Abstract
LKB1 is frequently mutated in non-small cell lung cancer (NSCLC). LKB1-mutated NSCLCs often have a dismal prognosis and receive lower benefit from the currently available therapies. LKB1 acts as a cell emergency brake in low-energy conditions, by modulating the activity of crucial anabolic enzymes. Thus, loss of LKB1 activity leads to the enhancement of tumor cell proliferation also under conditions of energy shortage. This unrestrained growth may be exploited as an Achilles heel in NSCLC, i.e., by inhibiting mitochondrial respiration. Recently, clinical trials have started to investigate the efficacy of metabolism-based treatments in NSCLCs. To date, enrollment of patients within these trials is based on LKB1 loss of function status, defined by mutation in the gene or by complete absence of immunohistochemical staining. However, LKB1 impairment could be the consequence of epigenetic regulations that partially or completely abrogate protein expression. These epigenetic regulations result in LKB1 wild-type tumors with aggressiveness and vulnerabilities similar to those of LKB1-mutated ones. In this review, we introduced the definition of the “LKB1less phenotype”, and we summarized all currently known features linked to this status, in order to optimize selection and treatment of NSCLC patients with impaired LKB1 function.
1. Introduction
STK11 (Liver Kinase B1—LKB1) is a tumor suppressor gene encoding an evolutionary conserved serine/threonine kinase. The LKB1 protein, which belongs to the calcium calmodulin family, consists of an N-terminal domain containing a nuclear localization signal and a phosphorylation site with unknown function at Serine 31, a central kinase domain, and a C-terminal domain. Intracellular localization and function of LKB1 are controlled by its interactions with STRAD and the armadillo repeat-containing mouse protein 25 (Mo25) [1]. LKB1 regulates the activity of 14 downstream kinases of the AMP-activated kinase catalytic subunit alpha 1 (AMPK) family [2]. AMPK is activated during metabolic stress, and in particular, in conditions of increased AMP/ATP and ADP/ATP ratios as a consequence of impaired glycolysis, mitochondrial metabolism, or other pathways, resulting in the inhibition of ATP biosynthesis. Once activated, AMPK catalyzes phosphorylation events that lead to the inhibition of acetyl-CoA carboxylase alpha (ACC1), 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), and the mammalian target of rapamycin (mTOR) kinases [3], which are involved in fatty acid, cholesterol, and protein biosynthesis, respectively. By restraining the activity of these crucial anabolic enzymes, the LKB1–AMPK axis promotes energy storage and preserves intracellular ATP levels, while at the same time activating catabolic processes, such as autophagy, which lead to increased intracellular metabolic reserves. In specific conditions leading to an impairment of intracellular bioenergetics, such as cell treatment with inhibitors of mitochondrial metabolism (e.g., metformin; phenformin), the LKB1–AMPK axis can result in the activation of glucose uptake and glycolysis, which can compensate for the lack of intracellular metabolites/energetic units by promoting glucose metabolism and ATP biosynthesis. On the other hand, genetic/epigenetic inactivation of LKB1 inactivation can make cells sensitive to metformin or other inhibitors of mitochondrial respiration by restraining their ability to upregulate glucose uptake and glycolysis upon inhibition of mitochondrial metabolism [4,5,6]. In the last two decades, LKB1 has emerged as a tumor suppressor protein. Indeed, germinal mutations in the LKB1 gene cause the Peutz–Jeghers syndrome (PJS) [7,8,9,10], an autosomal dominant inherited condition linked to an increased risk for the development of hamartomatous polyps in the digestive tract, and of melanosis. Of note, PJS patients have been reported to have a higher risk of developing pancreatic, cervical, and gastrointestinal cancers [11,12,13]. In addition, somatic LKB1 mutations leading to LKB1 inactivation have been reported in malignant melanoma [14], cervical cancer [15], pancreatic cancer [16], breast cancer [17], and lung cancer [18]. In particular, LKB1 is mutated in up to 30% of non-small cell lung cancers (NSCLC), and represents the third most frequently mutated gene in these tumors. Overall, more than 400 unique mutations have been described in the LKB1 locus, with 70% of them leading to LKB1 protein truncation, while the remaining 30% represents missense mutations. Notably, many of these missense mutations lead to a loss of LKB1 oncosuppressive activity by affecting LKB1 kinase activity or localization, as well as LKB1 protein stability [19]. Besides inactivating/truncating mutations, other mechanisms may have a role in the regulation of LKB1 expression, including increased protein degradation, as described in osteosarcomas [20], or LKB1 promoter hypermethylation [21,22,23]. Moreover, small non-coding RNA members of the miR-17~92 microRNA cluster have been reported to target the LKB1 3′UTR region in lymphoma models, thus leading to reduced LKB1 protein translation [24,25,26] (Figure 1). Therefore, mutation analysis of the LKB1 gene may not be sufficient to identify patients with impaired oncosuppressive LKB1 activity.
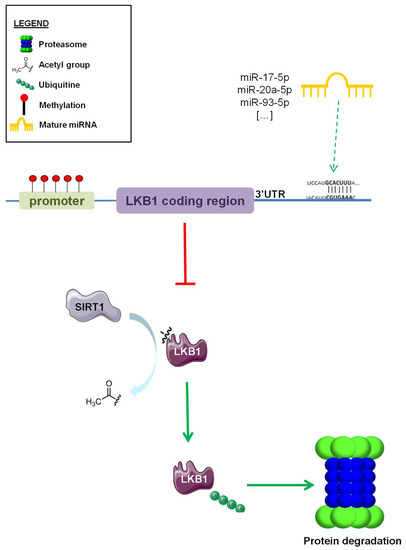
Figure 1.
Epigenetic regulation of LKB1 expression. Different systems of epigenetic regulation of LKB1 protein expression. Promoter hypermethylation, miRNA-mediated modulation of LKB1 translation (i.e., miR-17-5p, miR-20a-5p, and miR-93-5p), and SIRT1-induced deacetylation and subsequent ubiquitination and proteasome degradation of LKB1. Green arrows indicate ubiquitination and proteasome degradation, red “T” arrow indicate inhibition of protein expression.
