Effect of 6-Shogaol on the Glucose Uptake and Survival of HT1080 Fibrosarcoma Cells
Abstract
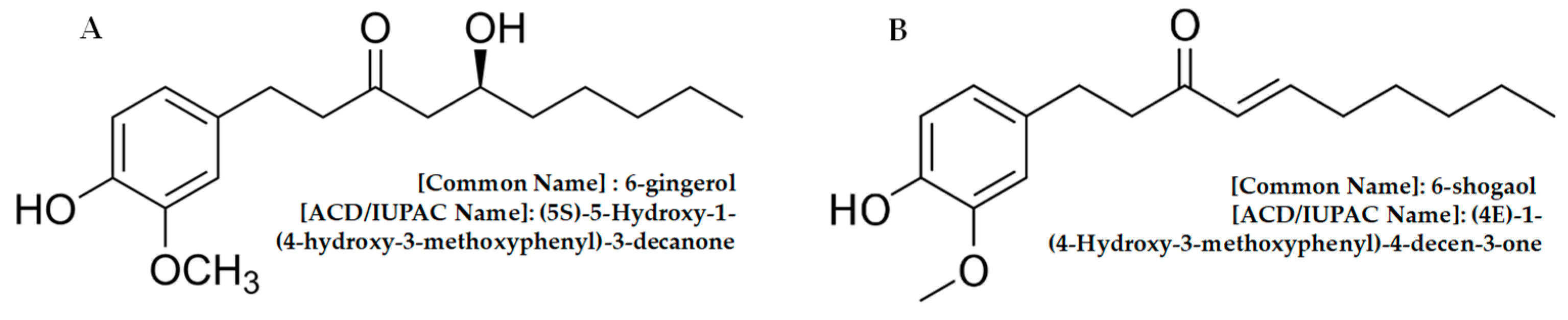
:1. Introduction
2. Results
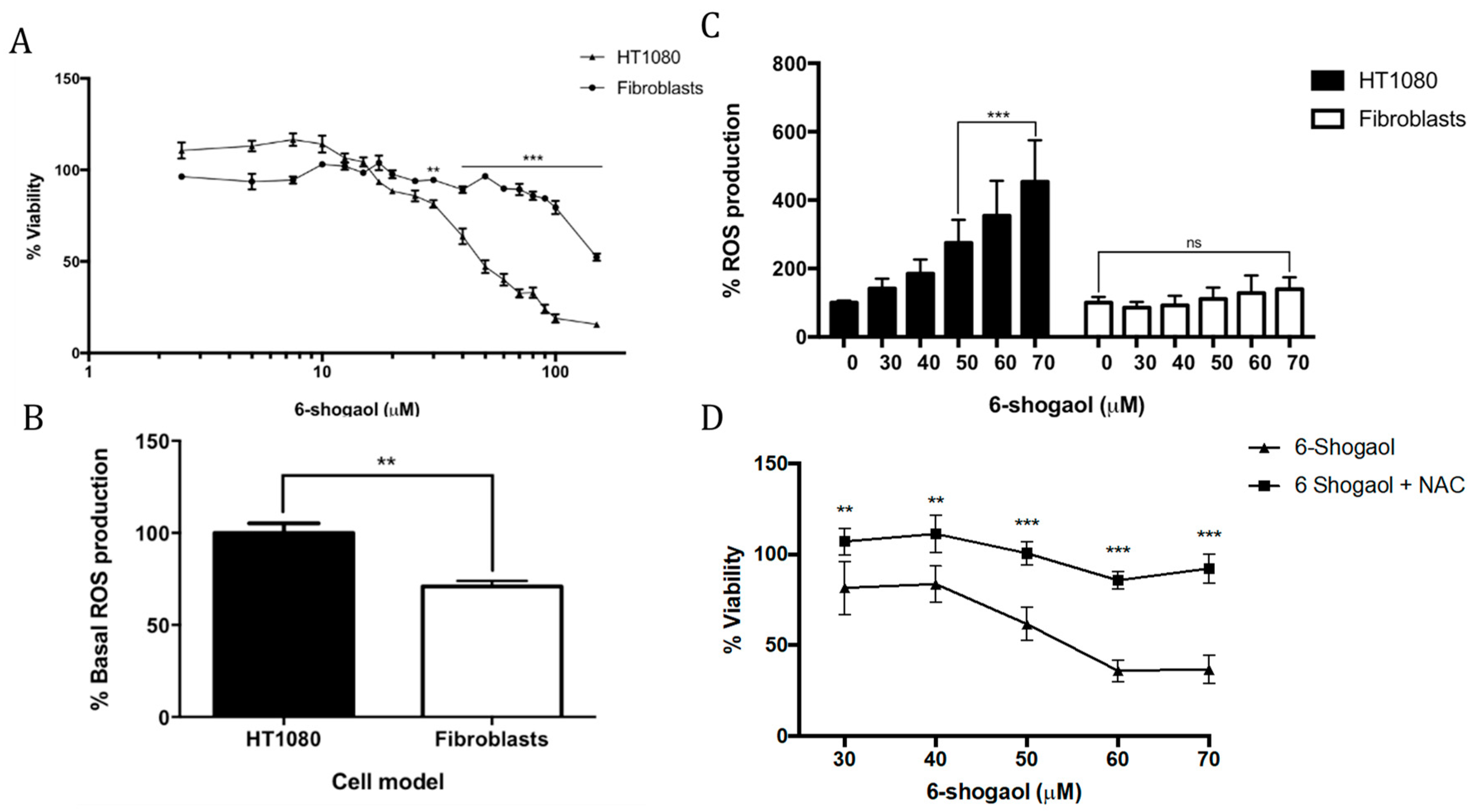
2.1. 6-Shogaol Induces Cell Death in Fibrosarcoma Cells
2.2. NAC Attenuates the Pro-Oxidant Effect of 6-Shogaol in Tumoral Model Cells (HT1080)
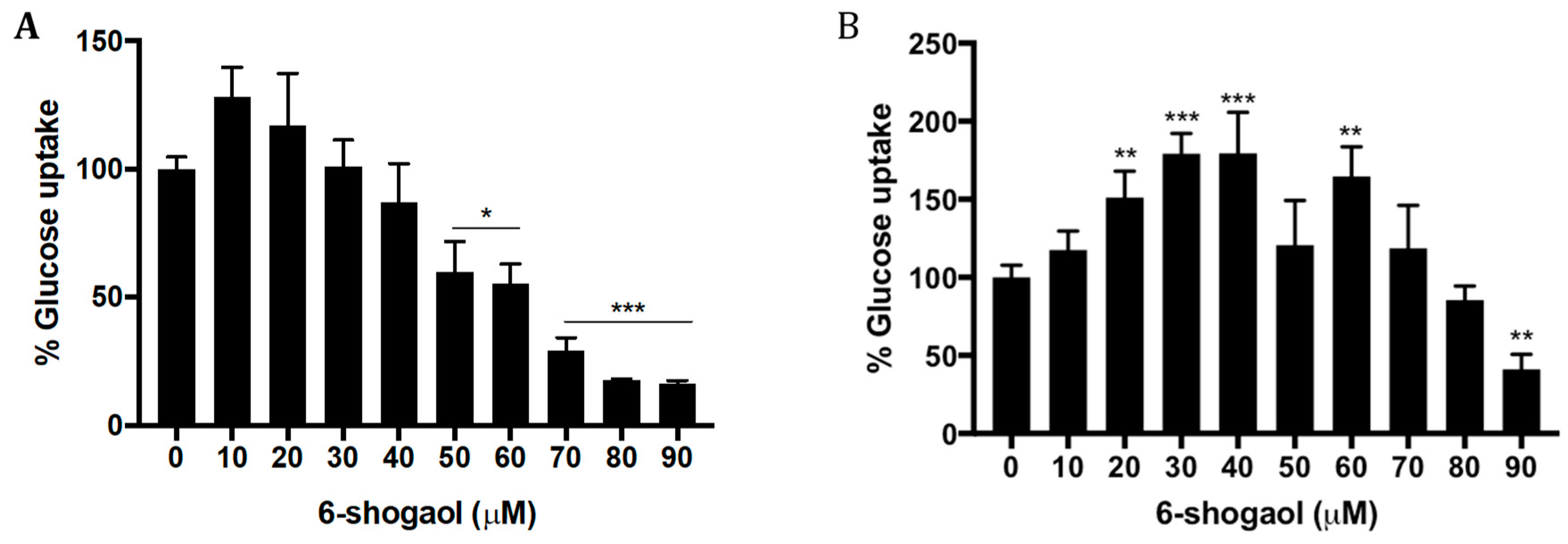
2.3. 6-Shogaol Decreases Glucose Uptake in HT1080 Cells
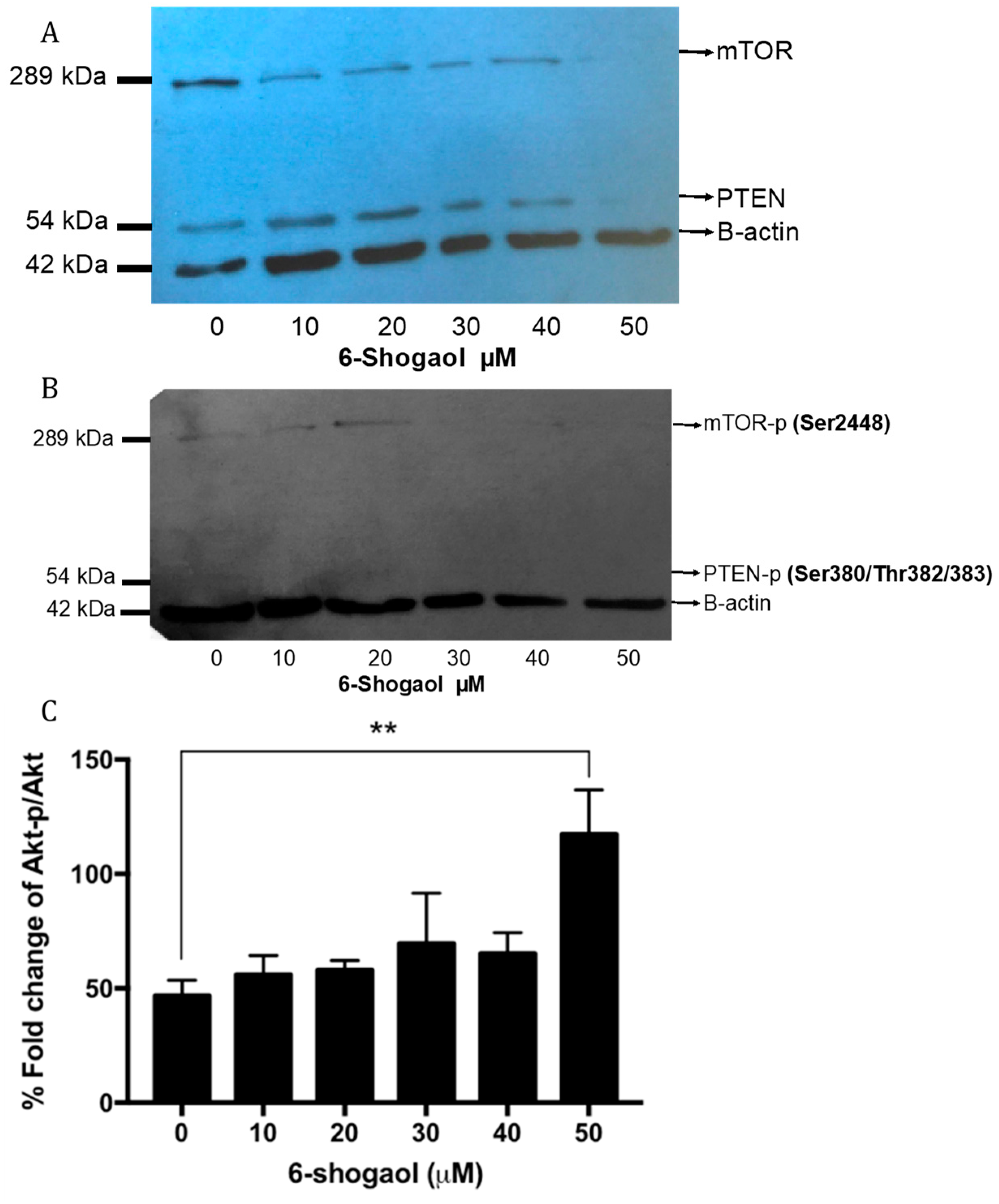
2.4. 6-Shogaol Produced a Survival Decrease through PI3K/AKt/mTOR Modulation on HT1080 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Reagents
4.2. Viability Assay
4.3. ROS Production Assay
4.4. Glucose Uptake Evaluation
4.5. Western Blot Analysis
4.6. Akt and Phospho-Akt Expression Analysis by Flow Cytometer
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive compounds and bioactivities of ginger (zingiber officinale roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tyagi, A.K. Ginger and its constituents: Role in prevention and treatment of gastrointestinal cancer. Gastroenterol. Res. Pract. 2015, 2015, 142979. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Arita, M.; Sakurai, H.; Ono, N.; Tezuka, Y. Analysis of chemical properties of edible and medicinal ginger by metabolomics approach. BioMed Res. Int. 2015, 2015, 671058. [Google Scholar] [CrossRef] [PubMed]
- Santos Braga, S. Ginger: Panacea or consumer’s hype? Appl. Sci. 2019, 9, 1570. [Google Scholar] [CrossRef]
- Afzalpour, M.; Nayebifar, S.; Kazemi, T.; Abtahi-Eivary, S.-H.; Mogharnasi, M. Determination of atherosclerosis markers changes after hiit and ginger consumption in response to acute exercise in overweight women. J. App. Pharm. Sci. 2016, 6, 78–84. [Google Scholar] [CrossRef]
