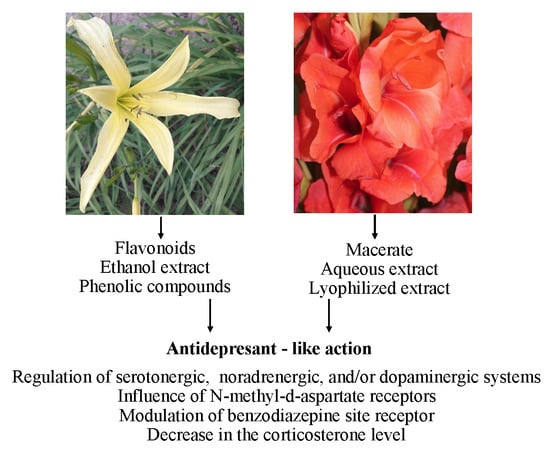
Recent Studies on Anti-Depressant Bioactive Substances in Selected Species from the Genera Hemerocallis and Gladiolus: A Systematic Review
Abstract
1. Introduction
1.1. Depression
1.1.1. Epidemiology with Its Relation to Pathogenesis
1.1.2. Symptoms and Their Organic Explanation
1.1.3. Neurotransmitters as the Key Pathogenetic Factors
1.2. Phytotherapy
2. Methodology
3. Antidepressant Action of Selected Plant Species from the Genera Hemerocallis and Gladiolus
3.1. Hemerocallis fulva and H. citrina
3.2. Gladiolus Dalenii
3.3. Synergistic Activity of Phytocompounds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ionescu, D.F.; Niciu, M.J.; Henter, I.D.; Zarate, C.A. Defining anxious depression: A review of the literature. CNS Spectr. 2013, 18, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Kupferberg, A.; Bicks, L.; Hasler, G. Social functioning in major depressive disorder. Neurosci. Biobehav. Rev. 2016, 69, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.E.R. Depression: A review of its definition. MOJ Addict. Med. Ther. 2018, 5, 6–7. [Google Scholar]
- Nesse, R.M. Is depression an adaptation? Arch. Gen. Psychiat. 2000, 57, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Karampampa, K.; Borgström, F.; Jönsson, B. Economic burden of depression of society. Medicographia 2011, 33, 163–168. [Google Scholar]
- McTernan, W.P.; Dollard, M.F.; LaMontagne, A.D. Depression in the workplace: An economic cost analysis of depression-related productivity loss attributable to job strain and bullying. Work Stress 2012, 27, 321–338. [Google Scholar] [CrossRef]
- Kessler, R.C.; Bromet, E.J. The epidemiology of depression across cultures. Annu. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Evans-Lacko, S.; Koeser, L.; Knapp, M.; Longhitano, C.; Zohar, J.; Kuhn, K. Evaluating the economic impact of screening and treatment for depression in the workplace. Eur. Neuropsychopharmacol. 2016, 26, 1004–1013. [Google Scholar] [CrossRef]
- De Lima, M.S.; De Oliveira Soares, B.G. Depression in developing countries. In Biology of Depression: From Novel Insights to Therapeutic Strategies, 1st ed.; Licinio, J., Wong, M.L., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 979–994. [Google Scholar]
- WHO (World Health Organization). 2018. Available online: http://www.who.int/en/news-room/fact-sheets/detail/depression (accessed on 19 October 2018).
- Pratt, L.A.; Druss, B.G.; Manderscheid, R.W.; Walker, E.R. Excess mortality due to depression and anxiety in the United States: Results from a nationally representative survey. Gen. Hosp. Psychiatry 2016, 39, 39–45. [Google Scholar] [CrossRef]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S. Depression: The disorder and the burden. Indian J. Psychol. Med. 2010, 32, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.J.; Charlson, F.J.; Norman, R.E.; Patten, S.B.; Freedman, G.; Murray, C.J.; Vos, T.; Whiteford, H.A. Burden of depressive disorders by country, sex, age, and year: Findings from the global burden of disease study 2010. PLoS Med. 2013, 10, e1001547. [Google Scholar] [CrossRef] [PubMed]
- Voinov, B.; Richie, W.D.; Bailey, R.K. Depression and chronic diseases: It is time for a synergistic mental health and primary care approach. Prim. Care Companion CNS Disord. 2013, 15, PCC.12r01468. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, J.; Carter, A.; Cornaby, L. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1603–1658. [Google Scholar] [CrossRef]
- Gurland, B.J.; Wilder, D.E.; Berkman, C. Depression and disability in the elderly: Reciprocal relations and changes with age. Int. J. Geriart. Psychiatry 1988, 3, 163–179. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Depression and Other Common Mental Disorders: Global Health Estimates; Rep. CC BY-NC-SA 3.0 IGO; World Health Organization (WHO reference number: WHO/MSD/MER/2017.2): Geneva, Switzerland, 2017; Available online: http://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf (accessed on 28 October 2018).
- Kessler, R.C. Epidemiology of women and depression. J. Affect. Disord. 2003, 74, 5–13. [Google Scholar] [CrossRef]
- Munce, S.E.; Stewart, D.E. Gender differences in depression and chronic pain conditions in a national epidemiologic survey. Psychosomatics 2007, 48, 394–399. [Google Scholar] [CrossRef]
- Albert, P.R. Why is depression more prevalent in women? J. Psychiatry Neurosci. 2015, 40, 219–221. [Google Scholar] [CrossRef]
- Flores-Ramos, M.; Salinas, M.; Carvajal-Lohr, A.; Rodríguez-Bores, L. The role of gamma-aminobutyric acid in female depression. Gac. Med. Mex. 2017, 153, 486–495. [Google Scholar] [CrossRef]
- Kuehner, C. Why is depression more common among women than among men? Lancet Psychiatry 2017, 4, 146–158. [Google Scholar] [CrossRef]
- Elavsky, S.; Gold, C.H. Depressed mood but not fatigue mediate the relationship between physical activity and perceived stress in middle-aged women. Maturitas 2009, 64, 235–240. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vagena, E.; Ryu, J.K.; Baeza-Raja, B.; Walsh, N.M.; Syme, C.; Day, J.P.; Houslay, M.D.; Baillie, G.S. A high-fat diet promotes depression-like behavior in mice by suppressing hypothalamic PKA signaling. Transl. Psychiatry 2019, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Speed, M.S.; Jefsen, O.H.; Børglum, A.D.; Speed, D.; Østergaard, S.D. Investigating the association between body fat and depression via Mendelian randomization. Transl. Psychiatry 2019, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Waclawiková, B.; El Aidy, S. Role of microbiota and tryptophan metabolites in the remote effect of intestinal inflammation on brain and depression. Pharmaceuticals 2018, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis in health and disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Nutt, D.J. Role of GABA in anxiety and depression. Depress. Anxiety 2007, 24, 495–517. [Google Scholar] [CrossRef]
- Mondelli, V.; Vernon, A.C.; Turkheimer, F.; Dazzan, P.; Pariante, C.M. Brain microglia in psychiatric disorders. Lancet Psychiatry 2017, 4, 563–572. [Google Scholar] [CrossRef]
- Ativie, F.; Komorowska, J.A.; Beins, E.; Albayram, Ö.; Zimmer, T.; Zimmer, A.; Tejera, D.; Heneka, M.; Bilkei-Gorzo, A. Cannabinoid 1 receptor signaling on hippocampal GABAergic neurons influences microglial activity. Front. Mol. Neurosci. 2018, 11, 295. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.; Luo, M.T.; Wang, H.; Li, Y.H. Gamma-aminobutyric acid levels in the anterior cingulate cortex of perimenopausal women with depression: A magnetic resonance spectroscopy study. Front. Neurosci. 2019, 13, 785. [Google Scholar] [CrossRef]
- Kurek, A.; Głombik, K.; Detka, J.; Basta-Kaim, A.; Kubera, M.; Lasoń, W.; Budziszewska, B. Regulators of glucocorticoid receptor function in an animal model of depression and obesity. J. Neuroendocrinol. 2018, 30, e12591. [Google Scholar] [CrossRef]
- Janik, M.K.; Wunsch, E.; Raszeja-Wyszomirska, J.; Krawczyk, M.; Milkiewicz, P. Depression: An overlooked villain in autoimmune hepatitis? Hepatology 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Birmaher, B.; Ryan, N.D.; Williamson, D.E.; Brent, D.A.; Kaufman, J.; Dahl, R.E.; Pereland, J.; Nelson, B. Childhood and adolescent depression: A review of the past 10 years. Part I. J. Am. Acad. Child. Adolesc. Psychiatry 1996, 35, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Birmaher, B.; Ryan, N.D.; Williamson, D.E.; Brent, D.A.; Kaufman, J. Childhood and adolescent depression: A review of the past 10 years. Part II. J. Am. Acad. Child. Adolesc. Psychiatry 1996, 35, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Mirowsky, J. Age and the gender gap in depression. J. Health Soc. Behav. 1996, 37, 362–380. [Google Scholar] [CrossRef]
- Weissman, M.M.; Wolk, S.; Goldstein, R.B.; Moreau, D.; Adams, P.; Greenwald, S.; Klier, C.M.; Ryan, N.D.; Dahl, R.E.; Wickramaratne, P. Depressed adolescents grown up. JAMA 1999, 281, 1707–1713. [Google Scholar] [CrossRef]
- Kessler, R.C.; Amminger, G.P.; Aguilar-Gaxiola, S.; Alonso, J.; Lee, J.S.; Ustun, T.B. Age of onset of mental disorders: A review of recent literature. Curr. Opin. Psychiatr. 2007, 20, 359–364. [Google Scholar] [CrossRef]
- Wilson, S.; Hicks, B.M.; Foster, K.T.; McGue, M.; Iacono, W.G. Age of onset and course of major depressive disorder: Associations with psychosocial functioning outcomes in adulthood. Psychol. Med. 2014, 45, 505–514. [Google Scholar] [CrossRef]
- Wang, H.; Lin, S.L.; Leung, G.M.; Schooling, C.M. Age at onset of puberty and adolescent depression: “children of 1997” Birth Cohort. Pediatrics 2016, 137, e20153231. [Google Scholar] [CrossRef]
- Weitz, E.; Kleiboer, A.; van Straten, A.; Cuijpers, P. The effects of psychotherapy for depression on anxiety symptoms: A meta-analysis. Psychol. Med. 2018, 48, 2140–2152. [Google Scholar] [CrossRef]
