Germinated Soybean Embryo Extract Ameliorates Fatty Liver Injury in High-Fat Diet-Fed Obese Mice
Abstract
1. Introduction
2. Results
2.1. Concentration of Soyasaponin Ab in the GSEE
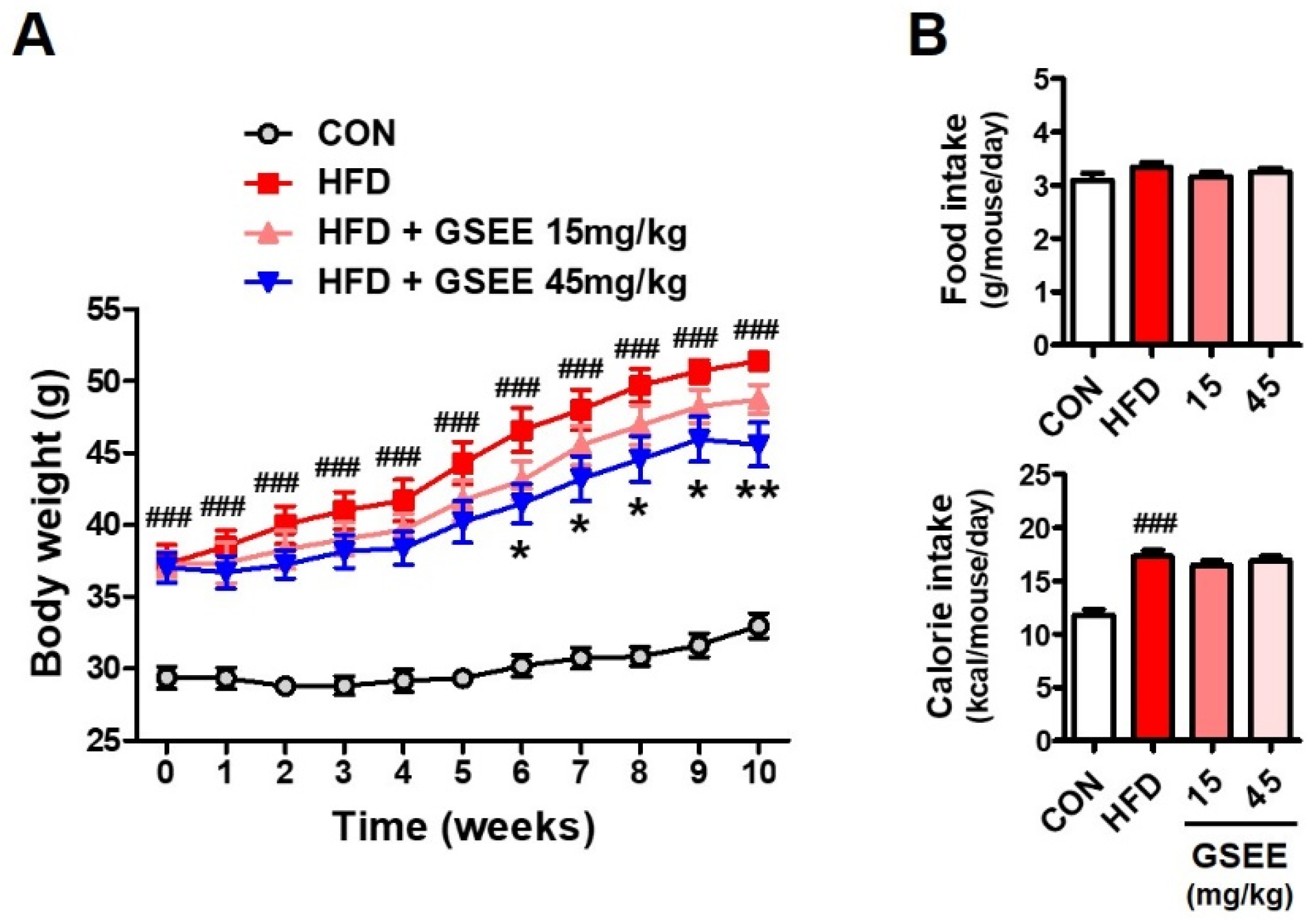
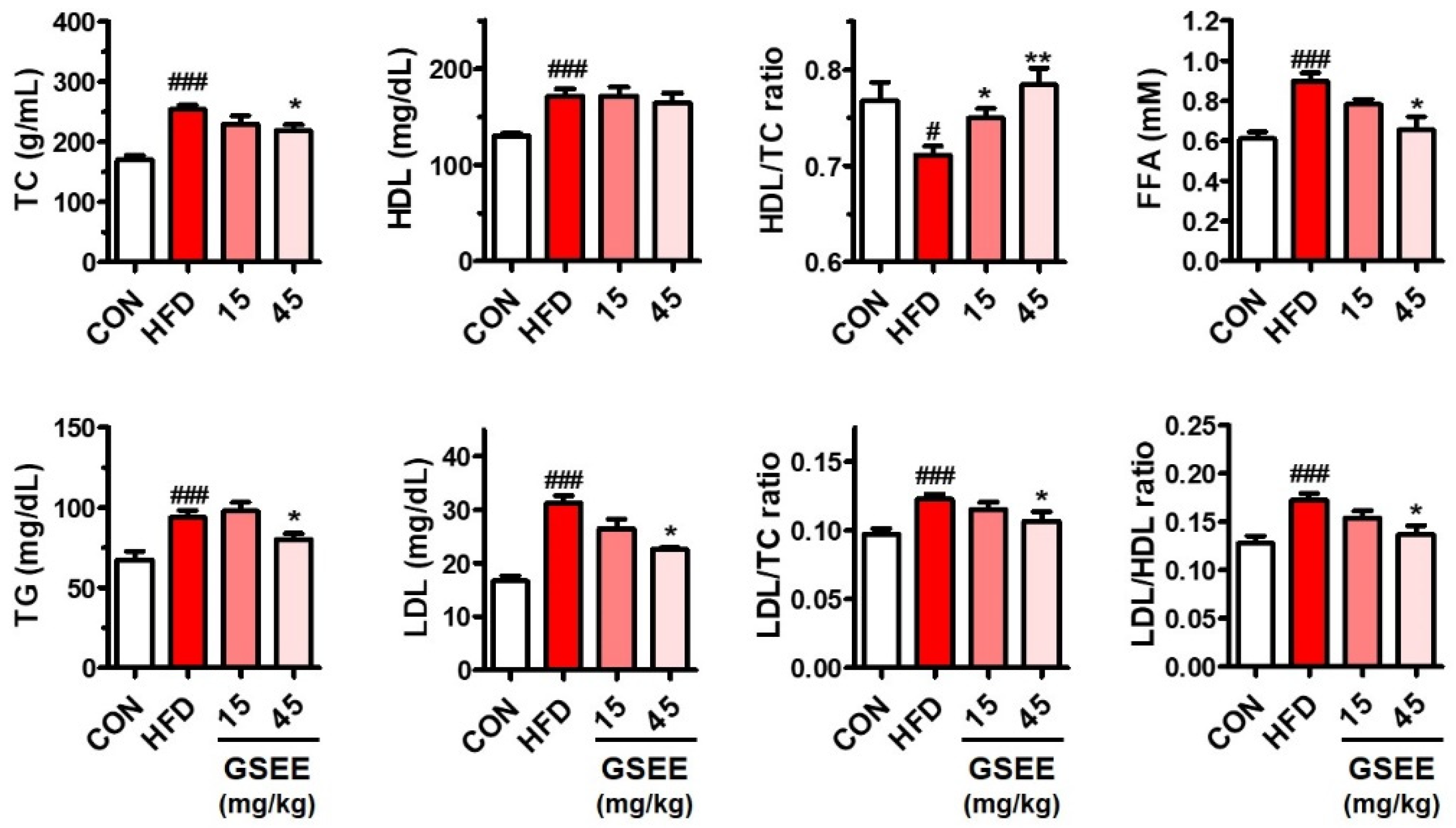
2.2. Anti-Obesity and Blood Lipid Lowering Effects of the GSEE in HFD-Fed Mice
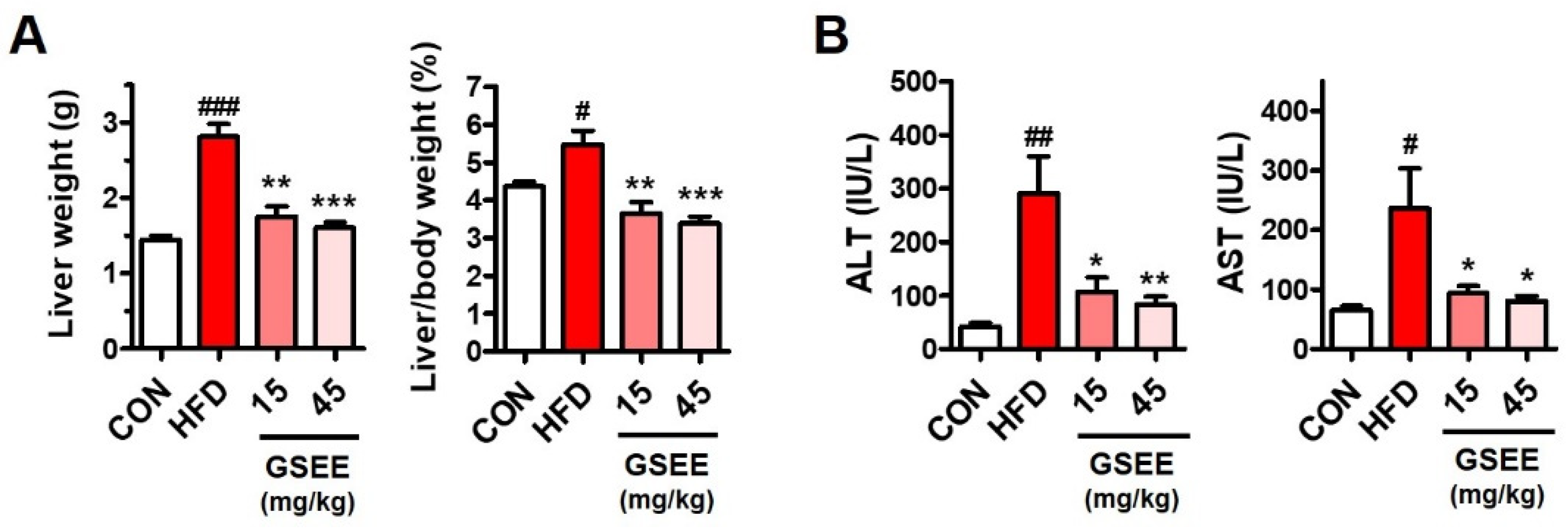
2.3. Effects of GSEE on HFD-Induced Liver Weight Changes and Hepatocellular Injury in Mice
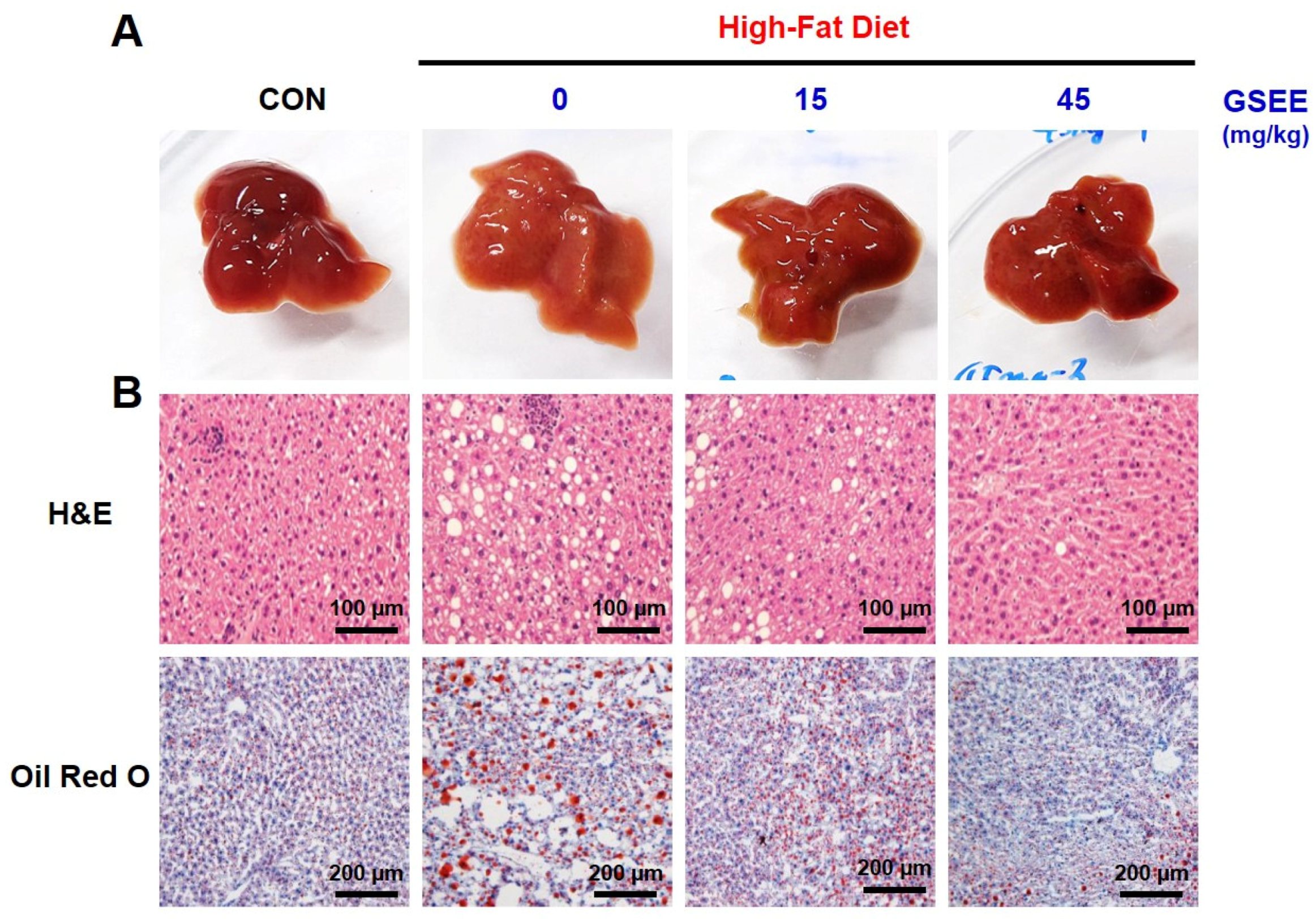
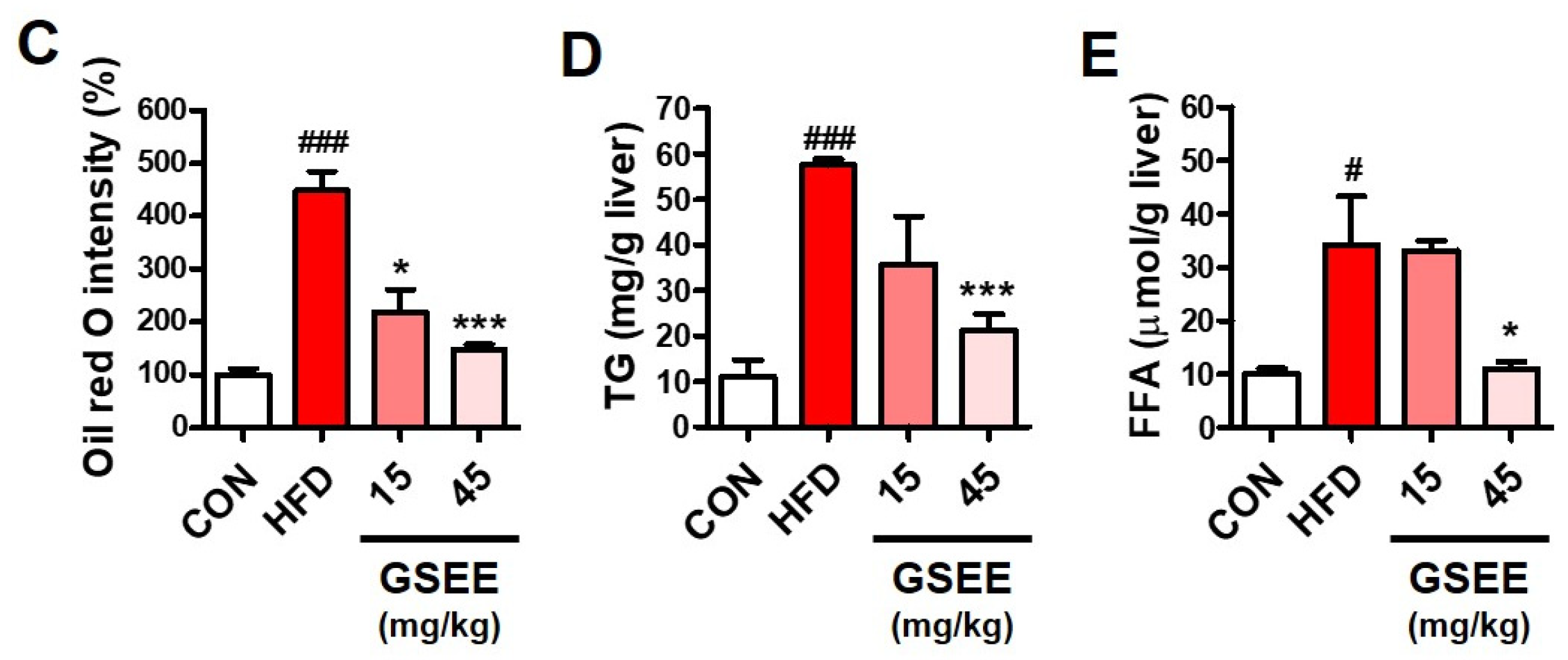
2.4. Inhibition of Hepatic Steatosis by GSEE in Mice
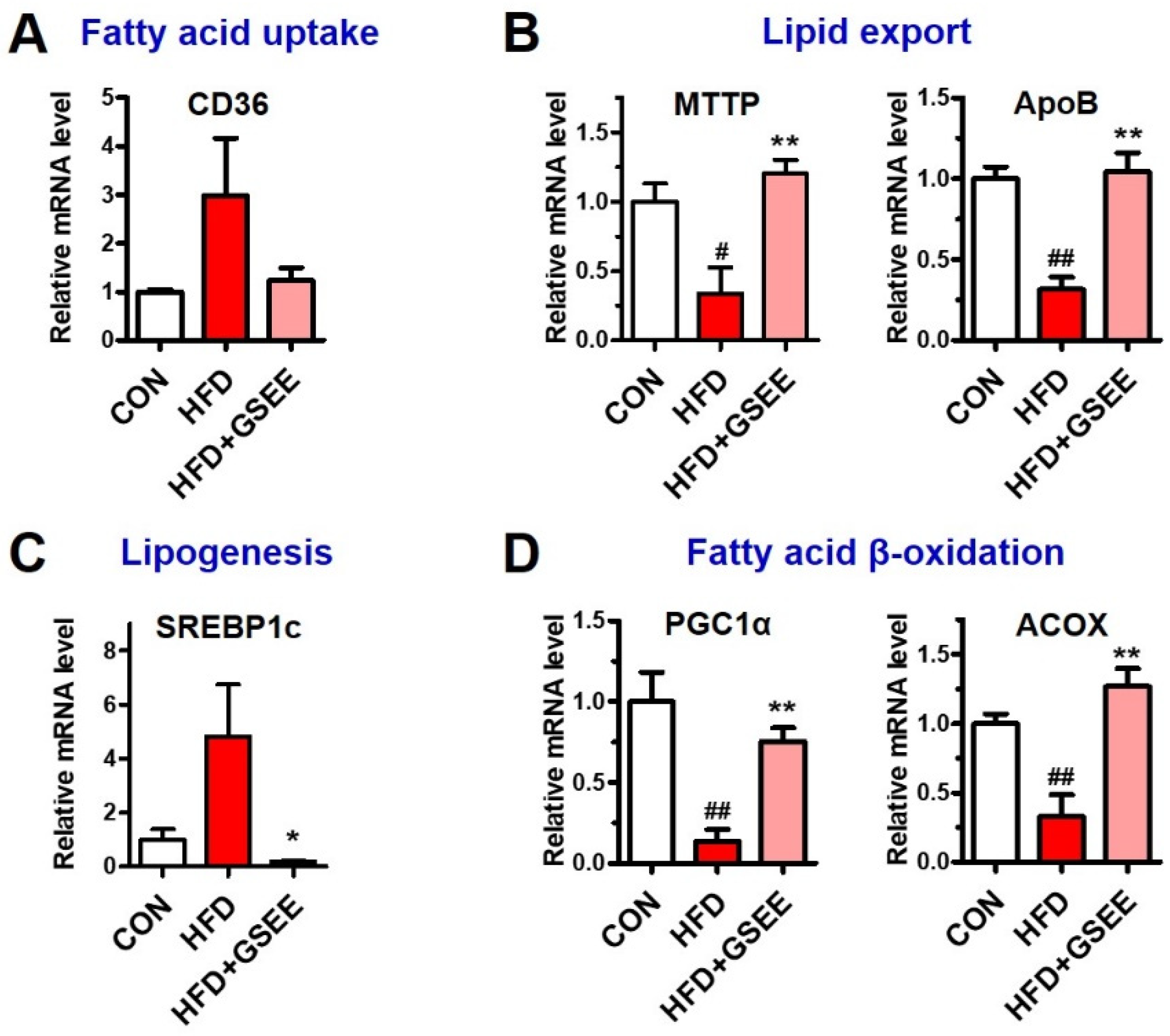
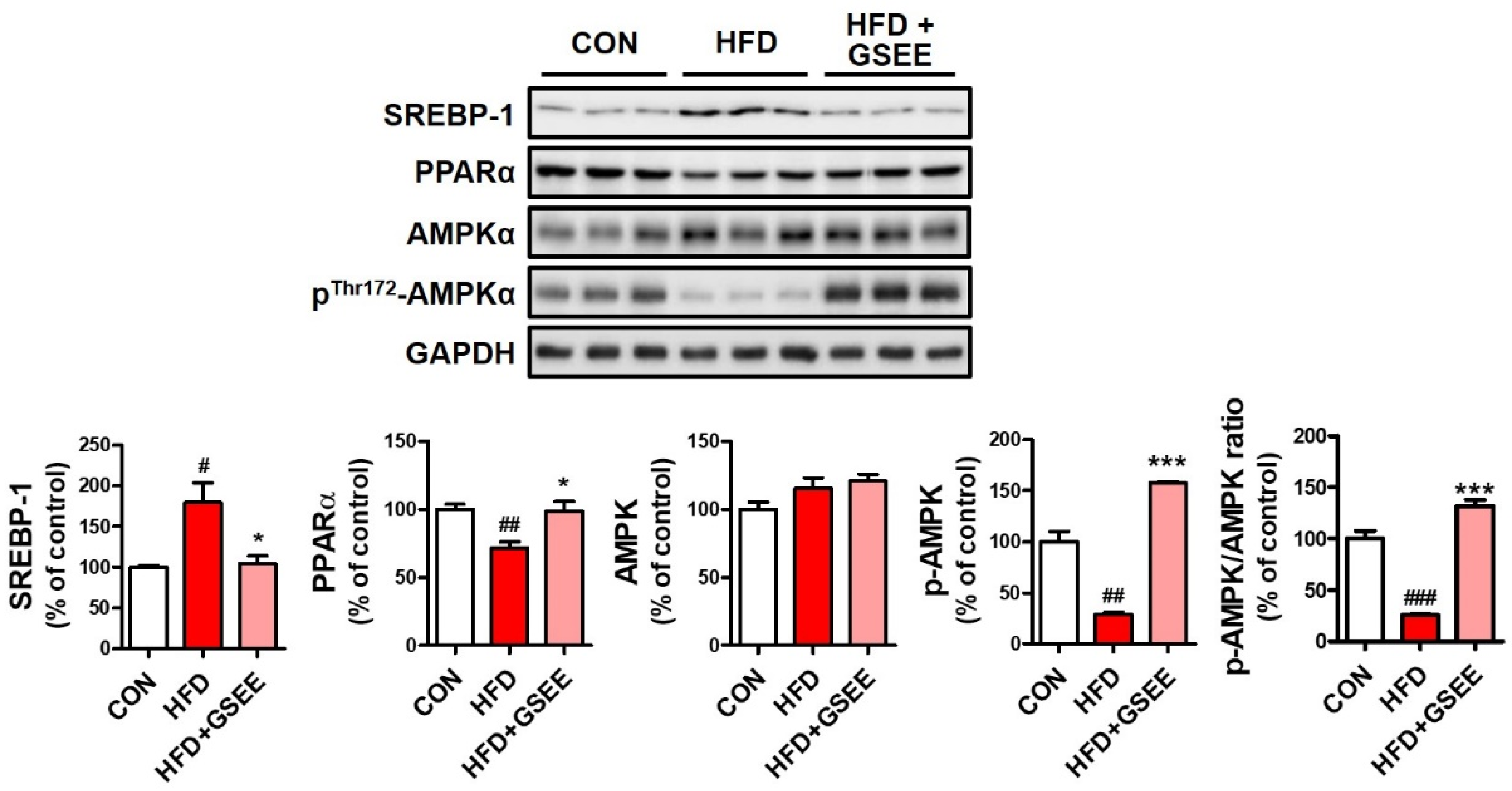
2.5. GSEE Restores the Dysregulated Hepatic Lipid Metabolism in HFD-Fed Mice
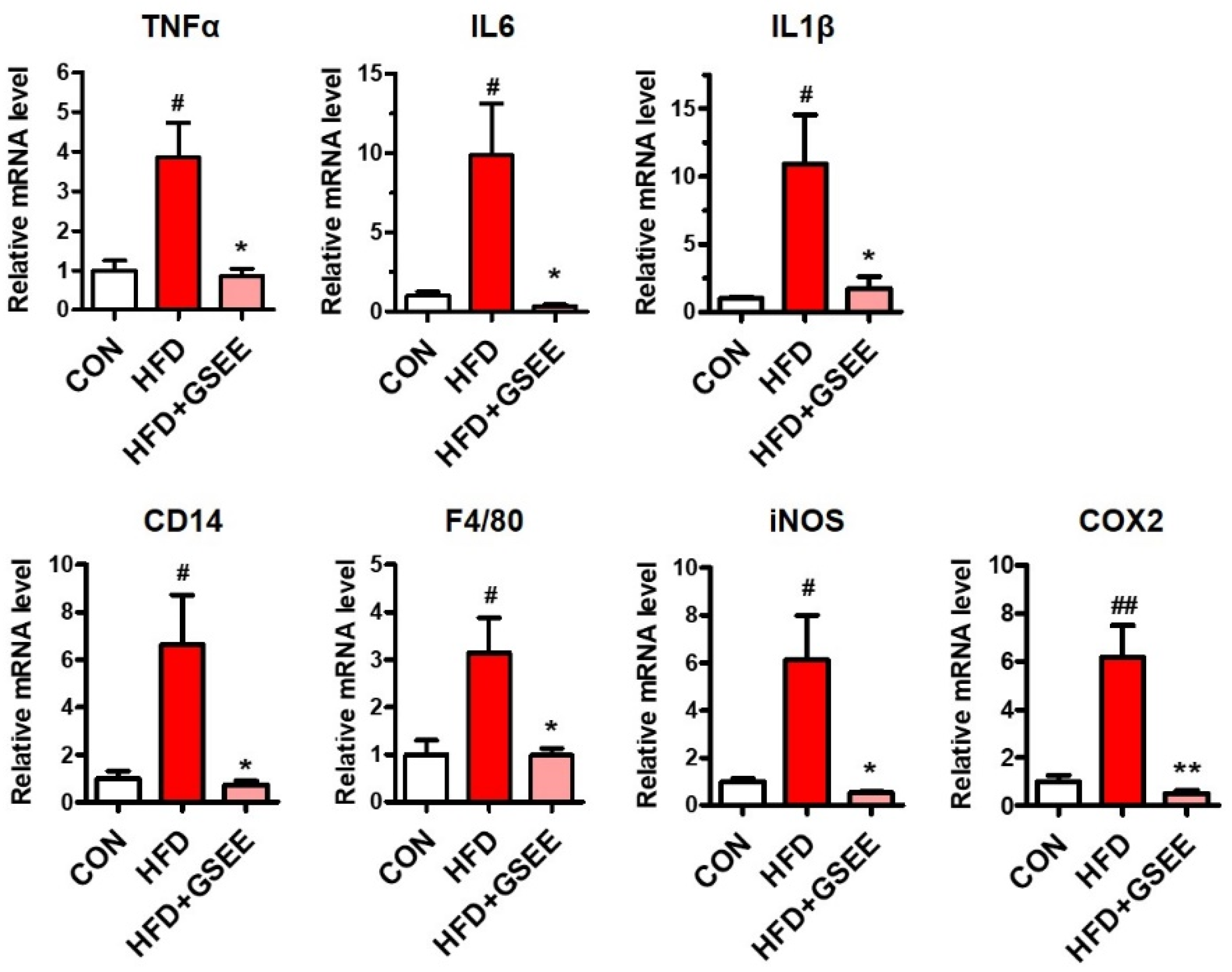
2.6. Anti-Inflammatory Effect of GSEE in HFD-Fed Mouse Livers
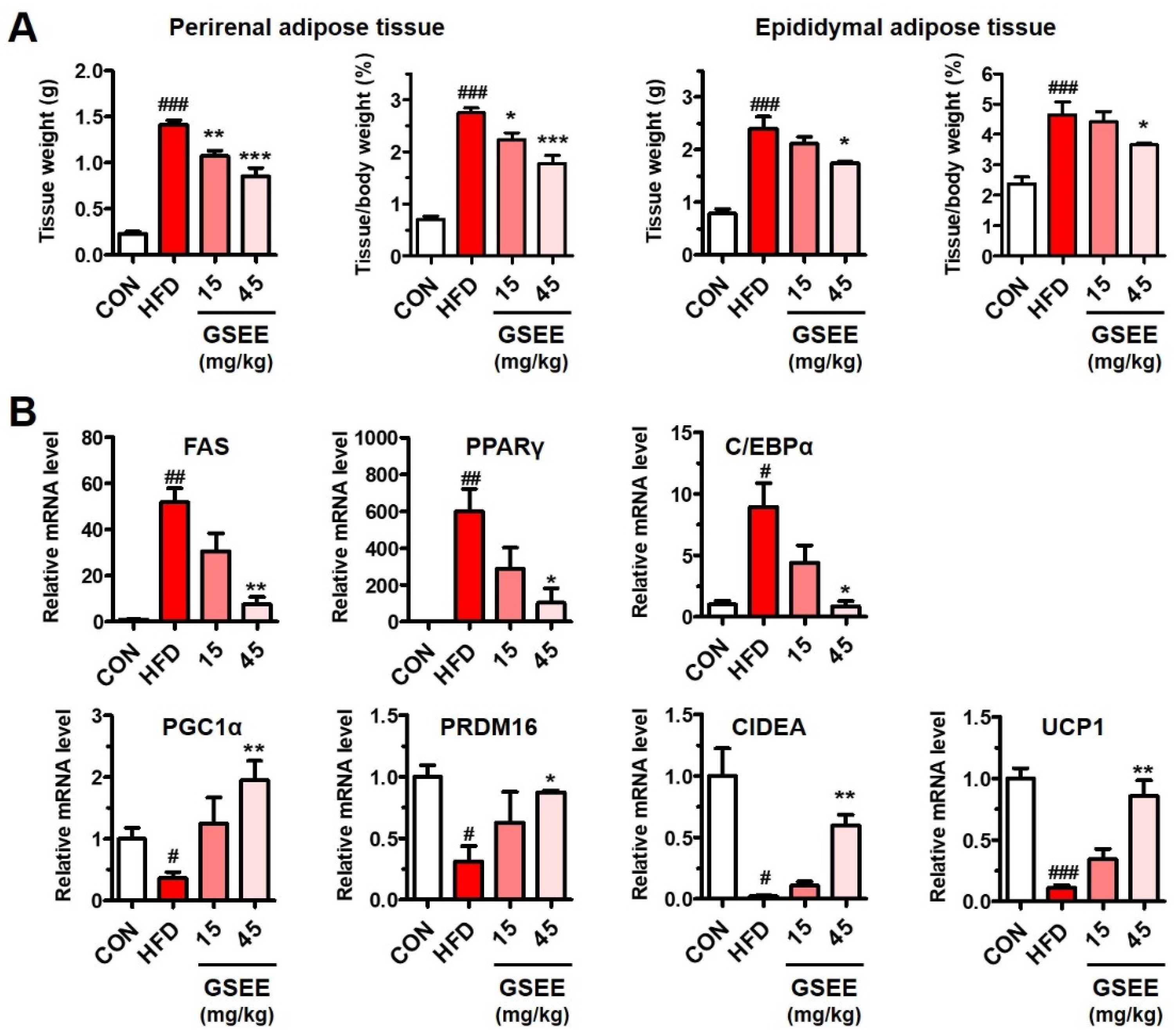
2.7. GSEE Inhibits White Adipocyte Differentiation but Stimulates Brown Adipocyte Differentiation in HFD-Fed Mouse Livers
3. Discussion
4. Materials and Methods
4.1. Preparation of the Germinated Soybean Embryo Extract (GSEE)
4.2. Quantification of the Soyasaponin Ab in the GSEE by HPLC/MS/MS
4.3. Animal Experiments
4.4. Serum Biochemical Analysis
4.5. Histological Examinations of the Mouse Liver Tissue
4.6. Hepatic Lipid Quantification
4.7. Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
4.8. Western Blotting
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [PubMed]
- Bessone, F.; Razori, M.V.; Roma, M.G. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell. Mol. Life Sci. 2019, 76, 99–128. [Google Scholar] [CrossRef] [PubMed]
- Messina, M. Soy and Health Update: Evaluation of the Clinical and Epidemiologic Literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, C.; Gleddie, S.; Xiao, C.W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef]
- Pabich, M.; Materska, M. Biological Effect of Soy Isoflavones in the Prevention of Civilization Diseases. Nutrients 2019, 11, 1660. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Bhathena, S.J. Role of dietary soy protein in obesity. Int. J. Med. Sci. 2007, 4, 72–82. [Google Scholar] [CrossRef]
- Ramdath, D.D.; Padhi, E.M.; Sarfaraz, S.; Renwick, S.; Duncan, A.M. Beyond the cholesterol-lowering effect of soy protein: A review of the effects of dietary soy and its constituents on risk factors for cardiovascular disease. Nutrients 2017, 9, 324. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Pan, M.H.; Ho, C.T. Anti-obesity molecular mechanism of soy isoflavones: Weaving the way to new therapeutic routes. Food Funct. 2017, 8, 3831–3846. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, S.; Tong, H.; Shi, S. Comprehensive evaluation of the role of soy and isoflavone supplementation in humans and animals over the past two decades. Phytother. Res. 2018, 32, 384–394. [Google Scholar] [CrossRef]
