Preparation and Characterization of Solid Dispersions Composed of Curcumin, Hydroxypropyl Cellulose and/or Sodium Dodecyl Sulfate by Grinding with Vibrational Ball Milling
Abstract
1. Introduction
2. Results and Discussion
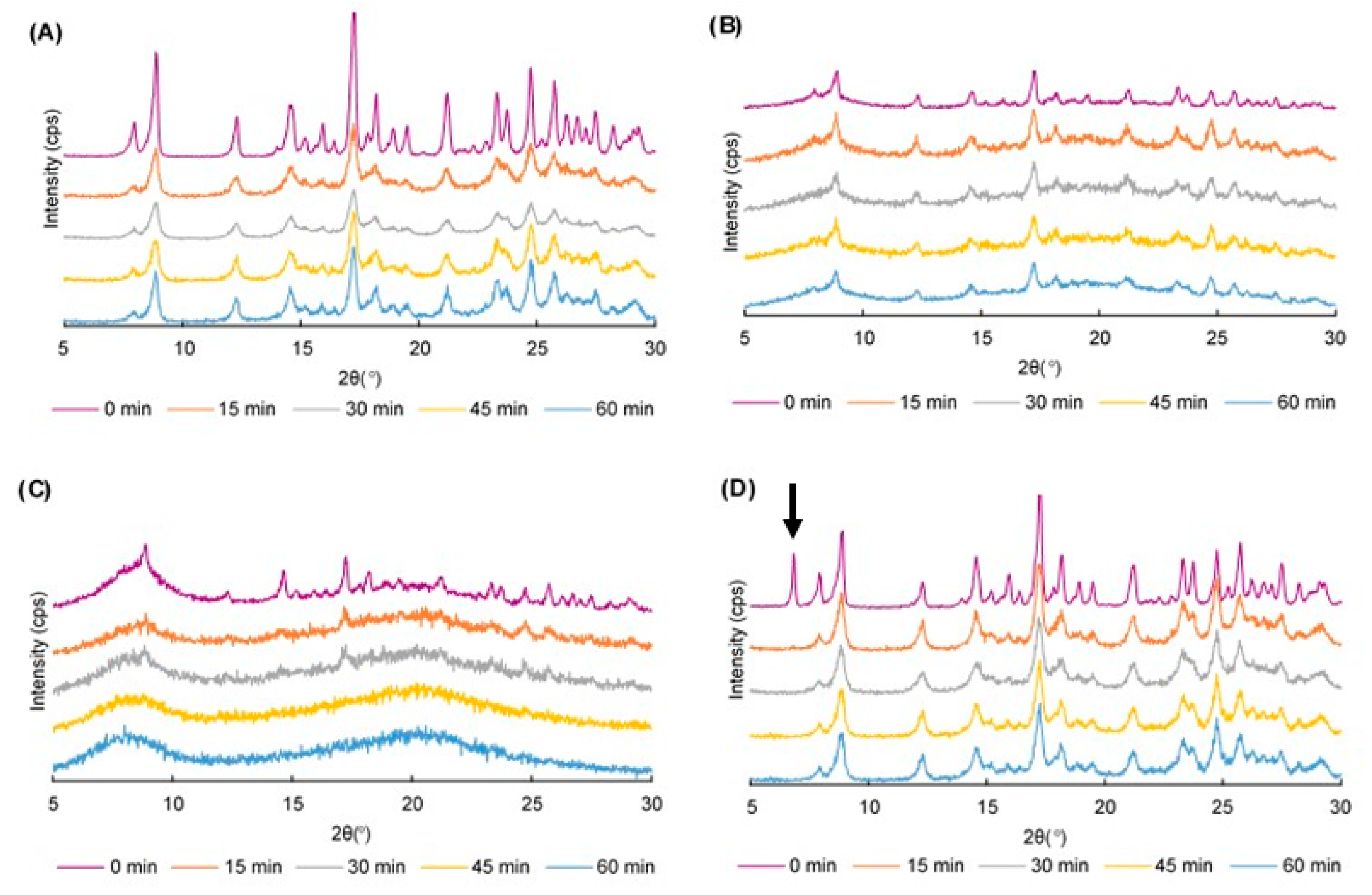
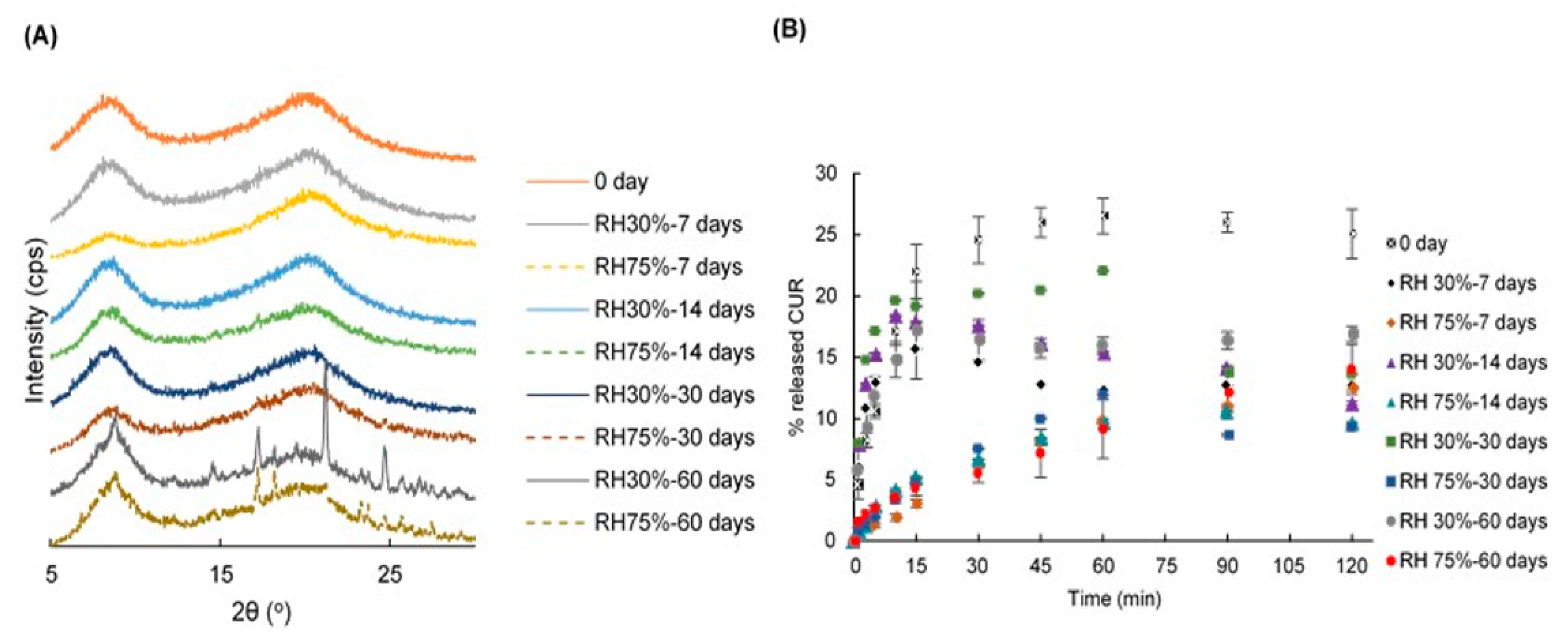
2.1. Power X-ray Diffraction (PXRD) Patterns
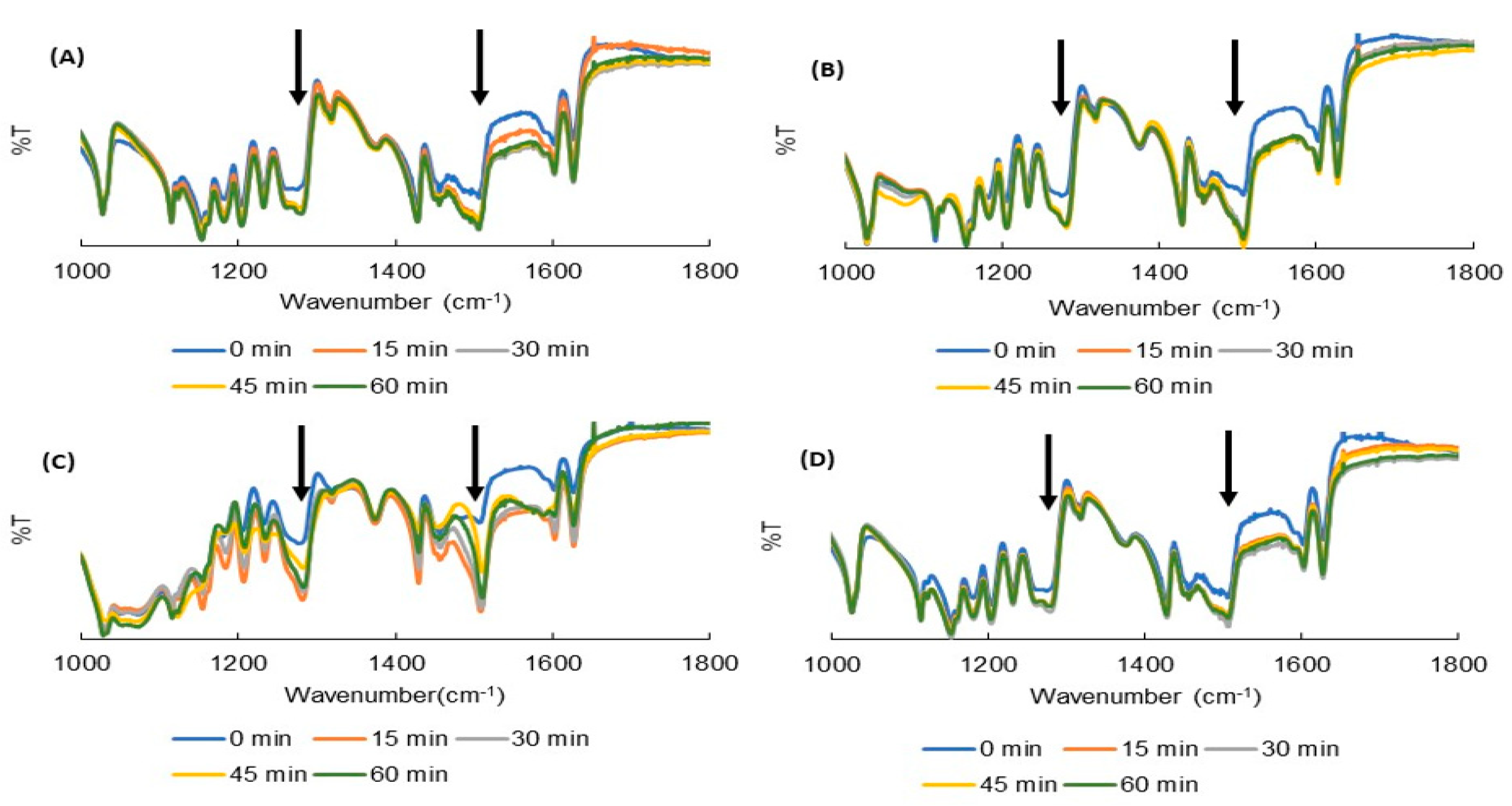
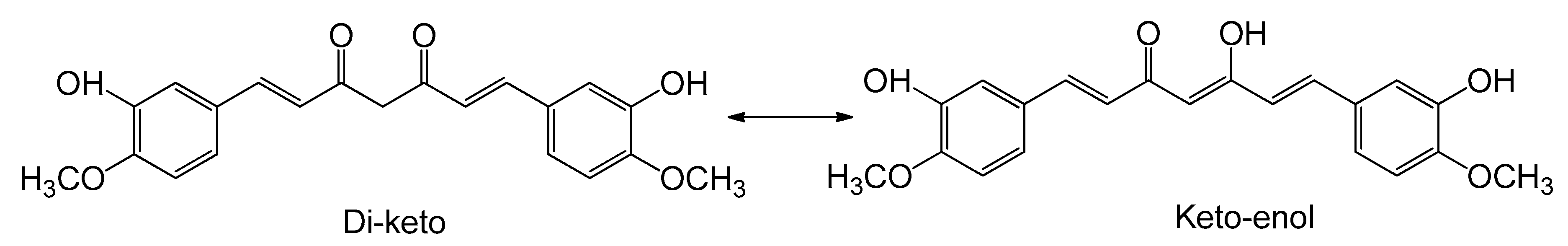
2.2. Fourier Transform Infrared (FTIR) Spectroscopy
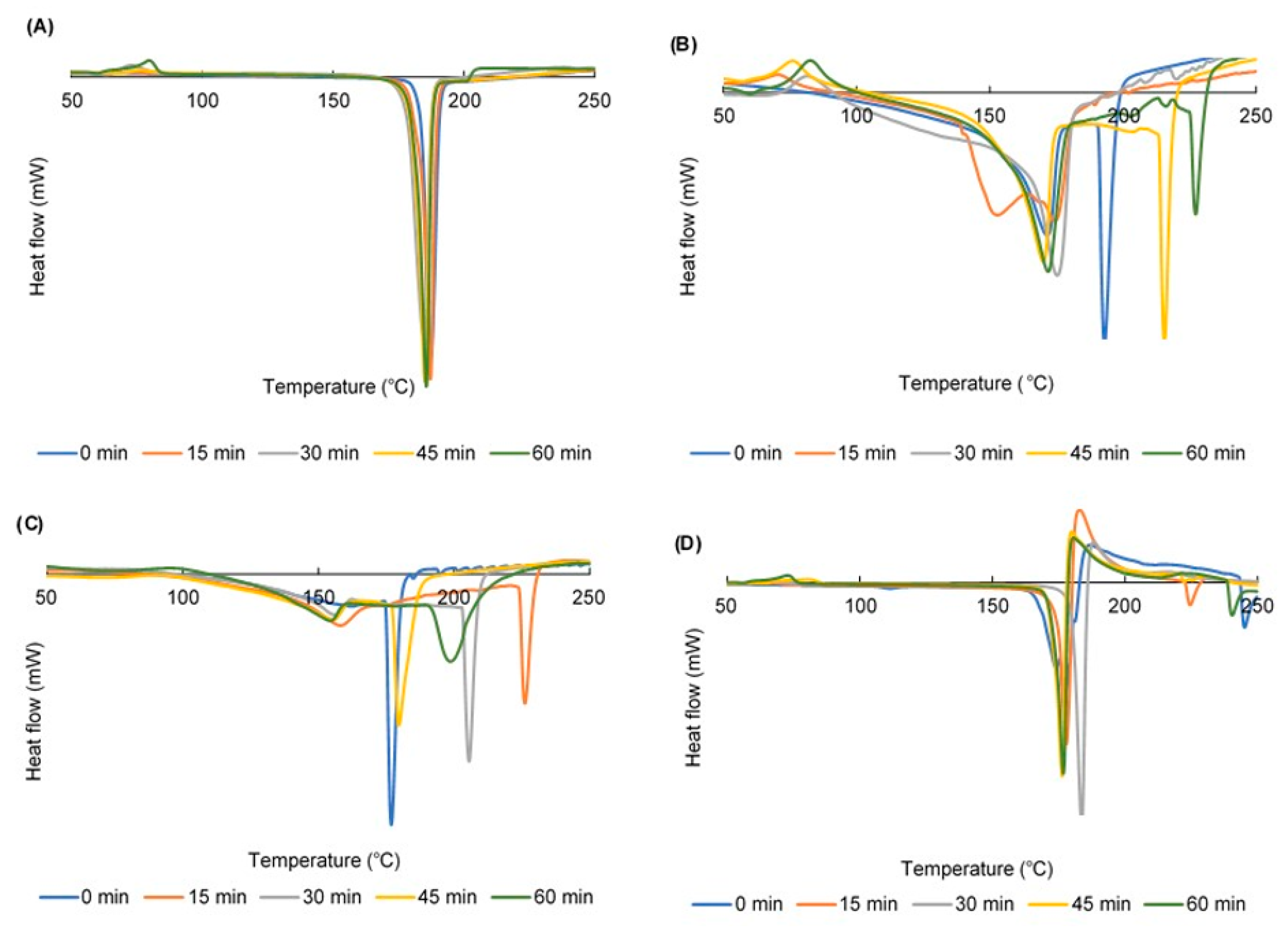
2.3. Differential Scanning Calorimetry (DSC) Curves
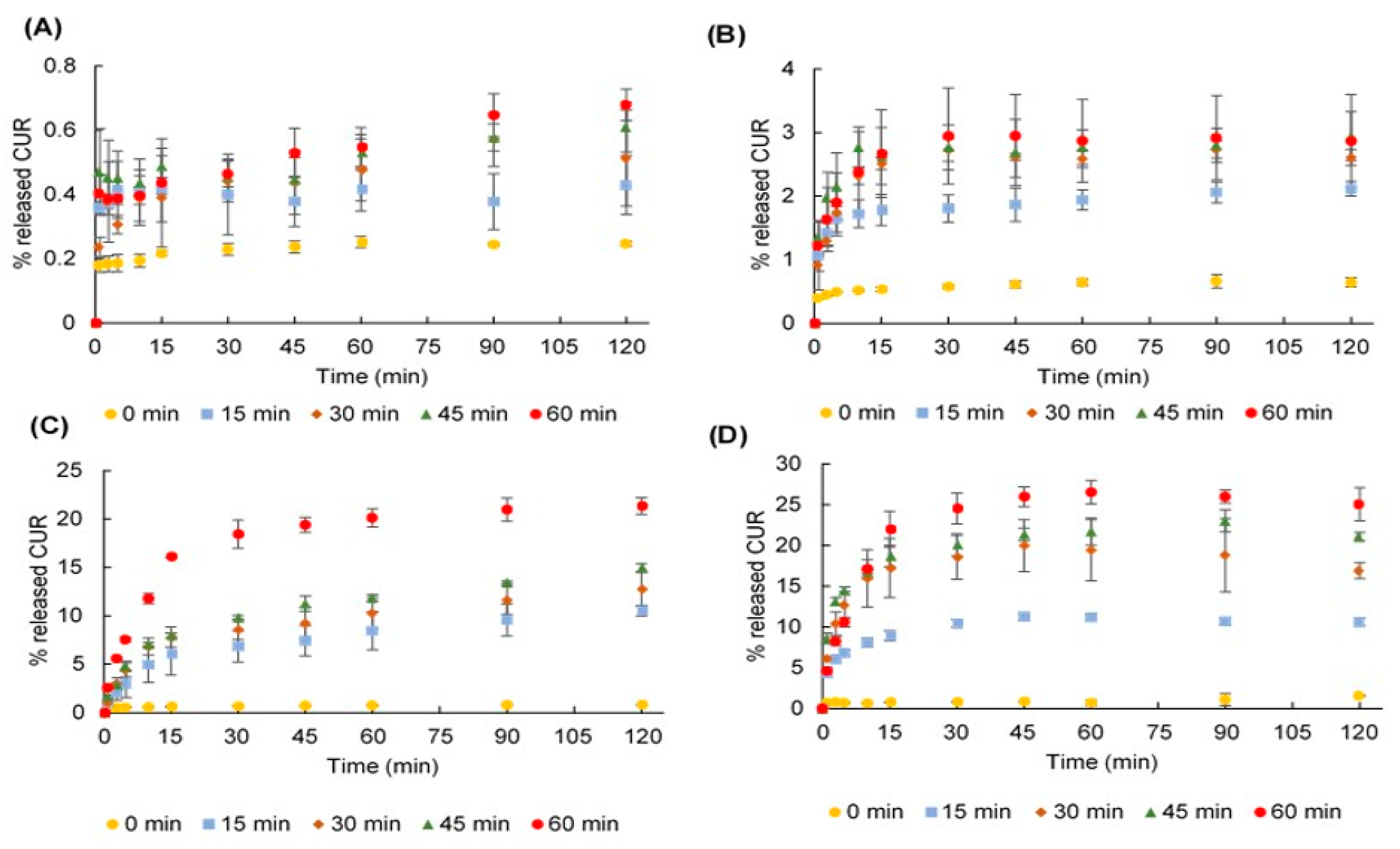
2.4. Dissolution Profiles
2.5. Fit Factors
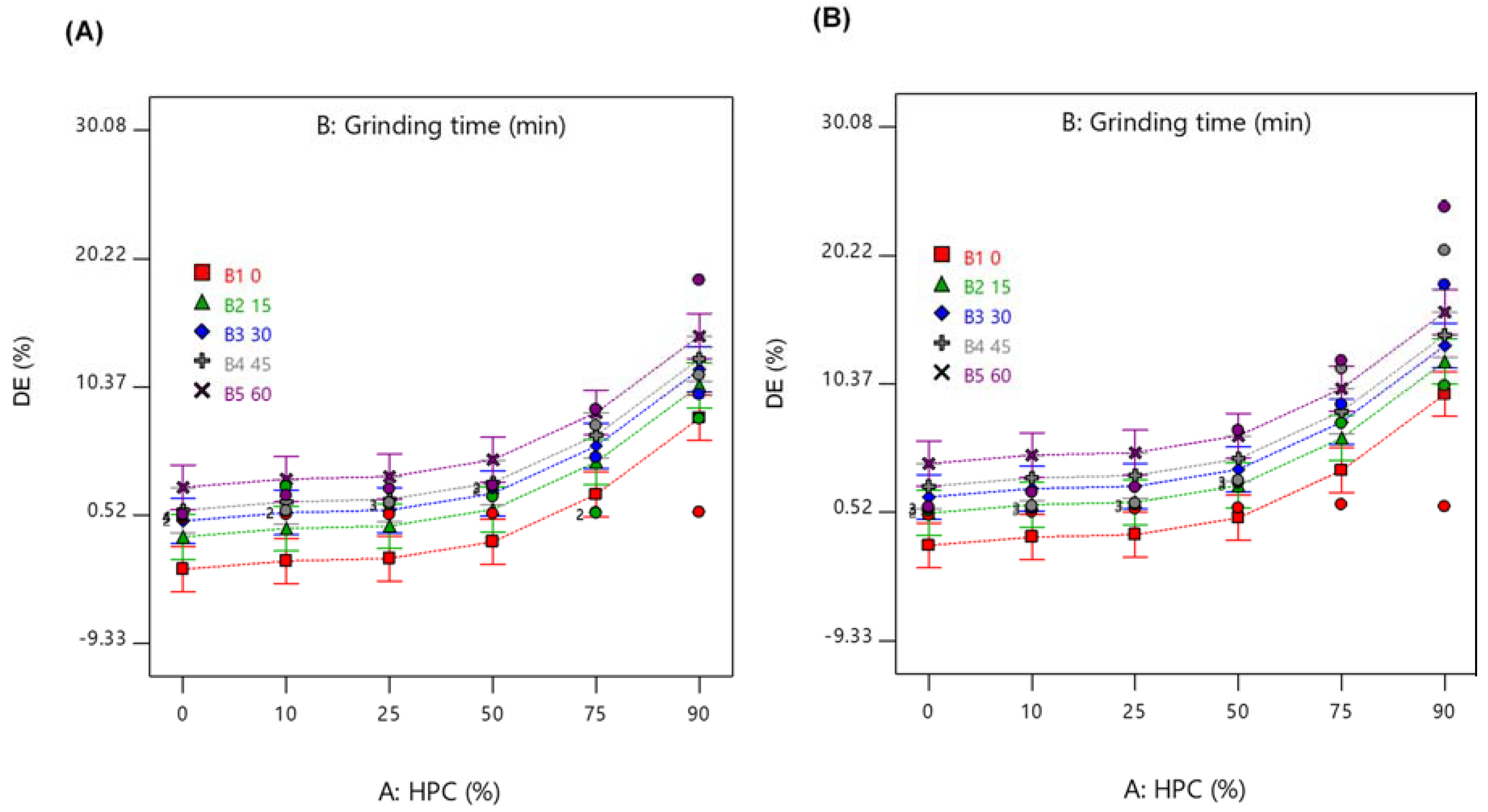
2.6. Dissolution Efficiency (DE)
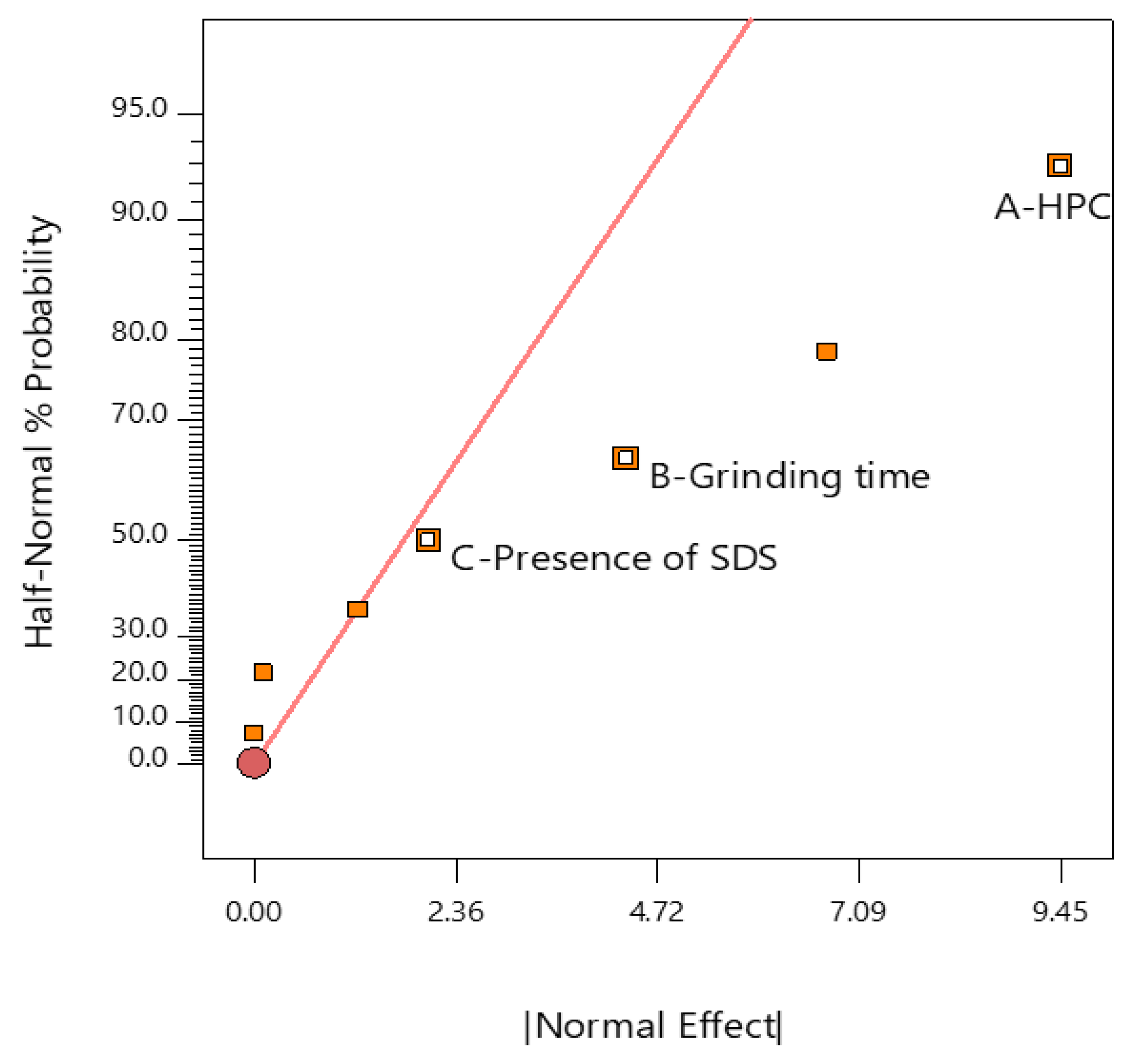
2.7. Analysis of DoE
2.8. Stability
3. Materials and Methods
3.1. Design of Experiments (DoE)
3.2. Preparation of Ground Mixtures (GMs)
3.3. Quantification of CUR
3.4. PXRD
3.5. FTIR
3.6. DSC
3.7. Dissolution Studies
3.8. Fit Factors
3.9. DE
