Abstract
Lymphomas (cancers of the lymphatic system) account for 12% of malignant diseases worldwide. Burkitt’s lymphoma (BL) is a rare form of non-Hodgkin’s lymphoma in which the cancer starts in the immune B-cells. We report the synthesis and preliminary studies on the antiproliferative activity of a library of 9,10-dihydro-9,10-ethanoanthracene based compounds structurally related to the antidepressant drug maprotiline against BL cell lines MUTU-1 and DG-75. Structural modifications were achieved by Diels-Alder reaction of the core 9-(2-nitrovinyl)anthracene with number of dienophiles including maleic anhydride, maleimides, acrylonitrile and benzyne. The antiproliferative activity of these compounds was evaluated in BL cell lines EBV− MUTU-1 and EBV+ DG-75 (chemoresistant). The most potent compounds 13j, 15, 16a, 16b, 16c, 16d and 19a displayed IC50 values in the range 0.17–0.38 μM against the BL cell line EBV− MUTU-1 and IC50 values in the range 0.45–0.78 μM against the chemoresistant BL cell line EBV+ DG-75. Compounds 15, 16b and 16c demonstrated potent ROS dependent apoptotic effects on the BL cell lines which were superior to the control drug taxol and showed minimal cytotoxicity to peripheral blood mononuclear cells (PBMCs). The results suggest that this class of compounds merits further investigation as antiproliferative agents for BL.
1. Introduction
Burkitt’s lymphoma (BL) is an aggressive non-Hodgkin lymphoma occurring with high incidence in developing areas such as equatorial Africa and Papua New Guinea [1]. Within these tropical regions of high incidence (40–50 per 106), BL accounts for approximately 50% of childhood cancers and up to 90% of diagnosed childhood lymphomas [2]. The treatment of BL in these regions is difficult and the development of new, safe and cost-effective therapeutics are of current interest. For developed countries, the sporadic form represents 1–2% of adult lymphomas [3]. In contrast, the endemic BL is commonly associated with infection by the oncogenic Epstein-Barr virus (EBV), which interrupts cellular pathways regulating cell proliferation and thus prevents apoptosis [4]. Treatment of BL includes the monoclonal antibody rituximab which binds to the cell surface protein CD20 of malignant and normal B lymphocytes [5,6]. Chemotherapy drugs used in combination with rituximab include vincristine, methotrexate, doxorubicin and cyclophosphamide, which result in approximately 90% survival rates in children [7]. However, due to reported development of resistance to chemotherapy drugs and increased incidences of immunodeficient HIV-associated BL [8,9], the development of selective and potent treatments for BL is required.
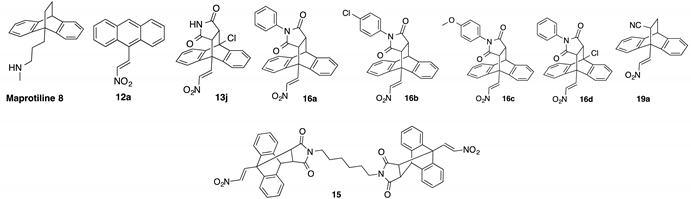
Among the compounds with reported biochemical effects in BL are a series of N-heteroarylhydrazones (e.g., compound 1, EPH116) which demonstrated antiproliferative effects and inhibition of ribonucleotide reductase in BL cells [10], Figure 1. The sesquiterpene β-elemene 2 effectively inhibited the growth and induced the apoptosis of BL cells through upregulation of p53 upregulated modulator of apoptosis (PUMA) expression and modulating PUMA related apoptotic signalling pathway [11]. A phase I clinical trial of lenalidomide 3 (G2M arrest) and blinatumomab combination in treating patients with relapsed non-Hodgkin lymphoma including relapsed and refractory Burkitt lymphoma is currently in progress [12]. The natural naphthoquinone shikonin 4 suppressed cellular proliferation and induced caspase-dependent apoptosis in BL cells with inhibition of c-MYC and suppression of the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway [13]. Phenothiazines such as chlorpromazine 5, trifluoroperazine and thioridazine were noted to both suppress proliferation and induce apoptosis in BL cells [14], while the novel indole based compound NecroX-7 6 is a reactive oxygen species scavenger and has been shown to induce G2/M arrest in BL cell lines [15,16]. Amidinopiperidine-based serine protease inhibitor 7 has been reported as a selective inducer of apoptosis in BL cells [17]. The functional overexpression and the pathogenetic role of the MYC proto-oncogene in BL is established [18], indicating the potential role of direct and indirect MYC inhibitors as new experimental therapies [19].
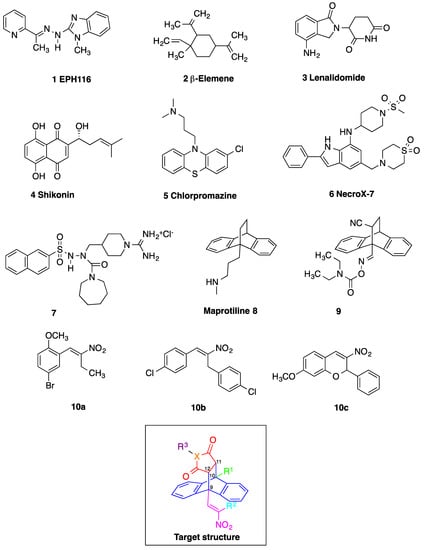
Figure 1.
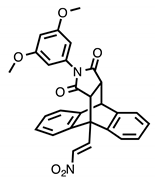
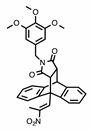
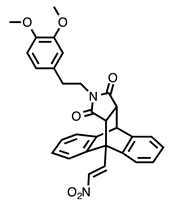
Chemical structures of compounds with reported activity against Burkitt’s lymphoma: compounds 1–7, maprotiline 8, ethanoanthracene 9 and nitrostyrene lead compounds 10a–c with target ethanoanthracene structure.
Our previous research reported the antidepressant drug maprotiline 8 (Figure 1) as an anti-proliferative and pro-apoptotic agent in BL cell lines MUTU-I and DG-75 [20,21]. The serotonin transporter (SERT) has been identified in B-cell malignancies; subsequently antidepressants and structurally related compounds were investigated for potential antileukemia/antilymphoma activity [22]. Induction of apoptosis was demonstrated by the selective serotonin reuptake inhibitor (SSRI) citalopram and the antidepressants imipramine and clomipramine in HL-60 acute myeloid leukaemia, and human T-lymphocytes [23,24,25]. Although these compounds act as non-selective SERT ligands, the pro-apoptotic activity of these drugs appear to be independent of SERT. In addition, fluoxetine [20,21,22], 3,4-methylenedioxymethamphetamine (MDMA) and analogues [22,26], fenfluramine [22], clomipramine [22] and the norepinephrine transporter (NET) targeting maprotiline and analogues have demonstrated proapoptotic effects in BL cell lines [20,21,27]. Our subsequent work involved the generation of a compound library structurally related to the tetracyclic antidepressant maprotiline. A biological screen of this library identified a number of lead compounds in BL cell lines (MUTU-I and DG-75) [27]. From this study we identified the 9,10-dihydro-9,10-ethanoanthracene scaffold e.g., compound 9 as favourable for anti-proliferative activity in these cell lines while the (E)-9-(2-nitrovinyl)anthracene was also shown to elicit a potent pro-apoptotic effect [27]. The ethanoanthracene scaffold was previously reported to demonstrate activity as L-type Ca2+ channel blockers [28], glucocorticoid and cannabinoid receptor modulators [29,30], inhibitors of drug efflux in multidrug resistance [31,32] together with antimicrobial [33] antimalarial [34], anti-inflammatory [35] and cytotoxic activity [36].
Nitrostyrenes and related nitrovinyl compounds induce anti-cancer effects and stimulate an apoptotic response in cancer cell lines e.g., oral and colon cancers, osteosarcoma and Erlich ascetic tumour cell lines [37,38,39,40,41,42], modulating tumorigenesis in colon and breast cancer via reactive oxygen species (ROS) mediated pathways [43,44,45]. Inhibition of telomerase, protein tyrosine phosphatase (PTP), phospholipase A2 and tubulin have been demonstrated for simple nitrostyrenes, while antimicrobial, anti-inflammatory and immunosuppressive effects are also reported [37,46,47]. Nitrostyrenes also act as Retinoid X receptor alpha (RXRα) ligands which inhibit tumor necrosis factor alpha (TNFα) activation of nuclear factor kappa B (NF–κΒ) [48]. We have previously investigated the synthesis, characterisation and biochemical evaluation of a series of structurally diverse nitrostyrenes (e.g., compound 10a, Figure 1) and related nitrovinyl compounds in the MUTU-I and DG-75 cell lines and identified the potent apoptotic effect induced by (E)-1,3-bis(aryl)-2-nitro-1-propenes (e.g., compound 10b, Figure 1) [49] and related heterocycles containing the nitrovinyl pharmacophore e.g., 3-nitro-2-phenyl-2H-chromene (10c), (Figure 1) with antiproliferative effects superior to the cancer therapeutics fludarabine and taxol [50]. A subset of these lead nitrostyrene compounds were also shown to elicit a potent anti-proliferative and pro-apoptotic response towards a range of malignant cell lines e.g., MCF-7 (ER positive breast cancer), HL-60 (acute promyelocytic leukemia) and HeLa (human cervical cancer) [51]. We have also reported the pro-apoptotic effect of selected examples of these nitrostyrene compounds in chronic lymphocytic leukemia (CLL) cell lines and also in ex vivo CLL patient samples [51].
In the present work, a structurally diverse library of 9,10-dihydro-9,10-ethanoanthracene compounds related in structure to the previous lead nitrostyrene compounds 10a–c and the tetracyclic antidepressant maprotiline 8 were synthesised. This approach will facilitate the identification of potent and selective compounds which may be useful in the design of proapoptotic agents. These compounds are synthesised by Diels-Alder cycloaddition reaction of the required anthracene-diene system and hence modification of the ethano-bridgehead could be achieved by variation of the dienophile employed. The dienophiles chosen for the study included maleic anhydride, maleimide and N-substituted maleimides together with benzyne, acrylate esters and acrylonitrile. Variation of the anthracene substitution at C-9 from nitrovinyl to alternative double bonded systems such as cyanovinyl, imine and oxime are also of interest for biological activity, as is the introduction of maleimide linkers and aryl-substituted maleimides. Structural modifications of the nitrovinyl unit by reduction and extension of the alkyl chain length at C-2 were also explored, (see target structure, Figure 1). The compounds were evaluated in the EBV− MUTU-1 cell line and chemoresistant EBV+ DG-75 cell line to establish the structure-activity relationships for these ethanoanthraenes and to optimize the antiproliferative and proapoptotic effects in BL cell lines.
2. Chemistry
The synthesis of the required compounds was achieved by Diels-Alder cycloaddition reaction of the 9-(2-nitrovinyl)anthracene dienes with selected dienophiles. The maleimide-based dienophiles were first prepared by reaction of maleic anhydride with a series of amines (alkyl, benzyl, aryl amines and a diamine) and involved generation of the corresponding amic acid intermediates, (step (a), Scheme 1). Subsequent intramolecular cyclisation of the amic acid with sodium acetate and acetic anhydride, results in the formation of the related maleimide or N-substituted maleimide compounds 11a–s, (step (b), Scheme 1). The (E)-9-(2-nitrovinyl)anthracenes 12a–f were prepared by Henry-Knoevenagel condensation of the nitroalkane with various 9-anthraldehydes giving access to a number of diverse diene systems (step (c), Scheme 1). 9-(2-Nitroethyl)anthracene 12g, was obtained by reduction of 12a with sodium borohydride, (step (d), Scheme 1). A series of (E)-11,12-substiuted-9-(2-nitrovinyl)-9,10-dihydro-9,10-ethanoanthracenes was synthesised by Diels-Alder reaction of the (E)-9-(2-nitrovinyl)anthracenes 12a–f and the dienophiles 11a–s.
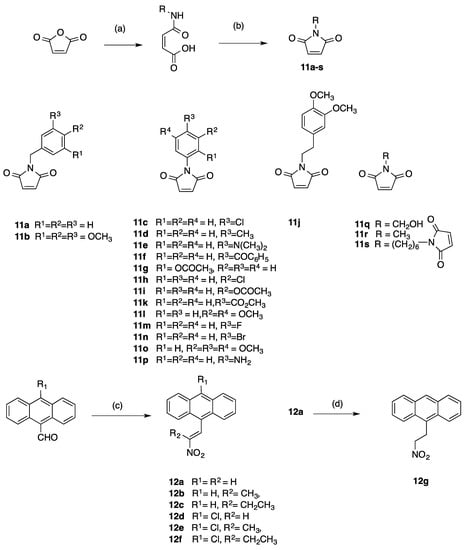
Scheme 1.
Synthesis of maleimides 11a–s and nitrovinylanthracenes 12a–f and nitroethylanthracene 12g. Reagents and conditions: (a) RNH2, diethyl ether, reflux, 1 h. (b) NaOAc, Ac2O, 120 oC, 30 min. (15–70%); (c) Piperidine acetate, excess nitroalkane (CH3NO2, CH3CH2NO2 or CH3CH2CH2NO2), 90 oC, N2, 1.5 h. (50–99%); (d) NaBH4, (CH3)2CHOH, CH2Cl2, RT, 24 h, 85%.
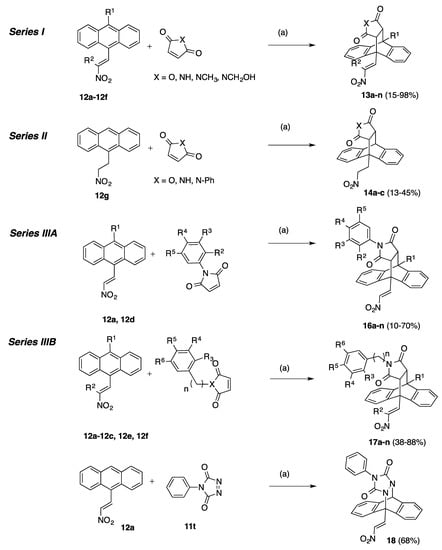
A summary of these Diels-Alder reactions is shown in Scheme 2, Scheme 3, Scheme 4 and Scheme 5. The novel adducts synthesised are arranged as follows:
Scheme 3.
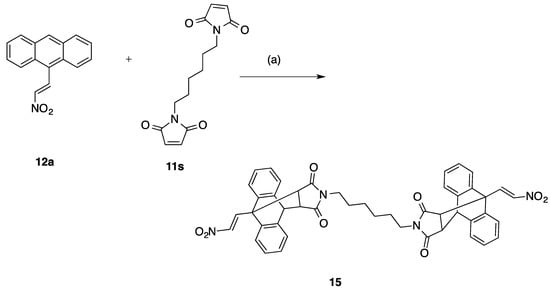
Synthesis of ethanoanthracene dimer 15 (Series 1, Table 1): Reagents and conditions: (a) Toluene, 90 °C, 48 h., (10%).
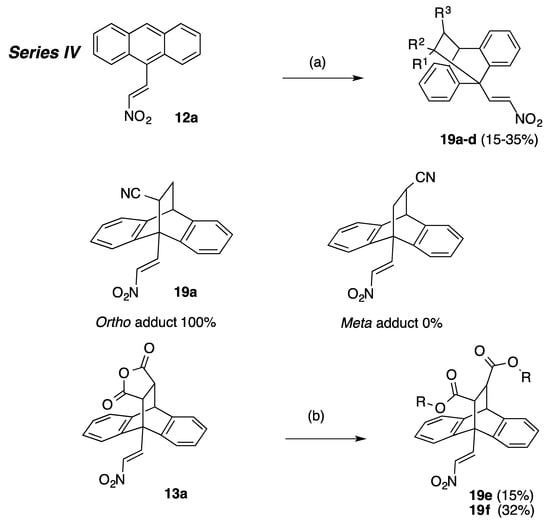
Scheme 4.
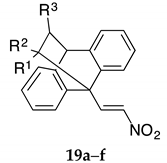
Synthesis of ethanoanthracenes 19a–f (Series IV, Table 6). Reagents and conditions: (a) R1R2C=CHR3, (NCC=CH2, CH3CH2OCOC=CH2, CH3OCOC=CH2, CH3OCO(CN)C=CH2), Toluene, 90 °C, 48 h; (b) H2SO4, R-OH, (R = CH3, CH2CH3), reflux, 6 h.
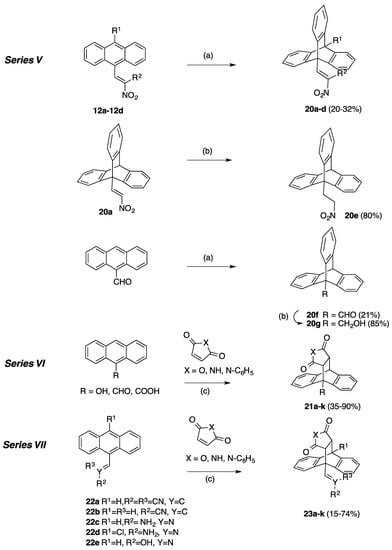
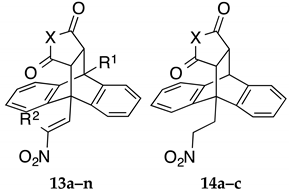
Series I (Scheme 2 and Scheme 3, Table 1): (E)-9-(2-Nitrovinyl)-9,10-dihydro-9,10-ethanoanthracenes 13a–n including maleic anhydride and maleimide Diels-Alder adducts and dimer 15.

Table 1.
Yields and preliminary cell viability data for compounds 13a–n, 15 (Series I) and 14a–c (Series II) in MUTU-1 and DG-75 Burkitt lymphoma cell lines a.
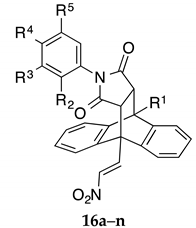
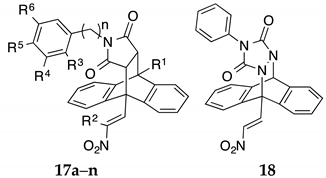
Series III (Scheme 2, Table 2, Table 3, Table 4 and Table 5): (E)-9-(2-Nitrovinyl)-9,10-dihydro-9,10-ethanoanthracenes (16a–n, Series IIIA and 17a–n, Series IIIB) including substituted aryl and benzyl maleimide Diels-Alder adducts and triazole adduct 18.

Table 2.
Yields and preliminary cell viability data for compounds 16a–n (Series IIIA) in MUTU-1 and DG-75 Burkitt lymphoma cell lines.

Table 3.
Yields and preliminary cell viability data for compounds 17a–n, 18 (Series IIIB) in MUTU-1 and DG-75 Burkitt lymphoma cell lines a.

Table 4.
X-ray crystallography data for compounds 16c, 16j, 17l, 17n, 19a and 20a.

Table 5.
Crystal Data and Structure Refinement Parameters for compounds 16c, 16j, 17l, 17n, 19a and 20a.
Series IV (Scheme 4, Table 6): (E)-9-(2-Nitrovinyl)-9,10-dihydro-9,10-ethanoanthracenes 19a–f, substituted at C-11 and C-12.

Table 6.
Yields and preliminary cell viability data for compounds 19a–f (Series IV) in MUTU-1 and DG-75 Burkitt lymphoma cell lines a.

Table 7.
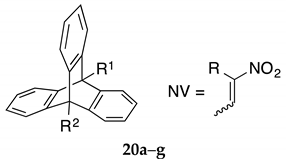
Yields and preliminary cell viability data for compounds 20a–g (Series V) in MUTU-1 and DG-75 Burkitt lymphoma cell lines a.
Series VI (Scheme 5, Table 8): 9,10-Dihydro-9,10-ethanoanthracene Diels-Alder adducts 21a–k substituted at C-9.

Table 8.
Yields and preliminary cell viability data for compounds 21a–k (Series VI) in MUTU-1 and DG-75 Burkitt lymphoma cell lines a.
Series VII (Scheme 5, Table 9): 9,10-Dihydro-9,10-ethanoanthracene Diels-Alder adducts 23a–k containing acrylonitrile, oxime and imine functional groups at C-9.

Table 9.
Yields and preliminary cell viability data for compounds 23a–k (Series VII) in MUTU-1 and DG-75 Burkitt lymphoma cell lines a.
The compounds 13a–n and 14a–c (Series I and II) encompasses maleic anhydride, maleimide and simple maleimide Diels-Alder adducts of 9-(2-nitrovinyl)anthracenes while the 16a–n, 17a–n products (Series IIIA and IIIB) include substituted phenyl and benzyl maleimide Diels-Alder adducts of 9-(2-nitrovinyl)anthracenes. All products were prepared by cycloaddition reaction of the anthracene dienes with the appropriate dienophile at 90 °C in toluene for 48 h and afforded compounds 13a–n (15–98%), 14a–c (30–45%), 16a–n (10–70%) and 17a–n (38–88%), Scheme 2. Variation of alkyl chain length at the C-2 and C-10 substitution was achieved by utilising different nitroalkanes for the Henry-Knoevenagel condensation with substituted 9-anthraldehydes giving access to a number of diverse diene systems. 9-(2-Nitroethyl)anthracene 12g was also reacted with maleic anhydride, maleimide and phenyl-maleimide to give the corresponding saturated Diels-Alder adducts (14a–c), Table 1, Scheme 2. In the 1H NMR spectrum of the novel maleic anhydride adduct 13a the signal at 3.82 ppm appears as a double doublet (J = 9.16, 3.66 Hz) and is assigned to H-11 due to interaction with H-10 and H-12 protons which appear as doublets at 4.98 ppm and 4.20 ppm respectively. The doublets occurring at 8.11 ppm and 8.28 ppm (J = 14.04 Hz) were assigned to the trans coupled protons of the nitrovinyl unit. The assignments were confirmed from the heteronuclear multiple bond correlation (HMBC) and carbon-hydrogen correlation spectroscopy (C-H COSY) NMR spectra, (Supplementary Information). The novel dimer compound 15 was obtained by cycloaddition reaction of (E)-9-(2-nitrovinyl)anthracene 12a and the dimaleimide 11s, Scheme 3. The 1H NMR spectrum of 15 was analogous with the above data for 13a: the ethano bridge protons at 3.36 ppm (double-doublet), 3.76 ppm (double-doublet) and 4.86–4.92 ppm (multiplet) as expected. High resolution mass spectrometry confirmed the required molecular ion for the dimer 775.2771, C46H39N4O8 [M+ + H].
The (E)-9-(2-nitrovinyl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-diones Series IIIA (16a–16n) and Series IIIB (17a–17n) were prepared from the appropriately substituted (E)-9-(2-nitrovinyl)anthracenes via Diels-Alder cycloaddition with substituted benzyl and aryl maleimides with varied aryl substitutions including methoxy, halogen (bromine, chlorine, fluorine), alkyl, hydroxy, ester and amines, (Table 2 and Table 3). The characteristic 1H NMR spectrum of compound 16j shows a double doublet at 3.53 ppm (J = 8.55, 3.05 Hz) assigned to H11. Doublets occurring at 3.92 ppm (J = 8.55 Hz) and 4.95 ppm (J = 3.05 Hz) were assigned to H12 and H10, respectively. The assignments were confirmed from the C-H COSY and DEPT 90 NMR spectra, (See Supplementary Information).
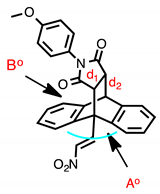
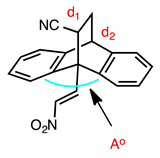
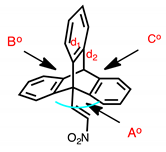
Single crystal X-ray structure determination was completed on (E)-9-(2-nitrovinyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-diones 16c, 16j, 17l and 17n (Figure 2) and selected data is summarised in Table 4 and Table 5. The core dihydroethanoanthracene moiety is rigid, possessing analogous conformations in the different structures; the packing structure displayed by the products is centrosymmetric and monoclinic. From the asymmetric unit, it was possible to confirm the (E)-configuration of the nitrovinyl unit. The angle Ao was calculated for each compound as the angle between the centroid of phenyl ring 1 and the centroid of phenyl ring 2—ranging from 122.05° to 129.35°. This angle is 119.46° in the crystal structure of maprotiline [52]. The flat succinimide ring is fused to the ethano bridge, so that it is tilted through almost exactly 60° with respect to the plane of the C- (4)-C(18)-C(22)-C(11) bridging atoms. The angle Bo (maleimide substituent centroid to centroid of main axis) was also calculated and ranged from 118.05–121.71°. The distance between the carbons in the ethano bridge (d1) ranged from 1.542–1.563 Å, comparing well to that of maprotiline 1.54 Å [52] and related inclusion complexes [53]. The distance between the carbon at C-10 and the nearest carbon of the ethano bridge (d2) for the series was 1.555–1.573 Å, while in maprotiline, this distance is 1.546 Å [52].
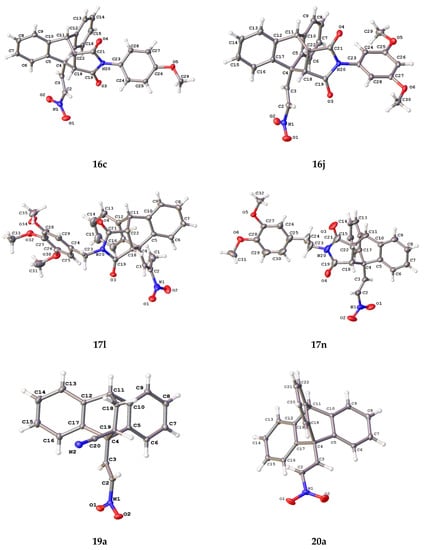
Figure 2.
X-ray crystallographic molecular structures of 16c, 16j, 17l, 17n, 19a and 20a with atomic displacement shown at 50% probability. Only major disordered moiety shown for 17l. Non-hydrogen atoms labelled where possible.
Reaction of the dienophile 4-phenyl-3H-1,2,4-triazole-3,5(4H)-dione 11t with the (E)-9-(2-nitrovinyl)anthracene 12a afforded the adduct 18 in 68% yield (Scheme 2) and allowed direct comparison of the nitrogen-nitrogen bridge system in 18 with the carbon-carbon bridge in compound 16a. The substituted simple (E)-9-(2-nitrovinyl)-9,10-dihydro-9,10-ethanoanthracenes 19a–f, (Series IV) (Scheme 4, Table 6) were prepared from 12a and the corresponding dienophiles (1-cyanovinyl acetate, methyl acrylate, ethyl acrylate and cyanoacrylate) affording yields of 15–35% (19a–d) or via a ring opening reaction (19e,f) in yields of 15–32% (Scheme 4, Table 6). Two products are possible from the Diels-Alder addition of the above dienophiles to the anthracene diene: an ‘ortho’ and a ‘meta’ addition product, (Scheme 4). The Diels-Alder addition of cyanoacrylate to diene systems such as 9-anthraldehyde and (E)-3-(anthracen-9-yl)acrylonitrile has been previously documented as forming the ortho addition adduct only [54]. The preference for the ‘ortho’ adduct is attributed to the stabilising molecular orbital overlap between the nitrile and the carbonyl group. In the present work, we have confirmed the exclusive formation of the novel ortho adduct 19a from the reaction of acrylonitrile and (E)-9-(2-nitrovinyl)anthracene 12a using 1H NMR and X-ray crystallography, (Table 4, Figure 2). In the 1H NMR spectrum of 19a, the diastereotopic protons H-12a and H-12b were identified as multiplets at 1.96 ppm and 2.30 ppm. The bridging protons H-11 and H-10 were identified at 3.69 ppm as a double doublet (J = 10.68, 3.97 Hz) and 4.63 ppm as a singlet respectively. Interestingly, although coupling was demonstrated for H-12a with H-10, (J = 2.44 Hz), this is not observed from its singlet signal. The assignments were confirmed from the C-H COSY and Distortionless Enhancement by Polarization Transfer (DEPT 90) NMR spectra, see Supplementary Information. The esters 19e and 19f were prepared from the anhydride 13a using acid catalysed ring opening conditions Scheme 4, [55].
X-ray crystallographic analysis of the novel Diels-Alder adduct (E)-10-(2-nitrovinyl)-9,10-dihydro-9,10-ethanoanthracene-11-carbonitrile (19a) confirmed the regioisomer obtained, (Figure 2, Table 4 and Table 5), and the packing structure of the product was centrosymmetric and monoclinic. The angle Ao between the centroid of phenyl ring 1 and the centroid of phenyl ring 2 was determined as 126.94o. This angle is reported as 119.46o in the crystal structure of maprotiline [52]. The distance between the carbons in the ethano bridge (d1) was 1.565 Å, comparing well to that of maprotiline 1.540 Å. The distance between the carbon at C-10 and the nearest carbon of the ethano bridge (d2) was 1.556 Å, in maprotiline this distance is 1.546 Å [52].
To investigate the effect of increased rigidity on the ethanoanthracene bridge, the (E)-9-(2-nitrovinyl)anthracenes (12a–12d) were converted to the corresponding (E)-9-(2-nitrovinyl)-9,10-dihydro-9,10-[1,2]benzenoanthracenes (20a–d) in modest yields (Scheme 5, Series V, Table 7). The Diels-Alder reaction was achieved with benzyne as the dienophile generated in situ by thermal decomposition of benzenediazonium-2-carboxylate, (prepared from anthranilic acid and isoamyl nitrite) [56,57,58,59,60]. Diphenyl and triphenyl side products have been reported using in situ benzyne generation [57], but these were not isolated during the current work. The benzyne adduct 20f was similarly prepared from anthracene carboxaldehyde. Sodium borohydride reduction of 20a and 20f afforded the alcohol and nitroalkane analogues 20e and 20g, respectively. The library of novel triptycene analogues synthesised are summarised in Table 7. The 1H NMR spectrum of compound 20d shows two multiplets occurring at 7.58–7.69 ppm and 7.72–7.82 ppm both integrating for three protons each. These signals were assigned to the two groups of three equivalent aromatic protons H-1, H-8, H-3″ and H-4, H-5, H-6″ respectively. The assignments were confirmed from the C-H COSY, HMBC and DEPT 90 NMR spectra, (see Supplementary Information).