- Torre-Villalvazo, I.; Tovar, A.R.; Ramos-Barragán, V.E.; Cerbón-Cervantes, M.A.; Torres, N. Soy protein ameliorates metabolic abnormalities in liver and adipose tissue of rats fed a high fat diet. J. Nutr. 2008, 138, 462–468. [Google Scholar] [CrossRef]
- Ronis, M.J.; Chen, Y.; Badeaux, J.; Badger, T.M. Dietary soy protein isolate attenuates metabolic syndrome in rats via effects on PPAR, LXR, and SREBP signaling. J. Nutr. 2009, 139, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, S.; Packirisamy, R.M.; Bobby, Z.; Jacob, S.E.; Sridhar, M.G. Soy isoflavones (Glycine max) ameliorate hypertriglyceridemia and hepatic steatosis in high fat-fed ovariectomized Wistar rats (an experimental model of postmenopausal obesity). J. Nutr. Biochem. 2016, 38, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhong, H.; Leng, L.; Jiang, Z. Effects of soy isoflavone on hepatic steatosis in high fat-induced rats. J. Clin. Biochem. Nutr. 2017, 61, 85–90. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guang, C.; Chen, J.; Sang, S.; Cheng, S. Biological functionality of soyasaponins and soyasapogenols. J. Agric. Food Chem. 2014, 62, 8247–8255. [Google Scholar] [CrossRef]
- Su, S.J.; Chow, N.H.; Kung, M.L.; Hung, T.C.; Chang, K.L. Effects of soy isoflavones on apoptosis induction and G2-M arrest in human hepatoma cells involvement of caspase-3 activation, Bcl-2 and Bcl-XL downregulation, and Cdc2 kinase activity. Nutr. Cancer 2003, 45, 113–123. [Google Scholar] [CrossRef]
- Yang, X.; Dong, C.; Ren, G. Effect of soyasaponins-rich extract from soybean on acute alcohol-induced hepatotoxicity in mice. J. Agric. Food Chem. 2011, 59, 1138–1144. [Google Scholar] [CrossRef]
- Huang, Q.; Huang, R.; Zhang, S.; Lin, J.; Wei, L.; He, M.; Zhuo, L.; Lin, X. Protective effect of genistein isolated from Hydrocotyle sibthorpioides on hepatic injury and fibrosis induced by chronic alcohol in rats. Toxicol. Lett. 2013, 217, 102–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, H.; Lv, Z.; Zhang, M.; Zhang, T.; Li, Q.; Li, K. Anti-hepatitis B virus and anti-cancer activities of novel isoflavone analogs. Eur. J. Med. Chem. 2013, 62, 158–167. [Google Scholar] [CrossRef]
- Lijie, Z.; Ranran, F.; Xiuying, L.; Yutang, H.; Bo, W.; Tao, M. Soyasaponin Bb protects rat hepatocytes from alcohol-induced oxidative stress by inducing heme oxygenase-1. Pharmacogn. Mag. 2016, 12, 302–306. [Google Scholar] [CrossRef]