3.10. Stability Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rams-Baron, M.; Jachowicz, R.; Boldyreva, E.; Zhou, D.; Jamroz, W.; Paluch, M. Amorphous drug solubility and absorption enhancement. In Amorphous Drugs; Springer International Publishing Co.: Cham, Switzerland, 2018; pp. 41–68. [Google Scholar]
- Ngono, F.; Willart, J.F.; Cuello, G.J.; Jimenez-Ruiz, M.; Yelles, C.H.; Affouard, F. Impact of amorphization methods on the physico-chemical properties of amorphous lactulose. Mol. Pharm. 2020, 17, 1–9. [Google Scholar] [CrossRef]
- Zeng, Y.; Alzate-Vargas, L.; Li, C.; Graves, R.; Brum, J.; Strachan, A.; Koslowski, M. Mechanically induced amorphization of small molecule organic crystals. Model. Simul. Mater. Sci. Eng. 2019, 27, 1–19. [Google Scholar] [CrossRef]
- Shen, W.; Wang, X.; Jia, F.; Tong, Z.; Sun, H.; Wang, X.; Song, F.; Ai, Z.; Zhang, L.; Chai, B. Amorphization enables highly efficient anaerobic thiamphenicol reduction by zero-valent iron. Appl. Catal. B 2020, 264, 118550. [Google Scholar] [CrossRef]
- Nuno, F.C.; João, F.P.; Ana, I.F. Co-amorphization of olanzapine for solubility enhancement. Ann. Med. 2019, 51, 87. [Google Scholar]
