Developments in Carbohydrate-Based Cancer Therapeutics
Abstract
1. Introduction
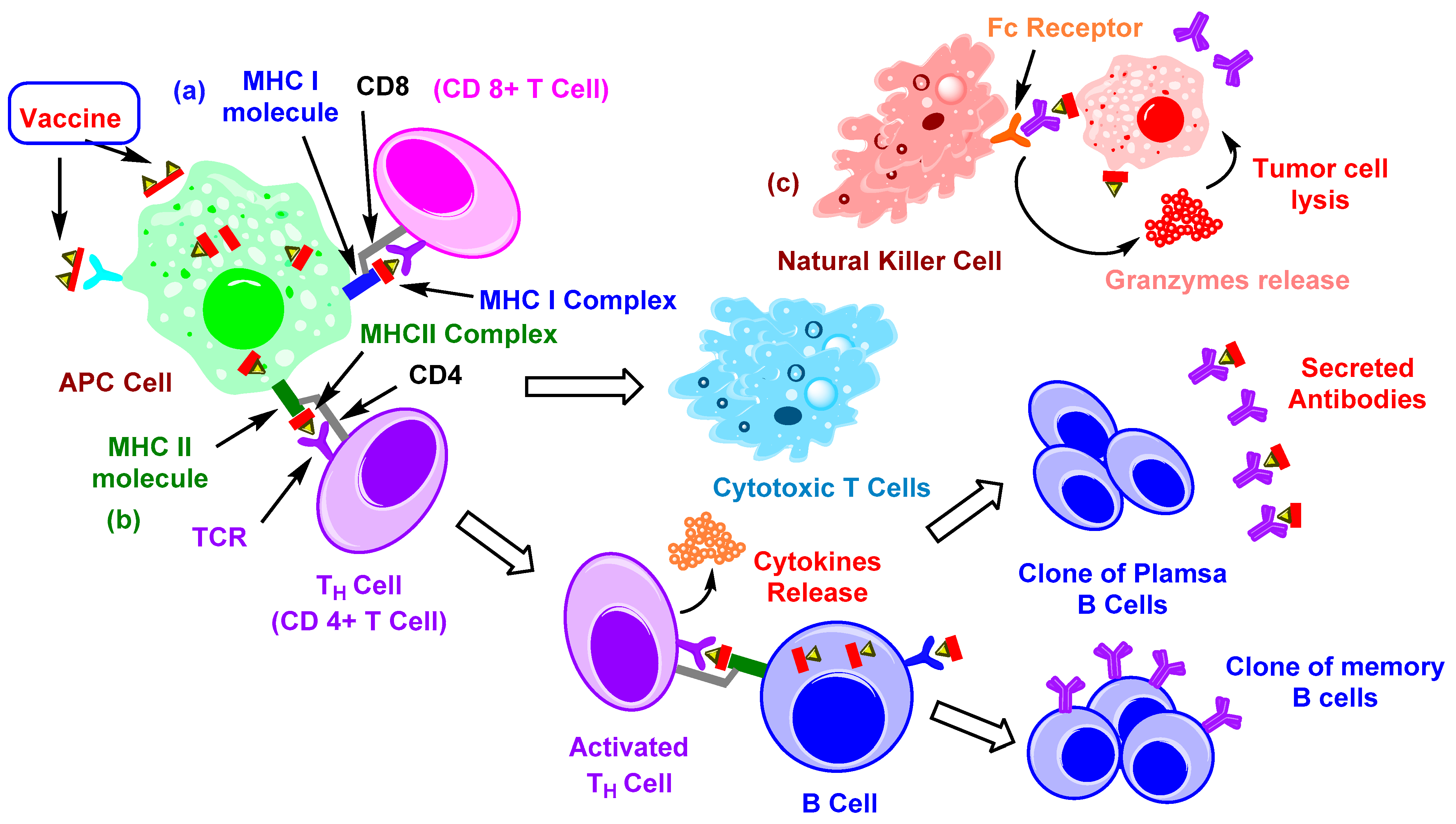
2. Immune Therapy with Carbohydrate-Based Vaccines
2.1. TACAs and Their Immune Response
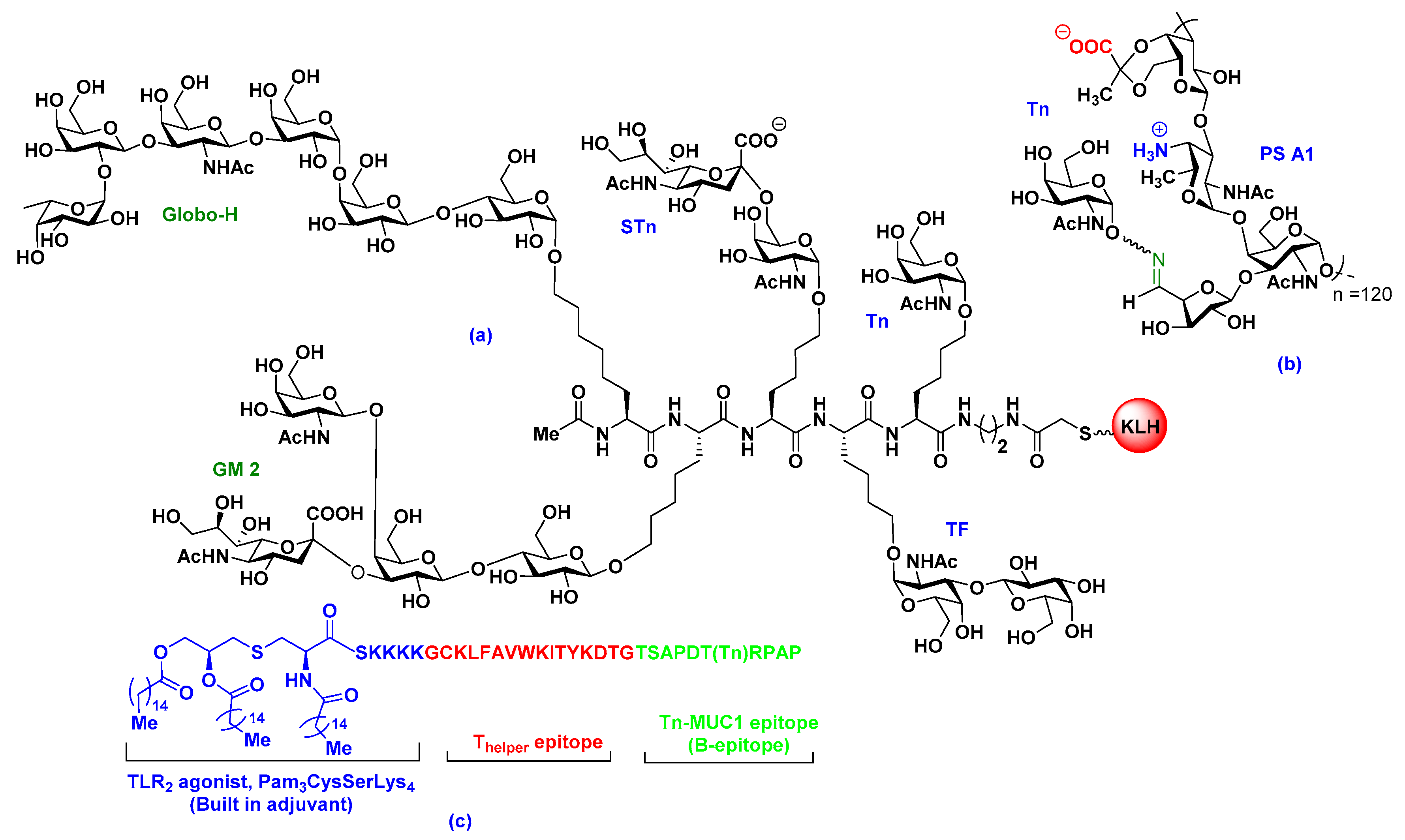
2.2. Carrier-Based Carbohydrate Conjugates
2.3. Fully Synthetic Carbohydrate Vaccines
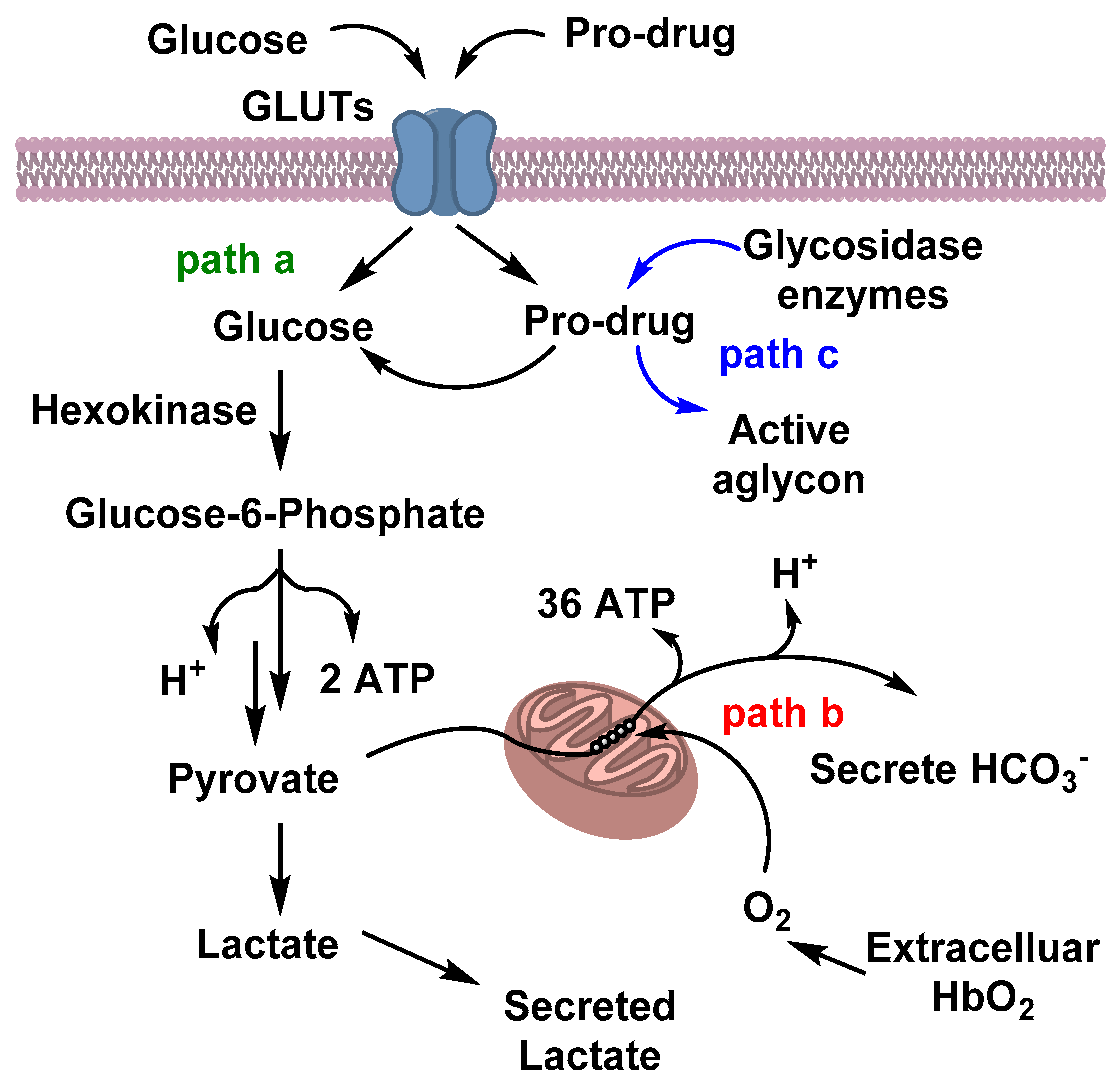
3. Glycosylation for Specific Anticancer Drug Delivery
3.1. Glucose Metabolism in Cancer Cells and Warburg Effects
3.2. Carbohydrate-Based Prodrugs for Specific Targeting
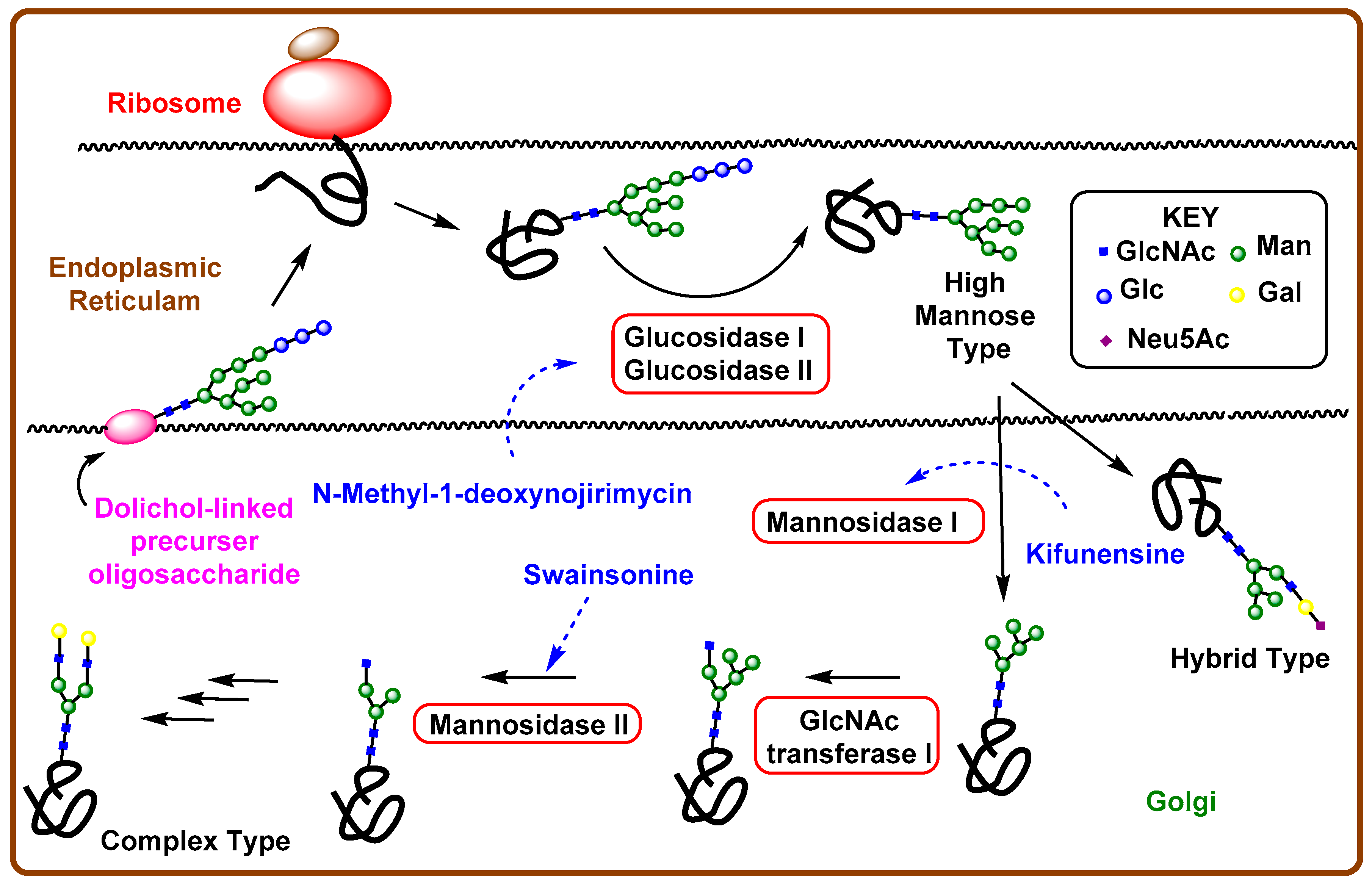
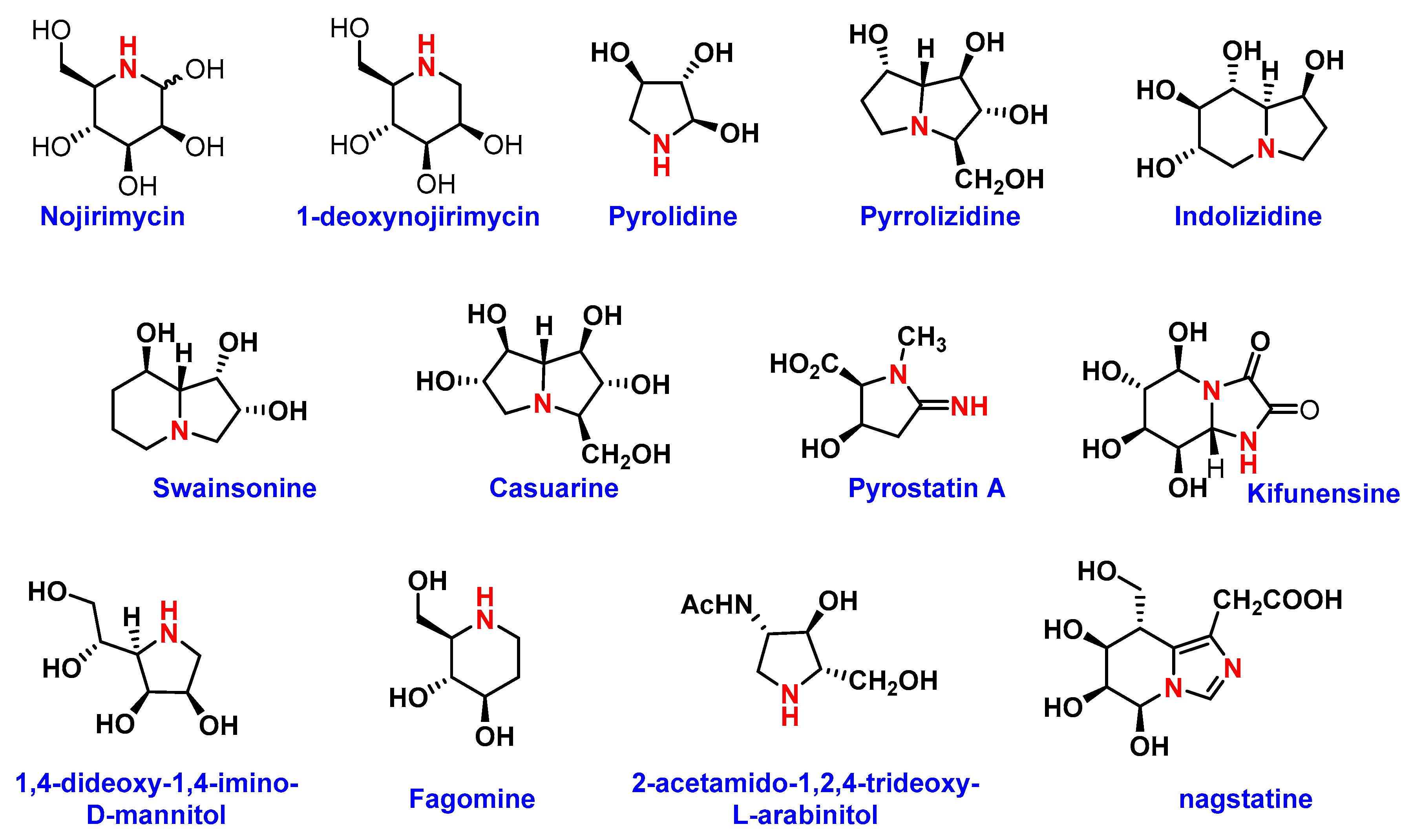
4. Iminosugar Analogs for Cancer Therapy
4.1. Aberrant N-Linked Glycosylation and Inhibition of Glycosidase Enzyme
4.2. Iminosugars as Enzyme Inhibitors
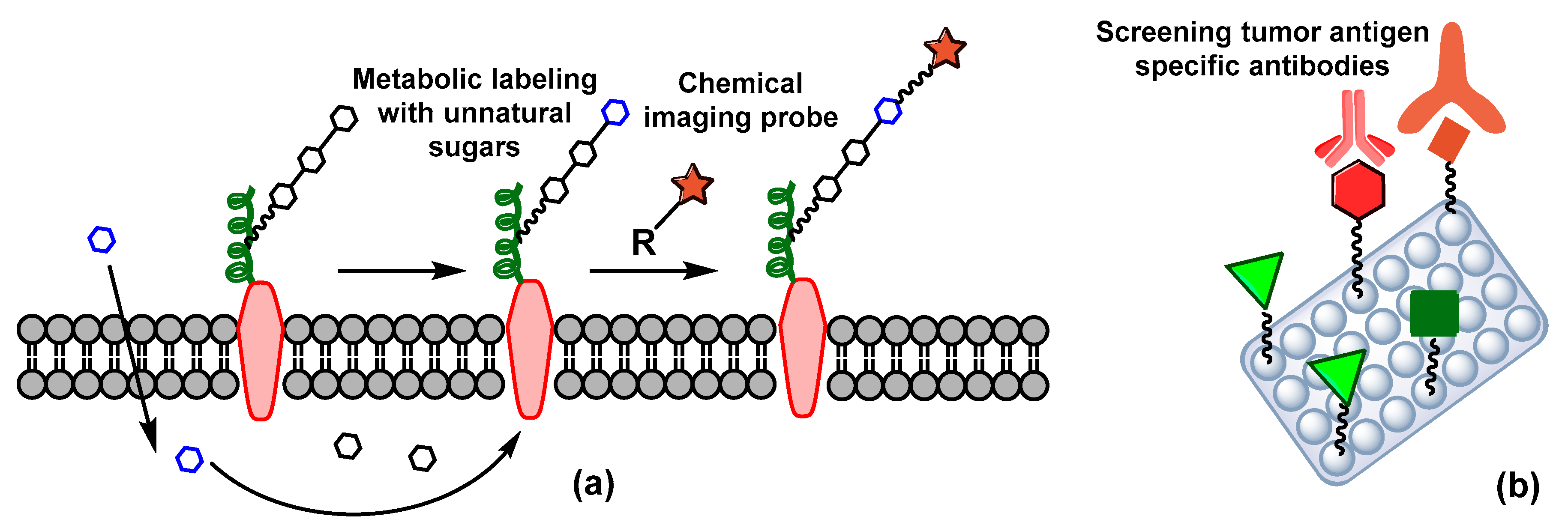
5. Carbohydrate-Based Diagnosis
6. Conclusion
Funding
Conflicts of Interest
References
- Brandley, B.K.; Schnaar, R.L. Cell-Surface Carbohydrates in Cell Recognition and Response. J. Leukocyte Biol. 1986, 40, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Tejada, A.; Canada, F.J.; Jimenez-Barbero, J. Glycans in Medicinal Chemistry: An Underexploited Resource. Chem. Med. Chem. 2015, 10, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Shaikh, A.S.; Wang, F. Recent Advance in Tumor-Associated Carbohydrate Antigens (TACAs)-based Antitumor Vaccines. ACS Chem. Biol. 2016, 11, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, Q. Recent Development in Carbohydrate-Based Cancer Vaccines. Curr. Opin. Chem. Biol. 2009, 13, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Nativi, C.; Renaudet, O. Recent Progress in Antitumoral Synthetic Vaccines. ACS Med. Chem. Lett. 2014, 5, 1176–1178. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Kleski, K.A.; Trabbic, K.R.; Bourgault, J.-P.; Andreana, P.R. Sialyl-Tn Polysaccharide A1 as an Entirely Carbohydrate Immunogen: Synthesis and Immunological Evaluation. J. Am. Chem. Soc. 2016, 138, 14264–14272. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayanan, V.; Thompson, P.; Wolfert, M.A.; Buskas, T.; Bradley, J.M.; Pathangey, L.B.; Madsen, C.S.; Cohen, P.A.; Gendler, S.J.; Boons, G.-J. Immune Recognition of Tumor-Associated Mucin MUC1 is Achieved by a Fully Synthetic Aberrantly Glycosylated MUC1 Tripartite Vaccine. Proc. Natl. Acad. Sci. USA 2012, 109, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Patronov, A.; Doytchinova, I. T-cell Epitope Vaccine Design by Immunoinformatics. Open Biol. 2013, 3, 120139. [Google Scholar] [CrossRef]
- Slovin, S.F.; Ragupathi, G.; Fernandez, C.; Diani, M.; Jefferson, M.P.; Wilton, A.; Kelly, W.K.; Morris, M.; Solit, D.; Clausen, H.; et al. A Polyvalent Vaccine for High-risk Prostate Patients: “Are More Antigens Better?”. Cancer Immunol. Immunother. 2007, 56, 1921–1930. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Calvaresi, E.C.; Hergenrother, P.J. Glucose Conjugation for the Specific Targeting and Treatment of Cancer. Chem. Sci. 2013, 4, 2319–2333. [Google Scholar] [CrossRef] [PubMed]
