Perspective of SGLT2 Inhibition in Treatment of Conditions Connected to Neuronal Loss: Focus on Alzheimer’s Disease and Ischemia-Related Brain Injury
Abstract
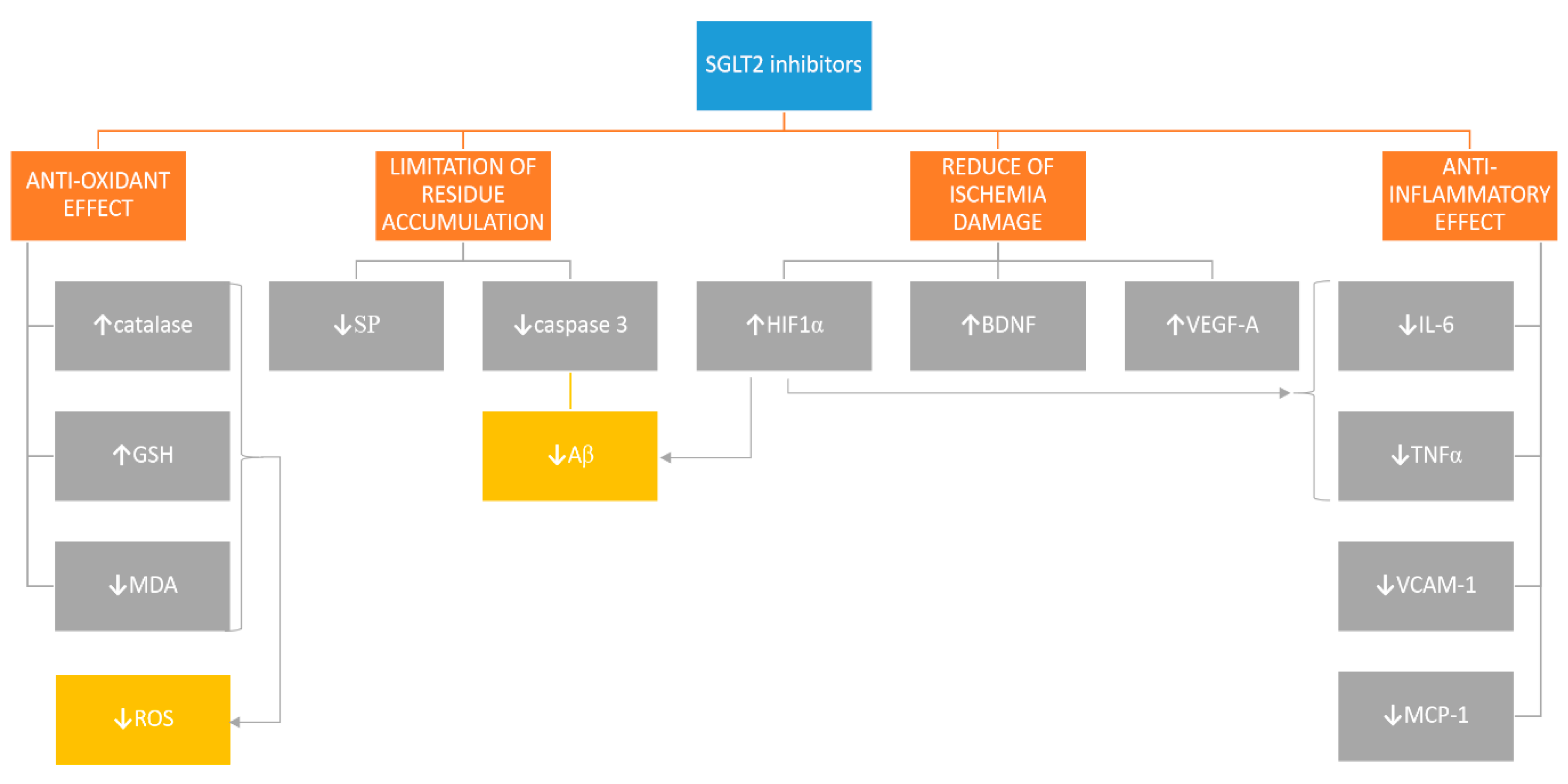
1. SGLT2 Receptor May Be a Potential Target in Therapies of CNS Disorders
2. SGLT2i Are Another DM-Registered Treatment That May Have an Influence on Alzheimer’s Disease Pathology
3. SGLT2i May Prove to Enhance Angiogenesis and Neurogenesis and Prevent Ischemia-Related Cerebral Damage
4. Anti-Inflammatory Properties of SGLT2i May Slow Down Atherogenesis and Prevent Neuronal Loss Related to Oxidative Stress
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nauck, M.A. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des. Dev. Ther. 2014, 8, 1335–1380. [Google Scholar] [CrossRef]
- Heise, T.; Seewaldt-Becker, E.; Macha, S.; Hantel, S.; Pinnetti, S.; Seman, L.; Woerle, H.J. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes. Metab. 2013, 15, 613–621. [Google Scholar] [CrossRef]
- Shah, K.; DeSilva, S.; Abbruscato, T. The Role of Glucose Transporters in Brain Disease: Diabetes and Alzheimer’s Disease. Int. J. Mol. Sci. 2012, 13, 12629–12655. [Google Scholar] [CrossRef]
- Yu, A.S.; Hirayama, B.A.; Timbol, G.; Liu, J.; Basarah, E.; Kepe, V.; Satyamurthy, N.; Huang, S.-C.; Wright, E.M.; Barrio, J.R. Functional expression of SGLTs in rat brain. Am. J. Physiol. Physiol. 2010, 299, C1277–C1284. [Google Scholar] [CrossRef]
- Yu, A.S.; Hirayama, B.A.; Timbol, G.; Liu, J.; Diez-Sampedro, A.; Kepe, V.; Satyamurthy, N.; Huang, S.-C.; Wright, E.M.; Barrio, J.R. Regional distribution of SGLT activity in rat brain in vivo. Am. J. Physiol. Physiol. 2013, 304, C240–C247. [Google Scholar] [CrossRef] [PubMed]
- Poppe, R.; Karbach, U.; Gambaryan, S.; Wiesinger, H.; Lutzenburg, M.; Kraemer, M.; Witte, O.W.; Koepsell, H. Expression of the Na+-D-glucose cotransporter SGLT1 in neurons. J. Neurochem. 1997, 69, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Enerson, B.E.; Drewes, L.R. The Rat Blood—Brain Barrier Transcriptome. Br. J. Pharmacol. 2005, 26, 959–973. [Google Scholar] [CrossRef] [PubMed]
- Jurcovicova, J. Glucose transport in brain—Effect of inflammation. Endocr. Regul. 2014, 48, 35–48. [Google Scholar] [CrossRef]
- Sa-Nguanmoo, P.; Tanajak, P.; Kerdphoo, S.; Jaiwongkam, T.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S. SGLT2-inhibitor and DPP-4 inhibitor improve brain function via attenuating mitochondrial dysfunction, insulin resistance, inflammation, and apoptosis in HFD-induced obese rats. Toxicol. Appl. Pharmacol. 2017, 333, 43–50. [Google Scholar] [CrossRef]
- Lin, B.; Koibuchi, N.; Hasegawa, Y.; Sueta, D.; Toyama, K.; Uekawa, K.; Ma, M.; Nakagawa, T.; Kusaka, H.; Kim-Mitsuyama, S. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc. Diabetol. 2014, 13, 1–15. [Google Scholar] [CrossRef]