- Zhang, W.; Zhao, Y.; Xu, L.; Song, X.; Yuan, X.; Sun, J.; Zhang, J. Superfine grinding induced amorphization and increased solubility of α-chitin. Carbohydr. Polym. 2020, 237, 116145. [Google Scholar] [CrossRef]
- Brough, C.; Williams, R.O., 3rd. Amorphous solid dispersions and nano-crystal technologies for poorly water-soluble drug delivery. Int. J. Pharm. 2013, 453, 157–166. [Google Scholar] [CrossRef]
- DiNunzio, J.C.; Miller, D.A.; Yang, W.; McGinity, J.W.; Williams, R.O., 3rd. Amorphous compositions using concentration enhancing polymers for improved bioavailability of itraconazole. Mol. Pharm. 2008, 5, 968–980. [Google Scholar] [CrossRef]
- Hancock, B.C.; Parks, M. What is the true solubility advantage for amorphous pharmaceuticals? Pharm. Res. 2000, 17, 397–404. [Google Scholar] [CrossRef]
- Vo, C.L.N.; Park, C.; Lee, B.J. Current Trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2013, 85, 799–813. [Google Scholar] [CrossRef]
- Shaikh, A.; Bhide, P.; Nachinolkar, R. Solubility enhancement of celecoxib by solid dispersion technique and incorporation into topical gel. Asian J. Pharm. Clin. Res. 2019, 12, 294–300. [Google Scholar] [CrossRef]