- De Freitas Junior, J.C.; Morgado-Diaz, J.A. The Role of N-glycans in Colorectal Cancer Progression: Potential Biomarkers and Therapeutic Applications. Oncotarget 2016, 7, 19395–19413. [Google Scholar] [PubMed]
- Wrodnigg, T.M.; Steiner, A.J.; Ueberbacher, B.J. Natural and Synthetic Iminosugars as Carbohydrate Processing Enzyme Inhibitors for Cancer Therapy. Anticancer Agents Med. Chem. 2008, 8, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Meany, D.L.; Sokoll, L.J.; Chan, D.W. Early Detection of Cancer: Immunoassays for Plasma Tumor Markers. Expert Opin. Med. Diagn. 2009, 3, 597–605. [Google Scholar] [CrossRef]
- Wang, C.-C.; Huang, Y.-L.; Ren, C.-T.; Lin, C.-W.; Hung, J.-T.; Yu, J.-C.; Yu, A.L.; Wu, C.-Y.; Wong, C.-H. Glycan Microarray of Globo H and Related Structures for Quantitative Analysis of Breast Cancer. Proc. Natl. Acad. Sci. USA 2008, 105, 11661. [Google Scholar] [CrossRef]
- Hevey, R.; Ling, C.C. Recent Advances in Developing Synthetic Carbohydrate-based Vaccines for Cancer Immunotherapies. Future Med. Chem. 2012, 4, 545–584. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, Z.; Yang, H.; Jiang, Y.; Wei, W.; Li, Q.; Mo, Q.; Liu, J. Beta-Glucosidase Inhibition Sensitizes Breast Cancer to Chemotherapy. Biomed. Pharmacother. 2017, 91, 504–509. [Google Scholar] [CrossRef]
- Nishat, S.; Andreana, P. Entirely Carbohydrate-Based Vaccines: An Emerging Field for Specific and Selective Immune Responses. Vaccines 2016, 4, 19. [Google Scholar] [CrossRef]
- Ju, T.; Lanneau, G.S.; Gautam, T.; Wang, Y.; Xia, B.; Stowell, S.R.; Willard, M.T.; Wang, W.; Xia, J.Y.; Zuna, R.E.; et al. Human Tumor Antigens Tn and Sialyl Tn Arise from Mutations in Cosmc. Cancer Res. 2008, 68, 1636–1646. [Google Scholar] [CrossRef]
- Ju, T.; Aryal, R.P.; Stowell, C.J.; Cummings, R.D. Regulation of Protein O-Glycosylation by the Endoplasmic Reticulum-Localized Molecular Chaperone Cosmc. J. Cell Biol. 2008, 182, 531–542. [Google Scholar] [CrossRef]
- Mong, T.K.; Lee, H.K.; Duron, S.G.; Wong, C.H. Reactivity-Based One-Pot Total Synthesis of Fucose GM1 Oligosaccharide: a Sialylated Antigenic Epitope of Small-Cell Lung Cancer. Proc. Natl. Acad. Sci. USA 2003, 100, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Seeberger, P.H. Automated Oligosaccharide Synthesis. Chem. Soc. Rev. 2008, 37, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Buskas, T.; Thompson, P.; Boons, G.J. Immunotherapy for Cancer: Synthetic Carbohydrate-Based Vaccines. Chem. Commun. 2009, 5335–5349. [Google Scholar] [CrossRef] [PubMed]
- Maddaly, R.; Pai, G.; Balaji, S.; Sivaramakrishnan, P.; Srinivasan, L.; Sunder, S.S.; Paul, S.F.D. Receptors and Signaling Mechanisms for B-lymphocyte Activation, Proliferation and Differentiation—Insights from Both in vivo and in vitro Approaches. FEBS Lett. 2010, 584, 4883–4894. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sette, A.; Sidney, J.; Gendler, S.J.; Franco, A. Tumor-Associated Carbohydrate Antigens: A Possible Avenue for Cancer Prevention. Immunol. Cell Biol. 2005, 83, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gendler, S.J.; Franco, A. Designer Glycopeptides for Cytotoxic T cell-Based Elimination of Carcinomas. J. Exp. Med. 2004, 199, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Cretney, E.; Kelly, J.M.; Westwood, J.A.; Street, S.E.; Yagita, H.; Takeda, K.; van Dommelen, S.L.; Degli-Esposti, M.A.; Hayakawa, Y. Activation of NK Cell Cytotoxicity. Mol. Immunol. 2005, 42, 501–510. [Google Scholar] [CrossRef]
- Wang, W.; Erbe, A.K.; Hank, J.A.; Morris, Z.S.; Sondel, P.M. NK Cell-Mediated Antibody-Dependent Cellular Cytotoxicity in Cancer Immunotherapy. Front. Immunol. 2015, 6, 368. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Wu, D.; Ragupathi, G.; Lu, S.; Williams, L.; Hwu, W.-J.; Johnson, D.; Livingston, P.O. Sequential Immunization of Melanoma Patients with GD3 Ganglioside Vaccine and Anti-Idiotypic Monoclonal Antibody That Mimics GD3 Ganglioside. Clin. Cancer. Res. 2004, 10, 4717. [Google Scholar] [CrossRef]
- Chapman, P.B.; Morrisey, D.; Panageas, K.S.; Williams, L.; Lewis, J.J.; Israel, R.J.; Hamilton, W.B.; Livingston, P.O. Vaccination with a Bivalent GM2 and GD2 Ganglioside Conjugate Vaccine: A Trial Comparing Doses of GD2-Keyhole Limpet Hemocyanin. Clin. Cancer. Res. 2000, 6, 4658. [Google Scholar]
- Ragupathi, G.; Koide, F.; Livingston, P.O.; Cho, Y.S.; Endo, A.; Wan, Q.; Spassova, M.K.; Keding, S.J.; Allen, J.; Ouerfelli, O.; et al. Preparation and Evaluation of Unimolecular Pentavalent and Hexavalent Antigenic Constructs Targeting Prostate and Breast Cancer: A Synthetic Route to Anticancer Vaccine Candidates. J. Am. Chem. Soc. 2006, 128, 2715–2725. [Google Scholar] [CrossRef] [PubMed]
- Danishefsky, S.J.; Allen, J.R. From the Laboratory to the Clinic: A Retrospective on Fully Synthetic Carbohydrate-Based Anticancer Vaccines Frequently used Abbreviations are Listed in the Appendix. Angew. Chem. Int. Ed. Engl. 2000, 39, 836–863. [Google Scholar] [CrossRef]
- Huang, Y.L.; Hung, J.T.; Cheung, S.K.; Lee, H.Y.; Chu, K.C.; Li, S.T.; Lin, Y.C.; Ren, C.T.; Cheng, T.J.; Hsu, T.L.; et al. Carbohydrate-Based Vaccines with a Glycolipid Adjuvant for Breast Cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 2517–2522. [Google Scholar] [CrossRef] [PubMed]
- De Silva, R.A.; Wang, Q.; Chidley, T.; Appulage, D.K.; Andreana, P.R. Immunological response from An Entirely Carbohydrate Antigen: Design of Synthetic Vaccines Based on Tn-PS A1 Conjugates. J. Am. Chem. Soc. 2009, 131, 9622–9623. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Wall, K.A. Immunological Evaluation of Recent MUC1 Glycopeptide Cancer Vaccines. Vaccines 2016, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Duke, J.A.; Avci, F.Y. Immunological Mechanisms of Glycoconjugate Vaccines. In Carbohydrate-Based Vaccines: From Concept to Clinic; ACS Symposium Series: Columbus, OH, USA, 2018; pp. 61–74. [Google Scholar]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An Immunomodulatory Molecule of Symbiotic Bacteria Directs Maturation of the Host Immune System. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef]