- Berhow, M.A.; Kong, S.B.; Vermillion, K.E.; Duval, S.M. Complete quantification of group A and group B soyasaponins in soybeans. J. Agric. Food Chem. 2006, 54, 2035–2044. [Google Scholar] [CrossRef]
- Pereira, I.R.; Faludi, A.A.; Aldrighi, J.M.; Bertolami, M.C.; Saleh, M.H.; Silva, R.A.; Nakamura, Y.; Campos, M.F.; Novaes, N.; Abdalla, D.S. Effects of soy germ isoflavones and hormone therapy on nitric oxide derivatives, lowdensity lipoprotein oxidation, and vascular reactivity in hypercholesterolemic postmenopausal women. Menopause 2006, 13, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Li, Z.; Yeung, V.; Xu, R.J. Dietary supplementation of soy germ phytoestrogens or estradiol improves spatial memory performance and increases gene expression of BDNF, TrkB receptor and synaptic factors in ovariectomized rats. Nutr. Metab. 2010, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.L.; Kim, Y.H.; Jung, G.H. Isolation of isoflavones and soyasaponins from the germ of soybean. Korean J. Crop. Sci. 2013, 58, 149–160. [Google Scholar] [CrossRef]
- Hong, J.; Kim, S.; Kim, H.S. Hepatoprotective Effects of Soybean Embryo by Enhancing Adiponectin-Mediated AMP-Activated Protein Kinase α Pathway in High-Fat and High-Cholesterol Diet-Induced Nonalcoholic Fatty Liver Disease. J. Med. Food 2016, 19, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.L.; Lee, J.E.; Kwon, Y.U.; Kim, W.H.; Jung, G.H.; Kim, D.W.; Lee, C.K.; Lee, Y.Y.; Kim, M.J.; Kim, Y.H.; et al. Introduction and nutritional evaluation of germinated soy germ. Food Chem. 2013, 136, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.W.; Choi, S.W.; Kim, H.J.; Lee, K.S.; Kim, S.H.; Kim, S.L.; Do, S.H.; Seo, W.D. Germinated soy germ with increased soyasaponin Ab improves BMP-2-induced bone formation and protects against in vivo bone loss in osteoporosis. Sci. Rep. 2018, 8, 12970. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, E.J.; Kim, H.S.; Choi, C.W.; Choi, S.W.; Kim, S.L.; Seo, W.D.; Do, S.H. Germinated soy germ extract ameliorates obesity through beige fat activation. Food Funct. 2019, 10, 836–848. [Google Scholar] [CrossRef]
- Charlton, M.; Sreekumar, R.; Rasmussen, D.; Lindor, K.; Nair, K.S. Apolipoprotein synthesis in nonalcoholic steatohepatitis. Hepatology 2002, 35, 898–904. [Google Scholar] [CrossRef]
- Fujita, K.; Nozaki, Y.; Wada, K.; Yoneda, M.; Fujimoto, Y.; Fujitake, M.; Endo, H.; Takahashi, H.; Inamori, M.; Kobayashi, N.; et al. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology 2009, 50, 772–780. [Google Scholar] [CrossRef]
