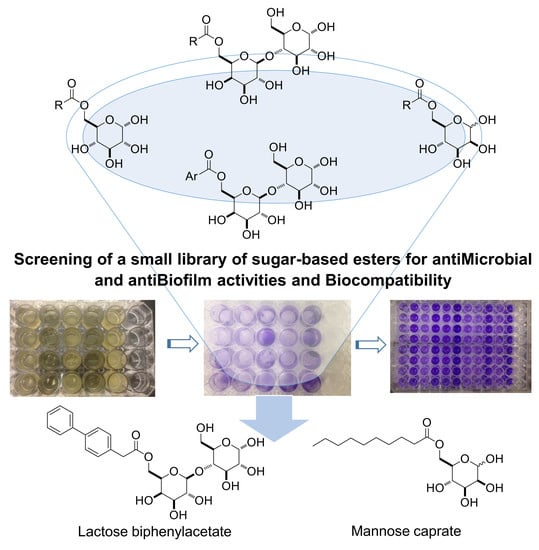
Synthesis and Evaluation of Saccharide-Based Aliphatic and Aromatic Esters as Antimicrobial and Antibiofilm Agents †
Abstract
1. Introduction
2. Results and Discussion
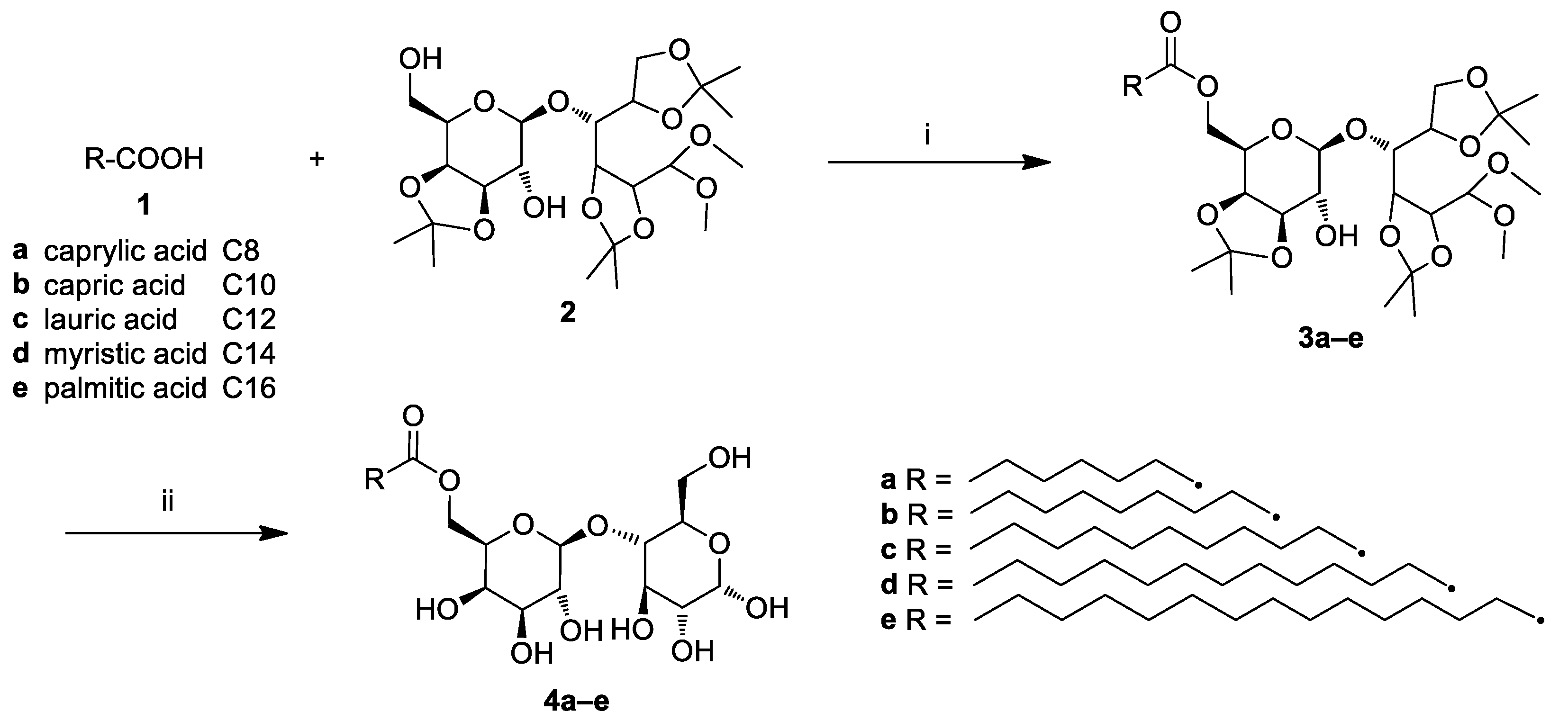
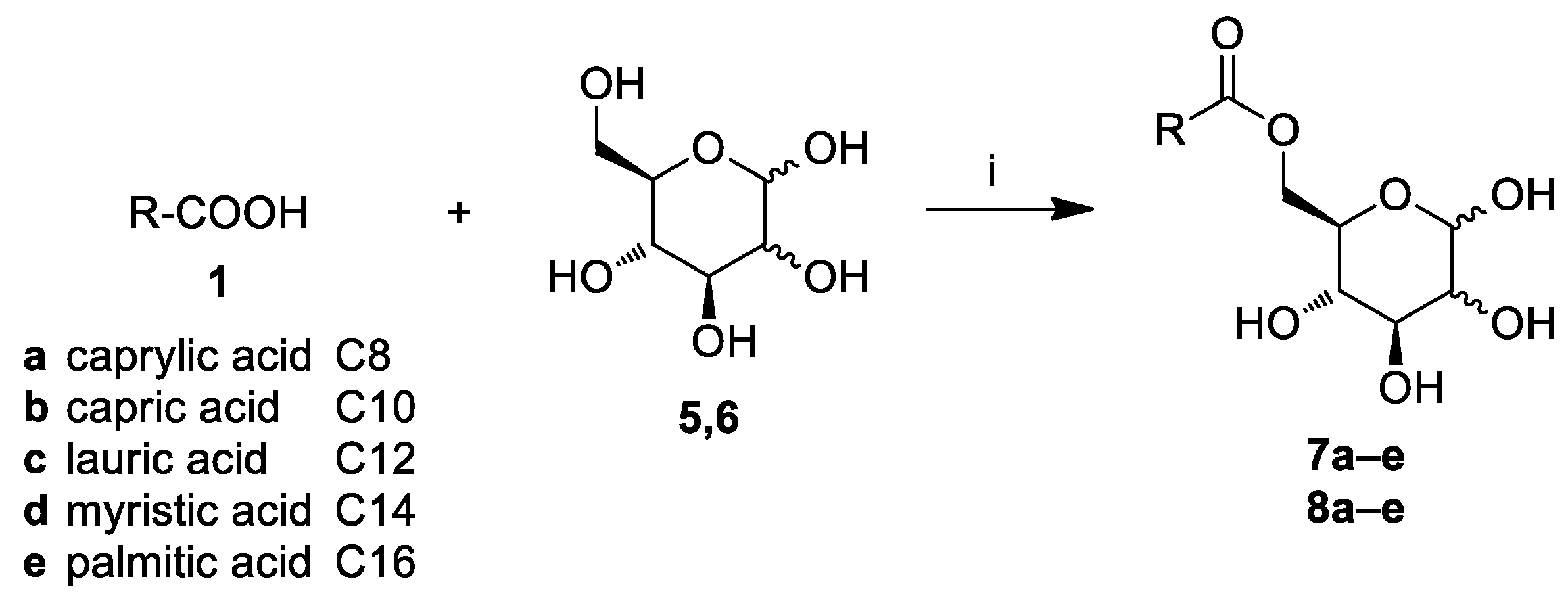
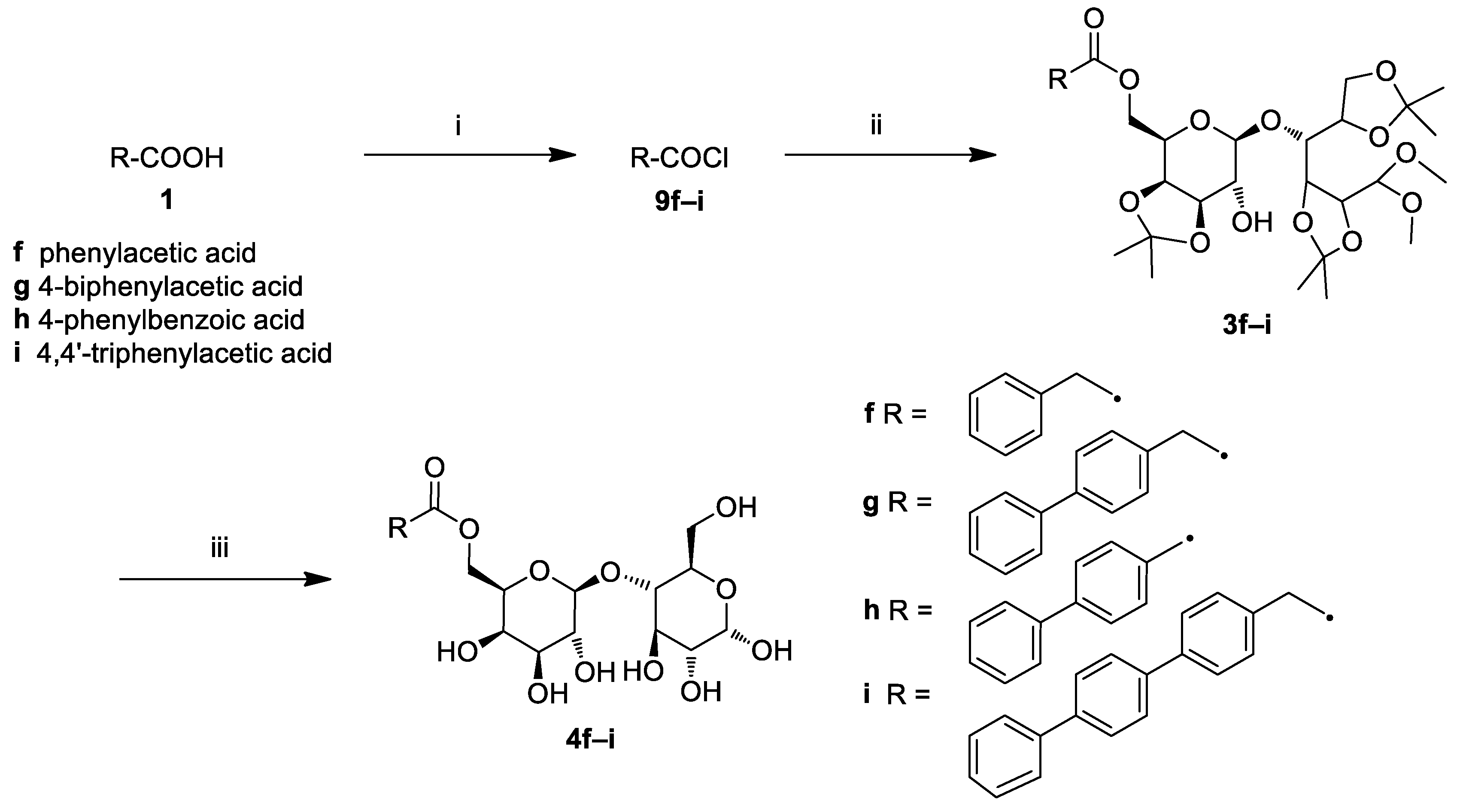
2.1. Chemistry
2.2. Antimicrobial Activity
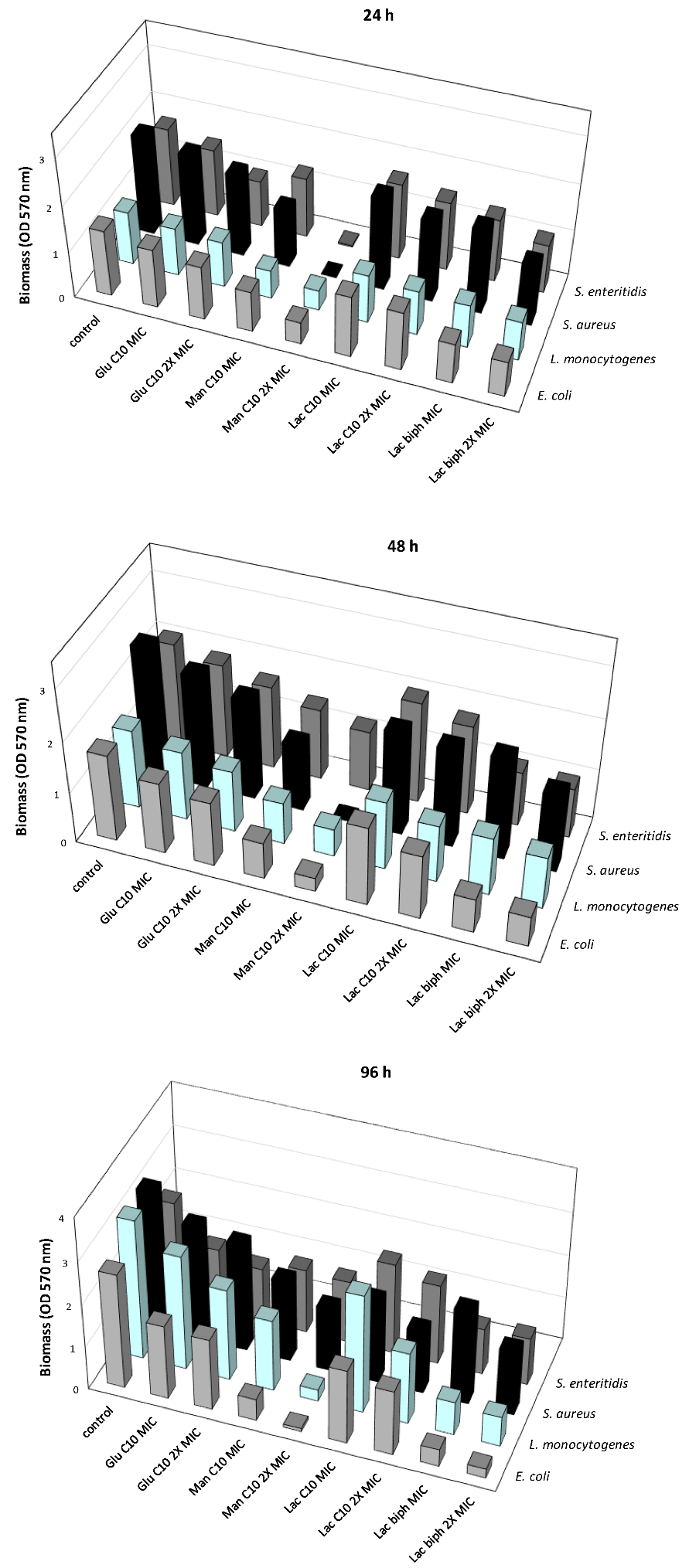
2.3. Inhibition of the Biofilm Formation at Different Times of Development
2.4. Inhibition of the Biofilm Formation Induced by Each Examined Sugar-Based Ester
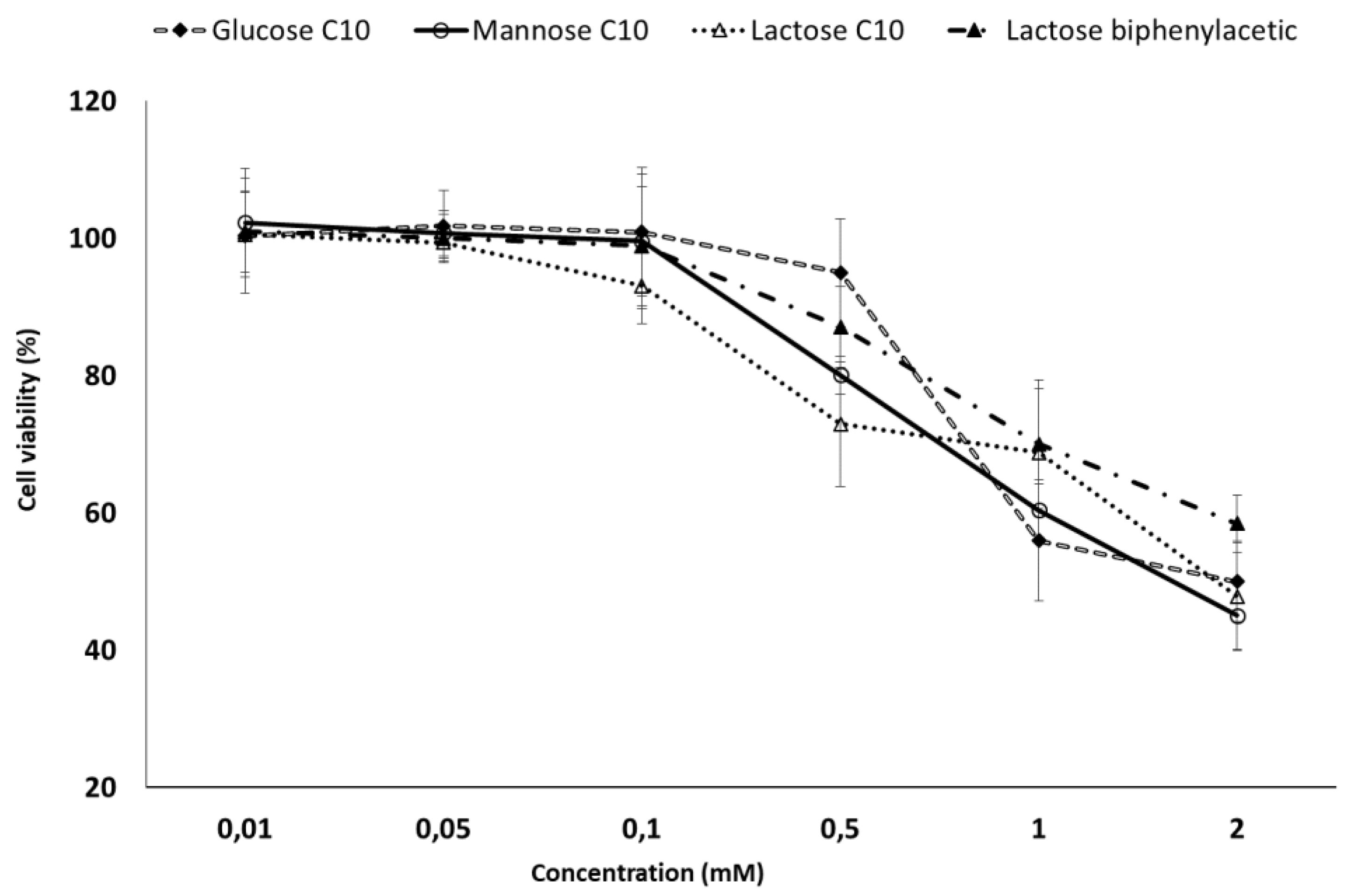
2.5. MTT Toxicity Assay
3. Materials and Methods
