In Vitro Characterization and Stability Profiles of Antibody–Fluorophore Conjugates Derived from Interchain Cysteine Cross-Linking or Lysine Bioconjugation
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
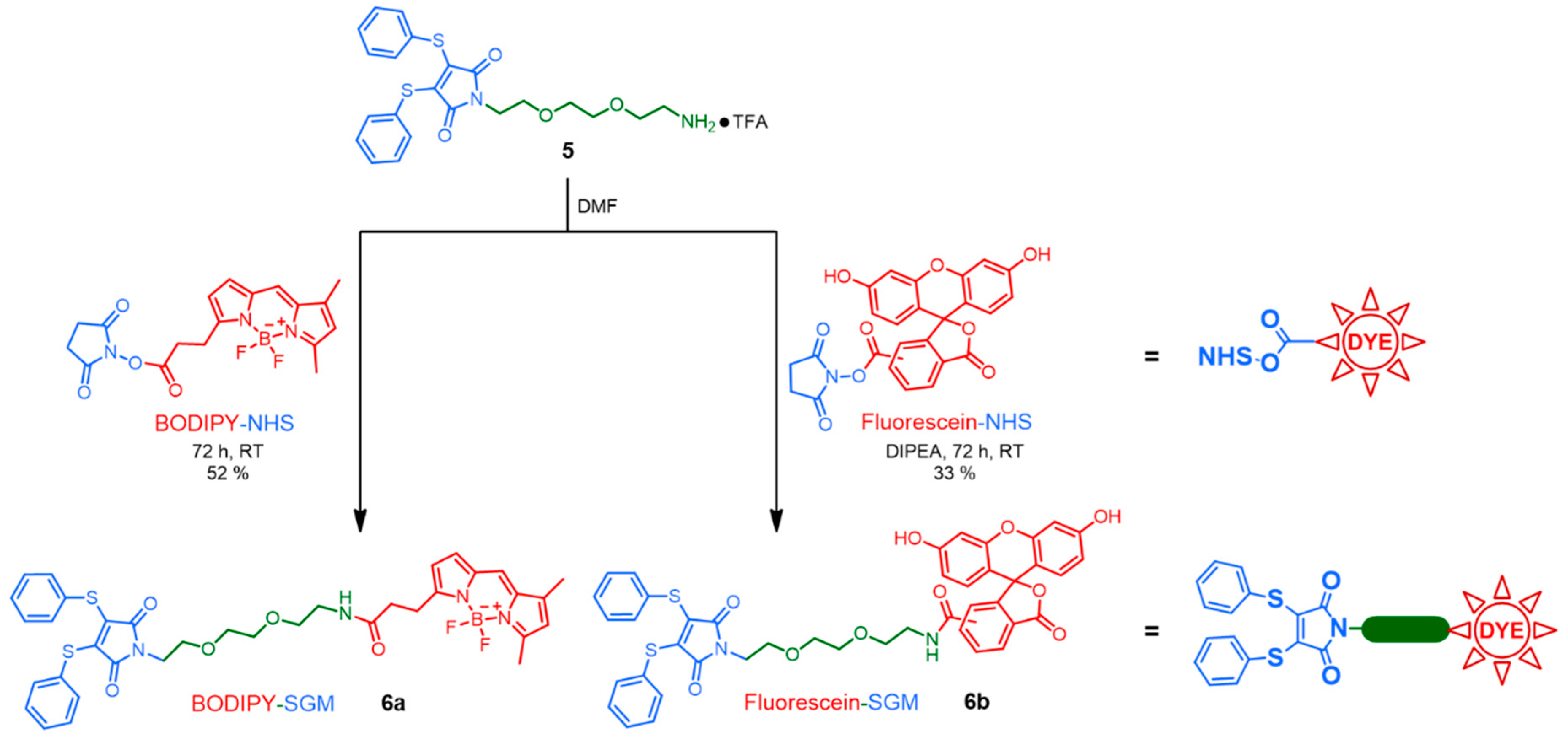
2.1.1. 3,4-Dithiophenoylmaleimide-N-8-amino-3,6-dioxaoctane-BODIPY 6a
2.1.2. 3,4-Dithiophenoylmaleimide-N-8-amino-3,6-dioxaoctane-FLU 6b
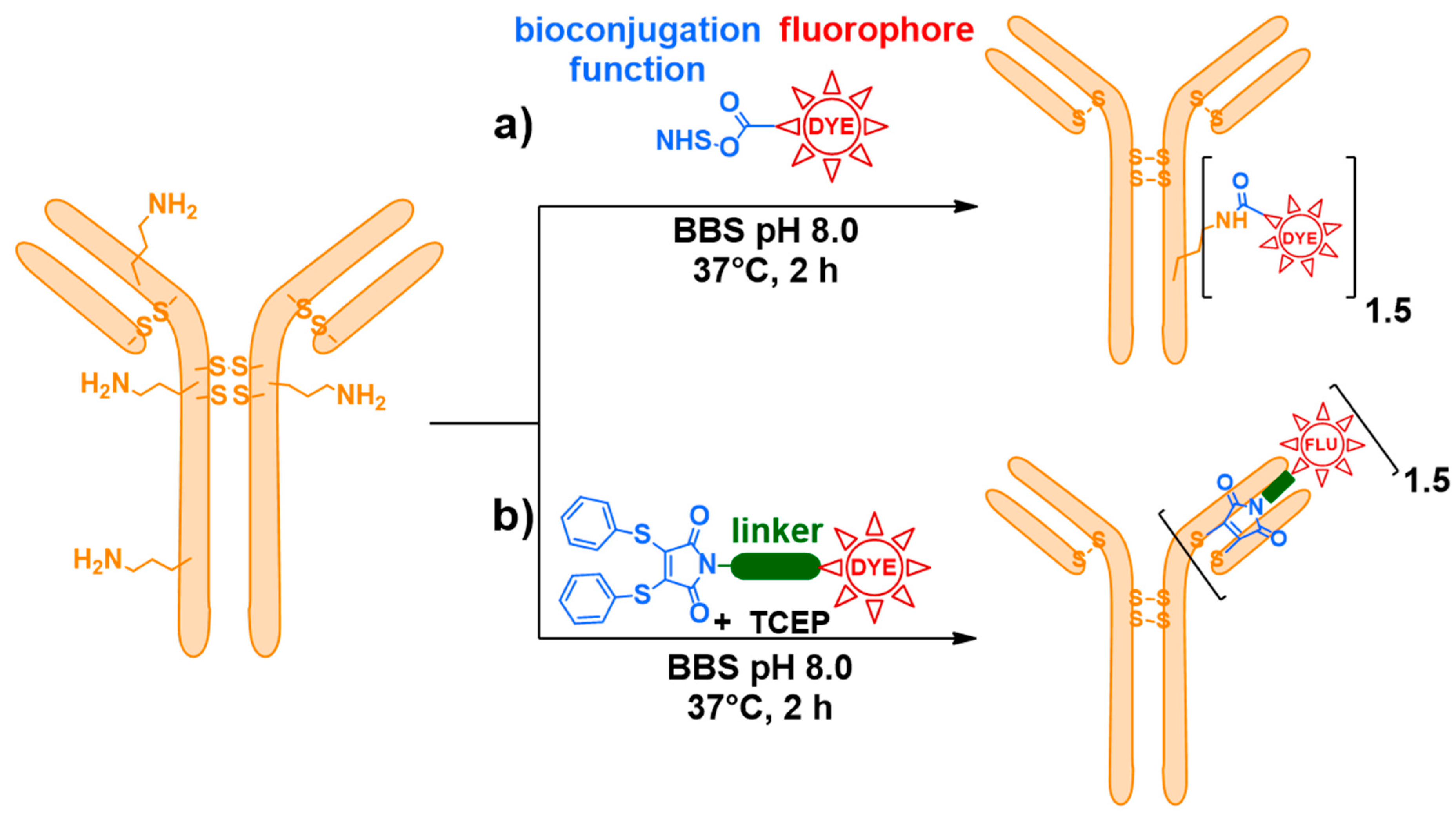
2.2. Bioconjugation
2.3. Mass Spectrometry
2.4. HER2 Binding by ELISA
2.5. CD20 Binding by Flow Cytometry
2.6. Fluorescence Emission
3. Results and Discussion
3.1. Synthesis of Linkers
3.2. Bioconjugation onto mAbs
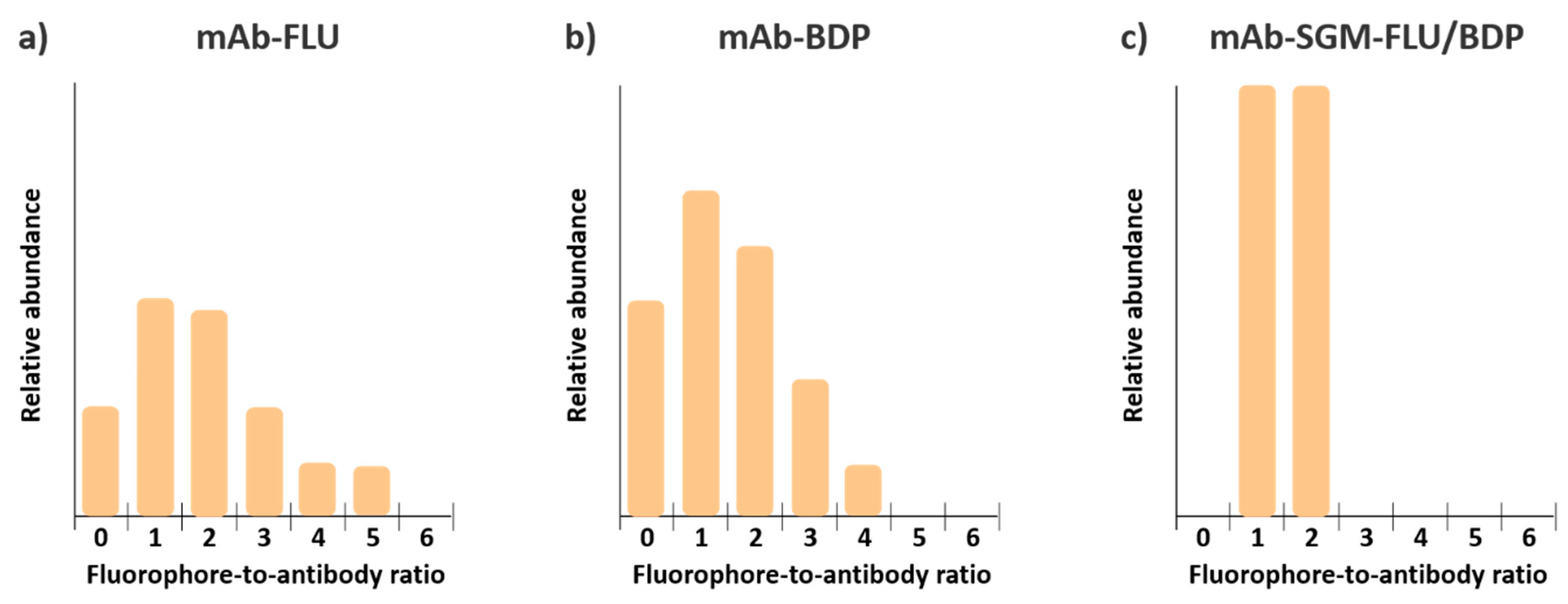
3.3. Mass Spectrometry
3.4. Antigen Binding
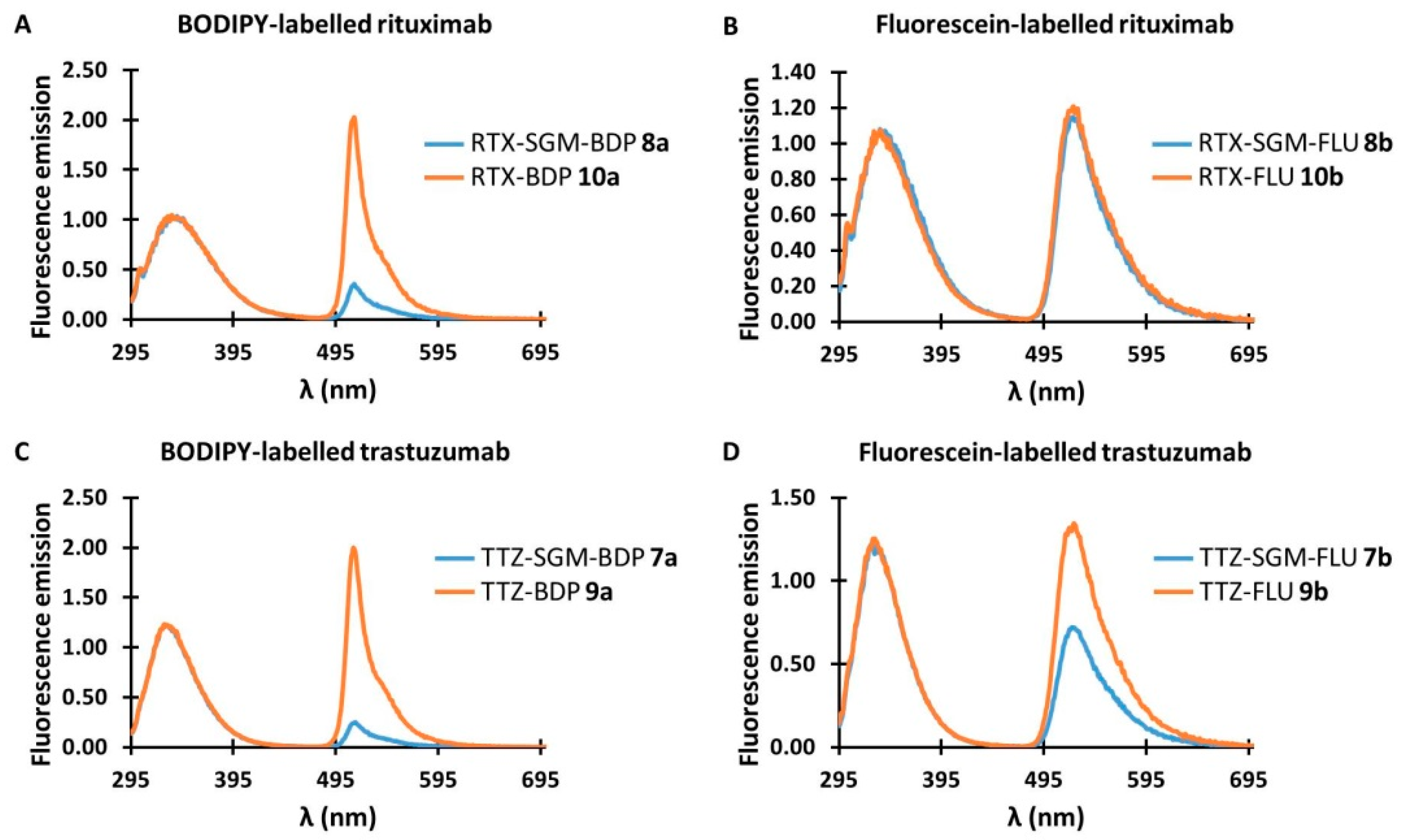
3.5. Comparative Fluorescence Emission between AFCs
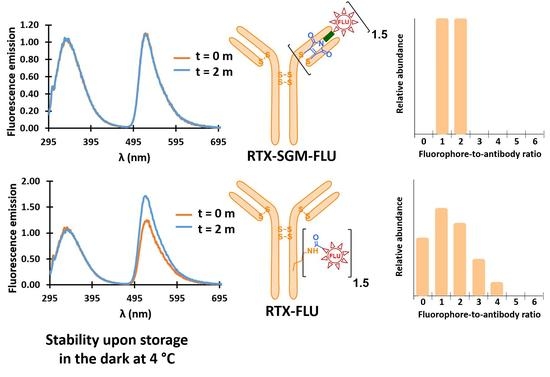
3.6. Stability of AFCs upon Storage
3.7. Stability of AFCs to pH Variation
4. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Brinkley, M. A brief survey of methods for preparing protein conjugates with dyes, haptens, and cross-linking reagents. Bioconjug. Chem. 1992, 3, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Acchione, M.; Kwon, H.; Jochheim, C.M.; Atkins, W.M. Impact of linker and conjugation chemistry on antigen binding, Fc receptor binding and thermal stability of model antibody-drug conjugates. MAbs 2012, 4, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Viehweger, K.; Barbaro, L.; García, K.P.; Joshi, T.; Geipel, G.; Steinbach, J.; Stephan, H.; Spiccia, L.; Graham, B. EGF receptor-targeting peptide conjugate incorporating a near-IR fluorescent dye and a novel 1,4,7-triazacyclononane-based 64Cu(II) chelator assembled via click chemistry. Bioconjug. Chem. 2014, 25, 1011–1022. [Google Scholar] [CrossRef]
