Raman Microspectroscopy as a Tool to Elucidate the Efficacy of Topical Formulations Containing Curcumin
Abstract
1. Introduction
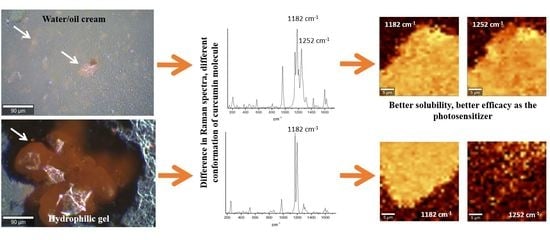
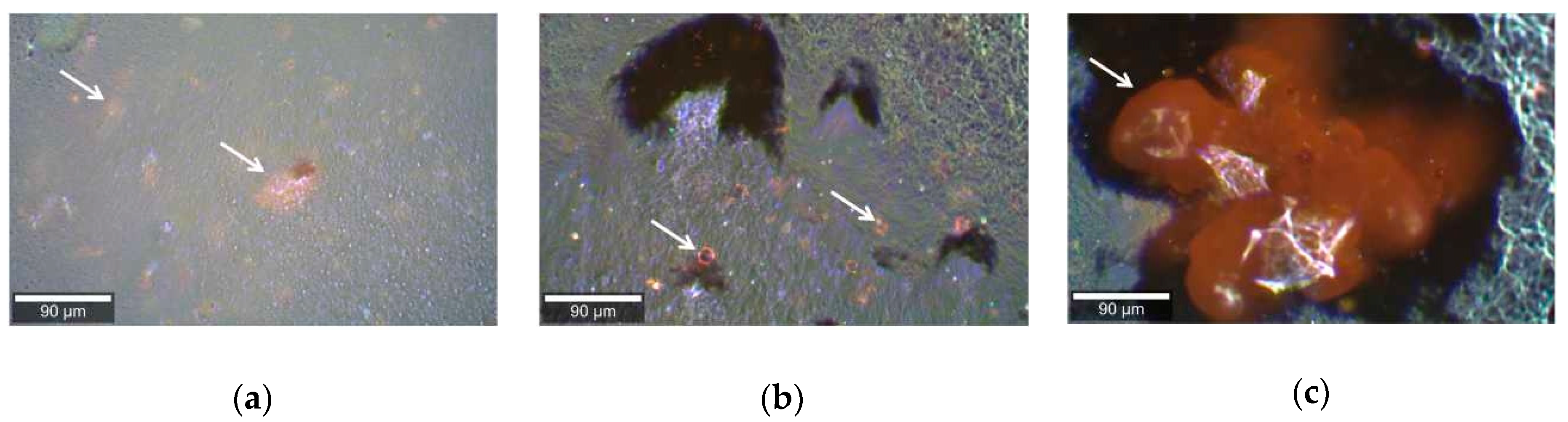
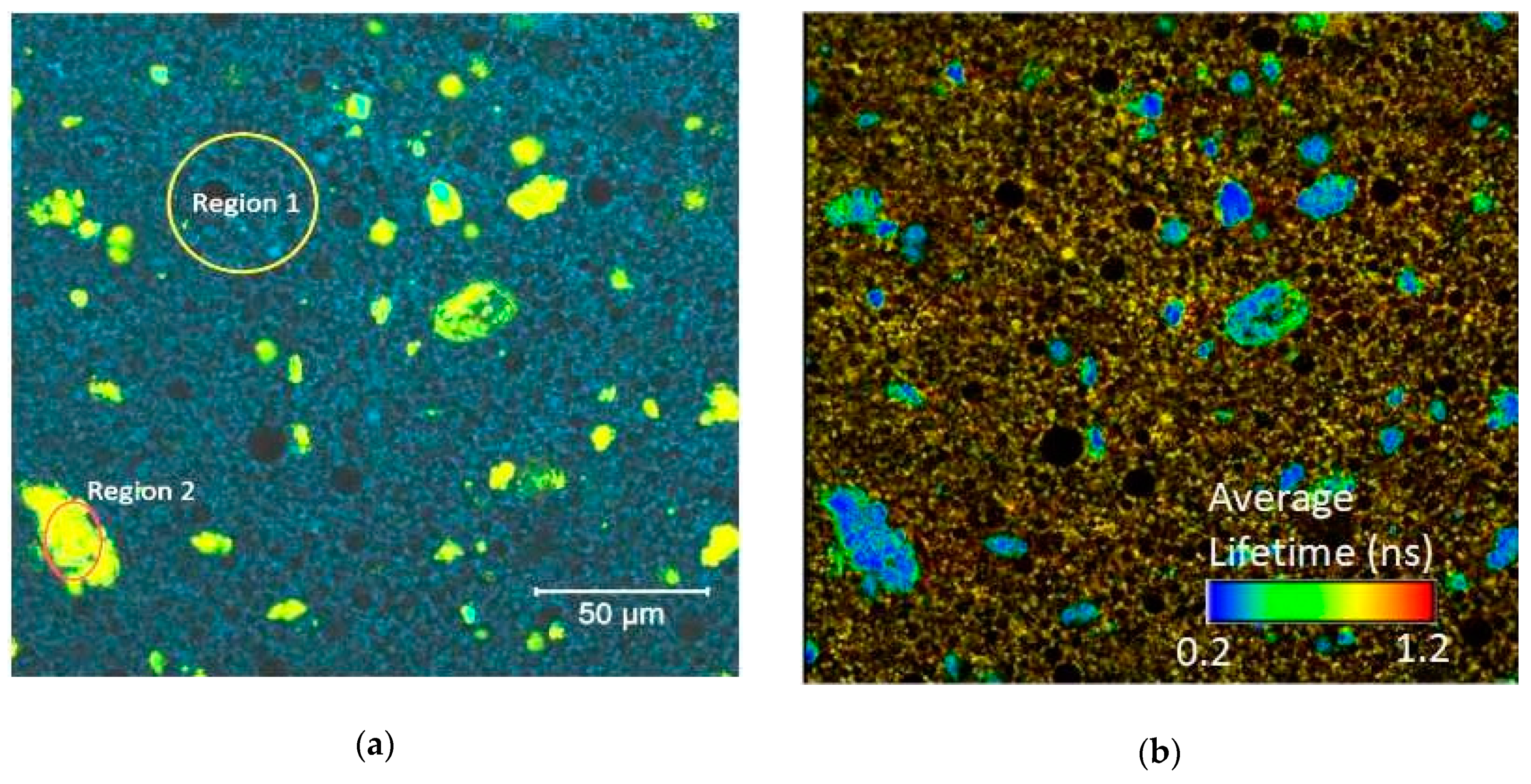
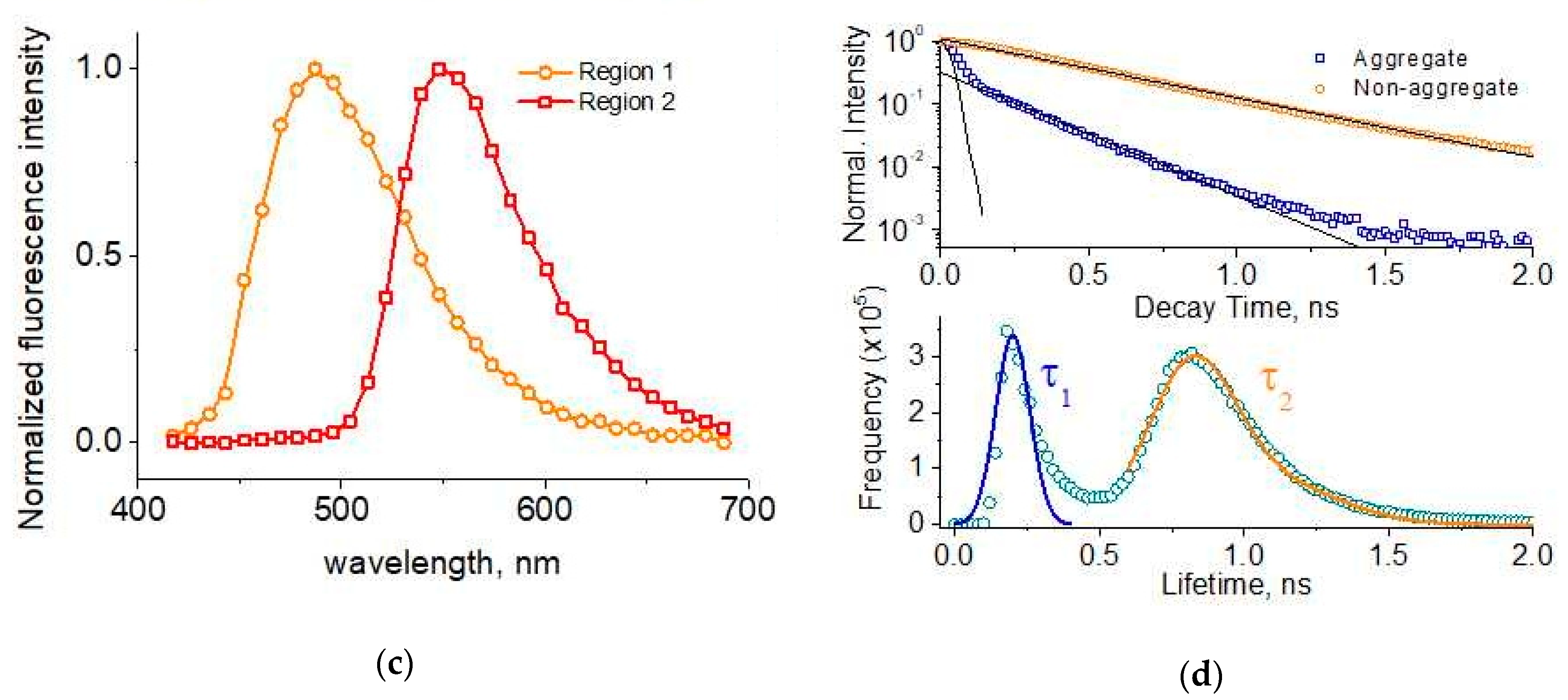
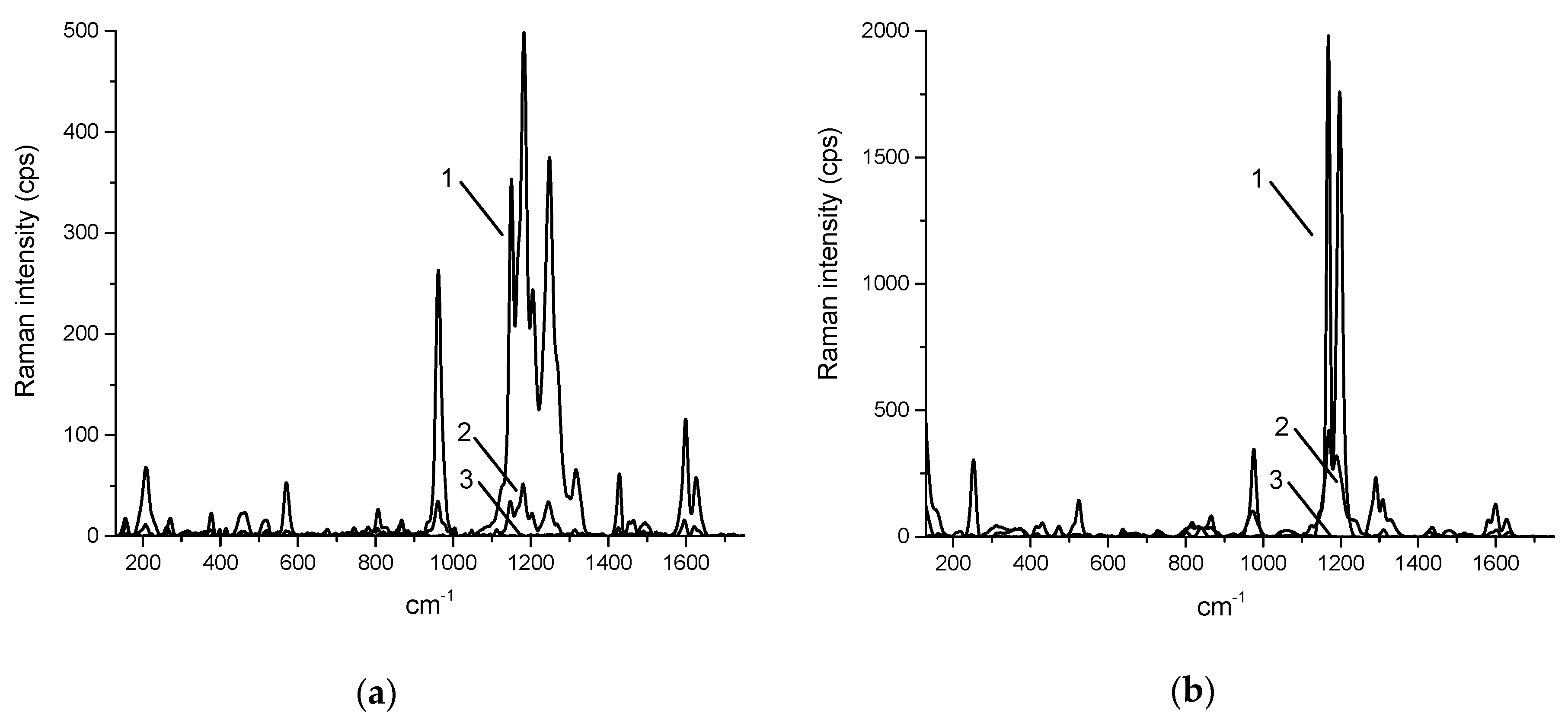
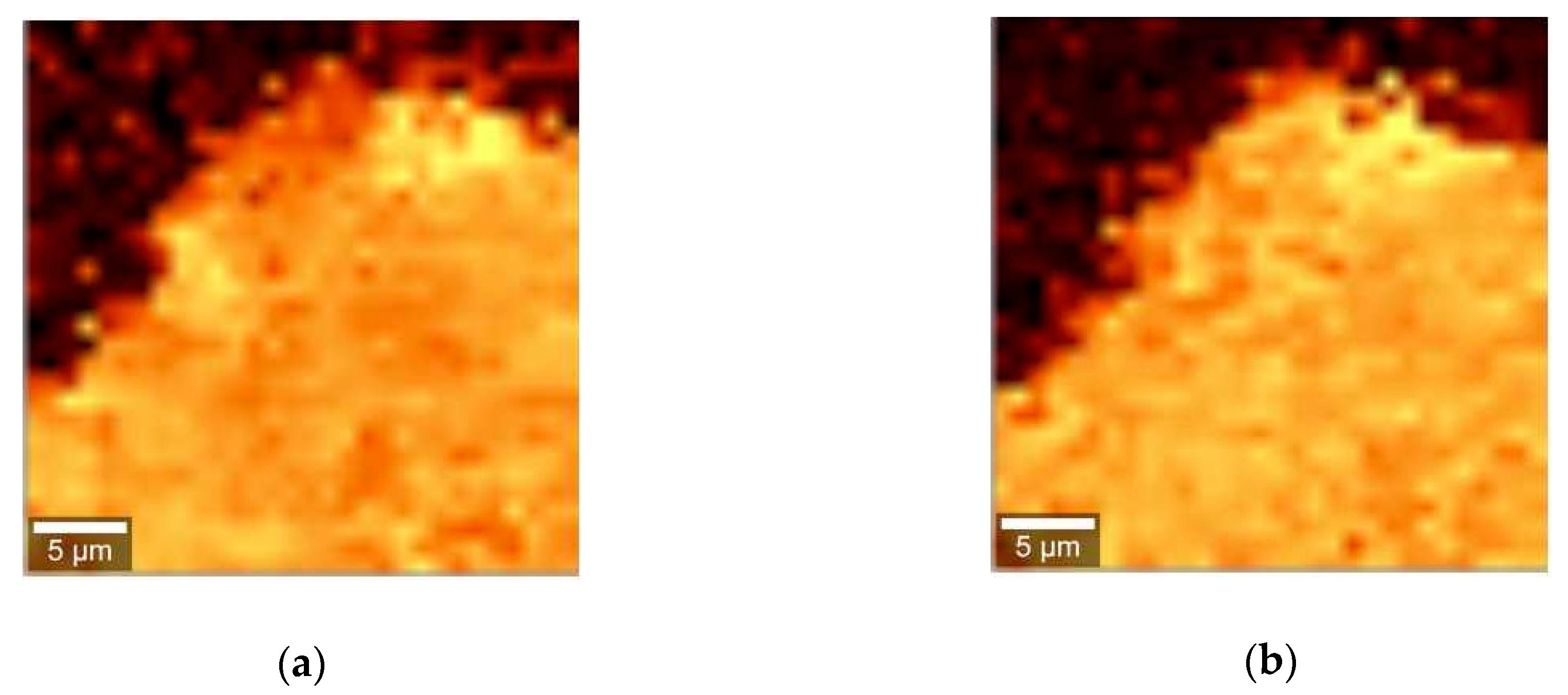
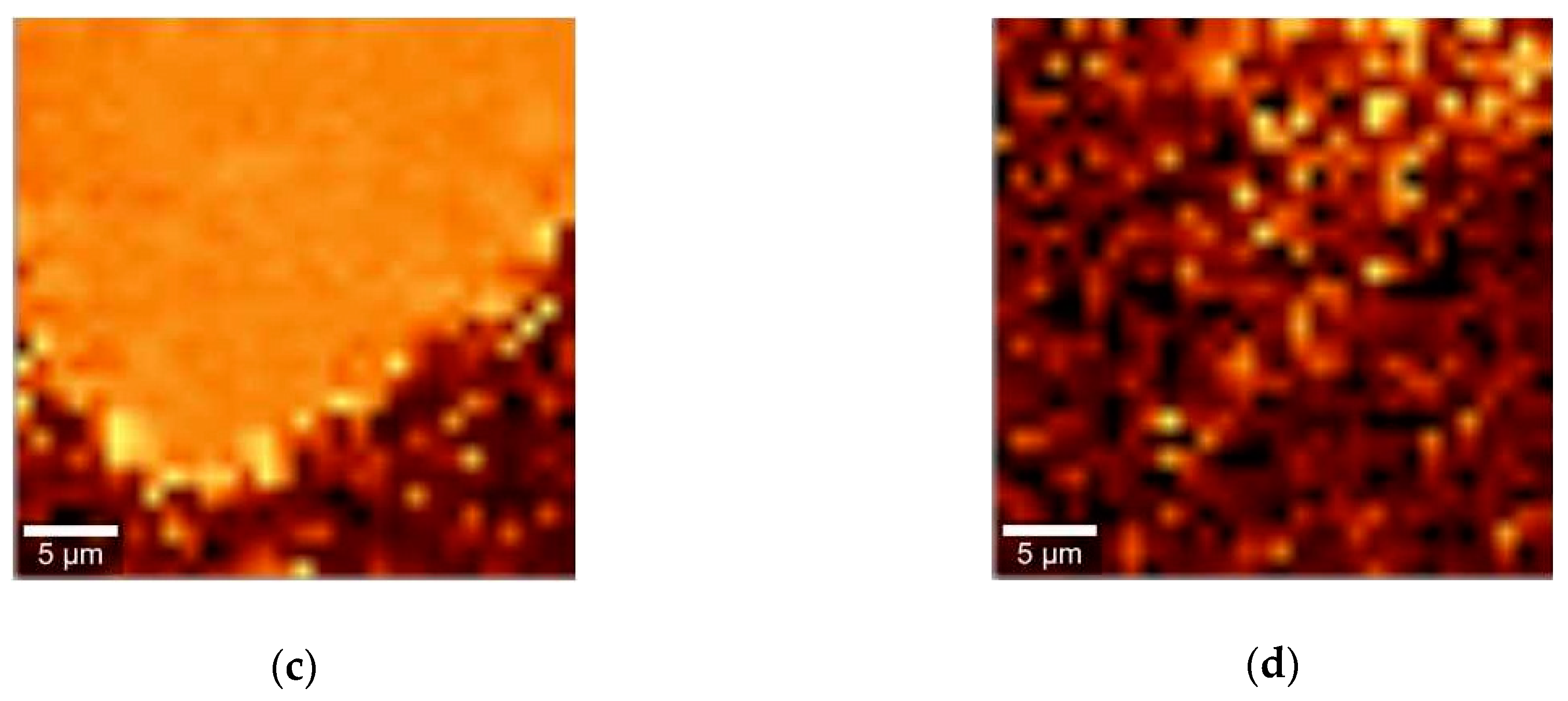
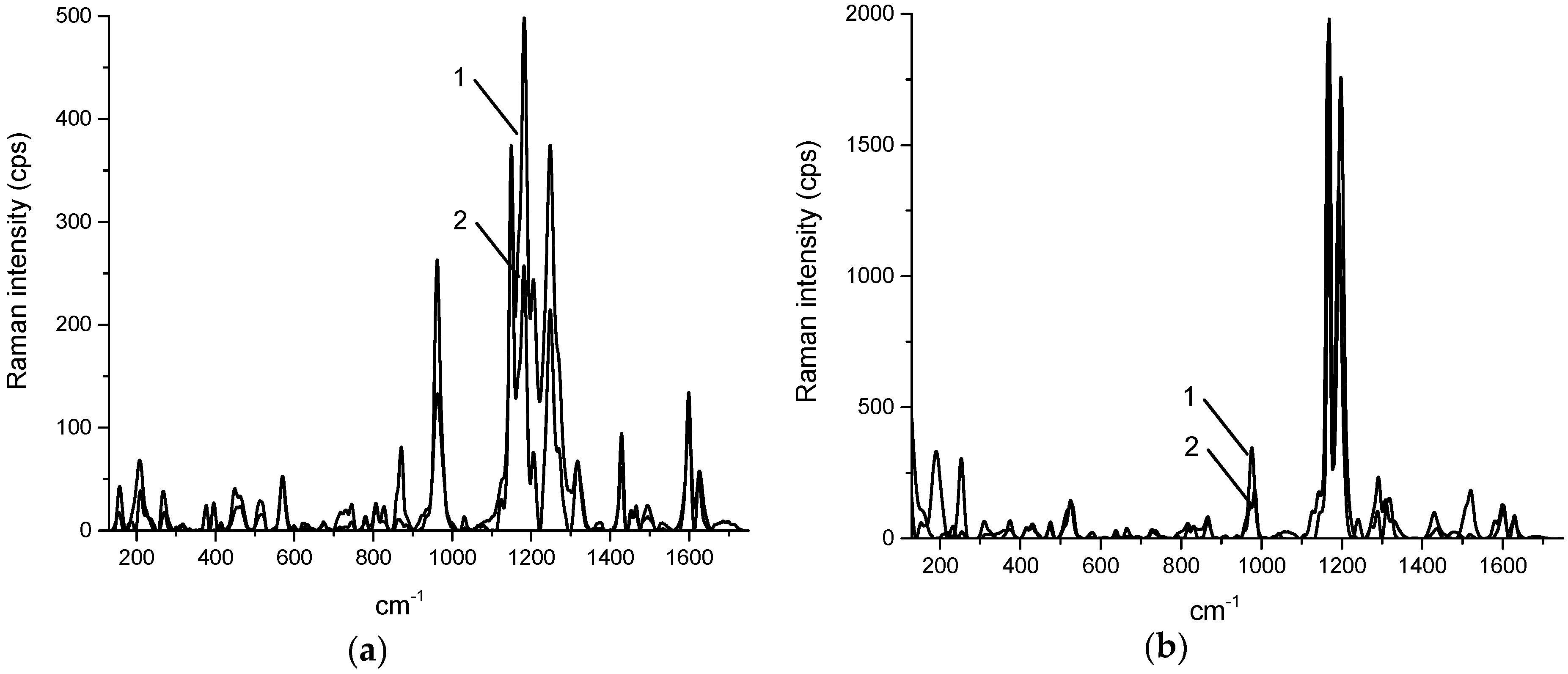
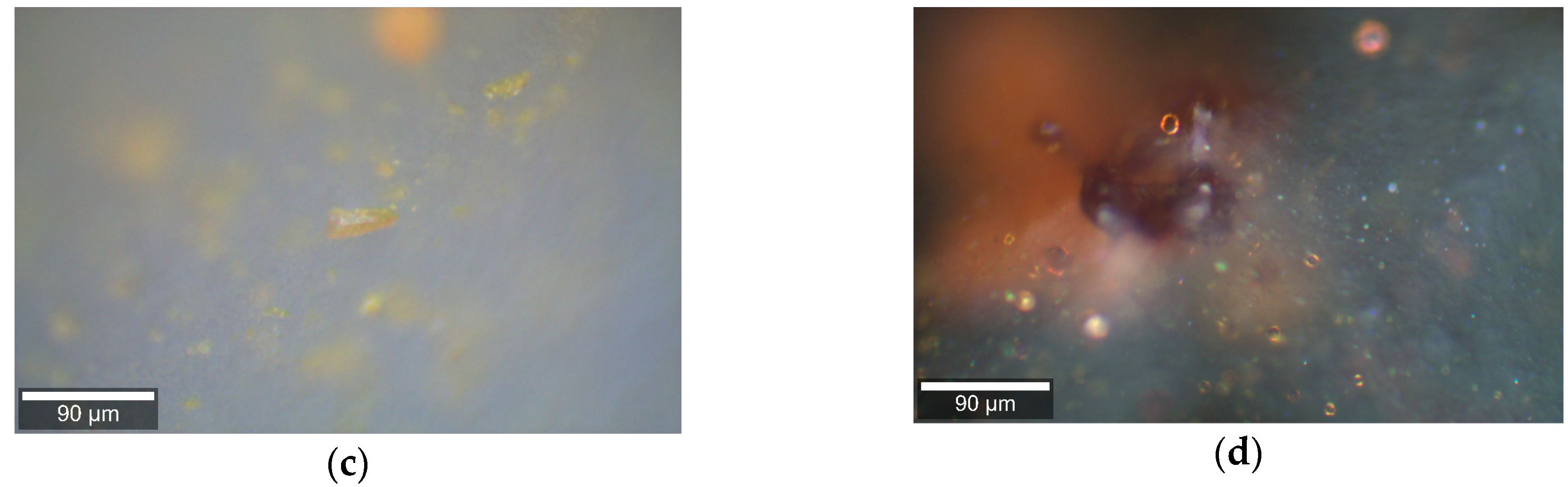
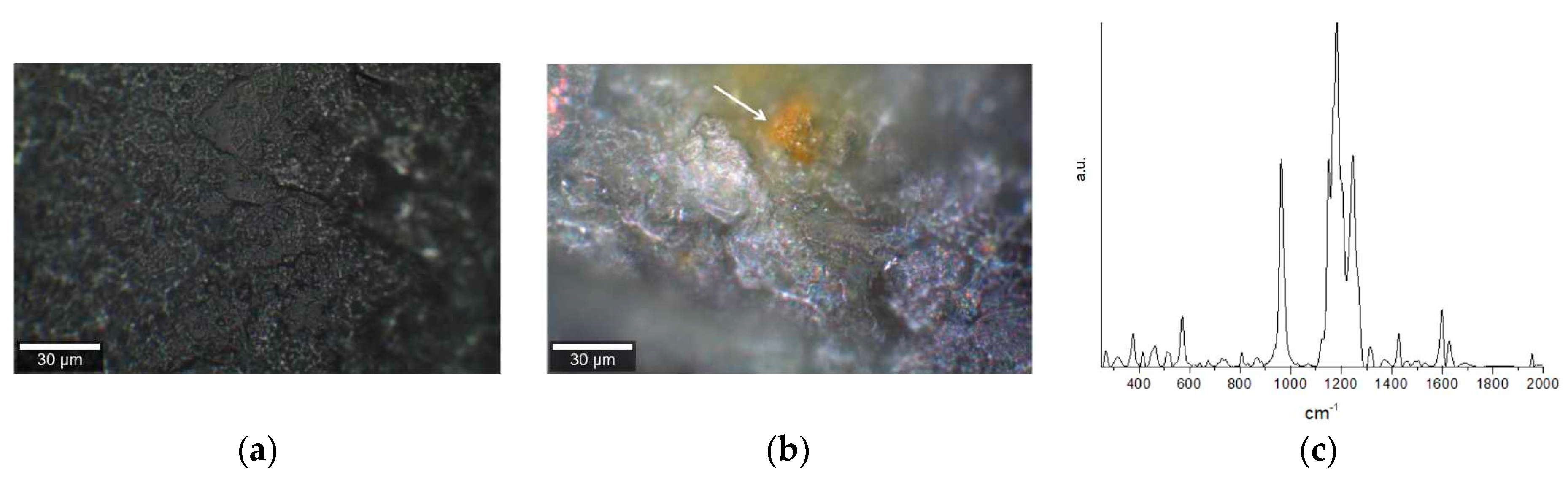
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Raman Microspectroscopy
4.3. Fluorescence Lifetime Imaging Microscopy
4.4. Photodynamic Therapy
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, A.K.; Jain, H.C.; Lynde, C.W.; MacDonald, P.; Cooper, E.A.; Summerbell, R.C. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: A multicenter Canadian survey of 15,000 patients. J. Am. Acad. Dermatol. 2000, 43, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Albert, S.F.; Weis, Z.H. Management of onychomycosis with topicals. Clin. Podiatr. Med. Surg. 2004, 21, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.A.; Guerrer, L.V.; Cunha, K.C.; Soares, M.M.C.N.; de Almeida, M.T.G. Onychomycosis: Clinical, epidemiological and mycological study in the municipality of São José do Rio Preto. Rev. Soc. Bras. Med. Trop. 2007, 40, 596–598. [Google Scholar] [CrossRef] [PubMed]