- Abdelgadir, E.; Rashid, F.; Bashier, A.; Ali, R. SGLT-2 Inhibitors and Cardiovascular Protection: Lessons and Gaps in Understanding the Current Outcome Trials and Possible Benefits of Combining SGLT-2 Inhibitors With GLP-1 Agonists. J. Clin. Med. Res. 2018, 10, 615–625. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McMurray, J.J.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Ndefo, U.A.; Anidiobi, N.O.; Basheer, E.; Eaton, A.T. Empagliflozin (Jardiance): A Novel SGLT2 Inhibitor for the Treatment of Type-2 Diabetes. Pharm. Ther. 2015, 40, 364–368. [Google Scholar]
- Monami, M.; Nardini, C.; Mannucci, E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: A meta-analysis of randomized clinical trials. Diabetes Obes. Metab. 2013, 16, 457–466. [Google Scholar] [CrossRef] [PubMed]
- DeSouza, C.V.; Gupta, N.; Patel, A. Cardiometabolic Effects of a New Class of Antidiabetic Agents. Clin. Ther. 2015, 37, 1178–1194. [Google Scholar] [CrossRef]
- Erondu, N.; Desai, M.; Ways, K.; Meininger, G. Diabetic Ketoacidosis and Related Events in the Canagliflozin Type 2 Diabetes Clinical Program. Diabetes Care 2015, 38, 1680–1686. [Google Scholar] [CrossRef]
- Hilaire, R.S.; Costello, H. Prescriber beware: Report of adverse effect of sodium-glucose cotransporter 2 inhibitor use in a patient with contraindication. Am. J. Emerg. Med. 2015, 33, 604. [Google Scholar] [CrossRef]
- Peters, A.L.; Buschur, E.O.; Buse, J.B.; Cohan, P.; Diner, J.C.; Hirsch, I.B. Euglycemic Diabetic Ketoacidosis: A Potential Complication of Treatment With Sodium–Glucose Cotransporter 2 Inhibition. Diabetes Care 2015, 38, 1687–1693. [Google Scholar] [CrossRef]
- Taylor, S.I.; Blau, J.E.; Rother, K.I. Perspective: SGLT2 inhibitors may predispose to ketoacidosis. J. Clin. Endocrinol. Metab. 2015, 100, 2849–2852. [Google Scholar] [CrossRef]
- Wang, Z.H.; Kihl-Selstam, E.; Eriksson, J.W. Ketoacidosis occurs in both Type1 and Type2 diabetes a population-based study from Northern Sweden. Diabet. Med. 2008, 25, 867–870. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kamboj, P.; Goswami, K.; Ahuja, K. Pathophysiology and management of alzheimer’s disease: An overview. J. Anal. Pharm. Res. 2018, 7, 1. [Google Scholar] [CrossRef]
- Bird, T.D.; Miller, B.L. Alzheimer’s disease and primary dementias. In Harrison’s Principles of Internal Medicine; Fauci, A.S., Braunwald, E., Eds.; McGraw-Hill: New York, NY, USA, 2008; pp. 2393–2406. [Google Scholar]
- Emedina, M.; Avila, J. The role of extracellular Tau in the spreading of neurofibrillary pathology. Front. Cell. Neurosci. 2014, 8, 113. [Google Scholar] [CrossRef]
- Holtzman, D.M.; Morris, J.C.; Goate, A.M. Alzheimer’s Disease: The Challenge of the Second Century. Sci. Transl. Med. 2011, 3, 77. [Google Scholar] [CrossRef] [PubMed]
