Baricitinib: A 2018 Novel FDA-Approved Small Molecule Inhibiting Janus Kinases
Abstract
:1. Introduction
2. Baricitinib
2.1. Names and Structure
2.2. Uses
2.3. Targets
2.4. In Vitro Studies, Rodent Models, and Clinical Trials
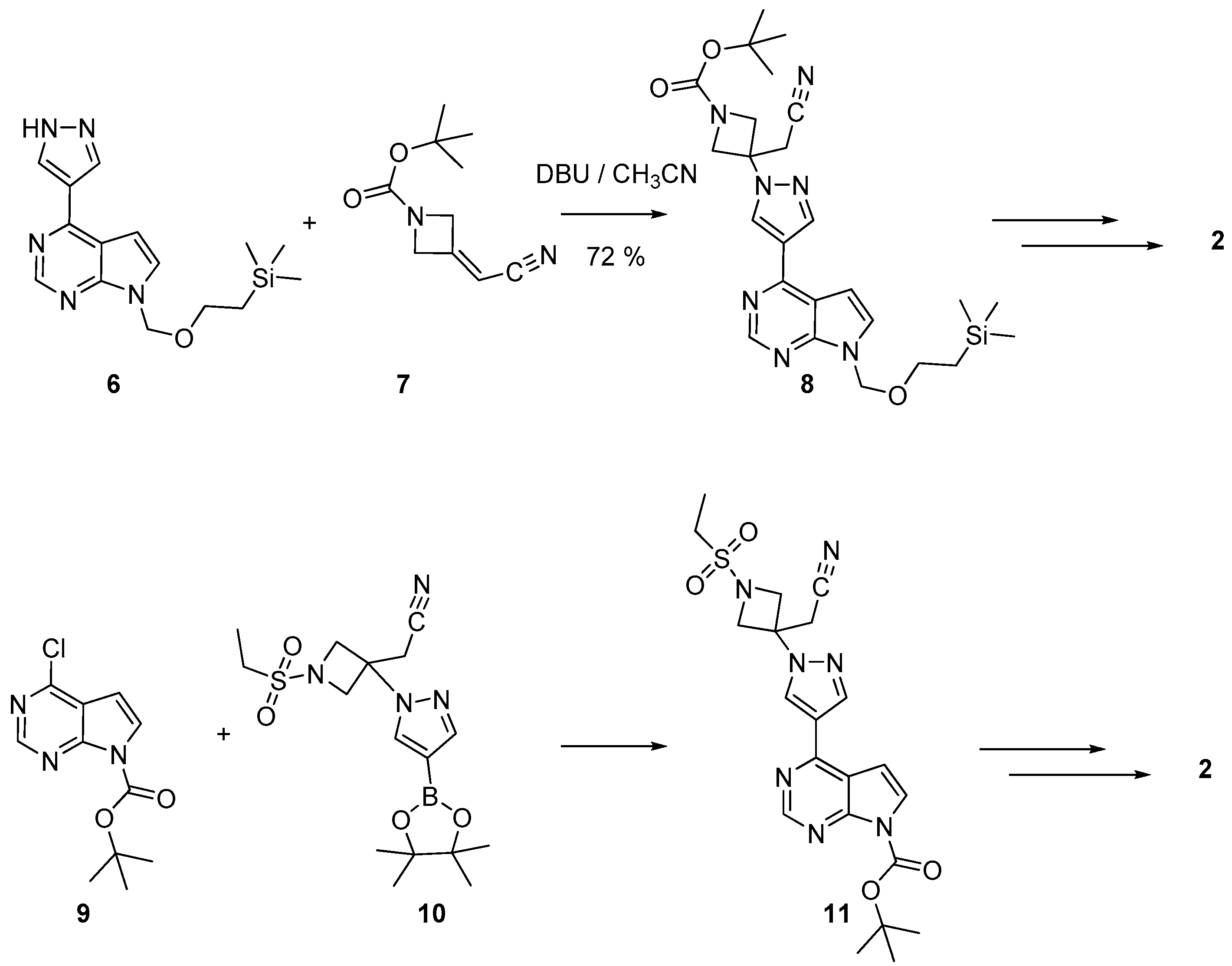
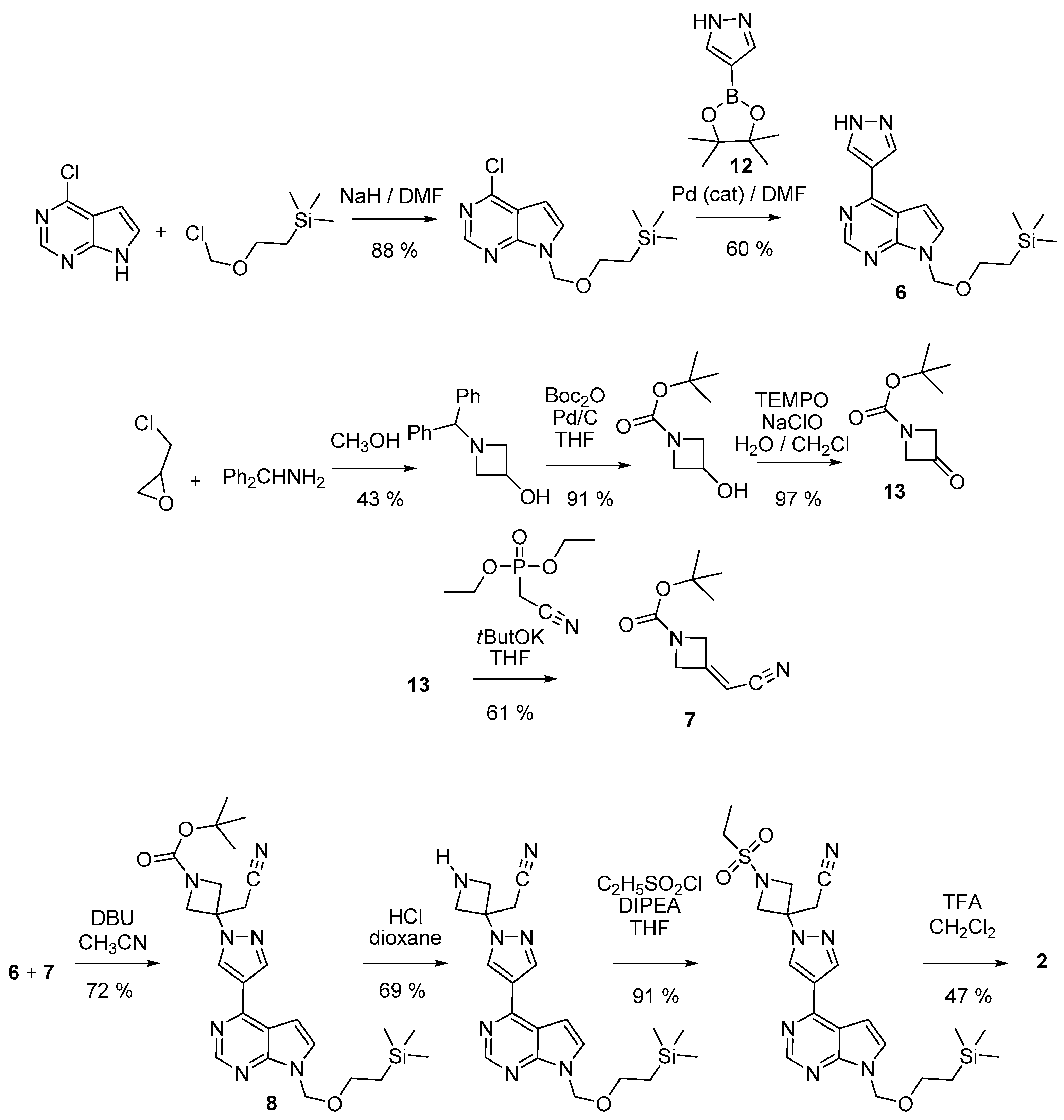
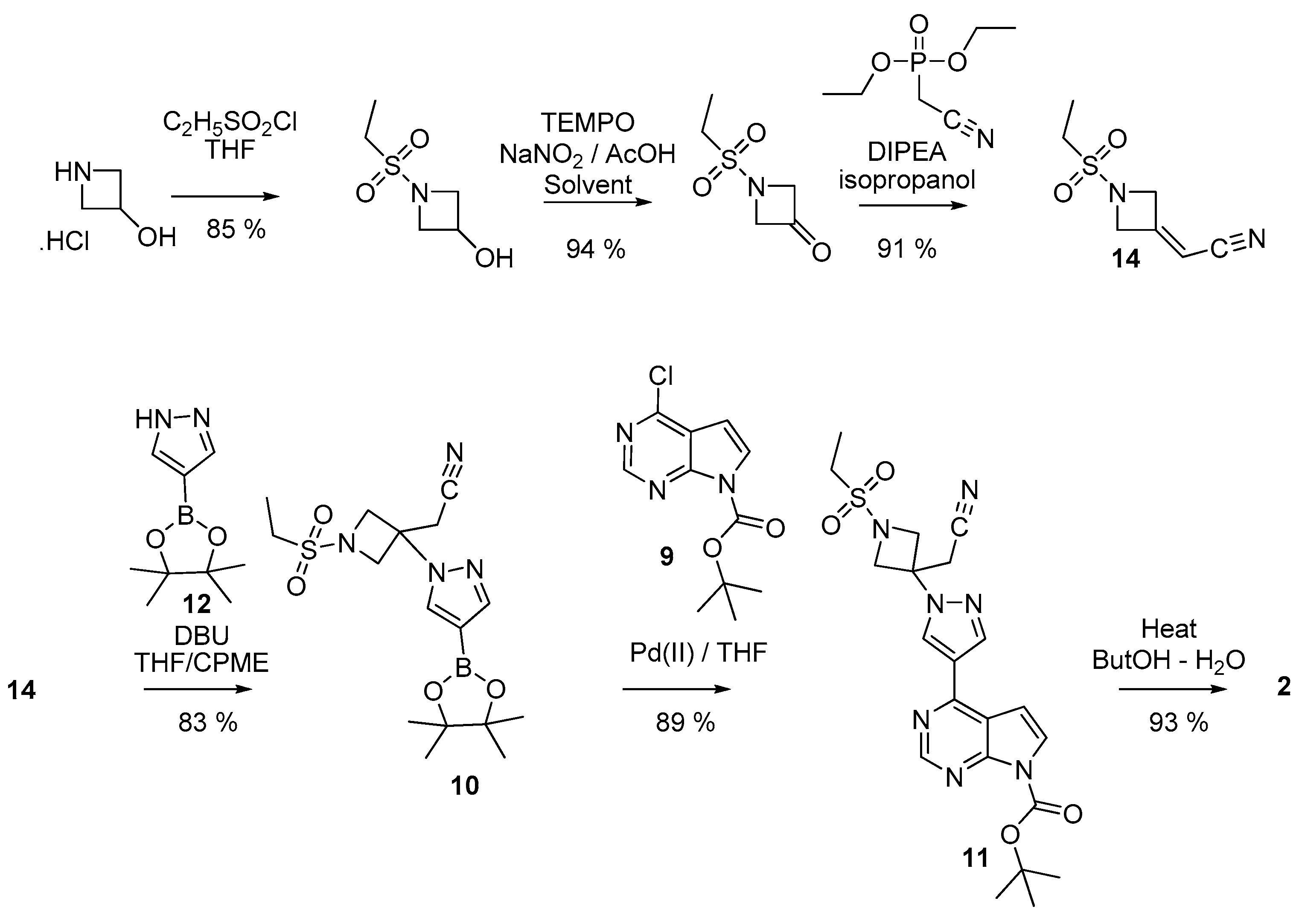
2.5. Syntheses
3. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Wu, P.; Nielsen, T.E.; Clausen, M.H. FDA-Approved Small-molecules Kinase Inhibitors. Trends Pharmacol. Sci. 2015, 36, 422–439. [Google Scholar] [CrossRef]
- CenterWatch. 2001 FDA Approved Drugs. Available online: https://www.centerwatch.com/drug-information/fda-approved-drugs/year/2001 (accessed on 5 March 2019).