- Zisook, S.; Lesser, I.; Stewart, J.W.; Wisniewski, S.R.; Balasubramani, G.K.; Fava, M.; Gilmer, W.S.; Dresselhaus, T.R.; Thase, M.E.; Nierenberg, A.A.; et al. Effect of age at onset on the course of major depressive disorder. Am. J. Psychiatry 2007, 164, 1539–1546. [Google Scholar] [CrossRef]
- Fiske, A.; Wetherell, J.L.; Gatz, M. Depression in older adults. Annu. Rev. Clin. Psychol. 2009, 5, 363–389. [Google Scholar] [CrossRef] [PubMed]
- Gournellis, R.; Oulis, P.; Rizos, E.; Chourdaki, E.; Gouzaris, A.; Lykouras, L. Clinical correlates of age of onset in psychotic depression. Arch. Gerontol. Geriatr. 2011, 52, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.H. The link between depression and physical symptoms. Prim. Care Companion. J. Clin. Psychiatry 2004, 6 (Suppl. 1), 12–26. [Google Scholar] [PubMed]
- Gruenberg, A.M.; Goldstein, R.D.; Pincus, H.A. Classification of depression: Research and diagnostic criteria: DSM-IV and ICD-10. In Biology of Depression: From Novel Insights to Therapeutic Strategies, 1st ed.; Licinio, J., Wong, M.L., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 1–12. [Google Scholar]
- Kapfhammer, H.P. Somatic symptoms in depression. Dialogues Clin. Neurosci. 2006, 8, 227–239. [Google Scholar]
- Bholownik, D.; Kumar, K.S.; Srivastava, S.; Paswan, S.; Dutta, A.S. Depression-symptoms, causes, medications and therapies. Pharm. Innov. 2012, 1, 37–51. [Google Scholar]
- Darcet, F.; Gardier, A.M.; Gaillard, R.; David, D.J.; Guilloux, J.P. Cognitive dysfunction in major depressive disorder. A translational review in animal models of the disease. Pharmaceuticals 2016, 9, 9. [Google Scholar] [CrossRef]
- Kalkman, H.O. Novel Treatment targets based on insights in the etiology of depression: Role of IL-6 trans-signaling and stress-induced elevation of glutamate and ATP. Pharmaceuticals 2019, 12, 113. [Google Scholar] [CrossRef]
- Konsman, J.P. Inflammation and depression: A nervous plea for psychiatry to not become immune to interpretation. Pharmaceuticals 2019, 12, 29. [Google Scholar] [CrossRef]
- Dey, A.; Hankey Giblin, P. Insights into macrophage heterogeneity and cytokine-induced neuroinflammation in major depressive disorder. Pharmaceuticals 2018, 11, 64. [Google Scholar] [CrossRef]
- Nutt, D.J. Relationship of neurotransmitters to the symptoms of major depressive disorder. J. Clin. Psychiat. 2008, 69 (Suppl. E1), 4–7. [Google Scholar]
- Cowen, P.J.; Browning, M. What has serotonin to do with depression? World Psychiatry 2015, 14, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, C.; Zhu, D.; Wang, X.; Fang, L.; Zhong, J.; Mao, Q.; Sun, L.; Gong, X.; Xia, J.; et al. Serotonin-1A receptor alterations in depression: A meta-analysis of molecular imaging studies. BMC Psychiatry 2016, 16, e319. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.X.; Xia, J.J.; Deng, F.L.; Liang, W.W.; Wu, J.; Yin, B.M.; Dong, M.X.; Chen, J.J.; Ye, F.; Wang, H.Y.; et al. Diagnosis of major depressive disorder based on changes in multiple plasma neurotransmitters: A targeted metabolomics study. Transl. Psychiat. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Prado, E.L.; Dewey, K.G. Nutrition and brain development in early life. Nutr. Rev. 2014, 72, 267–284. [Google Scholar] [CrossRef]
- Grosso, G.; Pajak, A.; Marventano, S.; Castellano, S.; Galvano, F.; Bucolo, C.; Drago, F.; Caraci, F. Role of omega-3 fatty acids in the treatment of depressive disorders: A comprehensive meta-analysis of randomized clinical trials. PloS ONE 2014, 9, e96905. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Wisner, K.L. Nutrition and depression: Implications for improving mental health among childbearing-aged women. Biol. Psychiatry 2005, 58, 679–685. [Google Scholar] [CrossRef]
- Crowther, C.A.; Hiller, J.E.; Moss, J.R.; McPhee, A.J.; Jeffries, W.S.; Robinson, J.S. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N. Engl. J. Med. 2005, 352, 2477–2486. [Google Scholar] [CrossRef]
- Thachil, A.F.; Mohan, R.; Bhugra, D. The evidence base of complementary and alternative therapies in depression. J. Affect. Disord. 2007, 97, 23–35. [Google Scholar] [CrossRef]
- Van der Watt, G.; Laugharne, J.; Janca, A. Complementary and alternative medicine in the treatment of anxiety and depression. Curr. Opin. Psychiatry 2008, 21, 37–42. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Vieira, K.F. Nutritional and herbal supplements for anxiety and anxiety-related disorders: Systematic review. Nutr. J. 2010, 14, 1–14. [Google Scholar] [CrossRef]
- Sarris, J.; Moylan, S.; Camfield, D.A.; Pase, M.P.; Mischoulon, D.; Berk, M.; Jacka, F.N.; Schweitzer, I. Complementary medicine, exercise, meditation, diet, and lifestyle modification for anxiety disorders: A review of current evidence. Evid. Based Complement. Alternat. Med. 2012, 2012, 809653. [Google Scholar] [CrossRef] [PubMed]
- Johansson, R.; Björklund, M.; Hornborg, C.; Karlsson, S.; Hesser, H.; Ljótsson, B.; Andersson, G. Affect-focused psychodynamic psychotherapy for depression and anxiety through the Internet: A randomized controlled trial. Peer J. 2013, 1, e102. [Google Scholar] [CrossRef] [PubMed]
- Nordanskog, P. On Electroconvulsive Therapy in Depression: Clinical, Cognitive and Neurobiological Aspects. Doctoral’s Dissertation, Faculty of Health Sciences, Department of Medical and Health Science, Linköping University, Linköping, Sweden, 2015; p. 68. [Google Scholar]
- Almeida, F.; Monteiro, I.S.; Moreira, D. Depression and psychotherapy: The importance of a psychotherapeutic approach focused on logical reasoning and functioning. Ann. Depress. Anxiety 2016, 3, 1074. [Google Scholar]
- Maina, G.; Mauri, M.; Rossi, A. Anxiety and depression. J. Psychopathol. 2016, 22, 236–250. [Google Scholar]
- O’Donnell, J.M.; Bies, R.R.; Shelton, R.C. Drug therapy of depression and anxiety disorders (section II neuropharmacology, chapter 15). In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 13th ed.; Brunton, L.L., Hilal-Dandan, R., Knollmann, B.C., Eds.; McCraw-Hill Education: Sydney, Australia, 2017; pp. 397–415. [Google Scholar]
- Thabrew, H.; Stasiak, K.; Hetrick, S.E.; Wong, S.; Huss, J.H.; Merry, S.N. Psychological therapies for anxiety and depression in children and adolescents with long-term physical conditions. Cochrane Database Syst. Rev. 2018, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Hyman, S.E. Neurotransmitters. Curr. Biol. 2005, 15, R154–R158. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.H.; Ferris, C.D. Novel neurotransmitters and their neuropsychiatric relevance. Am. J. Psychiatry 2000, 157, 1738–1751. [Google Scholar] [CrossRef]
- Molina, E.M.B.; Peña, A.B.; Perera, O.H. Neurotransmitters, their effects on the human organism. Anatomy Physiol. Biochem. Int. J. 2017, 2, 555–581. [Google Scholar]
- Ayano, G. Common neurotransmitters: Criteria for neurotransmitters, key locations, classifications and functions. Am. J. Psychiatry Neurosci. 2016, 4, 91–95. [Google Scholar]
- Dale, E.; Bang-Andersen, B.; Sanchez, C. Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs. Biochem. Pharmacol. 2015, 95, 81–97. [Google Scholar] [CrossRef]
- Drevets, W.C.; Price, J.L.; Furey, M.L. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct. Funct. 2008, 213, 93–118. [Google Scholar] [CrossRef] [PubMed]
- Hasler, G. Pathophysiology of depression: Do we have any solid evidence of interest to clinicians? World Psych. 2010, 9, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Li, H.; Zhao, Y.; Cai, E.; Zhu, H.; Gao, Y.; Liu, S.; Yang, H.; Zhang, L.; Tangand, G.; et al. Ergosteryl 2-naphthoate, an ergosterol derivative, exhibits antidepressant effects mediated by the modification of GABAergic and glutamatergic systems. Molecules 2017, 22, 565. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, S.; Yamada, M.; Takano, H.; Nagashima, T.; Takahata, K.; Yokokawa, K.; Ito, T.; Ishii, T.; Kimura, Y.; Zhang, M.R.; et al. Norepinephrine transporter in major depressive disorder: A pet study. Am. J. Psychiat. 2017, 174, 36–41. [Google Scholar] [CrossRef]
- Dunlop, B.W.; Nemeroff, C.B. The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatry 2007, 64, 327–337. [Google Scholar] [CrossRef]
- Goddard, A.W.; Ball, S.G.; Martinez, J.; Robinson, M.J.; Yang, C.R.; Russell, J.M.; Shekhar, A. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress. Anxiety 2010, 27, 339–350. [Google Scholar] [CrossRef]
- Moret, C.; Briley, M. The importance of norepinephrine in depression. Neuropsychiatr. Dis. Treat. 2011, 7 (Suppl. 1), 9–13. [Google Scholar]
- Chandley, M.J.; Ordway, G.A. Noradrenergic dysfunction in depression and suicide. In The Neurobiological Basis of Suicide, 1st ed.; Dwivedi, Y., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2012; Chapter 3. Available online: https://www.ncbi.nlm.nih.gov/books/NBK107205/ (accessed on 29 October 2018).