- Naumann, S.; Meyer, J.P.; Kiesow, A.; Mrestani, Y.; Wohlrab, J.; Neubert, R.H.H. Controlled nail delivery of a novel lipophilic antifungal agent using various modern drug carrier systems as well as in vitro and ex vivo model systems. J. Control. Release 2014, 180, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Van Laborde, S.; Scher, R.K. Developments in the treatment of nail psoriasis, melanonychia striata, and onychomycosis. A review of the literature. Dermatol. Clin. 2000, 18, 37–46. [Google Scholar] [CrossRef]
- Lim, E.H.; Kim, H.R.; Park, Y.O.; Lee, Y.; Seo, Y.J.; Kim, C.D.; Lee, J.H.; Im, M. Toenail onychomycosis treated with a fractional carbon-dioxide laser and topical antifungal cream. J. Am. Acad. Dermatol. 2014, 70, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.P.; Carbinatto, F.M.; Bagnato, V.S.; Inada, N.M. New strategies for treatment of onychomycosis. Photodiagnosis Photodyn. Ther. 2015, 12, 325. [Google Scholar] [CrossRef]
- Da Silva, A.P.; Chiandrone, D.J.; Tinta, J.W.R.; Kurachi, C.; Inada, N.M.; Bagnato, V.S. Development and comparison of two devices for treatment of onychomycosis by photodynamic therapy. J. Biomed. Opt. 2015, 20, 61109. [Google Scholar] [CrossRef] [PubMed]
- Ledon, J.A.; Savas, J.; Franca, K.; Chacon, A.; Nouri, K. Laser and light therapy for onychomycosis: A systematic review. Lasers Med. Sci. 2014, 29, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Paula Da Silva, A.; Carbinatto, F.M.; Bagnato, V.S.; Inada, N.M. A Promising Strategy for the Treatment of Onychomycosis with Curcumin and Photodynamic Therapy. J. Pharm. Pharmacol. 2015, 3, 434–437. [Google Scholar] [CrossRef]
