The Effects of Hydration Parameters and Co-Surfactants on Methylene Blue-Loaded Niosomes Prepared by the Thin Film Hydration Method
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Conflicts of Interest
References
- Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Esposito, S.; Carafa, M. Niosomes from 80s to present: The state of the art. Adv. Colloid Interface Sci. 2014, 205, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Mahale, N.B.; Thakkar, P.D.; Mali, R.G.; Walunj, D.R.; Chaudhari, S.R. Niosomes: Novel sustained release nonionic stable vesicular systems—An overview. Adv. Colloid Interface Sci. 2012, 183–184, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Khoee, S.; Yaghoobian, M. Niosomes: A novel approach in modern drug delivery systems. In Nanostructures for Drug Delivery; Andronescu, E., Grumezescu, A.M., Eds.; A Volume in Micro and Nano Technologies; Elsevier Publisher: Amsterdam, The Netherlands, 2017; pp. 207–237. [Google Scholar]
- Uchegbu, I.F.; Vyas, S.P. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int. J. Pharm. 1998, 172, 33–70. [Google Scholar] [CrossRef]
- Chaw, C.S.; Kim, K.Y. Effect of formulation compositions on niosomal preparations. Pharm. Dev. Technol. 2013, 18, 667–672. [Google Scholar] [CrossRef] [PubMed]
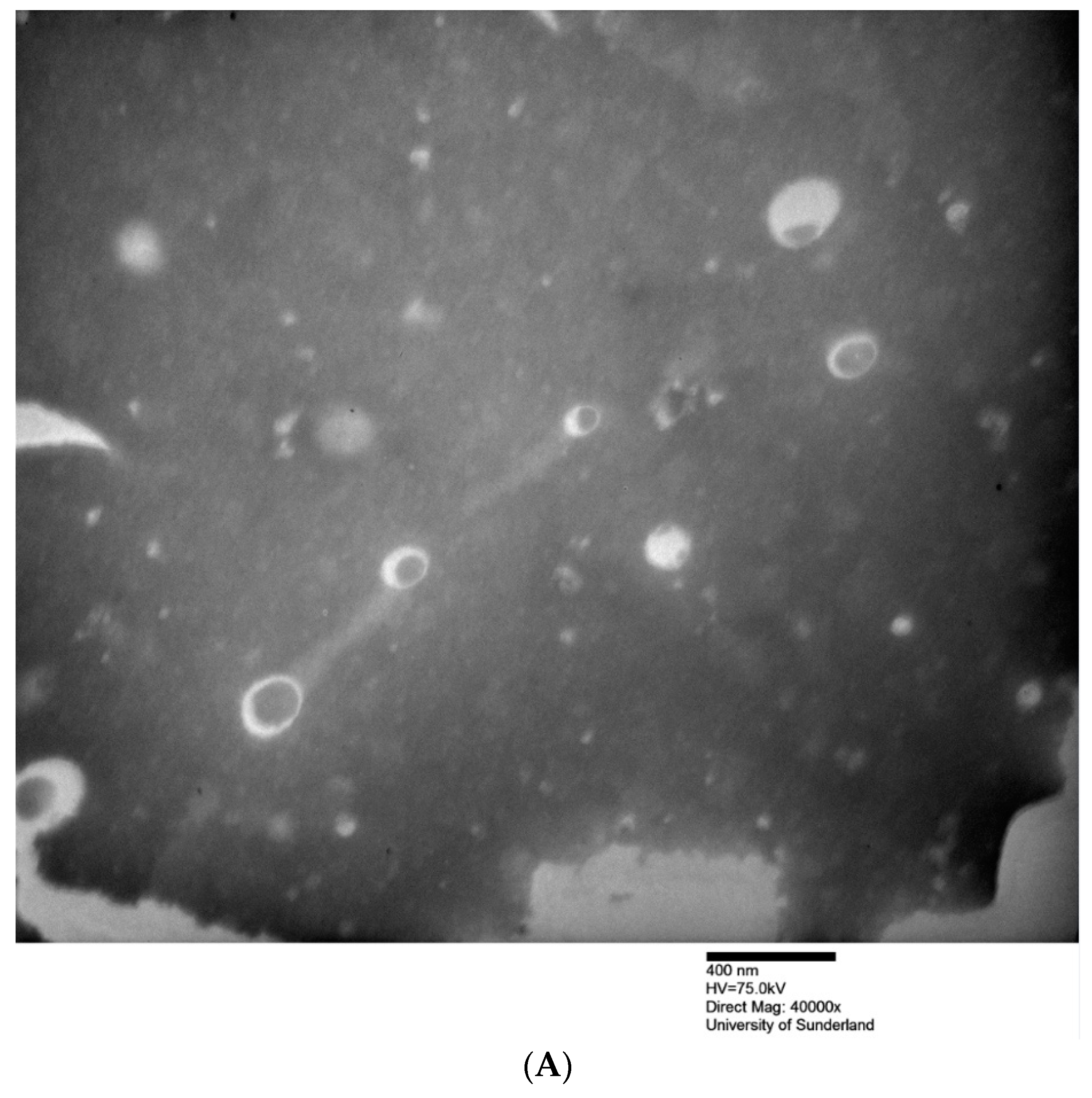
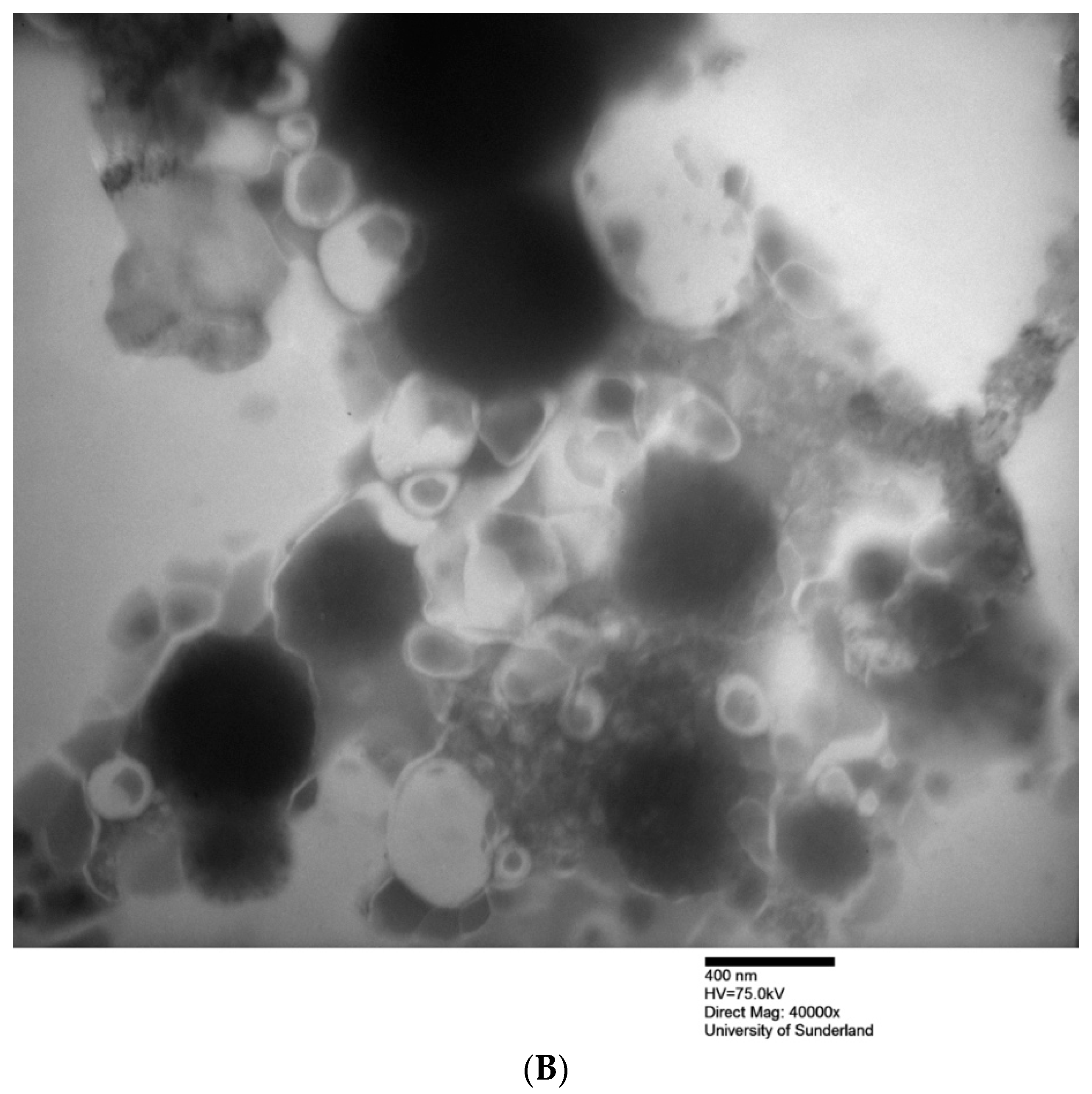
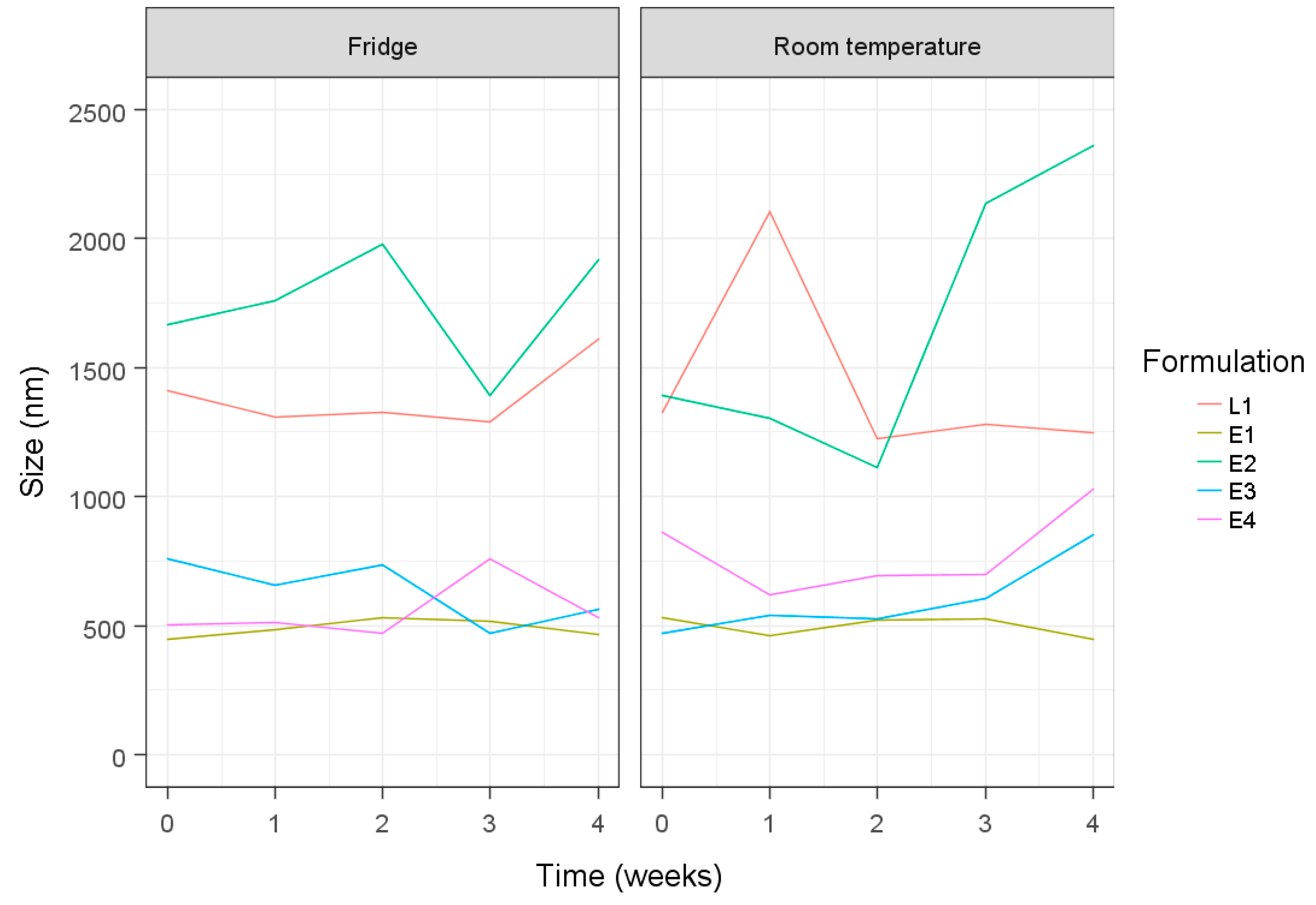
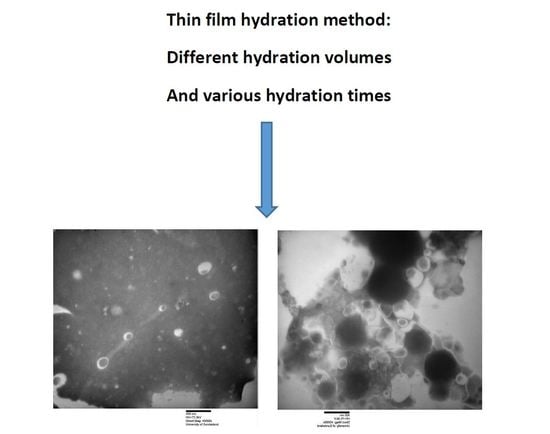
| Formulation a | Hydration Volume (mL) | Hydration Time (min) | Niosome Size (nm) | PDI | ||
|---|---|---|---|---|---|---|
| Before Sonication | After Sonication | Before Sonication | After Sonication | |||
| E1 S60: Cho: ELP b | 20 | 60 | 804.0 ± 294.8 | 272.9 ± 105.1 | 0.56 ± 0.28 | 0.39 ± 0.24 |