- Palanisamy, V.; Sanphui, P.; Prakash, M.; Chernyshev, V. Multicomponent solid forms of the uric acid reabsorption inhibitor lesinurad and cocrystal polymorphs with urea: DFT simulation and solubility study. Acta Crystallogr. Sect. C 2019, 75, 1102–1117. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Mandal, P. Design, formulation, and evaluation of solid dispersion tablets of poorly water-soluble antidiabetic drug using natural polymer. Asian J. Pharm. Clin. Res. 2019, 12, 195–207. [Google Scholar]
- Kapourani, A.; Vardaka, E.; Katopodis, K.; Kachrimanis, K.; Barmpalexis, P. Crystallization tendency of APIs possessing different thermal and glass related properties in amorphous solid dispersions. Int. J. Pharm. 2020, 579, 119149. [Google Scholar] [CrossRef]
- Thais, F.R.A.; Cecília, T.B.; Denicezar, B.; Venâncio, A.A.; Mirella, S.; Carolina, S.; Patrícia, S.; Marco, V.C. Preparation, characterization and ex vivo intestinal permeability studies of ibuprofen solid dispersion. J. Disper. Sci. Technol. 2019, 40, 546–554. [Google Scholar]
- Uchiyama, H.; Wada, Y.; Hatanaka, Y.; Hirata, Y.; Taniguchi, M.; Kadota, K.; Tozuka, Y. Solubility and permeability improvement of quercetin by an interaction between α-glucosyl stevia nano-aggregates and hydrophilic polymer. J. Pharm. Sci. 2019, 108, 2033–2040. [Google Scholar] [CrossRef]
- Trapani, A.; Catalano, A.; Carocci, A.; Carrieri, A.; Mercurio, A.; Rosato, A.; Mandracchia, D.; Tripodo, G.; Schiavone, B.I.P.; Franchini, C.; et al. Effect of methyl-β-cyclodextrin on the antimicrobial activity of a new series of poorly water-soluble benzothiazoles. Carbohydr. Polym. 2019, 207, 720–728. [Google Scholar] [CrossRef]
- Curcuma longa in India Materia. In Indian Materia Medica; Nadkarni, K.M., Ed.; Popular Prakashan Co.: Mumbai, India, 1976; pp. 414–418. [Google Scholar]
- Narayanacharyulu, R.; Krishna, S.C.; Mudit, D. Design and development of sustained release tablets using solid dispersion of beclomethasone dipropionate. Res. Rev. J. Drug Formulation Dev. Prod. 2015, 2, 30–41. [Google Scholar]
- Niederau, C.; Göpfert, E. The effect of chelidonium- and turmeric root extract on upper abdominal pain due to functional disorders of the biliary system. Results from a placebo-controlled double-blind study. Med. Klin 1999, 94, 425–430. [Google Scholar] [CrossRef]