- Silva, A.P.d.; Kurachi, C.; Bagnato, V.S.; Inada, N.M. Fast elimination of onychomycosis by hematoporphyrin derivative-photodynamic therapy. Photodiagnosis Photodyn. Ther. 2013, 10, 328–330. [Google Scholar] [CrossRef]
- Sotiriou, E.; Koussidou-Ermonti, T.; Chaidemenos, G.; Apalla, Z.; Ioannides, D. Photodynamic therapy for distal and lateral subungual toenail onychomycosis caused by Trichophyton rubrum: Preliminary results of a single-centre open trial. Acta Derm. Venereol. 2010, 90, 216–217. [Google Scholar] [CrossRef] [PubMed]
- Sharman, W.M.; Allen, C.M.; van Lier, J.E. Photodynamic therapeutics: Basic principles and clinical applications. Drug Discov. Today 1999, 4, 507–517. [Google Scholar] [CrossRef]
- Leite, I.S.; Geralde, M.C.; Salina, A.C.G.; Medeiros, A.I.; Dovigo, L.N.; Bagnato, V.S.; Inada, N.M. Near–infrared photodynamic inactivation of S. pneumoniae and its interaction with RAW 264.7 macrophages. J. Biophotonics 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Pavarina, A.C.; Ribeiro, A.P.D.; Dovigo, L.N.; de Andrade, C.R.; de Souza Costa, C.A.; Vergani, C.E. Photodynamic Therapy to Eradicate Tumor Cells. In Cell Metabolism; Bubulya, P., Ed.; IntechOpen Limited: London, UK, 2011; p. 149. [Google Scholar]