- Demchenko, A.P. Optimization of fluorescence response in the design of molecular biosensors. Anal. Biochem. 2005, 343, 1–22. [Google Scholar] [CrossRef]
- Sato, K.; Nagaya, T.; Nakamura, Y.; Harada, T.; Nani, R.R.; Shaum, J.B.; Gorka, A.P.; Kim, I.; Paik, C.H.; Choyke, P.L.; et al. Impact of C4′-O-Alkyl Linker on in Vivo Pharmacokinetics of Near-Infrared Cyanine/Monoclonal Antibody Conjugates. Mol. Pharm. 2015, 12, 3303–3311. [Google Scholar] [CrossRef]
- Hoogstins, C.E.S.; Boogerd, L.S.F.; Sibinga Mulder, B.G.; Mieog, J.S.D.; Swijnenburg, R.J.; van de Velde, C.J.H.; Farina Sarasqueta, A.; Bonsing, B.A.; Framery, B.; Pèlegrin, A.; et al. Image-Guided Surgery in Patients with Pancreatic Cancer: First Results of a Clinical Trial Using SGM-101, a Novel Carcinoembryonic Antigen-Targeting, Near-Infrared Fluorescent Agent. Ann. Surg. Oncol. 2018, 25, 3350–3357. [Google Scholar] [CrossRef] [PubMed]
- Baslé, E.; Joubert, N.; Pucheault, M. Protein Chemical Modification on Endogenous Amino Acids. Chem. Biol. 2010, 17, 213–227. [Google Scholar] [CrossRef]
- Badescu, G.; Bryant, P.; Bird, M.; Henseleit, K.; Swierkosz, J.; Parekh, V.; Tommasi, R.; Pawlisz, E.; Jurlewicz, K.; Farys, M.; et al. Bridging disulfides for stable and defined antibody drug conjugates. Bioconjug. Chem. 2014, 25, 1124–1136. [Google Scholar] [CrossRef]
