1. Introduction
Since ancient times, the plant kingdom has been an important source for the discovery of new drugs, and several therapeutic agents have been isolated from a variety of plant species [
1,
2,
3,
4,
5,
6]. The document “Strategies on Traditional Medicine” from the World Health Organization (WHO), promotes the strengthening of quality assurance, safety, and proper use of medicinal plants, which suggests the regulation of products and practices associated with plants used in folk medicine [
7]. This is a necessity because the natural products derived from plant species may not contain the active component in sufficient quantities, due to environmental influences. Additionally, the identity of the active compound is often unknown [
8]. This may be a limitation since it is known that molecular weight, charge density, solubility, and/or elementary distribution all play an important role in the possible biological activities of these products [
9]. Thus, although the population uses several plant-derived products due to their therapeutic properties, obtaining purified compounds for high-precision identification is still necessary to further characterize their composition [
10].
One plant with great cultural and medicinal value is
Anadenanthera colubrina var cebil (Griseb.) Altschul, a plant from the Fabaceae family. In particular,
A. colubrina is considered one of the most essential medicinal plants of the Caatinga and Cerrado area (the Brazilian semi-arid ecosystems), where it is popularly known as the red angico tree [
11,
12].
A. colubrina stands out due its medicinal properties, which include the application for treatment of inflammatory disorders in different tissues [
13,
14]. Several studies have shown the various pharmacological properties of products derived from
A. colubrina (mostly extracts and fractions from the leaves and roots) that include antimicrobial [
12,
15,
16], antioxidant [
17,
18,
19,
20], wound-healing [
21,
22], anti-inflammatory [
23], antinociceptive [
24], and antiproliferative properties [
18,
25]. Despite the therapeutic potential, little information is available regarding the chemical properties and biological activities of the biopolymer extracted from the trunk exudate of
A. colubrina, the red angico gum (RAG), of northeast Brazil. Previous studies [
26,
27,
28] have reported that high performance liquid chromatography’s composition of RAG was high in arabinose (67.8%), followed by galactose (24.1%), and a small amount of rhamnose (2.0%), being a high arabinose heterosaccharide with a main chain composed of β- D-Galp, also including α-L-Araf and β-L-Arap [
26]. Ethnopharmacological studies show that RAG is widely used in folk medicine. Lorenzi [
29] reported that this exudate has been consumed since ancient times by the locals, and water-soluble preparations are commonly used as a pectoral emollient, in broncho-pulmonary disorders, including cough, bronchitis, asthma, and pharyngitis, thereby facilitating sputum expulsion. This same study also describes the use of the RAG exudate for the treatment of gastrointestinal diseases, including diarrhea.
Natural gums are polysaccharides obtained from the exudates of tree trunks, seeds, algae, or by microbiological fermentation. They tend to hydrate in cold or hot water, forming colloidal dispersions, highly viscous solutions, or even gels and have numerous technological and medicinal applications [
30]. The polysaccharides extracted have potential to be used as novel bioactive products for therapeutic treatments. Previous studies performed by our research group have shown beneficial effects alleviating gastro-intestinal complaints, such as naproxen-induced gastric ulcers [
31] and diarrhea [
32], by using these exudates. Furthermore, polysaccharides are not absorbed by the intestinal mucosa, remaining there for long periods without producing systemic effects, providing a substantial advantage in comparison to the majority of standard drugs [
33]. However, there is still a lack of evidence-based studies on the effects of RAG in diarrhea, as well as its mechanism of action that justify the use of red angico tree exudate in folk medicine by some local communities of northeastern Brazil.
Diarrhea is a common symptom associated with gastrointestinal disorders and characterized by an increased frequency of defecation (three or more times per day), increased fluidity of the feces, and/or the presence of blood and mucus, which can lead to water and electrolyte imbalance [
34]. In the majority of developing countries, diarrhea remains one of the most common causes of hospitalization, morbidity, and mortality, mainly among children less than five years of age, worldwide [
35]. Generally, in developing countries, infectious diarrhea, including enterotoxin-producing bacteria (
Vibrio cholerae and
Escherichia coli), parasites (
Entamoeba histolytica), and viruses (Rotavirus) are the major causes of this clinical manifestation. The transmission of these infectious agents occurs through physical contact or contaminated water and food, and treatment with oral rehydration solutions (ORS) are the first line therapy for diarrhea worldwide [
36]. However, currently available ORS are unable to reduce the duration and severity of diarrhea [
37].
Considering the biological activity of polysaccharides obtained from closely related tree trunk exudates and the diarrhea pathophysiology [
32], evaluation of the antidiarrheal action of RAG, the polysaccharide extracted from the trunk exudate of
A. colubrina (RAG) is highly relevant. The results obtained could potentially lead to the adoption of new therapies and prophylactic measures.
Therefore, a major aim of the present work was to perform a chemical and biological analysis of the biopolymer extracted from the trunk exudate of A. colubrina, RAG. We additionally investigated whether RAG has antidiarrheal activity in experimental mouse models induced by castor oil, cholera toxin, and E. coli and conducted a safety assessment to determine if RAG can be used as an effective natural medication in endemic areas.
3. Materials and Methods
3.1. Chemicals
The following materials were obtained from commercial sources: castor oil, prostaglandin E2, charcoal, atropine, carbachol (CCh), Splittgerber’s reagent, cholera toxin, monosialoganglioside-GM1, and 3,3’,5’,50-Tetramethylbenzidine, from Sigma-Aldrich, Inc. (St Louis, MO, USA); bethanechol chloride, loperamide hydrochloride, and naloxone hydrochloride from Janssen-Cilag Pharmaceutics LTDA, Brazil and CRISTÁLIA Pharmaceutical Chemicals products LTDA, Brazil; Xylazine hydrochloride and ketamine hydrochloride, were obtained from Syntec (Cotia, SP, Brazil). All other chemicals used were of analytical grade and obtained from standard commercial suppliers. All drugs were dissolved in saline or phosphate-buffered saline (PBS).
3.2. Plant Material
Crude polysaccharide samples of RAG were collected in 2016 by the Biotechnology and Biodiversity Center Research–BIOTEC, Federal University of the Parnaíba Delta, Parnaíba-PI, Brazil, from the trunks of native red angico trees (A. colubrina var. cebil (Griseb.) Altschul) in the municipality of Simplício Mendes, Piauí, Brazil. Additionally, a voucher of this specimen was deposited in herbarium of the Parnaíba Delta (HDELTA), Parnaíba-PI, Brazil, (nº 3618).
3.3. Extraction and Purification of RAG
RAG was purified as a sodium salt using a previously described method [
91]. Crude samples of exudate were selected and dissolved in distilled water at 25 °C for 24 h, providing a 5% (w/v) solution. The pH of the solution was adjusted to approximately 7.0 by addition of diluted aqueous NaOH. For precipitation, RAG was added to a beaker containing 30 mL of ethanol during a 24-hour refrigeration period. The precipitate formed on the bottom was separated from the liquid and washed twice with ethanol to remove any water and impurities. The product was macerated and washed again with ethanol, followed by acetone to further remove any impurities and water. The washed precipitate was dried and macerated with exposure to frequent hot air flow until the gum was obtained [
82].