- Kessel, D. Adventures in photodynamic therapy: 1976–2008. J. Porphyr. Phthalocyanines 2008, 12, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Ramirez, D.P.; Kurachi, C.; Inada, N.M.; Moriyama, L.T.; Salvio, A.G.; Vollet Filho, J.D.; Pires, L.; Buzzá, H.H.; de Andrade, C.T.; Greco, C.; et al. Experience and BCC subtypes as determinants of MAL-PDT response: Preliminary results of a national Brazilian project. Photodiagnosis Photodyn. Ther. 2014, 11, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Geralde, M.C.; Leite, I.S.; Inada, N.M.; Salina, A.C.G.; Medeiros, A.I.; Kuebler, W.M.; Kurachi, C.; Bagnato, V.S. Pneumonia treatment by photodynamic therapy with extracorporeal illumination—An experimental model. Physiol. Rep. 2017, 5, e13190. [Google Scholar] [CrossRef] [PubMed]
- Stritt, A.; Merk, H.F.; Braathen, L.R.; Von Felbert, V. Photodynamic therapy in the treatment of actinic keratosis. Photochem. Photobiol. 2008, 84, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Inada, N.M.; da Costa, M.M.; Guimarães, O.C.C.; da Silva Ribeiro, E.; Kurachi, C.; Quintana, S.M.; Lombardi, W.; Bagnato, V.S. Photodiagnosis and treatment of condyloma acuminatum using 5-aminolevulinic acid and homemade devices. Photodiagnosis Photodyn. Ther. 2012, 9, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Inada, N.M.; Lombardi, W.; Leite, M.F.M.; Trujillo, J.R.; Kurachi, C.; Bagnato, V.S. Photodynamic therapy of cervical intraepithelial neoplasia. In Proceedings of the Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy XXIII, San Francisco, CA, USA, 1–2 February 2014; Volume 8931, pp. 89310X-1–89310X-7. [Google Scholar]
