-Line Triple Therapy for Helicobacter Pylori Eradication in Korea: Is It Time to Change? J. Korean Med. Sci. 2014, 29, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Takahashi, S.; Suzuki, H.; Sasaki, H.; Nagahara, A.; Asaoka, D.; Matsuhisa, T.; Masaoaka, T.; Nishizawa, T.; Suzuki, M.; et al. Changes in the First Line Helicobacter Pylori Eradication Rates Using the Triple Therapy-a Multicenter Study in the Tokyo Metropolitan Area (Tokyo Helicobacter Pylori Study Group). J. Gastroenterol. Hepatol. 2014, 29 (Suppl. 4), 29–32. [Google Scholar] [CrossRef]
- Ayala, G.; Escobedo-Hinojosa, W.I.; de la Cruz-Herrera, C.F.; Romero, I. Exploring Alternative Treatments for Helicobacter Pylori Infection. World J. Gastroenterol. 2014, 20, 1450–1469. [Google Scholar] [CrossRef]
- Myllyluoma, E.; Veijola, L.; Ahlroos, T.; Tynkkynen, S.; Kankuri, E.; Vapaatalo, H.; Rautelin, H.; Korpela, R. Probiotic Supplementation Improves Tolerance to Helicobacter Pylori Eradication Therapy—A Placebo-Controlled, Double-Blind Randomized Pilot Study. Aliment. Pharmacol. Ther. 2005, 21, 1263–1272. [Google Scholar] [CrossRef]
- Yang, Y.X.; Lohakare, J.; Chae, B. Effects of Lacquer (Rhus Verniciflua) Meal Supplementation on Layer Performance. Asian-Australas. J. Anim. Sci. 2007, 20. [Google Scholar] [CrossRef]
- Hatada, K.; Kitayama, T.; Nishiura, T.; Nishimoto, A.; Simonsick, W.J.; Vogl, O. Structural Analysis of the Components of Chinese Lacquer “Kuro-Urushi”. Macromol. Chem. Phys. 1994, 195, 1865–1870. [Google Scholar] [CrossRef]
- Hong, S.H.; Suk, K.T.; Choi, S.H.; Lee, J.W.; Sung, H.T.; Kim, C.H.; Kim, E.J.; Kim, M.J.; Han, S.H.; Kim, M.Y.; et al. Anti-Oxidant and Natural Killer Cell Activity of Korean Red Ginseng (Panax Ginseng) and Urushiol (Rhus Vernicifera Stokes) on Non-Alcoholic Fatty Liver Disease of Rat. Food Chem. Toxicol. 2013, 55, 586–591. [Google Scholar] [CrossRef]
- Kim, M.; Choi, Y.; Kim, W.; Kwak, S. Antioxidative Activity of Urushiol Derivatives from the Sap of Lacquer Tree (Rhus Vernicifera Stokes). Korean J. Plant Res. 1997, 10, 227–230. [Google Scholar]
- Kim, D.; Jeon, S.; Seo, J. The Preparation and Characterization of Urushiol Powders (YPUOH) Based on Urushiol. Prog. Org. Coat. 2013, 76, 1465–1470. [Google Scholar] [CrossRef]
- Kim, H.S.; Yeum, J.H.; Choi, S.W.; Lee, J.Y.; Cheong, I.W. Urushiol/Polyurethane-Urea Dispersions and Their Film Properties. Prog. Org. Coat. 2009, 65, 341–347. [Google Scholar] [CrossRef]
- Choi, J.Y.; Park, C.S.; Choi, J.O.; Rhim, H.S.; Chun, H.J. Cytotoxic Effect of Urushiol on Human Ovarian Cancer Cells. J. Microbiol. Biotechnol. 2001, 11, 399–405. [Google Scholar]
- Suk, K.T.; Baik, S.K.; Kim, H.S.; Park, S.M.; Paeng, K.J.; Uh, Y.; Jang, I.H.; Cho, M.Y.; Choi, E.H.; Kim, M.J.; et al. Antibacterial Effects of the Urushiol Component in the Sap of the Lacquer Tree (Rhus Verniciflua Stokes) on Helicobacter Pylori. Helicobacter 2011, 16, 434–443. [Google Scholar] [CrossRef]
- Ma, X.M.; Lu, R.; Miyakoshi, T. Recent Advances in Research on Lacquer Allergy. Allergol. Int. 2012, 61, 45–50. [Google Scholar] [CrossRef]
