Safety Pharmacological Evaluation of the Coffee Component, Caffeoylquinic Acid, and Its Metabolites, Using Ex Vivo and In Vitro Profiling Assays
Abstract
1. Introduction
2. Results
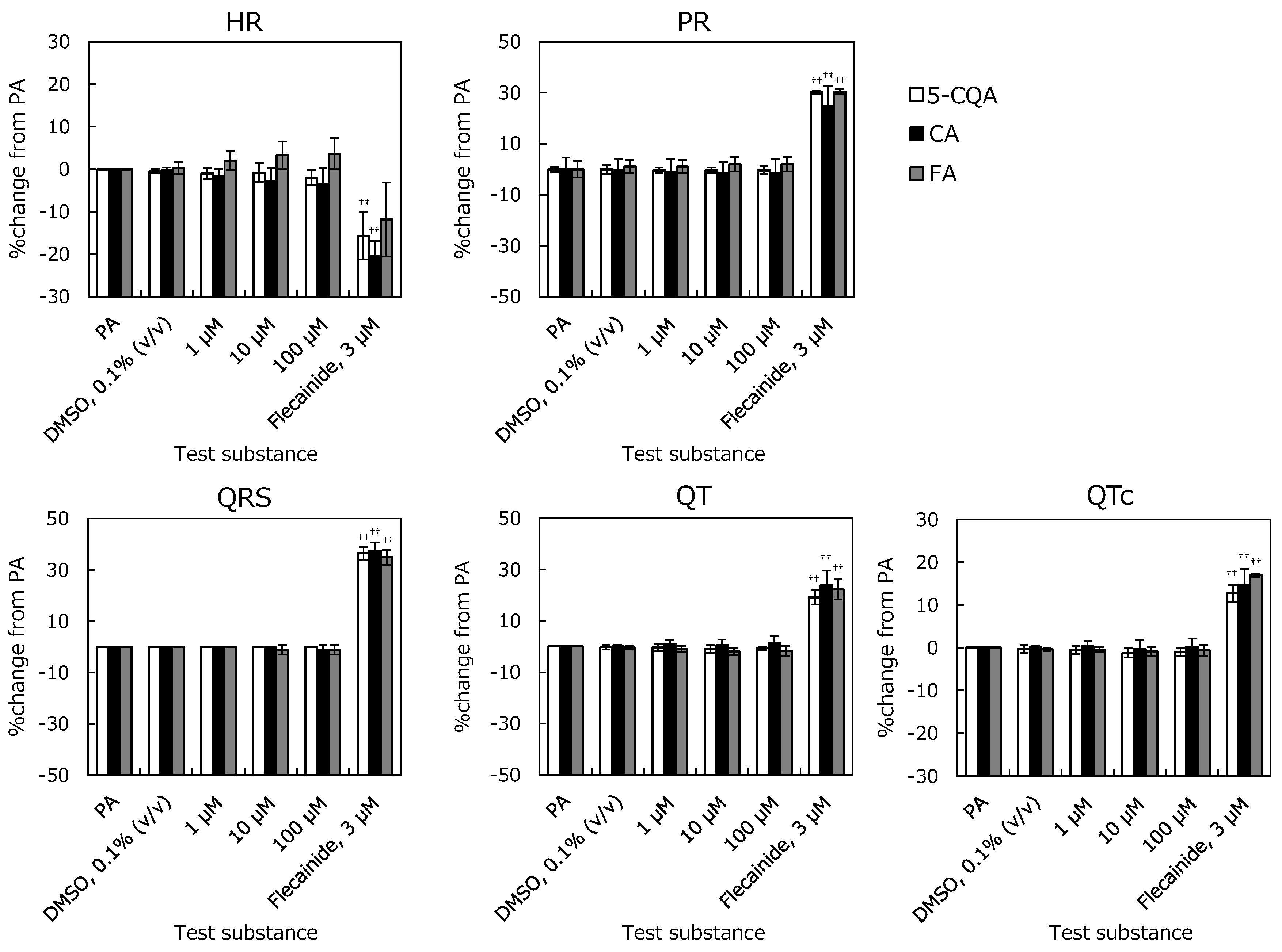
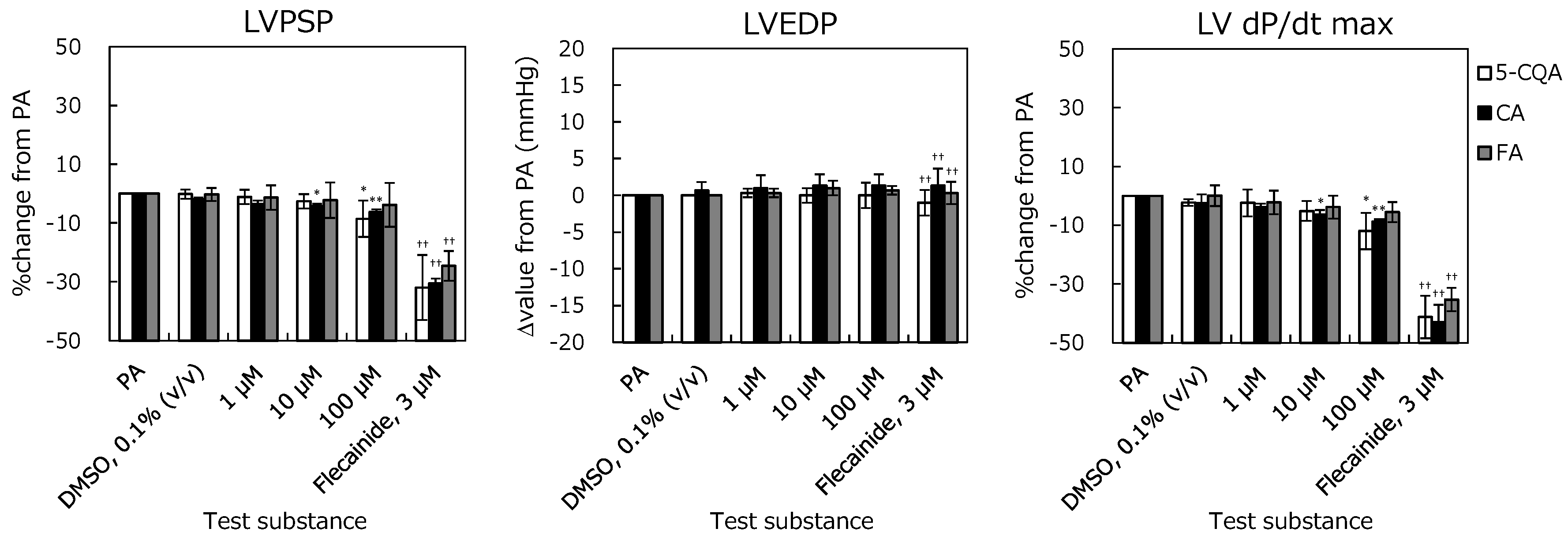
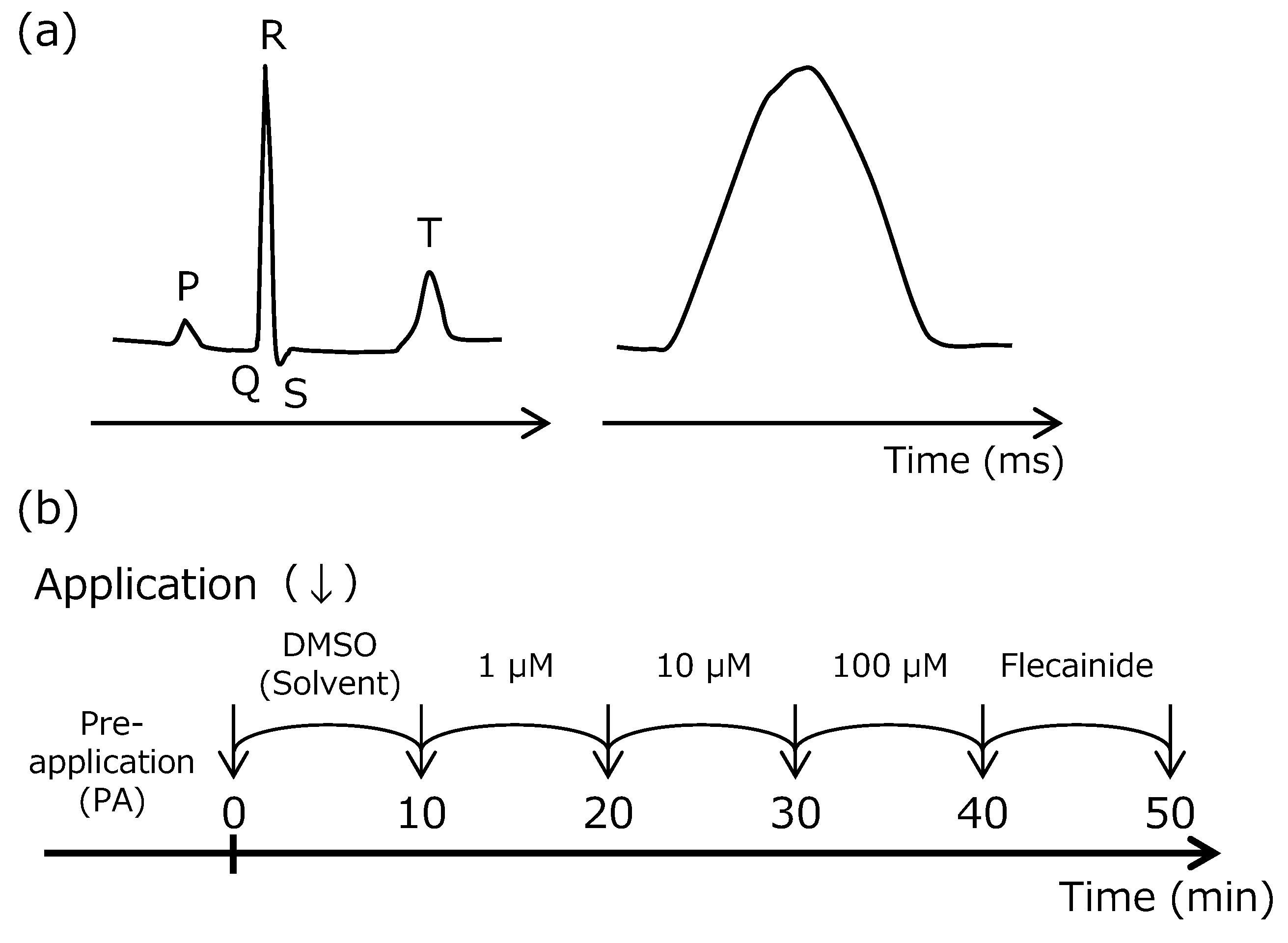
2.1. Langendorff Perfused Heart Assay
2.2. Electrophysiological Assays of Rat Hippocampal Slices
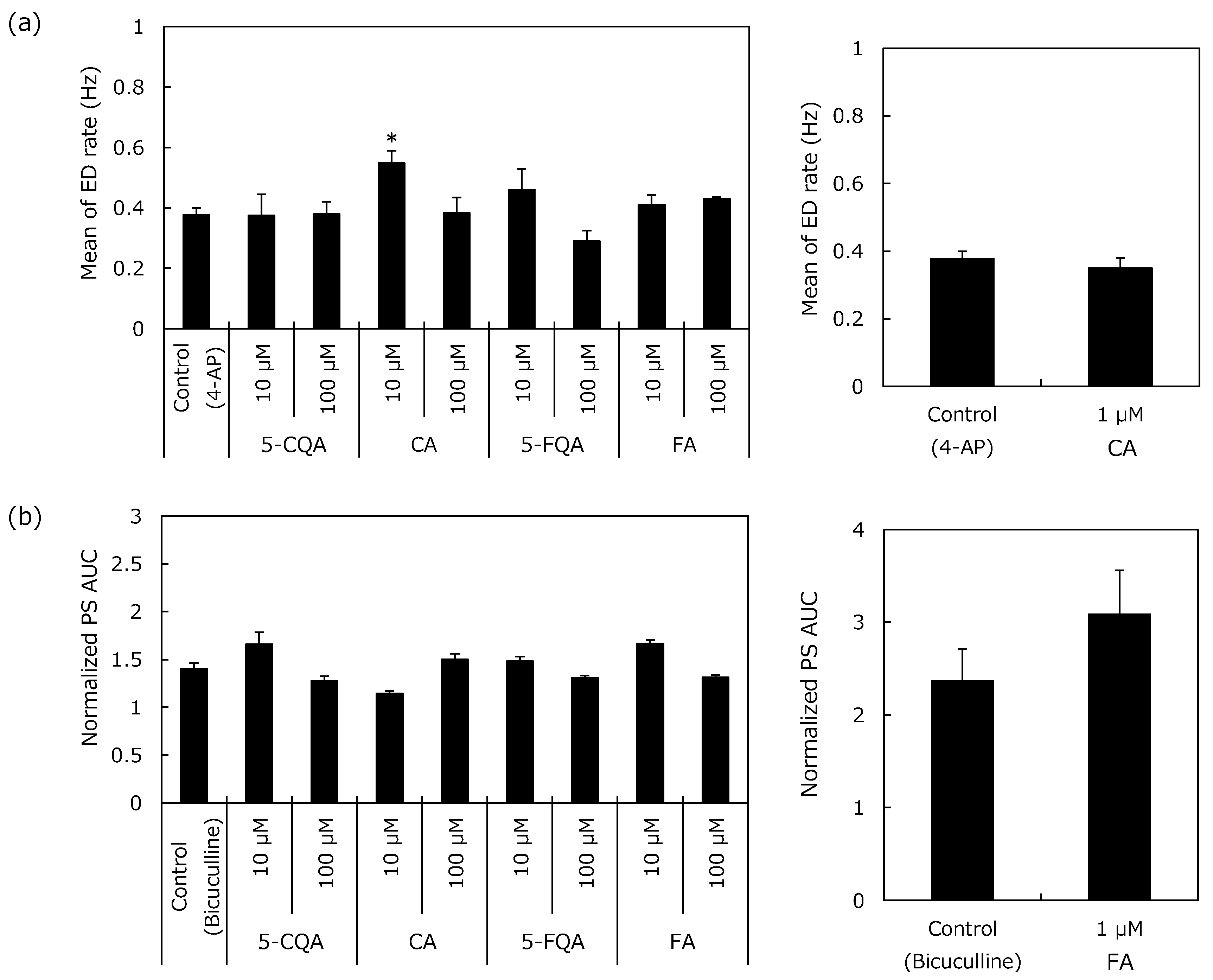
2.2.1. Epileptiform Discharges (ED) Rate
2.2.2. Population Spikes (PS) Recording
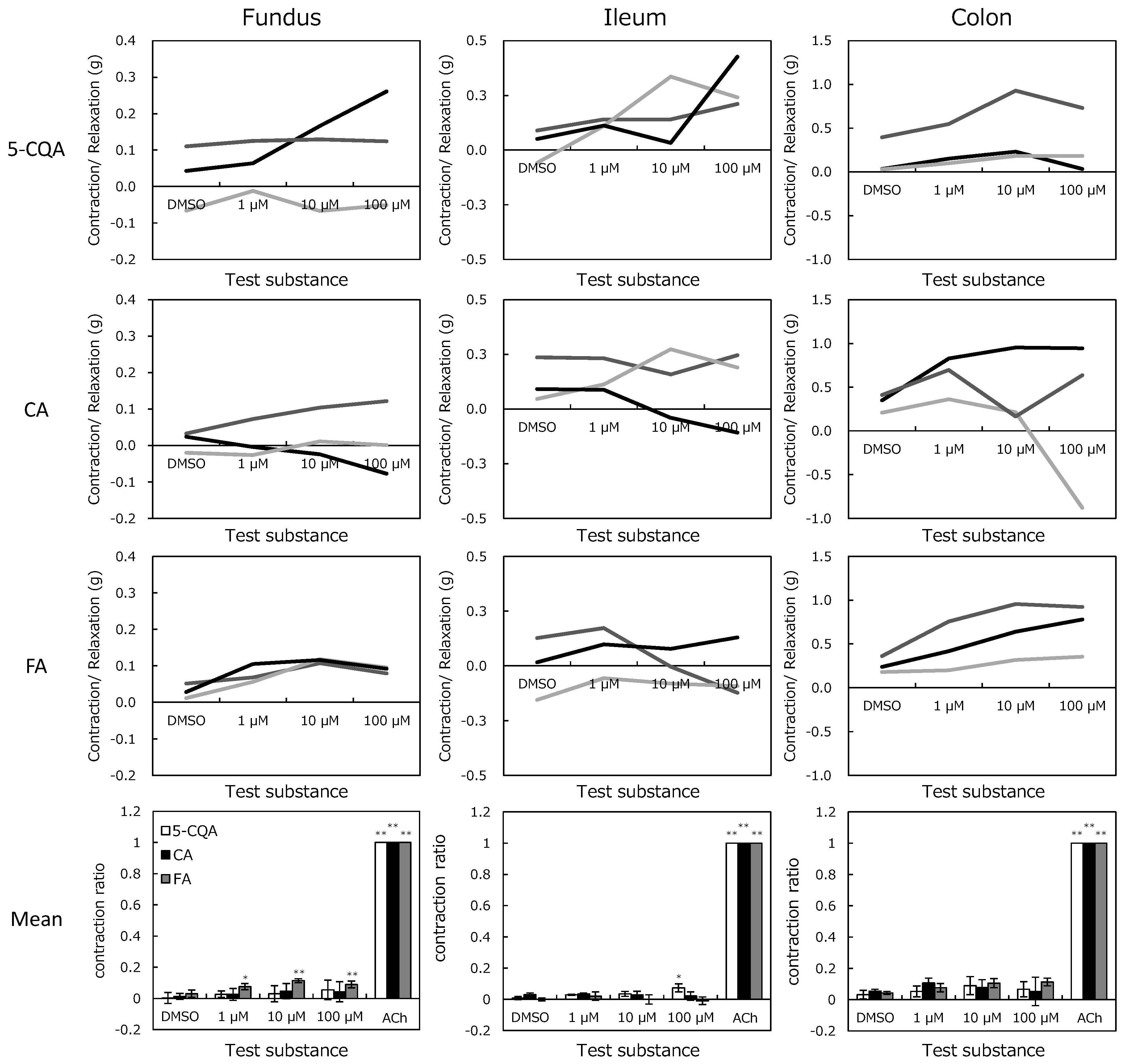
2.3. In Vitro Magnus Assay
2.4. In Vitro Profiling Assay
3. Discussion
4. Materials and Methods
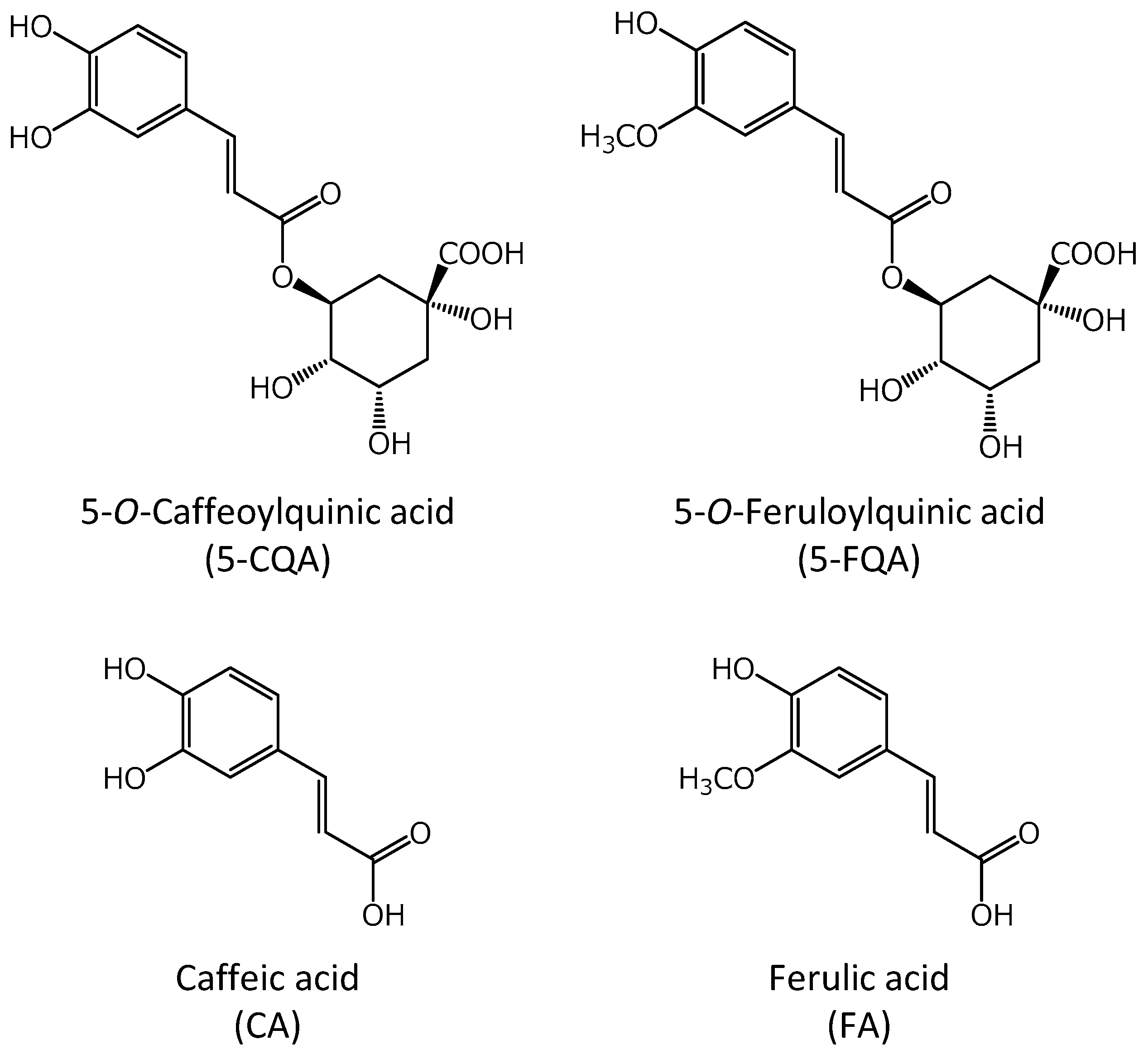
4.1. Chemical Agents
4.2. Ex Vivo Assays
4.2.1. Langendorff Perfused Heart Assay
4.2.2. Electrophysiological Assays
Preparation of the Hippocampal Slices
ED Recording
PS Recording
4.3. In Vitro Magnus Assay
4.4. In Vitro Profiling Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, A.C. An overview of herb and dietary supplement efficacy, safety and government regulations in the United States with suggested improvements. Part 1 of 5 series. Food Chem. Toxicol. 2017, 107, 449–471. [Google Scholar] [CrossRef] [PubMed]
- Lystrup, R.M.; Leggit, J.C. Caffeine Toxicity Due to Supplement Use in Caffeine—Naïve Individual: A Cautionary Tale. Mil. Med. 2015, 180, e936–e940. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Expert committee on Novel Foods, Japan. Basic Approach of the Safety Assessment of Food for Specified Health Uses (FOSHU). 2004; pp. 1–6. Available online: https://www.fsc.go.jp/senmon/sinkaihatu/index.data/FOSHU_for_HP_180530.pdf (accessed on 1 July 2019).