- Oliveira, E.R.d.; Inada, N.M.; Ramirez, D.P.; Bagnato, V.S.; Salvio, A.G. Photodynamic therapy for widespread actinic keratosis of the upper limbs: Comparison of pain and response using aminolevulinic acid 15% and methyl aminolevulinate 15% through a new light source device. Photodiagnosis Photodyn. Ther. 2015, 12, 375. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.A.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin-from molecule to biological function. Angew. Chem. Int. Ed. 2012, 51, 5308–5332. [Google Scholar] [CrossRef] [PubMed]
- Bruzell, E.M.; Morisbak, E.; Tønnesen, H.H. Studies on curcumin and curcuminoids. XXIX. Photoinduced cytotoxicity of curcumin in selected aqueous preparations. Photochem. Photobiol. Sci. 2005, 4, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Wikene, K.O.; Hegge, A.B.; Bruzell, E.; Tonnesen, H.H. Formulation and characterization of lyophilized curcumin solid dispersions for antimicrobial photodynamic therapy (aPDT): Studies on curcumin and curcuminoids LII. Drug Dev. Ind. Pharm. 2015, 41, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using Raman spectroscopy to characterize biological materials. Nat. Protoc. 2016, 11, 664–687. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Wright, K.L.; Ashton, L. Raman spectroscopy: An evolving technique for live cell studies. Analyst 2016, 141, 3590–3600. [Google Scholar] [CrossRef] [PubMed]