- Safitri, D.; Kurniati, N.F.; Adharani, S.; Suciyati, S.W.; Adnyana, I.K. The study of red ginger rhizomes ethanol extract (zingiber officinale roscoe var. Sunti val.) on hyperlipidemic-induced rats. Pharmacologyonline 2016, 3, 15–21. [Google Scholar]
- Dhanik, J.; Arya, N.; Nand, V. A review on zingiber officinale. J. Pharmacog. Phytochem. 2017, 6, 174–184. [Google Scholar]
- Mohd Sahardi, N.F.N.; Makpol, S. Ginger (zingiber officinale roscoe) in the prevention of ageing and degenerative diseases: Review of current evidence. Evid. Based. Complement. Alternat. Med. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Lee, C.C.; Chiou, L.Y.; Wang, J.Y.; Chou, S.Y.; Lan, J.C.; Huang, T.S.; Huang, K.C.; Wang, H.M. Functional ginger extracts from supercritical fluid carbon dioxide extraction via in vitro and in vivo assays: Antioxidation, antimicroorganism, and mice xenografts models. Sci. World J. 2013, 2013, 210845. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef]
- Choi, J.G.; Kim, S.Y.; Jeong, M.; Oh, M.S. Pharmacotherapeutic potential of ginger and its compounds in age-related neurological disorders. Pharmacol. Therapeut. 2018, 182, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Gao, M.; Cui, W.; Zeng, M.; Cheng, Y.; Li, J. Nine new gingerols from the rhizoma of zingiber officinale and their cytotoxic activities. Molecules 2018, 23, 315. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Forero, M.; Sequeda-Castañeda, L.G.; Grismaldo, A.; Iglesias, J.; Celis-Zambrano, C.A.; Schuler, I.; Morales, L. Effect of ginger extract on membrane potential changes and akt activation on a peroxide-induced oxidative stress cell model. J. King Saud. Univ. Sci. 2018, 30, 263–269. [Google Scholar] [CrossRef]