In this paper, we review the state of the art of the impact of LKB1 inactivation on the prognosis of NSCLC patients, highlighting the peculiar and aggressive features of this subtype of NSCLC. Then, we focus on tumor and microenvironment (TME)-related characteristics which can result in impaired LKB1 activity in parallel with inactivating LKB1 mutations. In particular, the definition of a LKB1less phenotype might be useful to improve the selection of NCSLC patients with poor clinical outcomes as a result of impaired LKB1–AMPK axis activation, and who may be candidate to receive experimental systemic treatments. Finally, we describe ongoing clinical trials aiming to target LKB1 alterations in NSCLC patients.
2. Role of LKB1 in NSCLC Tumor Cell Biology
LKB1 is involved in many energy-related cellular processes. Besides its “classical” role as a regulator of fatty acid, cholesterol, and protein biosynthesis, the LKB1/AMPK axis is involved in the translocation of intracellular GLUT4 to the cell surface, therefore regulating glucose uptake and modulating glycolysis [4]. Enhanced turnover of macromolecules and organelles via autophagy to produce amino acids is another process that relies on LKB1–AMPK axis activity, and which takes part in cells’ response to nutrient starvation and bioenergetic stress [27]. Moreover, LKB1 is involved in the regulation of angiogenesis by modulating the expression of genes taking part in reactive oxygen species (ROS) homeostasis, such as NADPH oxidase 1 (NOX1) [28]. The connection between LKB1 and angiogenesis is witnessed also by its role in suppressing the expression of VEGF, bFGF, MMP-2, and MMP-9 [29]. Furthermore, LKB1 loss has been linked to induction of epithelial-to-mesenchymal transition (EMT) [30] through the regulation of cell polarity and motility, and the modulation of FAK phosphorylation and CDC42 activation [31].
LKB1 has been reported to have tumor suppressor activity also in AMPK-independent ways. Indeed, LKB1 loss leads to the upregulation of the EMT transcription factor SNAIL1. This effect is mediated by LKB1-induced suppression of SNAIL1 expression in a way that depends on AMPK-related kinases MARK1 and MARK4, but not on AMPK itself [32]. LKB1 has been reported to activate two other AMPK-related kinases, namely NUAK1 and NUAK2, which are involved in cell adhesion through the regulation of myosin phosphatase complexes [33]. Recently, the role of salt-inducible kinases (SIK1, 2, and 3) as downstream effectors of LKB1 has been also highlighted. The LKB1–SIK axis, which plays a key role in regulating gluconeogenesis in the liver [34], has been identified as a metastasis suppressor pathway acting as a key modulator of P53-dependent anoikis [35]. Interestingly, LKB1- and SIK-deficient lung tumors showed similar gene expression profiles, and a SIK-ness gene-expression signature has been found to be enriched in LKB1-mutated human lung adenocarcinomas. Moreover, the SIK-ness gene signature was reported to be regulated by LKB1 in vitro [36]. To note, the presence of characteristic patterns of gene expression seems to be a hallmark of LKB1 loss of function [37,38,39,40]. A great challenge to improve LKB1less status definition is represented by the difficulty to integrate these gene expression signatures with IHC staining of LKB1 and downstream effectors, as well as with LKB1 mutational status.
3. Role of LKB1 Mutations in the Interactions with Tumor Microenvironment (TME)
Intelligence can be described as the ability to retain information perceived from an environment, and to apply this knowledge for adaptive behaviors. This definition seems to fit to NSCLC with impaired LKB1 function. Indeed, NSCLCs with inactive LKB1 seem to be perfectly adapted to grow in harsh conditions and to defend themselves from immune system attacks as well as from almost all therapeutic interventions. Thus, investigating the crosstalk between LKB1-inactive cancer cells and cells in their environment may offer important insights to fully characterize this subset of NSCLCs and to finally achieve a clearer definition of the LKB1less phenotype (Figure 2).
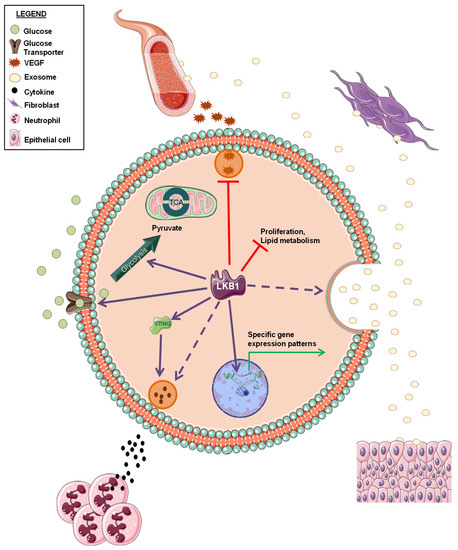
Figure 2.
Schematic representation of LKB1 biological functions within tumor cells and in the interactions with tumor microenvironment. Solid and dashed arrows indicate a positive regulation by LKB1 (already published or not, respectively). Red “T” arrows indicate a negative regulation by LKB1.
LKB1 alterations have been reported to be marker of tumor resistance to immune checkpoint blockade [41,42]. Interestingly, LKB1 loss has been related to a specific immune microenvironment, characterized by production of pro-inflammatory cytokines, a decrease in tumor-infiltrating lymphocytes and PD-L1 expression on tumor cells, and increase in neutrophils recruitment [43]. Moreover, Kitajima and colleagues demonstrated that LKB1 loss leads to the suppression of stimulator of interferon genes (STING) [44], whose activation is critical for anticancer immune response [45].