- Palonpon, A.F.; Sodeoka, M.; Fujita, K. Molecular imaging of live cells by Raman microscopy. Curr. Opin. Chem. Biol. 2013, 17, 708–715. [Google Scholar] [CrossRef]
- Surmacki, J.; Musial, J.; Kordek, R.; Abramczyk, H. Raman imaging at biological interfaces: Applications in breast cancer diagnosis. Mol. Cancer 2013, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Matthäus, C.; Chernenko, T.; Quintero, L.; Miljković, M.; Milane, L.; Kale, A.; Amiji, M.; Torchilin, V.; Diem, M. Raman Micro-spectral Imaging of Cells and Intracellular Drug Delivery Using Nanocarrier Systems. In Confocal Raman Microscopy; Dieing, T., Hollricher, O., Toporski, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 137–163. ISBN 978-3-642-12522-5. [Google Scholar]
- Lee, A.Y.; Erdemir, D.; Myerson, A.S. Crystal Polymorphism in Chemical Process Development. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 259–280. [Google Scholar] [CrossRef] [PubMed]
- Chemburkar, S.R.; Bauer, J.; Deming, K.; Spiwek, H.; Patel, K.; Morris, J.; Henry, R.; Spanton, S.; Dziki, W.; Porter, W.; et al. Dealing with the impact of ritonavir polymorphs on the late stages of bulk drug process development. Org. Process Res. Dev. 2000, 4, 413–417. [Google Scholar] [CrossRef]