3.1. Chemicals, Materials and Methods
3.2. General Procedure for the Synthesis of Lactose Fatty Acid Ester Derivatives (3a–e)
3.3. General Procedure for the Synthesis of Glucose and Mannose Fatty Acid Ester Derivatives (7a–e, 8a–e)
3.4. General Procedure for the Synthesis of Lactose Aromatic Fatty Acid Ester Derivatives (4f–i)
3.5. Synthesis of Triphenylacetic Acid (1i)
3.6. Bacterial Strains and Culture Conditions
3.7. Determination of MIC
3.8. Formation of the Biofilm on 24-Well Polystyrene Plates
3.9. Evaluation of the Antibiofilm Activity of Sugar-Based Esters at Different Stage of Biofilm Formation
3.10. Caco-2 Cell Culture
3.11. MTT Cell Proliferation Colorimetric Assay
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Scharff, R.L. Economic burden from health losses due to foodborne illness in the United States. J. Food Protec. 2012, 75, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Martinović, T.; Andjelković, U.; Gajdošik, M.Š.; Rešetar, D.; Josić, D. Foodborne pathogens and their toxins. J. Proteom. 2016, 147, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, A.A.; Papenbrock, J. Mycotoxins: Producing fungi and mechanisms of phytotoxicity. Agriculture 2015, 5, 492–537. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States-major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Scallan, E.; Griffin, P.M.; Angulo, F.J.; Tauxe, R.V.; Hoekstra, R.M. Foodborne illness acquired in the United States-unspecified agents. Emerg. Infect. Dis. 2011, 17, 16–22. [Google Scholar] [CrossRef]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Ann. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–632. [Google Scholar] [CrossRef]
- Pfuntner, A. Sanitizers and Disinfectants: The Chemicals of Prevention. Food Saf. Mag. 2011. Available online: https://www.foodsafetymagazine.com/magazine-archive1/augustseptember-2011/sanitizers-and-disinfectants-the-chemicals-of-prevention/ (accessed on 14 December 2019).