- Smolen, J.S.; Genovese, M.C.; Takeuchi, T.; Hyslop, D.L.; Macias, W.L.; Rooney, T.; Chen, L.; Dickson, C.L.; Riddle Camp, J.; Cardillo, T.E.; et al. Safety Profile of Baricitinib in Patients with Active Rheumatoid Arthritis with over 2 Years Median Time in Treatment. J. Rheumatol. 2019, 46, 7–18. [Google Scholar] [CrossRef]
- Drugbank. Baricitinb. Available online: https://www.drugbank.ca/drugs/DB11817 (accessed on 5 March 2019).
- Clarck, J.D.; Flanagan, M.E.; Telliez, J.-B. Discovery and Development of Janus Kinase (JAK) Inhibitors for Inflammatory Diseases. J. Med. Chem. 2014, 57, 5023–5038. [Google Scholar] [CrossRef] [PubMed]
- Villarino, A.V.; Kanno, Y.; O’Shea, J.J. Mechanisms and Consequences of Jak–STAT Signaling in the Immune System. Nat. Immunol. 2017, 18, 374–384. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 16, 843–862. [Google Scholar] [CrossRef] [PubMed]
- Drugbank. Ruxolitinib. Available online: https://www.drugbank.ca/drugs/DB08877 (accessed on 5 March 2019).
- Drugbank. Tofacitinib. Available online: https://www.drugbank.ca/drugs/DB08895 (accessed on 5 March 2019).
- Flanagan, M.E.; Blumenkopf, T.A.; Brissette, W.H.; Brown, M.F.; Casavant, J.M.; Shang-Poa, C.; Doty, J.L.; Elliott, E.A.; Fisher, M.B.; Hines, M.; et al. Discovery of CP-690,550: A Potent and Selective Janus Kinase (JAK) Inhibitor for the Treatment of Autoimmune Diseases and Organ Transplant Rejection. J. Med. Chem. 2010, 53, 8468–8484. [Google Scholar] [CrossRef] [PubMed]
- Furumoto, Y.; Gadina, M. The Arrival of JAK Inhibitors: Advancing the Treatment of Immune and Hematologic Disorders. BioDrugs 2013, 27, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.M.; Bonelli, M.; Gadina, M.; O’Shea, J.J. Type I/II Cytokines, JAKs, and New Strategies for Treating Autoimmune Diseases. Nat. Rev. Rheumatol. 2016, 12, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Fridman, J.S.; Scherle, P.A.; Collins, R.; Burn, T.C.; Li, Y.; Li, J.; Covington, M.B.; Thomas, B.; Collier, P.; Favata, M.F.; et al. Selective Inhibition of JAK1 and JAK2 is Efficacious in Rodent Models of Arthritis: Preclinical Characterization of INCB028050. J. Immunol. 2010, 184, 5298–5307. [Google Scholar] [CrossRef]
- NIH US National Library of Medicine. ClinicalTrials.gov. Baricitinib. Available online: https://www.clinicaltrials.gov/ct2/results?term=baricitinib&age_v=&gndr=&type=&rslt=&Search=Apply (accessed on 5 March 2019).