- Hédoux, A.; Guinet, Y.; Paccou, L.; Danède, F.; Derollez, P. Polymorphic transformation of anhydrous caffeine upon grinding and hydrostatic pressurizing analyzed by low-frequency raman spectroscopy. J. Pharm. Sci. 2013, 102, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Piqueras, S.; Duponchel, L.; Tauler, R.; De Juan, A. Monitoring polymorphic transformations by using in situ Raman hyperspectral imaging and image multiset analysis. Anal. Chim. Acta 2014, 819, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.S.; Williams, A.C.; York, P. Particle size dependent molecular rearrangements during the dehydration of trehalose dihydrate-in situ FT-Raman spectroscopy. Pharm. Res. 1998, 15, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hornedo, N.; Nehm, S.J.; Seefeldt, K.F.; Pagán-Torres, Y.; Falkiewicz, C.J. Reaction crystallization of pharmaceutical molecular complexes. Mol. Pharm. 2006, 3, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Lestari, M.L.A.D.; Indrayanto, G. Curcumin; Academic Press: Burlington, NJ, USA, 2014; Volume 39, ISBN 9780128001738. [Google Scholar]
- Van Nong, H.; Hung, L.X.; Thang, P.N.; Chinh, V.D.; Vu, L.V.; Dung, P.T.; Van Trung, T.; Nga, P.T. Fabrication and vibration characterization of curcumin extracted from turmeric (Curcuma longa) rhizomes of the northern Vietnam. Springerplus 2016, 5, 1147. [Google Scholar] [CrossRef]
- Kolev, T.M.; Velcheva, E.A.; Stamboliyska, B.A.; Spiteller, M. DFT and experimental studies of the structure and vibrational spectra of curcumin. Int. J. Quantum Chem. 2005, 102, 1069–1079. [Google Scholar] [CrossRef]