- Diehl, J.F. Food irradiation-past, present and future. Radiat. Phys. Chem. 2002, 63, 211–215. [Google Scholar] [CrossRef]
- Holah, J.T. Special needs for disinfectants in food-handling establishments. Rev. Sci. Tech. 1995, 14, 95–104. [Google Scholar] [CrossRef]
- Simoes, M.; Pereira, M.O.; Vieira, M.J. Effect of mechanical stress on biofilms challenged by different chemicals. Water Res. 2005, 39, 5142–5152. [Google Scholar] [CrossRef]
- Tuncel, A.T.; Ruppert, T.; Wang, B.T.; Okun, J.G.; Kölker, S.; Morath, M.A.; Sauer, S.W. Maleic acid–but not structurally related methylmalonic acid–interrupts energy metabolism by impaired calcium homeostasis. PLoS ONE 2015, 10, e0128770. [Google Scholar] [CrossRef] [PubMed]
- Mamur, S.; Yüzbasioglu, D.; Ünal, F.; Yılmaz, S. Does potassium sorbate induce genotoxic or mutagenic effects in lymphocytes? Toxicol. Vitr. 2010, 24, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Campana, R.; Casettari, L.; Fagioli, L.; Cespi, M.; Bonacucina, G.; Baffone, W. Activity of essential oil-based microemulsions against Staphylococcus aureus biofilms developed on stainless steel surface in different culture media and growth conditions. Int. J. Food Microbiol. 2017, 241, 132–140. [Google Scholar] [CrossRef]
- Bridier, A.; Sanchez-Vizuete, P.; Guilbaud, M.; Piard, J.C.; Naïtali, M.; Briandet, R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015, 45, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Cruces, M.A.; Bernabe, M.; Ballesteros, A.; Plou, F.J. Lipase catalyzed regioselective acylation of sucrose in two-solvent mixtures. Biotechnol. Bioeng. 1999, 65, 10–16. [Google Scholar] [CrossRef]
- Plat, T.; Linhardt, R.J. Syntheses and applications of sucrose-based esters. J. Surfactants Deterg. 2001, 4, 415–421. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Lucarini, S.; Fagioli, L.; Campana, R.; Vllasaliu, D.; Duranti, A.; Casettari, L. Lactose oleate as new biocompatible surfactant for pharmaceutical applications. Eur. J. Pharm. Biopharm. 2018, 124, 55–62. [Google Scholar] [CrossRef]
- Hayes, D.G. Bioprocessing methods to prepare biobased surfactants for pharmaceutical products. Am. Pharm. Rev. 2011, 14, 8–16. [Google Scholar]
- Gumel, A.M.; Annuar, M.S.M.; Heidelberg, T.; Chisti, Y. Lipase mediated synthesis of sugar fatty acid esters. Process Biochem. 2011, 46, 2079–2090. [Google Scholar] [CrossRef]
- Alama, T.; Katayama, T.; Hirai, S.; Ono, S.; Kajiyama, A.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Enhanced oral delivery of alendronate by sucrose fatty acids esters in rats and their absorption-enhancing mechanisms. Int. J. Pharm. 2016, 515, 476–489. [Google Scholar] [CrossRef]
- Liang, M.Y.; Chen, Y.; Banwell, M.G.; Wang, Y.; Lan, P. Enzymatic preparation of a homologous series of long-chain 6-O-acylglucose esters and their evaluation as emulsifiers. J. Agr. Food Chem. 2018, 66, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.Y.; Banwell, M.G.; Wang, Y.; Lan, P. Effect of Variations in the Fatty Acid Residue of Lactose Monoesters on Their Emulsifying Properties and Biological Activities. J. Agr. Food Chem. 2018, 66, 12594–12603. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Lamsal, B.P. Synthesis of some glucose-fatty acid esters by lipase from Candida Antarctica and their emulsion functions. Food Chem. 2017, 214, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W.C. Calculation of HLB values of non-ionic surfactants. J. Soc. Cosmet. Chem. 1954, 5, 249–256. [Google Scholar]
- Organic Chemistry Portal. Available online: https://www.organic-chemistry.org/prog/ (accessed on 22 November 2019).