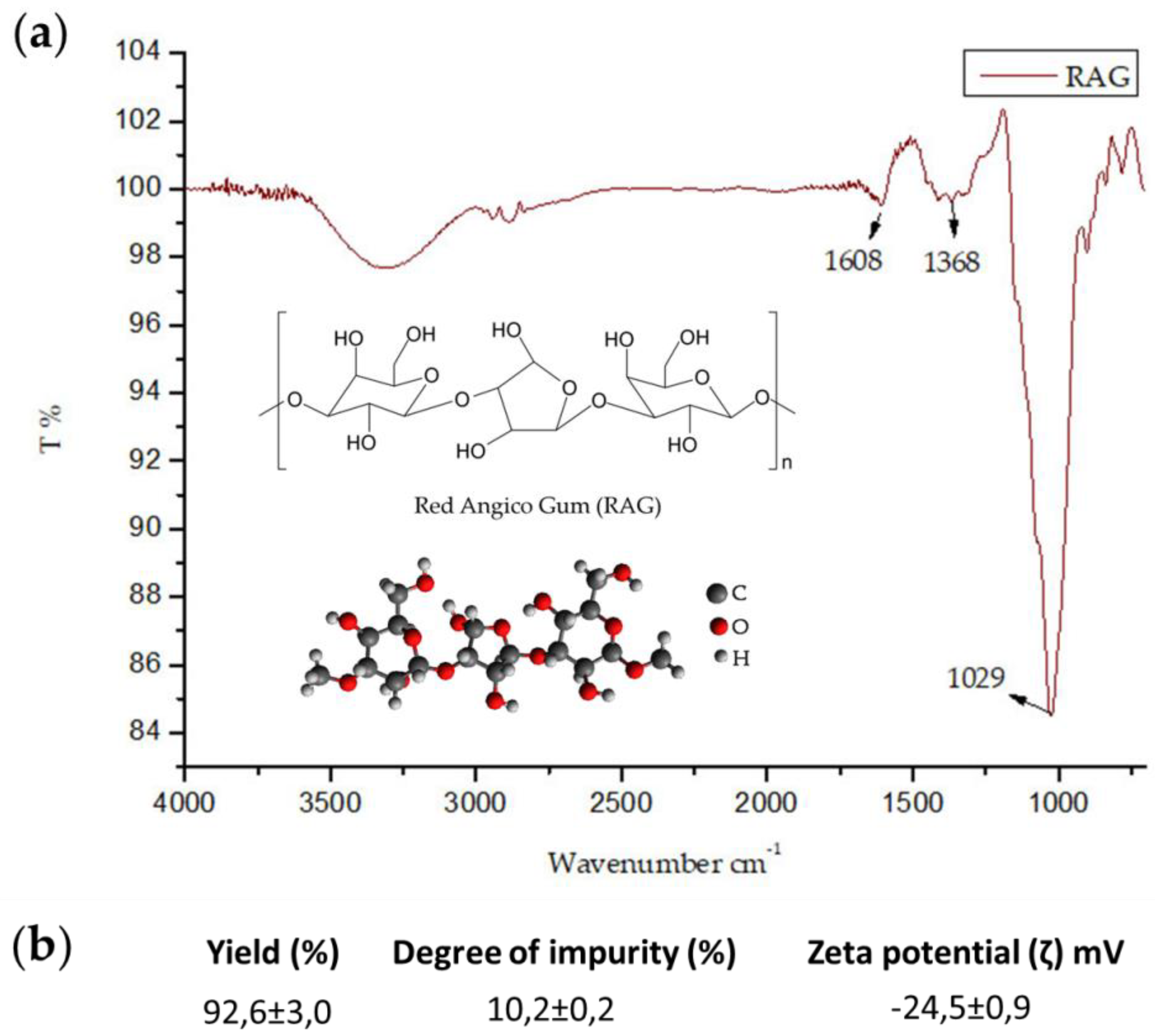
3.4. Characterization of RAG by Infrared Spectroscopy, Zeta Potential, Elemental Analysis, Molar Weight, Solubility and Impurity Determination
The Fourier transform infrared spectra (FT-IR) of the biopolymer were recorded with a Shimadzu IR spectrophotometer (Affinity-1S-ATR-attenuated Total Reflectance, ZnS crystal, model 8300) between 700 and 4000 cm−1. Samples were analyzed by absorbance in attenuated total reflection (ATR). All analyses were performed with 45 scans on zinc selenide crystal for the identification of the functional groups present in RAG. Zeta potential was measured using the Malvern Zetasizer Nano ZS90 with the following settings: dispersant: water, temperature: 25 °C, calibration time: 2 min, number of scans: 173, scan time: 20 min, and a 10 mg/mL aqueous solution was used. The measurements were carried out in triplicate to determine biopolymer load. The elemental analyses for carbon, hydrogen, and nitrogen composition were performed using a Perkin Elmer 2400 series CHNS analyzer with a thermal conductivity detector.
The molar mass distribution was determined by the chromatography of gel permeation using the Shimadzu LC-20AD coupled to a refractive index (RID-10A). For the analysis, we used a linear polysep column, 300 × 7.8 mm, using 0.1 mol/L NaNO3 (aq) as the eluent. Solubility was measured at 30 °C and a flow rate of 1 mL/min with an injected sample volume of 50 µL. A mass of 0.1 g RAG was applied to 1 mL distilled water at different pHs (2, 7, and 8), adjusted with HCl and 0.5 mol/L NaOH, and allowed to stir for 12 hours, the samples then underwent ultrasound for 15 min to aid in the solubilization process. Subsequently, centrifugation was performed for 10 min at 10,000 rpm for separation and precipitation. At weighing, samples were dried using vacuum centrifugation. The degree of impurity was determined by adding 0.1 g RAG to 10 mL acetone and stirring for 3 h, followed by centrifugation for 10 min at 3600 rpm. Samples were then dried and the supernatant centrifuged under vacuum for 5 h. All dry content was weighed.
3.5. Evaluation of Antidiarrheal Activity of RAG
3.5.1. Animals and Ethical Considerations
Mice (Swiss strain, 25–30 g) of both sexes were obtained from the Central Vivarium of Federal University do Piauí, Teresina, Piauí, Brazil. All animals were kept and maintained in cages under laboratory conditions at a temperature of 23 ± 1 °C under a 12 h light/12 h dark cycle with free access to food (standard pellet diet) and drinking water ad libitum. The animals were divided into groups of 6–8 animals per group for treatment with RAG, the reference drug, or saline-treated control. The animals were deprived of food for 18–24 h before the experiments, with free access to water. All experimental procedures and protocols used in this study were approved by the Ethics Committee in Research of the Federal University of Piauí (protocol no. 068/2014). This study was performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA) and the ARRIVE (Animal Research: Reporting of In Vivo Experiments) Guidelines.