- Magdelaine-Beuzelin, C.; Kaas, Q.; Wehbi, V.; Ohresser, M.; Jefferis, R.; Lefranc, M.-P.; Watier, H. Structure-function relationships of the variable domains of monoclonal antibodies approved for cancer treatment. Crit. Rev. Oncol. Hematol. 2007, 64, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Casadevall, A. The immunoglobulin constant region contributes to affinity and specificity. Trends Immunol. 2008, 29, 91–97. [Google Scholar] [CrossRef]
- Ternant, D.; Arnoult, C.; Pugnière, M.; Dhommée, C.; Drocourt, D.; Perouzel, E.; Passot, C.; Baroukh, N.; Mulleman, D.; Tiraby, G.; et al. IgG1 Allotypes Influence the Pharmacokinetics of Therapeutic Monoclonal Antibodies through FcRn Binding. J. Immunol. 2016, 196, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.Q.; Xu, K.; Liu, L.; Raab, H.; Bhakta, S.; Kenrick, M.; Parsons-Reponte, K.L.; Tien, J.; Yu, S.F.; Mai, E.; et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat. Biotechnol. 2012, 30, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, D.; Bagosi, A.; Szöllősi, J.; Jenei, A. Comparative study of the three different fluorophore antibody conjugation strategies. Anal. Bioanal. Chem. 2012, 404, 1449–1463. [Google Scholar] [CrossRef] [PubMed]
- Nath, N.; Godat, B.; Zimprich, C.; Dwight, S.J.; Corona, C.; McDougall, M.; Urh, M. Homogeneous plate based antibody internalization assay using pH sensor fluorescent dye. J. Immunol. Methods 2016, 431, 11–21. [Google Scholar] [CrossRef]
- Alley, S.C.; Benjamin, D.R.; Jeffrey, S.C.; Okeley, N.M.; Meyer, D.L.; Sanderson, R.J.; Senter, P.D. Contribution of linker stability to the activities of anticancer immunoconjugates. Bioconjug. Chem. 2008, 19, 759–765. [Google Scholar] [CrossRef]