- Zepter, K.; Häffner, A.; Soohoo, L.F.; De Luca, D.; Tang, H.P.; Fisher, P.; Chavinson, J.; Elmets, C.A. Induction of Biologically Active IL-1 Beta-Converting Enzyme and Mature IL-1 Beta in Human Keratinocytes by Inflammatory and Immunologic Stimuli. J. Immunol. 1997, 159, 6203–6208. [Google Scholar]
- Wakabayashi, T.; Hu, D.-L.; Tagawa, Y.-I.; Sekikawa, K.; Iwakura, Y.; Hanada, K.; Nakane, A. IFN-Gamma and TNF-Alpha Are Involved in Urushiol-Induced Contact Hypersensitivity in Mice. Immunol. Cell Biol. 2005, 83, 18–24. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, J.Y.; Ma, Y.K.; Lee, Y.G.; Moon, J.H. Nonallergenic Urushiol Derivatives Inhibit the Oxidation of Unilamellar Vesicles and of Rat Plasma Induced by Various Radical Generators. Free Radic. Biol. Med. 2014, 71, 379–389. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Park, K.Y.; Kim, S.-J.; Oh, S.; Moon, J.-H. Antimicrobial Activity of the Synthesized Non-Allergenic Urushiol Derivatives. Biosci. Biotechnol. Biochem. 2015, 79, 1915–1918. [Google Scholar] [CrossRef]
- Lee, Y.G. Absorption and Metabolism of an Urushiol Derivative, 3-Pentylcathechol, in Rat Plasma. Master’s Thesis, Chonnam National University, Gwangju, Korea, 2013. [Google Scholar]
- Jeong, H.Y. Comparison of Absorption, Metabolism, and Bioactivity among Chemically Synthesized 3-Pentylcatechol and Its Glucosides. Master’s Thesis, Chonnam National University, Gwangju, Korea, 2014. [Google Scholar]
- Noach, L.A.; Bosma, N.B.; Jansen, J.; Hoek, F.J.; van Deventer, S.J.; Tytgat, G.N. Mucosal Tumor Necrosis Factor-Alpha, Interleukin-1 Beta, and Interleukin-8 Production in Patients with Helicobacter Pylori Infection. Scand. J. Gastroenterol. 1994, 29, 425–429. [Google Scholar] [CrossRef]
- Olbe, L.; Hamlet, A.; Dalenbäck, J.; Fändriks, L. A Mechanism by Which Helicobacter Pylori Infection of the Antrum Contributes to the Development of Duodenal Ulcer. Gastroenterology 1996, 110, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Hong, Y.; Olczak, A.; Maier, S.E.; Maier, R.J. Dual Roles of Helicobacter Pylori NapA in Inducing and Combating Oxidative Stress. Infect. Immun. 2006, 74, 6839–6846. [Google Scholar] [CrossRef] [PubMed]
- Tzouvelekis, L.S.; Mentis, A.F.; Makris, A.M.; Spiliadis, C.; Blackwell, C.; Weir, D.M. In Vitro Binding of Helicobacter Pylori to Human Gastric Mucin. Infect. Immun. 1991, 59, 4252–4254. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.C.; Asim, M.; Verriere, T.G.; Piazuelo, M.B.; Suarez, G.; Romero-Gallo, J.; Delgado, A.G.; Wroblewski, L.E.; Barry, D.P.; Peek, R.M.J.; et al. Epidermal Growth Factor Receptor Inhibition Downregulates Helicobacter Pylori-Induced Epithelial Inflammatory Responses, DNA Damage and Gastric Carcinogenesis. Gut 2018, 67, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Berg, H.F.; Maraha, B.; Scheffer, G.-J.; Peeters, M.F.; Kluytmans, J.A.J.W. Effect of Clarithromycin on Inflammatory Markers in Patients with Atherosclerosis. Clin. Diagn. Lab. Immunol. 2003, 10, 525–528. [Google Scholar] [CrossRef]
