Enhancing Stability and Tooth Bleaching Activity of Carbamide Peroxide by Electrospun Nanofibrous Film
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparations and Characteristics of EFASs
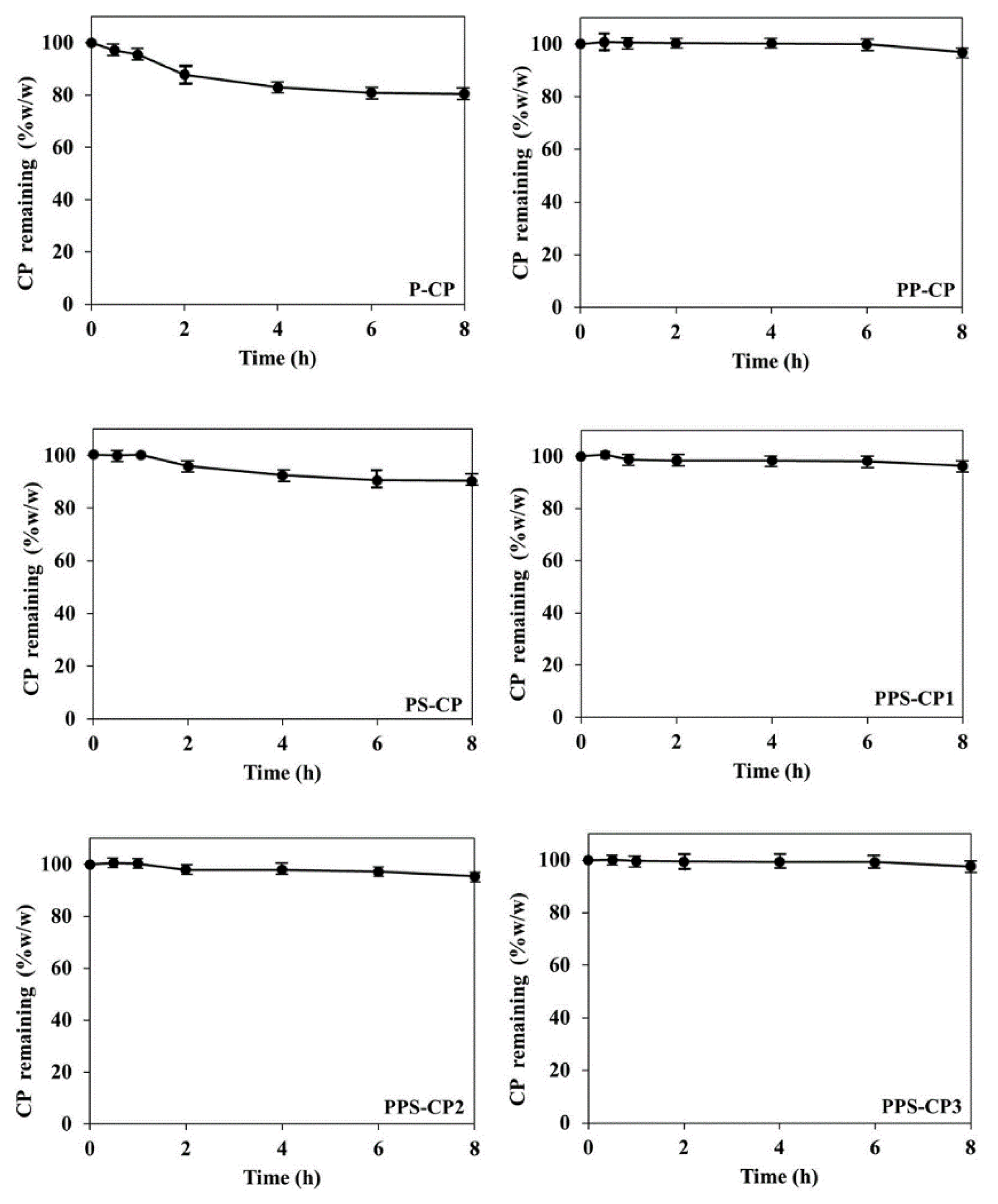
2.2. Stability of CP in EFASs
2.3. Fabrication and Characterization of ENFs
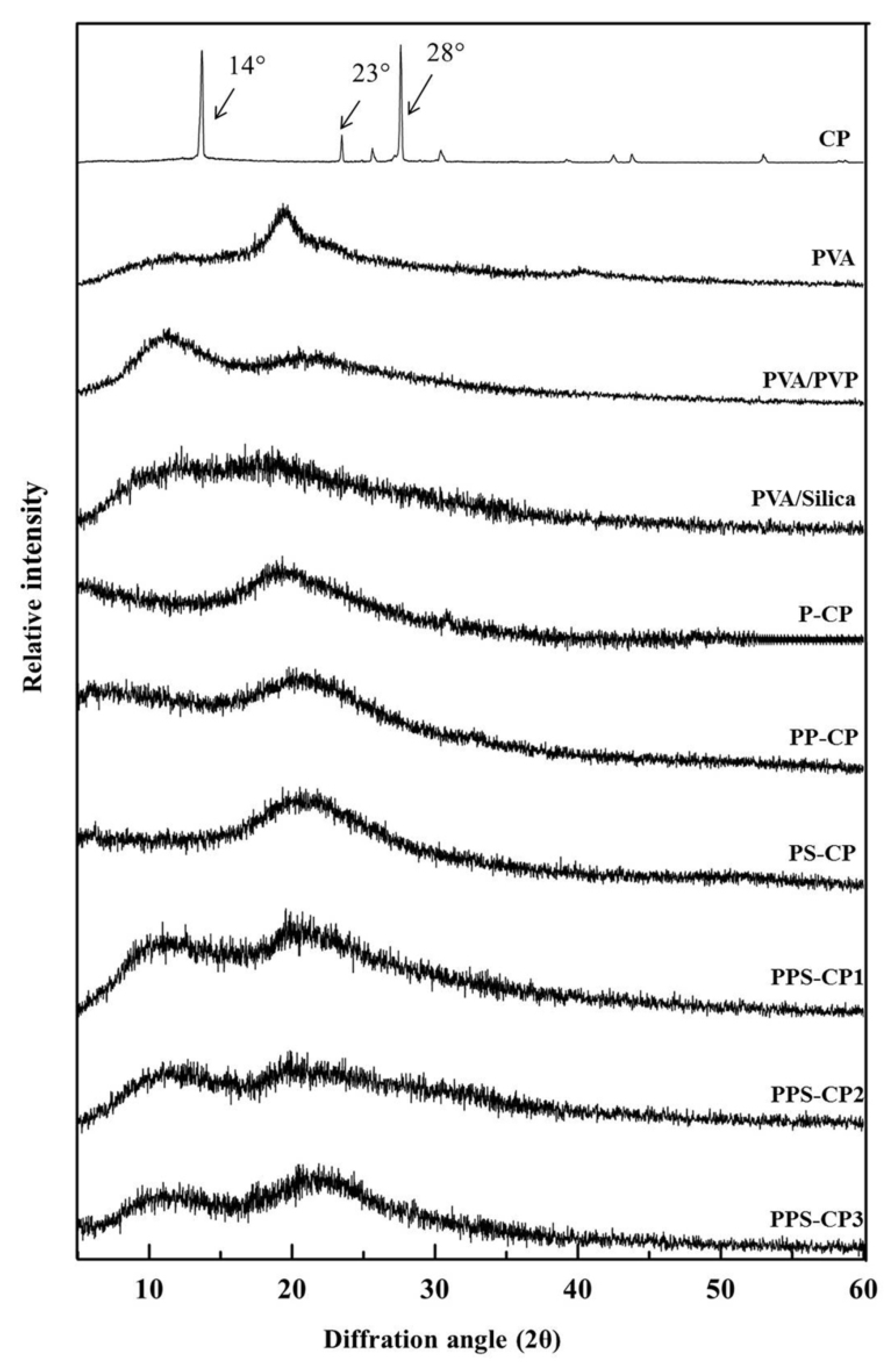
2.4. Solid State of ENFs
2.5. Adhesive Property and Entrapment Efficiency of ENFs
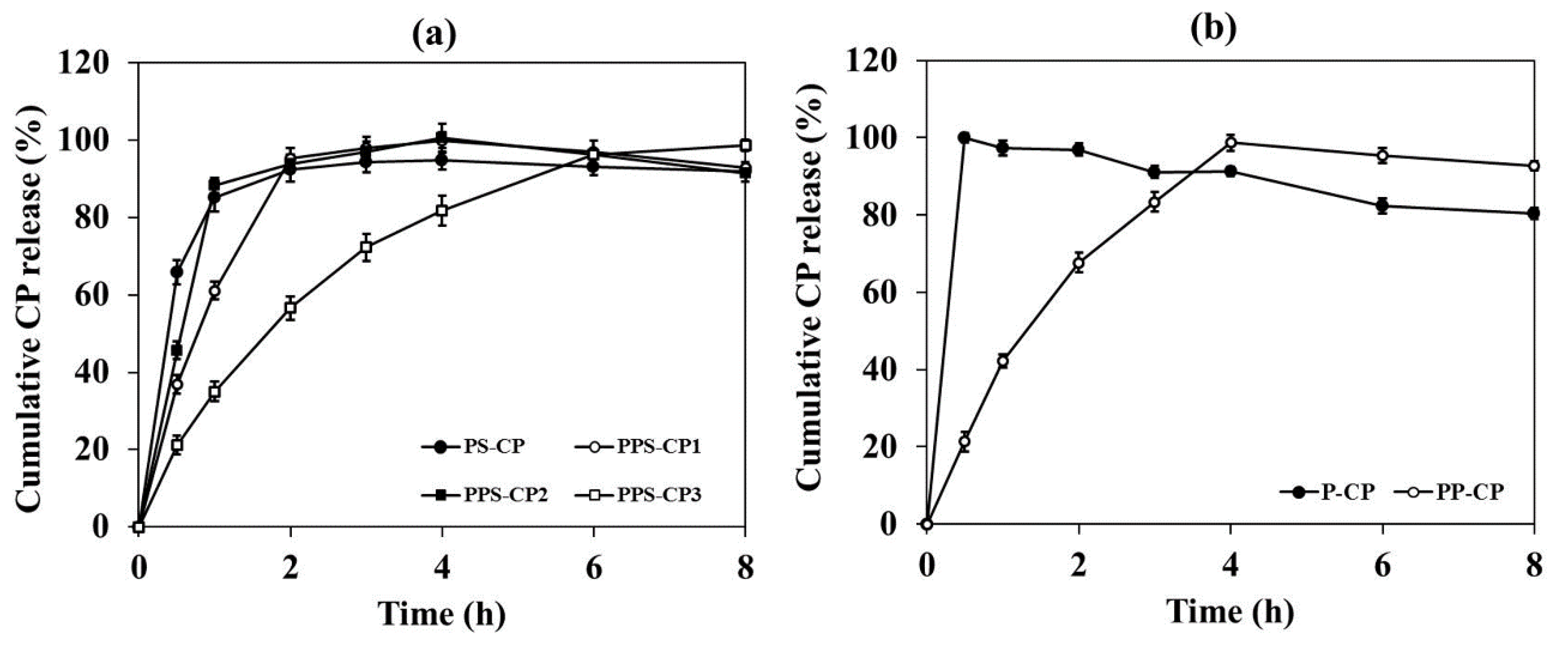
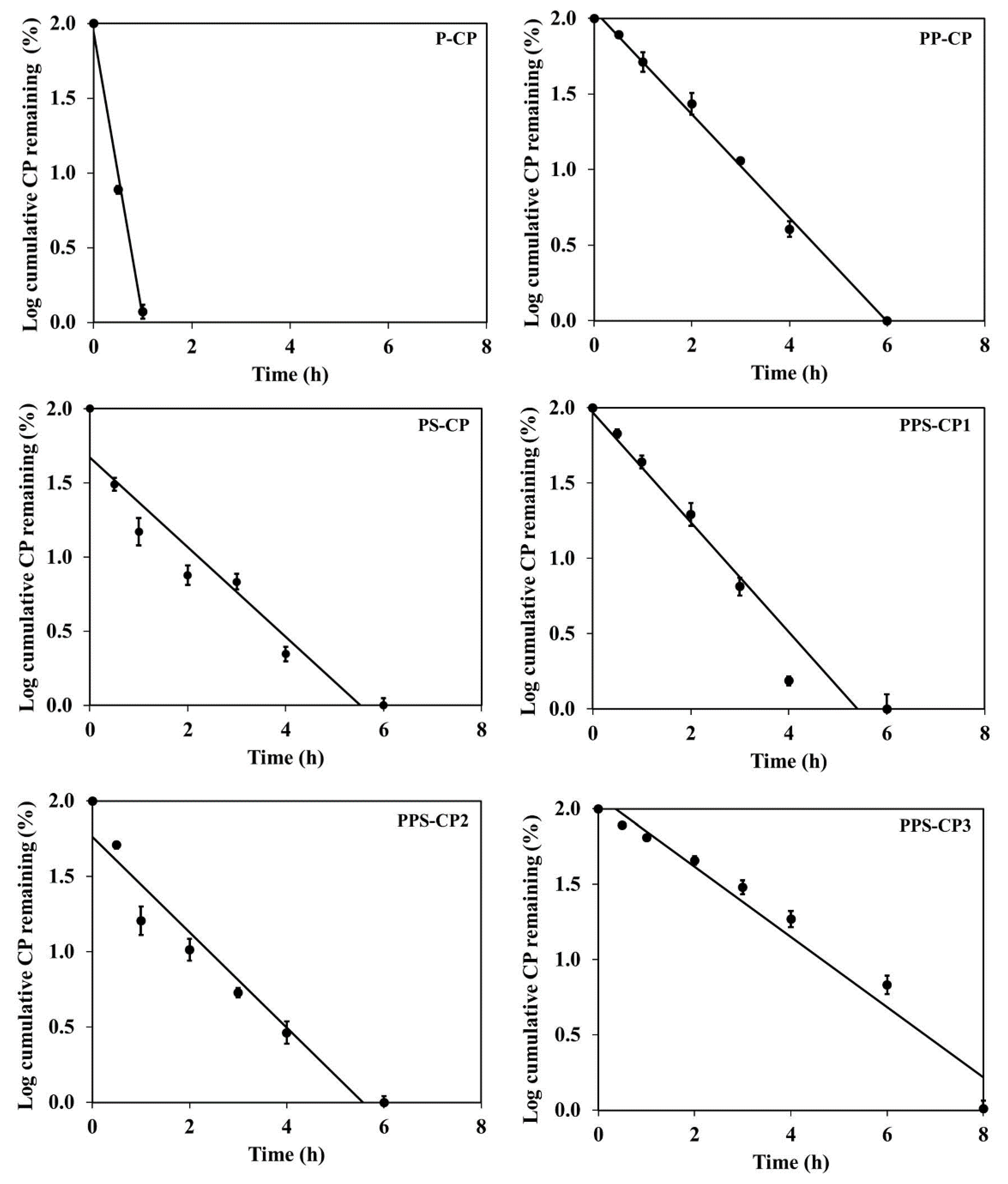
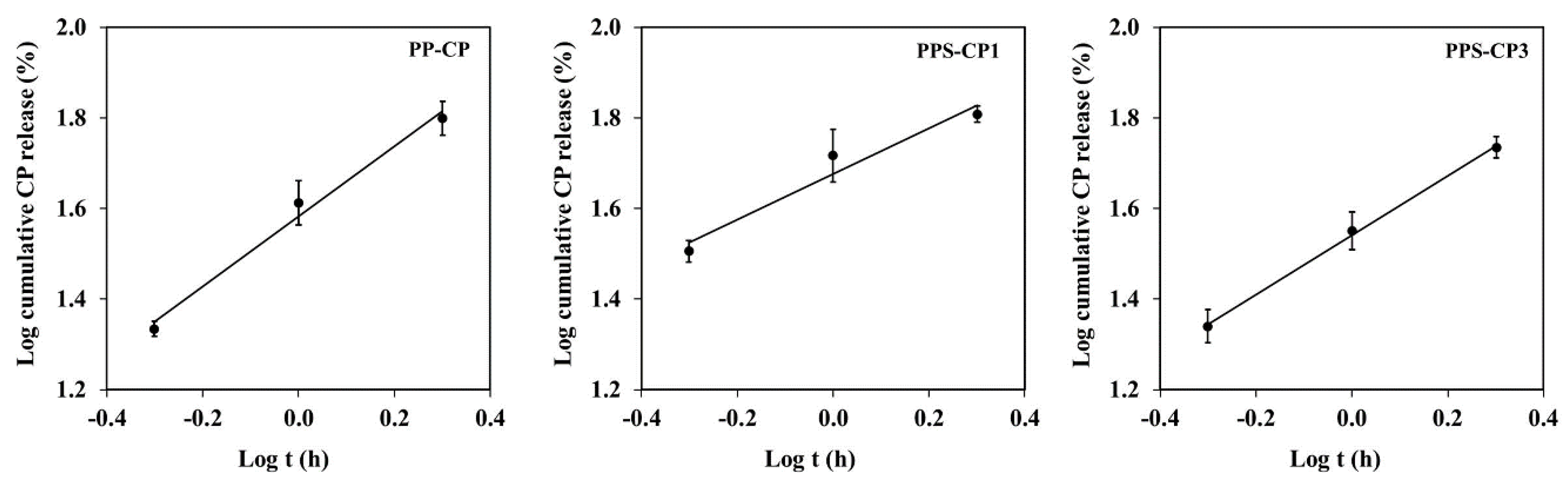
2.6. In Vitro Drug Release and Drug Release Kinetics
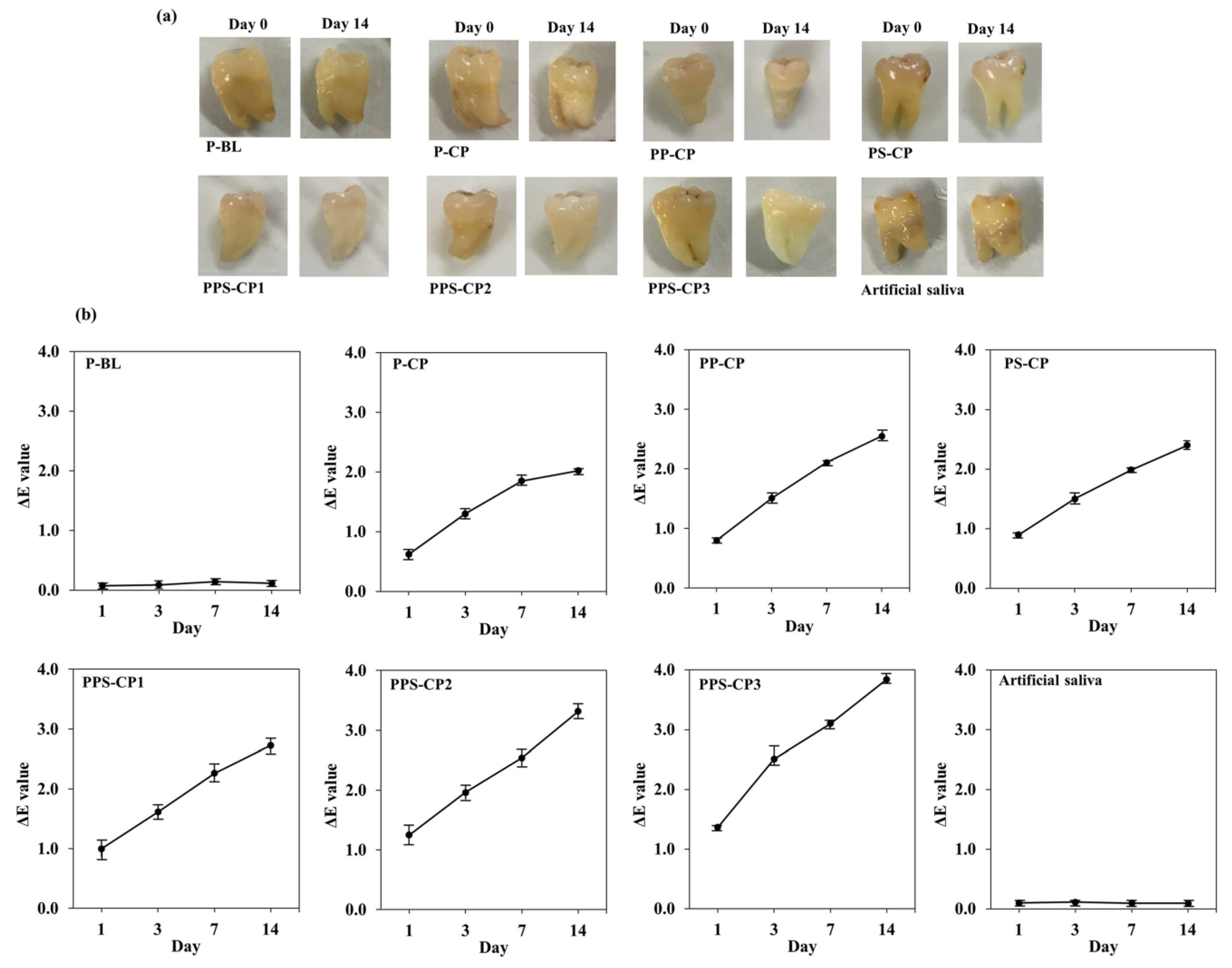
2.7. Ex Vivo Tooth Bleaching Efficiency
3. Materials and Methods
3.1. Materials
3.2. Preparation of EFASs
3.3. Stability of CP in EFASs
3.4. HPLC Analysis
3.5. Viscosity and Electrical Conductivity of EFASs
3.6. Fabrication of the ENF
3.7. Morphology Study
3.8. Investigation of Internal Solid State
3.9. Investigation of Adhesive Property
3.10. Determination of CP in CP-ENFs
3.11. Drug Release Kinetic
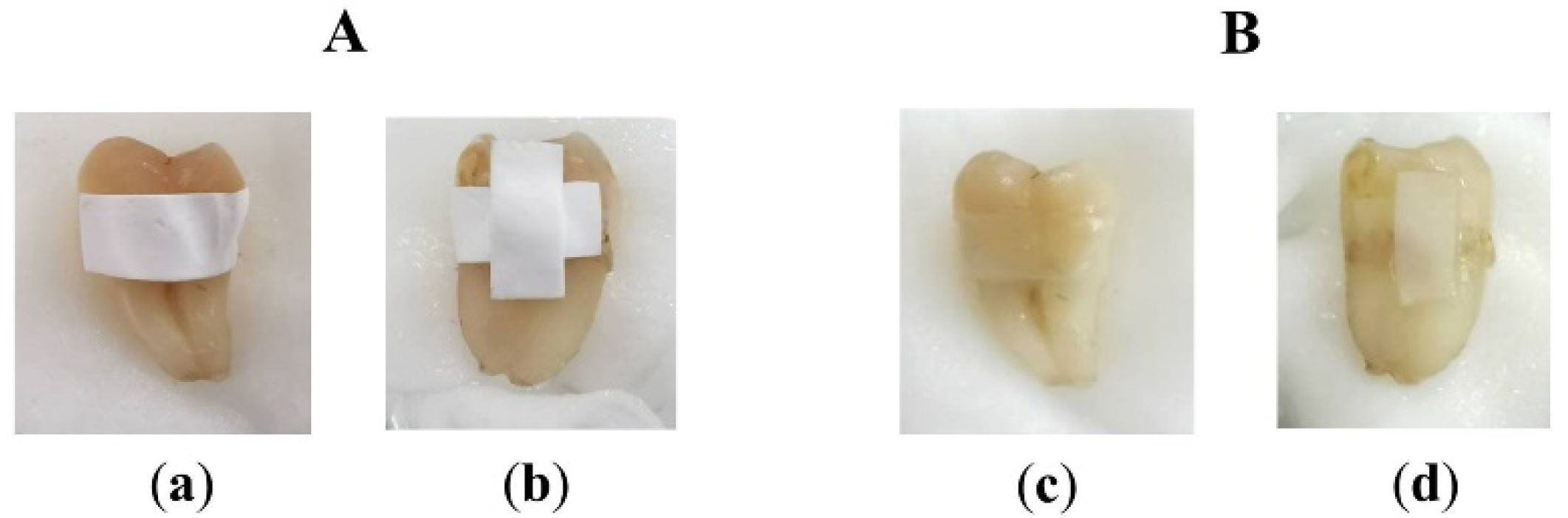
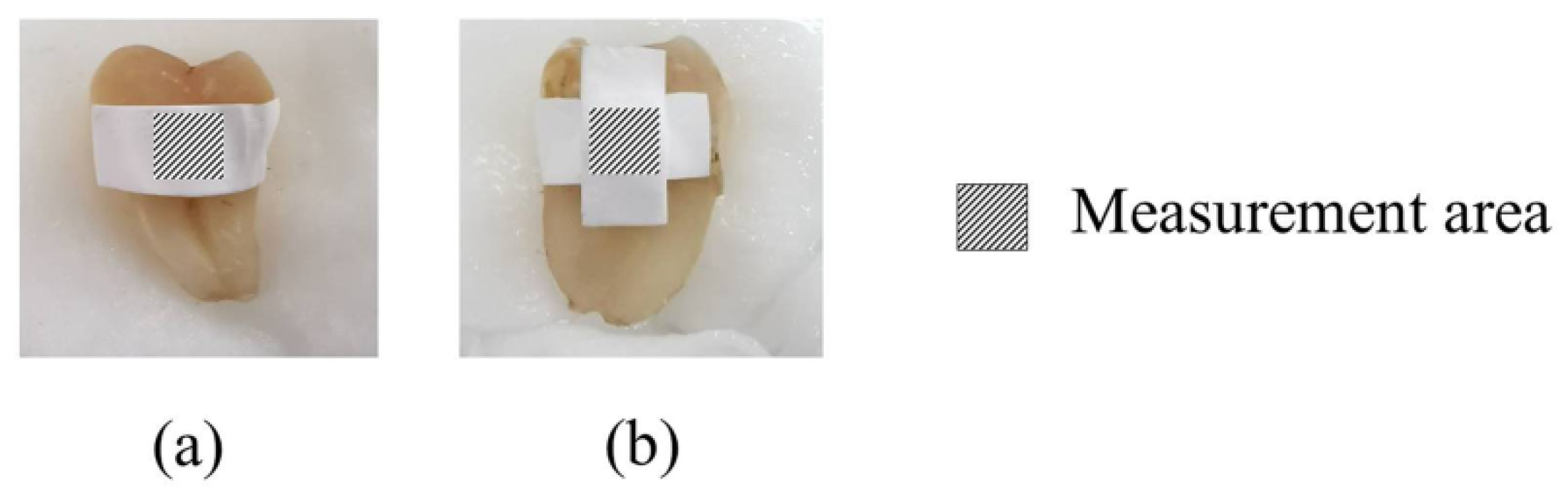
3.12. Ex Vivo Tooth Bleaching Assessment
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Féliz-Matos, L.; Hernández, L.M.; Abreu, N. Dental bleaching techniques; hydrogen-carbamide peroxides and light sources for activation, an update. mini review article. Open Dent. J. 2014, 8, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Pędziwiatr, P.; Mikołajczyk, F.; Zawadzki, D.; Mikołajczyk, K.; Bedka, A. Decomposition of hydrogen peroxide—kinetics and review of chosen catalysts. Acta Innov. 2018, 26, 45–52. [Google Scholar] [CrossRef]
- Hattab, F.N.; Qudeimat, M.A.; Al-Rimawi, H.S. Dental discoloration: An overview. J. Esthet. Restor. Dent. 1999, 11, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.E.; Pallesen, U. Tooth bleaching—A critical review of the biological aspects. Crit. Rev. Oral Biol. Med. 2003, 14, 292–304. [Google Scholar] [CrossRef]