Whilst some studies have highlighted the role of LKB1 loss in modulating a specific immune TME, and a role of LKB1/AMPK pathway in regulating angiogenesis has been reported, very little information is available about the involvement of LKB1 in the crosstalk between tumor cells and other components in TME. A prominent component of TME is represented by cancer-associated fibroblasts (CAFs). CAFs are a heterogeneous population of cells that play a key role in regulating tumorigenesis [46]. Investigating if the metabolic changes resulting from LKB1 loss may also affect TME cell function, including CAFs, could broaden our knowledge about the LKB1less phenotype, thus potentially suggesting new therapeutic strategies for LKB1-inactive NSCLC treatment. Interestingly, extracellular vesicles (EVs) have been also identified as players in the propagation of tumor cell signals to TME. Regarding the interaction with fibroblasts, Minciacchi et al. demonstrated that in preclinical models of prostate cancer, EV uptake induced a specific “reprogramming” of the fibroblasts towards a pro-tumorigenic phenotype [47]. Beside fibroblasts modulation, EVs’ role in metastasis formation [48] as well as in interactions between tumor cells and endothelial [49,50] or epithelial cells [51] have been described. Interestingly, recent data show that LKB1 alterations in cancer cells affects the release and the content of EVs [52,53]. However, the impact of these alterations on tumor cell–TME crosstalk has not been elucidated yet. Further investigation on the effect of LKB1 loss on EVs formation and cargo may uncover new EVs-mediated molecular mechanisms underlying the aggressiveness and malignancy of LKB1less tumors.
4. Evidence of LKB1 Impairment in Promoting Lung Tumorigenesis
LKB1 has well-known tumor-suppressive functions in lung cells. Indeed, an impaired function of LKB1 can promote oncogenic transformation of lung epithelial cells (LEC) to malignant cells. Interestingly, aberrant methylation of the LKB1 promoter was more frequently detected in smokers than in non-smokers, thus suggesting a role of LKB1 loss in smoke-induced lung cancer development [54]. Of note, LKB1 promoter methylation correlated with worse patient overall survival (OS). Data supporting the involvement of LKB1 inactivation in NSCLC development have been previously reported by Sasai and colleagues, who studied the in vivo capacity of Human Primary Small Airway Epithelial cells (HSAEC) to be xenografted in nude mice [55]. Interestingly, HSAECs showed enhanced in vivo tumorigenicity only when previously infected with a dominant-negative form of LKB1 together with retroviruses expressing KRAS, CDK4, hTERT, and a dominant-negative form of p53 [55]. Similar experiments carried out in human bronchial epithelial cells (HBECs) demonstrated that the overexpression of c-MYC greatly enhances the formation of murine malignancies, but only in the context of sh-p53+KRASV12 HBEC [56]. Together, these data highlight the essential function of metabolic regulators, like LKB1 or c-MYC, in the malignant transformation process of lung epithelial cells (LECs). Of note, this role is appreciable only in oncogene-driven genetic backgrounds (i.e., KRAS-mutated cells), thus suggesting that LKB1 loss might occur as a secondary oncogenic lesion that facilitates tumor progression in cells with one constitutively active oncogene. This hypothesis is supported by preclinical data indicating an implication of LKB1 loss in lung cancer initiation, differentiation, and metastasis formation [57]. Indeed, in a conditionally activatable Lox-Stop-Lox KrasG12D mouse model [58], loss of LKB1 function resulted in faster tumor development, expanded histological lung cancer spectrum (adeno-, squamous, and large-cell carcinoma) and higher metastasis frequency compared to mice lacking other oncosuppressor genes like p53 or Ink4a/Arf [57]. Interestingly, in these preclinical models, LKB1 loss alone did not result in tumor formation.
5. Clinical Significance of LKB1 Alterations in NSCLC
5.1. Prognostic Significance of LKB1 Status
A prognostic role of LKB1 loss, also in a KRAS-mutated background, in NSCLC patients has not yet been incontrovertibly proved. Calles et al. reported that KRAS-mutant NSCLC patients with concurrent loss of LKB1 immunohistochemistry (IHC) expression experienced a higher number of metastatic lesions (especially extrathoracic and brain metastases) at the time of tumor diagnosis; however, no significant differences, in terms of OS, were observed in patients whose tumors lost LKB1 IHC expression [59]. Our group recently performed a post hoc analysis in advanced NSCLC patients enrolled in the multicenter, open label, randomized phase III trial TAILOR; we found that patients with KRAS/LKB1 (KL) co-mutated tumors did not show significantly worse OS when compared to patients with KRAS-mutated neoplasms [60]. However, the lack of significant decrease in patient’s overall survival (OS) may be due to the extremely short OS of stage IV NSCLC in Calles’ study and to the selection of LKB1-mutated, rather than IHC-negative, tumors in our series.
5.2. Predictive Significance of LKB1
Clinical data regarding the impact of LKB1 inactivation on the efficacy of standard NSCLC treatment mainly derive from retrospective clinical series or from subgroup analysis of prospective studies. No data from specific prospective trials are available yet.
The majority of available data indicate that LKB1 inactivation is associated with lower benefit from immunotherapy in patients with advanced NSCLC. In a pooled analysis of different cohorts of patients with KRAS-mutated NSCLC, including 44 patients treated in the CheckMate-057 study, Skoulidis et al. showed that LKB1-deficient NSCLC patients treated with PD-1 axis inhibitors had significantly shorter PFS and OS when compared with patients with LKB1-proficient tumors. In this study, LKB1 deficiency was defined as the presence of inactivating LKB1 mutations, or as the lack of LKB1 protein expression at IHC analysis (H-score equal to zero). Interestingly, 17.6% of LKB1 wild-type tumors showed absence of LKB1 protein expression by IHC, thus confirming that genetic (i.e., mutational) LKB1 assessment might be insufficient to predict LKB1 functional status [41]. Another interesting finding of this study was the fact that LKB1-deficient tumors are characterized by lower PD-L1 expression, which could at least, in part, explain the low efficacy of anti-PD-1 immunotherapy agents.
In another study pulling data from several phase I/II trials, treatment with durvalumab (with or without tremelimumab) was associated with shorter patient OS and lower tumor response rates in patients with LKB1-mutated, advanced non-squamous NSCLC when compared to patients with LKB1 wild-type tumors [42].