3.5.2. Castor Oil-Induced Diarrhea
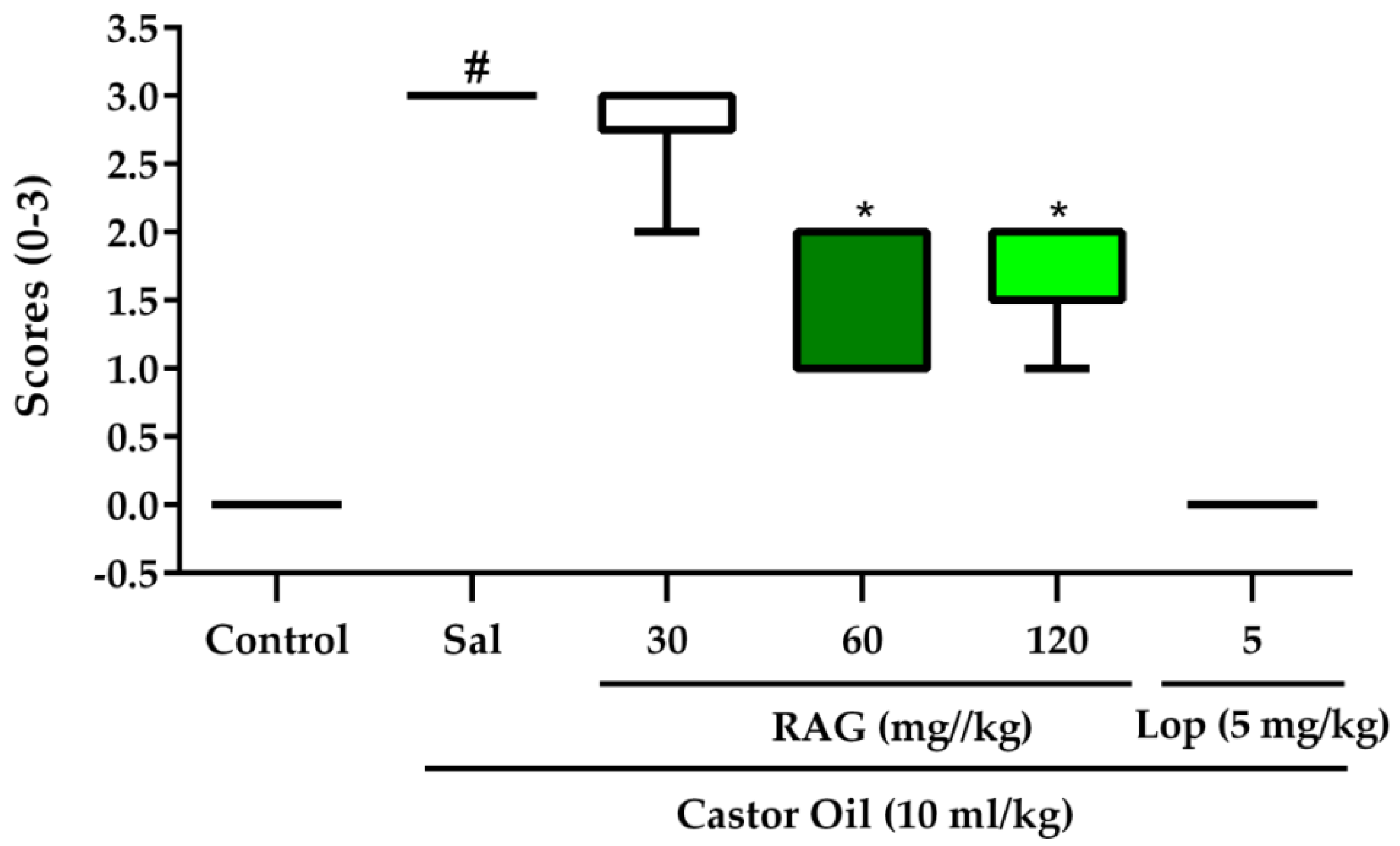
Castor oil was used to induce diarrhea according to a previously described method [
64], with some modifications. Mice were randomly allocated to groups (6–8 mice per group) and starved for 18 h before the experiment with free access to water. The treatment groups were allocated as follows: Saline, RAG 30, 60 and 120 mg/kg, loperamide (a standard antidiarrheal agent) 5 mg/kg, and vehicle only. All test substances were administered by gavage. After 1 h, diarrhea was induced in the experimental groups by administering castor oil (10 mL/kg p.o.), the vehicle group received saline only. Immediately after administration, the animals were placed in cages lined with adsorbent paper and were observed for 3 h to detect the presence of characteristic diarrheal droppings defined as watery (wet), unformed stools. The total mass of fecal output (mg) and the total number of diarrheic stools (mg) excreted by each group in that period of time were monitored. The severity of the castor-oil induced diarrhea was scored based on stool consistency and recorded. Scores were assigned using a previously described method [
63], which is as follows: normal stool (or lack of diarrhea) = 0, semi-solid stool = 1, pasty stool/feces in small/moderate amount = 2, and watery stool/feces in large amount = 3. The efficacy of each treatment was expressed as percent inhibition (%) of diarrhea compared to the control group result expressed as 100%. The percent inhibition of defecation and diarrhea was calculated by:
where A represents the mean mass of defecation caused by castor oil and B represents the mean mass of defecation after treatment with drug or RAG.
3.5.3. Castor Oil-Induced Intestinal Fluid Accumulation (Enteropooling)
Measurement of the castor oil-induced enteropooling was carried out according to the method previously described by Robert et al. [
92] with some modifications. Mice were deprived of food for 18 h before the experiment and randomly allocated into groups of 6–8 rats per group. Initially the control group received saline while the experimental groups were pre-treated with RAG (30, 60, and 120 mg/kg) or loperamide (5 mg/kg). After 1 h, diarrhea was induced in all groups with castor oil (10 mL/kg p.o.). All test substances were administered by gavage. After 3 h, the animals were euthanized using a combination of xylazine hydrochloride and ketamine, the small intestine from the pylorus to the caecum was then isolated, and the intestinal contents were measured in a graduated tube. The efficacy of each treatment was expressed as percent inhibition (%) of the volume of intestinal fluid and was calculated by:
where A represents the mean of the volume of fluid after castor oil administration alone; B represents the mean volume of fluid after pretreatment with Castor oil and treatment with drug or RAG.
3.5.4. PGE2-Induced Enteropooling
Prostaglandin E2 (PGE
2) was used to induce intestinal fluid accumulation according to the method previously described by Mukherjee et al. [
93], with some modifications. Mice were deprived of food for 18–24 h before the experiment. The control group received saline orally and the experimental groups received RAG (60 mg/kg) or loperamide (5 mg/kg) by gavage. Immediately after drug administration, intestinal fluid accumulation was induced in groups by oral administration of PGE2 (100 μg/kg; Sigma Aldrich, USA) to each mouse. The vehicle group received saline only. Thirty minutes after administration of PGE