- Hierro-Bujalance, C.; Infante-Garcia, C.; Del Marco, A.; Herrera, M.; Carranza-Naval, M.J.; Suarez, J.; Alves-Martinez, P.; Lubian-Lopez, S.; Garcia-Alloza, M. Empagliflozin reduces vascular damage and cognitive impairment in a mixed murine model of Alzheimer’s disease and type 2 diabetes. Alzheimer’s Res. Ther. 2020, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Holubova, M.; Hruba, L.; Popelova, A.; Bencze, M.; Prazienkova, V.; Gengler, S.; Kratochvílová, H.; Haluzík, M.; Železná, B.; Kuneš, J.; et al. Liraglutide and a lipidized analog of prolactin-releasing peptide show neuroprotective effects in a mouse model of beta-amyloid pathology. Neuropharmacology 2019, 144, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, C. The incretin hormones glucagonlike peptide 1 and glucose-dependent insulinotropic polypeptide are neuroprotective in mouse models of Alzheimer’s disease. Alzheimer’s Dement. 2014, 10, S47–S54. [Google Scholar] [CrossRef]
- Infante-Garcia, C.; Ramos-Rodriguez, J.J.; Galindo-Gonzalez, L.; Garcia-Alloza, M. Long-term central pathology and cognitive impairment are exacerbated in a mixed model of Alzheimer’s disease and type 2 diabetes. Psychoneuroendocrinology 2016, 65, 15–25. [Google Scholar] [CrossRef]
- Ma, D.-L.; Chen, F.-Q.; Xu, W.-J.; Yue, W.-Z.; Yuan, G.; Yang, Y. Early intervention with glucagon-like peptide 1 analog liraglutide prevents tau hyperphosphorylation in diabetic db/db mice. J. Neurochem. 2015, 135, 301–308. [Google Scholar] [CrossRef]
- Kosaraju, J.; Holsinger, R.M.D.; Guo, L.; Tam, K.Y. Linagliptin, a Dipeptidyl Peptidase-4 Inhibitor, Mitigates Cognitive Deficits and Pathology in the 3xTg-AD Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2017, 54, 6074–6084. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 6, 1164–1178. [Google Scholar] [CrossRef]
- Cunha, C.; Brambilla, R.; Thomas, K.L. A simple role for BDNF in learning and memory? Front. Mol. Neurosci. 2010, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, I.; Tweedie, D.; Li, Y.; Greig, N.H. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: An emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br. J. Pharmacol. 2012, 166, 1586–1599. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Socha, M.; Malinowski, B.; Wódkiewicz, E.; Walczak, M.; Górski, K.; Słupski, M.; Pawlak-Osińska, K. Liraglutide and its Neuroprotective Properties—Focus on Possible Biochemical Mechanisms in Alzheimer’s Disease and Cerebral Ischemic Events. Int. J. Mol. Sci. 2019, 20, 1050. [Google Scholar] [CrossRef] [PubMed]
- Arafa, N.M.S.; Ali, E.H.A.; Hassan, M.K. Canagliflozin prevents scopolamine-induced memory impairment in rats: Comparison with galantamine hydrobromide action. Chem. Interact. 2017, 277, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Rizvi, S.M.D.; Shakil, S.; Riyaz, S.; Biswas, D.; Jahan, R. Forxiga (dapagliflozin): Plausible role in the treatment of diabetes-associated neurological disorders. Biotechnol. Appl. Biochem. 2016, 63, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M.; Turk, E.; Martín, M.G. Molecular Basis for Glucose-Galactose Malabsorption. Cell Biophys. 2002, 36, 115–122. [Google Scholar] [CrossRef]
- Shakil, S. Molecular Interaction of Anti-Diabetic Drugs With Acetylcholinesterase and Sodium Glucose Co-Transporter 2. J. Cell. Biochem. 2017, 118, 3855–3865. [Google Scholar] [CrossRef]