- Wang, Q.; McLoughlin, R.M.; Cobb, B.A.; Charrel-Dennis, M.; Zaleski, K.J.; Golenbock, D.; Tzianabos, A.O.; Kasper, D.L. A Bacterial Carbohydrate Links Innate and Adaptive Responses through Toll-Like Receptor 2. J. Exp. Med. 2006, 203, 2853–2863. [Google Scholar] [CrossRef]
- Eradi, P.; Ghosh, S.; Andreana, P.R. Total Synthesis of Zwitterionic Tetrasaccharide Repeating Unit from Bacteroides fragilis ATCC 25285/NCTC 9343 Capsular Polysaccharide PS A1 with Alternating Charges on Adjacent Monosaccharides. Org. Lett. 2018, 20, 4526–4530. [Google Scholar] [CrossRef]
- Mesa, C.; Fernandez, L.E. Challenges Facing Adjuvants for Cancer Immunotherapy. Immunol. Cell Biol. 2004, 82, 644–650. [Google Scholar] [CrossRef]
- Yin, X.-G.; Chen, X.-Z.; Sun, W.-M.; Geng, X.-S.; Zhang, X.-K.; Wang, J.; Ji, P.-P.; Zhou, Z.-Y.; Baek, D.J.; Yang, G.-F.; et al. IgG Antibody Response Elicited by a Fully Synthetic Two-Component Carbohydrate-Based Cancer Vaccine Candidate with α-Galactosylceramide as Built-in Adjuvant. Org. Lett. 2017, 19, 456–459. [Google Scholar] [CrossRef]
- Richichi, B.; Thomas, B.; Fiore, M.; Bosco, R.; Qureshi, H.; Nativi, C.; Renaudet, O.; BenMohamed, L. A Cancer Therapeutic Vaccine based on Clustered Tn-Antigen Mimetics Induces Strong Antibody-Mediated Protective Immunit. Angew. Chem. Int. Ed. 2014, 53, 11917–11920. [Google Scholar] [CrossRef]
- Calvo, M.B.; Figueroa, A.; Pulido, E.G.; Campelo, R.G.; Aparicio, L.A. Potential Role of Sugar Transporters in Cancer and Their Relationship with Anticancer Therapy. Int. J. Endocrinol. 2010, 2010, 14. [Google Scholar] [CrossRef]
- Annibaldi, A.; Widmann, C. Glucose Metabolism in Cancer Cells. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 466–470. [Google Scholar] [CrossRef]
- Herrmann, K.; Benz, M.R.; Krause, B.J.; Pomykala, K.L.; Buck, A.K.; Czernin, J. (18)F-FDG-PET/CT in Evaluating Response to Therapy in Solid Tumors: Where We are and Where We Can Go. Q. J. Nucl. Med. Mol. Imaging 2011, 55, 620–632. [Google Scholar]
- Mahato, R.; Tai, W.; Cheng, K. Prodrugs for Improving Tumor Targetability and Efficiency. Adv. Drug Deliv. Rev. 2011, 63, 659–670. [Google Scholar] [CrossRef]
- Carvalho, K.C.; Cunha, I.W.; Rocha, R.M.; Ayala, F.R.; Cajaiba, M.M.; Begnami, M.D.; Vilela, R.S.; Paiva, G.R.; Andrade, R.G.; Soares, F.A. GLUT1 Expression in Malignant Tumors and its Use as an Immunodiagnostic Marker. Clinics 2011, 66, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Tungpradit, R.; Sinchaikul, S.; An, F.M.; Liu, D.Z.; Phutrakul, S.; Chen, S.T. Targeting the Delivery of Glycan-Based Paclitaxel Prodrugs to Cancer Cells via Glucose Transporters. J. Med. Chem. 2008, 51, 7428–7441. [Google Scholar] [CrossRef]
- Deng, D.; Xu, C.; Sun, P.; Wu, J.; Yan, C.; Hu, M.; Yan, N. Crystal Structure of the Human Glucose Transporter GLUT1. Nature 2014, 510, 121–125. [Google Scholar] [CrossRef]