2, mice were euthanized, and the small intestine from the pylorus to the caecum was resected. The content was collected in a graduated tube and the volume was measured. The efficacy of each treatment was expressed as percent inhibition (%) of the volume of intestinal fluid and was calculated identical to the castor oil-induced enteropooling model described above.
3.5.5. Assessment of Na+/K+-ATPase Activity
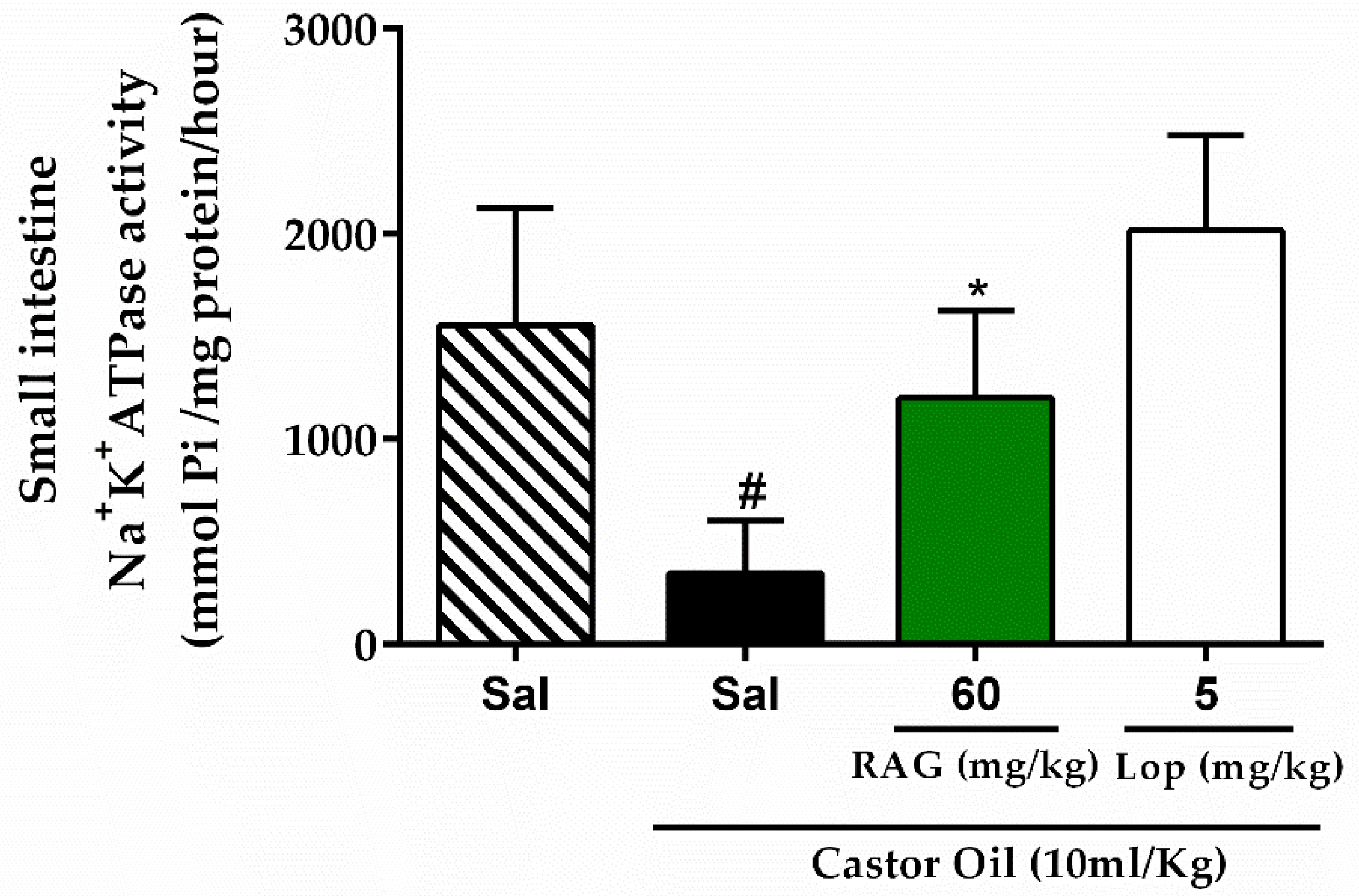
Mice were deprived of food for 18 h with free access to water. Castor oil was used to induce diarrhea as described above. The control group received saline, while experimental groups received RAG (60 mg/kg, p.o.) or loperamide (5 mg/kg, p.o.). After 1 h of pretreatment, castor oil (10 mL/kg p.o.) was administered to the experimental groups and the vehicle group received saline only. After 3 h, the mice were euthanized, laparatomized, and the small intestine (from the pylorus to the caecum) removed to evaluate Na+/K+-ATPase activity, as previously described [
94]. The activity of Na+/K+-ATPase was standardized to the protein concentration in the samples of the small intestine tissues using standard procedures, according to the manufacturers’ instructions (Labtest Diagnostica, Lagoa Santa, Brazil). Thereafter, the activity of Na+/K+-ATPase was calculated using the following equation:
where [Pi] represents the concentration of inorganic phosphate in nmoles (as obtained from the calibration curve, 2 represents the factor required to obtain the amount of Pi released per h, and 1000 represents the factor introduced to convert the Pi released to µmoles.
3.5.6. Gastrointestinal Transit Test (Charcoal Meal)
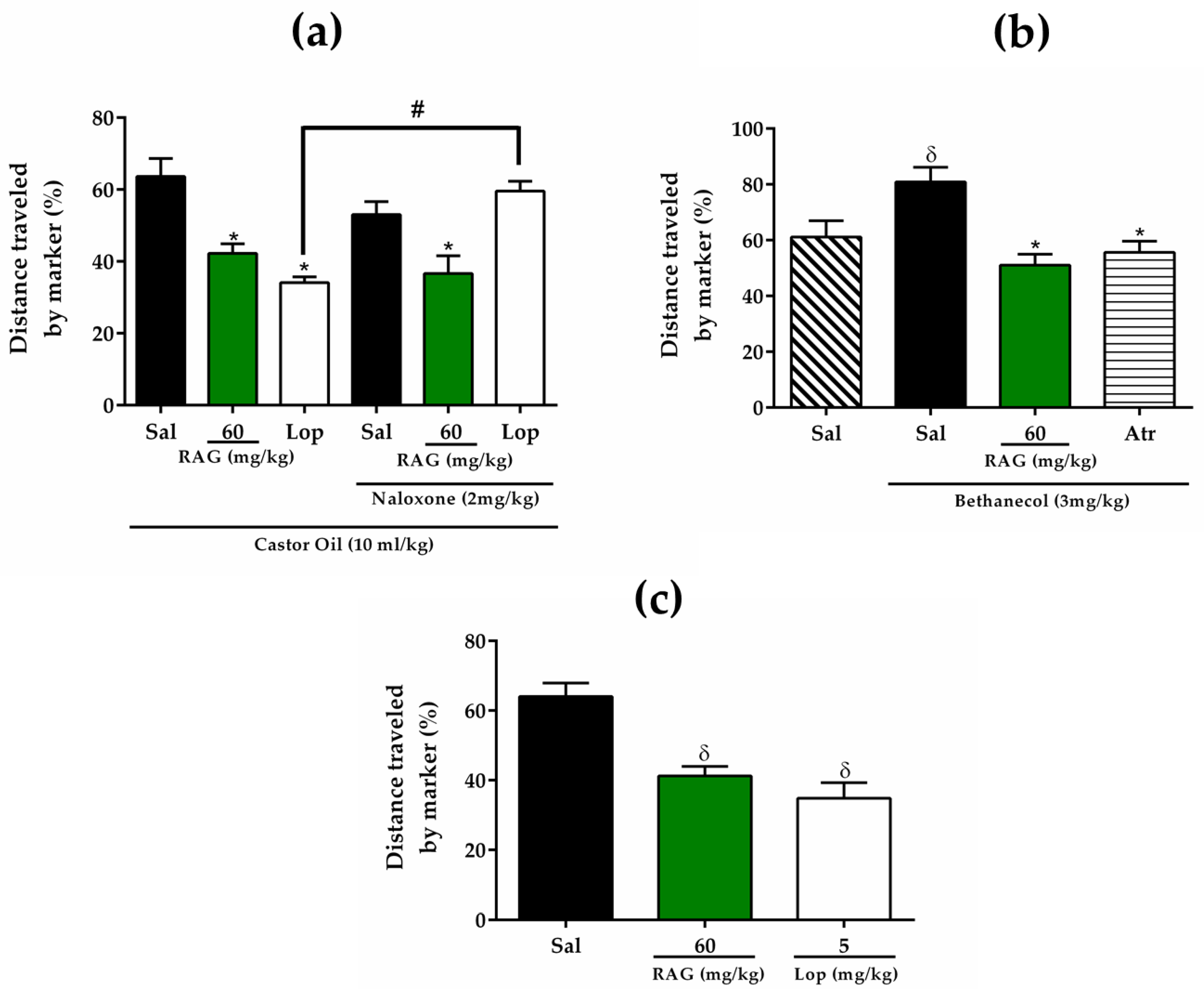
The effect of RAG on gastrointestinal transit was evaluated using a previously described procedure, whereby charcoal meal is used as a marker of the distance traveled [
63]. The experiment was performed in four stages separately, aiming to evaluate the possible involvement of opioid receptors or anticholinergic activity, on the effect of RAG under the inhibition of gastrointestinal transit. Initially, mice were randomly divided into groups of six animals and fasted for 18 h before the respective tests, but with free access to water.
In the first series of experiments the effect of RAG on the reduction of gastrointestinal transit was evaluated using the castor oil-induced diarrhea model. All animals received castor oil (10 mL/kg, p.o.) to induce diarrhea and after 1 h were treated orally with saline (2.5 mL/kg), RAG (60 mg/kg) or loperamide (5 mg/kg). One hour after drug administration, all animals orally received a charcoal meal (0.2 mL of 10% activated charcoal suspended in 5% gum acacia) by gavage. After 20 minutes, the animals were euthanized. The distance covered by the charcoal meal in the intestine from the pylorus to the caecum was measured and expressed as a percentage of distance covered where:
where A represents the mean of the distance traveled by the charcoal meal and B represents the mean total length of intestine.
In the second series of experiments, to examine the involvement of opioid receptors on the effect of RAG under reduced gastrointestinal motility, all mice were pre-treated with naloxone (2 mg/kg, s.c., opioid antagonist). After 30 minutes, the animals were treated with saline (2.5 mL/kg), RAG (60 mg/kg) or loperamide (5 mg/kg, opioid agonist). The steps following were performed as described above.