- Kurita, M. Noradrenaline plays a critical role in the switch to a manic episode and treatment of a depressive episode. Neuropsychiatr. Dis. Treat. 2016, 12, 2373–2380. [Google Scholar] [CrossRef]
- Gardier, A.M. Mutant mouse models and antidepressant drug research: Focus on serotonin and brain-derived neurotrophic factor. Behav. Pharmacol. 2009, 20, 18–32. [Google Scholar] [CrossRef]
- Artigas, F. Serotonin receptors involved in antidepressant effects. Pharmacol. Ther. 2013, 137, 119–131. [Google Scholar] [CrossRef]
- Mahar, I.; Bambico, F.R.; Mechawar, N.; Nobrega, J.N. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev. 2014, 38, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Samuels, B.A.; Mendez-David, I.; Faye, C.; David, S.A.; Pierz, K.A.; Gardier, A.M.; Hen, R.; David, D.J. Serotonin 1A and serotonin 4 receptors: Essential mediators of the neurogenic and behavioral actions of antidepressants. Neuroscientist 2016, 22, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Yohn, C.N.; Gergues, M.M.; Samuels, B.A. The role of 5-HT receptors in depression. Mol. Brain 2017, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Amidfar, M.; Colic, L.; Walter, M.; Kim, Y.K. Complex role of the serotonin receptors in depression: Implications for treatment. In Understanding Depression, 1st ed.; Kim, J.K., Ed.; Biomedical and Neurobiological Background; Springer: Singapore, 2018; Volume 1, pp. 83–95. [Google Scholar]
- Kamimura, M.; Aoba, A. Drug therapy for depression in Japan. Jpn. Med. Assoc. J. 2004, 45, 28–33. [Google Scholar]
- Pacher, P.; Kecskemeti, V. Trends in the development of new antidepressants. Is there a light at the end of the tunnel? Curr. Med. Chem. 2004, 11, 925–943. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S. Characteristics and use of new antidepressant drugs. Jpn. Med. Assoc. J. 2004, 47, 265–269. [Google Scholar]
- Zajecka, J.M.; Albano, D. SNRIs in the management of acute major depressive disorder. J. Clin. Psychiatry 2004, 65 (Suppl. 17), 11–18. [Google Scholar]
- Shelton, R.C. Serotonin norepinephrine reuptake inhibitors: Similarities and differences. Prim. Psychiatry 2009, 16 (Suppl. 4), 25–35. [Google Scholar]
- Higuchi, T. Major depressive disorder treatment guidelines in Japan. J. Clin. Psychiatry 2010, 71 (Suppl. E1), e05. [Google Scholar] [CrossRef]
- Machado, M.; Einarson, T.R. Comparison of SSRIs and SNRIs in major depressive disorder: A meta-analysis of head-to-head randomized clinical trials. J. Clin. Pharm. Ther. 2010, 35, 177–188. [Google Scholar] [CrossRef]
- Alev, L.; Lenox-Smith, A.; Altin, M.; Duenas, H. A review of the serotonin-norepinephrine reuptake inhibitors: Pharmacologic aspects and clinical implications for treatment of major depressive disorder and associated painful physical symptoms. Open J. Depress. 2013, 2, 54–63. [Google Scholar] [CrossRef]
- Jainer, A.K.; Kamatchi, R.; Marzanski, M.; Somashekar, B. Current advances in the treatment of major depression: Shift towards receptor specific drugs. In Mental Disorders-Theoretical and Empirical Perspectives, 1st ed.; Woolfolk, R., Allen, L., Eds.; InTech.: Rijeka, Croatia, 2013; pp. 269–288. [Google Scholar]
- Sansone, R.A.; Sansone, L.A. Serotonin norepinephrine reuptake inhibitors: A pharmacological comparison. Innov. Clin. Neurosci. 2014, 11, 37–42. [Google Scholar] [PubMed]
- Clevenger, S.S.; Malhotra, D.; Dang, J.; Vanle, B.; IsHak, W.W. The role of selective serotonin reuptake inhibitors in preventing relapse of major depressive disorder. Ther. Adv. Psychopharmacol. 2017, 8, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Dubovsky, S.L. What is new about new antidepressants? Psychother. Psychosom. 2018, 87, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Locher, C.; Koechlin, H.; Zion, S.R.; Werner, C.; Pine, D.S.; Kirsch, I.; Kessler, R.C.; Kossowsky, J. Efficacy and safety of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents: A systematic review and meta-analysis. JAMA Psychiatry 2017, 74, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Wang, L.; Wen, X.; Liu, Y.; Fan, Y.; Liu, Z. A meta-analysis of effects of selective serotonin reuptake inhibitors on blood pressure in depression treatment: Outcomes from placebo and serotonin and noradrenaline reuptake inhibitor controlled trials. Neuropsychiatr. Dis. Treat. 2017, 7, 2781–2796. [Google Scholar] [CrossRef]
- Sussman, N. SNRIs versus SSRIs: Mechanisms of action in treating depression and painful physical symptoms. Prim. Care Companion J. Clin. Psychiatry 2003, 5, 19–26. [Google Scholar]
- Bradley, A.J.; Lenox-Smith, A.J. Does adding noradrenaline reuptake inhibition to selective serotonin reuptake inhibition improve efficacy in patients with depression?. A systematic review of meta-analyses and large randomised pragmatic trials. J. Psychopharmacol. 2013, 27, 740–758. [Google Scholar] [CrossRef]
- Tabaka, J.M. Mechanisms of Action of Antidepressants and Their Combination for Major Depressive Disorder Treatment: A Theoretical and Cclinical Approach. Ph.D. Thesis, Department of Psychiatry, McGill University, Montreal, QC, Canada, 2013; p. 184. [Google Scholar]
- Santarsieri, D.; Schwartz, T.L. Antidepressant efficacy and side-effect burden: A quick guide for clinicians. Drugs Context. 2015, 4, 212–290. [Google Scholar] [CrossRef]
- Khushboo, S.B.; Sharma, B. Antidepressants: Mechanism of action, toxicity and possible amelioration. J. Appl. Biotechnol. Bioeng. 2017, 3, 1–13. [Google Scholar]
- Iversen, L. The monoamine hypothesis of depression. In Biology of Depression: From Novel Insights to Therapeutic Strategies, 1st ed.; Licinio, J., Wong, M.L., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 71–86. [Google Scholar]
- Stahl, S.M.; Felker, A. Monoamine oxidase inhibitors: A modern guide to an unrequited class of antidepressants. CNS Spectrums 2008, 13, 855–871. [Google Scholar] [CrossRef] [PubMed]
- Sacher, J.; Houle, S.; Parkes, J.; Rusjan, P.; Sagrati, S.; Wilson, A.A.; Meyer, J.H. Monoamine oxidase A inhibitor occupancy during treatment of major depressive episodes with moclobemide or St. John’s wort: An [11C]-harmine PET study. J. Psychiatry Neurosci. 2011, 36, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Blier, P.; El Mansari, M. Serotonin and beyond: Therapeutics for major depression. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120536. [Google Scholar] [CrossRef]
- Goldberg, J.S.; Bell, C.E., Jr.; Pollard, D.A. Revisiting the monoamine hypothesis of depression: A new perspective. Perspect. Med. Chem. 2014, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fasipe, O.J. Neuropharmacological classification of antidepressant agents based on their mechanisms of action. Arch. Med. Health Sci. 2018, 6, 81–94. [Google Scholar] [CrossRef]
- Hasler, G.; Neumeister, A.; van der Veen, J.W.; Tumonis, T.; Bain, E.E.; Shen, J.; Drevets, W.C.; Charney, D.S. Normal Prefrontal gamma-aminobutyric acid levels in remitted depressed subjects determined by proton magnetic resonance spectroscopy. Biol. Psychiatry 2005, 58, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Hasler, G.; van der Veen, J.W.; Tumonis, T.; Meyers, N.; Shen, J.; Drevets, W.C. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 2007, 64, 193–200. [Google Scholar] [CrossRef]
- Hasler, G.; Buchmann, A.; Haynes, M.; Müller, S.T.; Ghisleni, C.; Brechbühl, S.; Tuura, R. Association between prefrontal glutamine levels and neuroticism determined using proton magnetic resonance spectroscopy. Transl Psychiatry 2019, 9, 170. [Google Scholar] [CrossRef]
- Li, Z.; An, S.C.; Li, J.N. The interaction between gamma-aminobutyric acid and other related neurotransmitters in depression. Prog. Physiol. 2014, 45, 190–194. [Google Scholar]
- Gabbay, V.; Bradley, K.A.; Mao, X.; Ostrover, R.; Kang, G.; Shungu, D.C. Anterior cingulate cortex γ-aminobutyric acid deficits in youth with depression. Transl. Psychiatry 2017, 7, e1216. [Google Scholar] [CrossRef]
- Romeo, B.; Choucha, W.; Fossati, P.; Rotge, J.Y. Meta-analysis of central and peripheral γ-aminobutyric acid levels in patients with unipolar and bipolar depression. J. Psychiatry Neurosci. 2018, 43, 58–66. [Google Scholar] [CrossRef]
- Sanacora, G.; Mason, G.F.; Rothman, D.L.; Krystal, J.H. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am. J. Psychiatry 2002, 159, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Bhagwagar, Z.; Wylezinska, M.; Taylor, M.; Jezzard, P.; Matthews, P.M.; Cowen, P.J. Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am. J. Psychiatry 2004, 161, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Pinna, G.; Costa, E.; Guidotti, A. SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5-HT reuptake. Curr. Opin. Pharmacol. 2009, 9, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Kaupmann, K. Don’t worry ‘B’ happy!: A role for GABAB receptors in anxiety and depression. Trends Pharmacol. Sci. 2005, 26, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res. 2009, 61, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Zarate, C.; Machado-Vieira, R.; Henter, I.; Ibrahim, L.; Diazgranados, N.; Salvadore, G. Glutamatergic modulators: The future of treating mood disorders? Harv. Rev. Psychiatry 2010, 18, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Möhler, H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology 2012, 62, 42–45. [Google Scholar] [CrossRef]
- Tokita, K.; Yamaji, T.; Hashimoto, K. Roles of glutamate signaling in preclinical and/or mechanistic models of depression. Pharmacol. Biochem. Behav. 2012, 100, 688–704. [Google Scholar] [CrossRef]
- Wierońska, J.M.; Pałucha-Poniewiera, A.; Nowak, G.; Pilc, A. Depression viewed as a GABA/glutamate imbalance in the central nervous system. In Clinical, Research and Treatment Approaches to Affective Disorders, 1st ed.; Juruena, M.F., Ed.; InTech: Rijeka, Croatia, 2012; pp. 235–266. [Google Scholar]
- Henter, I.D.; de Sousa, R.T.; Zarate, C.A., Jr. Glutamatergic modulators in depression. Harv. Rev. Psychiat. 2018, 26, 307–319. [Google Scholar] [CrossRef]