- Arcos, J.A.; Bernabé, M.; Otero, C. Quantitative enzymatic production of 6-O-acylglucose esters. Biotechnol. Bioeng. 1998, 57, 505–509. [Google Scholar] [CrossRef]
- Bartolucci, S.; Bartoccini, F.; Righi, M.; Piersanti, G. Direct, regioselective, and chemoselective preparation of novel boronated tryptophans by Friedel-Crafts alkylation. Org. Lett. 2012, 14, 600–603. [Google Scholar] [CrossRef]
- AlFindee, M.N.; Zhang, Q.; Subedi, Y.P.; Shrestha, J.P.; Kawasaki, Y.; Grilley, M.; Takemoto, J.Y.; Chang, C.W.T. One-step synthesis of carbohydrate esters as antibacterial and antifungal agents. Bioorg. Med. Chem. 2018, 26, 765–774. [Google Scholar] [CrossRef]
- Jumina, J.; Mutmainah, M.; Purwono, B.; Kurniawan, Y.; Syah, Y.M. Antibacterial and antifungal activity of three monosaccharide monomyristate derivatives. Molecules 2019, 24, 3692. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Hao, T.; Li, S. In vitro antibacterial activities and mechanism of sugar fatty acid esters against five food-related bacteria. Food Chem. 2015, 187, 370–377. [Google Scholar] [CrossRef]
- Enayati, M.; Gong, Y.; Goddard, J.M.; Abbaspourrad, A. Synthesis and characterization of lactose fatty acid ester biosurfactants usingfree and immobilized lipases in organic solvents. Food Chem. 2018, 266, 508–513. [Google Scholar] [CrossRef]
- Enayati, M.; Gong, Y.; Abbaspourrad, A. Synthesis of lactose lauryl ester in organic solvents using aluminosilicate zeolite as a catalyst. Food Chem. 2019, 279, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Soliveri, J.; Plou, F.J.; López-Cortés, N.; Reyes-Duarte, D.; Christensen, M.; Copa-Patiño, J.L.; Ballesteros, A. Synthesis of sugar esters in solvent mixtures by lipases from Thermomyces lanuginosus and Candida antarctica B, and their antimicrobial properties. Enzym. Microb. Technol. 2005, 36, 391–398. [Google Scholar] [CrossRef]
- Xiao, D.; Ye, R.; Davidson, P.M.; Hayes, D.G.; Golden, D.A.; Zhong, Q. Sucrose monolaurate improves the efficacy of sodium hypochlorite against Escherichia coli O157:H7 on spinach. Int. J. Food Microbiol. 2011, 145, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Katayama, S.; Matsubara, M.; Honda, Y.; Kuwahara, M. Antibacterial carbohydrate monoesters suppressing cell growth of Streptococcus mutans in the presence of sucrose. Curr. Microbiol. 2000, 41, 210–213. [Google Scholar] [CrossRef]
- Devulapalle, K.S.; Gómez de Segura, A.; Ferrer, M.; Alcalde, M.; Mooser, G.; Plou, F.J. Effect of carbohydrate fatty acid esters on Streptococcus sobrinus and glucosyltransferase activity. Carbohydr. Res. 2004, 339, 1029–1034. [Google Scholar] [CrossRef]
- Karlová, T.; Polakova, L.; Šmidrkal, J.; Filip, V. Antimicrobial effects of fatty acid fructose esters. Czech J. Food Sci. 2010, 28, 146–149. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Mah, T.F.; Pitts, B.; Pellock, B.; Walker, G.C.; Stewart, P.S.; O’Toole, G.A. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 2003, 426, 306–310. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Stewart, P.S. Biofilms strike back. Nat. Biotechnol. 2005, 23, 1378–1379. [Google Scholar] [CrossRef]
- Monds, R.D.; O’Toole, G.A. The developmental model of microbial biofilms: Ten years of a paradigm up for review. Trends Microbiol. 2009, 17, 73–87. [Google Scholar] [CrossRef]
- Ito, A.; Taniuchi, A.; May, T.; Kawata, K.; Okabe, S. Increased antibiotic resistance of Escherichia coli in mature biofilms. Appl. Environ. Microbiol. 2009, 75, 4093–4100. [Google Scholar] [CrossRef]