- Abouhussein, D.M.N.; El Nabarawi, M.A.E.; Shalaby, S.H.; El-Bary, A.A. Sertraline-cyclodextrin complex orodispersible sublingual tablet: Optimization, stability, and pharmacokinetics. J. Pharm. Innov. 2019, 1–14. [Google Scholar] [CrossRef]
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water-soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef]
- Abd El-Bary, A.; Kamal, I.H.; Haza’a, B.S.; Al Sharabi, I. Formulation of sustained release bioadhesive minitablets containing solid dispersion of levofloxacin for once daily ocular use. Pharm. Dev. Technol. 2019, 24, 824–838. [Google Scholar] [CrossRef]
- Duke, J.A.; Bogenschutz-Godwin, M.J.; duCellier, J.; Duke, P.K. CRC Handbook of Medicinal Spices, 1st ed.; CRC Press LLC: Boca Raton, FL, USA, 2002; pp. 137–144. [Google Scholar]
- Li, C.; Li, L.; Luo, J.; Huang, N. Effect of turmeric volatile oil on the respiratory tract. Zhongguo Zhong Yao Za Zhi 1998, 23, 624–625. [Google Scholar]
- Curcuma longa (turmeric). Monograph. Altern. Med. Rev. 2001, 6, S62–S66.