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Bes-Rastrollo, M.; Galvano, F.; Martinez-Gonzalez, M.A. Long-term coffee consumption is associated with decreased incidence of new-onset hypertension: A dose–response meta-analysis. Nutrients 2017, 9, 890. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Bhupathiraju, S.N.; Satija, A.; Van Dam, R.M.; Hu, F.B. Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 2014, 129, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Solfrizzi, V.; Barulli, M.R.; Bonfiglio, C.; Guerra, V.; Osella, A.; Seripa, D.; Sabbà, C.; Pilotto, A.; Logroscino, G. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: A systematic review. J. Nutr. Health Aging 2015, 19, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Clifford, M.N.; Lean, M.E.J.; Ashihara, H.; Crozier, A. Coffee: Biochemistry and potential impact on health. Food Funct. 2014, 5, 1695–1717. [Google Scholar] [CrossRef] [PubMed]
- Vanderveen, J.E.; Armstrong, L.E.; Butterfield, G.E.; Chenoweth, W.L.; Dwyer, J.T.; Fernstrom, J.D.; Kanarek, R.B.; Levander, O.A.; Sternberg, E.M. Caffeine for the Sustainment of Mental Task Performance: Formulations for Military Operations; National Academy Press: Washington, DC, USA, 2001; ISBN 0309565944. [Google Scholar]
- Borota, D.; Murray, E.; Keceli, G.; Chang, A.; Watabe, J.M.; Ly, M.; Toscano, J.P.; Yassa, M.A. Post-study caffeine administration enhances memory consolidation in humans. Nat. Neurosci. 2014, 17, 201–203. [Google Scholar] [CrossRef]
- Heckman, M.A.; Weil, J.; de Mejia, E.G. Caffeine (1, 3, 7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 2010, 75, 77–87. [Google Scholar] [CrossRef]
- Maalik, A.; Bukhari, S.M.; Zaidi, A.; Shah, K.H.; Khan, F.A. Chlorogenic acid: A pharmacologically potent molecule. Acta Pol. Pharm. Drug Res. 2016, 73, 851–854. [Google Scholar]