- Bryden, F.; Martin, C.; Letast, S.; Lles, E.; Viéitez-Villemin, I.; Rousseau, A.; Colas, C.; Brachet-Botineau, M.; Allard-Vannier, E.; Larbouret, C.; et al. Impact of cathepsin B-sensitive triggers and hydrophilic linkers on in vitro efficacy of novel site-specific antibody-drug conjugates. Org. Biomol. Chem. 2018, 16, 1882–1889. [Google Scholar] [CrossRef]
- Aubrey, N.; Allard-Vannier, E.; Martin, C.; Bryden, F.; Letast, S.; Colas, C.; Lakhrif, Z.; Collinet, N.; Dimier-Poisson, I.; Chourpa, I.; et al. Site-Specific Conjugation of Auristatins onto Engineered scFv Using Second Generation Maleimide to Target HER2-positive Breast Cancer in Vitro. Bioconjug. Chem. 2018, 29, 3516–3521. [Google Scholar] [CrossRef]
- Hervé-Aubert, K.; Allard-Vannier, E.; Joubert, N.; Lakhrif, Z.; Alric, C.; Martin, C.; Viaud-Massuard, M.C.; Dimier-Poisson, I.; Aubrey, N.; Chourpa, I. Impact of Site-Specific Conjugation of ScFv to Multifunctional Nanomedicines Using Second Generation Maleimide. Bioconjug. Chem. 2018, 29, 1553–1559. [Google Scholar] [CrossRef]
- Chudasama, V.; Maruani, A.; Caddick, S. Recent Advances in the Construction of Antibody–Drug Conjugates. Nat. Chem. 2016, 8, 114–119. [Google Scholar] [CrossRef]
- Smith, M.E.B.; Schumacher, F.F.; Ryan, C.P.; Tedaldi, L.M.; Papaioannou, D.; Waksman, G.; Caddick, S.; Baker, J.R. Protein modification, bioconjugation, and disulfide bridging using bromomaleimides. J. Am. Chem. Soc. 2010, 132, 1960–1965. [Google Scholar] [CrossRef]