- Pohl, J.; Bertram, B.; Hilgard, P.; Nowrousian, M.R.; Stuben, J.; Wiessler, M. D-19575--a Sugar-Linked Isophosphoramide Mustard Derivative Exploiting Transmembrane Glucose Transport. Cancer Chemother. Pharmacol. 1995, 35, 364–370. [Google Scholar] [CrossRef]
- Briasoulis, E.; Judson, I.; Pavlidis, N.; Beale, P.; Wanders, J.; Groot, Y.; Veerman, G.; Schuessler, M.; Niebch, G.; Siamopoulos, K.; et al. Phase I trial of 6-hour Infusion of Glufosfamide, a New Alkylating Agent with Potentially Enhanced Selectivity for Tumors that Overexpress Transmembrane Glucose Transporters: a Study of the European Organization for Research and Treatment of Cancer Early Clinical Studies Group. J. Clin. Oncol. 2000, 18, 3535–3544. [Google Scholar]
- Blanchette, J.; Peppas, N.A. Oral Chemotherapeutic Delivery: Design and Cellular Response. Ann. Biomed. Eng. 2005, 33, 142–149. [Google Scholar] [CrossRef]
- Puranik, A.S.; Pao, L.P.; White, V.M.; Peppas, N.A. Synthesis and Characterization of pH-Responsive Nanoscale Hydrogels for Oral Delivery of Hydrophobic Therapeutics. Eur. J. Pharm. Biopharm. 2016, 108, 196–213. [Google Scholar] [CrossRef]
- Ranjbari, J.; Mokhtarzadeh, A.; Alibakhshi, A.; Tabarzad, M.; Hejazi, M.; Ramezani, M. Anti-Cancer Drug Delivery Using Carbohydrate-Based Polymers. Curr. Pharm. Des. 2018, 23, 6019–6032. [Google Scholar] [CrossRef]
- Posocco, B.; Dreussi, E.; De Santa, J.; Toffoli, G.; Abrami, M.; Musiani, F.; Grassi, M.; Farra, R.; Tonon, F.; Grassi, G.; et al. Polysaccharides for the Delivery of Antitumor Drugs. Materials 2015, 8, 2569–2615. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Lozano, D.; Colilla, M.; Vallet-Regí, M. Lectin-Conjugated pH-Responsive Mesoporous Silica Nanoparticles for Targeted Bone Cancer Treatment. Acta Biomater. 2018, 65, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Miot-Noirault, E.; Reux, B.; Debiton, E.; Madelmont, J.C.; Chezal, J.M.; Coudert, P.; Weber, V. Preclinical Investigation of Tolerance and Antitumour Activity of New Fluorodeoxyglucose-Coupled Chlorambucil Alkylating Agents. Invest. New Drug. 2011, 29, 424–433. [Google Scholar] [CrossRef]
- Mandai, T.; Okumoto, H.; Oshitari, T.; Nakanishi, K.; Mikuni, K.; Hara, K.; Hara, K.; Iwatani, W.; Amano, T.; Nakamura, K.; et al. Synthesis and Biological Evaluation of Water Soluble Taxoids Bearing Sugar Moieties. Heterocycles 2001, 54, 561–566. [Google Scholar] [CrossRef]
- Mikuni, K.; Nakanishi, K.; Hara, K.; Hara, K.; Iwatani, W.; Amano, T.; Nakamura, K.; Tsuchiya, Y.; Okumoto, H.; Mandai, T. In vivo Antitumor Activity of Novel Water-Soluble Taxoids. Biol. Pharm. Bull. 2008, 31, 1155–1158. [Google Scholar] [CrossRef]
- Fu, Y.; Li, S.; Zu, Y.; Yang, G.; Yang, Z.; Luo, M.; Jiang, S.; Wink, M.; Efferth, T. Medicinal Chemistry of Paclitaxel and its Analogues. Curr. Med. Chem. 2009, 16, 3966–3985. [Google Scholar] [CrossRef]
- Goff, R.D.; Thorson, J.S. Assessment of Chemoselective Neoglycosylation Methods Using Chlorambucil as a Model. J. Med. Chem. 2010, 53, 8129–8139. [Google Scholar] [CrossRef]