The structure of 20a was confirmed by X-ray crystallographic analysis (Figure 2, Table 4 and Table 5), showing that the packing structure assumed by the product was monoclinic and confirming the (E)-configuration of the nitrovinyl unit. The angles between the centroids of each of the three phenyl rings and the centroid of the central axis were calculated from the asymmetric unit, these were Ao (118.50o), Bo (121.24o) and Co (120.18o). This angle is 119.46o in the crystal structure of maprotiline. The distance between the carbons in the ethano bridge (d1) was 1.400 Å, in maprotiline this distance is 1.540 Å [52]. The distance between the carbon at C-10 and the nearest carbon of the ethano bridge (d2) was 1.518 Å, in maprotiline this distance is 1.546 Å [52], hence the X-ray structures indicate a comparable configuration for 20a and maprotiline.
Diels-Alder cycloaddition reactions with substituted anthracenes using maleic anhydride and maleimide dienophiles were further investigated to determine how the replacement of the nitrovinyl group by other functionalities at C-9 would affect the potency of the products. The 9-substituted Diels-Alder adducts 21a–k were obtained using with diene systems anthracene carboxylic acid, anthraldehyde and anthrone in yields of 35–90%, (Series VI, Scheme 5, Table 8). Similar Diels-Alder reaction with anthracene malononitrile (22a), acrylonitrile (22b) [27,61], hydrazines (22c, 22d) and oxime (22e) dienes afforded the cycloaddition products 23a–k in yields of 15–74%, (Series VII, Scheme 5, Table 9). We now report the first direct Diels-Alder reaction of (E)-(anthracen-9-ylmethylene)hydrazine (22c) and (E)-anthracene-9-carbaldehyde oxime (22e) with dienophiles maleic anhydride, maleimide and N-phenylmaleimide. Previous routes to these compounds required the reaction of Diels-Alder adducts of 9-anthraldehyde (e.g., 20f) with hydroxylamine [27,62,63,64] or hydrazine [65,66]. In the current work, the synthesis of the novel 2-(anthracen-9-ylmethylene)malononitrile Diels-Alder adducts 23d–f was also achieved. In the 1H NMR spectrum of the hydrazine 23g the characteristic signals for H-12 and H-11 were identified as doublets at 3.23 ppm and 3.44 ppm (J = 8.55 Hz) while the singlet at 4.72 ppm accounted for H-9, (see Supplementary Information).
A preliminary stability study of the representative ethanoanthracene compound 16a was carried out at acidic, neutral and basic conditions (pH 4, 7.4 and 9) using HPLC. The half-life (t½) was determined to be 11 h at pH 4, 10.5 h at pH 7.4 and greater than 24 h at pH 9. Based on this stability study the compound would be suitable for further preclinical investigation.
3. Biochemistry
3.1. Preliminary Evaluation of In Vitro Anti-Proliferative Activity of the Ethanoanthracenes in Burkitt Lymphoma EBV−MUTU-1 and EBV+DG-75 (Chemoresistant) Cell Lines
The panel of compounds synthesised (Series I–VII) based on the 9,10-dihydro-9,10-ethanoanthracene scaffold was then initially screened at two concentrations (1 μM and 10 μM) for antiproliferative activity in the BL EBV−MUTU-1 and EBV+DG-75 (chemoresistant) cell lines to determine the structure-activity relationship for these maprotiline analogues. The results obtained from this preliminary screen in the MUTU-I and DG-75 cell lines (at 10 µM and 1 µM) are displayed in Table 1, Table 2 and Table 3 and Table 6, Table 7, Table 8 and Table 9, with maprotiline and taxol used as the control drugs. Maprotiline induced a modest anti-proliferative effect at 10 µM in the MUTU-I and DG-75 BL cell lines, while taxol was more effective with 10% cell viability at 10 μM in both cell lines. The results obtained for these novel ethanoanthracene compounds (Series I-VII) are discussed by structural type.
3.1.1. Series I and II, Compounds 13a–n, 14a–c and 15
The initial lead 9-(2-nitrovinyl)anthracene 12a demonstrated activity (< 14% cell viability) in both BL cell lines at 10 µM, (Table 1). The effects of the maleic anhydride adducts 13a–f and maleimide adducts (13g–n) of the lead nitrovinyl anthracene compound 12a on cell viability were first investigated (Table 1). The 9-(2-nitrovinyl)-9,10-dihydro-9,10-ethanoanthracenes 13a, 13b produced a significant anti-proliferative effect at both 1 and 10 μM concentrations in the MUTU-I cell line (7–24% cell viability), (Table 1). In the DG-75 cell line the maleic anhydride derivative 13a gave slightly improved results compared to maprotiline at both concentrations. C-10 chloro substitution e.g., 13d resulted in decreased activity in both cell lines. The anhydride 13b and the imide 13g demonstrated potent antiproliferative effects in DG-75 cell line (1% cell viability at 1 μM), with the imide 13g more potent than the anhydride 13a. The 9-chlorosubstituted imide 13j, N-methylimide 13m and alcohol 13n demonstrated potent antiproliferative effects in the MUTU-1 cell line (< 1% cells remaining at 1 μM). Increasing alkyl chain length at the C-2 position decreased the anti-proliferative effect across most examples of the series (e.g., 13f, 13i, 13l). Reduction of the C-9 nitrostyrenes 13a, 13g to afford the C-9 nitroalkane substituted 14a and 14b (Series II) resulted in significant reduction in activity, indicating the essential requirement of the nitrostyrene functional group for activity. The dimer compound 15 was identified as a potential lead compound with significant activity in both cell lines (< 5% viable cells at 1 μM).
3.1.2. Series IIIA, Compounds 16a–n
Compounds 16a–n (Series IIIA, Table 2) were designed to investigate the effect of N-arylsubstitution (halogen, ether, phenol, ketone, ester, amine) on antiproliferative activity of the lead ethanoanthracene scaffold structure 13a. All analogues in the series elicited a very potent anti-proliferative action in the MUTU-I cell line at 1 µM (<5% cell viability, except 16a, 10%). In the DG-75 cell line, the most potent activity was produced by compounds 16a and 16b, with <6% viable cells remaining at 1 µM. The 9-chloro compound 16d also produced a promising result at 1 µM with <14% cell viability. Significantly reduced activity was observed when comparing compounds 16b (p-Cl) and 16k (m-Cl) in the DG-75 cell line. In a study of para phenyl substitution of this series of compounds 16a–16n in the DG-75 cell line, the unsubstituted compound 16a and p-chloro compound 16b were found to elicit more favourable antiproliferative effects than the bromo (16g), fluoro (16e), amino (16n), ketone (16m) and methoxy (16c) analogues (Table 2). Maleimide (13g) and phenyl maleimide (16a) adducts produce superior activity in the two BL cell lines than the maleic anhydride adduct 13a. C-9 chloro substituted compounds 13j and 16d possessed significant anticancer effects in both cell lines (0.4–13.5% cell viability).
3.1.3. Series IIIB, Compounds 17a–n
Compounds 17a–n were designed to investigate the effect of the alkyl substitution on the nitrostyrene group, and also to investigate the introduction of a benzyl or phenylethylamine substitution on the heterocyclic nitrogen (Table 3). The deactivating effect of extended alkyl chain length (methyl and ethyl) at C-2 on anticancer activity can once again be observed (70–90% cell viability). With the exception of compounds 17i (N-benzyl) and 17n (N-phenylethylamine), these analogues elicited poor anti-proliferative action in both MUTU-I and DG-75 cell lines confirming that alkyl substitution (methyl or ethyl) on the nitrostyrene group dramatically reduces activity e.g., comparing adducts 16a with 17a and 17b in both DG-75 and MUTU-I cell lines at 10 µM, (Table 3). Compound 18 was less potent at the lower concentration in both BL lines than 16a, indicating that the ethanoanthracene structure was more favourable than the triazole-anthracene bridged system for the desired anticancer effect.
3.1.4. Series IV, Compounds 19a–f
The effect of ethano-bridge substitution on antiproliferative effects was assessed in compounds 19a–f using a range of different dienophiles for the Diels-Alder reaction, together with the ring opening reaction of the anhydride 13a to introduce nitrile and ester groups on the ethano-bridge, Table 6. All but one of these simpler Diel-Alder adducts possessed enhanced anti-proliferative activity compared to the parent nitrovinylanthracene compound 12a. The most promising compounds identified from this cohort were 19a, 19c and 19f showing good anti-proliferative effects in both cell lines with cell viability of 0–14% at 10 μM. The inclusion of the ester group on the ethano-bridged system in 19d reduced the potency observed for compound 19a, (Table 6).
3.1.5. Series V, Compounds 20a–g
A series of triptycene based Diel-Alder adducts were prepared to assess the effect of the rigid triptycene moiety on the anti-proliferative activity (Table 7). The C-9 functionalities such as nitrovinyl 20a, 20b, 20c, 20d, nitroalkane 20e, aldehyde 20f and alcohol 20g were included to assess their impact on the anticancer properties of the series. The most promising results were obtained by nitrovinyl compounds 20a and 20d (10-chloro) having potent effects (<6% cell viability) in the MUTU-I cell line. A potent effect was also observed for 20d in DG-75 at 10 µM with no viable cells detected. Reduction of the nitrovinyl bond was once again detrimental to anti-proliferative effects in both cell lines (e.g., 20e). Alkyl substituent at C-2 of the nitrovinyl unit resulted in diminished activity demonstrated for methyl (20b) and ethyl (20c) compounds. The aldehyde based triptycene (20f) exhibited good activity at the higher concentration in the MUTU-I cell line (<5%), while alcohol (20g) was inactive (Table 7).
3.1.6. Series VI, Compounds 21a–21k
The 9,10-dihydro-9,10-ethanoanthracenes 21a–k which do not contain the nitrovinyl unit at C-9 demonstrated greatly reduced antiproliferative activity in both cell lines (>60% viability), indicating the C-9 nitrovinyl substitution was essential for enhanced anti-proliferative effect, Table 8. The 9,10-dihydro-9,10-ethanoanthracenes assessed include C-9 unsubstituted 21a–c, C-9 substituted aldehydes 21d–f, alcohols 21j,k and acids 21g–i, Table 8. Although these compounds possess anticancer activity exceeding the activity of maprotiline at 1 µM in the DG-75 cell line, the antiproliferative effect in the MUTU-I cell line was minimal (>80% cell viability).
3.1.7. Series VII, Compounds 23a–k
A group of C-9 substituted 9,10-dihydro-9,10-ethanoanthracenes 23a–k derived from maleic anhydride, maleimide and phenyl maleimide were next investigated for antiproliferative activity, to assess the effect of variation of the C-9 unsaturated substituent, Table 9. Of the C-9 acrylonitrile compounds 23a–f, the most promising was identified as compound 23b which demonstrated activities comparable to maprotiline in the MUTU-I (79% cell viability) and DG-75 (54% cell viability) cell lines, Table 9. Further C-9 substituted 9,10-dihydro-9,10-ethanoanthracenes included hydrazones (23g–i) and oximes (23j,k). The most potent compound in the DG-75 cell line was identified as the hydrazone 23i, (19% cell viability). In summary, introduction of alternative double bond functionality to replace the nitrovinyl e.g., cyanovinyl (23a–f), oxime (23j,k) and hydrazone (23g–i) were found to reduce the observed antiproliferative effects when compared with the C-9 nitrovinyl compounds 13a, 13f and 16a respectively, (Table 9).
In this initial evaluation of the ethanoanthracenes (Series I–VII) for antiproliferative activity, a number of key structural requirements were identified. The nitrovinyl pharmacophore was demonstrated to be critical for antiproliferative effect, epipyrrolo bridgeheads were also found to exert a more potent effect than simple ethano and furan-based bridgeheads. Introduction of a chloro substituent at C-9 of the anthracene core also contributed to potency for many compounds.
3.2. In Vitro Anti-Proliferative Activity of Selected Potent Ethanoanthracenes
Based on the results obtained from the cell viability study above, the following potent compounds were identified for further investigation in the MUTU-I cell line: maleimide 13j, N-hydroxymethylmaleimide 13n, and maleimide dimer 15 (Series I), N-arylmaleimides 16a–16j, 16m, 16n (Series IIIA), N-benzylmaleimide 17n (series IIIB), acrylonitrile adduct 19a, acrylate ester adduct 19c and diester 19f (Series IV) and triptycene 20d (Series V). The IC50 values were determined in the sub-micromolar range (0.09–0.55 μM), with compound 15 identified as the most potent (IC50 = 0.09 μM), Table 10. The MUTU-1 IC50 values combined with the data provided by the preliminary screen in the DG-75 cell line were used to select the following compounds for subsequent IC50 determination in the chemoresistant DG-75 cell line: 13j, 15 (Series I), 16a, 16b, 16c, 16d (Series II) and 19a (Series IV), Table 11.

Table 10.
IC50 values for selected ethanoanthracenes in the MUTU-I cell line (24 h) a.

Table 11.
IC50 values of selected compounds 12a, 13j, 15, 16a–d, 19a in BL cell lines DG-75 and MUTU-I Burkitt lymphoma cell lines.
The IC50 values of all the selected compounds 13j, 15, 16a–d and 19a were not only more potent than the lead 9-nitrovinylanthracene based compound 12a (MUTU-I; IC50 2.57 µM, DG-75; 2.08 µM) and maprotiline (MUTU-I; 15.8 µM, DG-75; 37.5 µM) in both BL cell lines but also these compounds exerted a more potent effect than taxol (MUTU-I; 0.32 µM, DG-75; 1.32 µM) with submicromolar IC50 values of 0.09–0.38 µM in the MUTU-I cell line and 0.24–0.78 µM in the chemoresistant DG-75 cell line. (Table 11). These novel compounds were selected for further investigation also based on analysis of their drug-like properties (Lipinski) from a Tier-1 profiling screen, together with predictions of blood brain barrier partition, permeability, plasma protein binding, metabolic stability and human intestinal absorption properties which confirmed that these compounds are moderately lipophilic-hydrophilic drugs and are suitable candidates for further investigation (Tables S1 and S2, Supporting information). Compounds 12a, 13j, 16a–d and 19a were found to satisfy all the Lipinski rule of five criteria with logP values in the range 3.31–5.26, indicating their potential as lead compounds for further development. The dimeric compound 15 was identified as the most potent analogue evaluated with an IC50 value of 0.09 µM in the MUTU-I cell line and an IC50 of 0.24 µM in the DG-75 cell line.
Examples of the potent compounds 13j, 16a, 16b and 19a (displayed as yellow in their respective overlays) were flexibly aligned with the lead compound maprotiline 8 (cyan) using MOE (Molecular Operating Environment) 2016.V8, (Figure 3). The close correspondence between overlays of these compounds with maprotiline highlights the presence of three main shared molecular features: the (E)-configuration nitrovinyl pharmacophore located at C-9, the 9,10-dihydroanthracene core structure and the presence of the 9,10-ethanoanthracene bridge, unsubstituted as in maprotiline, having a nitrile substituent as in the acrylonitrile adduct 19a or forming part of the heterocyclic structure as in the maleimide adducts 13j, 16a and 16b.
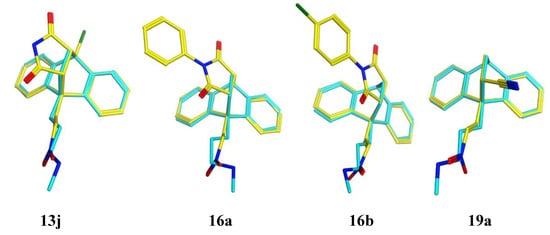
Figure 3.
Overlay of 9,10-dihydroethanoanthracene compounds 13j, 16a, 16b and 19a (yellow) with maprotiline 8 (cyan). The atoms are coloured by element type: oxygen = red, nitrogen = blue, chlorine = green.
3.3. Investigations into the Pro-Apoptotic Effect of the Most Potent Ethanoanthracenes-FITC Annexin V/PI FACS Analysis
The potential pro-apoptotic effects of the most potent maprotiline analogues were determined by Annexin V/PI FACS analysis of a subset of the most potent compounds identified e.g., compounds 12a, 13j, 15, 16a–d and 19a. The study was carried out over a concentration range (0.2–10 µM) in both the BL cell lines (DG-75 and MUTU-I), Figure 4. Taxol was used as a positive control. Taxol was found to elicit a pro-apoptotic effect in the MUTU-I at both 10 µM (87%) and 1 µM (63%). The initial anthracene-nitrostyrene compound 12a demonstrated potent apoptotic activity at 10 µM (90%), with little effect at 1 µM [27] (Figure 4A). Compound 16a induced over 80% apoptosis at 10 µM, 1 µM and 0.5 µM with 46% apoptosis at 0.2 µM (Figure 4A). The p-chlorophenyl compound 16b induced a more potent effect than the unsubstituted compound 16a with a response of >80% apoptosis at all concentrations in the MUTU-I cell line. The p-methoxyphenyl compound 16c induced >90% apoptosis at 0.5 µM. Similar activity was observed for the 10-chloro compound 16d with 63% apoptosis at 0.2 µM, (Figure 4B). Compounds 13j and 19a were also found to possess potent apoptotic activity at 0.5 µM > 90%. The most potent compound investigated was the dimer 15, demonstrating > 90% apoptosis at 0.2 μM, (Figure 4C). In summary, compounds 15 and 16b not only possessed the most potent antiproliferative activity but were found to induce the most favourable pro-apoptotic response in the MUTU-I cell line.
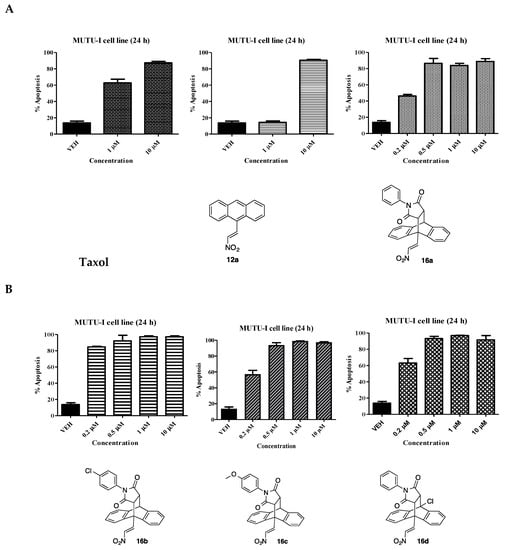
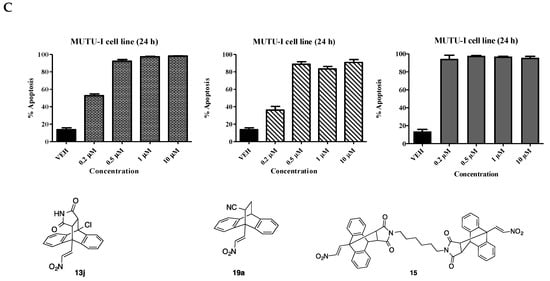
Figure 4.
Ethanoanthracene nitrostyrene compounds 16a–d, 13j, 19a, 15 induce apoptosis in Burkitt’s lymphoma MUTU-1 cell line. Induction of apoptosis in Burkitt’s lymphoma MUTU-1 cell line following treatment for 24 h with selected ethanoanthracene nitrostyrene compounds (0.2–10 μM) or a comparative control (taxol) in MUTU-1 cell line is determined using Annexin V and propidium iodide staining/FACS. (A) Taxol, 12, 16a; (B) 16b, 16c, 16d; (C) 13j, 19a, 15. Ethanol was used as the vehicle. Values represent the mean of three independent experiments.
In the chemoresistant DG-75 cell line, taxol was found to elicit a pro-apoptotic effect at both 10 µM (73%) and 1 µM (21%), (Figure 5A). Compound 12a elicits similar effects to taxol at both 10 µM (82%) and 1 µM (10%). The unsubstituted maleimide adduct 16a shows a potent apoptotic effect at 10 µM (92%) but is inactive at lower concentrations (<5% apoptosis). The p-chlorophenylmaleimide 16b and p-methoxyphenylmaleimide compound 16c exhibits improved pro-apoptotic activity when compared to the unsubstituted compound 16a in the DG-75 cell line. Compound 16b and 16c induce 61% and 29% apoptosis at 1 µM respectively. The 10-chloro compound 16d induced 44% apoptosis at 10 µM and a modest 15% at 1 µM (Figure 5B). The maleimide Diels-Alder adduct 13j and acrylonitrile adduct 19a induced 90% and 87% apoptosis at 10 µM. The leading compound 15 induced dose dependent apoptosis at all concentrations assayed with 95% (10 µM) in the DG-75 cell line, Figure 5C. Overall compounds 15, 16b and 16c were shown to induce a superior potent pro-apoptotic response in both the MUTU-I and DG-75 BL cell lines than other selected compounds in this grouping and merit further study.
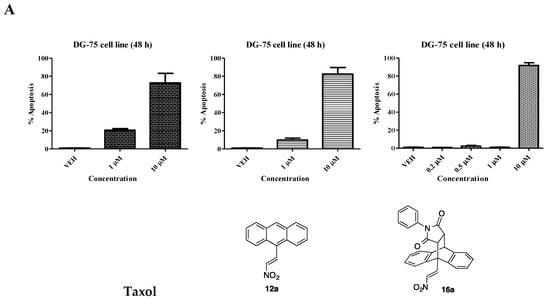
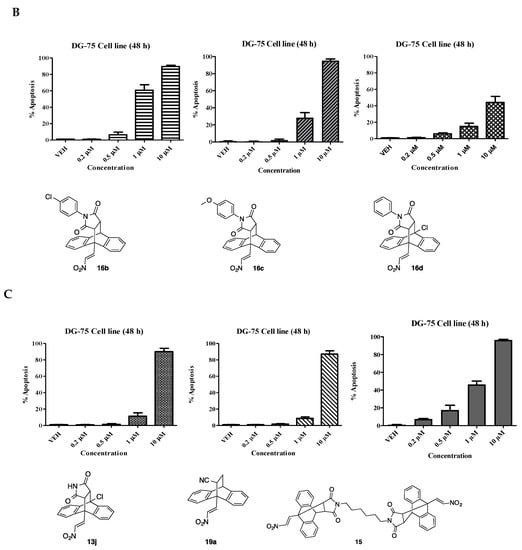
Figure 5.
Ethanoanthracene nitrostyrene compounds 16a–d, 13j, 19a, 15 induce apoptosis in Burkitt’s lymphoma DG-75 cell line. Induction of apoptosis in Burkitt’s lymphoma DG-75 cell line following treatment for 48 h with selected ethanoanthracene nitrostyrene compounds (0.2–10 μM) or a comparative control (taxol) in DG-75 cell line is determined by Annexin V and propidium iodide staining/FACS: (A) Taxol, 12, 16a; (B) 16b, 16c, 16d; (C) 13j, 19a, 15. Ethanol was used as the vehicle. Values represent the mean of three independent experiments.
3.4. Evaluation of In Vitro Cytotoxicity of Ethanoanthracenes
Compounds 15, 16b and 16c were found to elicit the most potent anti-proliferative and pro-apoptotic activity of the series. In vitro cytotoxicity of these compounds was evaluated using a lactate dehydrogenase (LDH) assay. LDH is released during mechanisms of cell death associated with loss of cell membrane integrity (necrosis). The MUTU-I and DG-75 BL cell lines were treated at 10 µM and 1 µM for the desired treatment period and the results are presented as percentage of total LDH release, (Figure 6). In the MUTU-I cell line low levels of LDH release were obtained (2–17%) at 10 µM and 1 µM concentration, indicating low cytotoxicity. The lowest LDH release was observed by compound 16b with 2% and 5% cytotoxicity (at 10 µM and 1 µM concentrations respectively). The compounds showed low to moderate cytotoxicity in the DG-75 cell line at 10 µM (25–41%) and 1 µM (27–30%). The lowest LDH release was observed for compound 16c with 25% (10 µM) and 17% (1 µM).
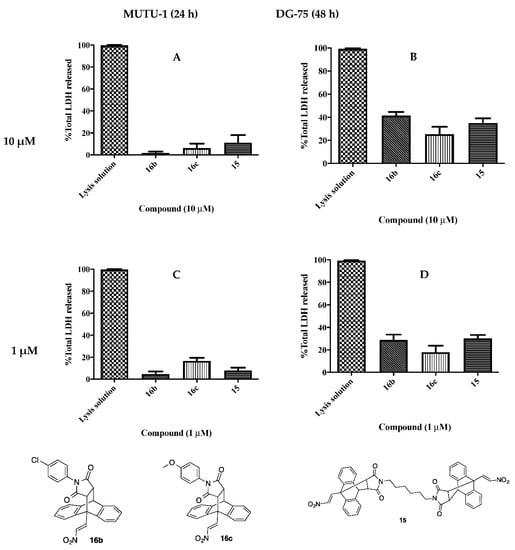
Figure 6.
Lactate dehydrogenase (LDH) assay of ethanoanthracenes 15, 16b and 16c. Cells were treated with ethanoanthracene compounds 15, 16b and 16c (1 μM and 10 μM) for 24 or 48 h. Induction of necrotic cell death was determined by measuring LDH release in MUTU-I (A,C) and DG-75 cell lines (B,D), with control lysis solution (100% necrosis). Values are shown the mean of three independent experiments.
3.5. Effect of Compounds 15, 16b and 16c on the Viability of PBMCs
The 2-nitrovinyl-9,10-dihydro-9,10-ethanoanthracenes compounds 15, 16b and 16c were evaluated for their toxicity on peripheral blood mononuclear cells (PBMCs) to determine the selective toxicity of these compounds on malignant BL cell lines over normal lymphatic cells. Compounds were evaluated at 1 µM and 0.5 µM over a 24 h treatment time. Compounds 15, 16b and 16c elicited minimal toxicity in the PBMCs at 0.5 µM (~99% viable cells) and low toxicity at 1 µM (72–81% viable cells), Figure 7. In comparison, compounds 15, 16b and 16c induced a potent anti-proliferative effect in the MUTU-I at 1 µM and 0.5 µM, (2–7% viable cells remaining). Compounds 5, 16b and 16c also induced a potent anti-proliferative effect in the DG-75 at 1 µM (6–28%) and 0.5 µM (18–57%). Comparing the results obtained from the BL cell lines and the PBMCs, the results suggest compounds 15, 16b and 16c exert a selectively toxic effect on BL cell lines.
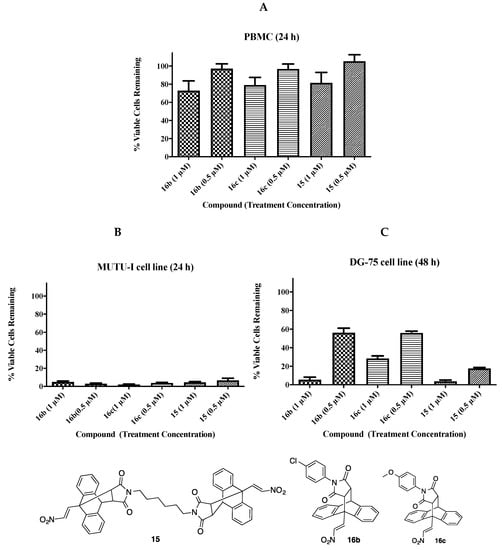
Figure 7.
In Vitro antiproliferative effect of compounds 15, 16b and 16c on (A) PBMCs (24 h), (B) MUTU-1 cell line (24 h) and (C) DG-75 cell line (48 h) at 1 μM and 0.5 μM.
3.6. Effect of Pre-Treatment with Antioxidants on Cellular Viability
Reactive oxygen species (ROS) are short lived diffusible entities containing oxygen such as hydroxy, nitroxyl, alkoxy, superoxide or peroxyl radicals. ROS are generated as metabolites of oxygen and are utilised for signalling events for essential cell functions. ROS are often associated with the induction of cell death and apoptosis. A potential role for ROS in the mechanism of cell death induced by selected potent compounds 15, 16b and 16c was investigated. A viability assay was used to investigate the effects of pre-incubation with an antioxidant on ROS levels in DG-75 BL cells with the compound of interest. DG-75 cells were pre-treated with N-acetylcysteine (NAC), a known ROS scavenger and subsequently treated with the selected compounds 15, 16b and 16. Viability was monitored using the alamarBlue assay, (Figure 8). From the results, obtained it is evident that in the presence of the reactive oxygen species inhibitor NAC—the anti-proliferative effects of compounds 15, 16b and 16 at 1 µM was reduced. Overall the anti-proliferative effects previously observed by compounds 16b, 15 and 16c increased from 6–23% viable cells to 73–83% viable cells in the presence of 5 mM NAC, indicating that ROS may be involved in the mechanism of cell death induced by these compounds, (Figure 8).