- Cilliers, C.; Nessler, I.; Christodolu, N.; Thurber, G.M. Tracking Antibody Distribution with Near-Infrared Fluorescent Dyes: Impact of Dye Structure and Degree of Labeling on Plasma Clearance. Mol. Pharm. 2017, 14, 1623–1633. [Google Scholar] [CrossRef]
- Castañeda, L.; Wright, Z.V.F.; Marculescu, C.; Tran, T.M.; Chudasama, V.; Maruani, A.; Hull, E.A.; Nunes, J.P.M.; Fitzmaurice, R.J.; Smith, M.E.B.; et al. A mild synthesis of N-functionalised bromomaleimides, thiomaleimides and bromopyridazinediones. Tetrahedron Lett. 2013, 54, 3493–3495. [Google Scholar] [CrossRef][Green Version]
- Goussu, C.; Vasseur, J.J.; Bazin, H.; Trinquet, E.; Maurin, F.; Morvan, F. Optimized synthesis of functionalized fluorescent oligodeoxynucleotides for protein labeling. Bioconjug. Chem. 2005, 16, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Gruber, H.J.; Hahn, C.D.; Kada, G.; Riener, C.K.; Harms, G.S.; Ahrer, W.; Dax, T.G.; Knaus, H.G. Anomalous fluorescence enhancement of Cy3 and Cy3.5 versus anomalous fluorescence loss of Cy5 and Cy7 upon covalent linking to IgG and noncovalent binding to avidin. Bioconjug. Chem. 2000, 11, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Vira, S.; Mekhedov, E.; Humphrey, G.; Blank, P.S. Fluorescent-labeled antibodies: Balancing functionality and degree of labeling. Anal. Biochem. 2010, 402, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Joubert, N.; Viaud-Massuard, M.C.; Respaud, R. Novel Antibody-Drug Conjugates and the Use of Same in Therapy. U.S. Patent No. WO2015004400, 15 January 2015. [Google Scholar]