- Akimoto, M.; Iizuka, M.; Kanematsu, R.; Yoshida, M.; Takenaga, K. Anticancer effect of ginger extract against pancreatic cancer cells mainly through reactive oxygen species-mediated autotic cell death. PLoS ONE 2015, 10, e0126605. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, V.; Hay, N. Molecular pathways: Reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin. Cancer Res. 2013, 19, 4309–4314. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, L.; Guo, H.; Wysham, W.Z.; Roque, D.R.; Willson, A.K.; Sheng, X.; Zhou, C.; Bae-Jump, V.L. Glucose promotes cell proliferation, glucose uptake and invasion in endometrial cancer cells via ampk/mtor/s6 and mapk signaling. Gynecol. Oncol. 2015, 138, 668–675. [Google Scholar] [CrossRef]
- Mohd Yusof, Y.A. Gingerol and its role in chronic diseases. Cham 2016, 929, 177–207. [Google Scholar]
- Shen, Y.; Zhang, H.; Cheng, L.; Wang, L.; Qian, H.; Qi, X. In vitro and in vivo antioxidant activity of polyphenols extracted from black highland barley. Food Chem. 2016, 194, 1003–1012. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, C.; Yang, G.; Yang, Y. Biological properties of 6-gingerol: A brief review. Nat. Prod. Commun. 2014, 9, 1027–1030. [Google Scholar] [CrossRef]
- Saha, A.; Blando, J.; Silver, E.; Beltran, L.; Sessler, J.; DiGiovanni, J. 6-shogaol from dried ginger inhibits growth of prostate cancer cells both in vitro and in vivo through inhibition of stat3 and nf-kappab signaling. Cancer Prev. Res. 2014, 7, 627–638. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Baghdadi, A.; Tayebi-Meigooni, A. Formation of 6-, 8- and 10-shogaol in ginger through application of different drying methods: Altered antioxidant and antimicrobial activity. Molecules 2018, 23, 1646. [Google Scholar] [CrossRef] [PubMed]
- RSC. Chemspider—Real Society of Chemistry. Available online: www.chemspider.com (accessed on 5 July 2019).