- Joiner, A. The bleaching of teeth: A review of the literature. J. Dent. 2006, 34, 412–419. [Google Scholar] [CrossRef]
- Rezende, M.; Ferri, L.; Kossatz, S.; Loguercio, A.D.; Reis, A. Combined bleaching technique using low and high hydrogen peroxide in-office bleaching gel. Oper. Dent. 2016, 41, 388–396. [Google Scholar] [CrossRef]
- Tredwin, C.J.; Naik, S.; Lewis, N.J.; Scully, C. Hydrogen peroxide tooth-whitening (bleaching) products: Review of adverse effects and safety issues. Br. Dent. J. 2006, 200, 371–376. [Google Scholar] [CrossRef]
- Barcellos, D.C.; Benetti, P.; Fernandes, V.V.B.; Valera, M.C. Effect of carbamide peroxide bleaching gel concentration on the bond strength of dental substrates and resin composite. Oper. Dent. 2010, 35, 463–469. [Google Scholar] [CrossRef]
- Meireles, S.S.; Fontes, S.T.; Coimbra, L.A.A.; Bona, Á.D.; Demarco, F.F. Effectiveness of different carbamide peroxide concentrations used for tooth bleaching: An in vitro study. J. Appl. Oral Sci. 2012, 20, 186–191. [Google Scholar] [CrossRef]
- Soares, D.G.; Basso, F.G.; Hebling, J.; de Souza Costa, C.A. Concentrations of and application protocols for hydrogen peroxide bleaching gels: Effects on pulp cell viability and whitening efficacy. J. Dent. 2014, 42, 185–198. [Google Scholar] [CrossRef]
- Jing, X.; Zhibing, Z. Physical, structural, and mechanical characterization of calcium–shellac microspheres as a carrier of carbamide peroxide. J. Appl. Polym. Sci. 2009, 113, 1619–1625. [Google Scholar]
- Adam, P.; Sasikanth, K.; Nama, S.; Suresh, S.; Brahmaiah, B. Nanofibers—A new trend in nano drug delivery. Pharma Innov. J. 2013, 2, 118–127. [Google Scholar]