More recently, a retrospective analysis in advanced NSCLC patients treated with first-line platinum-based chemotherapy with or without pembrolizumab revealed that patients with advanced LKB1-mutant non-squamous NSCLC do not achieve clinical benefit from pembrolizumab addition to chemotherapy. Consistent with previously discussed results, in this study, the authors found the percentage of tumors showing strongly positive PD-L1 expression (TPS ≥ 50%) to be significantly lower in LKB1-mutated than in LKB1 wild-type specimens (6.6% versus 21.7%, respectively) [61].
The negative prognostic role of LKB1 inactivation was confirmed in a large-scale, real-world, retrospective study conducted in 2407 patients with advanced NSCLC, which showed that patients with LKB1-mutated tumors (13.6% of the whole population) have worse PFS and OS when treated with either chemotherapy or immunotherapy, in both first- and in second-line settings [62].
Finally, in an exploratory analysis of the phase 3 MYSTIC trial, OS was shorter in patients with LKB1-mutated tumors when compared to patients with LKB1 wild-type tumors, irrespective of the treatment received (i.e., immunotherapy or chemotherapy). Additionally, in this study, the authors reported a positive correlation between LKB1 mutations and lower intratumor PD-L1 expression [63].
Together, these data consistently indicate that LKB1 inactivation in NSCLC specimens is associated with low PD-L1 expression and with lower chances to benefit from chemotherapy and/or immunotherapy in the advanced disease setting. Therefore, the possibility to avoid the use of immunotherapy in patients with LKB1-inactive NSCLC, while promoting experimental studies aimed at evaluating new treatment strategies, should be preferred in this clinical context.
To challenge this conclusion, recent data from a subgroup analysis from the phase 3 KEYNOTE-189 study showed that some patients with LKB1-mutated NSCLC can achieve clinical benefit from adding immunotherapy to first-line chemotherapy [64]. Even though the clinical benefit observed in this subset of patients is lower than observed in patients with LKB1 wild-type NSCLC, first-line chemo-immunotherapy remains the standard-of-care, first-line therapy for the majority of advanced LKB1-inactive NSCLC patients.
Overall, the available clinical evidence, and in data from large observational real-world genomic study and the two subgroup analyses from the phase 3 MYSTIC and KEYNOTE-189 trials, point to LKB1 inactivation as a negative prognostic factor, rather than a predictor of poor benefit from immunotherapy, in patients with advanced NSCLC.
6. Conclusions
Loss of oncosuppressive function of LKB1 confers peculiar characteristics to NSCLC, including clinical aggressiveness, resistance to immunotherapy, and enhanced sensitivity to metabolic stress. So far, it is not completely clear if LKB1 loss may be considered as a negative predictive factor for specific treatments, such as immunotherapy, or a negative prognostic factor tout court. However, especially when occurring together with KRAS hyperactivation, LKB1 loss is associated with dismal prognosis. Thus, broadening our knowledge on this particular subset of NSCLC is a compelling need.
To envisage an effective therapeutic intervention for tumors with inactive LKB1, the selection of LKB1less NSCLC patients is of pivotal importance. Since LKB1 expression also depends on epigenetic mechanisms that regulate LKB1 gene transcription, assessing LKB1 mutational status alone may underestimate the real number of tumors with impaired LKB1 functions. In this perspective, methods capable of assessing the expression of LKB1 protein, such as IHC, are as important as LKB1 gene sequencing to infer about the integrity of the LKB1 tumor suppressive axis. For instance, some tumors with wild-type LKB1 gene may show low or absent LKB1 protein expression as a result of epigenetic mechanisms, impairing LKB1 gene transcription. Conversely, missense LKB1 mutations that impair LKB1 activity without affecting LKB1 protein expression may not be detected as loss of LKB1 staining by IHC. Based on these considerations, a better definition of the LKB1less phenotype is mandatory to refine the selection of patients who could benefit from specific treatments (Figure 3). As previously described, these LKB1less tumors show phenotypic features comparable to those of LKB1-mutated ones. Accordingly, patients with LKB1less may be considered as LKB1-mutated tumors, and may benefit from the same treatments. Therefore, an in-depth comprehension of all features causing and linked to LKB1 loss of function is mandatory to increase the survival chances of NSCLC patients with both LKB1-mutated and LKB1less tumors.
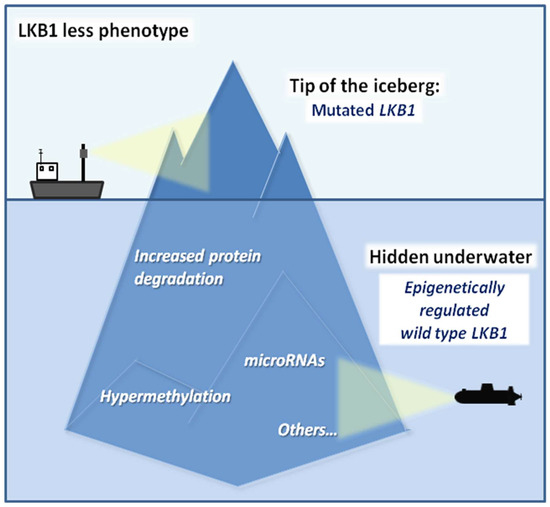
Figure 3.
Schematic representation of the current knowledge on the LKB1less phenotype. The tip of the iceberg represents LKB1 mutations. Hidden underwater, the less investigated conditions that led to the LKB1less phenotype in wild-type LKB1 patients (hypermethylation of the promoter, non-coding RNAs, increased protein degradation, etc.) are waiting to be further investigated.