- Ha, S.K.; Moon, E.; Ju, M.S.; Kim, D.H.; Ryu, J.H.; Oh, M.S.; Kim, S.Y. 6-shogaol, a ginger product, modulates neuroinflammation: A new approach to neuroprotection. Neuropharmacology 2012, 63, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Y.; Chiang, B.H. 6-shogaol induces autophagic cell death then triggered apoptosis in colorectal adenocarcinoma ht-29 cells. Biomed. Pharmacother. 2017, 93, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Nedungadi, D.; Binoy, A.; Pandurangan, N.; Pal, S.; Nair, B.G.; Mishra, N. 6-shogaol induces caspase-independent paraptosis in cancer cells via proteasomal inhibition. Exp. Cell Res. 2018, 364, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, G.; Kathiresan, S.; Kannappan, N. [6]-shogaol, a dietary phenolic compound, induces oxidative stress mediated mitochondrial dependant apoptosis through activation of proapoptotic factors in hep-2 cells. Biomed. Pharmacother. 2016, 82, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Wang, X.; Ji, R.; Liu, L.; Qiao, Y.; Lou, Z.; Ma, C.; Li, S.; Wang, H.; Ho, C.T. Occurrence, biological activity and metabolism of 6-shogaol. Food & function 2018, 9, 1310–1327. [Google Scholar]
- Zhu, Y.; Warin, R.F.; Soroka, D.N.; Chen, H.; Sang, S. Metabolites of ginger component [6]-shogaol remain bioactive in cancer cells and have low toxicity in normal cells: Chemical synthesis and biological evaluation. PLoS ONE 2013, 8, e54677. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.N. Comparative estudies in relation to the structure and biochemical properties of the active compounds in the volatile and nonvolatile fractions of turmeric (c. Longa) and ginger (z. Officinale). Stud. Nat. Prod. Chem. 2016, 48, 101–135. [Google Scholar]
- Kumari, S.; Badana, A.K.; G, M.M.; G, S.; Malla, R. Reactive oxygen species: A key constituent in cancer survival. Biomark. Insights 2018, 13, 1177271918755391. [Google Scholar] [CrossRef] [PubMed]