- Rizvi, S.; Shakil, S.; Biswas, D.; Shakil, S.; Shaikh, S.; Bagga, P.; Kamal, M.A. Invokana (Canagliflozin) as a Dual Inhibitor of Acetylcholinesterase and Sodium Glucose Co-Transporter 2: Advancement in Alzheimer’s Disease- Diabetes Type 2 Linkage via an Enzoinformatics Study. CNS Neurol. Disord. Drug Targets 2014, 13, 447–451. [Google Scholar] [CrossRef]
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef]
- Sanganalmath, S.K.; Gopal, P.; Parker, J.R.; Downs, R.K.; Parker, J.C.; Dawn, B. Global cerebral ischemia due to circulatory arrest: Insights into cellular pathophysiology and diagnostic modalities. Mol. Cell. Biochem. 2016, 426, 111–127. [Google Scholar] [CrossRef]
- Abdel-Latif, R.G.; Rifaai, R.A.; Amin, E.F. Empagliflozin alleviates neuronal apoptosis induced by cerebral ischemia/reperfusion injury through HIF-1α/VEGF signaling pathway. Arch. Pharmacal Res. 2020, 43, 514–525. [Google Scholar] [CrossRef]
- Carmeliet, P.; Dor, Y.; Herbert, J.-M.; Fukumura, D.; Brusselmans, K.; Dewerchin, M.; Neeman, M.; Bono, F.; Abramovitch, R.; Maxwell, P.H.; et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nat. Cell Biol. 1998, 394, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Lu, J. HIF-1α Activation Attenuates IL-6 and TNF-α Pathways in Hippocampus of Rats Following Transient Global Ischemia. Cell. Physiol. Biochem. 2016, 39, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Grosso, C.; Ng, G.; Roy, S.; Robertson, G.S. Caspase-cleaved amyloid precursor protein in Alzheimer’s disease. Brain Pathol. 2002, 12, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Rissman, R.A.; Poon, W.W.; Blurton-Jones, M.; Oddo, S.; Torp, R.; Vitek, M.P.; LaFerla, F.M.; Rohn, T.T.; Cotman, C.W. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J. Clin. Investig. 2004, 114, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Płóciennik, A.; Prendecki, M.; Zuba, E.; Siudzinski, M.; Dorszewska, J. Activated Caspase-3 and Neurodegeneration and Synaptic Plasticity in Alzheimer’s Disease. Adv. Alzheimer’s Dis. 2015, 4, 63–77. [Google Scholar] [CrossRef]
- Freitas, H.; Schaan, B.; David-Silva, A.; Sabino-Silva, R.; Okamoto, M.; Alves-Wagner, A.B.T.; Mori, R.; Machado, U. SLC2A2 gene expression in kidney of diabetic rats is regulated by HNF-1α and HNF-3β. Mol. Cell. Endocrinol. 2009, 305, 63–70. [Google Scholar] [CrossRef]
- Pontoglio, M.; Prié, D.; Cheret, C.; Doyen, A.; Leroy, C.; Froguel, P.; Velho, G.; Yaniv, M.; Friedlander, G. HNF1α controls renal glucose reabsorption in mouse and man. EMBO Rep. 2000, 1, 359–365. [Google Scholar] [CrossRef]
- Freitas, H.S.; Anhě, G.F.; Melo, K.F.S.; Okamoto, M.M.; Oliveira-Souza, M.; Bordin, S.; Machado, U.F. Na+-Glucose Transporter-2 Messenger Ribonucleic Acid Expression in Kidney of Diabetic Rats Correlates with Glycemic Levels: Involvement of Hepatocyte Nuclear Factor-1α Expression and Activity. Endocrinology 2007, 149, 717–724. [Google Scholar] [CrossRef]
- Thau-Zuchman, O.; Shohami, E.; Alexandrovich, A.G.; Leker, R.R. Vascular Endothelial Growth Factor Increases Neurogenesis after Traumatic Brain Injury. Br. J. Pharmacol. 2010, 30, 1008–1016. [Google Scholar] [CrossRef]