- Knoop, K.A.; McDonald, K.G.; Kulkarni, D.H.; Newberry, R.D. Antibiotics Promote Inflammation through the Translocation of Native Commensal Colonic Bacteria. Gut 2016, 65, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, T.F.; Farooqui, A.A. Molecular Mechanism Underlying the Therapeutic Activities of Propolis: A Critical Review. Curr. Nutr. Food Sci. 2010, 6, 186–199. [Google Scholar] [CrossRef]
- Johnston, D.E. Special Considerations in Interpreting Liver Function Tests. Am. Fam. Physician 1999, 59, 2223–2230. [Google Scholar]
- Lee, A.; O’Rourke, J.; De Ungria, M.C.; Robertson, B.; Daskalopoulos, G.; Dixon, M.F. A Standardized Mouse Model of Helicobacter Pylori Infection: Introducing the Sydney Strain. Gastroenterology 1997, 112, 1386–1397. [Google Scholar] [CrossRef]
- Lee, S.; Yang, M.; Kim, J.; Son, Y.; Kim, J.; Kang, S.; Ahn, W.; Kim, S.-H.; Kim, J.-C.; Shin, T.; et al. Involvement of BDNF/ERK Signaling in Spontaneous Recovery from Trimethyltin-Induced Hippocampal Neurotoxicity in Mice. Brain Res. Bull. 2016, 121, 48–58. [Google Scholar] [CrossRef]
- Go, D.-H.; Lee, Y.G.; Lee, D.-H.; Kim, J.-A.; Jo, I.-H.; Han, Y.S.; Jo, Y.H.; Kim, K.-Y.; Seo, Y.-K.; Moon, J.-H.; et al. 3-Decylcatechol Induces Autophagy-Mediated Cell Death through the IRE1α/JNK/P62 in Hepatocellular Carcinoma Cells. Oncotarget 2017, 8, 58790–58800. [Google Scholar] [CrossRef] [PubMed]





| Gene | Sequence | |
|---|---|---|
| Forward | Reverse | |
| Rplp0 | GTGCTGATGGGCAAGAAC | AGGTCCTCCTTGGTGAAC |
| Tnfα | CGAGTGACAAGCCTGTAGCC | AGCTGCTCCTCCACTTGGT |
| Il-1β | ATGAGAGCATCCAGCTTCAA | TGAAGGAAAAGAAGGTGCTC |
| cagA | CCGATCGATCCGAAATTTTA | CGTTCGGATTTGATTCCCTA |
| ureA | TGTTGGCGACAGACCGGTTCAAATC | GCTGTCCCGCTCGCAATGTCTAAGC |
| napA | CCATGTGCATAAAGCCACTG | GAGTTTGAGCGCTTCGGATA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, H.Y.; Lee, T.H.; Kim, J.G.; Lee, S.; Moon, C.; Truong, X.T.; Jeon, T.-I.; Moon, J.-H. 3-Pentylcatechol, a Non-Allergenic Urushiol Derivative, Displays Anti-Helicobacter pylori Activity In Vivo. Pharmaceuticals 2020, 13, 384. https://doi.org/10.3390/ph13110384
Jeong HY, Lee TH, Kim JG, Lee S, Moon C, Truong XT, Jeon T-I, Moon J-H. 3-Pentylcatechol, a Non-Allergenic Urushiol Derivative, Displays Anti-Helicobacter pylori Activity In Vivo. Pharmaceuticals. 2020; 13(11):384. https://doi.org/10.3390/ph13110384
Chicago/Turabian StyleJeong, Hang Yeon, Tae Ho Lee, Ju Gyeong Kim, Sueun Lee, Changjong Moon, Xuan Trong Truong, Tae-Il Jeon, and Jae-Hak Moon. 2020. "3-Pentylcatechol, a Non-Allergenic Urushiol Derivative, Displays Anti-Helicobacter pylori Activity In Vivo" Pharmaceuticals 13, no. 11: 384. https://doi.org/10.3390/ph13110384
APA StyleJeong, H. Y., Lee, T. H., Kim, J. G., Lee, S., Moon, C., Truong, X. T., Jeon, T.-I., & Moon, J.-H. (2020). 3-Pentylcatechol, a Non-Allergenic Urushiol Derivative, Displays Anti-Helicobacter pylori Activity In Vivo. Pharmaceuticals, 13(11), 384. https://doi.org/10.3390/ph13110384