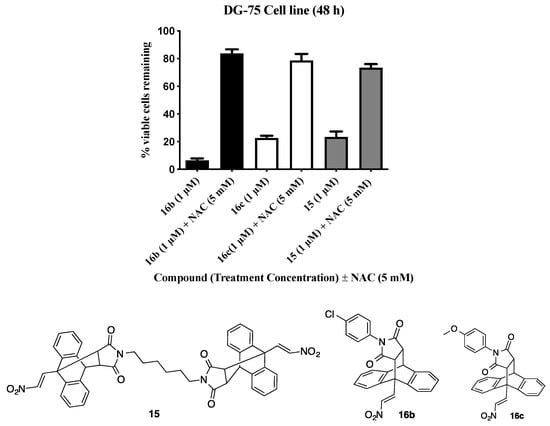
Figure 8.
Effect of antioxidant pre-treatment on viability of DG-75 cells treated with compounds 15, 16b and 16c.
Cell proliferation of MUTU-1 and DG-75 cells was determined with an alamarBlue assay (seeding density 1–5 × 104 cells/mL per well for 96-well plates). Compound concentrations of either 1 μM or 0.5 μM for 24 h (MUTU-1) or 48 h (DG-75) were used to treat the cells (in triplicate) with control wells containing vehicle ethanol (1% v/v). The mean value for three independent experiments is shown.
Cell proliferation of DG-75 cells was determined with an alamarBlue assay (seeding density 2 × 104 cells/mL per well for 96-well plates), with vehicle control ethanol 1% (v/v). Cells were retained for 24 h and then pre-treated with NAC (5 mM) for 1 h, followed by compounds 15, 16b and 16c at 1 μM for 48 h. Cell viability was measured by alamarBlue assay (mean of three independent experiments).
4. Conclusions
A series of 9,10-dihydro-9,10-ethanoanthracene based maprotiline analogues were synthesised and evaluated for potential antiproliferative activity in the MUTU-I and chemoresistant DG-75 BL cell lines. Substitution at C-9 and C-10 of the 9,10-dihydro-9,10-ethanoanthracene compounds was achieved by modification of the diene system to include functionalities such as nitrovinyl, nitroalkyl, aldehyde, imine, carboxylic acid, alcohol, oxime, cyanovinyl and hydrazone on the anthracene scaffold. The effect of a number of 9,10-dihydro-9,10-ethanoanthracene structural modifications on activity was also investigated; these modifications included ethano bridge modifications, phenyl substitutions, maleimide substitutions and extension of alkyl chain length at C-2 of the nitrovinyl unit. The most promising 9,10-dihydro-9,10-ethanoanthracene based maprotiline analogues were identified and all included a nitrovinyl substituent at C-9. The structure-activity relationships for the series of ethanoanthracenes synthesised in this study are summarised in Figure 9. The preliminary screen of the 9,10-dihydro-9,10-ethanoanthracenes identified the maleimide compounds 15, 16b and 16c as the lead compounds from this study. The dimer compound 15 displayed potent anticancer activity, in both BL lines with IC50 values of 0.09 µM (MUTU-I) and 0.24 µM (DG-75), while both compounds 16b and 16c elicited significant anti-proliferative activity in the BL cell lines. The present work has demonstrated the selectively toxic effect of compounds 15 and 16c towards the MUTU-I and DG-75 cell lines over PBMCs. These compounds were shown to induce a significant pro-apoptotic effect in MUTU-I cell and DG-75 BL cell lines and may target the stress response to ROS in DG-75 BL cell lines. The results suggest that this class of compounds merits further investigation as antiproliferative agents for BL. Further studies are in progress to investigate the role of these compounds in ROS mediated cell death and in the reversal of drug efflux in multidrug resistant cancer cells.
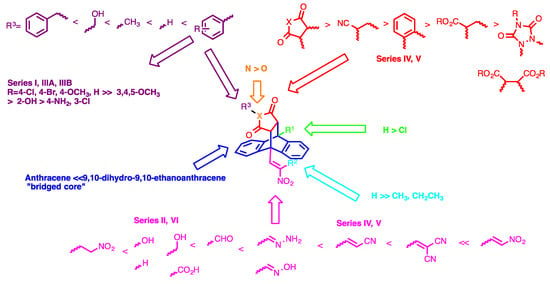
Figure 9.
Summary of SAR for 9,10-dihydro-9,10-ethanoanthracenes.
5. Experimental Section
5.1. Chemistry
All commercially available reagents were used without further purification. Solvents were dried prior to use; tetrahydrofuran (THF) by distillation from sodium/benzophenone under nitrogen, toluene was distilled from sodium, dichloromethane was distilled from calcium hydride. Melting points were recorded on a Gallenkamp SMP 11 melting point apparatus and are uncorrected. Infra-red (IR) spectra were obtained on a Perkin Elmer FT-IR Spectrum 100 spectrometer. 1H and 13C NMR spectra were obtained on a Bruker Avance DPX 400 spectrometer operating at 400.13 MHz, (1H) and 100.61 MHz (13C) at 20 °C in either CDCl3 or DMSO-d6 with appropriate solvent peaks as reference standards. Mass spectrometry (ESI-MS) was performed on a Micromass LCT instrument with mass measurement accuracies of <±5 ppm. Low resolution mass spectra (LRMS) were obtained on a Hewlett-Packard 5973 MSD GC-MS system. Preparative separations were performed using flash column chromatography on silica gel (Merck Kieselgel 60, particle size 0.040–0.063 mm). Chromatographic separations were also performed on Biotage SP4 instrument. All reactions and products were monitored on thin layer chromatography (TLC) using Merck silica gel 60 F254. HPLC was used to determine the purity of the compounds (2487 Dual Wavelength Absorbance detector (Waters), 1525 binary HPLC pump, In-Line Degasser AF and Waters 717plus Autosampler), together with a Varian Pursuit XRs C18 reverse phase 150 × 4.6 mm chromatography column. Samples were detected using a wavelength of 254 nm. Details for the preparation of compounds 11a–11n, 11p–11r, 20a, 20f, 20g, 21a–j, 22a–c, 22e is contained in the Supplementary Information.
5.2. General Procedure 1: Preparation of Phenyl and Benzyl Maleimides (11a–11s)
To a solution of maleic anhydride (20 mmol) in diethyl ether (25 mL) was added the appropriate benzyl or aryl amine (20 mmol) in diethyl ether (10 mL). The reaction mixture was stirred under reflux at 20 °C for 1 h. The precipitated solid was filtered and washed with diethyl ether. This solid was immediately used in the next step and placed in a conical flask containing sodium acetate (8.5 mmol, 0.7 g) and acetic anhydride (10 mL). The mixture was heated at 90 °C for 0.5 h, then poured over ice water (100 mL). The solid was filtered and recrystallised from ethanol.
5.2.1. 1-(3,4,5-Trimethoxyphenyl)-1H-pyrrole-2,5-dione (11o)
Compound 11o was prepared from maleic anhydride (20 mmol) and 3,4,5-trimethoxyaniline (20 mmol) following the general procedure 1. The precipitated solid filtered and recrystallised from ethanol, to afford the product as yellow crystals, 3.17 g (60%), Mp. 120–128 °C. IRVmax (KBr): 3085, 2964 (C-H), 1707 (C=O), 1509, 1470 (C=C), 1598 (C=C), 1127 (CN) cm−1. 1H NMR (400 MHz, CDCl3) δ 3.86 (s, 9 H, 3 x OCH3), 6.54 (s, 2 H, 2 x ArH), 6.84 (s, 2 H, 2 x =CH). 13C NMR (101 MHz, CDCl3) ppm 56.2 (OCH3), 60.8 (OCH3), 104.0 (CH), 126.6, 134.1 (CH), 137.7, 153.4, 169.5 (C=O). HRMS (APCI) calculated for C13H14NO5 [M+ + H] 264.0872: found 264.0867.
5.2.2. 1,1′-(Hexane-1,6-diyl)bis(1H-pyrrole-2,5-dione) (11s)
Compound 11s was prepared from maleic anhydride (20 mmol) and 1,4-diaminobenzene (20 mmol) following the general procedure 1. The product was obtained as yellow crystals (60%), Mp. 130–136 °C (lit. M.p. 136–141 °C [67]). IRVmax (ATR): 3103, 2939 (C-H), 1688 (C=O), 1587, 1453 (C=C), 1227 (CN) cm−1. 1H NMR (400 MHz, CDCl3) δ 1.09–1.34 (m, 4 H, 2 x CH2), 1.34–1.56 (m, 4 H, 2 x CH2), 3.39 (t, J = 7.26 Hz, 4 H, 2 x CH2), 6.60 (s, 3 H, 2 x =CH). 13C NMR (101 MHz, CDCl3) ppm 25.9 (CH2), 28.1 (CH2), 37.4 (CH2), 133.9 (=CH), 169.7 (C=O), 170.7 (C=O). HRMS (APCI) calculated for C14H17N2O4 [M+ + H] 277.1188: found 277.1183.
5.3. General Procedure 2: Preparation of Nitrovinyl Anthracenes 12a–12f
To a solution of 9-anthraldehyde (2 g, 9.7 mmol) in the appropriate nitroalkane (nitromethane, nitroethane, nitropropane) (15 mL) was added piperidinium acetate (1.5 g, 10.3 mmol). (Piperidinium acetate was prepared from piperidine 6.6 mL and acetic acid 3 mL). The solution was heated at 90 °C for 1.5 h under nitrogen for 1 h, then cooled to room temperature and poured onto 100 mL of ice cold H2O. Following DCM extraction, the organic layers were combined, dried (Na2SO4) and solvent removed. The product was recrystallised from an appropriate solvent.
5.3.1. (E)-9-(2-nitrovinyl)anthracene (12a)
Compound 12a was prepared from 9-anthraldehyde (9.7 mmol, 2 g) and nitromethane (15 mL) as outlined in the general procedure 2. The product was recrystallized from methanol and diethyl ether as red crystals 2.41 g (99%), Mp. 145–147 °C (lit. M.p. 142 °C [68]). IRVmax (KBr): 3050, 2948 (C-H), 1617, 1553 (C=C), 1498, 1330 (NO2), 1250 (C-N) cm−1. 1H NMR (400 MHz, CDCl3) δ 7.40–7.66 (m, 5 H, 4 x ArH, CH= x 1), 7.80–8.05 (m, 2 H, 2 x ArH), 8.05–8.25 (m, 2 H, 2 x ArH), 8.45 (br. s., 1 H, H10), 8.90 (d, J = 13.43 Hz, 1 H, CH= x 1). 13C NMR (101 MHz, CDCl3) ppm 124.3 (C9), 125.7 (CH), 127.5 (CH), 129.2 (CH), 129.2, 129.8, 130.4 (CH), 131.1, 135.6 (CH), 142.6 (C2′). HRMS (APCI) calculated for C16H12NO2 [M+ + H] 250.0868: found 250.0879.
5.3.2. (E)-9-(2-Nitroprop-1-en-1-yl)anthracene (12b)
Compound 12b was prepared from 9-anthraldehyde (9.7 mmol, 2 g) and nitroethane (15 mL) following the method in the general procedure 2. The product was recrystallized from ethanol and diethyl ether as orange crystals, 1.87 g (73%), Mp. 141–142 °C (lit. Mp. 142–143 °C [69]). IRVmax (KBr): 3052, 2825 (C-H), 1510, 1326 (NO2), 1622.16, 1442.74 (C=C), 1483 (C=C), 385 (CH3) cm−1. 1H NMR (400 MHz, CDCl3) δ 1.98 (s, 1 H, CH3), 7.42–7.63 (m, 4 H, 4 x ArH), 7.86 (d, J = 7.93 Hz, 2 H, 2 x ArH), 7.93–8.12 (m, 2 H, 2 x ArH), 8.48 (s, 1 H, H10), 8.71 (s, 1 H, H1′). 13C NMR (101 MHz, CDCl3) ppm 14.4 (C3′), 124.8 (C9), 125.5 (CH), 126.7, 128.6, 128.8 (CH), 129.0 (CH), 131.0, 131.3 (CH), 151.0 (C2′). HRMS (ESI) calculated for C17H14NO2 [M+ + H] 264.1025: found 264.1035.
5.3.3. (E)-9-(2-Nitrobut-1-en-1-yl)anthracene (12c)
Compound 12c was prepared from 9-anthraldehyde (9.7 mmol, 2 g) and nitropropane (15 mL) as described in the general procedure 2. The product was recrystallized from ethanol and diethyl ether as gold crystals, 1.62 g (60%), Mp. 159–160 °C [70]. IRVmax (KBr): 2982, 2937 C-H), 1622, 1427 (C=C), 1520, 1341 (NO2), 1444 (CH2), 1376 (CH3) cm−1. 1H NMR (400 MHz, CDCl3) δ 0.90 (t, J = 7.32 Hz, 3 H, CH3), 2.37 (q, J = 7.32 Hz, 2 H, CH2), 7.48–7.54 (m, 4 H,4 x ArH), 7.88–7.93 (m, 2 H, H4, H5), 8.01–8.05 (m, 2 H, H8, H1), 8.48 (s, 1 H, H10), 8.54 (s, 1 H, H1′). 13C NMR (101 MHz, CDCl3) ppm 11.6 (C3′), 21.4 (C2′), 124.9 (C9), 125.6 (CH), 125.7, 126.6 (CH), 128.3 (CH), 128.8, 128.9 (CH), 130.7 (CH), 131.1, 156.7 (C2′). HRMS (APCI) calculated for C18H16NO2 [M+ + H] 278.1181: found 278.1189.
5.3.4. (E)-9-Chloro-10-(2-nitrovinyl)anthracene (12d)
Compound 12d was prepared from 10-chloroanthracene-9-carbaldehyde (5 mmol, 1.2 g) and nitromethane (15 mL) as outlined in the general procedure 2. The product was recrystallized from methanol and diethyl ether as orange crystals, 1.01 g (71%), Mp. 232–234 °C. IRVmax (ATR): 3066, 2973 (C-H), 1623 (C=C), 1439 (C=C), 1538, 1326 (NO2), 1110 (C-N) cm−1. 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 14.04 Hz, 1 H, =CH), 7.60–7.77 (m, 4 H, 4 x ArH), 8.20 (d, J = 8.55 Hz, 2 H, 2 x ArH), 8.62 (d, J = 8.55 Hz, 2 H, 2 x ArH), 8.96 (d, J = 14.04 Hz, 1 H, =CH). 13C NMR (101 MHz, CDCl3) ppm 124.8, 125.8, 127.1, 127.6, 128.5, 130.1, 135.4 (C1), 143.3 (C2). HRMS (APCI) calculated for C16H11ClNO2 [M + H] 284.0478: found 284.0492.
5.3.5. (E)-9-Chloro-10-(2-nitroprop-1-en-1-yl)anthracene (12e)
Compound 12e was prepared from 10-chloroanthracene-9-carbaldehyde (5 mmol, 1.2 g) and nitroethane (15 mL) following general procedure 2. The product was recrystallized from methanol and diethyl ether as orange crystals 722 mg (50%), Mp. 158–160 °C. IRVmax (ATR): 3023, 2977 (C-H), 1622 (C=C), 1480, 1438 (C=C), 1512, 1327 (NO2), 1171 (C-N) cm−1. 1H NMR (400 MHz, CDCl3) δ 1.99 (s, 3 H, CH3), 7.53–7.77 (m, 4 H, 4 x ArH), 7.92 (d, J = 8.55 Hz, 2 H, 2 x ArH), 8.60 (d, J = 9.16 Hz, 2 H, 2 x ArH), 8.70 (s, 1 H, H1′). 13C NMR (101 MHz, CDCl3) ppm 14.4 (C3′), 125.2, 125.6, 127.0, 127.0, 128.4, 129.2, 130.8, 131.0 (C1′), 151.5 (C2′). HRMS (APCI) calculated for C17H13ClNO2 [M+ + H] 289.0635: found 289.0637.
5.3.6. (E)-9-Chloro-10-(2-nitrobut-1-en-1-yl)anthracene (12f)
Compound 12f was prepared from 10-chloroanthracene-9-carbaldehyde (5 mmol, 1.2 g) and nitropropane (15 mL) as described in general procedure 2. The product was recrystallized from methanol and diethyl ether as orange crystals 998 mg (64%), Mp. 157–159 °C. IRVmax (ATR): 3086, 2850 (C-H), 1621 (C=C), 1438, 1425 (C=C), 1553, 1328 (NO2), 1149 (C-N) cm−1. 1H NMR (400 MHz, CDCl3) δ 0.90 (t, J = 7.32 Hz, 3 H, CH3), 2.35 (q, J = 7.32 Hz, 2 H, CH2), 7.52–7.70 (m, 4 H, 4 x ArH), 7.94 (d, J = 8.55 Hz, 2 H, H4 & H6), 8.49 (s, 1 H, H1′), 8.57 (d, J = 8.55 Hz, 2 H, H1, H8). 13C NMR (101 MHz, CDCl3) ppm 11.5 (C4′), 21.4 (C3′) 125.3 (CH), 125.2 (CH), 125.4 (CH), 125.5, 126.9 (CH), 128.4, 129.1, 130.1 (C1′), 130.6, 157.0 (C2′). HRMS (APCI) calculated for C18H15ClNO2 [M+ + H] 312.0791: found 312.0797.
5.3.7. 9-(2-Nitroethyl)anthracene (12g)
To a stirred solution of (E)-9-(2-nitrovinyl)anthracene (12a) (100 mg, 0.4 mmol) in dichloromethane (10 mL) and isopropanol (2 mL) was added sodium borohydride (60 mg, 1.6 mmol). After 24 h stirring at room temperature, the reaction mixture neutralised using 1 M HCl. The solution was extracted with CH2Cl2, dried with sodium sulphate and solvent removed in vacuo. The product was recrystallized from methanol and diethyl ether as orange crystals, 85 mg (85%), Mp. 147–149 °C. IRVmax (ATR): 3053, 2974 (C-H), 1622, 1493 (C=C), 1546, 1377 (NO2), 1137 (C-N) cm−1. 1H NMR (400 MHz, CDCl3) δ 4.35–4.44 (m, 2 H, CH2), 4.70–4.78 (m, 2 H, CH2), 7.48–7.56 (m, 2 H, 2 x ArH), 7.57–7.65 (m, 2 H, 2 x ArH), 8.06 (d, J = 8.55 Hz, 2 H, 2 x ArH), 8.25 (d, J = 9.16 Hz, 2 H, 2 x ArH), 8.46 (s, 1 H, C10). 13C NMR (101 MHz, CDCl3) ppm 26.1 (C2′), 74.7 (C1′), 122.9 (CH), 125.2 (CH), 126.2, 126.9 (CH), 127.8, 129.6 (CH), 129.9, 131.5. HRMS (APCI) calculated for C16H12NO2 [M+ − H] 250.0874: found 250.0874.
5.4. General Procedure 3: Synthesis of Ethanoanthracenes
To a solution of the appropriate anthracene derivative (1 mmol) in toluene (2 mL) was added the required dienophile e.g., maleic anhydride, appropriate maleimide (1.3 mmol), 1-cyanovinyl acetate, methyl acrylate, ethyl acrylate and cyanoacrylate. The mixture was stirred and heated at 90 °C for 48 h, then cooled to RT and the product was obtained by filtration. The product was sequentially washed with toluene (2 mL) and diethyl ether (2 mL) and recrystallized from toluene.
5.4.1. (E)-9-(2-Nitrovinyl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (13a)
Compound 13a was prepared from (E)-9-(2-nitrovinyl)anthracene 12a (0.25 g, 1 mmol) and maleic anhydride (0.13 g, 1.3 mmol) following general procedure 3. The product was obtained as a colourless solid, 276 mg (80%), Mp. 244–245 °C. IRVmax (KBr): 3030, 2966 (C-H), 1860, 1778 (C=O), 1662 (C=C), 1484, 1467 (C=C), 1525, 1353 (NO2) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.81 (dd, J = 8.85, 3.36 Hz, 1 H, H11), 4.20 (d, J = 9.16 Hz, 1 H, H15), 4.98 (d, J = 3.66 Hz, 1 H, H10), 7.19–7.32 (m, 5 H, 5 x ArH), 7.32–7.40 (m, 1 H, ArH), 7.40–7.48 (m, 1 H,ArH), 7.57 (d, J = 6.71 Hz, 1 H, 1 x ArH), 8.10 (d, J = 14.04 Hz, 1 H, H1′), 8.28 (d, J = 13.43 Hz, 1 H, H2′). 13C NMR (101 MHz, DMSO-d6) ppm 44.2 (C10), 48.8 (C11), 49.2 (C9), 49.2 (C15), 123.1 (CH), 123.6 (CH), 124.6 (CH), 125.4 (CH), 126.7 (CH), 127.3 (CH), 127.4 (CH), 127.7 (CH), 136.3 (C2′), 138.2 (Cq), 138.4 (Cq), 140.5 (Cq), 140.8 (Cq), 145.3 (C1′), 170.1 (Cq, C=O, C12), 170.7 (Cq, C=O, C14). HRMS (APCI) calculated for C20H12NO5 [M+ − H] 346.0721: found 346.0721.
5.4.2. (E)-9-(2-Nitroprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (13b)
Compound 13b was prepared from (E)-9-(2-nitroprop-1-en-1-yl)anthracene 12b (0.26 g, 1 mmol) and maleic anhydride (0.13 g, 1.3 mmol) following general procedure 3. The product was obtained as a colourless solid 259 mg (72%), Mp. 256–258 °C. IRVmax (KBr): 3009, 2960 (C-H), 1831, 1771 (C=O), 1641, 1521 (C=C), 1529, 1390 (NO2), 1333 (CH3) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 1.81 (br. s., 3 H, -CH3), 3.65 (dd, J = 8.85, 2.75 Hz, 1 H, H11), 3.88 (d, J = 8.54 Hz, 1 H, H15), 4.95 (d, J = 2.44 Hz, 1 H, H10), 7.11–7.34 (m, 6 H, 6 x ArH), 7.41 (d, J = 6.71 Hz, 1 H, ArH), 7.56 (d, J = 7.32 Hz, 1 H, ArH), 8.16 (s, 1 H. H1′). 13C NMR (101 MHz, DMSO-d6) ppm 17.2 (C3′), 44.4 (C10), 48.5 (C11), 49.8 (C9), 51.8 (C15), 123.8 (CH), 124.0 (CH), 124.9 (CH), 125.4 (CH), 126.6 (CH), 127.2 (CH), 127.3 (CH), 127.8 (CH), 138.0 (C1′), 138.2 (Cq), 140.3 (Cq), 153.0 (C2′), 170.6 (C12), 170.7 (C14). HRMS (APCI) calculated for C21H14NO5 [M+ − H] 360.877: found 360.0865.
5.4.3. (E)-9-(2-Nitrobut-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (13c)
Compound 13c was prepared from (E)-9-(2-nitrobut-1-en-1-yl)anthracene 12c (0.27 g, 1 mmol) and maleic anhydride (0.13 g, 1.3 mmol) following general procedure 3. The product was obtained as a colourless solid, 336 mg (98%), Mp. 230–233 °C. IRVmax (KBr): 2981, 2943 (C-H), 1837, 1775 (C=O), 1528, 1340 (NO2), 1479 (C=C), 1458 (CH2), 1386 (CH3) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 0.92–1.04 (m, 3 H, -CH3) 2.19 (br. s., 2 H, CH2) 3.69 (d, J = 6.71 Hz, 1 H, H11) 3.93 (d, J = 8.55 Hz, 1 H, H15) 5.01 (d, J = 3.66 Hz, 1 H, H10) 7.19–7.35 (m, 6 H, 6 x ArH) 7.39–7.50 (m, 1 H, ArH) 7.52–7.66 (m, 1 H, ArH) 8.08 (s, 1 H, C1′). 13C NMR (101 MHz, DMSO-d6) ppm 9.5 (C4′), 23.4 (C3′), 44.6 (C10), 48.5 (C11), 49.9 (C9), 51.2 (C15), 123.3 (CH), 124.6 (CH), 125.4 (CH), 126.1 (CH), 126.5 (CH), 127.2 (CH), 127.3 (CH), 135.2 (C1′), 139.3 (Cq), 156.9 (C2′), 177.1 (C12), 177.3 (C14). HRMS (APCI) calculated for C22H16NO5 [M+ − H] 374.1034: found 374.1044.
5.4.4. (E)-9-Chloro-10-(2-nitrovinyl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (13d)
Compound 13d was prepared from (E)-9-chloro-10-(2-nitrovinyl)anthracene 12d (0.28 g, 1 mmol) and maleic anhydride (0.13 g, 1.3 mmol) following general procedure 3. The product was obtained as a colourless solid 58 mg (15%), Mp. 275–277 °C. IRVmax (ATR): 3049, 2957 (C-H), 1706 (C=O), 1599 (C=C), 1456, 1420 (C=C), 1530, 1350 (NO2) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.96 (d, J = 9.16 Hz, 1 H, H11), 4.44 (d, J = 9.16 Hz, 1 H, H15), 7.33–7.51 (m, 6 H, 6 x ArH), 7.71 (d, J = 7.32 Hz, 1 H, 1 x ArH), 7.83 (d, J = 7.94 Hz, 1 H, 1 x ArH), 8.12 (d, J = 14.04 Hz, 1 H, H1′), 8.28 (d, J = 14.04 Hz, 1 H, H2′). 13C NMR (101 MHz, DMSO-d6) ppm 48.3 (C10), 50.8 (C11), 55.0 (C15), 69.4 (C9), 122.5 (CH), 123.0 (CH), 123.4 (CH), 123.7 (CH), 127.8 (CH), 128.0 (CH), 128.3 (CH), 128.7 (CH), 135.1 (C2′), 136.7, 136.9, 139.4, 145.8 (C1′), 166.7 (C12), 168.8 (C14). HRMS (APCI) calculated for C20H13ClNO5 [M+ + H] 382.0482: found 382.0478.
5.4.5. (E)-9-Chloro-10-(2-nitroprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (13e)
Compound 13e was prepared from (E)-9-chloro-10-(2-nitroprop-1-en-1-yl)anthracene (0.298 g, 1 mmol) 12e and maleic anhydride (0.13 g, 1.3 mmol) following general procedure 3. The product was obtained as a colourless solid, 72 mg (18%), Mp. 270–272 °C. IRVmax (ATR): 3012, 2977 (C-H), 1781 (C=O), 1656 (C=C), 1474, 1459 (C=C), 1516, 1334 (NO2) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 1.82 (br. s., 3 H, CH3), 3.81 (d, J = 9.16 Hz, 1 H, H15), 4.18 (d, J = 8.55 Hz, 1 H, H11), 7.33–7.49 (m, 6 H, 6 x ArH), 7.74 (d, J = 7.32 Hz, 1 H, 1 x ArH), 7.86 (d, J = 7.94 Hz, 1 H, 1 x ArH), 8.20 (s, 1 H, H1′). 13C NMR (101 MHz, DMSO-d6) ppm 17.4 (C3′), 49.0 (C10), 53.1 (C11), 54.9 (C15), 69.7 (C9), 122.9 (CH), 123.1 (CH), 124.0 (CH), 127.8 (CH), 127.9 (CH), 128.3 (CH), 128.5 (CH), 136.7, 136.9, 139.0, 153.4 (C2′), 166.8 (C12, C14). HRMS (APCI) calculated for C21H14NClO5 [M+] 395.0561: found 395.0553.
5.4.6. (E)-9-Chloro-10-(2-nitrobut-1-en-1-yl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (13f)
Compound 13f was prepared from (E)-9-chloro-10-(2-nitrobut-1-en-1-yl)anthracene 12f (0.312 g, 1 mmol) and maleic anhydride (0.13 g, 1.3 mmol) following general procedure 3. The product was obtained as a colourless solid 224 mg (55%), Mp. 259–263 °C. IRVmax (KBr): 3065, 2951 (C-H), 1711 (C=O), 1610 (C=C), 1454 (C=C), 1530, 1346 (NO2) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 0.99 (br. s., 3 H, CH3) 2.02–2.23 (m, 2 H, CH2) 3.80 (br. s., 1 H, H15) 4.18 (d, J = 8.55 Hz, 1 H, H11) 7.29–7.50 (m, 6 H, 6 x ArH) 7.75 (d, J = 7.32 Hz, 1 H, 1 x ArH) 7.87 (d, J = 7.32 Hz, 1 H, 1 x ArH) 8.06 (br. s., 1 H, H1′). 13C NMR (101 MHz, DMSO-d6) ppm 55.0 (C10), 56.4 (C15, C11), 69.7 (C9), 115.6 (CH), 122.9 (CH), 123.1 (CH), 123.8 (CH), 127.9 (CH), 128.0 (CH), 128.5 (C1′), 137.1, 149.7 (C2′), 157.7 (C15, C11). HRMS (APCI) calculated for C22H16ClNO5 [M+] 409.0717: found 409.0717.
5.4.7. (E)-9-(2-Nitrovinyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (13g)
Compound 13g was prepared from (E)-9-(2-nitrovinyl)anthracene 12a (0.25 g, 1 mmol) and maleimide (0.13 g, 1.3 mmol) following general procedure 3. The product was obtained as a colourless solid 172 mg (50%), Mp. 176–178 °C. IRVmax (KBr): 3551 (N-H), 3058, 2963 (Ar C-H), 1722 (C=O), 1527, 1354 (NO2), 1167 (N-C) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.28 (br. s., 1 H, H11), 3.70 (d, J = 8.54 Hz, 1 H, H15), 4.79 (br. s., 1 H, H10), 6.99–7.36 (m, 7 H, 7 x ArH), 7.51 (d, J = 6.71 Hz, 1 H, ArH), 8.04 (d, J = 14.04 Hz, 1 H, H1′), 8.24 (d, J = 13.43 Hz, 1 H, H2′), 10.89 (br. s., 1 H, NH). 13C NMR (101 MHz, DMSO-d6) ppm 44.3 (C10), 48.6 (C11) 48.7 (C15), 49.2 (C9), 122.9 (CH), 123.3 (CH), 124.3 (CH), 125.3 (CH), 126.3 (CH), 126.8 (CH), 127.0 (CH), 127.2 (CH), 137.5 (C2′), 138.5 (Cq), 138.7 (Cq), 141.4 (Cq), 141.7 (Cq), 145.1 (C1′), 176.9 (C12), 177.1 (C14). HRMS (APCI) calculated for C20H13N2O4 [M+ − H] 345.0881: found 345.0896.