- Lee, H.J.; Lee, S.J.; Uthaman, S.; Thomas, R.G.; Hyun, H.; Jeong, Y.Y.; Cho, C.S.; Park, I.K. Biomedical applications of magnetically functionalized organic/inorganic hybrid nanofibers. Int. J. Mol. Sci. 2015, 16, 13661–13677. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.V.; Chen, C.; Tsai, S.; Wang, Y.J.; Lee, O.K. Growth of mesenchymal stem cells on electrospun type I. Stem Cells 2006, 24, 2391–2397. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.P.; Sell, S.A.; Boland, E.D.; Simpson, D.G.; Bowlin, G.L. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Adv. Drug Deliv. Rev. 2007, 59, 1413–1433. [Google Scholar] [CrossRef]
- Rieger, K.A.; Birch, N.P.; Schiffman, J.D. Designing electrospun nanofiber mats to promote wound healing-a review. J. Mater. Chem. B 2013, 1, 4531–4541. [Google Scholar] [CrossRef]
- Leung, V.; Ko, F. Biomedical applications of nanofibers. Polym. Adv. Technol. 2011, 22, 350–365. [Google Scholar] [CrossRef]
- Bajakova, J.; Chaloupek, J.; Lukaš, D.; Lacarin, M. Drawing—the production of individual nanofibers by experimental method. Int. Conf. Nanomater. Res. Appl. 2011, 9, 322–326. [Google Scholar]
- Feng, L.; Li, S.; Li, H.; Zhai, J.; Song, Y.; Jiang, L.; Zhu, D. Super-hydrophobic surface of aligned polyacrylonitrile nanofibers. Angew. Chemie Int. Ed. 2002, 41, 1221–1223. [Google Scholar] [CrossRef]
- Katsogiannis, K.A.G.; Vladisavljević, G.T.; Georgiadou, S. Porous electrospun polycaprolactone (PCL) fibres by phase separation. Eur. Polym. J. 2015, 69, 284–295. [Google Scholar] [CrossRef]
- Rolandi, M.; Rolandi, R. Self-assembled chitin nanofibers and applications. Adv. Colloid Interface Sci. 2014, 207, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Fujihara, K.; Teo, W.E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: Solving global issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nano fibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.H.; Mohamad, A.B.; Al-Amiery, A.A. Properties and applications of polyvinyl alcohol, halloysite nanotubes