- Maeng, S.; Zarate, C.A. The role of glutamate in mood disorders: Results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr. Psychiatry Rep. 2007, 9, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.J.; Shah, A.; Lapidus, K.; Clark, C.; Jarun, N.; Ostermeyer, B.; Murrough, J.W. Ketamine for treatment-resistant unipolar depression. CNS Drugs 2012, 26, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Ates-Alagoz, Z.; Adejare, A. NMDA receptor antagonists for treatment of depression. Pharmaceuticals 2013, 6, 480–499. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.K.; Budac, D.P.; Bisulco, S.; Lee, A.W.; Smith, R.A.; Beenders, B.; Kelley, K.W.; Dantzer, R. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 2013, 38, 1609–1916. [Google Scholar] [CrossRef] [PubMed]
- DeWilde, K.E.; Levitch, C.F.; Murrough, J.W.; Mathew, S.J.; Iosifescu, D.V. The promise of ketamine for treatment-resistant depression: Current evidence and future directions. Ann. N. Y. Acad. Sci. 2015, 1345, 47–58. [Google Scholar] [CrossRef]
- Newport, D.J.; Carpenter, L.L.; McDonald, W.M.; Potash, J.B.; Tohen, M.; Nemeroff, C.B. Ketamine and other NMDA antagonists: Early clinical trials and possible mechanisms in depression. Am. J. Psychiatry 2015, 172, 950–966. [Google Scholar] [CrossRef]
- Werma, S.S. Ketamine in treatment-resistant depression: A review of completed investigations. J. Mahatma Gandhi Inst. Med. Sci. 2015, 20, 55–59. [Google Scholar]
- Schwartz, J.; Murrough, J.W.; Iosifescu, D.V. Ketamine for treatment-resistant depression: Recent developments and clinical applications. Evid. Based Ment. Health 2016, 19, 35–38. [Google Scholar] [CrossRef]
- Levine, J.; Panchalingam, K.; Rapoport, A.; Gershon, S.; McClure, R.J.; Pettegrew, J.W. Increased cerebrospinal fluid glutamine levels in depressed patients. Biol. Psychiatry 2000, 47, 586–593. [Google Scholar] [CrossRef]
- Olajossy, M.; Olajossy, B.; Wnuk, S.; Potembska, E.; Urbańska, E. Blood serum concentrations of kynurenic acid in patients diagnosed with recurrent depressive disorder, depression in bipolar disorder, and schizoaffective disorder treated with electroconvulsive therapy. Psychiatry Pol. 2017, 51, 455–468. [Google Scholar] [CrossRef]
- Pytka, K.; Podkowa, K.; Rapacz, A.; Podkowa, A.; Żmudzka, E.; Olczyk, A.; Sapa, J.; Filipek, B. The role of serotonergic, adrenergic and dopaminergic receptors in antidepressant-like effect. Pharmacol. Rep. 2016, 68, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, A.; Bernasconi, N.; Koepp, M. Imaging Biomarkers in Epilepsy, 1st ed.; Cambridge University: Cambridge, UK; New York, NY, USA; Port Melbourne, Australia; New Delhi, India; Singapore, 2019. [Google Scholar]
- Bray, N.J.; O′Donovan, M.C. The genetics of neuropsychiatric disorders. Brain Neurosci Adv. 2019, 30, 2. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.D.; Hassel, S.; Arnott, S.R.; Harris, J.; Lam, R.W.; Milev, R.; Rotzinger, S.; Zamyadi, M.; Frey, B.N.; Minuzzi, L.; et al. White matter indices of medication response in major depression: A diffusion tensor imaging study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 913–924. [Google Scholar] [CrossRef]
- Ji, J.L.; Anticevic, A. Functional MRI in Psychiatric Disorders. In Functional MRI. Basic Principles and Emerging Clinical Applications in Anesthesiology and the Neurological Sciences, 1st ed.; Ramani, R., Ed.; Oxford University: New York, NY, USA, 2019; pp. 91–118. [Google Scholar]
- Jiang, B.; Petkova, E.; Tarpey, T.; Ogden, R.T. A Bayesian approach to joint modeling of matrix-valued imaging data and treatment outcome with applications to depression studies. Biometrics 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Fu, S.; Yin, Z.; Kang, J.; Wang, X.; Zhou, Y.; Wei, S.; Wu, F.; Kong, L.; Wang, F.; et al. Common and distinct neural activities in frontoparietal network in first-episode bipolar disorder and major depressive disorder: Preliminary findings from a follow-up resting state fMRI study. J. Affect. Disord. 2019, 260, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Karavasilis, E.; Parthimos, T.P.; Papatriantafyllou, J.D.; Christidi, F.; Papageorgiou, S.G.; Kapsas, G.; Papanicolaou, A.C.; Seimenis, I. The power of sample size through a multi-scanner approach in MR neuroimaging regression analysis: Evidence from Alzheimer’s disease with and without depression. Australas Phys. Eng. Sci. Med. 2019, 42, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Klok, M.P.C.; van Eijndhoven, P.F.; Argyelan, M.; Schene, A.H.; Tendolkar, I. Structural brain characteristics in treatment-resistant depression: Review of magnetic resonance imaging studies. BJPsych. Open. 2019, 5, e76. [Google Scholar] [CrossRef]
- Lacey, C.; Ohlhauser, L.; Gawryluk, J.R. Microstructural white matter characteristics in Parkinson’s disease with depression: A diffusion tensor imaging replication study. Front. Neurol. 2019, 10, 884. [Google Scholar] [CrossRef]
- Liang, Y.; Yao, Y.C.; Zhao, L.; Shi, L.; Chen, Y.K.; Mok, V.C.; Ungvari, G.S.; Chu, W.C.; Tang, W.K. Topological reorganization of the default mode network in patients with poststroke depressive symptoms: A resting-state fMRI study. J. Affect. Disord. 2019, 260, 557–568. [Google Scholar] [CrossRef]
- Piwowarska-Bilska, H.; Supińska, A.; Iwanowski, J.; Tyczyńska, A.; Birkenfeld, B. PET–Advanced nuclear imaging technology for medicine. Pomeranian J. Life Sci. 2019, 65, 45–53. [Google Scholar]
- Demyttenaere, K. Compliance during treatment with antidepressants. J. Affect. Disord. 1997, 43, 27–39. [Google Scholar] [CrossRef]
- Cascade, E.; Kalali, A.H.; Kennedy, S.H. Real-World data on SSRI antidepressant side effects. Psychiatry (Edgmont.) 2009, 6, 16–18. [Google Scholar] [PubMed]
- Ramachandraih, C.T.; Subramanyam, N.; Bar, K.J.; Baker, G.; Yeragani, V.K. Antidepressants: From MAOIs to SSRIs and more. Indian J. Psychiatry 2011, 53, 180–182. [Google Scholar] [PubMed]
- Hirsch, M.; Birnbaum, R.J. Monoamine Oxidase Inhibitors (MAOIs) for Treating Depressed Adults; Basow, D.S., Ed.; Up-ToDate: Waltham, MA, USA, 2019. [Google Scholar]
- Lan-lan, Z.; Liang, M.; Chuan-geng, M.; Mei-Zhen, F.; Yan, C.; Qin, J. Effects on animal models of depression of bioactive compounds from entomogenous fungi, a novel antioxidant. Chin. J. Integr. Med. 2004, 10, 221–225. [Google Scholar] [CrossRef]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Q.; Liu, Y.; Xia, C.; Shi, J.; Zheng, M. Effect of xanthone derivatives on animal models of depression. Curr. Ther. Res. Clin. 2014, 76, 45–50. [Google Scholar] [CrossRef][Green Version]
- Aquib, M.; Najmi, A.K.; Akhtar, M. Antidepressant effect of thymoquinone in animal models of depression. Drug Res. 2015, 65, 490–494. [Google Scholar] [CrossRef]
- Sarris, J.; Panossian, A.; Schweitzer, I.; Stough, C.; Scholey, A. Herbal medicine for depression, anxiety and insomnia: A review of psychopharmacology and clinical evidence. Eur. Neuropsychopharm. 2011, 21, 841–860. [Google Scholar] [CrossRef]
- Sarris, J. Nutrients and herbal supplements for mental health. Aust. Prescr. 2014, 37, 90–93. [Google Scholar] [CrossRef]
- Mao, J.J.; Xie, S.X.; Zee, J.; Soeller, I.; Li, Q.S.; Rockwell, K.; Amsterdam, J.D. Rhodiola rosea versus sertraline for major depressive disorder: A randomized placebo-controlled trial. Phytomedicine 2015, 22, 394–399. [Google Scholar] [CrossRef]
- Chen, G.; Guo, X. Neurobiology of Chinese herbal medicine on major depressive disorder. In Neurobiology of Chinese Herb Medicine Series International Review of Neurobiology, 1st ed.; Zeng, B.Y., Zhao, K., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 135, pp. 77–95. [Google Scholar]
- Cheng, D.; Murtaza, G.; Ma, S.; Li, L.; Li, X.; Tian, F.; Zheng, J.; Lu, Y. In silico prediction of the anti-depression mechanism of a herbal formula (Tiansi Liquid) containing Morinda officinalis and Cuscuta chinensis. Molecules 2017, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Bae, H. Therapeutic effects of phytochemicals and medicinal herbs on depression. BioMed Res. Int. 2017, 2017, e6596241. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.S.; Hernandez, M.; Mao, J.J.; Haviland, I.; Gubili, J. Herbal medicine for depression and anxiety: A systematic review with assessment of potential psycho-oncologic relevance. Phytother. Res. 2018, 32, 865–891. [Google Scholar] [CrossRef] [PubMed]
- Costa de Melo, N.; Sánchez-Ortiz, B.L.; dos Santos Sampaio, T.I.; Matias Pereira, A.C.; Pinheiro da Silva Neto, F.L.; Ribeiro da Silva, H.; Tavares Carvalho, J.C. Anxiolytic and antidepressant effects of the hydroethanolic extract from the leaves of Aloysia polystachya (Griseb.) Moldenke: A study on zebrafish (Danio rerio). Pharmaceuticals 2019, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Fallah-Pour, H.; Afkham, K.; Jamshidi, A.H.; Khalighi-Cigaroudi, F. Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: A pilot double-blind randomized trial [ISRCTN45683816]. BMC Complement. Altern. Med. 2004, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Basti, A.A.; Moshiri, E.; Noorbala, A.A.; Jamshidi, A.H.; Abbasi, S.H.; Akhondzadeh, S. Comparison of petal of Crocus sativus L. and fluoxetine in the treatment of depressed outpatients: A pilot double-blind randomized trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Moshiri, E.; Basti, A.A.; Noorbala, A.A.; Jamshidi, A.H.; Abbasi, S.H.; Akhondzadeh, S. Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: A double-blind, randomized and placebo-controlled trial. Phytomedicine 2006, 13, 607–611. [Google Scholar] [CrossRef]
- Hausenblas, H.A.; Saha, D.; Dubyak, P.J.; Anton, S.D. Saffron (Crocus sativus L.) and major depressive disorder: A meta-analysis of randomized clinical trials. J. Int. Med. 2013, 11, 377–383. [Google Scholar] [CrossRef]
- Shafiee, M.; Arekhi, S.; Omranzadeh, A.; Sahebkar, A. Saffron in the treatment of depression, anxiety and other mental disorders: Current evidence and potential mechanisms of action. J. Affect. Disord. 2018, 227, 330–337. [Google Scholar] [CrossRef]
- Ngoupaye, G.T.; Bum, E.N.; Daniels, W.M.U. Antidepressant-like effects of the aqueous macerate of the bulb of Gladiolus dalenii Van Geel (Iridaceae) in a rat model of epilepsy-associated depression. BMC Complement. Altern. Med. 2013, 13, 272–291. [Google Scholar] [CrossRef]