- Ota, N.; Soga, S.; Murase, T.; Shimotoyodome, A.; Hase, T. Consumption of Coffee Polyphenols Increases Fat Utilization in Humans. J. Health Sci. 2010, 56, 745–751. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Yamamoto, M.; Misawa, K.; Nishimura, H.; Misawa, K.; Ota, N.; Shimotoyodome, A. Coffee polyphenols prevent cognitive dysfunction and suppress amyloid β plaques in APP/PS2 transgenic mouse. Neurosci. Res. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.E.; Flury, A.; Marmet, C.; Poquet, L.; Rimoldi, S.F.; Sartori, C.; Rexhaj, E.; Brenner, R.; Allemann, Y.; Zimmermann, D.; et al. Mediation of coffee-induced improvements in human vascular function by chlorogenic acids and its metabolites: Two randomized, controlled, crossover intervention trials. Clin. Nutr. 2017, 36, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Ochiai, R.; Kozuma, K.; Sato, H.; Katsuragi, Y. Effect of Chlorogenic Acid Intake on Cognitive Function in the Elderly: A Pilot Study. Evid.-Based Complement. Altern. Med. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Satsu, H.; Bae, M.J.; Zhao, Z.; Ogiwara, H.; Totsuka, M.; Shimizu, M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chem. 2015, 168, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef]
- Tošović, J.; Marković, S.; Dimitrić Marković, J.M.; Mojović, M.; Milenković, D. Antioxidative mechanisms in chlorogenic acid. Food Chem. 2017, 237, 390–398. [Google Scholar] [CrossRef]
- Peng, J.; Leng, J.; Tian, H.; Yang, T.; Fang, Y.; Feng, Q.; Zhao, Y.; Hu, Y. Geniposide and Chlorogenic Acid Combination Ameliorates Non-alcoholic Steatohepatitis Involving the Protection on the Gut Barrier Function in Mouse Induced by High-Fat Diet. Front. Pharmacol. 2018, 9, 1399. [Google Scholar] [CrossRef]