In the third series of experiments, to evaluate a possible anticholinergic activity of RAG in the reduction of gastrointestinal transit, was performed the gastrointestinal transit model stimulated by bethanechol, a cholinergic agonist of muscarinic type 3 receptors [
95]. Mice were pre-treated orally with saline (2.5 mL/kg), RAG (60 mg/kg), or atropine (3 mg/kg, s.c., cholinergic antagonist). Thirty minutes later, the animals received bethanechol (3 m/kg, i.p.), and the negative control group received saline. One hour later, all animals orally received a charcoal meal (0.2 mL of 10% activated charcoal suspended in 5% acacia gum). Twenty minutes later, the mice were euthanized and the distance covered by the marker in the intestine, from the pylorus to the cecum, was measured as described above.
Additionally, to assess the effect of RAG on normal transit, mice were pre-treated orally with saline (2.5 mL/kg), RAG (60 mg/kg) or loperamide (5 mg/kg). One hour later, all animals orally received a charcoal meal by gavage. Twenty minutes later, mice were euthanized and the distance covered by the marker was measured as previously described.
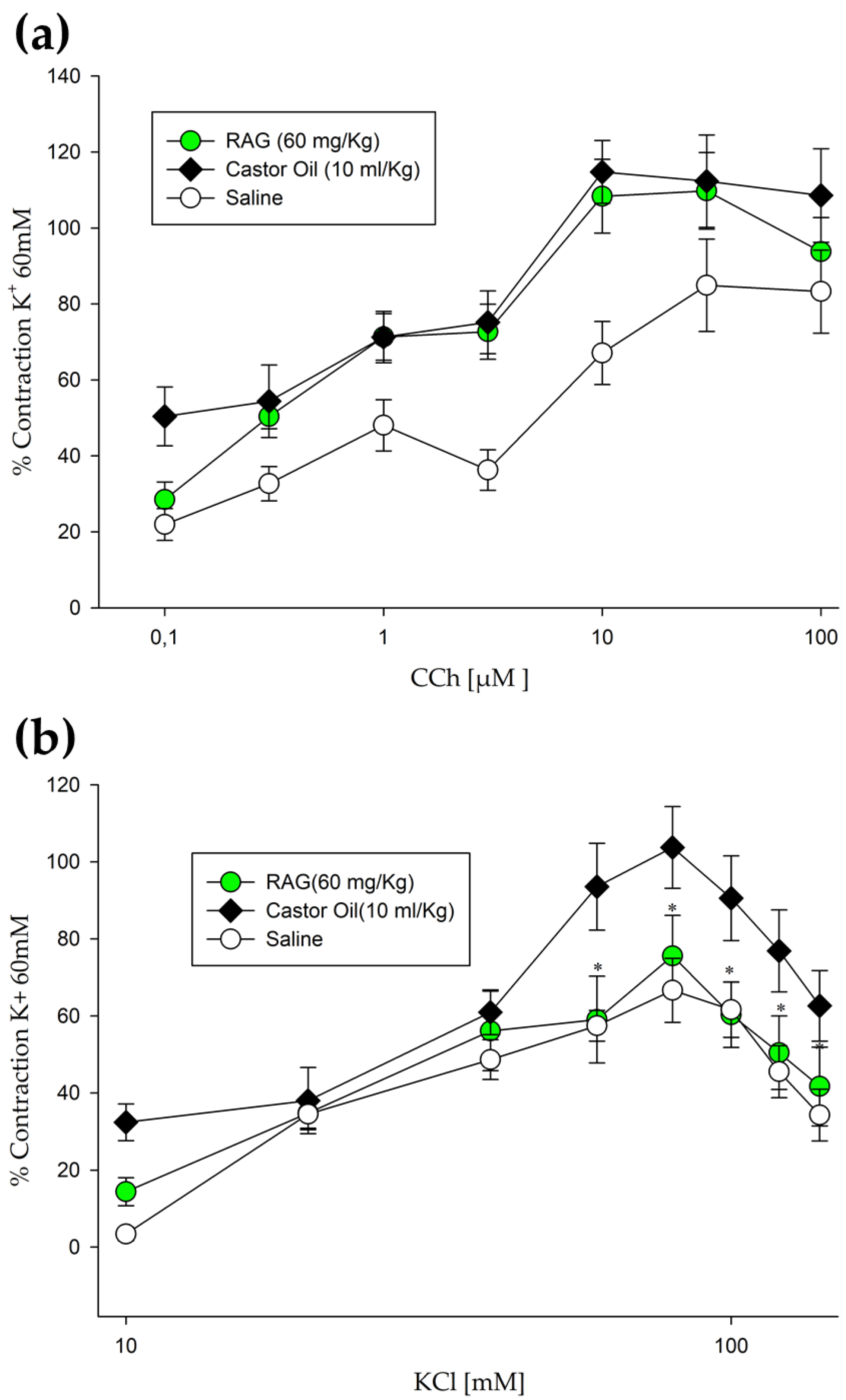
3.5.7. Contraction Induction of Isolated Mouse Jejunum-Ileum Ex Vivo
Evaluation of the effect of RAG on inducing contractions in isolated jejunum-ileum ex vivo was performed using previously described methods [
96], with some modifications. Mice were randomly allocated to groups and starved for 18 h, before the experiment, with free access to water. The treatment groups were as allocated as follows: saline, RAG (60 mg/kg) and vehicle only. After 1 h, diarrhea was induced in the experimental groups by administering castor oil (10 mL/kg, p.o.), the vehicle group received saline only. All test substances were administered by gavage. After 3 h, the mice were euthanized and gut preparations (jejunum- ileum) were obtained. The tissues were isolated after opening of the abdomen. Elongated segments (2–3 cm) were placed in a tissue bath (10 mL) comprising of Tyrode’s solution, kept at normal body temperature of 37 °C with constant aeration, a mixture of O
2 and CO
2 (Carbogen/95% + 5%). Tyrode’s solution (mM) comprises potassium chloride (KCl) 2.68, NaCl 136.9, MgCl
2 1.05, NaHCO
3 11.90, NaH
2PO
4 0.42, CaCl
2 1.8, and glucose 5.55 (pH 7.4). A preload of 1 g was used and contractions in the intestinal tissue were noted using a force displacement transducer (MLT0015) attached with bridge amplifier and Power Lab 4/25 data acquisition system coupled to a computer running Lab Chart 6 software (AD Instrument, Australia). The tissues were allowed to equilibrate for a period of 30 min, and then were repeatedly stimulated with 60 mM/L K
+ to evaluate the tissue viability. Preparations without reproducible contractions were discarded. These contractions served as a reference and allowed the comparison between different tissues, and the results presented in this study are generally expressed as a percentage of the last K
+-induced contraction. Contractile responses to carbachol (CCh; 0.1, 0.3, 1, 3, 10, 30, and 100 µM) and KCl (10, 20, 30, 40, 60, 80, 100, 120, and 140 mM) were recorded at 5-min intervals in animal samples, pretreated with or without RAG (60 mg/kg). The relaxant effects of RAG were observed when the spontaneous contractions of the preparation showed percent change, recorded directly before and after the adding of contraction inducing substances.