- Reinhard, J.; Eichhorn, U.; Wiessler, M.; Kaina, B. Inactivation of O(6)-methylguanine-DNA methyltransferase by glucose-conjugated inhibitors. Int. J. Cancer 2001, 93, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Shustov, G.; Liang, H.; Khlebnikov, V.; Zheng, W.; Yang, X.-H.; Cheeseman, C.; Wiebe, L.I. Design, Synthesis, and Preliminary Biological Evaluation of 6-O-Glucose–Azomycin Adducts for Diagnosis and Therapy of Hypoxic Tumors. J. Med. Chem. 2012, 55, 6033–6046. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Cui, S.; Li, S.; Du, C.; Tian, J.; Wan, S.; Qian, Z.; Gu, Y.; Chen, W.R.; Wang, G. Targeted Cancer Therapy with a 2-Deoxyglucose–Based Adriamycin Complex. Cancer Res. 2013, 73, 1362. [Google Scholar] [CrossRef]
- Cheng, H.; Cao, X.; Xian, M.; Fang, L.; Cai, T.B.; Ji, J.J.; Tunac, J.B.; Sun, D.; Wang, P.G. Synthesis and Enzyme-Specific Activation of Carbohydrate−Geldanamycin Conjugates with Potent Anticancer Activity. J. Med. Chem. 2005, 48, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Awuah, S.G.; Lippard, S.J. Chemical Approach to Positional Isomers of Glucose–Platinum Conjugates Reveals Specific Cancer Targeting through Glucose-Transporter-Mediated Uptake in Vitro and in Vivo. J. Am. Chem. Soc. 2016, 138, 12541–12551. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Kwon, J.-T.; Koh, M.; Cho, M.-H.; Park, S.B. Enhanced Efficacy of 7-hydroxy-3-methoxycadalene via Glycosylation in In vivo Xenograft Study. Bioorg. Med. Chem. Lett. 2007, 17, 6335–6339. [Google Scholar] [CrossRef] [PubMed]
- Gynther, M.; Ropponen, J.; Laine, K.; Leppänen, J.; Haapakoski, P.; Peura, L.; Järvinen, T.; Rautio, J. Glucose Promoiety Enables Glucose Transporter Mediated Brain Uptake of Ketoprofen and Indomethacin Prodrugs in Rats. J. Med. Chem. 2009, 52, 3348–3353. [Google Scholar] [CrossRef] [PubMed]
- Hwu, J.R.; Hsu, C.-I.; Hsu, M.-H.; Liang, Y.-C.; Huang, R.C.C.; Lee, Y.C. Glycosylated Nordihydroguaiaretic Acids as Anti-Cancer Agents. Bioorg. Med. Chem. Lett. 2011, 21, 380–382. [Google Scholar] [CrossRef]
- Ohtsubo, K.; Marth, J.D. Glycosylation in Cellular Mechanisms of Health and Disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef]
- Stanley, P. Golgi Glycosylation. Cold Spring Harb Perspect Biol. 2011, 3, 1–13. [Google Scholar] [CrossRef]
- Ho, W.-L.; Hsu, W.-M.; Huang, M.-C.; Kadomatsu, K.; Nakagawara, A. Protein Glycosylation in Cancers and its Potential Therapeutic Applications in Neuroblastoma. J. Hematol. Oncol. 2016, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Nash, R.J.; Kato, A.; Yu, C.Y.; Fleet, G.W. Iminosugars as Therapeutic Agents: Recent Advances and Promising Trends. Future Med. Chem. 2011, 3, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Fiaux, H.; Popowycz, F.; Favre, S.; Schütz, C.; Vogel, P.; Gerber-Lemaire, S.; Juillerat-Jeanneret, L. Functionalized Pyrrolidines Inhibit α-Mannosidase Activity and Growth of Human Glioblastoma and Melanoma Cells. J. Med. Chem. 2005, 48, 4237–4246. [Google Scholar] [CrossRef] [PubMed]