- Higuchi, N.; Kato, M.; Tanaka, M.; Miyazaki, M.; Takao, S.; Kohjima, M.; Kotoh, K.; Enjoji, M.; Nakamuta, M.; Takayanagi, R. Effects of insulin resistance and hepatic lipid accumulation on hepatic mRNA expression levels of apoB, MTP and L-FABP in non-alcoholic fatty liver disease. Exp. Ther. Med. 2011, 2, 1077–1081. [Google Scholar] [CrossRef]
- Moslehi, A.; Hamidi-Zad, Z. Role of SREBPs in Liver Diseases: A Mini-review. J. Clin. Transl. Hepatol. 2018, 6, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrinol. Metab. 2017, 28, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Marcos, P.J.; Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011, 93, 884S–890S. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as metabolic regulators in the liver: Lessons from liver-specific PPAR-null mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef]
- Morris, E.M.; Meers, G.M.; Booth, F.W.; Fritsche, K.L.; Hardin, C.D.; Thyfault, J.P.; Ibdah, J.A. PGC-1α overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G979–G992. [Google Scholar] [CrossRef]
- Braunersreuther, V.; Viviani, G.L.; Mach, F.; Montecucco, F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J. Gastroenterol. 2012, 18, 727–735. [Google Scholar] [CrossRef]
- Carvalho-Filho, M.A.; Ueno, M.; Hirabara, S.M.; Seabra, A.B.; Carvalheira, J.B.; de Oliveira, M.G.; Velloso, L.A.; Curi, R.; Saad, M.J. S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: A novel mechanism of insulin resistance. Diabetes 2005, 54, 959–967. [Google Scholar] [CrossRef]
- Wenfeng, Z.; Yakun, W.; Di, M.; Jianping, G.; Chuanxin, W.; Chun, H. Kupffer cells: Increasingly significant role in nonalcoholic fatty liver disease. Ann. Hepatol. 2014, 13, 489–495. [Google Scholar] [CrossRef]
- Lefere, S.; Tacke, F. Macrophages in obesity and non-alcoholic fatty liver disease: Crosstalk with metabolism. JHEP Rep. 2019, 1, 30–43. [Google Scholar] [CrossRef]
- Ogawa, Y.; Imajo, K.; Yoneda, M.; Kessoku, T.; Tomeno, W.; Shinohara, Y.; Kato, S.; Mawatari, H.; Nozaki, Y.; Fujita, K.; et al. Soluble CD14 levels reflect liver inflammation in patients with nonalcoholic steatohepatitis. PLoS ONE. 2013, 8, e65211. [Google Scholar] [CrossRef]
- Filliol, A.; Piquet-Pellorce, C.; Le Seyec, J.; Farooq, M.; Genet, V.; Lucas-Clerc, C.; Bertin, J.; Gough, P.J.; Dimanche-Boitrel, M.T.; Vandenabeele, P.; et al. RIPK1 protects from TNF-α-mediated liver damage during hepatitis. Cell Death Dis. 2016, 7, e2462. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Li, N.; Chen, M.; Zheng, J.; Qian, Z.; Wang, X.; Huang, C.; Xu, S.; Shi, G. Cyclooxygenase-2 promotes hepatocellular apoptosis by interacting with TNF-α and IL-6 in the pathogenesis of nonalcoholic steatohepatitis in rats. Dig. Dis. Sci. 2013, 58, 2895–2902. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: Pathophysiology and clinical implications. Gastroenterology 2012, 142, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Parker, R. The role of adipose tissue in fatty liver diseases. Liver. Res. 2018, 2, 35–42. [Google Scholar] [CrossRef]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Haakonsson, A.K.; Lazar, M.A.; Mandrup, S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 2014, 25, 293–302. [Google Scholar] [CrossRef]
- Linhart, H.G.; Ishimura-Oka, K.; DeMayo, F.; Kibe, T.; Repka, D.; Poindexter, B.; Bick, R.J.; Darlington, G.J. C/EBPalpha is required for differentiation of white, but not brown, adipose tissue. Proc. Natl. Acad. Sci. USA 2001, 98, 12532–12537. [Google Scholar] [CrossRef]
- Seale, P.; Kajimura, S.; Yang, W.; Chin, S.; Rohas, L.M.; Uldry, M.; Tavernier, G.; Langin, D.; Spiegelman, B.M. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007, 6, 38–54. [Google Scholar] [CrossRef]
- Jash, S.; Banerjee, S.; Lee, M.J.; Farmer, S.R.; Puri, V. CIDEA Transcriptionally Regulates UCP1 for Britening and Thermogenesis in Human Fat Cells. iScience 2019, 20, 73–89. [Google Scholar] [CrossRef]
- Lo, K.A.; Sun, L. Turning WAT into BAT: A review on regulators controlling the browning of white adipocytes. Biosci. Rep. 2013, 33, e00065. [Google Scholar] [CrossRef]
- Bays, H.E.; Toth, P.P.; Kris-Etherton, P.M.; Abate, N.; Aronne, L.J.; Brown, W.V.; Gonzalez-Campoy, J.M.; Jones, S.R.; Kumar, R.; La Forge, R.; et al. Obesity, adiposity, and dyslipidemia: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 304–383. [Google Scholar] [CrossRef] [PubMed]
- Rui, L. Energy Metabolism in the Liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; She, P.; Mozzoli, M.; Cheung, P.; Gumireddy, K.; Reddy, P.; Xiang, X.; Luo, Z.; Ruderman, N. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes 2005, 54, 3458–3465. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Pang, D.; Luo, Q.; Chen, X.; Gao, Q.; Shi, L.; Liu, W.; Zou, Y.; Li, L.; Chen, Z. Soy Isoflavones Regulate Lipid Metabolism through an AKT/mTORC1 Pathway in Diet-Induced Obesity (DIO) Male Rats. Molecules 2016, 21, 586. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Vinciguerra, M.; Gjinovci, A.; Kühne, F.; Klein, M.; Cederroth, M.; Caille, D.; Suter, M.; Neumann, D.; James, R.W.; et al. Dietary phytoestrogens activate AMP-activated protein kinase with improvement in lipid and glucose metabolism. Diabetes 2008, 57, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.Y.; Mao, L.M.; Lu, X.C.; Deng, H.; Ye, J.F.; Chu, X.W.; Sun, S.X.; Luo, H.J. Anti-inflammatory effect of soyasaponins through suppressing nitric oxide production in LPS-stimulated RAW 264.7 cells by attenuation of NF-κB-mediated nitric oxide synthase expression. Bioorg. Med. Chem. Lett. 2011, 21, 2415–2418. [Google Scholar] [CrossRef]