- Han, M.A.; Woo, S.M.; Min, K.J.; Kim, S.; Park, J.W.; Kim, D.E.; Kim, S.H.; Choi, Y.H.; Kwon, T.K. 6-shogaol enhances renal carcinoma caki cells to trail-induced apoptosis through reactive oxygen species-mediated cytochrome c release and down-regulation of c-flip(l) expression. Chem. Boil. Interact. 2015, 228, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.K.; Tsai, Y.H.; Korinek, M.; Hung, P.H.; El-Shazly, M.; Cheng, Y.B.; Wu, Y.C.; Hsieh, T.J.; Chang, F.R. 6-paradol and 6-shogaol, the pungent compounds of ginger, promote glucose utilization in adipocytes and myotubes, and 6-paradol reduces blood glucose in high-fat diet-fed mice. Int. J. mol. Sci. 2017, 18, 168. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Kim, N.; Lee, H.J.; Moon, J.W.; Lee, S.K.; Kim, S.J.; Kim, J.K.; Park, S.H.; Kim, H.S. [6]-gingerol affects glucose metabolism by dual regulation via the ampkalpha2-mediated as160-rab5 pathway and ampk-mediated insulin sensitizing effects. J. Cell. Biochem. 2015, 116, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Keating, E.; Martel, F. Antimetabolic effects of polyphenols in breast cancer cells: Focus on glucose uptake and metabolism. Front Nutr. 2018, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Blaszczak, W.; Barczak, W.; Masternak, J.; Kopczynski, P.; Zhitkovich, A.; Rubis, B. Vitamin c as a modulator of the response to cancer therapy. Molecules 2019, 24, 453. [Google Scholar] [CrossRef] [PubMed]
- Pinzón, C.E.; Serrano, M.L.; Sanabria, M.C. Papel de la vía fosfatidilinositol 3 kinasa (pi3k/akt) en humanos. Rev. Cienc. Salud 2009, 7, 47–66. [Google Scholar]
- Li, W.; Hou, J.Z.; Niu, J.; Xi, Z.Q.; Ma, C.; Sun, H.; Wang, C.J.; Fang, D.; Li, Q.; Xie, S.Q. Akt1 inhibition promotes breast cancer metastasis through egfr-mediated beta-catenin nuclear accumulation. Cell Commun Signal. 