- Behrens, C.R.; Ha, E.H.; Chinn, L.L.; Bowers, S.; Probst, G.; Fitch-Bruhns, M.; Monteon, J.; Valdiosera, A.; Bermudez, A.; Liao-Chan, S.; et al. Antibody-Drug Conjugates (ADCs) Derived from Interchain Cysteine Cross-Linking Demonstrate Improved Homogeneity and Other Pharmacological Properties over Conventional Heterogeneous ADCs. Mol. Pharm. 2015, 12, 3986–3998. [Google Scholar] [CrossRef] [PubMed]
- Guryev, O.; Abrams, B.; Lomas, C.; Nasraty, Q.; Park, E.; Dubrovsky, T. Control of the fluorescence of dye-antibody conjugates by (2-hydroxypropyl)-β-cyclodextrin in fluorescence microscopy and flow cytometry. Anal. Chem. 2011, 83, 7109–7114. [Google Scholar] [CrossRef]
- Pauli, J.; Grabolle, M.; Brehm, R.; Spieles, M.; Hamann, F.M.; Wenzel, M.; Hilger, I.; Resch-Genger, U. Suitable labels for molecular imaging—Influence of dye structure and hydrophilicity on the spectroscopic properties of IgG conjugates. Bioconjug. Chem. 2011, 22, 1298–1308. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer US: New York, NY, USA, 1983; ISBN 9781461576600. [Google Scholar]
- Würthner, F.; Kaiser, T.E.; Saha-Möller, C.R. J-Aggregates: From Serendipitous Discovery to Supramolecular Engineering of Functional Dye Materials. Angew. Chem. Int. Ed. 2011, 50, 3376–3410. [Google Scholar] [CrossRef]
- Vu, T.T.; Dvorko, M.; Schmidt, E.Y.; Audibert, J.F.; Retailleau, P.; Trofimov, B.A.; Pansu, R.B.; Clavier, G.; Méallet-Renault, R. Understanding the Spectroscopic Properties and Aggregation Process of a New Emitting Boron Dipyrromethene (BODIPY). J. Phys. Chem. C 2013, 117, 5373–5385. [Google Scholar] [CrossRef]