- Lin, J.; Cheng, Y.; Wang, T.; Tang, L.; Sun, Y.; Lu, X.; Yu, H. Soyasaponin Ab inhibits lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol. 2016, 30, 121–128. [Google Scholar] [CrossRef]
- Chen, J.; Ullah, H.; Zheng, Z.; Gu, X.; Su, C.; Xiao, L.; Wu, X.; Xiong, F.; Li, Q.; Zha, L. Soyasaponins reduce inflammation by downregulating MyD88 expression and suppressing the recruitments of TLR4 and MyD88 into lipid rafts. BMC Complement. Med. Ther. 2020, 20, 167. [Google Scholar] [CrossRef]
- Gao, B.; Tsukamoto, H. Inflammation in alcoholic and nonalcoholic fatty liver disease: Friend or foe? Gastroenterology 2016, 150, 1704–1709. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Y.; Keshaw, H.; Xu, L.Y.; Lam, K.S.; Cooper, G.J. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J. Clin. Investig. 2003, 112, 91–100. [Google Scholar] [CrossRef]
- Awazawa, M.; Ueki, K.; Inabe, K.; Yamauchi, T.; Kubota, N.; Kaneko, K.; Kobayashi, M.; Iwane, A.; Sasako, T.; Okazaki, Y.; et al. Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway. Cell Metab. 2011, 13, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Rideout, D.; Rakita, S.; Sajan, M.; Farese, R.; You, M.; Murr, M.M. Downregulation of adiponectin/AdipoR2 is associated with steatohepatitis in obese mice. J. Gastrointest. Surg. 2009, 13, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Handa, P.; Maliken, B.D.; Nelson, J.E.; Morgan-Stevenson, V.; Messner, D.J.; Dhillon, B.K.; Klintworth, H.M.; Beauchamp, M.; Yeh, M.M.; Elfers, C.T.; et al. Reduced adiponectin signaling due to weight gain results in nonalcoholic steatohepatitis through impaired mitochondrial biogenesis. Hepatology 2014, 60, 133–145. [Google Scholar] [CrossRef]
- Scheja, L.; Heeren, J. Metabolic interplay between white, beige, brown adipocytes and the liver. J. Hepatol. 2016, 64, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Kern, P.A.; Ranganathan, S.; Li, C.; Wood, L.; Ranganathan, G. Adipose tissue tumor necrosis factor and Interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E745–E751. [Google Scholar] [CrossRef]