and their nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhou, L.; Li, M.C.; Wu, Q.; Kojima, Y.; Zhou, D. Preparation and properties of electrospun poly (vinyl pyrrolidone)/cellulose nanocrystal/silver nanoparticle composite fibers. Materials (Basel) 2016, 9, 523. [Google Scholar] [CrossRef]
- Nasouri, K.; Shoushtari, A.M.; Mojtahedi, M.R.M. Effects of polymer/solvent systems on electrospun polyvinylpyrrolidone nanofiber morphology and diameter. Polym. Sci. Ser. A 2015, 57, 747–755. [Google Scholar] [CrossRef]
- Panarin, E.F.; Kalninsh, K.K.; Pestov, D.V. Complexation of hydrogen peroxide with polyvinylpyrrolidone: Ab initio calculations. Eur. Polym. J. 2001, 37, 375–379. [Google Scholar] [CrossRef]
- Kovačič, B.; Vrečer, F.; Planinšek, O. Solid dispersions of carvedilol with porous silica. Chem. Pharm. Bull. 2011, 59, 427–433. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun fibers and their application in drug controlled release, biological dressings, tissue repair, and enzyme immobilization. RSC Adv. 2019, 9, 25712–25729. [Google Scholar] [CrossRef]
- Zhong, L.; Lee, B.; Yang, S. Establishing vadose zone slow-release carbon sources for enhanced bioremediation using silica suspension. Vadose Zone J. 2018, 17, 1–10. [Google Scholar] [CrossRef]
- Sokolowska, D.; Dziob, D.; Gorska, U.; Kieltyka, B.; Moscicki, J.K. Electric conductivity percolation in naturally dehydrating, lightly wetted, hydrophilic fumed silica powder. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2013, 87, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hamad, D.; Dhib, R.; Mehrvar, M. Effects of hydrogen peroxide feeding strategies on the photochemical degradation of polyvinyl alcohol. Environ. Technol. 2016, 37, 2731–2742. [Google Scholar] [CrossRef]