- Brachet, G.; Respaud, R.; Arnoult, C.; Henriquet, C.; Dhommée, C.; Viaud-Massuard, M.C.; Heuze-Vourc’h, N.; Joubert, N.; Pugnière, M.; Gouilleux-Gruart, V. Increment in Drug Loading on an Antibody-Drug Conjugate Increases Its Binding to the Human Neonatal Fc Receptor in Vitro. Mol. Pharm. 2016, 13, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Bahou, C.; Love, E.A.; Leonard, S.; Spears, R.J.; Maruani, A.; Armour, K.; Baker, J.R.; Chudasama, V. Disulfide Modified IgG1: An Investigation of Biophysical Profile and Clinically Relevant Fc Interactions. Bioconjug. Chem. 2019, 30, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Hama, Y.; Urano, Y.; Koyama, Y.; Bernardo, M.; Choyke, P.L.; Kobayashi, H. A comparison of the emission efficiency of four common green fluorescence dyes after internalization into cancer cells. Bioconjug. Chem. 2006, 17, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.M.; Lindqvist, L. The pH dependence of fluorescein fluorescence. J. Lumin. 1975, 10, 381–390. [Google Scholar] [CrossRef]
| Antibody | Compound Used for Bioconjugation | AFC |
|---|---|---|
| Trastuzumab | BODIPY-SGM 6a | TTZ-SGM-BDP 7a |
| Trastuzumab | Fluorescein-SGM 6b | TTZ-SGM-FLU 7b |
| Rituximab | BODIPY-SGM 6a | RTX-SGM-BDP 8a |
| Rituximab | Fluorescein-SGM 6b | RTX-SGM-FLU 8b |
| Trastuzumab | BODIPY-NHS | TTZ-BDP 9a |
| Trastuzumab | Fluorescein-NHS | TTZ-FLU 9b |
| Rituximab | BODIPY-NHS | RTX-BDP 10a |