- Yun, N.; Kang, J.W.; Lee, S.M. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: Molecular evidence of its antioxidant and anti-inflammatory properties. J. Nutr. Biochem. 2012, 23, 1249–1255. [Google Scholar] [CrossRef]
- Belkaid, A.; Currie, J.C.; Desgagnés, J.; Annabi, B. The chemopreventive properties of chlorogenic acid reveal a potential new role for the microsomal glucose-6-phosphate translocase in brain tumor progression. Cancer Cell Int. 2006, 6, 1–12. [Google Scholar] [CrossRef]
- Lukitasari, M.; Nugroho, D.A.; Widodo, N. Chlorogenic Acid: The Conceivable Chemosensitizer Leading to Cancer Growth Suppression. J. Evid.-Based Integr. Med. 2018, 23, 1–6. [Google Scholar] [CrossRef] [PubMed]
- ICH Expert Working Group. Safety Pharmacology Studies for Human Pharmaceuticals S7A; ICH: Geneva, Switzerland, 2000. [Google Scholar]
- Schulz, M.; Iwersen-Bergmann, S.; Andresen, H.; Schmoldt, A. Therapeutic and toxic blood concentrations of nearly 1,000 drugs and other xenobiotics. Crit. Care 2012, 16, 1–69. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.; Obejero-Paz, C.A.; Myatt, G.; Kuryshev, Y.A.; Bruening-Wright, A.; Verducci, J.S.; Brown, A.M. MICE models: Superior to the HERG model in predicting torsade de pointes. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Dong, Z.; Guthrie, H. Validation of a guinea pig Langendorff heart model for assessing potential cardiovascular liability of drug candidates. J. Pharmacol. Toxicol. Methods 2009, 60, 130–151. [Google Scholar] [CrossRef] [PubMed]