- Ngoupaye, G.T.; Ngo Bum, E.; Ngah, E.; Talla, E.; Moto, F.C.O.; Taiwe, G.S.; Rakotonirina, A.; Rakotonirina, S.V. The anticonvulsant and sedative effects of Gladiolus dalenii extracts in mice. Epilepsy Behav. 2013, 28, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Ngoupaye, G.T.; Bum, E.N.; Taiwe, G.S.; Moto, F.C.O.; Talla, E. Antidepressant properties of aqueous acetate from Gladiolus dalenii corms. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 53–61. [Google Scholar] [PubMed]
- Gu, L.; Liu, Y.J.; Wang, Y.B.; Yi, L.T. Role for monoaminergic systems in the antidepressant-like effect of ethanol extracts from Hemerocallis citrine. J. Ethnopharmacol. 2012, 139, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Tang, X.; Liu, F.; Zhang, C.; Zhao, G.; Ren, F.; Leng, X. Antidepressant-like effects of the hydroalcoholic extracts of Hemerocallis citrina and its potential active components. BMC Complement. Altern. Med. 2014, 14, 326. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.R.; Mattei, R.; de Araújo Carlini, E.L. Activity of Hypericum brasiliense and Hypericum cordatum on the central nervous system in rodents. Fitoterapia 2002, 73, 462–471. [Google Scholar] [CrossRef]
- Mennini, T.T.; Gobbi, M. The antidepressant mechanism of Hypericum perforatum. Life Sci. 2004, 75, 1021–1027. [Google Scholar] [CrossRef]
- Filippini, R.; Piovan, A.; Borsarini, A.; Caniato, R. Study of dynamic accumulation of secondary metabolites in three subspecies of Hypericum perforatum. Fitoterapia 2010, 81, 115–119. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Knörle, R.; Appel, K.; Kammler, T.; Weiss, G. Pharmacological studies in an herbal drug combination of St. John’s Wort (Hypericum perforatum) and passion flower (Passiflora incarnata): In vitro and in vivo evidence of synergy between Hypericum and Passiflora in antidepressant pharmacological models. Fitoterapia 2011, 82, 474–480. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Kashani, L.; Fotouhi, A.; Jarvandi, S.; Mobaseri, M.; Moin, M.; Khani, M.; Jamshidi, A.H.; Baghalian, K.; Taghizadeh, M. Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: A double-blind, randomized trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 123–127. [Google Scholar] [CrossRef]
- Kasper, S.; Gastpar, M.; Müller, W.E.; Volz, H.P.; Möller, H.J.; Dienel, A.; Schläfke, S. Silexan, an orally administered Lavandula oil preparation, is effective in the treatment of ‘subsyndromal’anxiety disorder: A randomized, double-blind, placebo controlled trial. Int. Clin. Psychopharmacol. 2010, 25, 277–287. [Google Scholar] [CrossRef]
- Nikfarjam, M.; Parvin, N.; Assarzadegan, N.; Asghari, S. The effects of Lavandula angustifolia Mill infusion on depression in patients using citalopram: A comparison study. Iran. Red Crescent Med. J. 2013, 15, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Emamghoreishi, M.; Talebianpour, M.S. Antidepressant effect of Melissa officinalis in the forced swimming test. Daru 2009, 17, 42–47. [Google Scholar]
- Chehroudi, S.; Fatemi, M.J.; Isfeedvajani, M.S.; Salehi, S.H.; Akbari, H.; Samimi, R. Effects of Melissa officinalis L. on reducing stress, alleviating anxiety disorders, depression, and insomnia, and increasing total antioxidants in burn patients. Trauma Mont. 2016, 22, e33630. [Google Scholar] [CrossRef]
- Haybar, H.; Javid, A.Z.; Haghighizadeh, M.H.; Valizadeh, E.; Mohaghegh, S.M.; Mohammadzadeh, A. The effects of Melissa officinalis supplementation on depression, anxiety, stress, and sleep disorder in patients with chronic stable angina. Clin. Nutr. ESPEN 2018, 26, 47–52. [Google Scholar] [CrossRef]
- Hattesohl, M.; Feistel, B.; Sievers, H.; Lehnfeld, R.; Hegger, M.; Winterhoff, H. Extracts of Valeriana officinalis L. s.l. show anxiolytic and antidepressant effects but neither sedative nor myorelaxant properties. Phytomedicine 2008, 15, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.P.; Mathela, C.S.; Chopra, K. Antidepressant effect of Valeriana wallichii patchouli alcohol chemotype in mice: Behavioural and biochemical evidence. J. Ethnopharmacol. 2011, 135, 197–200. [Google Scholar] [CrossRef]
- Sah, S.P.; Mathela, C.S.; Chopra, K. Involvement of nitric oxide (NO) signalling pathway in the antidepressant activity of essential oil of Valeriana wallichii Patchouli alcohol chemotype. Phytomedicine 2011, 18, 1269–1275. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Karimi, G.; Niapoor, M. Antidepressant effects of Crocus sativus stigma extracts and their constituents, crocin and safranal, in mice. Acta Hortic. 2004, 650, 435–445. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Motamedshariaty, V.; Hadizadeh, F. Antidepressant effect of kaempferol, a constituent of saffron (Crocus sativus) petal, in mice and rats. Pharmacologyonline 2007, 2, 367–370. [Google Scholar]
- Gibon, J.; Deloulme, J.C.; Chevallier, T.; Ladevèze, E.; Abrous, D.N.; Bouron, A. The antidepressant hyperforin increases the phosphorylation of CREB and the expression of TrkB in a tissue-specific manner. Int. J. Neuropsychopharmacol. 2013, 16, 189–198. [Google Scholar] [CrossRef]
- Brown, R.P.; Gerbarg, P.L. Herbs and nutrients in the treatment of depression, anxiety, insomnia, migraine, and obesity. J. Psychiat. Pract. 2001, 7, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, A.V.; Whitten, D.L.; Hawrelak, J.A. Herbal medicines, other than St. John′s Wort, in the treatment of depression: A systematic review. Altern. Med. Rev. 2011, 16, 40–49. [Google Scholar] [PubMed]
- Zhang, Y.; Han, M.; Liu, Z.; Wang, J.; He, Q.; Liu, J. Chinese herbal formula xiao yao san for treatment of depression: A systematic review of randomized controlled trials. Evid. Based Complement. Altern. Med. 2012, 13, e931636. [Google Scholar] [CrossRef] [PubMed]
- Naoi, M.; Shamoto-Nagai, M.; Maruyama, W. Neuroprotection of multifunctional phytochemicals as novel therapeutic strategy for neurodegenerative disorders: Antiapoptotic and antiamyloidogenic activities by modulation of cellular signal pathways. Future Neurol. 2019, 14, 1–19. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nakamura, S.; Nakashima, S.; Ohta, T.; Yano, M.; Tsujihata, J.; Tsukioka, J.; Ogawa, K.; Fukaya, M.; Yoshikawa, M.; et al. γ-Lactam alkaloids from the flower buds of daylily. J. Nat. Med. 2016, 70, 376–383. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nakamura, S.; Ohta, T.; Fujimoto, K.; Yoshikawa, M.; Ogawa, K.; Matsuda, H. A rare glutamine derivative from the flower buds of daylily. Org. Lett. 2014, 16, 3076–3078. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.M.; Furkert, D.P.; Brimble, M.A. Total synthesis and stereochemical revision of the 2-formylpyrrole alkaloid hemerocallisamine I. J. Nat. Prod. 2017, 80, 1926–1929. [Google Scholar] [CrossRef]
- Griesbach, R.J.; Batdorf, L. Flower pigments within Hemerocallis fulva L. fm. Fulva, fm. Rosea, and fm. Disticha. HortScience 1995, 30, 353–354. [Google Scholar]
- McGarty, T.P. Flower Color and Patterning in the Genus Hemerocallis and Its Hybrids: A Mathematical Model and Experimental Analysis; MIT, Draft: Cambridge, MA, USA, 2009; pp. 1–13. [Google Scholar]
- Liao, J.; Wu, Y.; Yan, L. Biochemical characterization of the pollen tubulin from day lily (Hemerocallis fulva, Liliaceae). Acta Bot. Yunnan 2006, 28, 425–428. [Google Scholar]
- Liao, J.; Wu, Y.; Yan, L. Biophysical and pharmacological characterization of a dynamin-like protein from day-lily (Hemerocallis fulva, Liliaceae) pollens. Acta Bot. Yunnan 2007, 29, 247–250. [Google Scholar]
- Lin, Y.L.; Lu, C.K.; Huang, Y.J.; Chen, H.J. Antioxidative caffeoylquinic acids and flavonoids from Hemerocallis fulva flowers. J. Agric. Food Chem. 2011, 59, 8789–8795. [Google Scholar] [CrossRef] [PubMed]
- Cichewicz, R.H.; Nair, M.G. Isolation and characterization of stelladerol, a new antioxidant naphthalene glycoside, and other antioxidant glycosides from edible daylily (Hemerocallis) flowers. J. Agric. Food Chem. 2002, 50, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.Y.; Chen, B.H. Analysis and stability of carotenoids in the flowers of daylily (Hemerocallis disticha) as affected by various treatments. J. Agric. Food Chem. 2000, 48, 5962–5968. [Google Scholar] [CrossRef]
- Hsu, Y.W.; Tsai, C.F.; Chen, W.K.; Ho, Y.C.; Lu, F.J. Determination of lutein and zeaxanthin and antioxidant capacity of supercritical carbon dioxide extract from daylily (Hemerocallis disticha). Food Chem. 2011, 129, 1813–1818. [Google Scholar] [CrossRef]
- Chen, Q.; Fu, M.; Qu, Q.; Dai, H.; Zhao, S. Effect of blanching pre-treatment on antioxidant activities and involved compounds in fresh daylily (Hemerocallis fulva L.) flowers. Qual. Assur. Saf. Crop. 2014, 7, 287–293. [Google Scholar] [CrossRef]
- Fu, M.; He, Z.; Zhao, Y.; Yang, J.; Mao, L. Antioxidant properties and involved compounds of daylily flowers in relation to maturity. Food Chem. 2009, 14, 1192–1197. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, T.; Fan, B.; Zhang, L.; Lu, C.; Wang, D.; Wang, F. Advances in researches on chemical composition and functions of Hemerocallis plants. Med. Plant. 2018, 9, 16–21. [Google Scholar]
- Zhang, Y.; Cichewicz, R.H.; Nair, M.G. Lipid peroxidation inhibitory compounds from daylily (Hemerocallis fulva) leaves. Life Sci. 2004, 75, 753–763. [Google Scholar] [CrossRef]
- Liu, L.Y.; Chang, L.Y.; Chou, S.S.; Hsiao, Y.L.; Chien, Y.W. Studies on the antioxidant components and activities of the methanol extracts of commercially grown Hemerocallis fulva L. (daylily) in Taiwan. J. Food Biochem. 2010, 34, 90–104. [Google Scholar] [CrossRef]
- Que, F.; Mao, L.; Zheng, X. In vitro and vivo antioxidant activities of daylily flowers and the involvement of phenolic compounds. Asia Pac. J. Clin. Nutr. 2007, 16, 196–203. [Google Scholar]