| Rituximab | Fluorescein-NHS | RTX-FLU 10b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, C.; Brachet, G.; Colas, C.; Allard-Vannier, E.; Kizlik-Masson, C.; Esnault, C.; Respaud, R.; Denevault-Sabourin, C.; Chourpa, I.; Gouilleux-Gruart, V.; et al. In Vitro Characterization and Stability Profiles of Antibody–Fluorophore Conjugates Derived from Interchain Cysteine Cross-Linking or Lysine Bioconjugation. Pharmaceuticals 2019, 12, 176. https://doi.org/10.3390/ph12040176
Martin C, Brachet G, Colas C, Allard-Vannier E, Kizlik-Masson C, Esnault C, Respaud R, Denevault-Sabourin C, Chourpa I, Gouilleux-Gruart V, et al. In Vitro Characterization and Stability Profiles of Antibody–Fluorophore Conjugates Derived from Interchain Cysteine Cross-Linking or Lysine Bioconjugation. Pharmaceuticals. 2019; 12(4):176. https://doi.org/10.3390/ph12040176
Chicago/Turabian StyleMartin, Camille, Guillaume Brachet, Cyril Colas, Emilie Allard-Vannier, Claire Kizlik-Masson, Clara Esnault, Renaud Respaud, Caroline Denevault-Sabourin, Igor Chourpa, Valérie Gouilleux-Gruart, and et al. 2019. "In Vitro Characterization and Stability Profiles of Antibody–Fluorophore Conjugates Derived from Interchain Cysteine Cross-Linking or Lysine Bioconjugation" Pharmaceuticals 12, no. 4: 176. https://doi.org/10.3390/ph12040176
APA StyleMartin, C., Brachet, G., Colas, C., Allard-Vannier, E., Kizlik-Masson, C., Esnault, C., Respaud, R., Denevault-Sabourin, C., Chourpa, I., Gouilleux-Gruart, V., Viaud-Massuard, M.-C., & Joubert, N. (2019). In Vitro Characterization and Stability Profiles of Antibody–Fluorophore Conjugates Derived from Interchain Cysteine Cross-Linking or Lysine Bioconjugation. Pharmaceuticals, 12(4), 176. https://doi.org/10.3390/ph12040176