5.4.8. (E)-9-(2-Nitroprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (13h)
Compound 13h was prepared from (E)-9-(2-nitroprop-1-en-1-yl)anthracene 12b (0.26 g, 1 mmol) and maleimide (0.13 g, 1.3 mmol) following general procedure 3. The product was obtained as a colourless solid, 348 mg (97%), Mp. 296–300 °C. IRVmax (KBr): 3368 (N-H), 3090, 3008 (C-H), 1520, 1350 (NO2), 1771 (C=O), 1346 (CH3) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 1.80 (br. s., 3 H, CH3), 3.17 (dd, J = 8.54, 3.05 Hz, 1 H, H11), 3.35 (d, J = 7.93 Hz, 1 H, H15), 4.79 (d, J = 3.66 Hz, 1 H, H10), 7.13–7.27 (m, 6 H, 6 x ArH), 7.33 (d, J = 6.71 Hz, 1 H, ArH), 7.52 (d, J = 7.32 Hz, 1 H, ArH), 8.28 (s, 1 H, H1′), 10.83 (s, 1 H, NH). 13C NMR (101 MHz, DMSO-d6) ppm 17.2 (C3′), 44.5 (C10), 48.3 (C11), 49.8 (C9), 51.1 (C15), 123.4 (CH), 124.6 (CH), 125.3 (CH), 126.2 (CH), 126.6 (CH), 127.0 (CH), 127.2 (CH), 131.9 (C1′), 138.4 (Cq), 139.0 (Cq), 141.2 (Cq), 152.4 (C2′), 177.1 (C12), 177.4 (C14). HRMS (APCI) calculated for C21H16N2O4 [M+ − H] 359.1037: found 359.1024.
5.4.9. (E)-9-(2-Nitrobut-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (13i)
Compound 13i was prepared from (E)-9-(2-nitrobut-1-en-1-yl)anthracene 12c (0.27 g, 1 mmol) and maleimide (0.13 g, 1.3 mmol) according following general procedure 3. The product was isolated as a colourless solid, 283 mg (76%), Mp. 296–298 °C. IRVmax (KBr): 3182 (N-H), 3015, 2988 (C-H), 1707 (C=O), 1523, 1348 (NO2), 1457 (CH2) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.00 (br. s., 3 H, CH3), 2.12 (br. s., 2 H, CH2), 3.17 (br. s., 1 H, H11), 3.35 (br. s., 1 H, H15), 4.81 (d, J = 2.44 Hz, 1 H, H10), 7.09–7.27 (m, 6 H, 6 x ArH), 7.28–7.40 (m, 1 H, ArH), 7.53 (d, J = 6.10 Hz, 1 H, ArH), 8.13 (s, 1 H, H1′), 10.84 (s, 1 H). 13C NMR (101 MHz, DMSO-d6) ppm 9.5 (C4′), 23.4 (C3′), 44.6 (C10), 48.5 (C11), 49.8 (C9), 51.2 (C15), 123.3 (CH), 124.6 (CH), 125.4 (CH), 126.1 (CH), 126.5 (CH), 127.2 (CH), 127.3 (CH), 135.2, 139.2 (C1′), 156.9 (C2′), 177.1 (C12), 177.3 (C14). HRMS (APCI) calculated for C22H17N2O4 [M+ − H] 373.1194: found 373.1176.
5.4.10. (E)-9-Chloro-10-(2-nitrovinyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (13j)
Compound 13j was prepared from (E)-9-chloro-10-(2-nitrovinyl)anthracene 12d (0.28 g, 1 mmol) and maleimide (0.13 g, 1.3 mmol) as outlined in general procedure 3. The product was isolated as a colourless solid, 95 mg (25%), Mp. >300 °C. IRVmax (ATR): 3050, 2963 (C-H), 1707 (C=O), 1599 (C=C), 1457, 1419 (C=C), 1518, 1349 (NO2) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.51 (d, J = 8.55 Hz, 1 H, H11), 3.95 (d, J = 8.55 Hz, 1 H, H15), 7.25–7.49 (m, 6 H, 6 x ArH), 7.67 (d, J = 7.32 Hz, 1 H, 1 x ArH), 7.80 (d, J = 7.32 Hz, 1 H, 1 x ArH), 8.08 (d, J = 14.04 Hz, 1 H, H1′), 8.27 (d, J = 14.04 Hz, 1 H, H2′), 11.09 (br. s., 1 H, NH). 13C NMR (101 MHz, DMSO-d6) ppm 48.3 (C10), 50.4 (C11), 54.1 (C15), 70.2 (C9), 122.3 (CH), 123.0 (CH), 123.1 (CH), 123.3 (CH), 127.4 (CH), 127.6 (CH), 127.7, 128.1 (CH), 136.3 (C2′), 137.1, 137.3, 140.1, 140.4, 145.6 (C1′), 173.4 (C12), 175.5 (C14). HRMS (APCI) calculated for C20H12ClN2O4 [M+ − H] 379.0491: found 379.0478.
5.4.11. (E)-9-Chloro-10-(2-nitroprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (13k)
Compound 13k was prepared from (E)-9-chloro-10-(2-nitroprop-1-en-1-yl)anthracene 12e (0.298 g, 1 mmol) and maleimide (0.13 g, 1.3 mmol) as described in general procedure 3. The product was isolated as a colourless solid 197 mg (50%), Mp. 295–297 °C. IRVmax (ATR): 3368 (N-H), 3067, 2942 (C-H), 1711 (C=O), 1610 (C=C), 1455 (C=C), 1521, 1344 (NO2) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 1.81 (br. s., 3 H, CH3), 3.38 (d, J = 8.55 Hz, 1 H, H15), 3.60 (d, J = 7.94 Hz, 1 H, H11), 7.28–7.47 (m, 6 H, 6 x ArH), 7.70 (d, J = 7.94 Hz, 1 H, 1 x ArH), 7.84 (d, J = 7.94 Hz, 1 H, 1 x ArH), 8.32 (s, 1 H, H1′), 11.04 (s, 1 H, NH). 13C NMR (101 MHz, DMSO-d6) ppm 17.4 (C3′), 49.0 (C10), 52.6 (C11), 53.9 (C15), 70.4 (C9), 122.7 (CH), 123.0 (CH), 123.5 (CH), 124.1, 127.5 (CH), 127.6 (CH), 127.7 (CH), 127.9 (CH), 130.8, 135.1, 137.1, 137.6, 140.1, 152.8 (C2′), 173.5 (C12), 175.9 (C14). HRMS (APCI) calculated for C21H16ClN2O4 [M+ + H] 395.0799: found 395.0787.
5.4.12. (E)-9-Chloro-10-(2-nitrobut-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (13l)
Compound 13l was prepared from (E)-9-chloro-10-(2-nitrobut-1-en-1-yl)anthracene 12f (0.312 g, 1 mmol) and maleimide (0.13 g, 1.3 mmol) as described in general procedure 3. The product was isolated as a colourless solid 224 mg (55%), Mp. 294–298 °C. IRVmax (ATR): 3369 (N-H), 3022, 2942 (C-H), 1710 (C=O), 1608 (C=C), 1455, 1426 (C=C), 1519, 1342 (NO2) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 0.98 (br. s., 3 H, CH3), 2.11 (br. s., 2 H, CH2), 3.36 (d, J = 7.32 Hz, 1 H, H15), 3.48–3.76 (m, 1 H, H11), 7.26 (m, 1 H, 1 x ArH), 7.30–7.51 (m, 5 H, 5 x ArH), 7.71 (d, J = 7.32 Hz, 1 H, 1 x ArH), 7.85 (d, J = 7.94 Hz, 1 H, 1 x ArH), 8.16 (s, 1 H, H1′), 11.04 (br. s., 1 H, NH). 13C NMR (101 MHz, DMSO-d6) ppm 9.4 (C4′), 23.5 (C3′), 49.2 (C10), 52.6 (C11), 54.0 (C15), 70.4 (C9), 122.7 (CH), 123.1 (CH), 123.4 (CH), 127.5 (CH), 127.6 (CH), 127.8 (CH), 130.5, 137.8, 157.2 (C2′), 173.5 (C12), 175.9 (C14). HRMS (APCI) calculated for C22H18ClN2O4 [M+ − H] 409.0955: found 409.0974.
5.4.13. (E)-13-Methyl-9-(2-nitrovinyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (13m)
(E)-9-(2-nitrovinyl)anthracene 12a (0.25 g, 1 mmol) was reacted with 1-methyl-1H-pyrrole-2,5-dione 11r (0.144 g, 1.3 mmol) as described in general procedure 3 and isolated as a yellow solid 198 mg (55%), Mp. 267–268 °C. IRVmax (ATR): 3070, 2951 (C-H), 1713 (C=O), 1594 (C=C), 1481, 1450 (C=C), 1531, 1349 (NO2) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 2.35 (s, 3 H, CH3), 3.38 (dd, J = 8.24, 2.75 Hz, 1 H, H11), 3.78 (d, J = 8.55 Hz, 1 H, H15), 4.87 (d, J = 2.44 Hz, 1 H, H10), 7.15–7.36 (m, 7 H, 7 x ArH), 7.56 (d, J = 6.71 Hz, 1 H, 1 x ArH), 8.11 (d, J = 13.43 Hz, 1 H, H1′), 8.29 (d, J = 13.43 Hz, 1 H, H2′). 13C NMR (101 MHz, DMSO-d6) ppm 24.0 (CH3), 44.4 (C10), 47.5 (C11), 47.6 (C15), 49.3 (C9), 122.8 (CH), 123.4 (CH), 124.4 (CH), 125.1 (CH), 126.4 (CH), 126.8 (CH), 127.1 (CH), 127.2 (CH), 137.4 (CH), 138.2, 138.4, 141.1, 141.5, 145.1 (C1′), 175.4 (C12), 175.8 (C14). HRMS (APCI) calculated for C21H17N2O4 [M+ + H] 361.1188: found 361.1180.
5.4.14. (E)-13-(Hydroxymethyl)-9-(2-nitrovinyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (13n)
Compound 13n was prepared from (E)-9-(2-nitrovinyl)anthracene 12a 0.25 g (1 mmol) and 1-(hydroxymethyl)-1H-pyrrole-2,5-dione 11q 0.165 g (1.3 mmol) as described in general procedure 3 and isolated as a colourless solid, 301 mg (80%), Mp. 230–232 °C. IRVmax (KBr): 3463 (OH), 2964, 2899 (C-H), 1778 (C=O), 1698 (C=C), 1523 (NO), 1482, 1456 (C=C), 1198 (C-N stretch) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.43 (dd, J = 8.55, 3.05 Hz, 1 H, H11), 3.85 (d, J = 8.55 Hz, 1 H, H15), 4.19–4.31 (m, 2 H, CH2), 4.88 (d, J = 3.05 Hz, 1 H, H10), 6.12 (t, J = 7.02 Hz, 1 H, OH), 7.14–7.35 (m, 7 H, 7 x ArH), 7.57 (d, J = 7.32 Hz, 1 H, 1 x ArH), 8.13 (d, J = 14.04 Hz, 1 H, H1′), 8.30 (d, J = 14.04 Hz, 1 H, H2′). 13C NMR (101 MHz, DMSO-d6) ppm 44.4 (C10), 47.4 (C11), 47.4 (C15), 49.3 (C9), 60.2 (CH2), 122.8 (CH), 123.3 (CH), 124.4 (CH), 125.2 (CH), 126.4 (CH), 126.8 (CH), 127.1 (CH), 127.2 (CH), 137.4 (C2′), 138.2, 138.4, 141.3, 141.7, 145.1 (C1′), 174.9 (C12), 175.3 (C14). HRMS (APCI) calculated for C21H17N2O5 [M+ + H] 377.1137: found 377.1138.
5.4.15. 9-(2-Nitroethyl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione (14a)
Compound 14a was prepared from 9-(2-nitroethyl)anthracene 12a (0.25 g, 1 mmol) and maleic anhydride (0.13 g, 1.3 mmol) according to general procedure 3 to give the product as a colourless solid 150 mg (43%), Mp. 242–244 °C. IRVmax (ATR): 3065, 2974 (C-H), 1773 (C=O), 1581 (C=C), 1469, 1457 (C=C), 1546, 1338 (NO2) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.33–3.52 (m, 2 H, CH2), 3.60 (d, J = 9.16 Hz, 1 H, H11), 3.78 (d, J = 9.16 Hz, 1 H, H15), 4.88 (br. s., 1 H, H10), 5.06 (t, J = 14.65 Hz, 1 H, H1′a), 5.24 (t, J = 10.38 Hz, 1 H, H1′b), 7.25 (quin, J = 7.02 Hz, 4 H, 4 x ArH), 7.33–7.43 (m, 2 H, 2 x ArH), 7.53 (d, J = 6.71 Hz, 1 H, 1 x ArH), 7.49 (d, J = 7.32 Hz, 1 H, 1 x ArH). 13C NMR (101 MHz, DMSO-d6) ppm 25.6 (C1′), 44.4 (C10), 46.3 (C11), 48.4 (C9), 49.0 (C15), 72.8 (C2′), 122.6 (CH), 122.9 (CH), 125.3 (CH), 125.5 (CH), 127.1 (CH), 127.1, 127.7 (CH), 127.7, 139.7, 140.7, 141.3, 142.4, 170.9 (C12), 171.5 (C14). HRMS (APCI) calculated for C20H16NO5 [M+ + H] 350.1028: found 350.1030.
5.4.16. 9-(2-Nitroethyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (14b)
Compound 14b was prepared from 9-(2-nitroethyl)anthracene 11b (0.25 g, 1 mmol) and maleimide (0.13 g, 1.3 mmol) according to general procedure 3 to give the product as a colourless solid, 157 mg (45%), Mp. 295–297 °C. IRVmax (ATR): 3049, 2936 (C-H), 1702 (C=O), 1599 (C=C), 1456, 1419 (C=C), 1548, 1349 (NO2) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.16 (d, J = 8.55 Hz, 1 H, H2′a), 3.23–3.31 (m, 2 H, H2′b, H11), 3.44–3.62 (m, 1 H, H15), 4.72 (br. s., 1 H, C10), 5.15 (d, J = 7.32 Hz, 2 H, CH2), 7.11–7.37 (m, 6 H, 6 x ArH), 7.48 (t, J = 8.55 Hz, 2 H, 2 x ArH), 10.89 (br. s., 1 H, NH). 13C NMR (101 MHz, DMSO-d6) ppm 25.5 (C1′), 44.1 (C10), 45.6 (C11), 46.9 (C15), 48.3 (C9), 72.7 (C2′), 121.9, 122.1 (CH), 124.5 (CH), 124.9 (CH), 126.3 (CH), 126.3 (CH), 126.6 (CH), 126.7 (CH), 139.6, 140.8, 142.0, 142.8, 177.3 (C12), 177.6 (C14). HRMS (APCI) calculated for C20H17N2O5 [M+ + H] 349.1188: found 349.1181.
5.4.17. 9-(2-Nitroethyl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (14c)
Compound 14c was prepared from 9-(2-nitroethyl)anthracene 12b (0.25 g, 1 mmol) and phenylmaleimide (0.224 g, 1.3 mmol) according to general procedure 3, to give the product as a colourless solid, 127 mg (30%), Mp. 248–250 °C. IRVmax (ATR): 2964, 2939 (C-H), 1710 (C=O), 1595 (C=C), 1491, 1456 (C=C), 1541, 1388 (NO2) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.37 (d, J = 8.55 Hz, 2 H, H2′a), 3.49–3.60 (m, 2 H, H2′b, H15), 4.87 (br. s., 1 H, C10), 5.10–5.31 (m, 2 H, CH2), 6.44 (d, J = 5.49 Hz, 2 H, 2 x ArH), 7.20–7.42 (m, 9 H, 9 x ArH), 7.57 (d, J = 7.32 Hz, 1 H, 1 x ArH), 7.54 (d, J = 7.32 Hz, 1 H, 1 x ArH). 13C NMR (101 MHz, DMSO-d6) ppm 25.5 (C1′), 44.6 (C10), 46.2 (C11), 46.2 (C9), 47.4 (C15), 72.7 (C2′), 122.0 (CH), 122.3 (CH), 124.7 (CH), 125.0 (CH), 126.5 (CH), 126.5 (CH), 126.7 (CH), 126.8 (CH), 128.5 (CH), 128.9 (CH), 131.6, 139.4, 140.5, 141.6, 142.4, 175.0 (C12), 175.5 (C14). HRMS (APCI) calculated for C26H21NO4 [M+ + H] 425.1501: found 425.1512.
5.4.18. 13,13′-(Hexane-1,6-diyl)bis(9-((E)-2-nitrovinyl)-9,10-dihydro-9,10[3,4]epipyrroloanthracene-12,14-dione) (15)
Compound 15 was prepared from (E)-9-(2-nitrovinyl)anthracene 12a (0.25 g, 1 mmol) and the required maleimide 11s (0.5 mmol) following the general procedure 3 to afford the product as a colourless solid, 39 mg (10%), Mp. 186–188 °C. IRVmax (KBr): 3050, 2961 (Ar C-H), 1699 (C=O), 1533, 1358 (NO2), 1203 (N-C) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 0.43 (br. s., 4 H, 2 x CH2), 0.47–0.57 (m, 4 H, 2 x CH2), 2.86–2.94 (m, 4 H, 2 x NCH2), 3.36 (dd, J = 8.44, 3.30 Hz, 2 H, 2 x H11), 3.76 (dd, J = 8.44, 1.83 Hz, 2 H, 2 x H15), 4.86–4.92 (m, 2 H, 2 x H10), 7.18–7.31 (m, 12 H, 12 x ArH), 7.31–7.38 (m, 2 H, 2 x ArH), 7.54–7.59 (m, 2 H, 2 x ArH), 8.12 (d, J = 13.94 Hz, 2 H, 2 x H1′), 8.27–8.34 (m, 2 H, 2 x H2′). 13C NMR (101 MHz, DMSO-d6) ppm 25.14 (CH2), 26.17 (CH2), 37.67 (NCH2), 44.45 (C10, C10′), 47.24 (C11, C11′), 47.36 (C15, C15′), 49.35 (C9, C9′), 122.87 (CH), 123.32 (CH), 124.35 (CH), 125.19 (CH), 126.39 (CH), 126.79 (CH), 127.07 (CH), 137.33 (C2′), 138.44, 138.54, 141.29, 141.61, 145.15 (C1′), 175.35 (C11), 175.73 (C15). HRMS (APCI) calculated for C46H39N4O8 [M+ + H] 775.2768: found 775.2771.
5.4.19. (E)-9-(2-Nitrovinyl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (16a)
Compound 16a was prepared from (E)-9-(2-nitrovinyl)anthracene 12a (0.25 g, 1 mmol) and phenylmaleimide (0.23 g, 1.3 mmol) as described in general procedure 3, to give the product as a colourless solid 126 mg (30%), Mp. 256–257 °C. IRVmax (KBr): 3035, 2961 (C-H), 1709 (C=O), 1529, 1349 (NO2), 1596, 1457 (C=C), 1201 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.51 (m, J = 8.24, 2.75 Hz, 1 H, H11), 3.91 (d, J = 8.54 Hz, 1 H, H15), 4.86–4.98 (m, 1 H, H10), 6.28–6.46 (m, 2 H, H6″, H2″), 7.14–7.34 (m, 10 H, 10 x ArH), 7.58 (d, J = 6.71 Hz, 1 H, ArH), 8.08 (d, J = 14.04 Hz, 1 H, H1′), 8.28 (d, J = 14.04 Hz, 1 H, H2′). 13C NMR (101 MHz, DMSO-d6) ppm 44.8 (C10), 47.6 (C11), 47.9 (C15), 49.6 (C9), 123.1 (CH), 123.5 (CH), 124.5 (CH), 125.3 (CH), 126.5 (CH), 126.97 (CH), 127.16 (CH), 127.31 (CH), 128.6 (CH), 128.8 (CH), 131.5 (CH), 137.3 (C2′), 138.5 (Cq), 140.9 (Cq), 141.3 (Cq), 145.2 (C1′), 174.7 (C12), 175.1 (C14). HRMS (APCI) calculated for C26H19N2O4 [M+ + H] 423.1345: found 423.1364.
5.4.20. (E)-13-(4-Chlorophenyl)-9-(2-nitrovinyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (16b)
Compound 16b was prepared from (E)-9-(2-nitrovinyl)anthracene (0.25 g, 1 mmol) and 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione 11c (0.27 g, 1.3 mmol) following general procedure 3, and afforded the product as a colourless solid 233 mg (51%), Mp. 238–241 °C. IRVmax (KBr): 3035, 2946 (C-H), 1714 (C=O), 1659 (C=C), 1537, 1493 (C=C), 1191 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.55 (dd, J = 8.54, 3.05 Hz, 1 H, H11), 3.95 (d, J = 7.93 Hz, 1 H, H15), 4.97 (d, J = 3.05 Hz, 1 H, H10), 6.48 (d, J = 8.54 Hz, 2 H, H6″, H2″), 7.21–7.47 (m, 10 H, 10 x ArH), 7.61 (d, J = 6.71 Hz, 1 H, ArH), 8.12 (d, J = 14.04 Hz, 1 H, H1′), 8.32 (d, J = 14.04 Hz, 1 H, H2′). 13C NMR (101 MHz, DMSO-d6) ppm 44.8 (C10), 47.7 (C11), 48.0 (C15), 49.6 (C9), 123.0 (CH), 123.5 (CH), 124.5 (Cq), 125.3 (CH), 126.5 (CH), 127.0 (CH), 127.2 (Cq), 127.3 (CH), 128.1 (CH), 129.0 (CH), 130.3 (Cq), 133.1 (Cq), 137.2 (C2′), 138.4 (Cq), 138.5 (Cq), 140.9 (Cq), 141.2 (Cq), 145.2 (C1′), 174.5 (C12), 174.9 (C14). HRMS (APCI) calculated for C26H18ClN2O4 [M+ + H] 457.0955: found 457.0968.
5.4.21. (E)-13-(4-Methoxyphenyl)-9-(2-nitrovinyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (16c)
Compound 16c was prepared from (E)-9-(2-nitrobut-1-en-1-yl)anthracene (0.26 g, 1 mmol) and 1-(4-methoxyphenyl)-1H-pyrrole-2,5-dione (0.243 g, 1.3 mmol) following general procedure 3, and afforded the product as a tan solid, 317 mg (70%), Mp. 240–242 °C. IRVmax (KBr): 2988, 2938 (C-H), 1717 (C=O), 1515, 1349. (NO2), 1657, 1607 (C=C), 1250 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.51 (d, J = 6.71 Hz, 1 H, H11), 3.71 (br. s., 3 H, CH3), 3.91 (d, J = 7.93 Hz, 1 H, H15), 4.95 (br. s., 1 H, H10), 6.32 (d, J = 7.32 Hz, 2 H, H3″, H5″), 6.87 (d, J = 7.93 Hz, 2 H, H6″, H2″), 7.23–7.46 (m, 7 H, 7 x ArH), 7.61 (d, J = 6.10 Hz, 1 H, 1 x ArH), 8.11 (d, J = 13.43 Hz, 1 H, H2′), 8.31 (d, J = 14.04 Hz, 1 H, H1′). 13C NMR (101 MHz, DMSO-d6) ppm 44.8 (C10), 47.5 (C11), 47.8 (C15), 49.6 (C9), 55.3 (OCH3), 114.1, 123.1, 123.5, 124.0, 124.5, 125.3, 126.5, 126.9, 127.2, 127.3, 127.6, 137.3 (C2′), 138.5, 138.6, 141.0, 141.3, 145.2 (C1′), 159.0 (C4″), 174.9 (C12), 175.3 (C14). HRMS (APCI) calculated for C27H21N2O5 [M+ + H] 453.1450: found 453.1448.
5.4.22. (E)-9-Chloro-10-(2-nitrovinyl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (16d)
Compound 16d was prepared from (E)-9-chloro-10-(2-nitrovinyl)anthracene 12d (0.28 g, 1 mmol) and phenylmaleimide (0.22 g, 1.3 mmol) as described in general procedure 3, and gave the product as a colourless solid, 69 mg (15%). Mp. 263–264 °C. IRVmax (KBr): 3125, 3068 (C-H), 1705 (C=O), 1596 (C=C), 1494, 1453 (C=C), 1528, 1347 (NO2) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.71 (d, J = 8.55 Hz, 1 H, H11), 4.16 (d, J = 8.55 Hz, 1 H, H15), 6.38–6.49 (m, 2 H, 2 x ArH), 7.34 (br. s., 3 H, 3 x ArH), 7.37–7.52 (m, 6 H, 6 x ArH), 7.73 (d, J = 7.32 Hz, 1 H, 1 x ArH), 7.88 (d, J = 7.32 Hz, 1 H, 1 x ArH), 8.13 (d, J = 14.04 Hz, 1 H, H1′), 8.33 (d, J = 14.04 Hz, 1 H, H2′). 13C NMR (101 MHz, DMSO-d6) ppm 48.8 (C10), 49.7 (C11), 53.3 (C15), 70.4 (C9), 122.6 (CH), 123.0 (CH), 123.3 (CH), 123.6 (CH), 126.4 (CH), 127.6 (CH), 127.8 (CH), 127.9 (CH), 128.3 (CH), 128.7 (CH), 128.9 (CH), 129.0, 131.4, 136.2 (CH), 136.9 (C2′), 137.2, 139.7, 139.9, 145.7 (C1′), 171.4 (C12), 173.4 (C14). HRMS (APCI) calculated for C20H18ClN2O4 [M+ + H] 457.0955: found 457.0946.
5.4.23. (E)-13-(4-Fluorophenyl)-9-(2-nitrovinyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (16e)
Compound 16e was prepared from (E)-9-(2-nitrovinyl)anthracene 12a (0.25 g, 1 mmol) and 1-(4-fluorophenyl)-1H-pyrrole-2,5-dione 11m (0.248 g, 1.3 mmol) following general procedure 3, and afforded the product as a colourless solid, 120 mg (27%), Mp. 242–243 °C. IRVmax (KBr): 3099, 2892 (C-H), 1711 (C=O), 1688 (C=C), 1505, 1456 (C=C), 1156 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.55 (dd, J = 7.94, 3.05 Hz, 1 H, H11), 3.95 (d, J = 8.55 Hz, 1 H, H15), 4.97 (d, J = 3.05 Hz, 1 H, H10), 6.47 (dd, J = 8.55, 4.88 Hz, 2 H, 2 x ArH), 7.14–7.44 (m, 9 H, 9 x ArH), 7.61 (d, J = 7.32 Hz, 1 H, 1 x ArH), 8.12 (d, J = 14.04 Hz, 1 H, H1′), 8.33 (d, J = 14.04 Hz, 1 H, H2′). 13C NMR (101 MHz, DMSO-d6) ppm 45.2 (C10), 48.1 (C11), 48.4 (C15), 50.1 (C9), 116.0, 115.8, 123.5 (CH), 124.0 (CH), 125.0 (CH), 125.7 (CH), 127.0 (CH), 127.4 (CH), 127.6 (CH), 127.8 (CH), 128.9 (CH), 129.1 (CH), 135.1 (CH), 137.7 (C2′), 138.9, 139.0, 141.3, 141.7, 145.7 (C1′), 162.7, 160.3, 175.1 (C12), 175.5 (C14). HRMS (APCI) calculated for C26H18FN2O4 [M+ + H] 441.1251: found 441.1240.
5.4.24. (E)-9-(2-Nitrovinyl)-13-(3,4,5-trimethoxyphenyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (16f)
Compound 16f was prepared from (E)-9-(2-nitrovinyl)anthracene 12a (0.25 g, 1 mmol) and 1-(3,4,5-trimethoxyphenyl)-1H-pyrrole-2,5-dione 11o (0.341 g, 1.3 mmol) as outlined in general procedure 3, to obtain the product as a yellow solid, 52 mg (10%), Mp. 270–272 °C. IRVmax (ATR): 3049, 2964 (C-H), 1707 (C=O), 1660 (C=C), 1457, 1420 (C=C), 1518, 1349 (NO2), 1196 (C-N) cm−1. 1H NMR (400 MHz, CDCl3) δ 3.43–3.56 (m, 2 H, H11, H15), 3.71 (s, 6 H, 2 x OCH3), 3.78 (s, 3 H, p-OCH3), 4.90–5.03 (m, 1 H, H10), 5.58 (s, 2 H, H6″, H2″), 7.27–7.37 (m, 6 H, 6 x ArH), 7.43–7.55 (m, 2 H, 2 x ArH), 7.91 (d, J = 14.04 Hz, 1 H, H1′), 8.30 (d, J = 14.04 Hz, 1 H, H2′). 13C NMR (101 MHz, CDCl3) ppm 45.8 (C10), 48.0 (C11), 48.2 (C15), 50.4 (C9), 56.1 (C3″, C5″), 60.7 (C4″), 103.9 (C6″, C2″), 123.3 (CH), 123.4 (CH), 124.6 (CH), 125.9 (CH), 126.4 (CH), 127.2 (CH), 127.4 (CH), 127.7 (CH), 127.9 (CH), 136.7 (C2′), 138.2, 138.5, 140.4, 140.8 (C1′), 144.7, 153.5, 174.3 (C12), 174.9 (C14). HRMS (APCI) calculated for C29H25N2O7 [M+ + H] 513.1662: found 513.1643.