2018, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Al-Saffar, N.M.S.; Troy, H.; Wong Te Fong, A.C.; Paravati, R.; Jackson, L.E.; Gowan, S.; Boult, J.K.R.; Robinson, S.P.; Eccles, S.A.; Yap, T.A.; et al. Metabolic biomarkers of response to the akt inhibitor mk-2206 in pre-clinical models of human colorectal and prostate carcinoma. Br. J. Cancer 2018, 119, 1118–1128. [Google Scholar] [CrossRef]
- Chetram, M.A.; Bethea, D.A.; Odero-Marah, V.A.; Don-Salu-Hewage, A.S.; Jones, K.J.; Hinton, C.V. Ros-mediated activation of akt induces apoptosis via pvhl in prostate cancer cells. Mol. Cell. Biochem. 2013, 376, 63–71. [Google Scholar] [CrossRef]
- Huy, H.; Song, H.Y.; Kim, M.J.; Kim, W.S.; Kim, D.O.; Byun, J.E.; Lee, J.; Park, Y.J.; Kim, T.D.; Yoon, S.R.; et al. Txnip regulates akt-mediated cellular senescence by direct interaction under glucose-mediated metabolic stress. Aging Cell 2018, 17, e12836. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A.; Kumari, A.; Gulati, A.; Padwad, Y.; Sharma, R. Epigallocatechin gallate suppresses premature senescence of preadipocytes by inhibition of pi3k/akt/mtor pathway and induces senescent cell death by regulation of bax/bcl-2 pathway. Biogerontology 2018, 20, 171–189. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. Ros, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [PubMed]
- Graham, N.A.; Tahmasian, M.; Kohli, B.; Komisopoulou, E.; Zhu, M.; Vivanco, I.; Teitell, M.A.; Wu, H.; Ribas, A.; Lo, R.S.; et al. Glucose deprivation activates a metabolic and signaling amplification loop leading to cell death. Mol. Syst. Boil. 2012, 8, 589. [Google Scholar] [CrossRef] [PubMed]