- Tulloch, A.P. The composition of beeswax and other waxes secreted by insects. Lipids 1970, 5, 247–258. [Google Scholar] [CrossRef]
- Bogdanov, S. Beeswax: Production, Properties, Composition, Control. Bee Prod. Sci. 2016, 2, 1–17. [Google Scholar]
- March, J. Advanced Organic Chemistry; Wiley: New York, NY, USA, 1992; ISBN 9780387683508. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iermak, I.; da Silva, A.P.; Kurachi, C.; Bagnato, V.S.; Inada, N.M. Raman Microspectroscopy as a Tool to Elucidate the Efficacy of Topical Formulations Containing Curcumin. Pharmaceuticals 2019, 12, 44. https://doi.org/10.3390/ph12010044
Iermak I, da Silva AP, Kurachi C, Bagnato VS, Inada NM. Raman Microspectroscopy as a Tool to Elucidate the Efficacy of Topical Formulations Containing Curcumin. Pharmaceuticals. 2019; 12(1):44. https://doi.org/10.3390/ph12010044
Chicago/Turabian StyleIermak, Ievgeniia, Ana Paula da Silva, Cristina Kurachi, Vanderlei Salvador Bagnato, and Natalia Mayumi Inada. 2019. "Raman Microspectroscopy as a Tool to Elucidate the Efficacy of Topical Formulations Containing Curcumin" Pharmaceuticals 12, no. 1: 44. https://doi.org/10.3390/ph12010044
APA StyleIermak, I., da Silva, A. P., Kurachi, C., Bagnato, V. S., & Inada, N. M. (2019). Raman Microspectroscopy as a Tool to Elucidate the Efficacy of Topical Formulations Containing Curcumin. Pharmaceuticals, 12(1), 44. https://doi.org/10.3390/ph12010044