- Bowes, J.; Brown, A.J.; Hamon, J.; Jarolimek, W.; Sridhar, A.; Waldron, G.; Whitebread, S. Reducing safety-related drug attrition: the use of in vitro pharmacological profiling. Nat. Rev. Drug Discov. 2012, 11, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Mullen, W.; Barron, D.; Uchida, K.; Yokota, T.; Cavin, C.; Steiling, H.; Williamson, G.; Crozier, A. Metabolite profiling of hydroxycinnamate derivatives in plasma and urine after the ingestion of coffee by humans: identification of biomarkers of coffee consumption. Drug Metab. Dispos. 2009, 37, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Williamson, G.; Crozier, A. Impact of dose on the bioavailability of coffee chlorogenic acids in humans. Food Funct. 2014, 5, 1727–1737. [Google Scholar] [CrossRef]
- Figueira, I.; Tavares, L.; Jardim, C.; Costa, I.; Terrasso, A.P.; Almeida, A.F.; Govers, C.; Mes, J.J.; Gardner, R.; Becker, J.D.; et al. Blood–brain barrier transport and neuroprotective potential of blackberry-digested polyphenols: an in vitro study. Eur. J. Nutr. 2017, 58, 1–18. [Google Scholar] [CrossRef]
- Macedo, D.; Costa, I.; dos Santos, C.N.; Menezes, R.; Figueira, I. Polyphenols Beyond Barriers: A Glimpse into the Brain. Curr. Neuropharmacol. 2016, 15, 562–594. [Google Scholar]
- Badary, O.A.; Awad, A.S.; Sherief, M.A.; Hamada, F.M. In vitro and in vivo effects of ferulic acid on gastrointestinal motility: inhibition of cisplatin-induced delay in gastric emptying in rats. World J. Gastroenterol. 2006, 12, 5363–5367. [Google Scholar] [CrossRef]
- Zeiger, E. Review of Toxicological Literature. Chlorogenic Acid [327-97-9] and Caffeic Acid [331-39-5]. Natl. Inst. Environ. Health Sci. 1998, 1–120. [Google Scholar]
- Hagiwara, A.; Hirose, M.; Takahashi, S.; Ogawa, K.; Shirai, T.; Ito, N. Forestomach and Kidney Carcinogenicity of Caffeic Acid in F344 Rats and C57BL/6N × C3H/HeN F1 Mice. Cancer Res. 1991, 51, 5655–5660. [Google Scholar] [PubMed]
