Investigations of Physical Compatibilities of Commonly Used Intravenous Medications with and without Parenteral Nutrition in Pediatric Cardiovascular Intensive Care Unit Patients
Abstract
:1. Introduction
2. Methods and Materials
2.1. Materials
2.2. Design
2.3. Data Collection
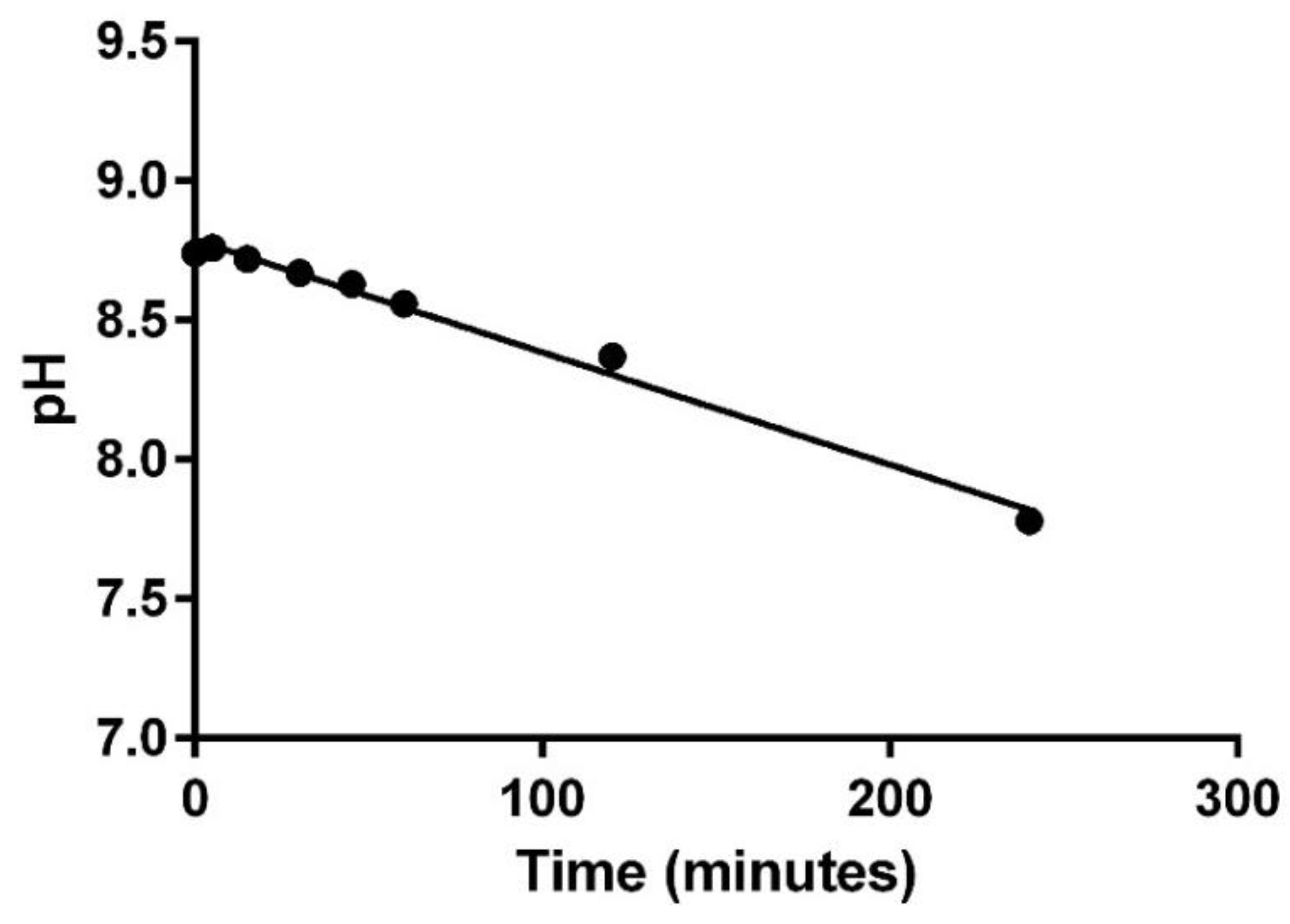
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Staven, V.; Wang, S.; Grønlie, I.; Tho, I. Development and evaluation of a test program for Y-site compatibility testing of total parenteral nutrition and intravenous drugs. Nutr. J. 2016, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Kanji, S.; Lam, J.; Johanson, C.; Singh, A.; Goddard, R.; Fairbairn, J.; Lloyd, T.; Monsour, D.; Kakal, J. Systematic review of physical and chemical compatibility of commonly used medications administered by continuous infusion in intensive care units. Crit. Care Med. 2010, 38, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Staven, V.; Iqbal, H.; Wang, S.; Grønlie, I.; Tho, I. Physical compatibility of total parenteral nutrition and drugs in Y-site administration to children from neonates to adolescents. J. Pharm. Pharmacol. 2017, 69, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.M.; Wilder, A.G.; Foushee, J.A. Physical compatibility of various drugs with neonatal total parenteral nutrient solution during simulated Y-site administration. Am. J. Health Syst. Pharm. 2013, 70, 520–524. [Google Scholar] [CrossRef]