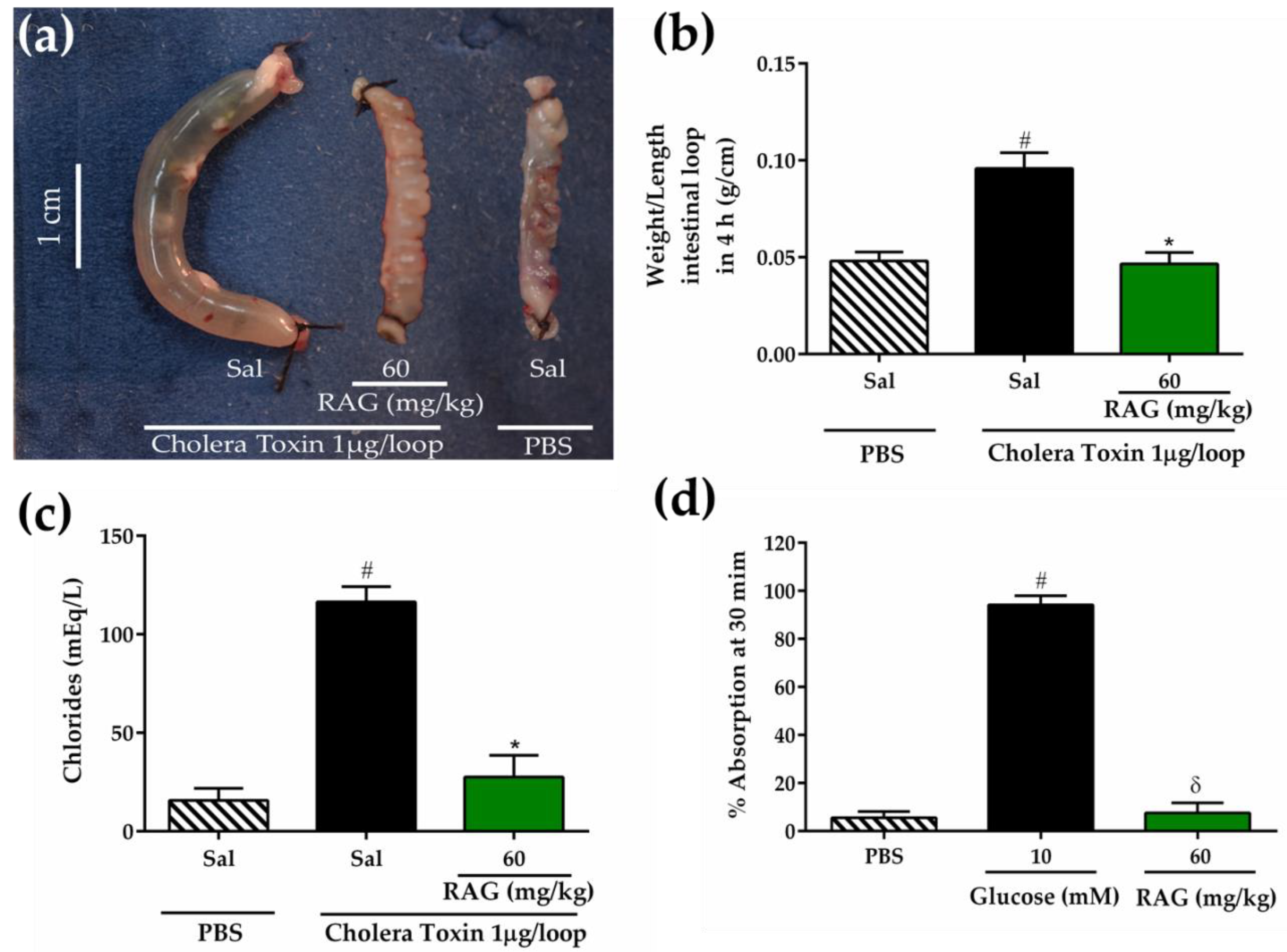
3.5.8. Cholera Toxin-Induced Secretory Diarrhea
The inhibitory effect of RAG on intestinal fluid secretion induced by cholera toxin (CT) inoculation in mice-intestinal closed loops was measured using a previously described method [
97]. The animals were given access to water but not food for 24 h before the experiment. Mice in the negative group and positive control group were pretreated orally with saline (2.5 mL/kg), while the test group (group 3) received RAG (60 mg/kg). After 1 h, the animals were anesthetized intraperitoneally with a combination of xylazine hydrochloride (5 mg/kg) and ketamine (60 mg/kg) and a median laparotomy was performed, where an abdominal incision was made to expose the small intestine, and closed jejunal loops (2–3 cm) were isolated using sutures. In this procedure, the body temperature was maintained during surgery at 36–38 °C using a heating pad. Intestinal loops were inoculated with 100 μL of phosphate-buffered saline (PBS) for negative group, and CT dissolved in PBS at a dose of 1 μg/loop for control and test groups. The abdominal incision was closed with sutures, and the animals were allowed to recover from anesthesia. At four hours, the mice were euthanized, and the closed loops were rapidly removed from the abdominal cavity. The fluid secretion was measured indirectly as the ratio of loop weight/length expressed in g/cm. The intestinal contents accumulated in each closed loop were collected separately to measure the concentration of chloride (Cl
-) ions according to the manufacturer’s instructions (Labtest, Lagoa Santa, Minas Gerais, Brazil).
3.5.9. Intestinal Fluid Absorption in Closed Loops
Initially, mice were fasted for 24 h before experiment. Jejunal loops were isolated as described in the previous section. For intestinal fluid absorption studies, loops were injected with 200 µL of PBS with or without 10 mM glucose or PBS containing RAG (60 mg/kg p.o.). The intestinal loops were returned to the abdominal cavity and the laparotomy was closed with sutures. Thirty minutes after inoculation, the intestinal loops were removed, and the percentage of fluid absorption was measured indirectly as the loop weight/length ratio.
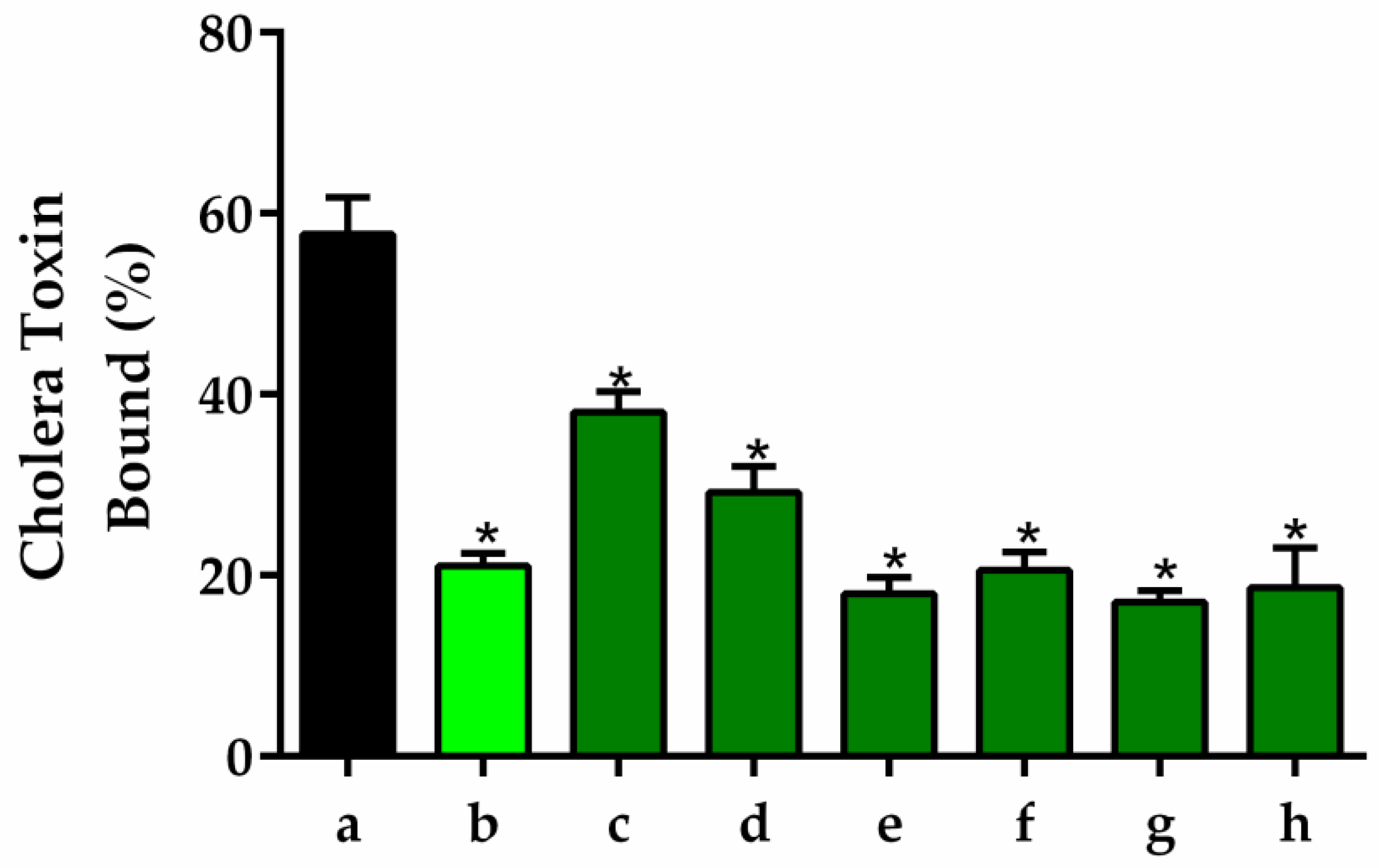
3.5.10. GM1 Ganglioside-Dependent ELISA
Wells containing monosialoganglioside 1 (GM1) with or without different concentrations of RAG were used for estimation of CT by GM1 ELISA. Samples containing cholera toxin (CT) with or without different concentrations of RAG (1 to 500 μg/mL) were serially diluted and added to microtiter plates containing immobilized GM1. ELISA was carried out as previously described by Saha et al. [
98].