- Lu, B.; Vayssade, M.; Miao, Y.; Chagnault, V.; Grand, E.; Wadouachi, A.; Postel, D.; Drelich, A.; Egles, C.; Pezron, I. Physico-chemical properties and cytotoxic effects of sugar-based surfactants: Impact of structural variations. Colloids Surf. B Biointerfaces 2016, 145, 79–86. [Google Scholar] [CrossRef]
- Hough, L.; Richardson, A.C.; Thelwall, L.A.W. Reaction of lactose with 2,2-dimethoxypropane: A tetraacetal of novel structure. Carbohydr. Res. 1979, 75, C11–C12. [Google Scholar] [CrossRef]
- Lucarini, S.; Fagioli, L.; Campana, R.; Cole, H.; Duranti, A.; Baffone, W.; Vllasaliu, D.; Casettari, L. Unsaturated fatty acids lactose esters: Cytotoxicity, permeability enhancement and antimicrobial activity. Eur. J. Pharm. Biopharm. 2016, 107, 88–96. [Google Scholar] [CrossRef]
- Sarney, D.B.; Kapeller, H.; Fregapane, G.; Vulfson, E.N. Chemo-enzymatic synthesis of disaccharide fatty acid esters. J. Am. Oil Chem. Soc. 1994, 71, 711–714. [Google Scholar] [CrossRef]
- Lucarini, S.; Fagioli, L.; Cavanagh, R.; Liang, W.; Perinelli, D.; Campana, M.; Stolnik, S.; Lam, J.; Casettari, L.; Duranti, A. Synthesis, structure–activity relationships and in vitro toxicity profile of lactose-based fatty acid monoesters as possible drug permeability enhancers. Pharmaceutics 2018, 10, 81. [Google Scholar] [CrossRef]
- Yosimoto, K.; Tahara, K.; Suzuki, S.; Sasaki, K.; Nishikawa, Y.; Tsuda, Y. Regioselective syntheses of mono-O-acylglucoses. Chem. Pharm. Bull. 1979, 27, 2661–2674. [Google Scholar] [CrossRef][Green Version]
- Shukla, S.K.; Rao, T.S. An improved crystal violet assay for biofilm quantification in 96-well microtitre plate. BioRxiv 2017. [Google Scholar] [CrossRef]
| Entry | Sugar Ester | MW | cHLB a | cLogP b | cTPSA c |
|---|---|---|---|---|---|
| 1 | Glucose C8 | 306.4 | 8.9 | 0.59 | 116.5 |
| 2 | Glucose C10 | 334.4 | 8.1 | 1.43 | 116.5 |
| 3 | Glucose C12 | 362.5 | 7.5 | 2.26 | 116.5 |
| 4 | Glucose C14 | 390.5 | 7.0 | 3.20 | 116.5 |
| 5 | Glucose C16 | 418.6 | 6.5 | 3.93 | 116.5 |
| 6 | Mannose C8 | 306.4 | 8.9 | 0.59 | 116.5 |
| 7 | Mannose C10 | 334.4 | 8.1 | 1.43 | 116.5 |
| 8 | Mannose C12 | 362.5 | 7.5 | 2.26 | 116.5 |
| 9 | Mannose C14 | 390.5 | 7.0 | 3.20 | 116.5 |
| 10 | Mannose C16 | 418.6 | 6.5 | 3.93 | 116.5 |
| 11 | Lactose C8 | 468.5 | 12.7 | −1.15 | 195.6 |
| 12 | Lactose C10 | 496.6 | 12.0 | −0.31 | 195.6 |
| 13 | Lactose C12 | 524.6 | 11.4 | 0.52 | 195.6 |
| 14 | Lactose C14 | 552.7 | 10.8 | 1.36 | 195.6 |
| 15 | Lactose C16 | 580.1 | 10.3 | 2.19 | 195.6 |
| 16 | Lactose phenylacetate | 460.4 | 12.9 | −2.05 | 195.6 |
| 17 | Lactose p-phenylbenzoate | 522.5 | 11.4 | −0.32 | 195.6 |
| 18 | Lactose biphenylacetate | 536.5 | 11.1 | −0.37 | 195.6 |
| 19 | Lactose triphenylacetate | 612.6 | 9.7 | 1.30 | 195.6 |
| E. coli O157:H7 ATCC 35150 | E. faecalis ATCC 29212 | L. monocytogenes ATCC 7644 | K. pneumoniae ATCC 13883 | P. aeruginosa ATCC 9027 | S. aureus ATCC 43387 | S. enteritidis ATCC 13076 | C. albicans ATCC 10231 | |
|---|---|---|---|---|---|---|---|---|
| Glucose C8 | 256 | 256 | 256 | >256 | >256 | >256 | 256 | 256 |
| Glucose C10 | 256 | 256 | 256 | 256 | 256 | 256 | 256 | 256 |
| Glucose C12 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 |
| Glucose C14 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 |
| Glucose C16 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 |
| Mannose C8 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 |
| Mannose C10 | 256 | 256 | 256 | 256 | 256 | 256 | 256 | 256 |
| Mannose C12 | 256 | 256 | 256 | 256 | 256 | 256 | 256 | 256 |
| Mannose C14 | >256 | 256 | >256 | 256 | 256 | >256 | >256 | 256 |
| Mannose C16 | >256 | 256 | >256 | 256 | >256 | >256 | >256 | >256 |