5.4.25. (E)-13-(4-Bromophenyl)-9-(2-nitrovinyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (16g)
Compound 16g was prepared from (E)-9-(2-nitrovinyl)anthracene 12a (0.25 g, 1 mmol) and 1-(4-bromophenyl)-1H-pyrrole-2,5-dione 11n (0.325 g, 1.3 mmol) as outlined in general procedure 3, to obtain the product as a tan solid, 330 mg (66%), Mp. 267–268 °C. IRVmax (KBr): 3059, 2965 (C-H), 1701 (C=O), 1524, 1351 (NO2), 1537, 1493 (C=C), 1174 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.55 (dd, J = 8.55, 3.05 Hz, 1 H, H11), 3.95 (d, J = 8.55 Hz, 1 H, H15), 4.97 (d, J = 3.05 Hz, 1 H, H10), 6.42 (d, J = 8.55 Hz, 2 H, 2 x ArH), 7.20–7.45 (m, 7 H, 7 x ArH), 7.51–7.65 (m, 3 H, 3 x ArH), 8.12 (d, J = 14.04 Hz, 1 H, H1′), 8.32 (d, J = 13.43 Hz, 1 H, H2′). 13C NMR (101 MHz, DMSO-d6) ppm 44.8 (C10), 47.7 (C11), 48.0 (C15), 49.7 (C9), 121.6, 123.0 (CH), 123.5 (CH), 124.5 (CH), 125.3 (CH), 126.5 (CH), 127.0 (CH), 127.2 (CH), 127.3 (CH), 128.4 (CH), 130.7, 131.9 (CH), 137.2 (C2′), 138.4, 138.5, 140.8, 141.2, 145.2 (C1′), 174.5 (C12), 174.8 (C14). HRMS (APCI) calculated for C26H18BrN2O4 [M+ + H] 501.0450: found 501.0468.
5.4.26. Methyl (E)-4-(9-(2-nitrovinyl)-12,14-dioxo-9,10-dihydro-9,10-[3,4]epipyrroloanthracen-13-yl)benzoate (16h)
Compound 16h was prepared from (E)-9-(2-nitrovinyl)anthracene 12a (0.25 g, 1 mmol) and methyl 4-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)benzoate 11k (0.3 g, 1.3 mmol) as outlined in general procedure 3, to obtain the product as a colourless solid, 288 mg (60%), Mp. 254–255 °C. IRVmax (KBr): 2989, 2901 (C-H), 1705 (C=O), 1524 (NO), 1459, 1433 (C=C), 1115 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.58 (dd, J = 8.24, 3.36 Hz, 1 H, H11), 3.83 (s, 3 H, OCH3), 3.97 (d, J = 8.55 Hz, 1 H, H15), 4.98 (d, J = 3.05 Hz, 1 H, H10), 6.66 (m, J = 8.55 Hz, 2 H, H6″, H2″), 7.24–7.34 (m, 5 H, 5 x ArH), 7.39 (td, J = 8.24, 4.88 Hz, 2 H, 2 x ArH), 7.62 (d, J = 6.71 Hz, 1 H, 1 x ArH), 7.92 (m, J = 8.55 Hz, 2 H, H3″, H5″), 8.13 (d, J = 14.04 Hz, 1 H, H1′), 8.33 (d, J = 14.04 Hz, 1 H, H2′). 13C NMR (101 MHz, DMSO-d6) ppm 44.8 (C10), 47.7 (C11), 48.1 (C15), 49.7 (C9), 52.3 (OCH3), 123.1 (CH), 123.5 (CH), 124.5 (CH), 125.3 (CH), 126.5 (CH), 126.6 (CH), 127.0 (CH), 127.2 (CH), 127.4 (CH), 129.5, 129.7 (CH), 135.5, 137.2 (C2′), 138.4, 138.5, 140.8, 141.2, 145.3 (C1′), 165.3 (Ester C=O), 174.4 (C12), 174.8 (C14). HRMS (APCI) calculated for C28H21N2O6 [M+ + H] 481.1400: found 481.1384.
5.4.27. (E)-13-(4-Benzoylphenyl)-9-(2-nitrovinyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (16i)
Compound 16i was prepared from (E)-9-(2-nitrovinyl)anthracene 12a (0.25 g, 1 mmol) and 1-(4-benzoylphenyl)-1H-pyrrole-2,5-dione 11f (0.36 g, 1.3 mmol) as outlined in general procedure 3, to obtain the product as a yellow solid, 210 mg (40%), Mp. 232–234 °C. IRVmax (ATR): 3071, 2966 (C-H), 1708 (C=O), 1660 (C=C), 1458, 1448 (C=C), 1529, 1353 (NO2), 1195 (C-N) cm−1.m1H NMR (400 MHz, DMSO-d6) δ 3.60 (dd, J = 8.55, 3.05 Hz, 1 H, H11), 3.99 (d, J = 8.55 Hz, 1 H, H15), 5.00 (d, J = 3.05 Hz, 1 H, H10), 6.69 (d, J = 8.55 Hz, 2 H, H6″, H2″), 7.22–7.35 (m, 5 H, 5 x ArH), 7.35–7.46 (m, 2 H, 2 x ArH), 7.50–7.60 (m, 2 H, 2 x ArH), 7.60–7.74 (m, 6 H, 6 x ArH), 8.14 (d, J = 14.04 Hz, 1 H, H1′), 8.34 (d, J = 14.04 Hz, 1 H, H2′).m13C NMR (101 MHz, DMSO-d6) ppm 44.8 (C10), 47.8 (C11), 48.1 (C15), 49.7 (C9), 123.1 (CH), 123.6 (CH), 124.5 (CH), 125.3 (CH), 126.4 (CH), 126.5 (CH), 127.1 (CH), 127.2 (CH), 127.4 (CH), 128.2, 128.6 (CH), 129.6 (CH), 130.2 (CH), 132.9 (CH), 134.9, 136.5, 136.9, 137.2 (C2′), 138.4, 138.5, 140.9, 141.2, 145.3 (C1′), 174.5 (C12), 174.8 (C14), 194.8 (C=O). HRMS (APCI) calculated for C33H23N2O5 [M+ + H] 527.1607: found 527.1599.
5.4.28. (E)-13-(3,5-Dimethoxyphenyl)-9-(2-nitrovinyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (16j)
Compound 16j was prepared from (E)-9-(2-nitrovinyl)anthracene 12a (0.25 g, 1 mmol) and 1-(3,5-dimethoxyphenyl)-1H-pyrrole-2,5-dione 11l (0.3 g, 1.3 mmol) following general procedure 3. The product was isolated as a colourless solid, 304 mg (63%), Mp. 268–271 °C. IRVmax (KBr): 2966, 2960 (C-H), 1714 (C=O), 1601 (C=C), 1531 (NO), 1474, 1457 (C=C), 1159 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.52 (dd, J = 8.55, 3.05 Hz, 1 H, H11), 3.64 (s, 6 H, 2 x OCH3), 3.92 (d, J = 8.55 Hz, 1 H, H15), 4.96 (d, J = 3.05 Hz, 1 H, H10), 5.49 (d, J = 1.83 Hz, 2 H, H1″, H6″), 6.42–6.49 (m, 1 H, H4″), 7.19–7.45 (m, 7 H, 7 x ArH), 7.61 (d, J = 6.71 Hz, 1 H, 1 x ArH), 8.12 (d, J = 14.04 Hz, 1 H, H1′), 8.32 (d, J = 13.43 Hz, 1 H, H2′). 13C NMR (101 MHz, DMSO-d6) ppm 44.9 (C10), 47.6 (C11), 47.9 (C15), 49.7 (C9), 55.4 (OCH3), 100.5 (C2″, C6″), 105.0 (C4″), 123.2 (CH), 123.5 (CH), 124.5 (CH), 125.3 (CH), 126.5 (CH), 126.9 (CH), 127.2 (CH), 127.3 (CH), 133.2, 137.3 (CH), 138.5, 138.6, 140.9, 141.2, 145.2 (C1′), 160.3 (C3″, C5″), 174.5 (C12), 174.9 (C14). HRMS (APCI) calculated for C28H23N2O6 [M+ + H] 483.1556: found 483.1543.
5.4.29. (E)-13-(3-Chlorophenyl)-9-(2-nitrovinyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (16k)
Compound 16k was prepared from (E)-9-(2-nitrovinyl)anthracene 12a (0.25 g, 1 mmol) and 1-(3-chlorophenyl)-1H-pyrrole-2,5-dione 11h (0.27 g, 1.3 mmol) following general procedure 3. The product was isolated as a tan solid 265 mg (58%), Mp. 258–261 °C. IRVmax (ATR): 3069, 2966 (C-H), 1713 (C=O), 1594 (C=C), 1481, 1434 (C=C), 1537, 1351 (NO2) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.57 (dd, J = 8.29, 3.32 Hz, 1 H, H11), 3.97 (d, J = 8.29 Hz, 1 H, H15), 4.98 (d, J = 3.32 Hz, 1 H, H10), 6.41–6.52 (m, 2 H, 2 x ArH), 7.23–7.42 (m, 9 H, 9 x ArH), 7.56–7.65 (m, 1 H, 1 x ArH), 8.13 (d, J = 13.68 Hz, 1 H, H1′), 8.34 (d, J = 13.68 Hz, 1 H, H2′). 13C NMR (101 MHz, DMSO-d6) ppm 44.8 (C10), 47.7 (C11), 48.1 (C15), 49.7 (C9), 123.1 (CH), 123.6 (CH), 124.5 (CH), 125.3 (CH), 126.4 (CH), 126.4 (CH), 126.5 (CH), 127.0 (CH), 127.2 (CH), 127.3 (CH), 128.7 (CH), 130.6 (CH), 132.8, 132.9, 134.7 (CH), 138.5, 138.5, 140.8, 141.2, 145.3 (C1″), 174.5 (C12), 174.8 (C14). HRMS (APCI) calculated for C26H18ClN2O4 [M+ + H] 457.0955: found 457.0951.
5.4.30. (E)-13-(2-Hydroxyphenyl)-9-(2-nitrovinyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (16l)
Compound 16l was prepared from (E)-9-(2-nitrovinyl)anthracene (0.25 g, 1 mmol) and 1-(2-hydroxyphenyl)-1H-pyrrole-2,5-dione (0.245 g, 1.3 mmol) following general procedure 3. The product was isolated as a colourless solid, 175 mg (40%), Mp. 203–206 °C. IRVmax (KBr): 3030, 2969 (C-H), 1710 (C=O), 1663 (C=C), 1527 (NO), 1495, 1456 (C=C), 1192 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.55 (dd, J = 8.55, 3.05 Hz, 1 H, H11), 3.96 (d, J = 8.55 Hz, 1 H, H15), 4.97 (d, J = 3.05 Hz, 1 H, H10), 6.41–6.50 (m, 2 H, 2 x ArH), 7.23–7.41 (m, 9 H, 9 x ArH), 7.61 (d, J = 7.32 Hz, 1 H, 1 x ArH), 8.12 (d, J = 14.04 Hz, 1 H, H1′), 8.32 (d, J = 14.04 Hz, 1 H, H2′). 13C NMR (101 MHz, DMSO-d6) ppm 44.8 (C10), 47.7 (C11), 48.1 (C15), 49.7 (C9), 123.1 (CH), 123.6 (CH), 124.5 (CH), 125.3 (CH), 126.4 (CH), 126.5 (CH), 127.0 (CH), 127.2 (CH), 127.3 (CH), 128.2 (CH), 128.7 (CH), 130.6 (CH), 132.8, 132.9, 137.2, 138.5, 138.5, 140.8, 141.2, 145.3 (C1′), 174.5 (C12), 174.8 (C14). HRMS (APCI) calculated for C26H19N2O5 [M+ + H] 439.1294: found 439.1306.
5.4.31. (E)-3-(9-(2-Nitrovinyl)-12,14-dioxo-9,10-dihydro-9,10-[3,4]epipyrroloanthracen-13-yl)phenyl acetate (16m)
Compound 16m was prepared from (E)-9-(2-nitrovinyl)anthracene (0.25 g, 1 mmol) and 3-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)phenyl acetate 11i (0.3 g, 1.3 mmol) following general procedure 3. The product was isolated as a colourless solid, 240 mg (50%), Mp. 246–248 °C. IRVmax (ATR): 3085, 3065 (C-H), 1715 (C=O), 1606 (C=C), 1484, 1482 (C=C), 1538, 1338 (NO2) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 2.25 (s, 3 H, CH3), 3.55 (dd, J = 8.24, 3.36 Hz, 1 H, H11), 3.95 (d, J = 8.55 Hz, 1 H, H15), 4.97 (d, J = 3.05 Hz, 1 H, H10), 6.25–6.36 (m, 2 H, C4″, C2″), 7.08–7.15 (m, 1 H, 1 x ArH), 7.21–7.43 (m, 8 H, 8 x ArH), 7.62 (d, J = 7.32 Hz, 1 H, 1 x ArH), 8.12 (d, J = 13.43 Hz, 1 H, H1′), 8.33 (d, J = 14.04 Hz, 1 H, H2′). 13C NMR (101 MHz, DMSO-d6) ppm 20.7 (CH3), 44.8 (C10), 47.7 (C11), 48.0 (C15), 49.7 (C9), 120.0 (CH), 122.2 (CH), 123.1 (CH), 123.5 (CH), 123.7 (CH), 124.5 (CH), 125.3 (CH), 126.5 (CH), 127.0 (CH), 127.2 (CH), 127.3 (CH), 128.2, 128.9, 129.6 (CH), 132.2, 137.2 (CH), 138.4, 138.5, 140.9, 141.2, 145.3 (C1′), 150.3 (C3″), 168.8 (Acetate C=O), 174.5 (C12), 174.8 (C14). HRMS (APCI) calculated for C28H21N2O6 [M+ + H] 481.1388: found 481.1394.
5.4.32. (E)-13-(4-Aminophenyl)-9-(2-nitrovinyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (16n)
Compound 16n was prepared from (E)-9-(2-nitrovinyl)anthracene 12a 0.25 g (1 mmol) and 1-(4-aminophenyl)-1H-pyrrole-2,5-dione 11p 0.244 g (1.3 mmol) following general procedure 3. The product was isolated as a brown solid, 197 mg (45%), Mp. >300 °C. IRVmax(ATR): 3070, 2953 (C-H), 1712 (C=O), 1595 (C=C), 1482, 1457 (C=C), 1518, 1349 (NO2) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.53 (dd, J = 7.94, 3.05 Hz, 1 H, H11), 3.93 (d, J = 8.55 Hz, 1 H, H15), 4.90–5.00 (m, 1 H, H10), 6.42 (s, 2 H, NH2), 7.12–7.42 (m, 11 H, 11 x ArH), 7.60 (d, J = 7.32 Hz, 1 H, 1 x ArH), 8.10 (d, J = 14.04 Hz, 1 H, H1′), 8.30 (d, J = 13.43 Hz, 1 H, H2′). 13C NMR (101 MHz, DMSO-d6) ppm 43.6 (C10), 44.7 (C11), 47.9 (C15), 49.7 (C9), 123.0 (CH), 123.5 (CH), 124.5 (CH), 125.2 (CH), 125.3 (CH), 126.5 (CH), 126.8 (CH), 127.2 (CH), 127.4 (CH), 128.2 (CH), 128.9 (CH), 131.3, 134.8 (C1′), 137.2, 138.4, 138.4, 140.8, 141.2, 145.2 (C1′), 174.5 (C12), 174.9 (C14). HRMS (APCI) calculated for C26H20N3O4 [M + H] 438.1454: found 438.1461.
5.4.33. (E)-9-(2-Nitroprop-1-en-1-yl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (17a)
Compound 17a was prepared from (E)-9-(2-nitroprop-1-en-1-yl)anthracene 12b (0.26 g, 1 mmol) and phenylmaleimide (0.22 g, 1.3 mmol) following general procedure 3. The product was isolated as a colourless solid, 384 mg (88%), Mp. 263–266 °C. IRVmax (KBr): 3045, 2934 (C-H), 1700 (C=O), 1598, 1460 (C=C), 1519 (C=C), 1523, 1390 (NO2), 1328 (CH3) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 1.86 (br. s., 3 H, CH3), 3.38–3.51 (m, 1 H, H11), 3.61 (d, J = 7.32 Hz, 1 H, H15), 4.97 (d, J = 3.66 Hz, 1 H, H10), 6.33–6.51 (m, 2 H, H6″, H2″), 7.19–7.41 (m, 10 H, 10 x ArH), 7.63 (d, J = 7.32 Hz, 1 H, ArH), 8.37 (s, 1 H, H1′). 13C NMR (101 MHz, DMSO-d6) ppm 17.2 (C3′), 44.9 (C11), 47.4 (C15), 50.2 (C9), 50.3 (C10), 123.7 (CH), 123.9 (CH), 124.8 (CH), 125.3 (CH), 125.4 (CH), 126.4 (CH), 126.8 (CH), 127.2 (CH), 127.4 (CH), 128.2 (CH), 128.5 (CH), 128.8 (CH), 131.5 (C1″), 131.8, 138.3 (C1′), 138.6, 140.7, 152.5 (C2′), 175.1 (C14, C12). HRMS (APCI) calculated for C27H21N2O4 [M+ + H] 437.1501: found 437.1513.
5.4.34. (E)-9-(2-Nitrobut-1-en-1-yl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (17b)
Compound 17b was prepared from (E)-9-(2-nitrobut-1-en-1-yl)anthracene 12c (0.27 g, 1 mmol) and phenylmaleimide (0.22 g, 1.3 mmol) following general procedure 3. The product was isolated as a colourless solid, 368 mg (82%), Mp. 259–263 °C. IRVmax (KBr): 2968, 2939 (C-H), 1707 (C=O), 1597 (C=C), 1500, 1456 (C=C), 1519, 1390 (NO2), 1464 (CH2), 1340 (CH3) cm−1. 1H NMR (400 MHz, CDCl3) δ 1.03 (br. s., 3 H, CH3), 2.28 (br. s., 2 H, CH2), 3.34 (br. s., 2 H, H15, H11), 4.95 (s, 1 H, H10), 6.47 (dd, J = 6.41, 2.75 Hz, 2 H, H6″, H2″), 7.19–7.45 (m, 11 H, 11 x ArH), 8.20 (s, 1 H, H1′). 13C NMR (101 MHz, CDCl3) ppm 9.8 (C4′), 23.9 (C3′), 46.0 (C10), 47.8 (C11), 49.7 (C9), 50.3 (C15), 123.5 (CH), 124.8 (CH), 125.8 (CH), 126.0, 126.2 (CH), 126.7 (CH), 127.1 (CH), 127.8 (CH), 127.9, 128.0 (CH), 128.8 (CH), 129.0 (C1′), 129.1, 129.9, 130.9, 134.2, 137.8, 139.0 (C2′), 174.9 (C12, C14). HRMS (APCI) calculated for C28H21N2O4 [M+ − H] 449.1507: found 449.1490.
5.4.35. (E)-13-(4-Chlorophenyl)-9-(2-nitroprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (17c)
Compound 17c was prepared from (E)-9-(2-nitroprop-1-en-1-yl)anthracene 12b (0.26 g, 1 mmol) and 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione 11c (0.27 g, 1.3 mmol) following general procedure 3. The product was isolated as a colourless solid, 282 mg (60%), Mp. 298–300 °C. IRVmax (KBr): 3003, 2967 (C-H), 1700 (C=O), 1524, 1390 (NO2), 1494 (C=C), 1460 (CH3), 1198 (C-N), 778.31 (Cl-C) cm−1. 1H NMR (400 MHz, CDCl3) δ 1.96 (br. s., 3 H, CH3), 3.35 (br. s., 2 H, H11, H15), 4.94 (br. s., 1 H, H10), 6.44 (d, J = 7.32 Hz, 2 H, H6″, H2″), 7.14–7.34 (m, 11 H, 11 x ArH), 8.45 (br. s., 1 H, H1′). 13C NMR (101 MHz, CDCl3) ppm 18.2 (C3′), 46.0 (C10), 47.7 (C11), 48.6 (C15), 50.6 (C9), 123.4, 124.9, 125.9, 126.9, 127.3, 127.4, 127.9, 128.0, 129.3, 137.2 (C2′), 174.7 (C14, C12). HRMS (APCI) calculated for C27H20ClN2O4 [M+ + H] 471.1112: found 471.1099.
5.4.36. (E)-13-(4-Chlorophenyl)-9-(2-nitrobut-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (17d)
Compound 17d was prepared from (E)-9-(2-nitrobut-1-en-1-yl)anthracene 12c (0.26 g, 1 mmol) and 1-(4-chlorophenyl)-1H-pyrrole-2,5-dione 11c (0.27 g, 1.3 mmol) following general procedure 3. The product was isolated as a colourless solid, 212 mg (44%), Mp. 275–279 °C. IRVmax (KBr): 3005, 2941 (C-H), 1702 (C=O), 1598 (C=C), 1458, 1429 (C=C), 1520, 1383.10 (NO2), 1341 (CH2), 1310 (CH3), 762 (Cl-C) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 0.99 (br. s., 3 H, CH3), 2.17 (br. s., 2 H, CH2), 3.42 (br. s., 1 H, H11), 3.62 (d, J = 6.10 Hz, 1 H, H15), 4.98 (d, J = 3.05 Hz, 1 H, H10), 6.47 (d, J = 9.16 Hz, 2 H, H6″, H2″), 7.17–7.48 (m, 10 H, 10 x ArH), 7.64 (d, J = 6.71 Hz, 1 H, ArH), 8.19 (s, 1 H, H1′). 13C NMR (101 MHz, DMSO-d6) ppm 8.4 (C3′), 23.4 (C4′), 39.8 (C10), 45.0 (C11), 47.5 (C15), 50.4 (C9), 123.6 (CH), 124.9 (CH), 125.4 (CH), 126.4 (CH), 126.8 (CH), 127.4 (CH), 128.1 (CH), 129.0 (CH), 130.2 (Cq), 133.1 (Cq), 138.8 (Cq), 142.6 (Cq), 157.0 (C2′), 174.8 (C14, C12). HRMS (APCI) calculated for C28H20ClN2O4 [M+ − H] 483.1117: found 483.1101.
5.4.37. (E)-13-(4-Methylphenyl)-9-(2-nitroprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (17e)
Compound 17e was prepared from (E)-9-(2-nitrobut-1-en-1-yl)anthracene 12c (0.26 g, 1 mmol) and 1-(4-methylphenyl)-1H-pyrrole-2,5-dione 11d (0.243 g, 1.3 mmol) as described in general procedure 3. The product was obtained as a colourless solid, 293 mg (65%), Mp. 276–277 °C. IRVmax (KBr): 3071, 3010 (C-H), 1700 (C=O), 1518, 1391 (NO2), 1198 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 1.85 (br. s., 3 H, CH3), 2.25 (s, 3 H, CH3), 3.41 (dd, J = 8.24, 2.75 Hz, 1 H, H11), 3.59 (d, J = 7.32 Hz, 1 H, H15), 4.96 (d, J = 3.05 Hz, 1 H, H10), 6.30 (d, J = 8.54 Hz, 2 H, H5″, H3″), 7.12 (d, J = 7.93 Hz, 2 H, H6″, H2″), 7.20–7.43 (m, 7 H, 7 x ArH), 7.63 (d, J = 6.71 Hz, 1 H,1 x ArH), 8.35 (s, 1 H, H1′). 13C NMR (101 MHz, DMSO-d6) ppm 21.0 (C3′), 45.4 (C10), 47.8 (C11), 49.9 (C15), 51.0 (C9), 55.0 (OCH3), 124.1, 125.2, 125.8, 126.6, 126.8, 127.2, 127.6, 127.7, 129.3, 129.7, 138.5, 139.1, 159.7 (C2′), 175.6 (C12, C14). HRMS (APCI) calculated for C28H23N2O4 [M + H] 451.1658: found 451.1666.
5.4.38. (E)-13-(4-Methylphenyl)-9-(2-nitrobut-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (17f)
Compound 17f was prepared from (E)-9-(2-nitrobut-1-en-1-yl)anthracene 12c (0.26 g, 1 mmol) and 1-(4-methyoxyphenyl)-1H-pyrrole-2,5-dione (0.243 g, 1.3 mmol) as described in general procedure 3. The product was obtained as a colourless solid, 251 mg (54%), Mp. 254–256 °C. IRVmax (KBr): 3006, 2962 (C-H), 1700 (C=O), 1523, 1387 (NO2), 1192 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 0.99 (br. s., 3 H, CH2-CH3), 2.17 (br. s., 2 H, CH2), 2.25 (s, 3 H, CH3), 3.40 (br. s., 1 H, H11), 3.58 (br. s., 1 H, H15), 4.97 (d, J = 3.05 Hz, 1 H, H10), 6.30 (d, J = 7.93 Hz, 2 H, H5″, H3″), 7.11 (d, J = 8.54 Hz, 2 H, H6″, H2″), 7.21–7.47 (m, 7 H, 7 x ArH), 7.63 (d, J = 6.71 Hz, 1 H, 1 x ArH), 8.20 (s, 1 H, H1′). 13C NMR (101 MHz, DMSO-d6) ppm 9.5 (C3′), 20.6 (OCH3), 23.4 (C4) 45.0 (C11), 47.4 (C15), 50.3 (C9), 109.5, 123.6, 124.8, 125.4, 126.1, 126.3, 126.7, 127.3, 127.5, 128.8, 129.2, 138.1, 138.9, 156.9 (C2′), 175.1 (C12, C14). HRMS (APCI) calculated for C29H25N2O5 [M+ + H] 465.1814: found 465.1831.
5.4.39. (E)-9-Chloro-10-(2-nitroprop-1-en-1-yl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (17g)
Compound 17g was prepared from (E)-9-chloro-10-(2-nitroprop-1-en-1-yl)anthracene 12e (0.298 g, 1 mmol) and phenylmaleimide (0.224 g, 1.3 mmol) as described in general procedure 3. The product was obtained as a colourless solid, 183 mg (39%), Mp. 268–271 °C. IRVmax (ATR): 3072, 2981 (C-H), 1713 (C=O), 1625 (C=C), 1499, 1455 (C=C), 1520, 1332 (NO2) cm−1. 1H NMR (400 MHz, CDCl3) δ 1.94 (br. s., 3 H, CH3), 3.46 (br. s., 2 H, H11, H15), 6.49 (dd, J = 6.10, 3.66 Hz, 2 H, 2 x ArH), 7.14–7.29 (m, 5 H, 5 x ArH), 7.29–7.45 (m, 4 H, 4 x ArH), 7.89 (d, J = 7.93 Hz, 1 H, 1 x ArH), 8.01 (d, J = 7.32 Hz, 1 H, 1 x ArH), 8.49 (s, 1 H, H1′). 13C NMR (101 MHz, CDCl3) ppm 17.8 (C3′), 52.0 (C10), 53.4 (C15, C11), 70.1 (C9), 122.8 (CH), 124.0 (CH), 124.2 (CH), 126.1 (CH), 127.9 (CH), 128.1 (CH), 128.2 (CH), 128.4 (CH), 128.9 (CH), 129.0 (CH), 130.8, 136.9, 137.3, 140.8 (C2′), 171.2 (C12, C14). HRMS (APCI) calculated for C27H20ClN2O4 [M+ + H] 471.1112: found 471.1101.
5.4.40. (E)-9-Chloro-10-(2-nitrobut-1-en-1-yl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (17h)
Compound 17h was prepared from (E)-9-chloro-10-(2-nitrobut-1-en-1-yl)anthracene 11f (0.312 g, 1 mmol) and phenyl maleimide (0.224 g, 1.3 mmol) as described in general procedure 3. The product was obtained as a colourless solid, 184 mg (38%), Mp. 261–262 °C. IRVmax (ATR): 3075, 2981, 2942 (C-H), 1712 (C=O), 1623 (C=C), 1498, 1455 (C=C), 1520, 1348 (NO2) cm−1. 1H NMR (400 MHz, CDCl3) δ 0.94–1.10 (m, 3 H, CH3), 2.26 (dd, J = 19.23, 7.02 Hz, 2 H, CH2), 3.44 (br. s., 2 H, H11, H15), 6.50 (dd, J = 5.80, 3.36 Hz, 2 H, 2 x ArH), 7.20–7.50 (m, 9 H, 9 x ArH), 7.90 (d, J = 7.94 Hz, 1 H, 1 x ArH), 8.02 (d, J = 7.93 Hz, 1 H, 1 x ArH), 8.21 (s, 1 H, H1′). 13C NMR (101 MHz, CDCl3) ppm 10.0 (C4′), 24.4 (C3′), 52.2 (C10), 53.5 (C15, C11), 70.1 (C9), 123.0 (CH), 123.9 (CH), 124.1 (CH), 126.1 (CH), 127.7 (CH), 128.1, 128.2 (CH), 128.4, 128.9 (CH), 129.0 (C1′), 130.7, 137.5 (C2′), 171.1 (C12, C14). HRMS (APCI) calculated for C28H22ClN2O4 [M+ − H] 485.1268: found 485.1248.