3.5.11. Enterotoxigenic Escherichia Coli- Induced Secretory Diarrhea
Enterotoxigenic
Escherichia coli (ETEC) cell suspension was used to induce diarrhea according to previously described methods by Bisson et al. [
99], with modifications. Mice were randomly allocated into groups with six animals per group and acclimated for 7 days before treatment. The animals were given access to water but not food 18 h before the experiment. Negative and positive controls received saline while the antibiotic group received gentamicin (8 mg/kg p.o.). The test group received RAG (60 mg/kg p.o.) for three days. ETEC was obtained from the Department of Microbiology Laboratory, Federal University of Ceará, Brazil. A cell suspension of ETEC was transferred to assay tubes containing NaCl sterile solution, and bacterial cultures were adjusted to a cell density of 2 × 10
8 colony forming units per mL (CFU/mL) by optical density (O.D.) measurements using the spectrophotometer. Diarrhea was induced in all groups except in the negative control group by single oral administration (5 mL/kg) of ETEC solution incubated at 37 °C. The negative group received a single oral dose (5 mL/kg) of physiological saline (vehicle) warmed at 37 °C. After treatment, the animals were placed in cages lined with absorbent material to detect the amount of diarrheal stool. The total amount of stool (g) and the total amount of diarrheal stool (g) excreted by each group were monitored for three days. The effect of each treatment was expressed as the percent inhibition of diarrhea, with the value for the control group set to 100%.
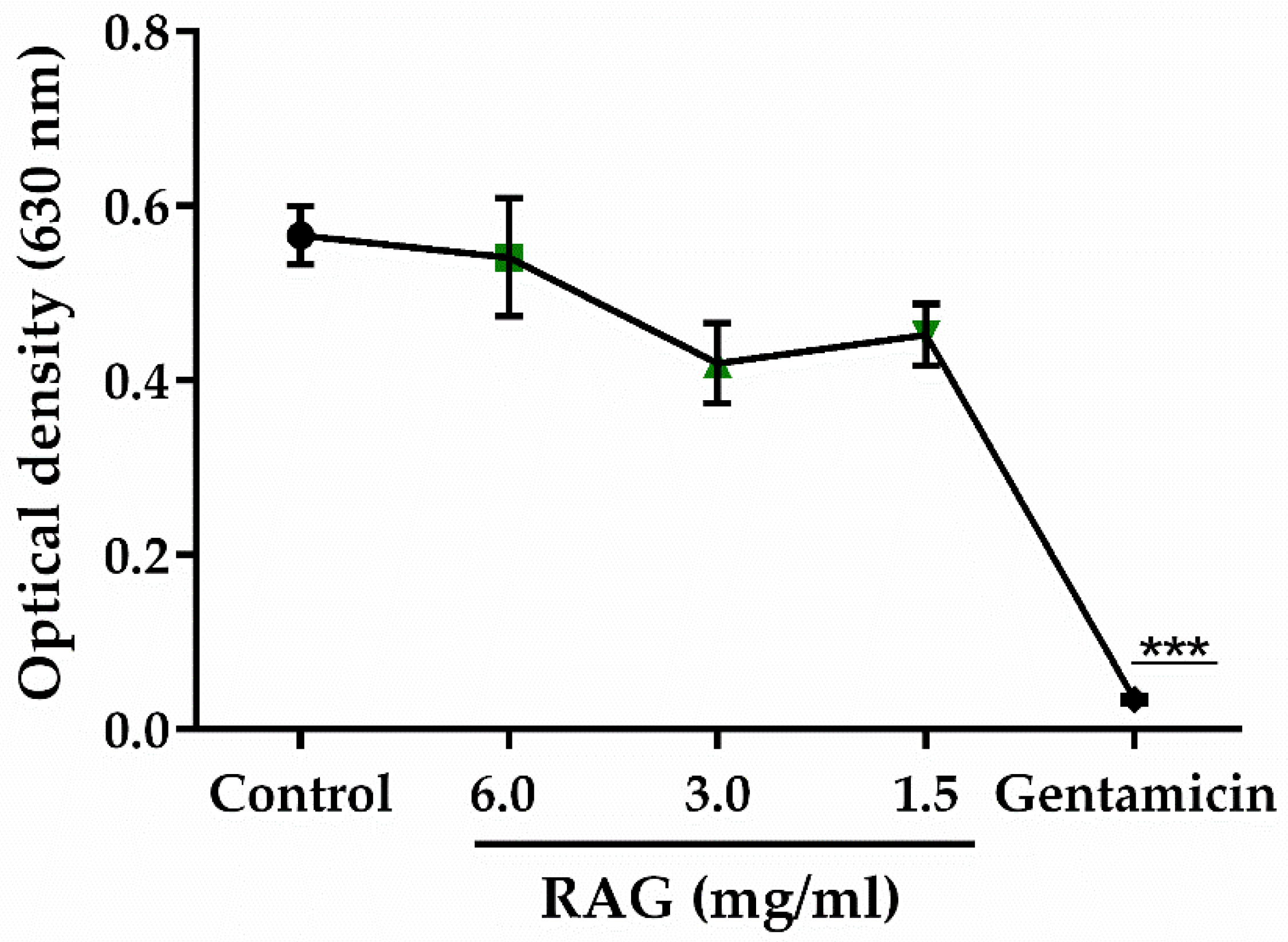
3.5.12. Test of the Antibacterial Activity of RAG
The minimum inhibitory concentration (MIC) of RAG against
Escherichia coli ATCC 25922 (at a concentration of 5 × 10
5 CFU/mL) was determined by microdilution in Mueller Hinton broth (BD Difco
™) with concentrations of RAG ranging from 0.05–6 mg/mL. The culture was placed in 96-well micro titer wells which were incubated at 35 ± 2 °C for 24 h under aerobic conditions, the growth was measured at 630 nm (OD630) using a microplate reader (Bioeasy). The MIC was defined as the lowest concentration that gives a close to zero absorbance reading (OD < 0.05). This test was carried out in triplicate and gentamicin (8–512 μg/mL) was used as the standard antibacterial agent [
100,
101].
3.6. Acute Toxicity Study of RAG
For the safety assessment of RAG, the acute toxic class method described by the Organization for Economic Cooperation and Development (OECD) (Guideline 423/2001) [
90] was performed. Female Swiss mice (three per group) were used for these procedures. Animals in the test group orally received a dose of RAG (2000 mg/kg) dissolved in saline and the control group received only saline.