| Lactose C8 | 256 | 256 | 256 | 256 | >256 | 256 | 256 | 256 |
| Lactose C10 | 256 | 128 | 256 | 256 | 256 | 256 | 256 | 128 |
| Lactose C12 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | 256 |
| Lactose C14 | >256 | 256 | >256 | >256 | >256 | >256 | >256 | 256 |
| Lactose C16 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | 256 |
| Lactose phenylacetate | 256 | 256 | 256 | 256 | >256 | >256 | 256 | 256 |
| Lactose biphenylacetate | 256 | 256 | 256 | 256 | 256 | 256 | 256 | 256 |
| Lactose p-phenylbenzoate | 256 | 256 | 256 | 256 | 256 | 256 | 256 | 256 |
| Lactose triphenylacetate | 256 | 256 | 256 | 256 | 256 | 256 | 256 | 256 |
| Gentamicin | 16 | 64 | 8 | 8 | 16 | 16 | 4 | 1 a |
| Parabens | >1024 | >1024 | >1024 | >1024 | >1024 | >1024 | >1024 | >1024 |
| 24 h | 48 h | 5 Days | ||||
|---|---|---|---|---|---|---|
| MIC | 2× MIC | MIC | 2× MIC | MIC | 2× MIC | |
| Glucose C10 | Glucose C10 | Glucose C10 | ||||
| E. coli O157:H7 ATCC 35150 | 10.77% | 18.71% | 18.27% | 25.94% | 35.46% | 37.90% |
| L. monocytogenes ATCC 7644 | 9.61% | 14.17% | 12.69% | 21.95% | 18.04% | 34.58% |
| S. aureus ATCC 43387 | 8.42% | 16.98% | 13.18% | 23.05% | 18.01% | 23.57% |
| S. enteritidis ATCC 13076 | 14.05% | 41.27% | 9.97% | 20.66% | 36.94% | 46.28% |
| Mannose C10 | Mannose C10 | Mannose C10 | ||||
| E. coli O157:H7 ATCC 35150 | 38.30% | 66.74% | 58.19% | 84.38% | 81.13% | 97.24% |
| L. monocytogenes ATCC 7644 | 46.19% | 62.32% | 47.15% | 65.45% | 49.05% | 91.80% |
| S. aureus ATCC 43387 | 41.49% | 99.58% | 48.72% | 99.88% | 42.16% | 54.61% |
| S. enteritidis ATCC 13076 | 23.78% | 98.54% | 32.16% | 42.71% | 37.04% | 38.61% |
| Lactose C10 | Lactose C10 | Lactose C10 | ||||
| E. coli O157:H7 ATCC 35150 | 7.54% | 11.69% | 9.66% | 26.75% | 35.46% | 43.60% |
| L. monocytogenes ATCC 7644 | 9.61% | 16.64% | 13.53% | 27.76% | 15.38% | 48.86% |
| S. aureus ATCC 43387 | 6.72% | 18.13% | 21.13% | 24.66% | 41.40% | 55.26% |
| S. enteritidis ATCC 13076 | 3.55% | 12.79% | 3.61% | 14.81% | 11.00% | 21.48% |
| Lactose biphenylacetate | Lactose biphenylacetate | Lactose biphenylacetate | ||||
| E. coli O157:H7 ATCC 35150 | 40.10% | 46.99% | 61.31% | 65.65% | 85.21% | 91.97% |
| L. monocytogenes ATCC 7644 | 19.42% | 27.12% | 26.98% | 34.66% | 76.14% | 78.77% |
| S. aureus ATCC 43387 | 15.35% | 39.00% | 22.46% | 40.27% | 33.03% | 53.91% |
| S. enteritidis ATCC 13076 | 20.58% | 38.35% | 45.41% | 52.22% | 48.12% | 53.27% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campana, R.; Merli, A.; Verboni, M.; Biondo, F.; Favi, G.; Duranti, A.; Lucarini, S. Synthesis and Evaluation of Saccharide-Based Aliphatic and Aromatic Esters as Antimicrobial and Antibiofilm Agents. Pharmaceuticals 2019, 12, 186. https://doi.org/10.3390/ph12040186
Campana R, Merli A, Verboni M, Biondo F, Favi G, Duranti A, Lucarini S. Synthesis and Evaluation of Saccharide-Based Aliphatic and Aromatic Esters as Antimicrobial and Antibiofilm Agents. Pharmaceuticals. 2019; 12(4):186. https://doi.org/10.3390/ph12040186
Chicago/Turabian StyleCampana, Raffaella, Alessio Merli, Michele Verboni, Francesca Biondo, Gianfranco Favi, Andrea Duranti, and Simone Lucarini. 2019. "Synthesis and Evaluation of Saccharide-Based Aliphatic and Aromatic Esters as Antimicrobial and Antibiofilm Agents" Pharmaceuticals 12, no. 4: 186. https://doi.org/10.3390/ph12040186
APA StyleCampana, R., Merli, A., Verboni, M., Biondo, F., Favi, G., Duranti, A., & Lucarini, S. (2019). Synthesis and Evaluation of Saccharide-Based Aliphatic and Aromatic Esters as Antimicrobial and Antibiofilm Agents. Pharmaceuticals, 12(4), 186. https://doi.org/10.3390/ph12040186