- Zhang, H.H.; Halbleib, M.; Ahmad, F.; Manganiello, V.C.; Greenberg, A.S. Tumor necrosis factor—A stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes 2002, 51, 2929–2935. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, Z.; Zhu, X.; Meng, M.; Li, L.; Shen, Y.; Chi, Q.; Wang, D.; Zhang, Z.; Li, C.; et al. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res. 2013, 23, 851–854. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; You, Y.; Meng, M.; Zheng, Z.; Dong, M.; Lin, J.; Zhao, Q.; Zhang, C.; Yuan, X.; et al. Brown adipose tissue transplantation reverses obesity in Ob/Ob mice. Endocrinology 2015, 156, 2461–2469. [Google Scholar] [CrossRef]
- Dang, Z.; Löwik, C.W. The balance between concurrent activation of ERs and PPARs determines daidzein-induced osteogenesis and adipogenesis. J. Bone Miner. Res. 2004, 19, 853–861. [Google Scholar] [CrossRef]
- Heim, M.; Frank, O.; Kampmann, G.; Sochocky, N.; Pennimpede, T.; Fuchs, P.; Hunziker, W.; Weber, P.; Martin, I.; Bendik, I. The phytoestrogen genistein enhances osteogenesis and represses adipogenic differentiation of human primary bone marrow stromal cells. Endocrinology 2004, 145, 848–859. [Google Scholar] [CrossRef]
- Park, H.J.; Della-Fera, M.A.; Hausman, D.B.; Rayalam, S.; Ambati, S.; Baile, C.A. Genistein inhibits differentiation of primary human adipocytes. J. Nutr. Biochem. 2009, 20, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, S.; Zha, D.; Wu, J.; Mao, L.; Deng, H.; Chu, X.; Luo, H.; Zha, L. Soyasaponins prevent H₂O₂-induced inhibition of gap junctional intercellular communication by scavenging reactive oxygen species in rat liver cells. Nutr. Cancer 2014, 66, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Yoshiki, Y.; Kahara, T.; Okubo, K.; Sakabe, T.; Yamasaki, T. Superoxide- and 1,1-diphenyl-2-picrylhydrazyl radical-scavenging activities of soyasaponin beta g related to gallic acid. Biosci. Biotechnol. Biochem. 2001, 65, 2162–2165. [Google Scholar] [CrossRef] [PubMed]
- Rimbach, G.; De Pascual-Teresa, S.; Ewins, B.A.; Matsugo, S.; Uchida, Y.; Minihane, A.M.; Turner, R.; VafeiAdou, K.; Weinberg, P.D. Antioxidant and free radical scavenging activity of isoflavone metabolites. Xenobiotica 2003, 33, 913–925. [Google Scholar] [CrossRef]
- Patel, R.P.; Boersma, B.J.; Crawford, J.H.; Hogg, N.; Kirk, M.; Kalyanaraman, B.; Parks, D.A.; Barnes, S.; Darley-Usmar, V. Antioxidant mechanisms of isoflavones in lipid systems: Paradoxical effects of peroxyl radical scavenging. Free Radic. Biol. Med. 2001, 31, 1570–1581. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F.; Chen, J.; Huang, S.; Chen, J.; Huang, J.; Li, N.; Sun, S.; Chu, X.; Zha, L. Soyasaponin Bb inhibits the recruitment of toll-like receptor 4 (TLR4) into lipid rafts and its signaling pathway by suppressing the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-dependent generation of reactive oxygen species. Mol. Nutr. Food Res. 2016, 60, 1532–1543. [Google Scholar] [CrossRef]