- Schoch, H.J. Hypoxia-induced vascular endothelial growth factor expression causes vascular leakage in the brain. Brain 2002, 125, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Larson, J.; Drew, K.L.; Folkow, L.P.; Milton, S.L.; Park, T.J. No oxygen? No problem! Intrinsic brain tolerance to hypoxia in vertebrates. J. Exp. Biol. 2014, 217, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Geiseler, S.J.; Morland, C. The Janus Face of VEGF in Stroke. Int. J. Mol. Sci. 2018, 19, 1362. [Google Scholar] [CrossRef]
- ElAli, A. The implication of neurovascular unit signaling in controlling the subtle balance between injury and repair following ischemic stroke. Neural Regen. Res. 2016, 11, 914–915. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R.; Grant, D.G.; Aroor, A.R.; Demarco, V.G. Ultrastructural Remodeling of the Neurovascular Unit in the Female Diabetic db/db Model–Part II: Microglia and Mitochondria. Neuroglia 2018, 1, 21. [Google Scholar] [CrossRef]
- Hayden, M.R.; Grant, D.G.; Aroor, A.R.; Demarco, V.G. Ultrastructural Remodeling of the Neurovascular Unit in the Female Diabetic db/db Model—Part III: Oligodendrocyte and Myelin. Neuroglia 2018, 1, 24. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef]
- Xing, C.; Hayakawa, K.; Lok, J.; Arai, K.; Lo, E.H. Injury and repair in the neurovascular unit. Neurol. Res. 2012, 34, 325–330. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, K.; Li, P.; Zhu, L.; Xu, J.; Yang, B.; Hu, X.; Lu, Z.; Chen, J. Dysfunction of the neurovascular unit in ischemic stroke and neurodegenerative diseases: An aging effect. Ageing Res. Rev. 2017, 34, 77–87. [Google Scholar] [CrossRef]
- Hayden, M.R.; Grant, D.G.; Aroor, A.R.; Demarco, V.G. Empagliflozin Ameliorates Type 2 Diabetes-Induced Ultrastructural Remodeling of the Neurovascular Unit and Neuroglia in the Female db/db Mouse. Brain Sci. 2019, 9, 57. [Google Scholar] [CrossRef]
- Bakris, G.L.; Fonseca, V.; Sharma, K.; Wright, E.M. Renal sodium–glucose transport: Role in diabetes mellitus and potential clinical implications. Kidney Int. 2009, 75, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, F.; Kanda, T. Disruption of the blood-brain barrier in inflammatory neurological diseases. Brain Nerve 2013, 65, 165. [Google Scholar] [PubMed]
- Amin, E.F.; Rifaai, R.A.; Abdel-Latif, R.G. Empagliflozin attenuates transient cerebral ischemia/reperfusion injury in hyperglycemic rats via repressing oxidative–inflammatory–apoptotic pathway. Fundam. Clin. Pharmacol. 2020, 34, 548–558. [Google Scholar] [CrossRef]
- Millar, P.; Pathak, N.; Parthsarathy, V.; Bjourson, A.J.; O’Kane, M.; Pathak, V.; Moffett, R.C.; Flatt, P.R.; Gault, V. Metabolic and neuroprotective effects of dapagliflozin and liraglutide in diabetic mice. J. Endocrinol. 2017, 234, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Sacco, R.L.; Smith, D.B.; Alberts, M.; Mustone-Alexander, L.; Rader, D.; Ross, J.L.; Raps, E.; Ozer, M.N.; Brass, L.M.; et al. Prevention of a First Stroke: A review of guidelines and a multidisciplinary consensus statement from the National Stroke Association. JAMA 1999, 281, 1112–1120. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef]