- Vallee, F.; Karaveg, K.; Herscovics, A.; Moremen, K.W.; Howell, P.L. Structural Basis for Catalysis and Inhibition of N-glycan Processing Class I alpha 1,2-mannosidases. J. Biol. Chem. 2000, 275, 41287–41298. [Google Scholar] [CrossRef] [PubMed]
- Popowycz, F.; Gerber-Lemaire, S.; Schütz, C.; Vogel, P. Syntheses and Glycosidase Inhibitory Activities of 2-(Aminomethyl)-5-(hydroxymethyl)pyrrolidine-3,4-diol Derivatives. Helv. Chim. Acta 2004, 87, 800–810. [Google Scholar] [CrossRef]
- Allan, G.; Ouadid-Ahidouch, H.; Sanchez-Fernandez, E.M.; Risquez-Cuadro, R.; Fernandez, J.M.; Ortiz-Mellet, C.; Ahidouch, A. New Castanospermine Glycoside Analogues Inhibit Breast Cancer Cell Proliferation and Induce Apoptosis Without Affecting Normal Cells. PLoS One 2013, 8, e76411. [Google Scholar] [CrossRef] [PubMed]
- Dal Piaz, F.; Vassallo, A.; Chini, M.G.; Cordero, F.M.; Cardona, F.; Pisano, C.; Bifulco, G.; De Tommasi, N.; Brandi, A. Natural Iminosugar (+)-Lentiginosine Inhibits ATPase and Chaperone Activity of Hsp90. PLOS ONE 2012, 7, e43316. [Google Scholar] [CrossRef][Green Version]
- Namikawa, T.; Kawanishi, Y.; Fujisawa, K.; Munekage, E.; Iwabu, J.; Munekage, M.; Maeda, H.; Kitagawa, H.; Kobayashi, M.; Hanazaki, K. Serum Carbohydrate Antigen 125 is a Significant Prognostic Marker in Patients with Unresectable Advanced or Recurrent Gastric Cancer. Surg. Today 2018, 48, 388–394. [Google Scholar] [CrossRef]
- Dube, D.H.; Bertozzi, C.R. Metabolic Oligosaccharide Engineering as a Tool for Glycobiology. Curr. Opin. Chem. Biol. 2003, 7, 616–625. [Google Scholar] [CrossRef]
- Evellyne de Oliveira, F.; Cassia Regina Albuquerque da, C.; Priscilla, B.S.A.; Raiana Apolinario de, P.; Mary Angela, A.-S.; Matheus Silva, A.; Adrielle, Z.; Maria, G.C.-d.-C.; Luis Claudio Nascimento da, S.; Maria Tereza dos Santos, C. Lectin-Carbohydrate Interactions: Implications for the Development of New Anticancer Agents. Curr. Med. Chem. 2017, 24, 3667–3680. [Google Scholar]
- Coulibaly, F.C.; Youan, B.-B. Current Status of Lectin-Based Cancer Diagnosis and Therapy. AIMS Mol. Sci. 2017, 4, 1–27. [Google Scholar]
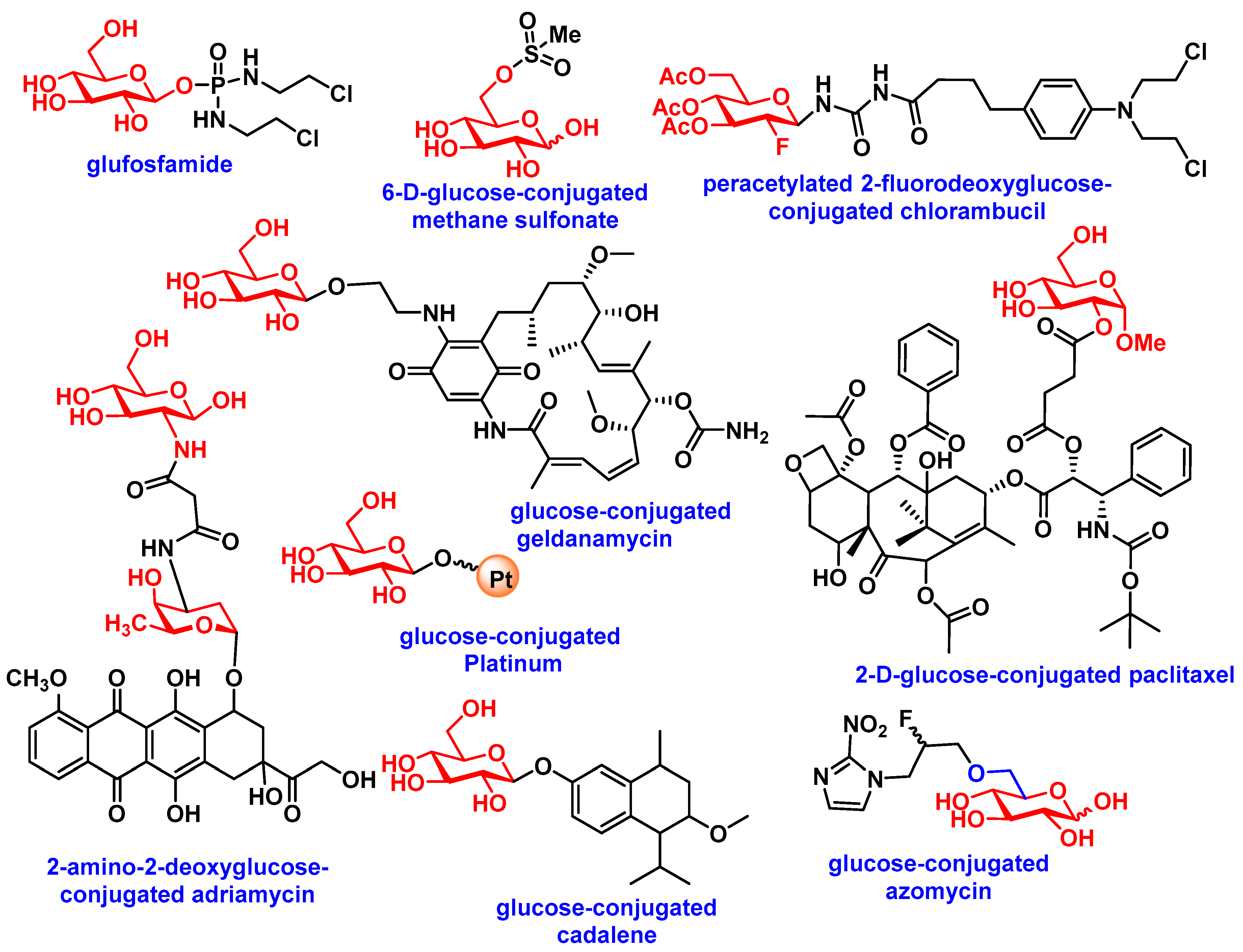
| Aglycons | Conjugated Sugars | Response of Glycoconjugates Compared to Aglycon in In-Vitro or In-Vivo | Transportation Mode | Ref(s) |
|---|---|---|---|---|
| Chlorambucil | Peracetylated 2-fluorodeoxyglucose | Human fibroblasts, MCF-7 (25-fold more active) and Mice (Increased in MTD) | - | [57] |
| Docetaxel | Glucose, galactose, mannose, xylose | B16 murine melanoma cells (3 to 18-fold more active) | - | [58] |
| Docetaxel | galactose | Syngeneic P388 murine leukemia tumor model (equivalent) | - | [59] |
| Paclitaxel | Glucose, glucuronic Acid | HUV-EC-C and CHO-K1, NCI-H838, Hep-3B, A498, MES-SA, HCT-116, NPC-TW01, MKN-45 (All less toxic) | Partially GLUT-1, /GLUT-3/GLUT-4 mediated | [48,60] |
| Chlorambucil | Amino derivatives of glucose, mannose, galactose, xylose, lyxose, D-threoside | NCI-H460, A549, Du145, SKOV3, Hep3b, SF268, MCF7, HT29, HCT15, H1299 (induce decrease in cell growth) | - | [61] |