- Jiang, J.-J.J.; Wang, X.-Y.; Zhang, Y.; Jin, Y.; Lin, J. Advances in the Inhibitors of Janus Kinases. Med. Chem. 2014, 4, 540–548. [Google Scholar] [CrossRef]
- Yamaoka, K. Janus Kinase Inhibitors for Rheumatoid Arthritis. Curr. Opin. Chem. Biol. 2016, 32, 29–33. [Google Scholar] [CrossRef]
- Tanaka, Y.; Emoto, K.; Cai, Z.; Aoki, T.; Schlichting, D.E.; Rooney, T.P.; Macias, W. Efficacy and Safety of Baricitinib in Japanese Patients with Active Rheumatoid Arthritis Receiving Background Methotrexate Therapy: A 12-week, Double-blind, Randomized Placebo-controlled Study. J. Rheumatol. 2016, 43, 3. [Google Scholar] [CrossRef]
- Keystone, E.C.; Taylor, P.C.; Drescher, E.; Schlichting, D.E.; Beattie, S.D.; Berclaz, P.-Y.; Lee, C.H.; Fidelus-Gort, R.K.; Luchi, M.E.; Rooney, T.P.; et al. Safety and Efficacy of Baricitinib at 24 Weeks in Patients with Rheumatoid Arthritis Who Have Had an Inadequate Response to Methotrexate. Ann. Rheum. Dis. 2015, 74, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Kuryia, B.; Cohen, M.D.; Keystone, E.C. Baricitinib in Rheumatoid Arthritis: Evidence-to-date and Clinical Potential. Ther. Adv. Musculoskelet. Dis. 2017, 9, 37–44. [Google Scholar] [CrossRef]
- Rodgers, J.; Shepard, S.; Maduskuie, T.; Wang, H.; Falahatpisheh, N.; Rafalski, M.; Arvanitis, A.; Storace, L.; Jalluri, R.; Fridman, J.; et al. Heteroaryl Substituted Pyrrolo[2,3-b]pyridines and Pyrrolo[2,3-b]pyrimidines as Janus Kinase Inhibitors. US20070135461A1, 14 June 2007. [Google Scholar]
- Rodgers, J.D.; Shepard, S.; Li, Y.-L.; Zhou, J.; Liu, P.; Meloni, D.; Xia, M. Azetidine and Cyclobutane Derivatives as JAK Inhibitors. U.S. Patent US20090233903A1, 17 September 2009. [Google Scholar]