Despite the twenty-year-old knowledge of LKB1/KRAS co-mutation in NSCLC patients, very few therapeutic interventions have been developed to specifically treat KL tumors. Only recently, some clinical trials aiming to exploit the specific vulnerabilities of KL NSCLC have been proposed. However, the current knowledge on the impact of LKB1 loss in tumor cell biology and in the interactions with TME, summarized in this review, is still too limited to allow the development of effective therapies. Thus, since “if you know yourself but not the enemy, for every victory gained you will also suffer a defeat” [65], further investigation of the causes and the implications of LKB1 loss in tumor biology is an essential point to face. Of note, no complete knowledge of our enemy, the highly aggressive KL NSCLC, may be reached without considering, both in preclinical studies and in clinical trials, the large amount of wild-type LKB1 tumors that partially or completely lost LKB1 activity: the so called “LKB1less tumors”.
Author Contributions
M.M. prepared the original draft. C.B., G.G., M.G., D.S., C.V., M.C.G. and G.S. reviewed and edited the draft. M.M. and C.B. prepared the figures. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by AIRC, grant numbers AIRC IG 20085 and AIRC IG 23244; and by Fondazione IRCCS Institutional Grant n. B.R.I D/16/01F CB was supported by the AIRC “Clara Borrelli” three-year fellowship.
Conflicts of Interest
Signorelli has received personal fees from AstraZeneca, Bristol Myers Squibb, and Lilly, and non-financial support from Merck Sharp & Dohme, Roche, and Pfizer. Garassino reported grants and personal fees from Eli Lilly, Otsuka Pharma, AstraZeneca, Novartis, BMS, Roche, Pfizer, Celgene, Incyte, Bayer, MSD, GlaxoSmithKline S.p.A., Spectrum Pharmaceuticals, and Blueprint Medicine; personal fees from Boehringer Ingelheim, Inivata, Takeda, Sanofi-Aventis, Seattle Genetics, Daiichi Sankyo, and Jannesen; grants from Tiziana Sciences, Clovis, Merck Serono, United Therapeutics Corporation, Merck KGaA, Turning Point, Ipsen, MedImmune, and Exelixis; non-financial support from MSD, Pfizer, and Eli-Lilly. All other authors declare no conflict of interest.
References
- Boudeau, J.; Scott, J.W.; Resta, N.; Deak, M.; Kieloch, A.; Komander, D.; Hardie, D.G.; Prescott, A.R.; van Aalten, D.M.F.; Alessi, D.R. Analysis of the LKB1-STRAD-MO25 complex. J. Cell Sci. 2004, 117, 6365–6375. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, J.M.; Göransson, O.; Toth, R.; Deak, M.; Morrice, N.A.; Boudeau, J.; Hawley, S.A.; Udd, L.; Mäkelä, T.P.; Hardie, D.G.; et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, D.B.; Shaw, R.J. The LKB1-AMPK pathway: Metabolism and growth control in tumour suppression. Nat. Rev. Cancer 2009, 9, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Chavez, J.A.; Roach, W.G.; Keller, S.R.; Lane, W.S.; Lienhard, G.E. Inhibition of GLUT4 translocation by Tbc1d1, a Rab GTPase-activating protein abundant in skeletal muscle, is partially relieved by AMP-activated protein kinase activation. J. Biol. Chem. 2008, 283, 9187–9195. [Google Scholar] [CrossRef]
- Shackelford, D.B.; Abt, E.; Gerken, L.; Vasquez, D.S.; Seki, A.; Leblanc, M.; Wei, L.; Fishbein, M.C.; Czernin, J.; Mischel, P.S.; et al. LKB1 Inactivation Dictates Therapeutic Response of Non-Small Cell Lung Cancer to the Metabolism Drug Phenformin. Cancer Cell 2013, 23, 143–158. [Google Scholar] [CrossRef]
- Parker, S.J.; Svensson, R.U.; Divakaruni, A.S.; Lefebvre, A.E.; Murphy, A.N.; Shaw, R.J.; Metallo, C.M. LKB1 promotes metabolic flexibility in response to energy stress. Metab. Eng. 2017, 43, 208–217. [Google Scholar] [CrossRef]
- Amos, C.I.; Bali, D.; Thiel, T.J.; Anderson, J.P.; Gourley, I.; Frazier, M.L.; Lynch, P.M.; Luchtefeld, M.A.; Young, A.; McGarrity, T.J.; et al. Fine Mapping of a Genetic Locus for Peutz-Jeghers Syndrome on Chromosome 19p. Cancer Res. 1997, 57, 3653–3656. [Google Scholar]
- Mehenni, H.; Blouin, J.L.; Radhakrishna, U.; Bhardwaj, S.S.; Bhardwaj, K.; Dixit, V.B.; Richards, K.F.; Bermejo-Fenoll, A.; Leal, A.S.; Raval, R.C.; et al. Peutz-Jeghers syndrome: Confirmation of linkage to chromosome 19p13.3 and Identification of a potential second locus, on 19q13.4. Am. J. Hum. Genet. 1997, 61, 1327–1334. [Google Scholar] [CrossRef]
- Hemminki, A.; Markie, D.; Tomlinson, I.; Avizienyte, E.; Roth, S.; Loukola, A.; Bignell, G.; Warren, W.; Aminoff, M.; Höglund, P.; et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 1998, 391, 184–187. [Google Scholar] [CrossRef]
- Jenne, D.E.; Reimann, H.; Nezu, J.I.; Friedel, W.; Loff, S.; Jeschke, R.; Müller, O.; Back, W.; Zimmer, M. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat. Genet. 1998, 18, 38–43. [Google Scholar] [CrossRef]