- Murase, T.; Yokoi, Y.; Misawa, K.; Ominami, H.; Suzuki, Y.; Shibuya, Y.; Hase, T. Coffee polyphenols modulate whole-body substrate oxidation and suppress postprandial hyperglycaemia, hyperinsulinaemia and hyperlipidaemia. Br. J. Nutr. 2012, 107, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Xia, E.; He, X.; Li, H.; Wu, S.; Li, S.; Deng, G. Biological Activities of Polyphenols from Grapes. Polyphen. Hum. Health Dis. 2013, 1, 47–58. [Google Scholar]
- Rasines-Perea, Z.; Teissedre, P.L. Grape Polyphenols’ effects in human cardiovascular diseases and diabetes. Molecules 2017, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, K.; Piche, M.; Tai, T.; Hollingsworth, A.; Khurana, S. Polyphenols: Benefits to the Cardiovascular System in Health and in Aging. Nutrients 2013, 5, 3779–3827. [Google Scholar]
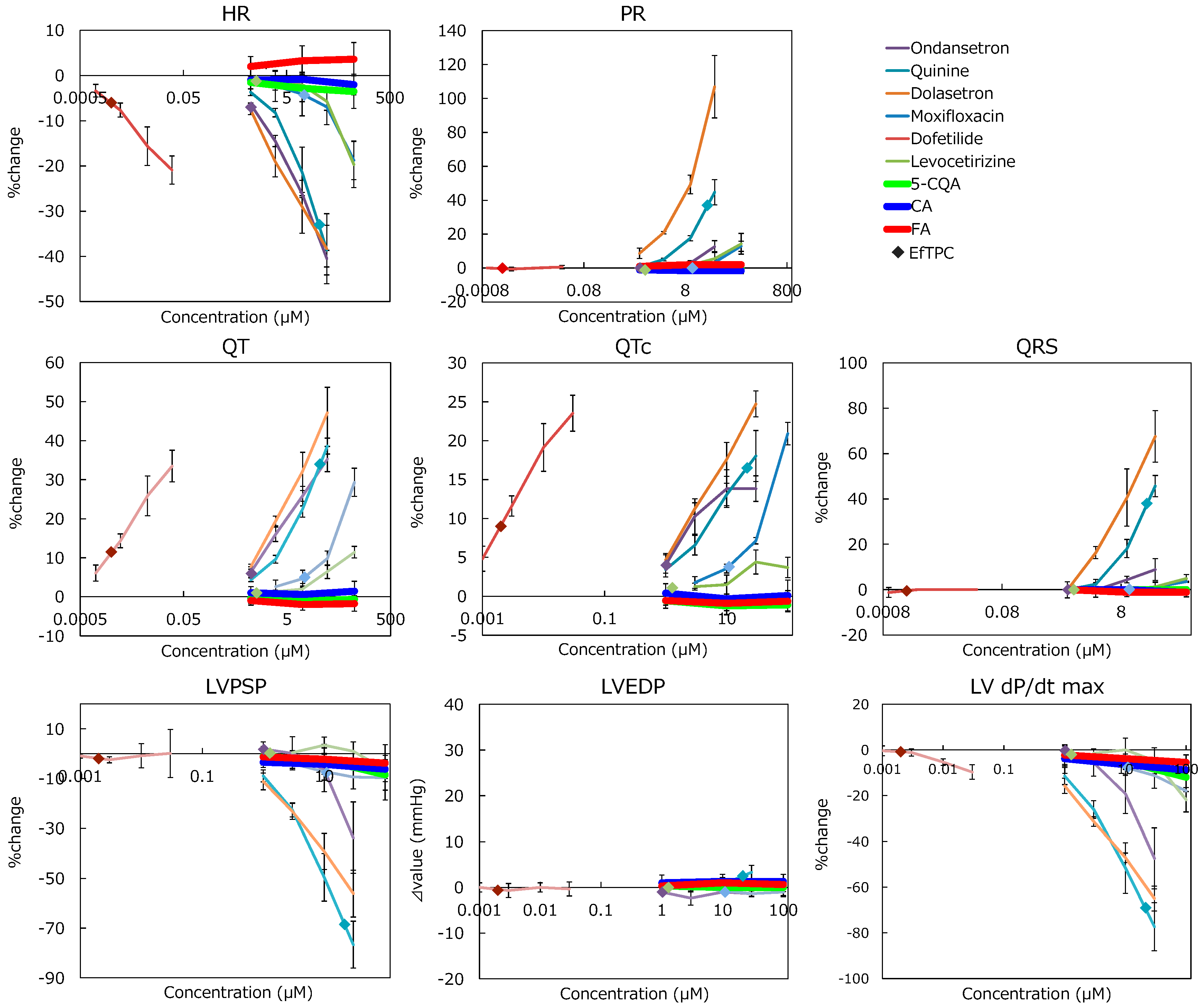
| Drug Name | HR | PR | QRS | QT | QTc | LVPSP | LVEDP | LV dP/dtmax | EfTPC |
|---|---|---|---|---|---|---|---|---|---|
| Ondansetron | 1 | 10 | 10 | 1 | 1 | 1 | 1 | 1 | 1.023 [25] |
| Quinine | 1 | 3 | 3 | 1 | 1 | 1 | 30 | 1 | 21.577 [25] |
| Dolasetron | 1 | 1 | 3 | 1 | 1 | 1 | - | 1 | - |
| Moxifloxacin | 30 | 30 | 10 | 3 | 3 | 3 | - | 100 | 10.960 [26] |
| Dofetilide | 0.001 | 0.01 | - | 0.001 | 0.001 | 0.03 | - | - | 0.020 [27] |
| Levocetirizine | 30 | 10 | 100 | 30 | 3 | 10 | - | 100 | 1.286 [25] |
| Target Name | Assay Type | Species | Main Organ Class or System | % Inhibition | |||
|---|---|---|---|---|---|---|---|
| CGA | CA | FQA | FA | ||||
| Adenosine A2A | B | human | CVS, CNS | −7 | 4 | 2 | −14 |
| Adrenergic α1A | B | rat | CVS, GI, CNS | 14 | 10 | 10 | −2 |