- Zhang, S.J.; Yu, H.Q. Radiation-induced degradation of polyvinyl alcohol in aqueous solutions. Water Res. 2004, 38, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.S.; Shanks, R.A. Hydrogen peroxide oxidation of poly(vinyl alcohol). J. Polym. Sci. Polym. Chem. Ed. 1979, 17, 1867–1869. [Google Scholar] [CrossRef]
- Chuangchote, S.; Sagawa, T.; Yoshikawa, S. Electrospinning of poly(vinyl pyrrolidone): Effects of solvents on electrospinnability for the fabrication of poly(p-phenylene) and TiO2 nanofiber. J. Appl. Polym. Sci. 2009, 114, 2777–2791. [Google Scholar] [CrossRef]
- Wasim, M.; Sabir, A.; Shafiq, M.; Jamil, T. Electrospinning: A fiber fabrication technique for water purification. In Nanoscale Materials in Water Purification; Thomas, S., Pasquini, D., Leu, S., Gopakuma, D., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 289–308. [Google Scholar]
- Shahreen, L.; Chase, G.G. Effects of electrospinning solution properties on formation of beads in TiO2 fibers with PdO particles. J. Eng. Fiber. Fabr. 2015, 10, 136–145. [Google Scholar] [CrossRef]
- Beachley, V.; Wen, X. Effect of electrospinning parameters on the nanofiber diameter and length. Mater. Sci. Eng. C 2009, 29, 663–668. [Google Scholar] [CrossRef]
- Jin, B.Z.; Dong, X.Q.; Xu, X.; Zhang, F.H. Development and in vitro evaluation of mucoadhesive patches of methotrexate for targeted delivery in oral cancer. Oncol. Lett. 2018, 15, 2541–2549. [Google Scholar] [CrossRef]
- Upendra, K.; Siddarth, D. Design and development of felodipine buccal mucoadhesive patches. Int. J. Curr. Pharm. Res. 2010, 2, 71–75. [Google Scholar]
- Yoon, Y.E.; Im, B.G.; Kim, J.S.; Jang, J.H. Multifunctional self-adhesive fibrous layered matrix (FiLM) for tissue glues and therapeutic carriers. Biomacromolecules 2017, 18, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. Intrinsic adhesion properties of poly(vinyl pyrrolidone) to pharmaceutical materials: Humidity effect. Macromol. Biosci. 2005, 5, 1085–1093. [Google Scholar] [CrossRef]