- Van Lier, M.G.F.; Westerman, A.M.; Wagner, A.; Looman, C.W.N.; Wilson, J.H.P.; De Rooij, F.W.M.; Lemmens, V.E.P.P.; Kuipers, E.J.; Mathus-Vliegen, E.M.H.; Van Leerdam, M.E. High cancer risk and increased mortality in patients with Peutz—Jeghers syndrome. Gut 2011, 60, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Riegert-Johnson, D.L.; Westra, W.; Roberts, M. High cancer risk and increased mortality in patients with Peutz-Jeghers syndrome. Gut 2012, 61, 322. [Google Scholar] [CrossRef] [PubMed]
- Resta, N.; Pierannunzio, D.; Lenato, G.M.; Stella, A.; Capocaccia, R.; Bagnulo, R.; Lastella, P.; Susca, F.C.; Bozzao, C.; Loconte, D.C.; et al. Cancer risk associated with STK11/LKB1 germline mutations in Peutz-Jeghers syndrome patients: Results of an Italian multicenter study. Dig. Liver Dis. 2013, 45, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Guldberg, P.; Straten, P.T.; Ahrenkiel, V.; Seremet, T.; Kirkin, A.F.; Zeuthen, J. Somatic mutation of the Peutz-Jeghers syndrome gene, LKB1/STK11, in malignant melanoma. Oncogene 1999, 18, 1777–1780. [Google Scholar] [CrossRef] [PubMed]
- McCabe, M.T.; Powell, D.R.; Zhou, W.; Vertino, P.M. Homozygous deletion of the STK11/LKB1 locus and the generation of novel fusion transcripts in cervical cancer cells. Cancer Genet. Cytogenet. 2010, 197, 130–141. [Google Scholar] [CrossRef]
- Morton, J.P.; Jamieson, N.B.; Karim, S.A.; Athineos, D.; Ridgway, R.A.; Nixon, C.; McKay, C.J.; Carter, R.; Brunton, V.G.; Frame, M.C.; et al. LKB1 haploinsufficiency cooperates with Kras to promote pancreatic cancer through suppression of p21-dependent growth arrest. Gastroenterology 2010, 139, 586–597. [Google Scholar] [CrossRef]
- Shen, Z.; Wen, X.F.; Lan, F.; Shen, Z.Z.; Shao, Z.M. The tumor suppressor gene LKB1 is associated with prognosis in human breast carcinoma. Clin. Cancer Res. 2002, 8, 2085–2090. [Google Scholar]
- Sanchez-Cespedes, M.; Parrella, P.; Esteller, M.; Nomoto, S.; Trink, B.; Engles, J.M.; Westra, W.H.; Herman, J.G.; Sidransky, D. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002, 62, 3659–3662. [Google Scholar]
- Granado-Martínez, P.; Garcia-Ortega, S.; González-Sánchez, E.; McGrail, K.; Selgas, R.; Grueso, J.; Gil, R.; Naldaiz-Gastesi, N.; Rhodes, A.C.; Hernandez-Losa, J.; et al. STK11 (LKB1) missense somatic mutant isoforms promote tumor growth, motility and inflammation. Commun. Biol. 2020, 3, 1–14. [Google Scholar] [CrossRef]
- Presneau, N.; Duhamel, L.A.; Ye, H.; Tirabosco, R.; Flanagan, A.M.; Eskandarpour, M. Post-translational regulation contributes to the loss of LKB1 expression through SIRT1 deacetylase in osteosarcomas. Br. J. Cancer 2017, 117, 398–408. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Song, G.; Luo, D. Prognostic significance of LKB1 promoter methylation in cutaneous malignant melanoma. Oncol. Lett. 2017, 14, 2075–2080. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M.; Avizienyte, E.; Corn, P.G.; Lothe, R.A.; Baylin, S.B.; Aaltonen, L.A.; Herman, J.G. Epigenetic inactivation of LKB1 in primary tumors associated with the Peutz-Jeghers syndrome. Oncogene 2000, 19, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Trojan, J.; Brieger, A.; Raedle, J.; Esteller, M.; Zeuzem, S. 5’-CpG island methylation of the LKB1/STK11 promoter and allelic loss at chromosome 19p13.3 in sporadic colorectal cancer. Gut 2000, 47, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Izreig, S.; Samborska, B.; Johnson, R.M.; Sergushichev, A.; Ma, E.H.; Lussier, C.; Loginicheva, E.; Donayo, A.O.; Poffenberger, M.C.; Sagan, S.M.; et al. The miR-17∼92 microRNA Cluster Is a Global Regulator of Tumor Metabolism. Cell Rep. 2016, 16, 1915–1928. [Google Scholar] [CrossRef]
- Jiang, C.; Bi, C.; Jiang, X.; Tian, T.; Huang, X.; Wang, C.; Fernandez, M.R.; Iqbal, J.; Chan, W.C.; McKeithan, T.W.; et al. The miR-17~92 cluster activates mTORC1 in mantle cell lymphoma by targeting multiple regulators in the STK11/AMPK/TSC/mTOR pathway. Br. J. Haematol. 2019, 185, 616–620. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Qi, P.; Ma, Z. Biology of MiR-17-92 Cluster and Its Progress in Lung Cancer. Int. J. Med. Sci. 2018, 15, 1443–1448. [Google Scholar] [CrossRef]
- Russell, R.C.; Yuan, H.X.; Guan, K.L. Autophagy regulation by nutrient signaling. Cell Res. 2014, 24, 42–57. [Google Scholar] [CrossRef]
- Zulato, E.; Ciccarese, F.; Nardo, G.; Pinazza, M.; Agnusdei, V.; Silic-Benussi, M.; Ciminale, V.; Indraccolo, S. Involvement of NADPH Oxidase 1 in Liver Kinase B1-Mediated Effects on Tumor Angiogenesis and Growth. Front. Oncol. 2018, 8, 195. [Google Scholar] [CrossRef]
- Zhuang, Z.G.; Di, G.H.; Shen, Z.Z.; Ding, J.; Shao, Z.M. Enhanced expression of LKB1 in breast cancer cells attenuates angiogenesis, invasion, and metastatic potential. Mol. Cancer Res. 2006, 4, 843–849. [Google Scholar] [CrossRef]