- Kobierski, M.E.; Kopach, M.E.; Martinelli, J.R.; Varie, D.L.; Wilson, T.M. Processes and Intermediates for the Preparation of {1(Ethylsulfonyl)-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1yl]azetidin-3-yl}acetonitrile. WO 2016/ 205487, 22 December 2016. [Google Scholar]
- Xu, J.; Cai, J.; Chen, J.; Zong, X.; Wu, X.; Ji, M.; Wang, P. An Efficient Synthesis of Baricitinib. J. Chem. Res. 2016, 40, 205–208. [Google Scholar] [CrossRef]
- Tung, R.D. Deuterated Baricitinib. U.S. Patent US20180221374A1, 9 August 2018. [Google Scholar]
- Hughes, D.L. Applications of Flow Chemistry in Drug Development: Highlights of Recent Patent Literature. Org. Process Res. Dev. 2018, 22, 13–20. [Google Scholar] [CrossRef]
- Rodgers, J.D.; Wang, H.; Combs, A.P.; Sparks, R.B. Pyrrolo[2,3-b]pyridin-4-yl-amines and Pyrrolo[2,3-b] pyrimidin-5-yl-amines as Janus Kinase Inhibitors. U.S. Patent US9879010B2, 30 January 2018. [Google Scholar]
- Hamaguchi, H.; Amano, Y.; Moritomo, A.; Shirakami, S.; Nakajima, Y.; Nakai, K.; Nomura, N.; Ito, M.; Higashi, Y.; Inoue, T. Discovery and Structural Characterization of Peficitinib (ASP015K) as a Novel and Potent JAK Inhibitor. Bioorg. Med. Chem. 2018, 26, 4971–4983. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, L.; Giovannoni, M.P.; Schepetkin, I.A.; Quinn, M.T.; Khlebnikov, A.I.; Cantini, N.; Guerrini, G.; Iacovone, A.; Teodori, E.; Vergelli, C. 1H-pyrrolo[2,3-b]pyridine: A New Scaffold for Human Neutrophil Elastase (HNE) Inhibitors. Bioorg. Med. Chem. 2018, 26, 5583–5585. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Duan, Y.; Wang, L.; Wang, M.; Ouyang, Y.; Wang, C.; Mei, H.; Tang, S.; Xiong, Y.; Zheng, P.; et al. Synthesis and Antiproliferative Activity of Pyrrolo[2,3-b]pyridine Derivatives Bearing the 1,8-Naphthyridin-2-one Moiety. Eur. J. Med. Chem. 2018, 143, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, S.; Duan, Y.; Liu, X.; Li, X.; Wang, C.; Zhao, B.; Zheng, P.; Zhu, W. Synthesis and Bioevaluation and Docking Study of 1H-pyrrolo[2,3-b]pyridine Derivatives Bearing Aromatic Hydrazone Moiety as c-Met Inhibitors. Eur. J. Med. Chem. 2018, 145, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Günther, M.; Laux, J.; Laufer, S. Synthesis and Structure-activity-relationship of 3,4-Diaryl-1H-pyrrolo[2,3-b]pyridines as Irreversible Inhibitors of Mutant EGFR-L858R/T790M. Eur. J. Pharm. Sci. 2019, 128, 91–96. [Google Scholar] [CrossRef] [PubMed]
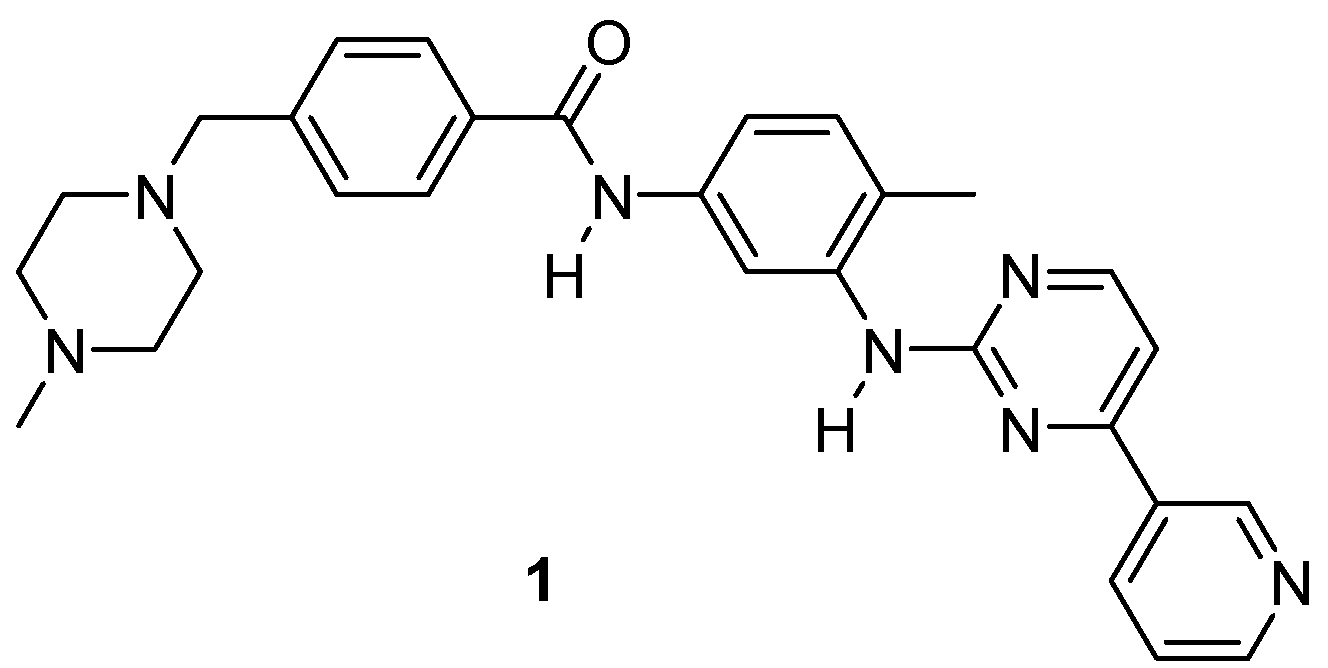
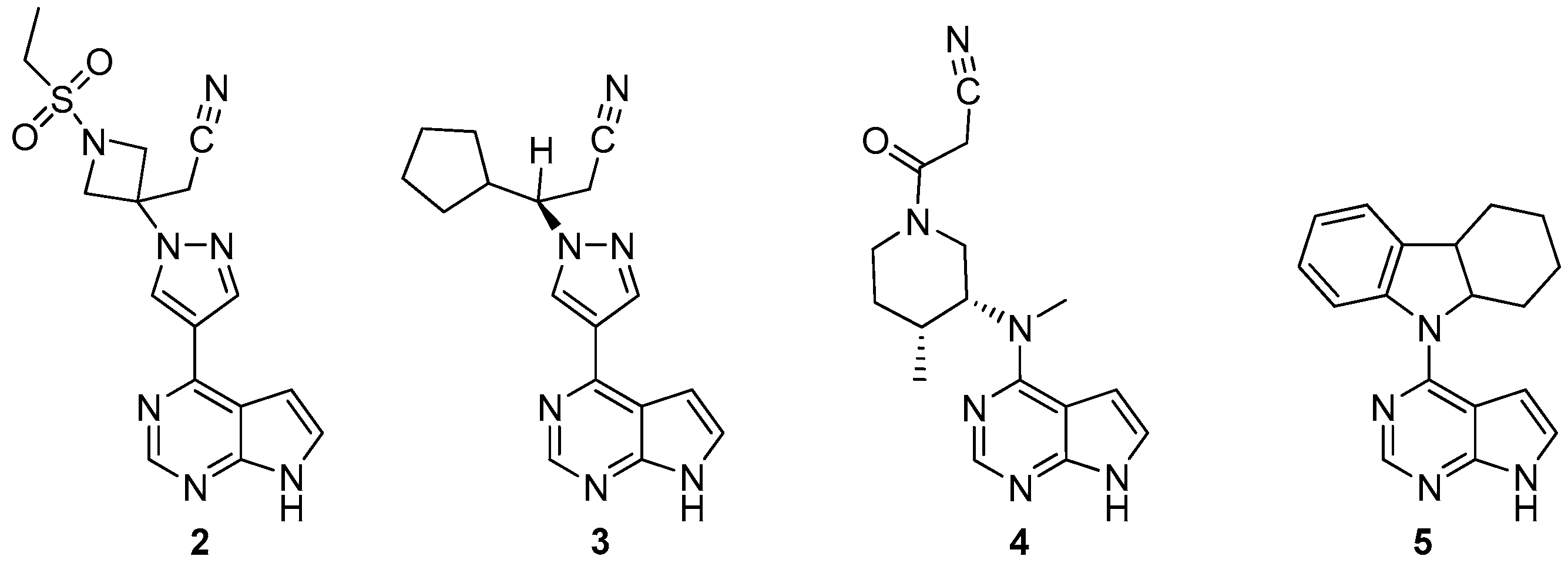
| Compound | IC50 Values, Enzyme Assay (nM) | |||
|---|---|---|---|---|
| JAK1 | JAK2 | JAK3 | TYK2 | |
| 2 | 5.9 | 5.7 | >400 | 53 |
| 3 | 3.3 | 2.8 | 323 | 19 |
| 4 | 3.2 | 4.1 | 1.6 | 34 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayence, A.; Vanden Eynde, J.J. Baricitinib: A 2018 Novel FDA-Approved Small Molecule Inhibiting Janus Kinases. Pharmaceuticals 2019, 12, 37. https://doi.org/10.3390/ph12010037
Mayence A, Vanden Eynde JJ. Baricitinib: A 2018 Novel FDA-Approved Small Molecule Inhibiting Janus Kinases. Pharmaceuticals. 2019; 12(1):37. https://doi.org/10.3390/ph12010037
Chicago/Turabian StyleMayence, Annie, and Jean Jacques Vanden Eynde. 2019. "Baricitinib: A 2018 Novel FDA-Approved Small Molecule Inhibiting Janus Kinases" Pharmaceuticals 12, no. 1: 37. https://doi.org/10.3390/ph12010037
APA StyleMayence, A., & Vanden Eynde, J. J. (2019). Baricitinib: A 2018 Novel FDA-Approved Small Molecule Inhibiting Janus Kinases. Pharmaceuticals, 12(1), 37. https://doi.org/10.3390/ph12010037