- Kundrat, V.; Cernekova, N.; Kovalcik, A.; Enev, V.; Marova, I. Drug release kinetics of electrospun PHB meshes. Materials (Basel) 2019, 12, 1924. [Google Scholar] [CrossRef] [PubMed]
- Barzegar-Jalali, M.; Adibkia, K.; Valizadeh, H.; Shadbad, M.R.; Nokhodchi, A.; Omidi, Y.; Mohammadi, G.; Nezhadi, S.H.; Hasan, M. Kinetic analysis of drug release from nanoparticles. J. Pharm. Pharm. Sci. 2008, 11, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. Drug Res. 2010, 67, 217–223. [Google Scholar]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. [Google Scholar]
- ADA Council on Scientific Affairs. Tooth Whitening/Bleaching: Treatment Considerations for Dentists and Their Patients; American Dental Association: Chicago, IL, USA, 2009; pp. 1–12. [Google Scholar]
- Alqahtani, M.Q. Tooth-bleaching procedures and their controversial effects: A literature review. Saudi Dent. J. 2014, 26, 33–46. [Google Scholar] [CrossRef]
- Bizhang, M.; Chun, Y.H.P.; Damerau, K.; Singh, P.; Raab, W.H.M.; Zimmer, S. Comparative clinical study of the effectiveness of three different bleaching methods. Oper. Dent. 2009, 34, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Matis, B.A.; Cochran, M.A.; Eckert, G. Review of the effectiveness of various tooth whitening systems. Oper. Dent. 2009, 34, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.M.; Miguez, P.A.; Oliveira, G.B.; Swift, E.J.; Farrell, S.; Anastasia, M.K.; Conde, E.; Walter, R. Safety and efficacy of a high-adhesion whitening strip under extended wear regimen. J. Dent. 2013, 41, e46–e52. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.J. Safety issues relating to the use of hydrogen peroxide in dentistry. Aust. Dent. J. 2000, 45, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S. An overview of vital teeth bleaching. J. Interdiscip. Dent. 2016, 6, 3–13. [Google Scholar] [CrossRef]