- Sykes, R.; McPherson, C.; Foulks, K.; Wade, J.; Gal, P. Aminophylline compatibility with neonatal total parenteral nutrition. J. Pediatr. Pharmacol. Ther. 2008, 13, 76–79. [Google Scholar] [PubMed]
- Kuensting, L.L.; DeBoer, S.; Holleran, R.; Shultz, B.L.; Steinmann, R.A.; Venella, J. Difficult venous access in children: Taking control. J. Emerg. Nurs. 2009, 35, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Gorman, G.; Miller, R.R.; Joiner, L.C.; Quattlebaum, C.L.; Benner, K. Multiport Y-site compatibility studies of a parenteral nutrition solution with routinely used pediatric CVICU medications. Advan. Crit. Care Med. 2017, 1, 1. [Google Scholar]
- Trissel’s 2 Clinical Pharmaceutics Database (Created by Lawrence A. Trissel). Lexicomp Website. Available online: http://www.crlonline.com.ezproxy.samford.edu/lco/action/ivcompatibility/trissels (accessed on 11 April 2019).
- Trissel’s 2 Clinical Pharmaceutics Database (Parenteral Compatibility). Micromedex Website. Available online: http://www.micromedexsolutions.com.ezproxy.samford.edu/micromedex2/librarian/PFDefaultActionId/evidencexpert.ShowIVCompResults (accessed on 11 April 2019).