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Bouvier, G.; Hergenhahn, M.; Polack, A.; Bornkamm, G.W.; Bartsch, H. Validation of two test systems for detecting tumor promoters and EBV inducers: Comparative responses of several agents in DR-CAT Raji cells and in human granulocytes. Carcinogenesis 1993, 14, 1573–1578. [Google Scholar] [CrossRef]
- Saikia, A.P.; Ryakala, V.K.; Sharma, P.; Goswami, P.; Bora, U. Ethnobotany of medicinal plants used by Assamese people for various skin ailments and cosmetics. J. Ethnopharmacol. 2006, 106, 149–157. [Google Scholar] [CrossRef]
- Sharma, R.A.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and pharmacodynamics of curcumin. Adv. Exp. Med. Biol. 2007, 595, 453–470. [Google Scholar]
- Kulac, M.; Aktas, C.; Tulubas, F.; Uygur, R.; Kanter, M.; Erboga, M.; Ozen, O.A. The effects of topical treatment with curcumin on burn wound healing in rats. J. Mol. Histol. 2012, 44, 83–90. [Google Scholar] [CrossRef]
- Cianfruglia, L.; Minnelli, C.; Laudadio, E.; Scirè, A.; Armeni, T. Side effects of curcumin: Epigenetic and antiproliferative implications for normal dermal fibroblast and breast cancer cells. Antioxidants 2019, 8, 382. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; Shi, H.; Li, X.; Li, Y.; Taha, A.; Xu, C. Protective effect of curcumin against ultraviolet A irradiation-induced photoaging in human dermal fibroblasts. Mol. Med. Rep. 2018, 17, 7227–7237. [Google Scholar] [CrossRef]
- Yamada, T.; Saito, N.; Imai, T.; Otagiri, M. Effect of Grinding with Hydroxypropyl Cellulose on the Dissolution and Particle Size of a Poorly Water-Soluble Drug. Chem. Pharm. Bull. 1999, 47, 1311–1313. [Google Scholar] [CrossRef]
- Sugimoto, S.; Niwa, T.; Nakanishi, Y.; Danjo, K. Novel ultra-cryo milling and co-grinding technique in liquid nitrogen to produce dissolution-enhanced nanoparticles for poorly water-Soluble drugs. Chem. Pharm. Bull. 2012, 60, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Madelung, P.; Østergaard, J.; Bertelsen, P.; Jørgensen, E.V.; Jacobsen, J.; Müllertz, A. Impact of sodium dodecyl sulphate on the dissolution of poorly soluble drug into biorelevant medium from drug-surfactant discs. Int. J. Pharm. 2014, 467, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mochalin, V.N.; Sagar, A.; Gour, S.; Gogotsi, Y. Manufacturing nanosized fenofibrate by salt assisted milling. Pharm. Res. 2009, 26, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Elder, R.L. Final report on the safety assessment of sodium lauryl sulfate and ammonium lauryl sulfate. J. Am. Coll. Toxicol. 1983, 2, 127–181. [Google Scholar]
- Li, B.; Konecke, S.; Wegiel, L.A.; Taylor, L.S.; Edgar, K.J. Both solubility and chemical stability of curcumin are enhanced by solid dispersion in cellulose derivative matrices. Carbohyd. Polym. 2013, 98, 1108–1116. [Google Scholar] [CrossRef]
- Seo, S.-W.; Han, H.-K.; Chun, M.-K.; Choi, H.-K. Preparation and pharmacokinetic evaluation of curcumin solid dispersion using Solutol® HS15 as a carrier. Int. J. Pharm. 2012, 424, 18–25. [Google Scholar] [CrossRef]
- Satomi, O.; Takahashi, H.; Kawabata, Y.; Seto, Y.; Hatanaka, J.; Timmermann, B.; Yamada, S. Formulation design and photochemical studies on nanocrystal solid dispersion of curcumin with improved oral bioavailability. J. Pharm. Sci. 2010, 99, 1871–1881. [Google Scholar]
- Wegiel, L.A.; Zhao, Y.; Mauer, L.J.; Edgar, K.J.; Taylor, L.S. Curcumin amorphous solid dispersions: The influence of intra and intermolecular bonding on physical stability. Pharm. Dev. Technol. 2013, 19, 976–986. [Google Scholar] [CrossRef]
- Colombo, I.; Grassi, G.; Grassi, M. Drug mechanochemical activation. J. Pharm. Sci. 2009, 98, 3961–3986. [Google Scholar] [CrossRef]
- Kolev, T.M.; Velcheva, E.A.; Stamboliyska, B.A.; Spiteller, M. DFT and experimental studies of the structure and vibrational spectra of curcumin. Int. J. Quantum Chem. 2005, 102, 1069–1079. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J.; Mostad, A. Structural studies of curcuminoids. I. The crystal structure of curcumin. Acta Chem. Scand. Ser. B 1982, 36, 475–479. [Google Scholar] [CrossRef]
- Mague, J.T.; Alworth, W.T.; Payton, F.L. Curcumin and derivatives. Acta Cryst. 2004, 60, 608–610. [Google Scholar] [CrossRef]
- Kararli, T.T.; Hurlbut, J.B.; Needham, T.E. Glass–rubber transitions of cellulosic polymers by dynamic mechanical analysis. J. Pharm. Sci. 1990, 79, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Allen, E. The melting point of impure organic compounds. J. Chem. Educ. 1942, 19, 278. [Google Scholar] [CrossRef]
- Mark, J.A.; Patrick, J.W. DoE Simplified, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Center for drug evaluation and research. Q2 (R1) Validation of Analytical Procedures: Text and Methodology; FDA: Beltsville, MD, USA, 1995.