| Benzylguanine | Glucose | HeLa S3 and HeLa MR cells (inhibition of O6-methyl-guanine-DNA methyltransferase, MGMT) | - | [62] |
| Azomycin | Glucose | Several immortalized murine and human cancer cells (improved selectivity towards hypoxic tumor as radiosensitizer) | GLUTs mediated | [63] |
| Adriamycin | 2-amino-2-deoxy-glucose | MCF-7, Bel-7402, HepG2, MDA-MB-231, U87MG, HELF, SKOV3, and S180, HELF and mice (enhance selectivity towards cancer cells) | GLUTs mediated | [64] |
| Geldanamycin | Glucose, lactose, galactose | SW620, HT29, MCF7, K562 (one showed 3- to 40-fold enhanced activity with β-galactosidase) | - | [65] |
| Platinum | Glucose | DU145, RWPE2 | GLUTs mediated | [66] |
| Cadalene | Glucose, lactose, galactose | In vitro (less toxic) and in vivo (reduced tumor size) | - | [67] |
| Ketoprofen | Glucose | Cross blood−brain barrier (BBB) | GLUTs mediated | [68] |
| Nordihydroguaiaretic acid | Galactose, glucose | NCI/ADR-RES, Hep3B, MCF-7, HT-29 | - | [69] |
| Amino Sugars | Glucosidase Inhibition | Other Anti-Tumor Activities | Ref (s) |
|---|---|---|---|
| Swainsonine | Lysosomal α-1-3- (IC50 0.70 nM) and α-1-6-mannosidase (Ki 40 nM) and Golgi α-mannosidase | Inhibits growth of tumor cells | [13] |
| 1,4-Dideoxy-1,4-imino-D-mannitol | α-mannosidase, Lysosomal Golgi α-mannosidase II, glycogen phosphorylase | Human Glioblastoma and Melanoma Cells | [74] |
| 1-Deoxymannojirimycin | α-1-2-mannosidase (IC50 0.02 mM), Golgi α-mannosidase II (IC50 400 µM) | Interact with recombinant tumor necrosis factor (rTNF) and recombinant interleukin 1 (rIL-1) | [75] |
| 2-aminomethyl-5-(hydroxymethyl) pyrrolidine3,4-diol derivative | Jack bean α-Mannosidase (IC50 55 µM) | Inhibits growth of human glioblastoma cells and melanoma cells, DNA, synthesis of proteins | [74,76] |
| Castanospermine | α- and β-glucosidases | Inhibitor of breast cancer | [77] |
| 1-deoxynojirimycin | Glucosidase I and II | Anti-metastatic activity, reduce adhesion of tumor cells to vascular endothelium, inhibit cellular transformation, prevent morphological differentiation of endothelial cells | [13] |
| (+)-Lentiginosine | amyloglucosidases | Inhibits ATPase and Chaperone Activity of Hsp90 | [78] |
| Siastatin B | β-glucuronidase, NAG-ase | Antimetastatic activity | [13] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, F.; Andreana, P.R. Developments in Carbohydrate-Based Cancer Therapeutics. Pharmaceuticals 2019, 12, 84. https://doi.org/10.3390/ph12020084
Hossain F, Andreana PR. Developments in Carbohydrate-Based Cancer Therapeutics. Pharmaceuticals. 2019; 12(2):84. https://doi.org/10.3390/ph12020084
Chicago/Turabian StyleHossain, Farzana, and Peter R. Andreana. 2019. "Developments in Carbohydrate-Based Cancer Therapeutics" Pharmaceuticals 12, no. 2: 84. https://doi.org/10.3390/ph12020084
APA StyleHossain, F., & Andreana, P. R. (2019). Developments in Carbohydrate-Based Cancer Therapeutics. Pharmaceuticals, 12(2), 84. https://doi.org/10.3390/ph12020084