- Trissel, L.A.; Gilbert, D.L.; Martinez, J.F.; Baker, M.B.; Walter, W.V.; Mirtallo, J.M. Compatibility of parenteral nutrient solutions with selected drugs during simulated Y-site administration. Am. J. Health-Syst. Pharm. 1997, 54, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Trissel, L.A.; Williams, K.Y.; Gilbert, D.L. Compatibility screening of linezolid injection during simulated y-site administration with other drugs and infusion solutions. J. Am. Pharm. Assoc. 2000, 40, 515–519. [Google Scholar]
- Dice, J.E. Physical compatibility of alprostadil with commonly used IV solutions and medications in the neonatal intensive care unit. J. Pediatr. Pharmacol. Ther. 2006, 11, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Jasti, B.R.; Saraf, P. Compatibility of parenteral furosemide with seventeen secondary drugs used in standard concentrations. Int. J. Pharmaceut. Compound. 2011, 15, 259–261. [Google Scholar]
- Faria, C.E.; Fiumara, K.; Patel NTran, D.C. Visual compatibility of furosemide with phenylephrine and vasopressin. Am. J. Health Syst. Pharm. 2006, 63, 906–908. [Google Scholar] [CrossRef] [PubMed]
| Drug/Vehicle | Supplied Concentration | Manufacturer | Lot Number | Expiration Date |
|---|---|---|---|---|
| Alprostadil | 500 mcg/mL | Pharmacia and Upjohn Co. | R75137 | 06/2019 |
| Calcium gluconate | 1 mg/mL | Fresenius Kabi | 6013761 | 04/2018 |
| Dexmedetomidine HCl | 400 mcg/mL | Hospira | 74160DD | 02/2019 |
| Dexmedetomidine | 200 mcg/2 mL | Intas Pharm. Limited | W08976 | 05/2019 |
| Epinephrine HCl | 1 mg/mL | Amphastar | DT020C7 | 02/2019 |
| Esmolol | 20 mg/mL | Baxter | Y225839 | 02/2019 |
| Furosemide | Bulk | Letco | 1502110214 | 02/2018 |
| Milrinone Lactate Inj | 1 mg/mL | Hikma West-Ward Pharmaceutical | 1510491 | 03/2018 |
| Milrinone Lactate Inj | 1 mg/mL | APP Pharmaceuticals | 6008428 | 08/2017 |
| Norepinephrine Bitartrate | 1 mg/mL | Hospira | 740653A | 08/2018 |
| Vasopressin | 20 units/mL | Par Pharmaceutical | 818725 | 08/2018 |
| 5% Dextrose in water | 5% | Baxter | P352880 | 02/2018 |
| Dextrose Anhydrous | Not Applicable | Letco | 1601050027 | 02/6/18 |
| Normal Saline | 0.9% | Baxter | Y230961 | 10/2018 |
| Sodium Hydroxide | 97% flakes | Letco | 1601050027 | 02/2018 |
| Ingredient | Concentration |
|---|---|
| Dextrose | 25% |
| Travasol 1 | 3% |
| Sodium | 150 mEq/L |
| Potassium | 80 mEq/L |
| Magnesium | 5 mEq/L |
| Calcium | 18 mEq/L |
| Chloride | 75 mEq/L |
| Phosphorus | 7 mmol/L |
| Acetate | 75 mEq/L |
| Infuvite Pediatric Multivitamin 2 | 5 mL |
| Selenium | 10 mcg/L |
| Multitrace-4 Concentrate 3 | 1 mL |
| Heparin | 1000 units/L |
| Phase I Combinations | ||
|---|---|---|
| Drug | Furosemide 1 mg/mL | Furosemide 10 mg/mL |
| Epinephrine 16 mcg/mL | Compatible * | Compatible |
| Epinephrine 100 mcg/mL | Compatible | Compatible |
| Norepinephrine 16 mcg/mL | Compatible | Compatible |
| Norepinephrine 100 mcg/mL ** | Compatible | Compatible |
| Vasopressin 1 unit/mL | Compatible | Compatible |
| Esmolol 20 mg/mL | Compatible | Not Tested |
| Alprostadil 10 mcg/mL | Compatible | Not Tested |
| Dexmedetomidine 4 mcg/mL | Not Tested | Compatible |
| Phase II Combinations * | |||||
|---|---|---|---|---|---|
| Drug 1 | Drug 2 | Drug 3 | Drug 4 | Drug 5 | Results |