5.4.41. (E)-13-Benzyl-9-(2-nitrovinyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (17i)
Compound 17i was prepared from (E)-9-(2-nitrovinyl)anthracene 12a (0.25 g, 1 mmol) and 1-benzyl-1H-pyrrole-2,5-dione 11a (0.24 g, 1.3 mmol) as described in general procedure 3. The product was obtained as a colourless solid 323 mg (74%), Mp 232–236 °C. IRVmax (KBr): 3033 2933 (C-H), 1702 (C=O), 1657 (C=C), 1496, 1464 (C=C), 1533, 1390 (NO2), 1464 (CH2), 1189 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.47 (d, J = 5.49 Hz, 1 H, H11), 3.86 (d, J = 7.93 Hz, 1 H, H15), 4.22 (br. s., 2 H, CH2), 4.90 (br. s., 1 H, H10), 6.31 (d, J = 6.71 Hz, 2 H, H2″, H6″), 7.01–7.39 (m, 10 H, 10 x ArH), 7.57 (d, J = 6.10 Hz, 1 H, 1 x ArH), 8.12 (d, J = 14.04 Hz, 1 H, H2′), 8.28 (d, J = 14.04 Hz, 1 H, H1′). 13C NMR (101 MHz, DMSO-d6) ppm 41.3 (C10), 43.6 (C11), 44.2 (C15), 47.5 (C16), 49.2 (C9), 109.5, 122.9, 123.3, 124.3, 125.3, 126.1, 126.4, 126.8, 127.0, 127.4, 128.2, 134.9, 137.2 (C1′), 138.5, 138.6, 141.4, 141.8, 145.2 (C2′), 175.3 (C12), 175.6 (C14). HRMS (APCI) calculated for C27H21N2O4 [M+ + H] 437.1501: found 437.1505.
5.4.42. (E)-13-Benzyl-9-(2-nitroprop-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (17j)
Compound 17j was prepared from (E)-9-(2-nitroprop-1-en-1-yl)anthracene 12b (0.26 g, 1 mmol) and 1-benzyl-1H-pyrrole-2,5-dione 11a (0.24 g, 1.3 mmol) as described in general procedure 3. The product was obtained as a colourless solid, 279 mg (62%), Mp 232–236 °C. IRVmax (KBr): 3007, 2960 (C-H), 1692 (C=O), 1586 (C=C), 1631, 1431 (Ar C=C), 1520, 1399 (NO2), 1457 (CH2), 1362 (CH3), 1175 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 1.80 (br. s., 3 H, CH3), 3.49 (d, J = 7.32 Hz, 1 H11), 4.13 (s, 2 H, CH2), 4.37 (d, J = 5.49 Hz, 1 H15), 4.88 (d, J = 3.05 Hz, 1 H, H10), 6.30 (d, J = 6.71 Hz, 2 H, H6′, H2′), 6.97–7.37 (m, 10 H, 10 x ArH), 7.55 (d, J = 6.71 Hz, 1 H, ArH), 8.33 (s, 1 H, H1′). 13C NMR (101 MHz, DMSO-d6) ppm 17.3 (C3′), 41.3 (C10), 42.6 (C11), 44.4 (C15), 47.2 (C16), 50.0 (C9), 123.5, 124.7, 125.3, 126.2, 126.3, 126.8, 126.8, 127.1, 127.2, 127.4, 127.6, 128.2, 128.4, 131.5, 132.4, 135.0, 137.9, 138.3, 139.1, 141.2, 152.6 (C2′), 165.2, 165.8, 175.7 (C12), 175.8 (C14). HRMS (APCI) calculated for C28H23N2O4 [M+ + H] 451.1658: found 451.1671.
5.4.43. (E)-13-Benzyl-9-(2-nitrobut-1-en-1-yl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (17k)
Compound 17k was prepared from (E)-9-(2-nitrobut-1-en-1-yl)anthracene 12c (0.26 g, 1 mmol) and 1-benzyl-1H-pyrrole-2,5-dione 11a (0.24 g, 1.3 mmol) as described in general procedure 3. The product was obtained as a colourless solid 232 mg (50%), Mp. 188–199 °C. IRVmax (KBr): 3002, 2943 (C-H), 1695 (C=O), 1496, 1456 (C=C), 1518, 1339 (NO2), 1400 (CH2), 1362 (CH3), 1175 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 0.98 (br. s., 3 H, CH3), 2.15 (br. s., 2 H, CH2), 3.52 (br. s., 1 H, H11), 4.20 (d, J = 2.44 Hz, 2 H, CH2 benzyl), 4.41 (d, J = 6.10 Hz, 1 H, H15), 4.93 (d, J = 3.05 Hz, 1 H, H10), 6.35 (s, 2 H, H2″, H6″), 7.07–7.32 (m, 10 H, 10 x ArH), 7.59 (d, J = 6.10 Hz, 1 H, ArH), 8.21 (s, 1 H, H1′). 13C NMR (101 MHz, DMSO-d6) ppm 9.9 (C4′), 23.9 (C3′) 41.3 (C10), 42.7 (C11), 44.4 (C15), 47.3 (C16), 49.9 (C9), 123.4, 124.7, 125.4, 126.2, 126.3, 126.8, 126.8, 127.2, 127.2, 127.6, 127.6, 128.2, 128.4, 131.5, 132.45, 135.0, 139.4, 157.1 (C2′), 165.2, 165.7, 175.6 (C12, C14). HRMS (APCI) calculated for C29H27N2O4 [M+ + H] 465.1814: found 465.1795.
5.4.44. (E)-9-(2-Nitroprop-1-en-1-yl)-13-(3,4,5-trimethoxybenzyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (17l)
Compound 17l was prepared from (E)-9-(2-nitroprop-1-en-1-yl)anthracene 12c (0.26 g, 1 mmol) and 1-(3,4,5-trimethoxybenzyl)-1H-pyrrole-2,5-dione 11b (0.36 g, 1.3 mmol) as described in general procedure 3. The product was obtained as yellow crystals, 243 mg (45%), Mp. 227–229 °C. IRVmax (KBr): 2961, 2997 (C-H), 1697 (C=O), 1592 (C=C), 1462, 1426 (C=C), 1522, 1330 (NO2), 1241, 1103 (C-O), 1126 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.21–3.29 (m, 1 H, H11), 3.45 (br. s., 1 H, H15), 3.60 (s, 3 H, p-OCH3), 3.66 (s, 6 H, 2 x OCH3), 3.95 (s, 2 H, CH2), 4.82 (d, J = 3.05 Hz, 1 H, H10), 6.18 (s, 2 H, H2″, H6″), 6.78–7.05 (m, 3 H, 3 x ArH), 7.10–7.32 (m, 4 H, 4 x ArH), 7.53 (d, J = 6.71 Hz, 1 H, ArH), 8.30 (s, 1 H, H1′). 13C NMR (101 MHz, DMSO-d6) ppm 17.6 (C3′), 41.5 (C10), 44.6 (C11), 47.0 (C15), 50.2 (C9), 55.9 (2 x OCH3), 59.8 (p-OCH3), 106.1, 123.6, 124.6, 124.9, 126.3, 126.8, 127.1, 130.9, 138.9, 141.0, 152.4 (C2′), 175.6 (C12), 175.7 (C14). HRMS (APCI) calculated for C31H29N2O7 [M+ + H] 541.1975: found 541.1953.
5.4.45. (E)-9-(2-Nitrobut-1-en-1-yl)-13-(3,4,5-trimethoxybenzyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (17m)
Compound 17m was prepared from (E)-9-(2-nitrobut-1-en-1-yl)anthracene 12b (0.26 g, 1 mmol) and 1-(3,4,5-trimethoxybenzyl)-1H-pyrrole-2,5-dione 11b (0.36 g, 1.3 mmol) as described in general procedure 3. The product was obtained as a tan powder, 299 mg (54%), Mp. 224–229 °C. IRVmax (KBr): 2940, 2836 (C-H), 1700 (C=O), 1593 (C=C), 1508, 1426 (C=C), 1521, 1340 (NO2), 1460 (CH2), 1401 (CH3), 1127 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 0.93 (br. s., 3 H, CH3), 2.09 (br. s., 2 H, CH2), 3.23–3.27 (m, 1 H, H11), 3.47 (d, J = 7.32 Hz, 1 H, H15), 3.60 (s, 3 H, p-OCH3), 3.67 (s, 6 H, 2 x OCH3), 3.94 (s, 2 H, CH2 benzyl), 4.84 (d, J = 3.05 Hz, 1 H, H10), 6.18 (s, 2 H, H2″, H6″), 6.93 (d, J = 6.10 Hz, 3 H, 3 x ArH), 7.12–7.30 (m, 4 H, 4 x ArH), 7.54 (d, J = 6.71 Hz, 1 H, ArH), 8.14 (s, 1 H, H1′). 13C NMR (101 MHz, DMSO-d6) ppm 9.5 (C4′), 23.4 (C3′), 41.5 (C10), 44.6 (C11), 47.1 (C15), 50.0 (C9), 55.9 (OCH3 x 2), 59.8 (p-OCH3), 106.1 (CH), 123.4, 124.7, 124.9, 126.2, 127.0, 127.3, 130.9, 137.1, 139.1, 152.5 (C2′), 156.9 (C4″), 175.6 (C12, C14). HRMS (APCI) calculated for C32H30N2O7 [M+ + H] 555.2131: found 555.2122.
5.4.46. (E)-13-(3,4-Dimethoxyphenethyl)-9-(2-nitrovinyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (17n)
Compound 17n was prepared from (E)-9-(2-nitrovinyl)anthracene 12a (0.25 g, 1 mmol) and 1-(3,4-dimethoxyphenethyl)-1H-pyrrole-2,5-dione 11j (0.34 g, 1.3 mmol) as described in general procedure 3. The product was obtained as a colourless solid, 393 mg (77%), Mp. 152–156 °C. IRVmax (KBr): 2941, 2837 (C-H), 1771 (C=O), 1698 (C=C), 1528 (NO), 1494, 1450 (C=C), 1155 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 1.62–1.81 (m, 2 H, CH2), 3.00–3.19 (m, 2 H, CH2), 3.40 (dd, J = 8.55, 3.05 Hz, 1 H, H11), 3.71 (s, 3 H, OCH3), 3.69 (s, 3 H, OCH3), 3.81 (d, J = 7.94 Hz, 1 H, H15), 4.91 (d, J = 3.05 Hz, 1 H, H10), 6.50 (d, J = 7.94 Hz, 1 H, H6″), 6.59 (s, 1 H, H2″), 6.80 (d, J = 8.55 Hz, 1 H, H5″), 7.21–7.39 (m, 7 H, 7 x ArH), 7.58 (d, J = 7.32 Hz, 1 H, 1 x ArH), 8.14 (d, J = 14.04 Hz, 1 H, H1′), 8.32 (d, J = 14.04 Hz, 1 H, H2′). 13C NMR (101 MHz, DMSO-d6) ppm 21.0 (CH2), 32.1 (CH2-N), 44.6 (C10), 47.3 (C11), 47.5 (C15), 49.5 (C9), 55.4 (OCH3), 55.5 (OCH3), 111.9 (CH), 111.9 (CH), 120.1 (CH), 123.0 (CH), 123.4 (CH), 124.4 (CH), 125.3 (CH), 126.5 (CH), 126.8 (CH), 127.1 (CH), 127.2 (CH), 128.2 (CH), 128.9 (CH), 129.9, 137.4 (C2′), 138.3, 138.5, 141.1, 141.4, 145.2 (C1′), 147.5 (C3″), 148.7 (C4″), 175.2 (C12), 175.5 (C14). HRMS (APCI) calculated for C30H27N2O6 [M+ + H] 511.1869: found 511.1881.
5.4.47. (E)-9-(2-Nitrovinyl)-14-phenyl-9,10-dihydro-9,10-[1,2]epitriazoloanthracene-13,15-dione (18)
Compound 18 was prepared from (E)-9-(2-nitrovinyl)anthracene 12a (0.25 g, 1 mmol) and 4-phenyl-3H-1,2,4-triazole-3,5(4H)-dione 11t (1.3 mmol) as outlined in general procedure 3, to give the product as a brown solid, 289 mg (68%), Mp. 168–169 °C. IRVmax (ATR): 3048, 2998 (C-H), 1730 (C=O), 1625 (C=C), 1555, 1486 (C=C) cm−1. 1H NMR (400 MHz, CDCl3) δ 6.39 (s, 1 H, H10), 7.10 (d, J = 7.93 Hz, 2 H, 2 x ArH), 7.34–7.45 (m, 9 H, 9 x ArH), 7.58 (d, J = 6.71 Hz, 2 H, 2 x ArH), 7.70 (d, J = 14.04 Hz, 1 H, H1′), 8.46 (d, J = 14.04 Hz, 1 H, H2′). 13C NMR (101 MHz, DMSO-d6) ppm 60.0 (C10), 67.1 (C9), 123.1 (CH), 124.5 (CH), 125.9 (CH), 128.3 (CH), 128.8 (CH), 129.0 (CH), 129.1 (CH), 130.4, 131.8 (C1′), 135.6, 136.3, 145.4 (C2′), 155.8 (C12/C14), 156.4 (C12/C14). HRMS (APCI) calculated for C24H17N4O4 [M+ + H] 425.1250: found 425.1251.
5.4.48. (E)-10-(2-Nitrovinyl)-9,10-dihydro-9,10-ethanoanthracene-11-carbonitrile (19a)
Compound 19a was prepared from (E)-9-(2-nitrovinyl)anthracene 12a (0.25 g, 1 mmol) and acrylonitrile (3 mmol) as outlined in general procedure 3, to give the product as orange crystals, 91 mg (30%), Mp. 223–225 °C. IRVmax (ATR): 3113, 3072, 2952 (C-H), 1658 (C=C), 1485, 1457 (C=C), 1526, 1354 (NO2), 1190 (CN) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 1.93 (dt, J = 12.82, 3.36 Hz, 1 H, H12a), 2.27 (ddd, J = 12.67, 10.53, 2.44 Hz, 1 H, H12b), 3.66 (dd, J = 10.68, 3.97 Hz, 1 H, H11), 4.60 (s, 1 H, C9), 7.11–7.31 (m, 5 H, 5 x ArH), 7.34–7.49 (m, 3 H, 3 x ArH), 8.04 (d, J = 13.43 Hz, 1 H, H1′), 8.33 (d, J = 14.04 Hz, 1 H, H2′). 13C NMR (101 MHz, DMSO-d6) ppm 31.2 (C11), 34.0 (C12), 41.7 (C9), 48.9 (C10), 121.0, 123.1 (CH), 123.2 (CH), 123.8 (CH), 124.2 (CH), 126.1 (CH), 126.3 (CH), 127.3 (CH), 127.4 (CH), 138.1 (C1′), 138.7, 139.9, 142.1, 142.5, 144.0 (C2′). HRMS (APCI) calculated for C19H14N2O2 [M+] 302.1055: found 302.1055.
5.4.49. Ethyl (E)-10-(2-nitrovinyl)-9,10-dihydro-9,10-ethanoanthracene-11-carboxylate (19b)
Compound 19b was prepared from (E)-9-(2-nitrovinyl)anthracene 12a (0.25 g, 1 mmol) and ethyl acrylate (1.3 mmol) following the general procedure 3. Purification by column chromatography (hexane: ethyl acetate 9:1) afforded the product as a yellow oil, 63 mg (18%). IRVmax (ATR): 3123, 2981 (C-H), 1731 (C=O), 1626 (C=C), 1486, 1443 (C=C), 1510, 1361 (NO2), 1120 (CN) cm−1. 1H NMR (400 MHz, CDCl3) δ 1.12 (t, J = 7.32 Hz, 3 H, CH3), 1.99 (d, J = 12.21 Hz, 1 H, H12a), 2.31 (t, J = 11.60 Hz, 1 H, H11), 3.01 (dd, J = 10.68, 4.58 Hz, 1 H, H12b), 4.00 (dd, J = 16.18, 7.63 Hz, 2 H, OCH2), 4.43 (br. s., 1 H, H9), 7.11–7.26 (m, 5 H), 7.28–7.42 (m, 3 H), 7.49 (d, J = 14.04 Hz, 1 H, H1′), 8.35 (d, J = 14.04 Hz, 1 H, H2′). 13C NMR (101 MHz, CDCl3) ppm 14.0 (C2″, CH3), 34.2 (C11), 43.7 (C9), 46.3 (C12), 49.3 (C10), 60.9 (C1″, OCH2), 122.2 (CH), 123.6 (CH), 123.6 (CH), 123.9 (CH), 126.0 (CH), 126.0 (CH), 126.8 (CH), 127.1 (CH), 139.0, 140.2 (C1′), 142.0, 142.5 (C2′), 142.8, 142.9, 172.7 (C=O). HRMS (APCI) calculated for C21H20NO4 [M+ + H] 350.1392: found 350.1389.
5.4.50. Methyl (E)-10-(2-nitrovinyl)-9,10-dihydro-9,10-ethanoanthracene-11-carboxylate (19c)
Compound 19c was prepared from (E)-9-(2-nitrovinyl)anthracene 12a (0.25 g, 1 mmol) and methyl acrylate (1.3 mmol) as described in general procedure 3. Purification by column chromatography (hexane: ethyl acetate 9:1) afforded the product as a gold oil, 50 mg (15%). IRVmax (ATR): 3086, 2990 (C-H), 1730 (C=O), 1625 (C=C), 1466, 1444 (C=C), 1555, 1361 (NO2), 1120 (CN) cm−1. 1H NMR (400 MHz, CDCl3) δ 1.97 (d, J = 12.21 Hz, 1 H, H12a), 2.33 (t, J = 11.29 Hz, 1 H, H11), 3.03 (dd, J = 10.07, 4.58 Hz, 1 H, H12b), 3.56 (s, 3 H, OCH3), 4.43 (br. s., 1 H, H9), 7.05–7.30 (m, 6 H, 6 x ArH), 7.30–7.48 (m, 4 H, 4 x ArH, H1′), 8.34 (d, J = 14.65 Hz, 1 H, H2′). 13C NMR (101 MHz, CDCl3) ppm 34.26 (C11), 43.68 (C9), 46.06 (C12), 49.24 (C10), 52.01 (OCH3), 122.19 (CH), 123.58 (CH), 123.73 (CH), 123.92 (CH), 126.05 (CH), 126.81 (CH), 127.13 (CH), 139.01, 140.29 (CH), 141.98, 142.41 (C2′), 142.76, 142.86, 173.20 (C=O). HRMS (APCI) calculated for C20H18NO4 [M+ + H] 336.1236: found 336.1233.
5.4.51. (E)-11-Cyano-10-(2-nitrovinyl)-9,10-dihydro-9,10-ethanoanthracen-11-yl acetate (19d)
Compound 19d was prepared from (E)-9-(2-nitrovinyl)anthracene 12a (0.25 g, 1 mmol) and 1-cyanovinyl acetate (1.3 mmol) following the general procedure 3. The crude product was purified by flash column chromatography (hexane: ethyl acetate 9:1), and afforded a yellow solid, 127 mg (35%), Mp.168–170 °C. IRVmax (ATR): 3062, 2956 (C-H), 1753 (C=O), 1650 (C=C), 1484, 1422 (C=C), 1534, 1351 (NO2), 1193 (CN) cm−1. 1H NMR (400 MHz, CDCl3) δ 2.08 (dd, J = 14.65, 2.44 Hz, 1 H, H12a), 2.94 (dd, J = 14.65, 3.05 Hz, 1 H, H12b), 4.40 (t, J = 2.75 Hz, 1 H, C9), 7.22–7.30 (m, 5 H, 5 x ArH), 7.33–7.41 (m, 3 H, 3 x ArH), 7.60 (d, J = 14.65 Hz, 1 H, H1′), 8.11 (d, J = 14.04 Hz, 1 H, H2′). 13C NMR (101 MHz, CDCl3) ppm 20.7 (CH3), 42.8 (C10), 45.2 (C12), 55.1 (C9), 74.8 (C11), 117.1 (CN), 123.8 (CH), 124.2 (CH), 124.8 (CH), 124.9 (CH), 126.8 (CH), 127.2 (CH), 128.1 (CH), 128.6 (CH), 134.9 (C1′), 135.9, 136.0, 141.8, 142.0, 144.5 (C2′), 168.5 (C=O). HRMS (APCI) calculated for C21H17N2O4 [M+ + H] 361.1188: found 361.1191.
5.4.52. Dimethyl (E)-9-(2-nitrovinyl)-9,10-dihydro-9,10-ethanoanthracene-11,12-dicarboxylate (19e)
(E)-9-(2-Nitrovinyl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione 13a (150 mg, 0.5 mmol) was heated at reflux in methanol (10 mL) and concentrated hydrochloric acid (2 drops) for 6 h. Following cooling to room temperature and removal of the solvent, the residue was taken up in dichloromethane, washed with 10% NaOH solution, water and dried (anhydrous sodium sulphate). The solvent was evaporated and the residue purified by column chromatography (hexane: ethyl acetate 9:1). Recrystallization from ethanol yielded a white solid 126 mg (32%), Mp. 141–143 °C. IRVmax (ATR): 3031, 2948 (C-H), 1746 (C=O), 1662 (C=C), 1467, 1422 (C=C), 1539, 1353 (NO2), 1211 (CN) cm−1. 1H NMR (400 MHz, CDCl3) δ 3.30 (d, J = 10.99 Hz, 1 H, H11), 3.43 (d, J = 10.99 Hz, 1 H, H12), 3.46–3.50 (m, 3 H, OCH3), 3.54 (s, 3 H, OCH3), 4.71 (s, 1 H, H10), 7.05–7.31 (m, 6 H, 6 x ArH), 7.36 (d, J = 7.32 Hz, 1 H, 1 x ArH), 7.41–7.54 (m, 2 H, 1 x ArH, H1′), 8.24 (d, J = 14.04 Hz, 1 H, H2′). 13C NMR (101 MHz, CDCl3) ppm 45.7 (C10), 49.2 (C11), 49.6 (C12), 49.7 (C9), 51.9 (OCH3), 52.0 (OCH3), 122.6 (CH), 123.3 (CH), 123.9 (CH), 126.0 (CH), 126.3 (CH), 126.5 (CH), 126.8 (CH), 127.4 (CH), 139.2 (C1′), 139.2, 139.6, 141.7, 142.3, 142.7 (C2′), 170.6 (C=O), 170.9 (C=O). HRMS (APCI) calculated for C22H20NO6 [M+ + H] 394.1291: found 394.1285.
5.4.53. Diethyl (E)-9-(2-nitrovinyl)-9,10-dihydro-9,10-ethanoanthracene-11,12-dicarboxylate (19f)
(E)-9-(2-nitrovinyl)-9,10-dihydro-9,10-[3,4]furanoanthracene-12,14-dione 13a (150 mg, 0.5 mmol) was refluxed in ethanol (10 mL) and concentrated hydrochloric acid (2 drops) for 6 h. Following cooling to room temperature and removal of the solvent, the residue was taken up in dichloromethane, washed with 10% NaOH solution, water and dried (anhydrous sodium sulphate). The solvent was evaporated and the residue purified by column chromatography (hexane: ethyl acetate 9:1). Recrystallization from ethanol yielded a white solid, 64 mg (15%), Mp. 151–152 °C. IRVmax (ATR): 2980, 2928 (C-H), 1745 (C=O), 1655 (C=C), 1467, 1442 (C=C), 1522, 1347 (NO2), 1175 (CN) cm−1. 1H NMR (400 MHz, CDCl3) δ 1.04 (t, J = 7.32 Hz, 3 H, CH3), 1.14 (t, J = 7.02 Hz, 3 H, CH3), 3.26 (d, J = 10.99 Hz, 1 H, H12), 3.43 (d, J = 10.99 Hz, 1 H, H11), 3.79–4.09 (m, 4 H, 2 x OCH2), 4.71 (s, 1 H, H10), 7.04–7.30 (m, 6 H, 6 x ArH), 7.35 (d, J = 6.71 Hz, 1 H, 1 x ArH), 7.46 (d, J = 6.71 Hz, 1 H, 1 x ArH), 7.58 (d, J = 14.65 Hz, 1 H, H1′), 8.24 (d, J = 14.04 Hz, 1 H, H2′). 13C NMR (101 MHz, CDCl3) ppm 13.7 (CH3), 13.9 (CH3), 45.6 (C10), 49.2 (C11), 49.8 (C12), 49.8 (C9), 60.9 (OCH2), 61.0 (OCH2), 122.6 (CH), 123.2 (CH), 123.8 (CH), 126.1 (CH), 126.2 (CH), 126.5 (CH), 126.7 (CH), 127.3 (CH), 129.8, 139.1 (C1′), 139.5, 139.8, 141.7, 142.6, 142.9 (C2′), 169.9 (C=O), 170.6 (C=O). HRMS (APCI) calculated for C24H24NO6 [M+ + H] 422.1604: found 422.1596.
5.5. General Procedure 4: Preparation of Triptycene Compounds (20a–20d, 20f)
(i) Preparation of benzenediazonium-2-carboxylate: A solution of anthranilic acid (5.4 g, 0.04 mmol) and trichloroacetic acid (0.06 g) in tetrahydrofuran (60 mL) in a 250 mL conical flask was stirred and cooled on an ice-water bath. Isoamyl nitrite (10 mL) was added portion-wise over 1 min and the mixture warmed to RT over 1.5 h. The mixture was cooled and the tan solid was washed with ice cold tetrahydrofuran. The yield of air dried benzenediazonium-2-carboxylate was 78–80%. The benzenediazonium-2-carboxylate was washed with toluene and stored in solution with toluene (60 mL). (ii) Preparation of Triptycene compounds. To a boiling solution of the appropriate anthracene derivative (4 mmol) in toluene (60 mL) was slowly added a slurry of benzenediazonium-2-carboxylate (prepared from 5.4 g (0.04 mmol) of anthranilic acid) in toluene over the course of 1 h. The mixture was heated to reflux for 1 h, then cooled and the solvent removed. The residual oil was purified by column chromatography (dichloromethane: hexane 1:1) and recrystallised from dichloromethane-hexane gave pure product.
5.5.1. (E)-9-(2-Nitroprop-1-en-1-yl)-9,10-dihydro-9,10-[1,2]benzenoanthracene (20b)
Compound 20b was prepared from (E)-9-(2-nitroprop-1-en-1-yl)anthracene 12b (1.05 g, 4 mmol) and benzenediazonium-2-carboxylate (prepared from anthranilic acid (5.4 g, 0.04 mmol)) according to general procedure 4, yielded the product as a yellow solid, 311 mg (23%), Mp. 226–228 °C. IRVmax (ATR): 3071, 3016, (C-H), 1594 (C=C), 1482, 1455 (C=C), 1526, 1328 (NO2) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 1.97 (s, 3 H, CH3), 5.75 (s, 1 H, C10), 6.97–7.14 (m, 6 H, 6 x ArH), 7.42 (d, J = 6.71 Hz, 3 H, 3 x ArH), 7.50–7.58 (m, 3 H, 3 x ArH), 8.51 (s, 1 H, H1′). 13C NMR (101 MHz, DMSO-d6) ppm 18.0 (C3′), 52.6 (C10), 54.0 (C9), 122.2 (CH), 124.3 (CH), 124.9 (CH), 125.7 (C1′), 126.8, 145.4, 154.3 (C2′). HRMS (APCI) calculated for C23H17NO2 [M+] 339.1259: found 339.1259.
5.5.2. (E)-9-(2-Nitrobut-1-en-1-yl)-9,10-dihydro-9,10-[1,2]benzenoanthracene (20c)
Compound 20c was prepared from (E)-9-(2-nitrobut-1-en-1-yl)anthracene 12c (1.1 g, 4 mmol) and benzenediazonium-2-carboxylate (prepared from anthranilic acid (5.4 g, 0.04 mmol)) as outlined in general procedure 4 yielded the product as a colourless solid 453 mg (32%), Mp. 210–211 °C. IRVmax (ATR): 3071, 2952 (C-H), 1713 (C=O), 1594 (C=C), 1481, 1459 (C=C), 1527, 1349 (NO2) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 1.00 (t, J = 7.32 Hz, 3 H, CH3), 2.25 (q, J = 7.12 Hz, 2 H, CH2), 5.72 (s, 1 H, C10), 6.99–7.10 (m, 6 H, 6 x ArH), 7.30–7.40 (m, 3 H, 3 x ArH), 7.46–7.53 (m, 3 H, 3 x ArH), 8.28 (s, 1 H, H1′). 13C NMR (101 MHz, DMSO-d6) ppm 9.4 (C4′), 24.1 (C3′), 49.7 (C9), 52.5 (C10), 122.0 (CH), 124.3 (CH), 124.8 (CH), 125.8 (CH), 126.1 (C1′), 142.8, 145.2, 158.9 (C2′). HRMS (APCI) calculated for C24H20NO2 [M+ + H] 354.1494: found 354.1490.
5.5.3. (E)-9-Chloro-10-(2-nitrovinyl)-9,10-dihydro-9,10-[1,2]benzenoanthracene (20d)
Compound 20d was prepared from (E)-9-chloro-10-(2-nitrovinyl)anthracene 12d (1.13 g, 4 mmol) and benzenediazonium-2-carboxylate (prepared from anthranilic acid (5.4 g, 0.04 mmol)) according to general procedure 4, yielded the product as a colourless solid, 430 mg (30%), Mp. 259–261 °C. IRVmax (ATR): 3070, 2953 (C-H), 1713 (C=O), 1648 (C=C), 1481, 1481 (C=C), 1540, 1350 (NO2) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.15–7.29 (m, 6 H, 6 x ArH), 7.58–7.69 (m, 3 H, 3 x ArH), 7.72–7.82 (m, 3 H, 3 x ArH), 8.12 (d, J = 14.04 Hz, 1 H, H1′), 8.50 (d, J = 14.65 Hz, 1 H, H2′). 13C NMR (101 MHz, DMSO-d6) ppm 52.9 (C10), 74.6 (C9), 121.4 (CH), 122.1 (CH), 126.2 (CH), 126.5 (CH), 128.9, 131.7 (C1′), 142.5, 143.4, 148.0 (C2′). HRMS (APCI) calculated for C22H14NO2Cl [M+] 359.0713: found 359.0712.