- Zhao, X.; Guo, Y.; Zhang, Y.; Xie, Y.; Yan, S.; Jin, H.; Zhang, W. Monoterpene derivatives from the flowers of the Hemerocallis minor Mill. Phytochem. Lett. 2017, 21, 134–138. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.C.; Xie, Y.G.; Fan, C.; Huang, Y.Y.; Yan, S.K.; Zhang, Y.; Jin, H.Z.; Zhang, W.D. Eight new γ-lactam alkaloids from the roots of the Hemerocallis minor Mill. Fitoterapia 2017, 118, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Cichewicz, R.H.; Kee-Chong, L.; McKerrow, J.H.; Nair, M.G. Kwanzoquinones A–G and other constituents of Hemerocallis fulva ‘Kwanzo’ roots and their activity against the human pathogenic trematode Schistosoma mansoni. Tetrahedron 2002, 58, 8597–8606. [Google Scholar] [CrossRef]
- Wang, D.Y.; Ye, Q.; Zhang, G.L.; Li, B.G. Note: New anthraquinones from Gladiolus gandavensis. J. Asian Nat. Prod. Res. 2003, 5, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, K.M. Chemical constituents of Gladiolus segetum Ker-gawl. Bull. Pharm. Sci. 2005, 28, 71–78. [Google Scholar]
- Ngamba, D.; Tane, P.; Bezabih, M.; Awouafack, M.; Abegaz, B. Two new anthraquinones from Gladiolus psittascinus. Biochem. Syst. Ecol. 2007, 35, 709–713. [Google Scholar] [CrossRef]
- Rao, T.; Raja, G.S.; Murti, R.; Challa, P. Cytokinins in gladiolus (Gladiolus grandiflorus) corms. Ann. Bot. 1983, 52, 703–710. [Google Scholar] [CrossRef]
- El-Shanawany, M.A.; Hassanean, H.A.; Mohamed, M.H.; Nafady, A.M. A new oleanene triterpene from Gladiolus segetum Ker-Gawl. Nat. Prod. Res. 2009, 23, 613–616. [Google Scholar] [CrossRef]
- Al-Jaber, H.I.; Al-Qudah, M.A.; Odeh, F.M.; Zarga, M.H.A. Two new 28-noroleanane type triterpenoids and other constituents from Gladiolus atroviolaceus growing wild in Jordan. Jordan J. Chem. 2019, 14, 11–16. [Google Scholar]
- Ali, A.A.; Abd-Allah, O.M.; Steglich, W. Anthraquinone derivatives from Gladiolus segetum. Phytochemistry 1989, 28, 281–282. [Google Scholar] [CrossRef]
- Abdessemed, D.; Fontanay, S.; Duval, R.E.; Mattar, D.L.; Dibi, A. Two new anthraquinone glycosides from Gladiolus segetum. Arab. J. Sci. Eng. 2011, 36, 57–62. [Google Scholar] [CrossRef]
- Abdessemed, D.; Alloui, N.; Dibi, A. Phytochemical studies on the toxic compounds of Gladiolus segetum. Asian J. Chem. 2011, 23, 609–613. [Google Scholar]
- Abdessemed, D.; Dibi, A. Secondary metabolite from Gladiolus segetum. J. Chem. Pharm. Res. 2013, 5, 939–941. [Google Scholar]
- Tai, Z.G.; Yang, X.Q.; Cai, L.; Sun, W.J.; Ding, Z.T.; Yang, Y.B. Studies on the chemical constituents from the aerial parts of Gladiolus gandavensis. J. Chin. Med. Mater. 2010, 33, 1257–1259. [Google Scholar]
- Tai, Z.G.; Cai, L.; Yang, Y.B.; Liu, C.S.; Xia, J.J.; Ding, Z.T. Three new oleanane-type triterpene saponins from Gladiolus gandavensis. Bull. Korean Chem. Soc. 2010, 31, 2786–2790. [Google Scholar] [CrossRef]
- Islam, S. Anthocyanin compositions in different colored gladiolus species: A source of natural food colorants. Am. J. Food Sci. Tech. 2016, 4, 109–114. [Google Scholar]
- Takemura, T.; Takatsu, Y.; Kasumi, M.; Marubashi, W.; Iwashina, T. Flavonoids and their distribution patterns in the flowers of Gladiolus cultivars. Acta Hortic 2005, 673, 487–493. [Google Scholar] [CrossRef]
- Cohen, A.; Akavia, N.; Umiel, N. The identification of anthocyanin pigments in the petals as an aid to the breeding of Gladiolus. Acta Hortic. 1985, 177, 375–384. [Google Scholar] [CrossRef]
- Takemura, T.; Takatsu, Y.; Kasumi, M.; Marubashi, W.; Iwashina, T. Anthocyanins of Gladiolus cultivars and their contribution to flower colors. J. Jpn. Soc. Hortic. Sci. 2008, 77, 80–87. [Google Scholar] [CrossRef][Green Version]
- Mao, L.C.; Pan, X.; Que, F.; Fang, X.H. Antioxidant properties of water and ethanol extracts from hot air-dried and freeze-dried daylily flowers. Eur. Food Res. Technol. 2006, 222, 236–241. [Google Scholar] [CrossRef]
- Taguchi, K.; Yamasaki, K.; Maesaki, H.; Tokuno, M.; Okazaki, S.; Moriuchi, H.; Takeshita, K.; Otagiri, M.; Seo, H. An evaluation of novel biological activity in a crude extract from Hemerocallis fulva L. var. sempervirens M. Hotta. Nat. Prod. Res. 2014, 28, 2211–2213. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yang, F.F.; Liu, C.Y.; Liu, X.M.; Pan, R.L.; Chang, Q.; Zhang, Z.S.; Liao, Y.H. Effects of phenolic constituents of daylily flowers on corticosterone and glutamate-treated PC12 cells. BMC Complement. Altern. Med. 2017, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.W.J.; Xu, S.F. Experimental observation of the sedative effect of Xuan-Cao flower on mice. J. Tradit. Chin. Med. 1996, 2, 40–41. [Google Scholar]
- Wang, C.Y.; Shi, M.; Li, Y.P.; Zhu, X.W.; Zhang, J.; Wang, J.P.; Xu, A.; Kuang, Y.S.; Gu, G.Q.; Hu, P.F.; et al. Clinical and experimental report on the treatment of insomnia with Xuan-Cao flower. Shanghai J. Tradit. Chin. Med. 1993, 8, 42–44. [Google Scholar]
- Uezu, E. A philological and experimental investigation of the effects of Hemerocallis as food in man and ddY mice. Bull. Coll. Educ. Unit. Ryukyrrs 1997, 51, 231–238. [Google Scholar]
- Uezu, E. Effects of Hemerocallis on sleep in mice. Psychiatry Clin. Neurosci. 1998, 52, 136–137. [Google Scholar] [CrossRef]
- Bor, J.Y.; Chen, H.Y.; Yen, G.C. Evaluation of antioxidant activity and inhibitory effect on nitric oxide production of some common vegetables. J. Agric. Food Chem. 2006, 54, 1680–1686. [Google Scholar] [CrossRef]
- Negishi, T.; Denpo, K.; Kamohara, S.; Kageyama, M. Efficacy of a dietary supplement on sleep disorder. Jpn. Pharmacol. Ther. 2015, 43, 815–826. [Google Scholar]
- Yoshihara, K.; Eguchi, N.; Doe, N. Composition Containing Hot-Water Extract of Plant of the Genus Hemerocallis and Having Antidepressant-Like Effects or Fatigue-Relieving Effects Based on Sleep Improvement. U.S. Patent Application No. 12/995,208, 31 March 2011. [Google Scholar]
- Fu, M.; Mao, L. In vitro antioxidant activities of five cultivars of daylily flowers from China. Nat. Prod. Res. 2008, 22, 584–591. [Google Scholar] [CrossRef]
- He, Y.H.Z.; Yang, J.; Yang, Y.; Wang, T.; Zhou, Y.Z. Experimental study on the antidepressant effects of Hemerocallis citrine. J. Ningxia Med. 2008, 30, 682–683. [Google Scholar]
- Lin, S.H.; Chang, H.C.; Chen, P.J.; Hsieh, C.L.; Su, K.P.; Sheen, L.Y. The antidepressant-like effect of ethanol extract of daylily flowers (金針花 Jīn Zhēn Huā) in rats. J. Tradit. Complement. Med. 2013, 3, 53–61. [Google Scholar] [CrossRef]
- Machado, D.G.; Bettio, L.E.; Cunha, M.P.; Santos, A.R.; Pizzolatti, M.G.; Brighente, I.M.; Rodrigues, A.L. Antidepressant-like effect of rutin isolated from the ethanolic extract from Schinus molle L. in mice: Evidence for the involvement of the serotonergic and noradrenergic systems. Eur. J. Pharmacol. 2008, 587, 163–168. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Arad, M.; Terrillion, C.E.; Piantadosi, S.C.; Gould, T.D. The mouse forced swim test. J. Vis. Exp. 2012, 59, e3638. [Google Scholar] [CrossRef]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The forced swim test as a model of depressive-like behavior. J. Vis. Exp. 2015, 97, e52587. [Google Scholar] [CrossRef]
- Rittenhouse, P.A.; López-Rubalcava, C.; Stanwood, G.D.; Lucki, I. Amplified behavioral and endocrine responses to forced swim stress in the Wistar-Kyoto rat. Psychoneuroendocrinology 2002, 27, 303–318. [Google Scholar] [CrossRef]
- Moghaddam, B.; Jackson, M. Effect of stress on prefrontal cortex function. Neurotox. Res. 2004, 6, 73–78. [Google Scholar] [CrossRef]
- Llorens-Martín, M.V.; Rueda, N.; Martínez-Cué, C.; Torres-Alemán, I.; Flórez, J.; Trejo, J.L. Both increases in immature dentate neuron number and decreases of immobility time in the forced swim test occurred in parallel after environmental enrichment of mice. Neuroscience 2007, 147, 631–638. [Google Scholar] [CrossRef][Green Version]
- Liu, X.L.; Luo, L.; Liu, B.B.; Li, J.; Geng, D.; Liu, Q.; Yi, L.T. Ethanol extracts from Hemerocallis citrina attenuate the upregulation of proinflammatory cytokines and indoleamine 2,3-dioxygenase in rats. J. Ethnopharmacol. 2014, 153, 484–490. [Google Scholar] [CrossRef]
- Yi, L.T.; Li, J.; Li, H.C.; Zhou, Y.; Su, B.F.; Yang, K.F.; Jiang, M.; Zhang, Y.T. Ethanol extracts from Hemerocallis citrina attenuate the decreases of brain-derived neurotrophic factor, TrkB levels in rat induced by corticosterone administration. J. Ethnopharmacol. 2012, 144, 328–334. [Google Scholar] [CrossRef]
- Li, C.F.; Chen, X.Q.; Chen, S.M.; Chen, X.M.; Geng, D.; Liu, Q.; Yi, L.T. Evaluation of the toxicological properties and anti-inflammatory mechanism of Hemerocallis citrina in LPS-induced depressive-like mice. Biomed. Pharmacother. 2017, 91, 167–173. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Liu, B.B.; Liu, Q.; Geng, D.; Weng, L.J.; Yi, L.T. Nobiletin ameliorates the deficits in hippocampal BDNF, TrkB, and synapsin I induced by chronic unpredictable mild stress. Evid-Based Compl. Altern. Med. 2013, 2013, 359682. [Google Scholar] [CrossRef]
- Li, C.F.; Chen, S.M.; Chen, X.M.; Mu, R.H.; Wang, S.S.; Geng, D.; Liu, Q.; Yi, L.T. ERK-dependent brain-derived neurotrophic factor regulation by hesperidin in mice exposed to chronic mild stress. Brain Res. Bull. 2016, 124, 40–47. [Google Scholar] [CrossRef]
- Zhai, J.L.; Li, M.Q.; Zhang, Z.S.; Liao, Y.H.; Chang, Q.; Pan, R.L.; Liu, X.M. Screen of active anti-depression ingredients from daylily. Chin. Food Addit. 2015, 140, 93–97. [Google Scholar]
- Xu, P.; Wang, K.Z.; Lu, C.; Dong, L.M.; Le Zhai, J.; Liao, Y.H.; Aibai, S.; Yang, Y.; Liu, X.M. Antidepressant-like effects and cognitive enhancement of the total phenols extract of Hemerocallis citrina Baroni in chronic unpredictable mild stress rats and its related mechanism. J. Ethnopharmacol. 2016, 194, 819–826. [Google Scholar] [CrossRef]
- Bandeira, S.O.; Gaspar, F.; Pagula, F.P. African ethnobotany and healthcare: Emphasis on Mozambique. Pharm. Biol. 2001, 39 (Suppl. 1), 70–73. [Google Scholar]
- Burkill, H.M. The Useful Plants of West. Tropical Africa, 2nd ed.; Royal Botanic Gardens: Kew, UK, 1985; p. 969. [Google Scholar]
- Goldblatt, P. Gladiolus of tropical Africa: Systematics, Biology and Evolution, 1st ed.; Timber Press: Portland, ON, USA, 2003; p. 338. [Google Scholar]
- Zyss, T. Similarities and differences between depression and epilepsy-a comparison trial (in Polish with English abstract). Psychiatr. Pol. 2009, 43, 513–527. [Google Scholar]