| Genes | Primer Sequences | |
|---|---|---|
| ACOX | F: GGAAGACTTCCAATCATGCGATAG | R: GACAACAAAGGCATGTAACCCG |
| ApoB | F: TTGGCAAACTGCATAGCATCC | R: TCAAATTGGGACTCTCCTTTAGC |
| C/EBPα | F: CAAGAACAGCAACGAGTACCG | R: GTCACTGGTCAACTCCAGCAC |
| CD14 | F: AAACTCGCTCAATCTGTCTTTCACT | R: TCCTATCCAGCCTGTTGTAACTGA |
| CD36 | F: CCTTGGCAACCAACCACAAA | R: ATCCACCAGTTGCTCCACAC |
| CIDEA | F: TGACATTCATGGGATTGCAGAC | R: CATGGTTTGAAACTCGAAAAGGG |
| COX2 | F: GCATTCTTTGCCCAGCACTT | R: AGACCAGGCACCAGACCAAAG |
| F4/80 | F: GTGACTCACCTTGTGGTCCT | R: CAGACACTCATCAACATCTGCG |
| FAS | F: AGGTGGTGATAGCCGGTATGT | R: TGGGTAATCCATAGAGCCCAG |
| IL1β | F: TTCACCATGGAATCCGTGTC | R: GTCTTGGCCGAGGACTAAGG |
| IL6 | F: TTGCCTTCTTGGGACTGATG | R: CCACGATTTCCCAGAGAACA |
| iNOS | F: CGAAACGCTTCACTTCCAA | R: TGAGCCTATATTGCTGTGGCT |
| MTTP | F: CTCTTGGCAGTGCTTTTTCTCT | R: GAGCTTGTATAGCCGCTCATT |
| PGC1α | F: TATGGAGTGACATAGAGTGTGCT | R: CCACTTCAATCCACCCAGAAAG |
| PPARγ | F: GGAAGACCACTCGCATTCCTT | R: GTAATCAGCAACCATTGGGTC |
| PRDM16 | F: CCACCAGCGAGGACTTCAC | R: GGAGGACTCTCGTAGCTCGAA |
| SREBP1c | F: GATGTGCGAACTGGACACAG | R: CATAGGGGGCGTCAAACAG |
| TNFα | F: GGCCTCTCTACCTTGTTGCC | R: CAGCCTGGTCACCAAATCAG |
| UCP1 | F: AGCCATCTGCATGGGATCAAA | R: GGGTCGTCCCTTTCCAAAGTG |
| β-actin | F: ACGTCGACATCCGCAAAGACCTC | R: TGATCTCCTTCTGCATCCGGTCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, D.; Kim, S.H.; Son, S.W.; Seo, J.; Jeong, T.B.; Kim, K.-M.; Jung, J.-C.; Jung, M.S.; Lee, Y.-H.; Jung, Y.-S. Germinated Soybean Embryo Extract Ameliorates Fatty Liver Injury in High-Fat Diet-Fed Obese Mice. Pharmaceuticals 2020, 13, 380. https://doi.org/10.3390/ph13110380
Kwon D, Kim SH, Son SW, Seo J, Jeong TB, Kim K-M, Jung J-C, Jung MS, Lee Y-H, Jung Y-S. Germinated Soybean Embryo Extract Ameliorates Fatty Liver Injury in High-Fat Diet-Fed Obese Mice. Pharmaceuticals. 2020; 13(11):380. https://doi.org/10.3390/ph13110380
Chicago/Turabian StyleKwon, Doyoung, Sou Hyun Kim, Seung Won Son, Jinuk Seo, Tae Bin Jeong, Kyung-Mi Kim, Jae-Chul Jung, Mi Sook Jung, Yun-Hee Lee, and Young-Suk Jung. 2020. "Germinated Soybean Embryo Extract Ameliorates Fatty Liver Injury in High-Fat Diet-Fed Obese Mice" Pharmaceuticals 13, no. 11: 380. https://doi.org/10.3390/ph13110380
APA StyleKwon, D., Kim, S. H., Son, S. W., Seo, J., Jeong, T. B., Kim, K.-M., Jung, J.-C., Jung, M. S., Lee, Y.-H., & Jung, Y.-S. (2020). Germinated Soybean Embryo Extract Ameliorates Fatty Liver Injury in High-Fat Diet-Fed Obese Mice. Pharmaceuticals, 13(11), 380. https://doi.org/10.3390/ph13110380