- Kaewpinta, A.; Khongkhunthian, S.; Chaijareenont, P.; Okonogi, S. Preparation and characterization of rice gels containing tooth bleaching agent. Drug Discov. Ther. 2018, 12, 275–282. [Google Scholar] [CrossRef]
- Tonglairoum, P.; Ngawhirunpat, T.; Rojanarata, T.; Panomsuk, S.; Kaomongkolgit, R.; Opanasopit, P. Fabrication of mucoadhesive chitosan coated polyvinylpyrrolidone/cyclodextrin/clotrimazole sandwich patches for oral candidiasis. Carbohydr. Polym. 2015, 132, 173–179. [Google Scholar] [CrossRef]
- Dionysopoulos, D.; Strakas, D.; Koliniotou-Koumpia, E.; Koumpia, E. Effect of Er,Cr:YSGG laser irradiation on bovine enamel surface during in-office tooth bleaching ex vivo. Odontology 2017, 105, 320–328. [Google Scholar] [CrossRef]
- Borges, B.C.D.; Borges, J.S.; de Melo, C.D.; Pinheiro, I.V.A.; dos Santos, A.J.S.; Braz, R.; Montes, M.A.J.R. Efficacy of a novel at-home bleaching technique with carbamide peroxides modified by CPP-ACP and its effect on the microhardness of bleached enamel. Oper. Dent. 2011, 36, 521–528. [Google Scholar] [CrossRef]

| EFASs | Composition (% w/w) | ||||
|---|---|---|---|---|---|
| PVA | PVP | Silica | CP | Water | |
| P-BL | 10 | - | - | - | 90 |
| P-CP | 9 | - | - | 1 | 90 |
| PP-CP | 6 | 3 | - | 1 | 90 |
| PS-CP | 8 | - | 1 | 1 | 90 |
| PPS-CP1 | 5 | 3 | 1 | 1 | 90 |
| PPS-CP2 | 4 | 3 | 2 | 1 | 90 |
| PPS-CP3 | 5.5 | 3 | 1 | 0.5 | 90 |
| EFASs | Viscosity (mPas) * | Conductivity (µS/cm) * |
|---|---|---|
| P-BL | 3.47 ± 0.42 a | 1.45 ± 0.05 e |
| P-CP | 2.74 ± 0.11 b | 2.13 ± 0.02 cd |
| PP-CP | 3.79 ± 0.20 a | 2.43 ± 0.05 a |
| PS-CP | 2.02 ± 0.51 c | 2.02 ± 0.03 d |
| PPS-CP1 | 2.52 ± 0.40 b | 2.24 ± 0.04 bc |
| PPS-CP2 | 1.75 ± 0.54 c | 2.02 ± 0.04 d |
| PPS-CP3 | 3.64 ± 0.40 a | 2.32 ± 0.06 ab |
| EFASs for ENFs | Adhesion Force (N) * | EE (%) * |
|---|---|---|
| P-BL | 0.77 ± 0.02 b | - |
| P-CP | 0.74 ± 0.02 c | 59.48 ± 2.25 e |
| PP-CP | 0.81 ± 0.02 a | 73.83 ± 1.37 d |