- Jackson, M.J.; Turkington, D. Depression and anxiety in epilepsy. J. Neurol. Neurosur. Ps. 2005, 76 (Suppl. 1), i45–i47. [Google Scholar] [CrossRef]
- Hoppe, C.; Elger, C.E. Depression in epilepsy: A critical review from a clinical perspective. Nat. Rev. Neurol. 2011, 7, 462–472. [Google Scholar] [CrossRef]
- Elger, C.E.; Johnston, S.A.; Hoppe, C. Diagnosing and treating depression in epilepsy. Seizure 2017, 44, 184–193. [Google Scholar] [CrossRef]
- Mula, M. Depression in epilepsy. Curr. Opin. Neurol. 2017, 30, 180–186. [Google Scholar] [CrossRef]
- Insel, B.J.; Ottman, R.; Heiman, G.A. Mood disorders in familial epilepsy: A test of shared etiology. Epilepsia 2018, 59, 431–439. [Google Scholar] [CrossRef]
- Ngoupaye, G.T.; Pahaye, D.B.; Ngondi, J.; Moto, F.C.O.; Bum, E.N. Gladiolus dalenii lyophilisate reverses scopolamine-induced amnesia and reduces oxidative stress in rat brain. Biomed. Pharmacother. 2017, 91, 350–357. [Google Scholar] [CrossRef]
- Fotsing, D.; Ngoupaye, G.T.; Ouafo, A.C.; Njapdounke, S.K.J.; Kenneth, Y.A.; Ngo Bum, E. Effects of Gladiolus dalenii on the stress-induced behavioral, neurochemical, and reproductive changes in rats. Front. Pharmacol. 2017, 8, 685. [Google Scholar] [CrossRef]
- Maes, M.; Galecki, P.; Chang, Y.S.; Berk, M.A. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro) degenerative processes in that illness. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 676–692. [Google Scholar]
- Duivis, H.E.; Vogelzangs, N.; Kupper, N.; de Jonge, P.; Penninx, B.W. Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: Findings from the Netherlands Study of Depression and Anxiety (NESDA). Psychoneuroendocrinology 2013, 38, 1573–1585. [Google Scholar] [CrossRef]
- Palazidou, E. The neurobiology of depression. Br. Med. Bull. 2012, 101, 127–145. [Google Scholar] [CrossRef]
- Rawdin, B.J.; Mellon, S.H.; Dhabhar, F.S.; Epel, E.S.; Puterman, E.; Su, Y.; Burke, H.M.; Reus, V.I.; Rosser, R.; Hamilton, S.P.; et al. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav. Immun. 2013, 31, 143–152. [Google Scholar] [CrossRef]
- Liu, T.; Zhong, S.; Liao, X.; Chen, J.; He, T.; Lai, S.; Jia, Y. A meta-analysis of oxidative stress markers in depression. PLoS ONE 2015, 10, e0138904. [Google Scholar] [CrossRef]
- Bakunina, N.; Pariante, C.M.; Zunszain, P.A. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 2015, 144, 365–373. [Google Scholar] [CrossRef]
- Bouayed, J.; Rammal, H.; Soulimani, R. Oxidative stress and anxiety: Relationship and cellular pathways. Oxid. Med. Cell. Longev. 2009, 2, 63–67. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Maker, G.L.; Hood, S.D.; Drummond, P.D. A review of peripheral biomarkers in major depression: The potential of inflammatory and oxidative stress biomarkers. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 102–111. [Google Scholar] [CrossRef]
- Palta, P.; Samuel, L.J.; Miller, E.R.; Szanton, S.L. Depression and oxidative stress: Results from a meta-analysis of observational studies. Psychosom. Med. 2014, 76, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W. Is depression associated with increased oxidative stress?. A systematic review and meta-analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef]
- Fedoce, A.D.G.; Ferreira, F.; Bota, R.G.; Bonet-Costa, V.; Sun, P.Y.; Davies, K.J. The role of oxidative stress in anxiety disorder: Cause or consequence? Free Radical Res. 2018, 52, 737–750. [Google Scholar] [CrossRef]
- Ochekpe, N.A.; Ameh, S.; Okoliko, I.; Olorunfemi, P.O. Antimicrobial and phytochemical properties of aqueous extracts of Gladiolus corm (family Iridaceae) from Benue State of Nigeria. J. Pharm. Biores. 2009, 6, 65–70. [Google Scholar] [CrossRef]
- Munyemana, F.; Mondego, A.P.; Cumbane, P. Qualitative phytochemical screening and antimicrobial activity evaluation of the bulb extracts of Gladiolus psittacinus Hook (Iridaceae). Int. Network Environ. Manag. Conflicts Santa Catarina-Brazil 2013, 2, 14–31. [Google Scholar]
- Pirvu, L.; Grigore, A.; Bubueanu, C.; Hlevca, C.; Colceru-Mihul, S. Plant compounds synergistic activity benefits on human health. Studia Universitatis “Vasile Goldiş” Seria Ştiinţele Vieţii 2014, 24 (Suppl. 1), 33–38. [Google Scholar]
- Wang, J.; Hu, D.; Hou, J.; Li, S.; Wang, W.; Li, J.; Bai, J. Ethyl acetate fraction of Hemerocallis citrina Baroni decreases tert-butyl hydroperoxide-induced oxidative stress damage in BRL-3A cells. Oxid. Med. Cell. Longev. 2018, 2018, 1526125. [Google Scholar] [CrossRef]
- Sonam, K.S.; Guleria, S. Synergistic antioxidant activity of natural products. Ann. Pharmacol. Pharm. 2017, 2, 1086. [Google Scholar]
- Spinella, M. The importance of pharmacological synergy in psychoactive herbal medicines. Altern. Med. Rev. 2002, 7, 130–137. [Google Scholar]
- Hussain, S. Patient counseling about herbal-drug interactions. Afr. J. Tradit. Complem. Altern. Med. 2011, 8, 152–163. [Google Scholar] [CrossRef] [PubMed]
| Group of Bioactive Compounds | Bioactive Compounds | Species | Author |
|---|---|---|---|
| Flower | |||
| Alkaloids | hemerocallisamine I–VII | Hemerocallis sp. | [201,202] |
| 2-formylopyrole hemerokallisamine I | H. fulva L. H. flava L. H. minor Mill. | [203] | |
| Anthocyanidins | cyanidin3-rutinoside; delphinidin-3-rutinoside | H. fulva L. | [204] |
| cyanidin; delphinidin; pelargonidin; peonidin; petunidin | Hemerocallis sp. | [205] | |
| Amino acids | tryptophan derivative; tyrosine | H. fulva L. | [206,207,208] |
| Amino acid amides | longitubanine a | [209] | |
| Protein | globulins | [206,207] | |
| Carotenoids | lutein, zeaxanthin; lutein; lutein-5,6-epoxide; neoxanthin; trans-β-carotene; violaxanthin; violeoxanthin; β-cryptoxanthin; zeaxanthin | H. disticha Donn | [210,211] |
| β-karoten, lutein; zeaxanthin | H. fulva L. | [204,212] | |
| carotene; lycopene | Hemerocallis sp. | [205] | |
| Flavonoids | agipenin; kaempferol; luteolin; myricetin; quercetin; rutin | [205,213] | |
| hesperidin; hyperoside; isoquercitrin; isorhamnetin 3-o-glucoside; kaempferol 3-rutinoside; kaempferol-3-o-galactoside; quercetin 3,7-o-β-d-diglucopyranoside; quercetin 3-o-β-d-xylopyranoside; rutin | H. citrina Baron | [214] | |
| chrysin; chrysoeriol 7-o-[β-d-glucuronopyranosyl(1→2)(2-o-trans-feruloyl)-β-d-glucuronopyranoside; hesperidin; isorhamnetin 3-o-glycosides; isorhamnetin-3-o-β-d-6′-acetylglucopyranoside; kaempferol 3-o-{α-l-rhamnopyranosyl(1→6)[α-l-rhamnopyranosyl(1→2)]}-β-d- galactopyranoside; kaempherol; myricetin; naringenin; naringin; n-butyl 4-trans-o-caffeoylquinate; pinocembrin; quercetin 3,7-o-β-d-diglucopyranoside; quercetin 3-o-α-l-rhamnopyransol-(1→6)-β-d-glucopyranosol-7-o-β-d-glucopyranoside; quercetin 3-o-β-d-glucoside; quercetin; rutin | H. fulva L. | [208,209,215,216] | |
| Glycosides | orcinol beta-d-glucopyranoside; phenethyl β-d-glucopyranoside; phloretin 2′-o-β-d-glucopyranoside; phloretin 2′-o-β-d-xylopyranosyl-(1→6)-β-d- glucopyranoside | [209] | |
| Phenolic acids | caffeoylquinic acid; gallic acid | [208,217] | |
| 4-o-p-coumaroylquinic acid; gallic acid | H. citrina Baron | [214] | |
| Naphthalene glycosides | stelladerol | H. fulva L. | [208,209] |
| Unsaturated polyhydroxy alcohols | ascorbic acid | [212] | |
| Nucleosides | adenosine; guanosine | [208] | |
| Phenol derivatives | hemeratrol a | H. minor Mill. | [218] |
| Phenylpropanoids | 4-o-caffeoylquinic acid; caffeic acid; chlorogenic acid | H. citrina Baron | [214] |
| Terpenes | hemerolides a–c | H. minor Mill. | [218] |
| Group of Bioactive Compounds | Bioactive Compounds | Species | Author |
|---|---|---|---|
| Leaves | |||
| Amino acid amides | pinnatanine | H. fulva L. | [209,215] |
| Catechins | catechin | Hemerocallis sp. | [213] |
| Glucoside | phlomuroside | H. fulva L. | [209,215] |
| Terpenoids | roseoside | ||
| Lignans | lariciresinol | [209,215] | |
| Nucleosides | adenosine | [209,215] | |
| Phenylpropanoids | chlorogenic acid | Hemerocallis sp. | [213] |
| roots | |||
| Alkaloids | hemerominory A-H; γ-lactam | H. minor Mill | [219] |
| Anthraquinones | 2-hydroksychrysophanol; kwanzoquinones A, B, C, D, E, F, G; rhein | H. fulva L. | [220] |
| Flavonols | 6-methylluteolin | ||
| Naphtalene glycosides | 5-hydroxydianellin; dianelin | ||
| Vitamins | α-tocopherol | ||
| Group of Bioactive Compounds | Bioactive Compounds | Species | Author |
|---|---|---|---|
| Anthraquinones | methyl trans-p-methoxycinnamate; methyl 8-hydroxy-3,6,7-trimethoxy-1-methylanthraquinone-2-carboxylate (gandavensin B); methyl 8-hydroxy-3,6-dimethoxy-1-methylanthraquinone-2-carboxylate; methyl 8-hydroxy-3-methoxy-6,7-methylenedioxy-1-methylanthraquinone-2-carboxylate (gandavensin A); 5,7-dimethoxy-2-methylchromone; 5-hydroxy-2-hydroxymethyl-7-methoxychromone | G. gandavensis Van Houtt. | [221] |
| deoxy-erythrolaccin; laccaic acid D methylester; physcion | G. segetum Ker-Gawl. | [222] | |
| 1,6,7-trihydroxy-3-methoxy-8-methyl-anthraquinone; 1-hydroxy-3,6,7-trimethoxy-8-methyl-anthraquinone | G. psittascinus Hook | [223] | |
| Cytokinins | isopentenyl adenine; zeatin | G. grandiflorus L. | [224] |
| Steroids | (–)-dehydrodiconiferyl alcohol; (+)-demethoxypinoresinol; (+)-pinoresinol monomethylether; (+)-pinoresinol; 6′-Opalmitoyl-3-O-sitosterol glucoside; neolignan; β-sitosterol-3-O-glucoside | G. segetum Ker-Gawl. | [222] |
| Terpenes | 2β, 3β, 16α, 28-tetrahydroxy-olean-12-ene-23-oic acid; medicagenic acid | [225] |
| Group of Bioactive Compounds | Species | Author | |
|---|---|---|---|
| Whole Plant | |||
| Anthraquinones | emodin | G. atroviolaceus Boiss | [226] |
| Flavonoids | kampferol-3-o-rhamnoside; kampferol-3-o- β-glucopyranoside; quercetin-3-o-rhanmnoside | ||
| Phytosterols | stigmasterol glucoside | ||
| Terpenoids | gladioloic acid A; gladioloic acid B | ||
| Aerial parts | |||
| Anthraquinones | 1-hydroxy-3,6,7-trimethoxy8-methylanthraquinone; 3,8-dihydroxy-4,7-dimethoxy-1-methylanthraquinone2-carboxylic acid methyl ester; 3,8-dihydroxy-6-methoxy-1-methylanthraquinone-2-carboxylic acid; 3,8-dimethoxy-1-methylanthraquinone-2-carboxylic acid methyl ester; desoxyerythrolaccin; methyl 3-methoxy-1-methyl-9; 10-dioxo-8-(beta-d-glucopyranosyloxy)-9,10-dihydroanthracene-2-carboxylate; methyl 8-hydroxy-4,7-dimethoxy-1-methyl-9,10-dioxo-3-(beta-d-glucopyranosyloxy)-9,10-dihydroanthracene-2-carboxylate | G. segetum Ker-Gawl | [227,228,229,230] |