- Roy, B.C.; Kohno, T.; Iwakawa, R.; Moriguchi, T.; Kiyono, T.; Morishita, K.; Sanchez-Cespedes, M.; Akiyama, T.; Yokota, J. Involvement of LKB1 in epithelial-mesenchymal transition (EMT) of human lung cancer cells. Lung Cancer 2010, 70, 136–145. [Google Scholar] [CrossRef]
- Zhang, S.; Schafer-Hales, K.; Khuri, F.R.; Zhou, W.; Vertino, P.M.; Marcus, A.I. The tumor suppressor LKB1 regulates lung cancer cell polarity by mediating cdc42 recruitment and activity. Cancer Res. 2008, 68, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.M.; Svensson, R.U.; Lou, H.J.; Winslow, M.M.; Turk, B.E.; Shaw, R.J. An AMPK-independent signaling pathway downstream of the LKB1 tumor suppressor controls snail1 and metastatic potential. Mol. Cell 2014, 55, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Zagórska, A.; Deak, M.; Campbell, D.G.; Banerjee, S.; Hirano, M.; Aizawa, S.; Prescott, A.R.; Alessi, D.R. New roles for the LKB1-NUAK pathway in controlling myosin phosphatase complexes and cell adhesion. Sci. Signal. 2010, 3, ra25. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Foretz, M.; Marion, A.; Campbell, D.G.; Gourlay, R.; Boudaba, N.; Tournier, E.; Titchenell, P.; Peggie, M.; Deak, M.; et al. The LKB1-salt-inducible kinase pathway functions as a key gluconeogenic suppressor in the liver. Nat. Commun. 2014, 5, 1–16. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, P.; Wang, Z.C.; Zou, L.; Santiago, S.; Garbitt, V.; Gjoerup, O.V.; Dirk Iglehart, J.; Miron, A.; Richardson, A.L.; et al. SIK1 couples LKB1 to p53-dependent anoikis and suppresses metastasis. Sci. Signal. 2009, 2, ra35. [Google Scholar] [CrossRef]
- Murray, C.W.; Brady, J.J.; Tsai, M.K.; Li, C.; Winters, I.P.; Tang, R.; Andrejka, L.; Ma, R.K.; Kunder, C.A.; Chu, P.; et al. An Lkb1-Sik axis suppresses lung tumor growth and controls differentiation. Cancer Discov. 2019, 9, 1590–1605. [Google Scholar] [CrossRef]
- Kaufman, J.M.; Amann, J.M.; Park, K.; Arasada, R.R.; Li, H.; Shyr, Y.; Carbone, D.P. LKB1 loss induces characteristic patterns of gene expression in human tumors associated with NRF2 activation and attenuation of PI3K-AKT. J. Thorac. Oncol. 2014, 9, 794–804. [Google Scholar] [CrossRef]
- Chen, L.; Engel, B.E.; Welsh, E.A.; Yoder, S.J.; Brantley, S.G.; Chen, D.T.; Beg, A.A.; Cao, C.; Kaye, F.J.; Haura, E.B.; et al. A sensitive nano string-based assay to score STK11 (LKB1) pathway disruption in lung adenocarcinoma. J. Thorac. Oncol. 2016, 11, 838–849. [Google Scholar] [CrossRef]
- Carretero, J.; Shimamura, T.; Rikova, K.; Jackson, A.L.; Wilkerson, M.D.; Borgman, C.L.; Buttarazzi, M.S.; Sanofsky, B.A.; McNamara, K.L.; Brandstetter, K.A.; et al. Integrative Genomic and Proteomic Analyses Identify Targets for Lkb1-Deficient Metastatic Lung Tumors. Cancer Cell 2010, 17, 547–559. [Google Scholar] [CrossRef]
- Kaufman, J.M.; Yamada, T.; Park, K.; Timmers, C.D.; Amann, J.M.; Carbone, D.P. A transcriptional signature identifies LKB1 functional status as a novel determinant of MEK sensitivity in lung adenocarcinoma. Cancer Res. 2017, 77, 153–163. [Google Scholar] [CrossRef]
- Skoulidis, F.; Goldberg, M.E.; Greenawalt, D.M.; Hellmann, M.D.; Awad, M.M.; Gainor, J.F.; Schrock, A.B.; Hartmaier, R.J.; Trabucco, S.E.; Gay, L.; et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS -Mutant Lung Adenocarcinoma. Cancer Discov. 2018, 8, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Jure-Kunkel, M.; Wu, S.; Xiao, F.; Abdullah, S.E.; Gao, G.; Englert, J.M.; Hsieh, H.-J.; Mukhopadhyay, P.; Gupta, A.K.; Dennis, P.A.; et al. Somatic STK11/LKB1 mutations to confer resistance to immune checkpoint inhibitors as monotherapy or in combination in advanced NSCLC. J. Clin. Oncol. 2018, 36, 3028. [Google Scholar] [CrossRef]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Aref, A.R.; Skoulidis, F.; Herter-Sprie, G.S.; Buczkowski, K.A.; Liu, Y.; Awad, M.M.; Denning, W.L.; et al. STK11/LKB1 deficiency promotes neutrophil recruitment and proinflammatory cytokine production to suppress T-cell activity in the lung tumor microenvironment. Cancer Res. 2016, 76, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, S.; Ivanova, E.; Guo, S.; Yoshida, R.; Campisi, M.; Sundararaman, S.K.; Tange, S.; Mitsuishi, Y.; Thai, T.C.; Masuda, S.; et al. Suppression of STING Associated with LKB1 Loss in KRAS-Driven Lung Cancer. Cancer Discov. 2019, 9, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; An, X.; Zhang, X.; Qiao, Y.; Zheng, T.; Li, X. STING: A master regulator in the cancer-immunity cycle. Mol. Cancer 2019, 18, 1–15. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 6, 582–598. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Spinelli, C.; Reis-Sobreiro, M.; Cavallini, L.; You, S.; Zandian, M.; Li, X.; Mishra, R.; Chiarugi, P.; Adam, R.M.; et al. MYC Mediates Large Oncosome-Induced Fibroblast Reprogramming in Prostate Cancer. Cancer Res. 2017, 77, 2306–2317. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef]