| PS-CP | 0.69 ± 0.01 d | 76.82 ± 1.83 d |
| PPS-CP1 | 0.71 ± 0.02 cd | 82.67 ± 1.57 c |
| PPS-CP2 | 0.63 ± 0.01 e | 88.25 ± 1.01 b |
| PPS-CP3 | 0.73 ± 0.02 c | 98.32 ± 1.87 a |
| EFASs for ENFs | Release Kinetics | ||||||
|---|---|---|---|---|---|---|---|
| Zero Order | First Order | Korsmeyer–Peppas | |||||
| r2 | k0 | r2 | k0 | r2 | kkp | n | |
| P-CP | 0.78 | 100.29 | 0.99 | 6.78 | - | - | - |
| PP-CP | 0.94 | 24.29 | 0.95 | 0.95 | 0.97 | 1.63 | 0.86 |
| PS-CP | 0.57 | 17.44 | 0.86 | 0.70 | - | - | - |
| PPS-CP1 | 0.90 | 24.23 | 0.94 | 0.84 | 0.96 | 1.75 | 0.58 |
| PPS-CP2 | 0.64 | 19.62 | 0.90 | 0.85 | - | - | - |
| PPS-CP3 | 0.86 | 11.58 | 0.95 | 0.56 | 0.98 | 1.54 | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okonogi, S.; Kaewpinta, A.; Rades, T.; Müllertz, A.; Yang, M.; Khongkhunthian, S.; Chaijareenont, P. Enhancing Stability and Tooth Bleaching Activity of Carbamide Peroxide by Electrospun Nanofibrous Film. Pharmaceuticals 2020, 13, 381. https://doi.org/10.3390/ph13110381
Okonogi S, Kaewpinta A, Rades T, Müllertz A, Yang M, Khongkhunthian S, Chaijareenont P. Enhancing Stability and Tooth Bleaching Activity of Carbamide Peroxide by Electrospun Nanofibrous Film. Pharmaceuticals. 2020; 13(11):381. https://doi.org/10.3390/ph13110381
Chicago/Turabian StyleOkonogi, Siriporn, Adchareeya Kaewpinta, Thomas Rades, Anette Müllertz, Mingshi Yang, Sakornrat Khongkhunthian, and Pisaisit Chaijareenont. 2020. "Enhancing Stability and Tooth Bleaching Activity of Carbamide Peroxide by Electrospun Nanofibrous Film" Pharmaceuticals 13, no. 11: 381. https://doi.org/10.3390/ph13110381
APA StyleOkonogi, S., Kaewpinta, A., Rades, T., Müllertz, A., Yang, M., Khongkhunthian, S., & Chaijareenont, P. (2020). Enhancing Stability and Tooth Bleaching Activity of Carbamide Peroxide by Electrospun Nanofibrous Film. Pharmaceuticals, 13(11), 381. https://doi.org/10.3390/ph13110381