| Flavonoids | apigenin-7-O-alpha-L-rhamnoside; astragalin-2”-O-beta-D-glucopyranoside kaempferol; glycerol-alpha-monohexacosanate; nicotiflorin; quercetin-3-O-(6”-O-Ecaffeoyl)-beta-D-glucopyranoside; tamarixetin-3-robinobioside | G. gandavensis Van Houtt. | [231] |
| 2, 5, 6- trihydroxy-2, 4-dimethyl-6-metoxy-1-benzofuran-3-one; kaempferol-3-O-β-D-glucopyranoside8; quercetin-3-O-β-D-glucopyranoside8 | G. segetum Ker-Gawl | [229,230] | |
| Phytosterols | β-sitosterol, daucosterol | G. gandavensis Van Houtt. | [232] |
| ergosterol, stigmasterol | G. segetum Ker-Gawl | [229] | |
| Terpenoids | 29-o-(β-d-glucopyranosyl)-2β,3βdihydroxyolean-12-en-28-oic acid; 3-o-(β-d-xylopyranosyl)-29-o-(β-d-glucopyranosyl)-12-en-28-oic acid; β-d-glucopyranosyl] ester | G. gandavensis Van Houtt | [232] |
| betulinic acid | G. segetum Ker-Gawl | [229] | |
| Fatty acyl glycosides of mono- and disaccharides | isopentyl gentiobioside | G. gandavensis Van Houtt. | [231] |
| Sterol lipoprotein | cholesterol | G. segetum Ker-Gawl | [229] |
| Nucleosides | adenosine | G. atroviolaceus Boiss. | [226] |
| Group of Bioactive Compounds | Bioactive Compounds | Species | Author |
|---|---|---|---|
| Leaf | |||
| Anthocyanins | cyaniding; delphinidin; malvidin; pelargonidin | Gladiolus “Green Star”, “Red Flair”, “Pink Event”, “Violetta”, “Ice Cap” | [233] |
| Flower | |||
| Flavonoids | flavonol glycosides; kaempferol; kaempferol 3-o-rutinoside; kaempferol 3-o-sophoroside; laricitrin; myricetin; quercetin; quercetin 3-o-rutinoside; syringetin | G. grandiflora “Ariake” | [234] |
| Anthocyanins | cyaniding; delphinidin; malvidin; pelargonidin; peonidin; petunidin | Gladiolus sp. | [235] |
| malvidin 3,5-di-o-glucoside (malvin); malvidin glycosides | G. grandiflora “Ariake” | [234] | |
| 3,5-di-o-glucosides of petunidin; 3-o-rutinoside-5-oglucosides of cyaniding; cyaniding; malvidin; malvidin 3-o-glucoside, pelargonidin 3-o-rutinoside; pelargonidin; peonidin | Gladiolus of 18 cultivars | [236] | |
| Plant Organ | Extract, Active Compound | Dosage and the Way of Administration/Biological Object | Main Results | Proposed Mechanism of Antidepressant Action | Author |
|---|---|---|---|---|---|
| Hemerocallis citrina Baroni | |||||
| Flower | ethanol extract | 90, 180 or 360 mg·kg−1, p.o./* | Reduced immobility time in FST and TST. Enhanced 5-HT and NA levels in the frontal cortex and hippocampus. Elevated DA levels in the frontal cortex | Via the serotonergic (5-HT1A and 5-HT2 receptors), noradrenergic (α1-, α2- and β-adrenoceptors) and dopaminergic (D-2 receptor) systems | [179] |
| Flower | hydroalcoholic extracts, flavonoids – rutin, hesperidin | 400 mg·kg−1, p.o./* | Reduced immobility time in TST and improvement of locomotor activity in OFT. Increase in the serotonin and dopamine levels in the central nervous system | Via the serotoninergic and dopaminergic systems. The presence of flavonoids with sub-additive interaction between rutin and hesperidin | [180] |
| Flower | phenolic (phenolic acid derivatives, flavonoids) and non-phenolic fractions of the hydroalcoholic extract | 24 h pretreatment with fractions 0.3–5.0 mg raw material/mL /*** | Neuroprotective effects against corticosterone and glutamate-induced damage in PC12 cells exerted by phenolics, but not non-phenolic fractions. Similar extent of the neuroprotective effect of phenolic acid derivatives and flavonoids, but quite different release of neurotransmitters | Regulation of neurotransmitters. Influence of phenolic acid derivatives on the release of dopamine DA and NA. Modulation of the release of 5-HT, NA, and ACh by flavonoids | [239] |
| Flower | ethanol extract | 130 mg kg−1 for four weeks via gavage/** | Amelioration of CUMS-induced depressive symptoms. Reversion of the decreased sucrose preference in SPT, inhibition of IL-1β, IL-6, and TNF-α expression, as well as IDO activity in the frontal cortex and hippocampus | Restoration or improvement of monoaminergic and neurotrophin systems due to the anti-inflammatory properties of daily flower extracts | [256] |
| Flower | ethanol extract | 32.5; 65 or 130 mg·kg−1 BW, p.o./** | Reversion of the corticosterone induced (40 mg/kg, s.c.) depression-like behaviors in SPT and FST | Via BDNF-TrkB (brain-derived neurotrophic factor and its receptor) signaling in the frontal cortex and hippocampus | [257] |
| Flower | ethanol extract | 180, 360, and 720 mg·kg−1 per eight weeks, p.o./* | Decreased total cholesterol levels without any significant histopathological changes in the liver and kidney. Reversion of the reduction of sucrose preference (SPT) with LPS. Normalization of NF-κB activation as well as the expression of iNOS and COX-2 in an LPS-induced depressive-like model | Inhibition of the NF-κB signaling pathway in the prefrontal cortex | [258] |
| Flower | total phenols extract | 10, 20, and 40 mg·kg−1 daily, via gastric gavage ** | Improvement of depression-like emotional status, amelioration of depression-related behavior in TST, and association of cognitive deficits in MWM induced by chronic unpredictable mild stress (CUMS) procedures due to HCPE, especially at 40 mg kg−1 | Regulation of neurotransmitters (5-HT, DA, and NE) and BDNF levels in the brain. Reduced CORT level in the serum. Alleviation of oxidative stress manifested by decreased MDA in the frontal cortex | [262] |
| Hemerocallis fulvaL. | |||||
| Flower | ethanol extract, flavonoid rutin | 3, 15, or 30 g·kg−1 BW for one or two weeks via oral gavage/** | Reduced immobility time and increased swimming time in FST. Increase in the serotonin, norepinephrine, and dopamine levels in the frontal cortex, hippocampus, striatum, and amygdala. DFEtoH elevated the serotonin level and reduced the serotonin turnover rate in these brain regions but not in the frontal cortex. | Regulation of the serotonergic system. Role of rutin in the antidepressant-like effects of DFEtoH through blockage of MAO and elevation of the synaptic neurotransmitter level | [249] |
| Plant Organ | Extract, Active Compound | Dosage and the Way of Administration/Biological Object | Main Results | Proposed Mechanism of Antidepressant Action | Author |
|---|---|---|---|---|---|
| Corm or bulbs | aqueous extract | 15 mg·kg−1 for 7 days, i.p./** | Counteraction of associated depressive states induced with pilocarpine combined with atropine pretreatment. Reduction of the immobility time assessed in FST and enhancement of spontaneous locomotor activity in OFT. Drop in the levels of ACTH, CORT, but not the adrenal gland weight. Increase in the level of BDNF in the hippocampus | Restoration of the activity of the HPA axis and an increase in the BDNF level in the hippocampus | [176] |
| 7.5; 15 and 150 mg kg−1, p.o./* | Reduction of the immobility time in FST and TST. Antagonization of the effect of N-methyl-D-aspartate (NMDA) after administration of the moderate and highest doses of the extract. Shortening of the immobility time at the sub-effective dose (7.5 mg kg−1) in combination with either D-(−)-2-amino-7-phosphonohepta- noic acid (D-AP7) (the NMDA receptor antagonist) or imipramine. Stronger therapeutic effect of GD than that of imipramine, fluoxetine, and D-AP7 | Interactions with NMDA, serotonin, and/or noradrenergic systems | [178] | ||
| Corm or bulbs | aqueous and lyophilized extract, macerate | 150 mg kg−1, p.o./* | Protection against pentylenetetrazol (PTZ)- and maximal electroshock (MES)-induced seizures. Additive effect of co-administration of GD with diazepam, opposite to the combination of GD with flumazenil or FG7142. Sedative activity of GD by shortening the latency time to sleep and an increase in the total duration of diazepam-induced sleep used for evaluation of the sedative properties | Via the benzodiazepine site receptor | [177] |
| aqueous extract | 7.5 or 15 mg kg−1, every day during 28 days, 5 min before induction of stress, p.o./** | Antagonization of the chronic immobilization of stress-induced behavioral, reproductive, and neurochemical changes in female albino rats by the GD extract. Increase in the number of entries and prolonged time of open arm exploration in the elevated plus maze (EPM). Reduction of the corticosterone, progesterone, and prolactin concentrations elevated due to chronic stress as well as normalization of the level of reproductive hormones and reversed unfavorable changes in the estrous cycle by GD | Possible role of the bioactive molecules and secondary metabolites (alkaloids, flavonoids) in the potential adaptogenic action of GD against a chronic restraint model in animals. Plausible mediation of GD action through interactions with NMDA, GABA, 5-HT and/ or NA systems | [273] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matraszek-Gawron, R.; Chwil, M.; Terlecka, P.; Skoczylas, M.M. Recent Studies on Anti-Depressant Bioactive Substances in Selected Species from the Genera Hemerocallis and Gladiolus: A Systematic Review. Pharmaceuticals 2019, 12, 172. https://doi.org/10.3390/ph12040172
Matraszek-Gawron R, Chwil M, Terlecka P, Skoczylas MM. Recent Studies on Anti-Depressant Bioactive Substances in Selected Species from the Genera Hemerocallis and Gladiolus: A Systematic Review. Pharmaceuticals. 2019; 12(4):172. https://doi.org/10.3390/ph12040172
Chicago/Turabian StyleMatraszek-Gawron, Renata, Mirosława Chwil, Paulina Terlecka, and Michał M. Skoczylas. 2019. "Recent Studies on Anti-Depressant Bioactive Substances in Selected Species from the Genera Hemerocallis and Gladiolus: A Systematic Review" Pharmaceuticals 12, no. 4: 172. https://doi.org/10.3390/ph12040172
APA StyleMatraszek-Gawron, R., Chwil, M., Terlecka, P., & Skoczylas, M. M. (2019). Recent Studies on Anti-Depressant Bioactive Substances in Selected Species from the Genera Hemerocallis and Gladiolus: A Systematic Review. Pharmaceuticals, 12(4), 172. https://doi.org/10.3390/ph12040172