- Song, W.; Yan, D.; Wei, T.; Liu, Q.; Zhou, X.; Liu, J. Tumor-derived extracellular vesicles in angiogenesis. Biomed. Pharmacother. 2018, 102, 1203–1208. [Google Scholar] [CrossRef]
- Ahmadi, M.; Rezaie, J. Tumor cells derived-exosomes as angiogenenic agents: Possible therapeutic implications. J. Transl. Med. 2020, 18, 249. [Google Scholar] [CrossRef]
- Borzi, C.; Calzolari, L.; Ferretti, A.M.; Caleca, L.; Pastorino, U.; Sozzi, G.; Fortunato, O. c-Myc shuttled by tumour-derived extracellular vesicles promotes lung bronchial cell proliferation through miR-19b and miR-92a. Cell Death Dis. 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- McAndrews, K.M.; Kalluri, R. Mechanisms associated with biogenesis of exosomes in cancer. Mol. Cancer 2019, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xiao, X.; Chen, M.; Aldharee, H.; Chen, Y.; Long, W. Liver kinase B1 restoration promotes exosome secretion and motility of lung cancer cells. Oncol. Rep. 2018, 39, 376–382. [Google Scholar] [PubMed]
- Sun, R.; Li, J.; Wang, B.; Guo, Y.; Ma, L.; Quan, X.; Chu, Z.; Li, T. Liver kinase B1 promoter CpG island methylation is related to lung cancer and smoking. Int. J. Clin. Exp. Med. 2015, 8, 14070–14074. [Google Scholar] [PubMed]
- Sasai, K.; Sukezane, T.; Yanagita, E.; Nakagawa, H.; Hotta, A.; Itoh, T.; Akagi, T. Oncogene-mediated human lung epithelial cell transformation produces adenocarcinoma phenotypes in vivo. Cancer Res. 2011, 71, 2541–2549. [Google Scholar] [CrossRef]
- Sato, M.; Larsen, J.E.; Lee, W.; Sun, H.; Shames, D.S.; Dalvi, M.P.; Ramirez, R.D.; Tang, H.; DiMaio, J.M.; Gao, B.; et al. Human lung epithelial cells progressed to malignancy through specific oncogenic manipulations. Mol. Cancer Res. 2013, 11, 638–650. [Google Scholar] [CrossRef]
- Ji, H.; Ramsey, M.R.; Hayes, D.N.; Fan, C.; McNamara, K.; Kozlowski, P.; Torrice, C.; Wu, M.C.; Shimamura, T.; Perera, S.A.; et al. LKB1 modulates lung cancer differentiation and metastasis. Nature 2007, 448, 807–810. [Google Scholar] [CrossRef]
- Jackson, E.L.; Willis, N.; Mercer, K.; Bronson, R.T.; Crowley, D.; Montoya, R.; Jacks, T.; Tuveson, D.A. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001, 15, 3243–3248. [Google Scholar] [CrossRef]
- Calles, A.; Sholl, L.M.; Rodig, S.J.; Pelton, A.K.; Hornick, J.L.; Butaney, M.; Lydon, C.; Dahlberg, S.E.; Oxnard, G.R.; Jackman, D.M.; et al. Immunohistochemical Loss of LKB1 Is a biomarker for more aggressive biology inKRAS-mutant lung Adenocarcinoma. Clin. Cancer Res. 2015, 21, 2851–2860. [Google Scholar] [CrossRef]
- Moro, M.; Caiola, E.; Ganzinelli, M.; Zulato, E.; Rulli, E.; Marabese, M.; Centonze, G.; Busico, A.; Pastorino, U.; De Braud, F.G.; et al. Metformin enhances cisplatin-induced apoptosis and prevents resistance to cisplatin in co-mutated KRAS/LKB1 Non-Small Cell Lung Cancer (NSCLC). J. Thorac. Oncol. Non-Small Cell Lung Cancer 2018, 13, 1692–1704. [Google Scholar] [CrossRef]
- Skoulidis, F.; Arbour, K.C.; Hellmann, M.D.; Patil, P.D.; Marmarelis, M.E.; Awad, M.M.; Murray, J.C.; Hellyer, J.; Gainor, J.F.; Dimou, A.; et al. Association of STK11/LKB1 genomic alterations with lack of benefit from the addition of pembrolizumab to platinum doublet chemotherapy in non-squamous non-small cell lung cancer. J. Clin. Oncol. 2019, 37, 102. [Google Scholar] [CrossRef]
- Shire, N.J.; Klein, A.B.; Golozar, A.; Collins, J.M.; Fraeman, K.H.; Nordstrom, B.L.; McEwen, R.; Hembrough, T.; Rizvi, N.A. STK11 (LKB1) mutations in metastatic NSCLC: Prognostic value in the real world. PLoS ONE 2020, 15, e0238358. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.; Cho, B.C.; Reinmuth, N.; Lee, K.H.; Luft, A.; Ahn, M.; Papadimitrakopoulou, V.; Heymach, J.; Scheuring, U.; Higgs, B.; et al. OA04.07 Mutations Associated with Sensitivity or Resistance to Immunotherapy in mNSCLC: Analysis from the MYSTIC Trial. J. Thorac. Oncol. 2019, 14, S217. [Google Scholar] [CrossRef]
- Gadgeel, S.M.; Rodriguez-Abreu, D.; Felip, E.F.; Esteban, E.; Speranza, G.; Reck, M.; Hui, R.; Boyer, M.; Garon, E.B.; Horinouchi, H.; et al. LB-397/21: Pembrolizumab Plus Pemetrexed and Platinum vs. Placebo Plus Pemetrexed and Platinum as First-Line Therapy for Metastatic Nonsquamous NSCLC: Analysis of KEYNOTE-189 by STK11 and KEAP1 status; AACR Abstract; American Association for Cancer Research: Philadelphia, PA, USA, 2020. [Google Scholar] [CrossRef]
- Tzu, S. The Art of War; Simon & Schuster: New York, NY, USA, 2020. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).