| Adrenergic α2A | B | human | CVS, CNS | 5 | −4 | −3 | −2 |
| Adrenergic β1 | B | human | CVS, GI | −1 | 3 | −4 | −2 |
| Adrenergic β2 | B | human | CVS | 1 | 2 | 6 | 2 |
| Calcium Channel L-Type, Benzothiazepine | B | rat | CVS | −3 | −3 | 6 | 6 |
| Calcium Channel L-Type, Dihydropyridine | B | rat | CVS | 1 | 7 | 6 | 1 |
| Calcium Channel L-Type, Phenylalkylamine | B | rat | CVS | 14 | 21 | 19 | 26 |
| Cannabinoid CB1 | B | human | CNS | −6 | −9 | −6 | −15 |
| Cholecystokinin CCK1 (CCKA) | B | human | GI | 10 | 12 | 7 | 1 |
| Cyclooxygenase COX-1 | F | human | GI | 2 | 6 | 11 | 5 |
| Cyclooxygenase COX-2 | F | human | CVS | 7 | 9 | 18 | 14 |
| Dopamine D1 | B | human | CVS, CNS | 7 | 5 | 22 | −1 |
| Dopamine D2L | B | human | CVS, CNS | 7 | 16 | −8 | 9 |
| Endothelin ETA | B | human | CVS | 3 | 4 | 1 | 1 |
| GABAA | B | rat | CNS | 8 | 18 | 15 | 1 |
| Glutamate, NMDA | B | rat | CNS | 9 | 5 | 10 | 16 |
| Histamine H1 | B | human | CVS | −12 | −11 | −22 | −9 |
| Histamine H2 | B | human | GI, CVS | 0 | −6 | −5 | −5 |
| Monoamine Oxidase MAO-A | F | human | CVS, CNS | −5 | 0 | 0 | 5 |
| Muscarinic M1 | B | human | CNS, GI, CVS | 0 | 5 | −5 | 0 |
| Muscarinic M2 | B | human | CVS | −1 | 23 | 7 | 17 |
| Muscarinic M3 | B | human | GI | 5 | −3 | 2 | −1 |
| Opiate δ1 | B | human | CNS, CVS | 5 | 11 | 2 | 16 |
| Opiate κ | B | human | GI, CNS, CVS | −3 | 3 | −7 | 3 |
| Opiate μ | B | human | CNS, GI, CVS | 8 | −6 | 5 | 4 |
| Peptidase, CTSG (Cathepsin G) | F | human | CVS | 8 | 1 | 2 | −2 |
| Phosphodiesterase PDE4 | F | human | CNS | −6 | 4 | 2 | 7 |
| Potassium Channel hERG | B | human | CVS | 3 | −19 | 6 | 7 |
| 5-HT1A | B | human | CNS | 12 | 9 | 11 | 2 |
| 5-HT1B | B | human | CVS, CNS | −1 | 3 | −10 | 4 |