5.5.4. 9-(2-Nitroethyl)-9,10-dihydro-9,10-[1,2]benzenoanthracene (20e)
Sodium borohydride (60 mg, 1.6 mmol) was added to a stirred solution of (E)-9-(2-nitrovinyl)-9,10-dihydro-9,10-[1,2]benzenoanthracene 20a (100 mg, 0.3 mmol) in dichloromethane (10 mL) and isopropanol (2 mL). After 24 h at room temperature, the solution was neutralised (1 M HCl) and then extracted with CH2Cl2, dried (sodium sulphate) and solvent removed in vacuo to give the product as a colourless solid, 79 mg (80%), Mp. 185–187 °C. IRVmax (ATR): 3069, 2951 (C-H), 1594 (C=C), 1482, 1450 (C=C), 1523, 1349 (NO2) cm−1. 1H NMR (400 MHz, CDCl3) δ 3.75–3.84 (m, 2 H, CH2), 5.25–5.36 (m, 2 H, CH2), 5.44 (s, 1 H, H10), 7.02–7.13 (m, 6 H, 6 x ArH), 7.35 (d, J = 7.32 Hz, 3 H, 3 x ArH), 7.46 (d, J = 6.71 Hz, 3 H, 3 x ArH). 13C NMR (101 MHz, CDCl3) ppm 25.7 (C1′), 52.3 (C9), 54.3 (C10), 72.8 (C2′), 121.2, 124.0, 125.2, 125.5, 146.5. HRMS (APCI) calculated for C22H17NO2 [M+] 327.1259: found 327.1255.
5.5.5. 9-Hydroxy-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (21k)
To a solution of anthrone (0.97 g, 5 mmol) in xylene (10 mL) was added maleimide (0.5 g, 5 mmol). The solution was heated at reflux for 1 h, then was cooled to room temperature, filtered and the precipitate was washed with toluene and hexane. The solid dried at room temperature and was recrystallized from toluene affording the product as a colourless solid, 846 mg (58%), Mp. 253–255 °C. IRVmax (ATR): 3474 (O-H), 3125, 2981 (C-H), 1752 (C=O), 1665 (C=C), 1571, 1478 (C=C) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.12 (d, J = 8.55 Hz, 1 H, H11), 3.32 (dd, J = 8.24, 2.75 Hz, 1 H, H10), 4.60–4.79 (m, 1 H, H15), 6.64 (s, 1 H, 1 x ArH), 7.13–7.29 (m, 4 H, 4 x ArH), 7.44 (d, J = 7.32 Hz, 1 H, 1 x ArH), 7.56 (t, J = 6.71 Hz, 2 H, 2 x ArH), 10.76 (br. s., 1 H, NH). 13C NMR (101 MHz, DMSO-d6) ppm 43.5 (C11), 49.0 (C10), 51.5 (C15), 76.6 (C9), 120.7 (CH), 121.2 (CH), 123.5 (CH), 124.3 (CH), 126.0 (CH), 126.2 (CH), 126.3 (CH), 126.4 (CH), 137.6, 140.2, 141.5, 145.1, 176.5 (C=O), 177.7 (C=O). HRMS (APCI) calculated for C18H14NO3 [M+ + H] 292.0974: found 292.0974.
5.5.6. (E)-((10-Chloroanthracen-9-yl)methylene)hydrazine (22d)
To a stirred solution of 10-chloro-9-anthraldehyde (1.16 g, 4.85 mmol) in DCM (20 mL) and ethanol 10 mL, was added dropwise hydrazine (1 g, 20 mmol). After 24 h at RT, the solvent evaporated and the residual solid was recrystallized from dichloromethane/hexane. The product was isolated as an orange solid 1.05 g (85%), Mp. 205–207 °C. IRVmax (KBr): 3351 (N-H), 3038, 2904 (C-H), 1621 (C=C), 1483, 1438 (C=C) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.30 (s, 2 H, NH2), 7.58–7.67 (m, 2 H, 2 x ArH), 7.67–7.76 (m, 2 H, 2 x ArH), 8.45 (d, J = 8.55 Hz, 2 H, 2 x ArH), 8.63 (d, J = 8.55 Hz, 2 H, 2 x ArH), 8.82 (s, 1 H, C1′). 13C NMR (101 MHz, DMSO-d6) ppm 109.6 (C10), 124.3 (CH), 126.2 (CH), 126.3 (CH), 127.0, 127.4 (CH), 127.9, 128.7, 129.4, 135.5 (C1′). HRMS (APCI) calculated for C15H12N2Cl [M+ + H] 255.0689: found 255.0684.
5.5.7. (E)-3-(12,14-Dioxo-9,10-[3,4]furanoanthracen-9(10H)-yl)acrylonitrile (23a)
Compound 23a was prepared from (E)-3-(anthracen-9-yl)acrylonitrile 22a (0.23 g, 1 mmol) and maleic anhydride (0.13 g, 1.3 mmol) according to general procedure 3, to yield the product as a colourless solid, 241 mg (74%), Mp. 215–219 °C. IRVmax (KBr): 3054, 2965 (C-H), 2218 (CN), 1863, 1700 (C=O), 1626 (C=C), 1458, 1436, 1403 (C=C), 1235 (C-O) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.77 (dd, J = 8.85, 3.36 Hz, 1 H, H11), 4.06 (d, J = 8.55 Hz, 1 H, H15), 4.94 (d, J = 3.05 Hz, 1 H, H10), 6.54 (d, J = 17.09 Hz, 1 H, H2′), 7.15–7.32 (m, 5 H, 5 x ArH), 7.33–7.47 (m, 2 H, 2 x ArH), 7.55 (d, J = 6.10 Hz, 1 H, 1 x ArH), 7.95 (d, J = 17.09 Hz, 1 H, H1′). 13C NMR (101 MHz, DMSO-d6) ppm 44.3 (C10), 48.8 (C11), 49.6 (C9), 51.6 (C15), 106.6 (CH), 117.6 (CN), 123.1 (CH), 123.5 (CH), 124.6 (CH), 125.3 (CH), 126.6 (CH), 127.2 (CH), 127.3 (CH), 127.6 (CH), 138.3, 138.6, 140.6, 141.0, 149.2 (C2′), 169.9 (C12), 170.8 (C14). HRMS (APCI) calculated for C21H12NO3 [M+ − H] 326.0823: found 326.0817.
5.5.8. (E)-3-(12,14-Dioxo-9,10-[3,4]epipyrroloanthracen-9(10H)-yl)acrylonitrile (23b)
Compound 23b was prepared from (E)-3-(anthracen-9-yl)acrylonitrile 22c (0.23 g, 1 mmol) and maleimide anhydride (0.13 g, 1.3 mmol) according to general procedure 3, to give the product as a colourless solid, 123 mg (38%), Mp. 289–300 °C. IRVmax (KBr): 3238 (N-H), 3064, 2970 (C-H), 2222 (CN), 1717 (C=O), 1636 (C=C), 1482, 1457.43 (C=C), 1341 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.26–3.38 (m, 1 H, H11), 3.63 (d, J = 8.54 Hz, 1 H, H15), 4.78 (d, J = 3.05 Hz, 1 H, H10), 6.51 (d, J = 17.09 Hz, 1 H, H1′), 6.88 (s, 1 H, H10), 7.17–7.27 (m, 3 H, 3 x ArH), 7.27–7.40 (m, 2 H, 2 x ArH), 7.52 (d, J = 5.49 Hz, 1 H, ArH), 7.91 (d, J = 17.09 Hz, 1 H, H2′), 10.88 (s, 1 H, NH). 13C NMR (101 MHz, DMSO-d6) ppm 44.4 (C10), 48.3 (C11), 48.7 (C15), 48.8 (C9), 106.1, 117.9 (CN), 122.9 (CH), 123.2 (CH), 124.2 (CH), 125.2 (CH), 126.2 (CH), 126.6 (CH), 126.8 (CH), 127.0 (CH), 135.2 (CH), 138.7, 139.0, 141.6, 141.9, 150.3 (C2′), 172.7 (C14*/C12*), 176.8 (C12), 177.1 (C14). HRMS (APCI) calculated for C21H13N2O2 [M+ − H] 325.0983: found 325.0975.
5.5.9. (E)-3-(12,14-Dioxo-13-phenyl-9,10-[3,4]epipyrroloanthracen-9(10H)-yl)acrylonitrile (23c)
Compound 23c was prepared from (E)-3-(anthracen-9-yl)acrylonitrile 22c (0.23 g, 1 mmol) and phenylmaleimide (0.22 g, 1.3 mmol) according to general procedure 3, to obtain the product as a colourless solid, 81 mg (20%), Mp. 274–281 °C. IRVmax (KBr): 3024, 2940 (C-H), 2221 (CN), 1714 (C=O), 1638, 1500 (C=C), 1595 (C=C), 1383 (CN) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.51 (dd, J = 8.54, 3.05 Hz, 1 H, H11), 3.82 (d, J = 8.54 Hz, 1 H, H15), 4.92 (d, J = 3.05 Hz, 1 H, H10), 6.38–6.45 (m, 2 H, H6″, H2″), 6.55 (d, J = 17.09 Hz, 1 H, H1′), 7.23–7.44 (m, 10 H, 10 x ArH), 7.59 (d, J = 5.49 Hz, 1 H, ArH), 7.95 (d, J = 17.09 Hz, 1 H, H2′). 13C NMR (101 MHz, DMSO-d6) ppm 45.1 (C10), 47.6 (C11), 47.6 (C15), 52.3 (C9), 106.6 (CN), 123.3 (CH), 123.6 (CH), 124.6 (CH), 125.4 (CH), 126.6 (CH), 126.7 (CH), 127.0 (CH), 127.2 (CH), 127.3 (CH), 128.7 (CH), 129.1 (C1′), 131.8, 138.8, 139.0, 141.3, 141.7, 150.3 (C2′), 174.8 (C12), 175.4 (C14). HRMS (APCI) calculated for C27H17N2O2 [M+ − H] 401.1296: found 401.1285.
5.5.10. 2-((12,14-Dioxo-9,10-[3,4]furanoanthracen-9(10H)-yl)methylene)malononitrile (23d)
Compound 23d was prepared from 2-(anthracen-9-ylmethylene)malononitrile 22a (0.35 g, 1 mmol) and maleic anhydride (0.13 g, 1.3 mmol) according to general procedure 3, to isolate the product as a colourless solid, 53 mg (15%), Mp. 196–198 °C. IRVmax (KBr): 2941, 2970 (C-H), 2231 (CN), 1864, 1776 (C=O), 1623 (C=C), 1551, 1457.45, (C=C), 1078 (C-O) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.74 (br. s., 1 H, H11), 4.12 (br. s., 1 H, H15), 5.00 (d, J = 3.05 Hz, 1 H, H10), 7.20–7.38 (m, 4 H, 4 x ArH), 7.44 (t, J = 6.41 Hz, 2 H, 2 x ArH), 7.52–7.65 (m, 2 H, 2 x ArH), 8.80 (br. s., 1 H, H1′). 13C NMR (101 MHz, DMSO-d6) ppm 44.3 (C10), 48.5 (C11), 50.2 (C15), 51.1 (C9), 113.1 (CN), 123.5 (CH), 125.3 (CH), 125.6 (CH), 126.6 (CH), 127.1 (CH), 127.7 (CH), 128.1 (CH), 163.6 (C1′), 170.4 (C14, C12). HRMS (APCI) calculated for C22H11N2O3 [M+ − H] 351.0775: found 351.0788.
5.5.11. 2-((12,14-Dioxo-9,10-[3,4]epipyrroloanthracen-9(10H)-yl)methylene)malononitrile (23e)
Compound 23e was prepared from 2-(anthracen-9-ylmethylene)malononitrile 22a (0.35 g, 1 mmol) and maleimide (0.13 g, 1.3 mmol) according to general procedure 3, to obtain the product as a colourless solid, 147 mg (42%), Mp. 281–284 °C. IRVmax (KBr): 3338 (N-H), 3063, 3010 (C-H), 2239 (CN), 1719 (C=O), 1467, 1457 (C=C), 1336 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.20–3.32 (m, 1 H, H11), 3.67 (br. s., 1 H H15), 4.83 (d, J = 2.44 Hz, 1 H, H10), 7.17–7.43 (m, 6 H, 6 x ArH), 7.43–7.69 (m, 2 H, 2 x ArH), 8.84 (br. s., 1 H, H1′), 10.95 (s, 1 H, NH).13C NMR (101 MHz, DMSO-d6) ppm 44.4 (C10), 48.1 (C11), 50.4 (C15), 52.3 (C9), 111.8 (CN), 113.3 (CN), 123.2 (CH), 123.4 (CH), 125.0 (CH), 125.5 (CH), 126.2 (CH), 126.5 (CH), 127.4 (CH), 127.5 (CH), 138.1, 140.9, 165.3 (C1′), 177.1 (C14, C12). HRMS (APCI) calculated for C22H12N3O2 [M+ − H] 350.0935: found 350.0925.
5.5.12. 2-((12,14-Dioxo-13-phenyl-9,10-[3,4]epipyrroloanthracen-9(10H)-yl)methylene)malononitrile (23f)
Compound 23f was prepared from 2-(anthracen-9-ylmethylene)malononitrile 22a (0.35 g, 1 mmol) and phenylmaleimide (0.22 g, 1.3 mmol) according to general procedure 3, to give the product as a colourless solid, 188 mg (44%), Mp. 255–258 °C. IRVmax (KBr): 3039, 2970 (C-H), 2240 (CN), 1712 (C=O), 1597 (C=C), 1470, 1457 (C=C), 1387 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.48 (br. s., 1 H, H11), 3.84 (br. s., 1 H, H15), 4.95 (br. s., 1 H, H10), 6.32–6.54 (m, 2 H, H2″, H6″), 7.20–7.70 (m, 11 H, 11 x ArH), 8.87 (br. s., 1 H, H1′). 13C NMR (101 MHz, DMSO-d6) ppm 44.9 (C10), 47.2 (C11), 49.6 (C15), 52.6 (C9), 113.2 (CN), 123.4 (CH), 125.2 (CH), 125.3 (CH), 125.6 (CH), 126.4 (CH), 126.7 (CH), 127.5 (CH), 127.6 (CH), 128.2, 128.6 (CH), 128.8 (CH), 131.4, 165.0 (C1′), 175.0 (C14, C12). HRMS (APCI) calculated for C28H18N3O2 [M+ + H] 428.1399: found 428.1382.
5.5.13. (E)-9-(Hydrazonomethyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (23g)
Compound 23g was prepared from (E)-(anthracen-9-ylmethylene)hydrazine 22c (0.25 g, 1 mmol) and maleimide (0.13 g, 1.3 mmol) according to general procedure 3, to yield the product as a colourless solid, 200 mg (63%), Mp. 245–247 °C. IRVmax (ATR): 3369 (NH), 3062, 2972 (C-H), 1710 (C=O), 1609 (C=C), 1483, 1457 (C=C), 1213 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.23 (d, J = 8.55 Hz, 1 H, H11), 3.44 (d, J = 8.55 Hz, 1 H, H15), 4.72 (br. s., 1 H, H10), 6.82 (br. s., 2 H, NH2), 7.16 (br. s., 4 H, 4 x ArH), 7.25–7.38 (m, 2 H, 2 x ArH), 7.46 (d, J = 5.49 Hz, 1 H, 1 x ArH), 7.53 (d, J = 6.10 Hz, 1 H, 1 x ArH), 8.06 (s, 1 H, H1′), 10.75 (br. s., 1 H, NH). 13C NMR (101 MHz, DMSO-d6) ppm 44.7 (C10), 48.6 (C11), 49.8 (C15), 50.2 (C9), 123.6 (CH), 123.9 (CH), 124.9 (CH), 125.7 (CH), 126.1 (CH), 126.2 (CH), 126.3 (CH), 137.9 (C1′), 139.1, 140.3, 142.0, 144.0, 177.0 (C12), 177.6 (C14). HRMS (APCI) calculated for C19H16N3O2 [M+ + H] 318.1243: found 318.1237.
5.5.14. (E)-9-(Hydrazonomethyl)-13-phenyl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (23h)
Compound 23h was prepared from (E)-(anthracen-9-ylmethylene)hydrazine 22c (0.25 g, 1 mmol) and phenylmaleimide (0.224 g, 1.3 mmol) according to general procedure 3, to give the product as a colourless solid, 171 mg (54%), Mp. 275–277 °C. IRVmax (ATR): 3630 (NH), 3072, 2981 (C-H), 1711 (C=O), 1628 (C=C), 1498, 1455 (C=C), 1203 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.44 (d, J = 7.94 Hz, 1 H, H11), 3.64 (d, J = 7.94 Hz, 1 H, H15), 4.85 (br. s., 1 H, H10), 6.42 (d, J = 5.49 Hz, 2 H, 2 x ArH), 6.84 (s, 2 H, NH2), 7.15–7.40 (m, 10 H, 10 x ArH), 7.58 (d, J = 6.71 Hz, 1 H, 1 x ArH), 7.53 (d, J = 6.10 Hz, 1 H, 1 x ArH), 8.09 (s, 1 H, H1′). 13C NMR (101 MHz, DMSO-d6) ppm 45.1 (C10), 47.6 (C11), 48.9 (C15), 50.6 (C9), 123.7 (CH), 124.0 (CH), 124.1 (CH), 124.9 (CH), 125.9 (CH), 126.3 (CH), 126.4 (CH), 126.4 (CH), 126.5 (CH), 128.4 (CH), 128.8 (CH), 131.8, 137.5 (C1′), 139.0, 140.1, 141.5, 143.7, 174.7 (C12), 175.5 (C14). HRMS (APCI) calculated for C19H16N3O2 [M+ + H] 318.1243: found 318.1237.
5.5.15. (E)-9-Chloro-10-(hydrazonomethyl)-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-dione (23i)
Compound 23i was prepared from (E)-((10-chloroanthracen-9-yl)methylene)hydrazine 22c (0.25 g, 1 mmol) and maleimide (0.13 g, 1.3 mmol) according to general procedure 3, yielding the product as a colourless solid 119 mg (34%), Mp. 241–244 °C. IRVmax (ATR): 3635 (NH), 3066, 2980 (C-H), 1713 (C=O), 1597 (C=C), 1498, 1455 (C=C), 1185 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.41 (d, J = 8.55 Hz, 1 H, H11), 3.63 (d, J = 8.55 Hz, 1 H, H15), 6.90 (br. s., 2 H, NH2), 7.24–7.36 (m, 4 H, 4 x ArH), 7.40 (d, J = 7.32 Hz, 1 H, 1 x ArH), 7.62 (br. s., 2 H, 2 x ArH), 7.74 (d, J = 7.32 Hz, 1 H, 1 x ArH), 8.03 (s, 1 H, H1′), 10.91 (br. s., 1 H, NH). 13C NMR (101 MHz, DMSO-d6) ppm 49.2 (C10), 51.5 (C11), 54.1 (C15), 70.7 (C9), 122.0 (CH), 122.6 (CH), 123.6 (CH), 126.7 (CH), 126.8 (CH), 127.0 (CH), 127.4 (CH), 136.7 (C1′), 137.9, 138.7, 141.0, 142.6, 173.9 (C12), 175.6 (C14). HRMS (APCI) calculated for C19H15ClN3O2 [M+ + H] 352.0853: found 352.0848.
5.5.16. (E)-12,14-Dioxo-9,10-[3,4]epipyrroloanthracene-9(10H)-carbaldehyde oxime (23j)
Compound 23j was prepared from (E)-anthracene-9-carbaldehyde oxime 22e (0.22 g, 1 mmol) and maleimide (0.13 g, 1.3 mmol) following general procedure 3. The product was obtained as a colourless solid, 137 mg (43%), Mp. 272–273 °C. IRVmax (ATR): 3043, 2939 (C-H), 1710 (C=O), 1595 (C=C), 1491, 1456 (C=C), 1192 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.28 (dd, J = 8.55, 3.05 Hz, 1 H, H11), 3.54 (d, J = 8.55 Hz, 1 H, H15), 4.77 (d, J = 3.05 Hz, 1 H, H10), 7.06–7.26 (m, 4 H, 4 x ArH), 7.33 (d, J = 4.88 Hz, 2 H, 2 X ArH), 7.45–7.62 (m, 2 H, 2 x ArH), 8.44 (s, 1 H, H1′), 10.83 (s, 1 H, NH), 11.49 (s, 1 H, =N-OH). 13C NMR (101 MHz, DMSO-d6) ppm 44.7 (C10), 48.4 (C11), 49.4 (C9), 49.9 (C15), 123.6 (CH), 124.1 (CH), 125.1 (CH), 125.9 (CH), 126.3 (CH), 126.6 (CH), 126.6 (CH), 139.0, 139.3, 141.8, 142.9, 147.5 (C1′), 177.0 (C12), 177.5 (C14). HRMS (APCI) calculated for C19H15N2O3 [M+ + H] 319.1083: found 319.1077.
5.5.17. (E)-12,14-Dioxo-13-phenyl-9,10-[3,4]epipyrroloanthracene-9(10H)-carbaldehyde oxime (23k)
Compound 23k was prepared from (E)-anthracene-9-carbaldehyde oxime 22e (0.22 g, 1 mmol) and phenylmaleimide (0.22 g, 1.3 mmol) following general procedure 3, The product was obtained as a colourless solid, 260 mg (66%), Mp. 238–242 °C. IRVmax (ATR): 3380 (OH), 3053, 2972 (C-H), 1760 (C=O), 1598 (C=C), 1483, 1457 (C=C), 1210 (C-N) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 3.48 (dd, J = 8.55, 3.05 Hz, 1 H, H11), 3.75 (d, J = 8.55 Hz, 1 H, H15), 4.90 (d, J = 3.05 Hz, 1 H, H10), 6.34–6.51 (m, 2 H, 2 x ArH), 7.14–7.43 (m, 9 H, 9 x ArH), 7.50–7.65 (m, 2 H, 2 x ArH), 8.49 (s, 1 H, H1′), 11.51 (s, 1 H, =N-OH). 13C NMR (101 MHz, DMSO-d6) ppm 45.0 (C10), 47.3 (C11), 49.0 (C9), 49.9 (C15), 123.7 (CH), 124.3 (CH), 125.1 (CH), 126.1 (CH), 126.5 (CH), 126.5 (CH), 126.7 (CH), 126.8 (CH), 128.5 (CH), 128.8 (CH), 131.7, 138.8, 139.0, 141.3, 142.5, 147.3 (C1′), 174.7 (C12), 175.4 (C14). HRMS (APCI) calculated for C25H19N2O3 [M+ + H] 395.1396: found 395.1390.
5.6. Stability Study of Compound 16a
The HPLC stability studies for compound 16a were performed using a Symmetry® column (C18, 5 µm, 4.6 × 150 mm), detector Waters 2487 Dual Wavelength Absorbance, HPLC pump: Waters 1525 binary and Autosampler: Waters 717 plus (Waters Corporation, Milford, MA, USA). Samples were detected at wavelength of 254 nm. The chromatography was performed with the following optimised conditions: mobile phase: (acetonitrile (80%): water (20%)), run time: 10 min, flow rate: 1 mL/min, with detection at 254 nm. Compound 16a (5 mg) was dissolved in the mobile phase (10 mL) to provide the stock solution for the experiment. The phosphate buffers were prepared following the British Pharmacopoeia monograph 2019 at the selected pH 4, pH 7.4 and pH 9. To a volume of the appropriate buffer (1 mL), stock solution (30 μL) was added, and the solution was shaken and then used immediately for the experiment. Samples of the solution were then analysed over the following 48 h period at selected time intervals (t = 0 min, 5 min, 30 min, 60 min, 90 min, 120 min, 24 h and 48 h).
5.7. Molecular Modelling
Using MOE (Molecular Operating Environment) 2016.V8 [71], the structures of interest compounds 13j, 16a, 16b and 19a were flexibly aligned with maprotiline 1 for structural comparison. 13j, 16a, 16b and 19a (displayed as yellow in their respective overlays) were flexibly aligned with the lead compound Maprotiline 1 (cyan), Figure 8. The molecular structures were processed using the MMFF94s force field, commonly used for small molecule modelling. Flexible alignment was conducted on each compound at 1000 iterations per run. The chirality of the stereogenic centres of the compounds was not defined. Default parameters were utilised for other settings. The proposed alignments featured as the top ranked alignment of generated poses, ranked in order of ascending S score (flexible alignment score comprising of both molecular strain energy and configuration similarity inputs).
5.8. X-ray Crystallography
Data for samples 16c, 16j, 17l, 17n, 19a and 20a were collected on a Bruker APEX DUO Kappa system with Mo Kα (λ = 0.71073 Å). Samples were mounted on a MiTeGen microloop and data collected at 100(2) K using an Oxford Instruments Cobra low temperature device. Bruker APEX [72] software was used for collection and reducing data and determination of the space group. Structures were solved employing direct methods (XT [73]) and subsequently refined using least squares minimization procedures (XL [74]) in Olex2 [75]. SADABS [76] was used to apply absorption corrections. Details of the crystal data, data collection and refinement are presented in Table S1. The trimethoxyphenyl group are disordered in 17l. The phenyl ring was modelled in two positions with the ipso carbon constrained by EXYZ/EADP. Occupancies are 65:35%. Two of the methoxy groups were further disordered and modelled in three positions in total. O30/O30c were constrained to the same position using EXYZ/EADP with O30/C31 34%; O30c/C31c 31% and O30b/C31b 34% occupied and restrained with DFIX, SIMU. O32/C32 34%; O32c/C32c 32%; O32b/C32b 34% occupied and restrained with DFIX and SIMU. The disordered trimethoxyphenyl atoms were restrained by SIMU. In 17n, the diffuse contribution of approximately 4 MeOH molecules per unit cell have been removed from the overall scattering by using SQUEEZE/PLATON. [77] CCDC 1938150–1938155 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
5.9. Biochemistry
5.9.1. Materials
The DG-75 BL cell line was kindly provided by Dr. Dermot Walls (School of Biotechnology, Dublin City University, Ireland). The MUTU-I (c179) cell line was provided by Professor Martin Rowe, (Division of Cancer Studies, The University of Birmingham, Birmingham, UK). alamarBlue was obtained from BioSource, Belgium and Fetal Bovine Serum (FBS) was sourced from Invitrogen, U.K. RPMI 1640 medium, HEPES and sodium pyruvate were sourced from Biosciences, Ireland. Cell culture consumables were purchased from Greiner Bio-One Ltd., U.K., while all reagents used were obtained from Sigma-Aldrich, Arklow, Ireland.
5.9.2. Cell Culture
The DG-75 Burkitt’s lymphoma cell line used in these experiments is a B-lymphocyte cell line which is derived from a metastatic pleural effusion (lung) isolated from a sporadic case of Burkitt’s lymphoma. The MUTU-I (c179) cell line is an isogenic stable group I BL cell line derived from a BL biopsy. MUTU-I and chemoresistant DG-75 cell lines were cultured in RPMI 1640 (Glutamax) medium which contains phenol red and is supplemented with 10% (v/v) foetal bovine serum (FBS), L-glutamine (2 mM) and 50 µg/mL penicillin/streptomycin. The MUTU-I cell line is supplemented with alpha-thioglycerol (5 mM in phosphate buffered saline (PBS) with 20 μM bathocuprione disulfonic acid), sodium pyruvate (100 mM) and HEPES (1 mM). The cells were grown in a humidified environment (95% O2 with 5% CO2) and passaged twice weekly as required depending on their levels of confluency.
5.9.3. AlamarBlue Viability Assay
BL cells were seeded in a 96-well plate (1–5 × 104 cells per 200 µL in well). Cells were treated with the desired drug concentration for a predetermined time frame and incubated as required. alamarBlue (20 µL) was added to each well and the cells were incubated at 37 °C in the dark for 4 h. Plates were then recorded on a fluorescence plate reader (SpectraMax Gemini, Molecular Devices) at 544 nm (excitement) and 590 nm (emission). Experiments were performed in triplicate using controls of reagent, medium and vehicle (100% viability). Taxol (10 μM) was used as the control drug (90% cytotoxicity) for both cell lines. The experiments were performed in triplicate and the mean value for three independent experiments was calculated.
5.9.4. Flow Cytometry
BL cells DG-75 and MUTU-1 (750,000) were treated with the specified concentration of the selected compound and incubated for 24 h (MUTU-1) or 48 h (DG-75) with taxol as the positive control. Samples were harvested by centrifugation and the pellets were rinsed with 0.5 mL of Ca2+ Annexin V binding buffer (0.1 M HEPES, pH 7.4; 1.4 M NaCl; 25 mM CaCl2) each and pelleted again by centrifugation. Supernatants were decanted and the pellets were suspended again in 50 µL FITC Annexin V (diluted 1:33 in Ca2+ Annexin V binding buffer), the samples were then placed on ice and incubated for 10 min in the dark. After the incubation period 0.5 mL of Ca2+ Annexin V binding buffer was added and samples were pelleted by centrifugation. The supernatant was decanted and the pellet was suspended in PI solution (500 µL; 0.5 µg/mL). After 1 h, the samples were recorded using a CyAn ADP flow cytometer (Beckman Coulter Inc., Brea, CA, USA). 10,000 cells were analysed with the Flowjo software package. Taxol was used as a positive control for cell death.