- Elwood, E.; Lim, Z.; Naveed, H.; Galea, I. The effect of systemic inflammation on human brain barrier function. Brain Behav. Immun. 2017, 62, 35–40. [Google Scholar] [CrossRef]
- Dziedzic, T. Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev. Neurother. 2015, 15, 523–531. [Google Scholar] [CrossRef]
- Chitnis, T.; Weiner, H.L. CNS inflammation and neurodegeneration. J. Clin. Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef]
- Holmes, C. Review: Systemic inflammation and Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2013, 39, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-W.; Zhang, X.; Huang, W.-J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Oh, T.J.; Lee, G.; Maeng, H.J.; Lee, D.H.; Kim, K.M.; Choi, S.H.; Jang, H.C.; Lee, H.S.; Park, K.S.; et al. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE−/− mice fed a western diet. Diabetologia 2017, 60, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Intiso, D.F.; Zarrelli, M.; Lagioia, G.; Di Rienzo, F.; De Ambrosio, C.C.; Simone, P.; Tonali, P.; Cioffi†, R.P. Tumor necrosis factor alpha serum levels and inflammatory response in acute ischemic stroke patients. Neurol. Sci. 2004, 24, 390–396. [Google Scholar] [CrossRef]
- Waje-Andreassen, U.; Krakenes, J.; Ulvestad, E.; Thomassen, L.; Myhr, K.-M.; Aarseth, J.; Vedeler, C.A. IL-6: An early marker for outcome in acute ischemic stroke. Acta Neurol. Scand. 2005, 111, 360–365. [Google Scholar] [CrossRef]
- McCusker, S.M.; Curran, M.D.; Dynan, K.B.; McCullagh, C.D.; Urquhart, D.D.; Middleton, D.; Patterson, C.C.; Mcilroy, S.P.; Passmore, A.P. Association between polymorphism in regulatory region of gene encoding tumour necrosis factor α and risk of Alzheimer’s disease and vascular dementia: A case-control study. Lancet 2001, 357, 436–439. [Google Scholar] [CrossRef]
- Skoog, T.; Dichtl, W.; Boquist, S.; Skoglund-Andersson, C.; Karpe, F.; Tang, R.; Bond, M.; De Faire, U.; Nilsson, J.; Eriksson, P.; et al. Plasma tumour necrosis factor-α and early carotid atherosclerosis in healthy middle-aged men. Eur. Heart J. 2002, 23, 376–383. [Google Scholar] [CrossRef]
- Cui, G.; Wang, H.; Li, R.; Zhang, L.; Li, Z.; Wang, Y.; Hui, R.; Ding, H.; Wang, D.W. Polymorphism of tumor necrosis factor alpha (TNF-alpha) gene promoter, circulating TNF-alpha level, and cardiovascular risk factor for ischemic stroke. J. Neuroinflamm. 2012, 9, 235. [Google Scholar] [CrossRef]
- Jefferis, B.J.; Whincup, P.H.; Welsh, P.; Wannamethee, S.G.; Rumley, A.; Lennon, L.; Thomson, A.G.; Carson, C.; Ebrahim, S.; Lowe, G.D. Circulating TNFα levels in older men and women do not show independent prospective relations with MI or stroke. Atherosclerosis 2009, 205, 302–308. [Google Scholar] [CrossRef]
- Pennig, J.; Scherrer, P.; Gissler, M.C.; Anto-Michel, N.; Hoppe, N.; Füner, L.; Härdtner, C.; Stachon, P.; Wolf, D.; Hilgendorf, I.; et al. Glucose lowering by SGLT2-inhibitor empagliflozin accelerates atherosclerosis regression in hyperglycemic STZ-diabetic mice. Sci. Rep. 2019, 9, 17937. [Google Scholar] [CrossRef]