3.6.1. Clinical Observation
All animals were observed after administration (15 min, 30 min, and 1 h) and periodically during the first 24 h, with special attention given during the first 4 h, and daily thereafter, for 14 days at scheduled times. Any symptoms of illness or abnormal behavior were taken into account. Parameters of toxic signs (Hippocratic screening) were studied according to OECD guideline 423 [
52], which includes tremors, salivation, convulsions, lethargy, diarrhea, sleep, and coma. Skin and fur, eyes and mucous membranes, respiratory, circulatory, autonomic and central nervous systems, somatomotor activity, and behavior patterns were also evaluated. Furthermore, the body weight of the animals, consumption of water and feed and production of excreta was monitored throughout the study period.
3.6.2. Biochemical and Hematological Parameters
At the end of monitoring for toxic signs, the 14th day, all animals were anaesthetized with a combination of xylazine hydrochloride (5 mg/kg, i.p.) and ketamine (60 mg/kg, i.p.). Blood samples were then collected by cardiac puncture and stored in tubes with ethylenediaminetetraacetic acid (EDTA) for the hematological analysis. Biochemistry analysis was then performed on the plasma using the Semi-automatic biochemical analyzer TEKNA (Labtest Diagnosis, São Paulo, Brazil) for the following parameters: aspartate aminotransferase (AST), alanine aminotransferase (ALT), total proteins, creatinine, and urea, according to the manufacturer’s specifications.
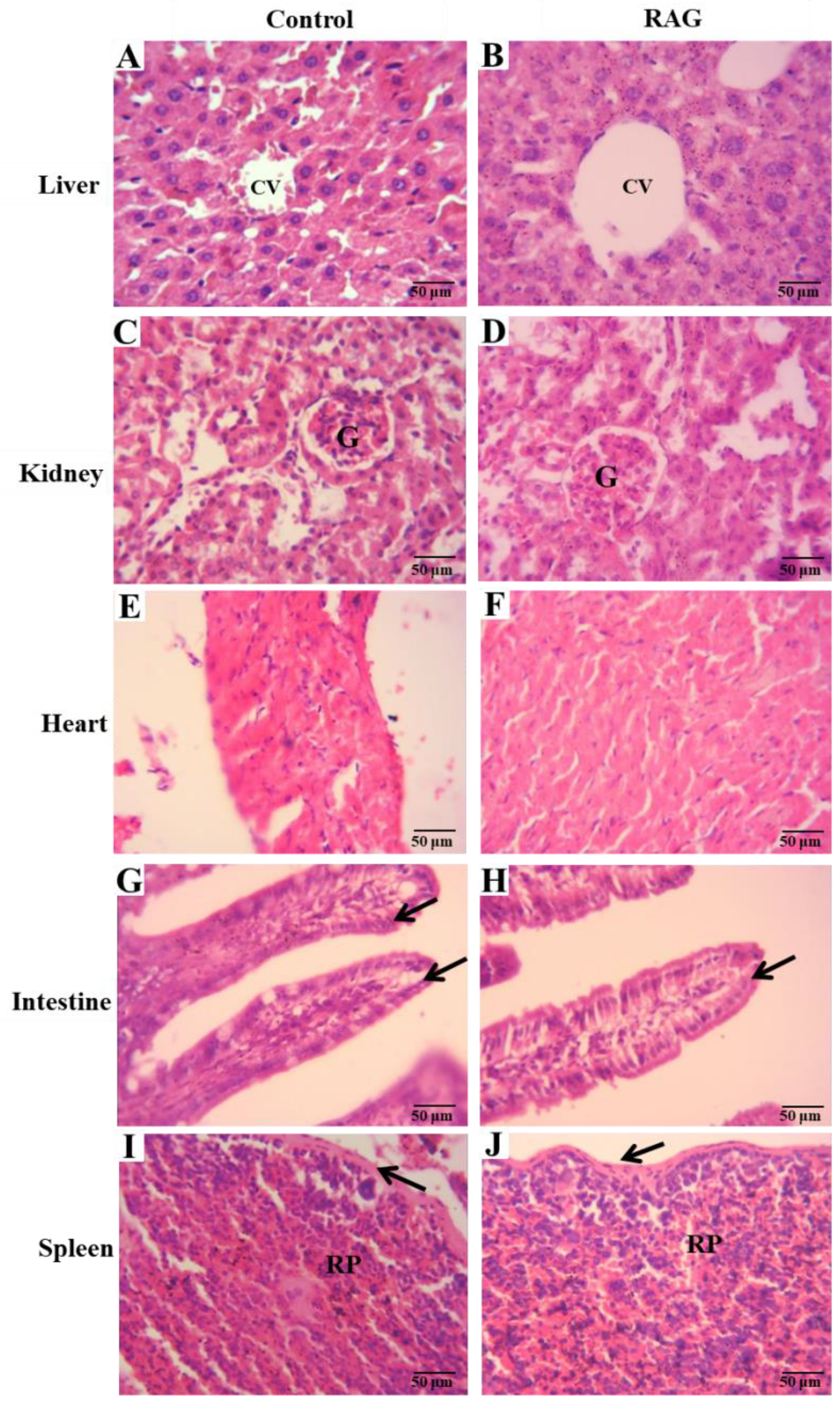
3.6.3. Organ Weights and Histological Analysis
On the 14th day, after cardiac puncture and subsequent euthanasia, mice were then laparatomized and organs (liver, kidney, heart, spleen and small intestine) were removed and weighed. The relative weight of each organ was calculated using the following expression: organ weight/body weight after treatment ×100. For histological analysis, a portion of 3 cm of the small intestine (jejunum) and organs mentioned above were fixed in alcohol, cleared in xylene, and embedded in paraffin. The blocks were in 10% buffered formalin. After fixing, the tissues were dehydrated and sliced into 5-µm sections, stained with hematoxylin-eosin (HE), and observed under a light microscope.
3.7. Data and Statistical Analysis
Data are presented as the mean (±SEM) for the animals in each group (n = 6–8). Results from saline-treated control mice were used as baseline values. In all cases, the results obtained from RAG- or reference drug-treated test groups were compared with those obtained from saline-treated controls. The in vitro test data represent the mean ± SEM from three independent triplicate experiments. Statistical tests were performed using GraphPad Prism (version 6.0) software. The statistical significance of the differences between groups was determined by one-way analysis of variance (ANOVA) and the Tukey post-hoc test. To study the fluidity of feces and histological analysis, the Kruskal–Wallis nonparametric test was used, followed by Dunn’s post-hoc test for multiple comparisons. For the toxicological analysis, the differences between the control group and the test were determined by Student’s t test. Differences were considered to be significant when p < 0.05. To determine the significance of the in vitro results one- or two-way analysis of variance (ANOVA) was used and, when significant, was followed by the Holm–Sidak multiple comparisons test.
4. Conclusions
In this study, the polysaccharide extracted from the trunk exudate of A. colubrina (RAG) was successfully purified and characterized with results corroborating that of the literature. FT-IR of RAG revealed a chemical identity compatible with the polysaccharides, with a molar mass of 1.89 × 105 g/mol and a negative zeta potential. Furthermore, RAG showed high yield and solubility at different pHs with a low degree of impurity.
The results obtained in the biological assays suggest that the antidiarrheal activity of RAG is probably due to its ability to 1) alter fecal parameters, 2) reduce gastrointestinal motility, possibly through physical blocking of extracellular ion influx in the mucosa, and thus 3) inhibit intestinal smooth muscle contractions. Additionally, RAG displayed effective antisecretory activity against CT- and ETEC-induced diarrhea, likely due to blocking the binding of these toxins to the GM1 receptor.
These results are substantiated by the low acute oral toxicity observed, providing scientific support for the traditional use of RAG tree exudate for the treatment of diarrhea by the local communities of northeastern Brazil. In conclusion, since there is an increasing need for new antidiarrheal treatments that do not exhibit side effects similar to that of standard drugs, further studies could evaluate other parameters involved in the antidiarrheal activity of RAG to validate its use as a novel natural antidiarrheal agent.