5.9.5. Cytotoxicity Assay
The CytoTox 96 non-radioactive cytotoxicity assay (Promega Corporation; 2800 Woods Hollow Road, Madison, WI, USA) was used to determine the cyctotoxicity of selected compounds. BL cells were incubated for 24 hr and then treated with the selected compounds (at 10 μM concentration) 15, 16b and 16c as in the cell viability assay. After 24 or 48 h as appropriate, the lysis solution (10X), (20 μL) was added. The cells were incubated for a further 1 h to ensure complete death. The supernatant (50 μL) was removed and transferred to a new 96-well plate. “Substrate mix″ (50 μL) was added and the plate was retained in the dark at RT for 30 min. “Stop solution″ (50 μL) was added to each well and the absorbance was recorded at 490 nm with a Dynatech MR5000 plate reader and the cell death was calculated.
5.9.6. Generation of Human Peripheral Blood Mononuclear Cells (PBMCs)
Blood was obtained from healthy donors (n = 2) after informed consent was received. The blood was then placed into a 50 mL falcon tube and diluted with phosphate-buffered saline (PBS), 1:2. The blood was separated into red blood cells, white blood cell ring and serum using LymphoPrep. The blood was slowly added to Ficoll pague plus (20 mL). The tubes containing the blood were then centrifuged for 30 min at 1700 g. Following transfer of the white blood cell ring to a new 50 mL tube, the volume was adjusted to 50 mL. The samples were again centrifuged for 10 min at 1700 g. The, supernatant liquid was removed; and this step was repeated. The resultant pellet was then resuspended in 10 mL of complete Iscove’s Modified Dulbecco’s (IMDM) media (10% FBS, 0.1% Ciprofloxacin (10 mg/mL)) for use in the cell viability experiments.
5.9.7. Inhibitor Studies: Reactive Oxygen Species
N-Acetylcysteine (Sigma) was dissolved in sterile water (100 mM). Fresh solutions were prepared for each experiment. A 96 well plate was seeded with 2 × 104 cells/well for DG-75 cells. After 23 h, the DG-75 cells were then pre-treated with NAC (5 mM) for 1 h before compound treatment. The wells were then treated with the desired drug concentration (1 µM) for a predetermined time frame (48 h). After treatment and incubation, each well was treated with 20 µL of alamarBlue (37 °C) and the plate was incubated in the dark at 37 °C for 4–6 h. Fluorescence of the 96 well plates was then recorded at 590 nm (excitation 544 nm), as described for ‘alamarBlue viability assay’ (above). The percentage of viable cells remaining with NAC pre-treatment was compared to the percentage of viable cells remaining without NAC pre-treatment in order to determine the implication of ROS in the mechanism of cell death induced.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-8247/13/1/16/s1, Details of synthesis and spectroscopic characterization data for compounds 11a–11n, 11p–11r, 20a, 20f, 20g, 21a–j, 22a, 22b, 22c, 22e; 1H and 13C NMR spectra for compounds 13a, 16j, 19a, 20d and 23g (Figures S1–S23); Tier 1 Profiling screen of selected ethanoanthracenes and related compounds, (Table S1); Lipinski properties for selected ethanoanthracenes and related compounds, (Table S2).
Author Contributions
A.J.B. synthesised and characterised compounds in the studies according to Scheme 1, Scheme 2, Scheme 3, Scheme 4 and Scheme 5, performed cell studies and generated data in Table 1, Table 2, Table 3, Table 6, Table 7, Table 8, Table 9, Table 10 and Table 11, performed data analysis and interpreted data. J.P.M. synthesised and characterised some molecules in Scheme 1 and Scheme 2 and performed the molecular modelling studies in Figure 3. D.C.W. and S.A.B. designed and supervised the biochemical studies. S.A.B. designed and performed biochemical experiments and generated the data in Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8, performed data analysis and interpreted data. J.E.O. provided analysis and interpretation of the NMR data, Figures S1–S23. D.F. generated the Tier-1 profiling screen and ADMETox data in Tables S1 and S2. X-ray Crystallographic structures were determined by B.T. (Figure 2, Table 4 and Table 5). M.J.M. designed the chemistry studies, wrote drafts of the manuscript and submitted the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Postgraduate research scholarships from Trinity College Dublin (A.J.B., J.P.M.) are gratefully acknowledged.
Acknowledgments
We thank Dermot Walls (School of Biotechnology, Dublin City University, Ireland) for the gift of the DG-75 BL cell line. The contribution from Thomas Fraisse (HPLC) is also appreciated. The Trinity Biomedical Sciences Institute (TBSI) is supported by a capital infrastructure investment from Cycle 5 of the Irish Higher Education Authority’s Programme for Research in Third Level Institutions (PRTLI). D.F. thanks the software vendors for their continuing support of academic research efforts, in particular the contributions of Biovia, the Chemical Computing Group, and OpenEye Scientific. The support and provisions of Dell Ireland, the Trinity Centre for High Performance Computing (TCHPC) and the Irish Centre for High-End Computing (ICHEC) are also gratefully acknowledged.
Conflicts of Interest
The authors confirm that this article content has no conflict of interest.
Abbreviations
| Abbreviations | Full Name |
| AKT | protein kinase B |
| BL | Burkitt’s lymphoma |
| C-H COSY | Carbon-Hydrogen Correlation Spectroscopy |
| CLL | Chronic lymphocytic leukaemia |
| DEPT | Distortionless Enhancement by Polarization Transfer |
| EBV | Epstein Barr virus |
| ER | Estrogen Receptor |
| HMBC | Heteronuclear Multiple Bond Correlation |
| LDH | Lactose dehydrogenase |
| MS | Mass Spectrometry |
| mTOR | mammalian target of rapamycin |
| NAC | N-Acetylcysteine |
| NET | Norepinephrine transporter |
| NF–κΒ | nuclear factor kappa B |
| NMR | Nuclear Magnetic Resonance |
| MOE | Molecular Operating Environment |
| PBMCs | Peripheral blood mononuclear cells |
| PBS | Phosphate-buffered saline |
| PI3K | Phosphatidylinositol 3-kinase |
| PTP | protein tyrosine phosphatase |
| PUMA | p53 upregulated modulator of apoptosis |
| ROS | Reactive oxygen species |
| RXRα | Retinoid X receptor alpha |
| SAR | Structure activity relationship |
| SERT | Serotonin reuptake transporter |
| SSRI | Selective serotonin reuptake inhibitor |
| TNFα | tumor necrosis factor alpha |
References
- Blum, K.A.; Lozanski, G.; Byrd, J.C. Adult Burkitt leukemia and lymphoma. Blood 2004, 104, 3009–3020. [Google Scholar] [CrossRef] [PubMed]
- Dozzo, M.; Carobolante, F.; Donisi, P.M.; Scattolin, A.; Maino, E.; Sancetta, R.; Viero, P.; Bassan, R. Burkitt lymphoma in adolescents and young adults: Management challenges. Adolesc. Health Med. Ther. 2017, 8, 11–29. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Registry Ireland. Cancer in Ireland 1994–2016 with Estimates for 2016–2018: Annual Report of the National Cancer Registry; NCRI: Cork, Ireland, 2018. [Google Scholar]
- Anderton, E.; Yee, J.; Smith, P.; Crook, T.; White, R.E.; Allday, M.J. Two Epstein-Barr virus (EBV) oncoproteins cooperate to repress expression of the proapoptotic tumour-suppressor bim: Clues to the pathogenesis of Burkitt’s lymphoma. Oncogene 2008, 27, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Phase II Study of Dose-Adjusted EPOCH-Rituximab in Adults with Untreated Burkitt Lymphoma and C-Myc+ Diffuse Large B-Cell Lymphoma-Full Text View-Clinicaltrials. Gov. Available online: Http://clinicaltrials.Gov/ct2/show/nct01092182?Term=burkitt+lymphoma&rank=1 (accessed on 24 October 2019).
- Chemotherapy Plus Rituximab Combination for Adult Lymphoblastic Leukemia (B-ALL) and Burkitt’s Non-Hodgkin Lymphoma-Full Text View-Clinicaltrials. Gov. Available online: Http://clinicaltrials.Gov/ct2/show/nct01290120?Term=burkitt+lymphoma&rank=2 (accessed on 24 October 2019).
- Thomas, D.A.; Faderl, S.; O’Brien, S.; Bueso-Ramos, C.; Cortes, J.; Garcia-Manero, G.; Giles, F.J.; Verstovsek, S.; Wierda, W.G.; Pierce, S.A.; et al. Chemoimmunotherapy with hyper-cvad plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer 2006, 106, 1569–1580. [Google Scholar] [CrossRef]
- Grogg, K.L.; Miller, R.F.; Dogan, A. HIV infection and lymphoma. J. Clin. Pathol. 2007, 60, 1365–1372. [Google Scholar] [CrossRef]
- Guech-Ongey, M.; Simard, E.P.; Anderson, W.F.; Engels, E.A.; Bhatia, K.; Devesa, S.S.; Mbulaiteye, S.M. Aids-related Burkitt lymphoma in the United States: What do age and CD4 lymphocyte patterns tell us about etiology and/or biology? Blood 2010, 116, 5600–5604. [Google Scholar] [CrossRef]
- Easmon, J.; Puerstinger, G.; Roth, T.; Fiebig, H.H.; Jenny, M.; Jaeger, W.; Heinisch, G.; Hofmann, J. 2-benzoxazolyl and 2-benzimidazolyl hydrazones derived from 2-acetylpyridine: A novel class of antitumor agents. Int. J. Cancer 2001, 94, 89–96. [Google Scholar] [CrossRef]
- Hu, T.; Gao, Y. Beta-elemene against Burkitt’s lymphoma via activation of PUMA mediated apoptotic pathway. Biomed. Pharmacother. 2018, 106, 1557–1562. [Google Scholar] [CrossRef]
- Lenalidomide and Blinatumomab in Treating Patients with Relapsed Non-Hodgkin Lymphoma. Available online: https://clinicaltrials.gov/ct2/show/NCT02568553 (accessed on 24 October 2019).
- Ni, F.; Huang, X.; Chen, Z.; Qian, W.; Tong, X. Shikonin exerts antitumor activity in Burkitt’s lymphoma by inhibiting C-MYC and PI3K/AKT/mTOR pathway and acts synergistically with doxorubicin. Sci. Rep. 2018, 8, 3317. [Google Scholar] [CrossRef]
- Zhelev, Z.; Ohba, H.; Bakalova, R.; Hadjimitova, V.; Ishikawa, M.; Shinohara, Y.; Baba, Y. Phenothiazines suppress proliferation and induce apoptosis in cultured leukemic cells without any influence on the viability of normal lymphocytes. Phenothiazines and leukemia. Cancer Chemother. Pharmacol. 2004, 53, 267–275. [Google Scholar] [CrossRef]
- Park, J.P.E.; Ahn, B.H.; Kim, H.J.; Park, J.H.; Koo, S.Y.; Kwak, H.S.; Park, H.S.; Kim, D.W.; Song, M.; Yim, H.J.; et al. Necrox-7 prevents oxidative stress-induced cardiomyopathy by inhibition of nadph oxidase activity in rats. Toxicol. Appl. Pharmacol. 2012, 263, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-J.; Lee, E.S.; Im, K.-I.; Kim, S.H.; Lim, J.-Y.; Kim, N.; Cho, S.-G. In vitro evaluation of a novel chemotherapeutic agent, NecroX-7, in human Burkitt lymphoma cells. Cancer Res. 2014, 74, 2720. [Google Scholar]
- Gobec, M.; Obreza, A.; Prijatelj, M.; Brus, B.; Gobec, S.; Mlinaric-Rascan, I. Selective cytotoxicity of amidinopiperidine based compounds towards Burkitt’s lymphoma cells involves proteasome inhibition. PLoS ONE 2012, 7, e41961. [Google Scholar] [CrossRef] [PubMed]
- Cortiguera, M.G.; Batlle-López, A.; Albajar, M.; Delgado, M.D.; León, J. MYC as therapeutic target in leukemia and lymphoma. Blood Lymphat. Cancer Targets Ther. 2015, 5, 75–91. [Google Scholar]
- Poole, C.J.; Zheng, W.; Lee, H.; Young, D.; Lodh, A.; Chadli, A.; van Riggelen, J. Targeting the MYC oncogene in Burkitt lymphoma through HSP90 inhibition. Cancers 2018, 10, 448. [Google Scholar] [CrossRef]
- Cloonan, S.M.; Drozgowska, A.; Fayne, D.; Williams, D.C. The antidepressants maprotiline and fluoxetine have potent selective antiproliferative effects against Burkitt lymphoma independently of the norepinephrine and serotonin transporters. Leuk. Lymphoma 2010, 51, 523–539. [Google Scholar] [CrossRef]
- Cloonan, S.M.; Williams, D.C. The antidepressants maprotiline and fluoxetine induce type II autophagic cell death in drug-resistant Burkitt’s lymphoma. Int. J. Cancer 2011, 128, 1712–1723. [Google Scholar] [CrossRef]
- Meredith, E.J.; Holder, M.J.; Chamba, A.; Challa, A.; Drake-Lee, A.; Bunce, C.M.; Drayson, M.T.; Pilkington, G.; Blakely, R.D.; Dyer, M.J.; et al. The serotonin transporter (SLC6A4) is present in B-cell clones of diverse malignant origin: Probing a potential anti-tumor target for psychotropics. FASEB J. 2005, 19, 1187–1189. [Google Scholar] [CrossRef]
- Xia, Z.; Bergstrand, A.; DePierre, J.W.; Nassberger, L. The antidepressants imipramine, clomipramine, and citalopram induce apoptosis in human acute myeloid leukemia HL-60 cells via caspase-3 activation. J. Biochem. Mol. Toxicol. 1999, 13, 338–347. [Google Scholar] [CrossRef]
- Xia, Z.; DePierre, J.W.; Nassberger, L. Modulation of apoptosis induced by tricyclic antidepressants in human peripheral lymphocytes. J. Biochem. Mol. Toxicol. 1998, 12, 115–123. [Google Scholar] [CrossRef]
- Xia, Z.; Karlsson, H.; DePierre, J.W.; Nassberger, L. Tricyclic antidepressants induce apoptosis in human T lymphocytes. Int. J. Immunopharmacol. 1997, 19, 645–654. [Google Scholar] [CrossRef]
- Gandy, M.N.; McIldowie, M.; Lewis, K.; Wasik, A.M.; Salomonczyk, D.; Wagg, K.; Millar, Z.A.; Tindiglia, D.; Huot, P.; Johnston, T.; et al. Redesigning the designer drug ecstasy: Non-psychoactive mdma analogues exhibiting Burkitt’s lymphoma cytotoxicity. MedChemComm 2010, 1, 287–293. [Google Scholar] [CrossRef]
- McNamara, Y.M.; Bright, S.A.; Byrne, A.J.; Cloonan, S.M.; McCabe, T.; Williams, D.C.; Meegan, M.J. Synthesis and antiproliferative action of a novel series of maprotiline analogues. Eur. J. Med. Chem. 2014, 71, 333–353. [Google Scholar] [CrossRef] [PubMed]
- Bova, S.S.S.; Rampa, A.; Gobbi, S.; Cima, L.; Fusi, F.; Sgaragli, G.; Cavalli, M.; de los Rios, C.; Striessnig, J.; Bisi, A.; et al. Anthracene based compounds as new L-type Ca2+ channel blockers: Design, synthesis, and full biological profile. J. Med. Chem. 2009, 52, 1259–1262. [Google Scholar] [CrossRef]
- Yang, B.V.; Vaccaro, W.; Doweyko, A.M.; Doweyko, L.M.; Huynh, T.; Tortolani, D.; Nadler, S.G.; McKay, L.; Somerville, J.; Holloway, D.A.; et al. Discovery of novel dihydro-9,10-ethano-anthracene carboxamides as glucocorticoid receptor modulators. Bioorg. Med. Chem. Lett. 2009, 19, 2139–2143. [Google Scholar] [CrossRef]
- Bisi, A.; Mokhtar Mahmoud, A.; Allara, M.; Naldi, M.; Belluti, F.; Gobbi, S.; Ligresti, A.; Rampa, A. Polycyclic maleimide-based scaffold as new privileged structure for navigating the cannabinoid system opportunities. ACS Med. Chem. Lett. 2019, 10, 596–600. [Google Scholar] [CrossRef]
- Bisi, A.; Gobbi, S.; Merolle, L.; Farruggia, G.; Belluti, F.; Rampa, A.; Molnar, J.; Malucelli, E.; Cappadone, C. Design, synthesis and biological profile of new inhibitors of multidrug resistance associated proteins carrying a polycyclic scaffold. Eur. J. Med. Chem. 2015, 92, 471–480. [Google Scholar] [CrossRef]
- Alibert, S.; Santelli-Rouvier, C.; Castaing, M.; Berthelot, M.; Spengler, G.; Molnar, J.; Barbe, J. Effects of a series of dihydroanthracene derivatives on drug efflux in multidrug resistant cancer cells. Eur. J. Med. Chem. 2003, 38, 253–263. [Google Scholar] [CrossRef]
- Bonvicini, F.; Manet, I.; Belluti, F.; Gobbi, S.; Rampa, A.; Gentilomi, G.A.; Bisi, A. Targeting the bacterial membrane with a new polycyclic privileged structure: A powerful tool to face staphylococcus aureus infections. ACS Infect. Dis. 2019, 5, 1524–1535. [Google Scholar] [CrossRef]
- Alibert, S.; Santelli-Rouvier, C.; Pradines, B.; Houdoin, C.; Parzy, D.; Karolak-Wojciechowska, J.; Barbe, J. Synthesis and effects on chloroquine susceptibility in plasmodium falciparum of a series of new dihydroanthracene derivatives. J. Med. Chem. 2002, 45, 3195–3209. [Google Scholar] [CrossRef]
- Arya, S.K.S.; Rani, R.; Kumar, N.; Roy, P.; Sondhi, S.M. Synthesis, anti-inflammatory, and cytotoxicity evaluation of 9,10-dihydroanthracene-9,10-α, β-succinimide and bis-succinimide derivatives. Med. Chem. Res. 2013, 22, 4278–4285. [Google Scholar] [CrossRef]
- Rossiter, S.; Kirton, S.B.; Camara, R. Preparation of Substituted 3,4-(9′, 10′-dihydroanthracene-9′,10′-diyl) Succinimides as Inhibitors of S100P/RAGE Interaction for Treating Pancreatic Cancer. PCT Int. Appl. (2016). WO 2016181120 A1, 17 November 2016. [Google Scholar]
- Pettit, R.K.; Pettit, G.R.; Hamel, E.; Hogan, F.; Moser, B.R.; Wolf, S.; Pon, S.; Chapuis, J.C.; Schmidt, J.M. E-combretastatin and E-resveratrol structural modifications: Antimicrobial and cancer cell growth inhibitory beta-E-nitrostyrenes. Bioorg. Med. Chem. 2009, 17, 6606–6612. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Rastogi, N.; Namboothiri, I.N.; Mobin, S.M.; Panda, D. Synthesis and evaluation of alpha-hydroxymethylated conjugated nitroalkenes for their anticancer activity: Inhibition of cell proliferation by targeting microtubules. Bioorg. Med. Chem. 2006, 14, 8073–8085. [Google Scholar] [CrossRef] [PubMed]
- Kaap, S.; Quentin, I.; Tamiru, D.; Shaheen, M.; Eger, K.; Steinfelder, H.J. Structure activity analysis of the pro-apoptotic, antitumor effect of nitrostyrene adducts and related compounds. Biochem. Pharmacol. 2003, 65, 603–610. [Google Scholar] [CrossRef]
- Calgarotto, A.K.; da Silva Pereira, G.J.; Bechara, A.; Paredes-Gamero, E.J.; Barbosa, C.M.; Hirata, H.; de Souza Queiroz, M.L.; Smaili, S.S.; Bincoletto, C. Autophagy inhibited Ehrlich ascitic tumor cells apoptosis induced by the nitrostyrene derivative compounds: Relationship with cytosolic calcium mobilization. Eur. J. Pharmacol. 2012, 678, 6–14. [Google Scholar] [CrossRef]
- Wang, L.G.; Liu, X.M.; Kreis, W.; Budman, D.R. The effect of antimicrotubule agents on signal transduction pathways of apoptosis: A review. Cancer Chemother. Pharmacol. 1999, 44, 355–361. [Google Scholar] [CrossRef]
- Reddy, M.A.; Jain, N.; Yada, D.; Kishore, C.; Vangala, J.R.P.; Surendra, R.; Addlagatta, A.; Kalivendi, S.V.; Sreedhar, B. Design and synthesis of resveratrol-based nitrovinylstilbenes as antimitotic agents. J. Med. Chem. 2011, 54, 6751–6760. [Google Scholar] [CrossRef]
- Tsai, C.H.; Hung, A.C.; Chen, Y.Y.; Chiu, Y.W.; Hsieh, P.W.; Lee, Y.C.; Su, Y.H.; Chang, P.C.; Hu, S.C.; Yuan, S.F. 3′-Hydroxy-4′-methoxy-beta-methyl-beta-nitrostyrene inhibits tumorigenesis in colorectal cancer cells through ROS-mediated DNA damage and mitochondrial dysfunction. Oncotarget 2017, 8, 18106–18117. [Google Scholar] [CrossRef]
- Hung, A.C.; Tsai, C.H.; Hou, M.F.; Chang, W.L.; Wang, C.H.; Lee, Y.C.; Ko, A.; Hu, S.C.; Chang, F.R.; Hsieh, P.W.; et al. The synthetic beta-nitrostyrene derivative CYT-Rx20 induces breast cancer cell death and autophagy via ROS-mediated MEK/ERK pathway. Cancer Lett. 2016, 371, 251–261. [Google Scholar] [CrossRef]
- Hsieh, P.W.; Chang, Y.T.; Chuang, W.Y.; Shih, H.C.; Chiang, S.Z.; Wu, C.C. The synthesis and biologic evaluation of anti-platelet and cytotoxic beta-nitrostyrenes. Bioorg. Med. Chem. 2010, 18, 7621–7627. [Google Scholar] [CrossRef]
- He, Y.; Varadarajan, S.; Munoz-Planillo, R.; Burberry, A.; Nakamura, Y.; Nunez, G. 3,4-methylenedioxy-beta-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J. Biol. Chem. 2014, 289, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.C.; Finnon, Y.S.; Daeid, N.N.; Robson, D.C.; Waddell, R. The effect of nitrostyrene on cell proliferation and macrophage immune responses. Immunopharmacol. Immunotoxicol. 2002, 24, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Sun, Z.; Huang, M.; Zhang, W.; Liu, J.; Chen, L.; Chen, F.; Zhou, Y.; Lin, J.; Huang, F.; et al. Nitrostyrene derivatives act as RXRalpha ligands to inhibit TNFalpha Activation of NF-kappaB. Cancer Res. 2015, 75, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- McNamara, Y.M.; Cloonan, S.M.; Knox, A.J.; Keating, J.J.; Butler, S.G.; Peters, G.H.; Meegan, M.J.; Williams, D.C. Synthesis and serotonin transporter activity of 1,3-bis(aryl)-2-nitro-1-propenes as a new class of anticancer agents. Bioorg. Med. Chem. 2011, 19, 1328–1348. [Google Scholar] [CrossRef]
- Byrne, A.J.; Bright, S.A.; Fayne, D.; McKeown, J.P.; McCabe, T.; Twamley, B.; Williams, C.; Meegan, M.J. Synthesis, antiproliferative and pro-apoptotic effects of nitrostyrenes and related compounds in burkitt’s lymphoma. Med. Chem. 2018, 14, 181–199. [Google Scholar] [CrossRef]
- Bright, S.A.; Byrne, A.J.; Vandenberghe, E.; Browne, P.V.; McElligott, A.M.; Meegan, M.J.; Williams, D.C. Selected nitrostyrene compounds demonstrate potent activity in chronic lymphocytic leukaemia cells, including those with poor prognostic markers. Oncol. Rep. 2019, 41, 3127–3136. [Google Scholar] [CrossRef]
- Research Council of Lithuania, Crystallography Open Database. 2010. Available online: http://www.crystallography.net/cod/2003498.html (accessed on 24 October 2019).
- Weber, E.; Finge, S.; Csoregh, I. Modular design of hosts involving a rigid succinimide framework and N-bonded lateral groups. Crystalline inclusion properties and crystal structures of inclusion compounds with dioxane, MeOH, and DMF. J. Org. Chem. 1991, 56, 7281–7288. [Google Scholar] [CrossRef]
- Alston, P.V.; Ottenbrite, R.M.; Newby, J. Regioselectivity in the Diels-Alder reaction of 9-substituted anthracenes. J. Org. Chem. 1979, 44, 4939–4943. [Google Scholar] [CrossRef]
- Russell, G.A.; Whittle, P.R.; Keske, R.G.; Holland, G.; Aubuchon, C. Application of electron spin resonance spectroscopy to problems of structure and conformation. XXIV. Aliphatic semidiones. XIX. Polycyclic derivatives of cyclobutanesemidione. J. Am. Chem. Soc. 1972, 94, 1693–1698. [Google Scholar] [CrossRef]
- Yamamoto, G.; Koseki, A.; Sugita, J.; Mochida, H.; Minoura, M. Deamination of 1-alkyl-9-aminomethyltriptycenes. Participation of a neighboring 1-alkyl substituent. Bull. Chem. Soc. Jpn. 2006, 79, 1585–1600. [Google Scholar] [CrossRef]
- Logullo, F.M.; Seitz, A.H.; Friedman, L. Benzenediazonium-2-carboxylate and biphenylene. Org. Synth. 1968, 48, 12–17. [Google Scholar]
- Friedman, L.; Logullo, F.M. Benzynes via aprotic diazotization of anthranilic acids: A convenient synthesis of triptycene and derivatives. J. Am. Chem. Soc. 1963, 85, 1549. [Google Scholar] [CrossRef]
- Gouda, M.A.; Berghot, M.A.; Elattar, K.M.; Khalil, A.E.G.M. Chemistry of dibenzobarallene. Turk. J. Chem. 2011, 35, 663–697. [Google Scholar]
- Brunovlenskaya, I.I.; Sengal, N.; Skvarchenko, V.R. Deamination of certain amines with amino group at the substituent in the head of a triptycene molecule bridge. Dokl. Akad. Nauk. SSSR 1975, 225, 1323. [Google Scholar] [CrossRef]
- Wilhelm, M.; Schmidt, P. Synthesis and properties of 1-aminoalkyl-dibenzo(b,e)bicyclo(2,2,2) octadienes. Helv. Chim. Acta 1969, 52, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.D.; Soerensen, H.; Sandros, K. Photochemical isomerization and dimerization of 1-(9-anthryl)-2-nitroethylene. J. Org. Chem. 1986, 51, 3223–3226. [Google Scholar] [CrossRef]
- Yamamoto, G.; Mochida, H. Deamination of 1-alkyl-9-aminomethyltriptycenes: Participation of the α-C-H bond of the 1-alkyl group. Chem. Lett. 2000, 29, 454–455. [Google Scholar] [CrossRef]
- Becker, H.D.; Hansen, L.; Andersson, K. Synthesis and photochemical isomerization of 1,2-di-9-anthrylethanol and 1,2-di-9-anthrylethanone. J. Org. Chem. 1986, 51, 2956–2961. [Google Scholar] [CrossRef]
- Ishii, A.; Kawai, T.; Noji, M.; Nakayama, J. Synthesis and reactions of a monosubstituted dithiirane 1-oxide, 3-(9-triptycyl) dithiirane 1-oxide. Tetrahedron 2005, 61, 6693–6699. [Google Scholar] [CrossRef]
- Tomioka, H.; Nakajima, J.; Mizuno, H.; Iiba, E.; Hirai, K. Spectroscopic studies on effects of triptycyl group on structures and reactivities of triplet arylcarbenes. Can. J. Chem. 1999, 77, 1066–1076. [Google Scholar] [CrossRef]
- Sauers, C.K.; Cotter, R.J. Preparation of Maleimides. U.S. Patent 3018290 A, 23 January 1962. [Google Scholar]
- Koremura, M.; Oku, H.; Shono, T.; Nakanishi, T. Relation between chemical structure and antimicrobial and insecticidal activities in organonitro compounds. VIII. Synthesis of heterocyclic nitroalkene derivatives and their activities. Takamine Kenkyusho Nenpo 1961, 13, 216–221. [Google Scholar]
- Becker, H.D.; Skelton, B.W.; Sorensen, H.; White, A.H. Photochemical cis-trans isomerization of 2-substituted 9-(2-nitroethenyl)anthracenes. X-ray structure analyses of (E)-and (Z)-9-(2-nitro-2-phenylethenyl)anthracene. Aust. J. Chem. 1989, 42, 593–601. [Google Scholar] [CrossRef]
- Nakajima, S.; Osuka, A. Synthesis of a tetrakis(9-anthryl) substituted porphyrin and intramolecular charge-transfer emission in its dication. Tetrahedron Lett. 1995, 36, 8457–8460. [Google Scholar] [CrossRef]
- Molecular Operating Environment (MOE), 2015.10; 1010 Sherbooke St. West, Suite #910; Chemical Computing Group Inc.: Montreal, QC, Canada, 2015; Available online: https://www.chemcomp.com/Products.html (accessed on 16 January 2020).
- Bruker APEX2 v2012.12-0; Bruker AXS Inc.: Madison, WI, USA, 2012; Available online: https://www.bruker.com/nc/search.html?q=Bruker%20APEX2%202012.12-0;&resetPager=1 (accessed on 16 January 2020).
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELX. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- SADABS, Area Detector Absorption Correction Program, Sheldrick, G.M. University of Göttingen, Germany, 2014. Available online: https://journals.iucr.org/e/services/stdswrefs.html (accessed on 16 January 2020).
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).