| 5-HT2A | B | human | CVS, CNS | 3 | 7 | 8 | 8 |
| 5-HT2B | B | human | CVS | −16 | 1 | −5 | 5 |
| 5-HT3 | B | human | GI | −9 | −5 | −3 | −13 |
| Sodium Channel | B | rat | CVS | 19 | 1 | 10 | 5 |
| Transporter, Dopamine (DAT) | B | human | CNS | −10 | 2 | 6 | 0 |
| Transporter, Norepinephrine (NET) | B | human | CNS, CVS | −3 | 0 | −1 | 2 |
| Vasopressin V1A | B | human | CVS | 0 | 9 | 16 | 6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amano, Y.; Honda, H.; Nukada, Y.; Ikeda, N.; Yamane, M.; Nakano, K.; Kameyama, A.; Morita, O. Safety Pharmacological Evaluation of the Coffee Component, Caffeoylquinic Acid, and Its Metabolites, Using Ex Vivo and In Vitro Profiling Assays. Pharmaceuticals 2019, 12, 110. https://doi.org/10.3390/ph12030110
Amano Y, Honda H, Nukada Y, Ikeda N, Yamane M, Nakano K, Kameyama A, Morita O. Safety Pharmacological Evaluation of the Coffee Component, Caffeoylquinic Acid, and Its Metabolites, Using Ex Vivo and In Vitro Profiling Assays. Pharmaceuticals. 2019; 12(3):110. https://doi.org/10.3390/ph12030110
Chicago/Turabian StyleAmano, Yuto, Hiroshi Honda, Yuko Nukada, Naohiro Ikeda, Masayuki Yamane, Koji Nakano, Akiyo Kameyama, and Osamu Morita. 2019. "Safety Pharmacological Evaluation of the Coffee Component, Caffeoylquinic Acid, and Its Metabolites, Using Ex Vivo and In Vitro Profiling Assays" Pharmaceuticals 12, no. 3: 110. https://doi.org/10.3390/ph12030110
APA StyleAmano, Y., Honda, H., Nukada, Y., Ikeda, N., Yamane, M., Nakano, K., Kameyama, A., & Morita, O. (2019). Safety Pharmacological Evaluation of the Coffee Component, Caffeoylquinic Acid, and Its Metabolites, Using Ex Vivo and In Vitro Profiling Assays. Pharmaceuticals, 12(3), 110. https://doi.org/10.3390/ph12030110