- Dimitriadis, G.K.; Nasiri-Ansari, N.; Agrogiannis, G.; Kostakis, I.D.; Randeva, M.S.; Nikiteas, N.; Patel, V.H.; Kaltsas, G.; Papavassiliou, A.G.; Randeva, H.S.; et al. Empagliflozin improves primary haemodynamic parameters and attenuates the development of atherosclerosis in high fat diet fed APOE knockout mice. Mol. Cell. Endocrinol. 2019, 494, 110487. [Google Scholar] [CrossRef] [PubMed]
- Ganbaatar, B.; Fukuda, D.; Shinohara, M.; Yagi, S.; Kusunose, K.; Yamada, H.; Soeki, T.; Hirata, K.-I.; Sata, M. Empagliflozin ameliorates endothelial dysfunction and suppresses atherogenesis in diabetic apolipoprotein E-deficient mice. Eur. J. Pharmacol. 2020, 875, 173040. [Google Scholar] [CrossRef]
- Behnammanesh, G.; Durante, Z.E.; Peyton, K.J.; Martinez-Lemus, L.A.; Brown, S.M.; Bender, S.B.; Durante, W. Canagliflozin Inhibits Human Endothelial Cell Proliferation and Tube Formation. Front. Pharmacol. 2019, 10, 362. [Google Scholar] [CrossRef] [PubMed]
- Obikane, H.; Abiko, Y.; Ueno, H.; Kusumi, Y.; Esumi, M.; Mitsumata, M. Effect of endothelial cell proliferation on atherogenesis: A role of p21Sdi/Cip/Waf1 in monocyte adhesion to endothelial cells. Atherosclerosis 2010, 212, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Devineni, D.; Polidori, D.; Curtin, C.R.; Murphy, J.; Wang, S.-S.; Stieltjes, H.; Wajs, E. Pharmacokinetics and pharmacodynamics of once- and twice-daily multiple-doses of canagliflozin, a selective inhibitor of sodium glucose co-transporter 2, in healthy participants. Int. J. Clin. Pharmacol. Ther. 2015, 53, 438–446. [Google Scholar] [CrossRef]
- Kasichayanula, S.; Chang, M.; Hasegawa, M.; Liu, X.; Yamahira, N.; LaCreta, F.P.; Imai, Y.; Boulton, D.W. Pharmacokinetics and pharmacodynamics of dapagliflozin, a novel selective inhibitor of sodium-glucose co-transporter type 2, in Japanese subjects without and with type 2 diabetes mellitus. Diabetes Obes. Metab. 2011, 13, 357–365. [Google Scholar] [CrossRef]
- Brand, T.; Macha, S.; Mattheus, M.; Pinnetti, S.; Woerle, H.J. Pharmacokinetics of Empagliflozin, a Sodium Glucose Cotransporter-2 (SGLT-2) Inhibitor, Coadministered with Sitagliptin in Healthy Volunteers. Adv. Ther. 2012, 29, 889–899. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Tehrani, H.S.; Moosavi-Movahedi, A.A. Catalase and its mysteries. Prog. Biophys. Mol. Biol. 2018, 140, 5–12. [Google Scholar] [CrossRef]
- Hines, M.; Blum, J. Bend propagation in flagella. II. Incorporation of dynein cross-bridge kinetics into the equations of motion. Biophys. J. 1979, 25, 421–441. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.H.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. New Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Imprialos, K.P.; Boutari, C.; Stavropoulos, K.; Doumas, M.; Karagiannis, A. Stroke paradox with SGLT-2 inhibitors: A play of chance or a viscosity-mediated reality? J. Neurol. Neurosurg. Psychiatry 2016, 88, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; De Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