- van Tonder, A.; Joubert, A.M.; Cromarty, A.D. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2h-tetrazolium bromide (mtt) assay when compared to three commonly used cell enumeration assays. BMC Res. Notes 2015, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Wang, Y.; Shen, Z. 2-nbdg as a fluorescent indicator for direct glucose uptake measurement. J. Biochem. Bioph. Meth. 2005, 64, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Romero-Arias, A.C. Efecto del 6-shogaol sobre la captación de glucosa y supervivencia de células tumorales derivadas de fibrosarcoma humano (ht1080). Doctorado En Ciencias Biológicas, Pontificia Universidad Javeriana, Bogotá, Colombia, 2018. [Google Scholar]
- Huang, H.; Li, H.; Shi, K.; Wang, L.; Zhang, X.; Zhu, X. Trektraak twopore domain potassium channels protect human retinal pigment epithelium cells from oxidative stress. Int. J. Mol. Med. 2018, 42, 2584–2594. [Google Scholar] [PubMed]
- Myers, J.L.; Well, A.D.; Lorch, R.F. Research Design and Statistical Analysis, 3rd ed.; Chapman and Hall: London, UK, 2013; pp. 167–306. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Arias, A.C.; Sequeda-Castañeda, L.G.; Aristizábal-Pachón, A.F.; Morales, L. Effect of 6-Shogaol on the Glucose Uptake and Survival of HT1080 Fibrosarcoma Cells. Pharmaceuticals 2019, 12, 131. https://doi.org/10.3390/ph12030131
Romero-Arias AC, Sequeda-Castañeda LG, Aristizábal-Pachón AF, Morales L. Effect of 6-Shogaol on the Glucose Uptake and Survival of HT1080 Fibrosarcoma Cells. Pharmaceuticals. 2019; 12(3):131. https://doi.org/10.3390/ph12030131
Chicago/Turabian StyleRomero-Arias, Angie C., Luis G. Sequeda-Castañeda, Andres F. Aristizábal-Pachón, and Ludis Morales. 2019. "Effect of 6-Shogaol on the Glucose Uptake and Survival of HT1080 Fibrosarcoma Cells" Pharmaceuticals 12, no. 3: 131. https://doi.org/10.3390/ph12030131
APA StyleRomero-Arias, A. C., Sequeda-Castañeda, L. G., Aristizábal-Pachón, A. F., & Morales, L. (2019). Effect of 6-Shogaol on the Glucose Uptake and Survival of HT1080 Fibrosarcoma Cells. Pharmaceuticals, 12(3), 131. https://doi.org/10.3390/ph12030131