| E2 S60: Cho: ELP b | 5 | 15 | 1514.3 ± 203.5 | 292.4 ± 12.3 | 0.40 ± 0.17 | 0.38 ± 0.23 |
| E3 S60: Cho: ELP b | 5 | 60 | 615.7 ± 126.8 | 362.2 ± 138.3 | 0.71 ± 0.25 | 0.45 ± 0.17 |
| E4 S60: Cho: ELP b | 2.5 | 60 | 619.7 ± 191.2 | 311.7 ± 27.4 | 0.72 ± 0.16 | 0.36 c 0.16 |
| L1 S60: Cho: L90 b | 5 | 60 | 1463.4 ± 62.4 | 256.9 ± 51.9 | 0.46 ± 0.06 | 0.34 ± 0.13 |
| Formulation a | Final Lipid Content (mg/mL) | %EE | |
|---|---|---|---|
| Before Sonication | After Sonication | ||
| E1 S60: Cho: ELP b | 10 | 22.1 ± 12.7 | 14.3 ± 1.3 |
| E2 S60: Cho: ELP b | 40 | 32.9 ± 10.1 | 16.3 ± 3.5 |
| E3 S60: Cho: ELP b | 40 | 40.1 ± 7.9 | 11.6 ± 4.5 |
| E4 S60: Cho: ELP b | 80 | 27.9 ± 15.8 | 18.0 ± 14.5 |
| L1 S60: Cho: L90 b | 40 | 10.5 ± 2.4 | 27.1 ± 1.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, L.K.; Chaw, C.S.; Elkordy, A.A. The Effects of Hydration Parameters and Co-Surfactants on Methylene Blue-Loaded Niosomes Prepared by the Thin Film Hydration Method. Pharmaceuticals 2019, 12, 46. https://doi.org/10.3390/ph12020046
Yeo LK, Chaw CS, Elkordy AA. The Effects of Hydration Parameters and Co-Surfactants on Methylene Blue-Loaded Niosomes Prepared by the Thin Film Hydration Method. Pharmaceuticals. 2019; 12(2):46. https://doi.org/10.3390/ph12020046
Chicago/Turabian StyleYeo, Li Key, Cheng Shu Chaw, and Amal Ali Elkordy. 2019. "The Effects of Hydration Parameters and Co-Surfactants on Methylene Blue-Loaded Niosomes Prepared by the Thin Film Hydration Method" Pharmaceuticals 12, no. 2: 46. https://doi.org/10.3390/ph12020046
APA StyleYeo, L. K., Chaw, C. S., & Elkordy, A. A. (2019). The Effects of Hydration Parameters and Co-Surfactants on Methylene Blue-Loaded Niosomes Prepared by the Thin Film Hydration Method. Pharmaceuticals, 12(2), 46. https://doi.org/10.3390/ph12020046