| Authors | Subject of Study | Type of SGLT2i | Dose of SGLT2i | Result |
|---|---|---|---|---|
| Abdel-Latif et al. [29] | Wistar rats | empagliflozin | 1 and 10 mg/kg, p.o. twice after 1 and 24 h of reperfusion | ↓ neurological defects, ↑ HIF-1α, ↑ VEGF-A, ↓ caspase-3, ↓ infract volume |
| Amin et al. [41] | Wistar rats | empagliflozin | 1 and 10 mg/kg, p.o. twice after 1 and 24 h of reperfusion | ↓ infract volume, ↓ MDA, ↑ catalase, ↑ GSH compared to gliclazide |
| Arafa et al. [22] | Wistar rats | canagliflozin | 10 mg/kg p.o. once | ↓ memory dysfunction |
| Behnammanesh et al. [63] | HUVECs, HAECs, MAECs | canagliflozin, empagliflozin, dapagliflozin | 1–50 μM | ↓ ECs proliferation |
| Ganbaatar et al. [62] | ApoE-/- mice | empagliflozin | 20 mg/kg/day for 8 or 12 weeks | ↓ size of atherosclerosis lesions, ↓ lipid deposition, ↓ macrophage accumulation, ↓ MCP-1 |
| Han et al. [53] | ApoE-/- mice | empagliflozin | 3 mg/kg/day for 8 weeks | ↓ TNF-α, ↓ IL-6, ↓ MCP-1, ↓ atherosclerotic plaque |
| Hayden et al. [75] | db/db mouse | empagliflozin | 10 mg/kg/day for 10 weeks | prevented NVU’s cell and myelin US remodeling |
| Hierro-Bujalance et al. [11] | APP/PS1xdb/db mice | empagliflozin | 10 mg/kg/day for 22 weeks | ↓ SP, ↓ Aβ, ↓ NOD, ↑ MWM, ↓ microglia burden |
| Lin et al. [17] | db/db mice | empagliflozin | diet containing 0.03% empagliflozin for 7 days or for 10 weeks | prevented cognitive function impairment, ↑ BDNF |
| Pennig et al. [60] | C57BL/6J mice | empagliflozin | 35 mg/kg/day for 3 weeks | ↓ size of atherosclerotic plaques, ↓ lipid and ↑ collagen content in atherosclerotic plaques structure |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiciński, M.; Wódkiewicz, E.; Górski, K.; Walczak, M.; Malinowski, B. Perspective of SGLT2 Inhibition in Treatment of Conditions Connected to Neuronal Loss: Focus on Alzheimer’s Disease and Ischemia-Related Brain Injury. Pharmaceuticals 2020, 13, 379. https://doi.org/10.3390/ph13110379
Wiciński M, Wódkiewicz E, Górski K, Walczak M, Malinowski B. Perspective of SGLT2 Inhibition in Treatment of Conditions Connected to Neuronal Loss: Focus on Alzheimer’s Disease and Ischemia-Related Brain Injury. Pharmaceuticals. 2020; 13(11):379. https://doi.org/10.3390/ph13110379
Chicago/Turabian StyleWiciński, Michał, Eryk Wódkiewicz, Karol Górski, Maciej Walczak, and Bartosz Malinowski. 2020. "Perspective of SGLT2 Inhibition in Treatment of Conditions Connected to Neuronal Loss: Focus on Alzheimer’s Disease and Ischemia-Related Brain Injury" Pharmaceuticals 13, no. 11: 379. https://doi.org/10.3390/ph13110379
APA StyleWiciński, M., Wódkiewicz, E., Górski, K., Walczak, M., & Malinowski, B. (2020). Perspective of SGLT2 Inhibition in Treatment of Conditions Connected to Neuronal Loss: Focus on Alzheimer’s Disease and Ischemia-Related Brain Injury. Pharmaceuticals, 13(11), 379. https://doi.org/10.3390/ph13110379