- Dhanoa, M.S.; Lister, S.J.; Sanderson, R.; Barnes, R.J. The link between multiplicative scatter correction (MSC) and standard normal variate (SNV) transformations of NIR spectra. J. Near Infrared Spec. 1994, 2, 43–47. [Google Scholar] [CrossRef]
- Moore, W.; Flanner, H.H. Mathematical comparison of curves with an emphasis on in vitro dissolution profiles. Pharm. Technol. 1996, 20, 64–74. [Google Scholar]
- Polli, J.E.; Rekhi, G.S.; Augsburger, L.L.; Shah, V.P. Methods to compare dissolution profiles and a rationale for wide dissolution specifications for metoprolol tartarate tablets. J. Pharm. Sci. 1997, 86, 690–700. [Google Scholar] [CrossRef]
- Khan, K.A.; Rhodes, C.T. Effect of compaction pressure on the dissolution efficiency of some direct compression systems. Pharm. Acta Helv. 1972, 47, 594–700. [Google Scholar]
| Without SDS | ||||||||||||
| Grinding Time (min) | HPC 0% | HPC 10% | HPC 25% | HPC 50% | HPC 75% | HPC 90% | ||||||
| f1 | f2 | f1 | f2 | f1 | f2 | f1 | f2 | f1 | f2 | f1 | f2 | |
| 15 min | 54.8 | 99.6 | 48.4 | 99.1 | 70.8 | 93.7 | 77.2 | 88.5 | 81.7 | 82.3 | 91.9 | 59.8 |
| 30 min | 56.6 | 99.5 | 58.3 | 98.2 | 66.0 | 92.3 | 82.0 | 83.3 | 90.3 | 68.2 | 93.5 | 54.9 |
| 45 min | 63.5 | 99.2 | 59.8 | 97.8 | 72.9 | 92.6 | 84.0 | 81.3 | 93.1 | 59.9 | 94.3 | 51.8 |
| 60 min | 63.2 | 99.2 | 81.6 | 87.7 | 84.8 | 82.2 | 83.7 | 81.5 | 93.8 | 57.0 | 96.6 | 40.6 |
| With SDS | ||||||||||||
| Grinding time (min) | HPC 0% | HPC 10% | HPC 25% | HPC 50% | HPC 75% | HPC 90% | ||||||
| f1 | f2 | f1 | f2 | f1 | f2 | f1 | f2 | f1 | f2 | f1 | f2 | |
| 15 min | 59.1 | 98.8 | 54.8 | 98.3 | 47.8 | 97.6 | 78.2 | 81.8 | 66.2 | 65.9 | 92.0 | 53.5 |
| 30 min | 67.4 | 97.7 | 55.1 | 98.2 | 54.9 | 96.4 | 78.9 | 80.9 | 71.1 | 60.8 | 95.5 | 40.4 |
| 45 min | 73.2 | 96.8 | 63.4 | 96.8 | 54.5 | 96.5 | 79.0 | 80.8 | 75.7 | 54.7 | 96.1 | 37.5 |
| 60 min | 74.0 | 96.5 | 82.5 | 86.8 | 78.1 | 84.5 | 90.8 | 61.5 | 76.4 | 53.4 | 96.3 | 34.9 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 1314.82 | 10 | 131.48 | 13.77 | <0.0001 | significant |
| A—HPC | 1008.40 | 5 | 201.68 | 21.12 | <0.0001 | |
| B—Grinding time | 266.59 | 4 | 66.65 | 6.98 | 0.0002 | |
| C—Presence of SDS | 39.84 | 1 | 39.84 | 4.17 | 0.0465 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, N.N.S.; Otsuka, Y.; Kawano, Y.; Hanawa, T. Preparation and Characterization of Solid Dispersions Composed of Curcumin, Hydroxypropyl Cellulose and/or Sodium Dodecyl Sulfate by Grinding with Vibrational Ball Milling. Pharmaceuticals 2020, 13, 383. https://doi.org/10.3390/ph13110383
Mai NNS, Otsuka Y, Kawano Y, Hanawa T. Preparation and Characterization of Solid Dispersions Composed of Curcumin, Hydroxypropyl Cellulose and/or Sodium Dodecyl Sulfate by Grinding with Vibrational Ball Milling. Pharmaceuticals. 2020; 13(11):383. https://doi.org/10.3390/ph13110383
Chicago/Turabian StyleMai, Nguyen Ngoc Sao, Yuta Otsuka, Yayoi Kawano, and Takehisa Hanawa. 2020. "Preparation and Characterization of Solid Dispersions Composed of Curcumin, Hydroxypropyl Cellulose and/or Sodium Dodecyl Sulfate by Grinding with Vibrational Ball Milling" Pharmaceuticals 13, no. 11: 383. https://doi.org/10.3390/ph13110383
APA StyleMai, N. N. S., Otsuka, Y., Kawano, Y., & Hanawa, T. (2020). Preparation and Characterization of Solid Dispersions Composed of Curcumin, Hydroxypropyl Cellulose and/or Sodium Dodecyl Sulfate by Grinding with Vibrational Ball Milling. Pharmaceuticals, 13(11), 383. https://doi.org/10.3390/ph13110383