| Furosemide 10 mg/mL | Epinephrine 100 mcg/mL | Milrinone 1000 mcg/mL | NA | NA | Incompatible |
| Furosemide 10 mg/mL | Epinephrine 100 mcg/mL | Milrinone 1000 mcg/mL | Dexmedetomidine 4 mcg/mL | NA | Incompatible |
| Furosemide 10 mg/mL | Epinephrine 100 mcg/mL | Milrinone 1000 mcg/mL | Dexmedetomidine 4 mcg/mL | Vasopressin 1 unit/mL | Incompatible |
| Phase III Combination | |||||
|---|---|---|---|---|---|
| Nutrition | Drug 1 concentration, vehicle | Drug 2 concentration, vehicle | Drug 3 concentration, vehicle | Drug 4 concentration, vehicle | Result |
| Lipid-free TPN * | Epinephrine 100 mcg/mL, NS | Milrinone 1000 mcg/mL | Vasopressin 1 unit/mL, D5W | Calcium gluconate 100 mg/mL | Compatible |
| Combination | Visual Changes | Turbidity Changes (NTU) | pH Changes (pH Units) | Odor | Evolution of Gas |
|---|---|---|---|---|---|
| Phase I | |||||
| 1 | None | 0.06 (0.48–0.54) | −0.06 (4.90–4.96) | None | None |
| 2 | None | 0.14 (0.97–1.11) | 0.31 (9.19–9.50) | None | None |
| 3 | None | 0.24 (0.55–0.79) | −0.10 (6.41–6.51) | None | None |
| 4 | Clear, light yellow | 0.21 (0.9–1.11) | 0.28 (9.55–9.83) | None | None |
| 5 | None | −0.37 (0.43–0.80) | −0.96 (7.78–8.74) | None | None |
| 6 | Clear, light yellow | 0.15 (0.75–0.90) | 0.11 (9.41–9.52) | None | None |
| 7 | None | −0.18 (0.34–0.52) | 0.11 (5.31–5.42) | None | None |
| 8 | None | 0.01 (0.87–0.88) | −0.07 (9.71–9.78) | None | None |
| 9 | None | −0.10 (0.33–0.43) | −0.43 (8.56–8.99) | None | None |
| 10 | None | 0.30 (1.78–2.08) | −0.57 (8.99–9.56) | None | None |
| 11 | None | 0.01 (0.93–0.94) | −0.07 (9.74–9.81) | None | None |
| 12 | None | −0.11 (0.96–1.07) | −0.08 (9.67–9.75) | None | None |
| 13 | None | 0.01 (0.34–0.35) | −0.19 (6.63–6.82) | None | None |
| Phase II | |||||
| 1 | White precipitate formation | 3.6 (3.88–7.48) a | −0.09 (4.68–4.77) | None | None |
| 2 | White precipitate formation | 6.14 (0.84–6.98) b | 0.09 (4.73–4.82) | None | None |
| 3 | White precipitate formation | 22.14 (1.06–23.3) b | 4.67 | None | None |
| Phase III | |||||
| 1 | None | −0.08 (0.54–0.62) | 0.08 (5.55–5.63) | None | None |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greenhill, K.; Hornsby, E.; Gorman, G. Investigations of Physical Compatibilities of Commonly Used Intravenous Medications with and without Parenteral Nutrition in Pediatric Cardiovascular Intensive Care Unit Patients. Pharmaceuticals 2019, 12, 67. https://doi.org/10.3390/ph12020067
Greenhill K, Hornsby E, Gorman G. Investigations of Physical Compatibilities of Commonly Used Intravenous Medications with and without Parenteral Nutrition in Pediatric Cardiovascular Intensive Care Unit Patients. Pharmaceuticals. 2019; 12(2):67. https://doi.org/10.3390/ph12020067
Chicago/Turabian StyleGreenhill, Katherine, Erin Hornsby, and Greg Gorman. 2019. "Investigations of Physical Compatibilities of Commonly Used Intravenous Medications with and without Parenteral Nutrition in Pediatric Cardiovascular Intensive Care Unit Patients" Pharmaceuticals 12, no. 2: 67. https://doi.org/10.3390/ph12020067
APA StyleGreenhill, K., Hornsby, E., & Gorman, G. (2019). Investigations of Physical Compatibilities of Commonly Used Intravenous Medications with and without Parenteral Nutrition in Pediatric Cardiovascular Intensive Care Unit Patients. Pharmaceuticals, 12(2), 67. https://doi.org/10.3390/ph12020067





