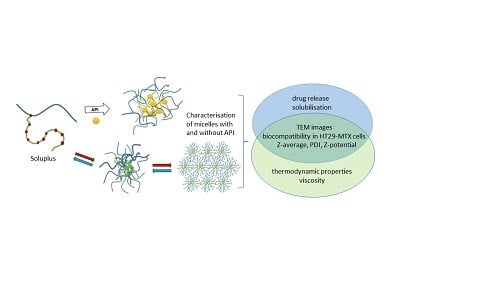
Micellisation Mechanism and Behaviour of Soluplus®–Furosemide Micelles: Preformulation Studies of an Oral Nanocarrier-Based System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
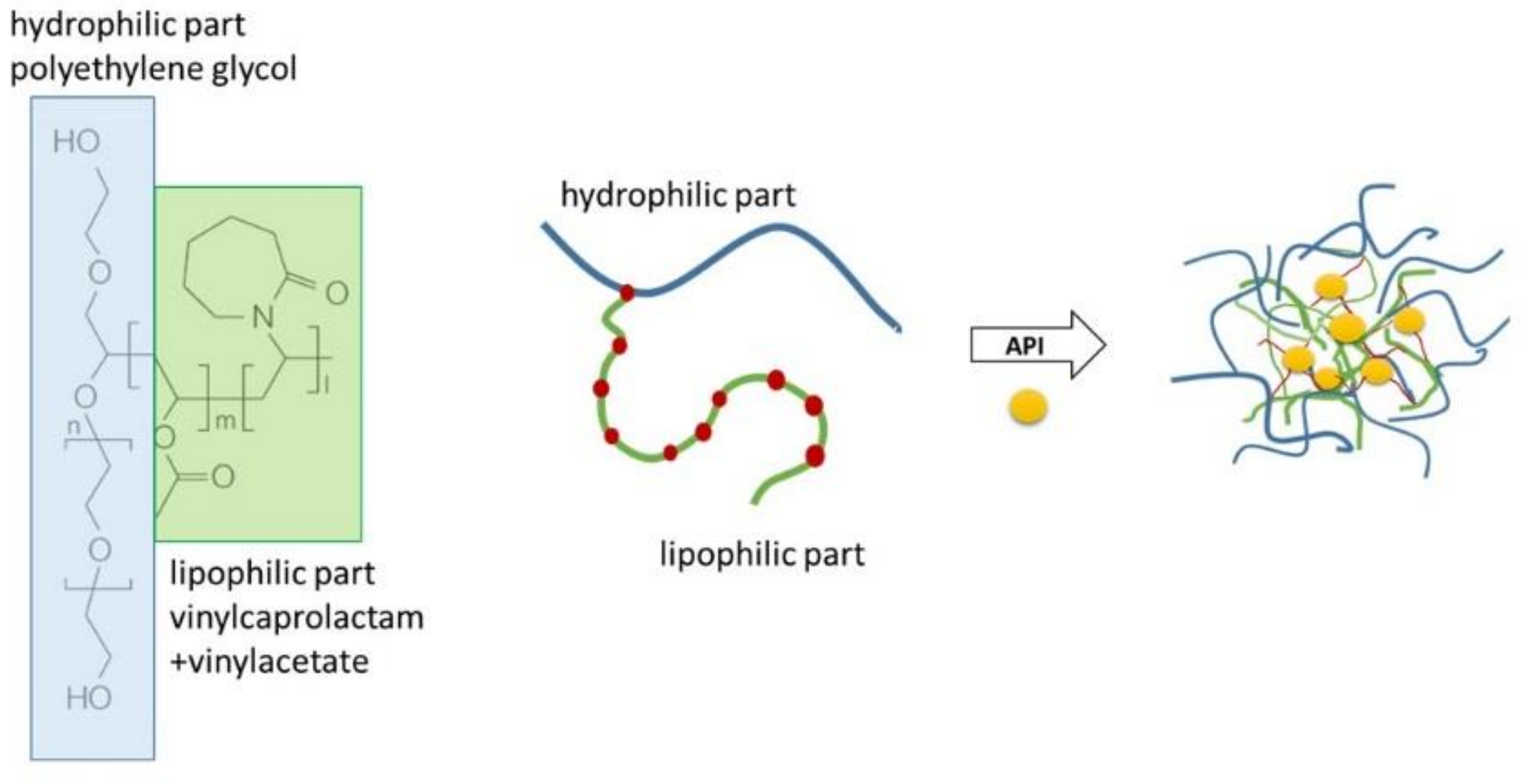
2.2. Preparation of Micellar Dispersions
2.3. Isothermal Titration Calorimetry (ITC)
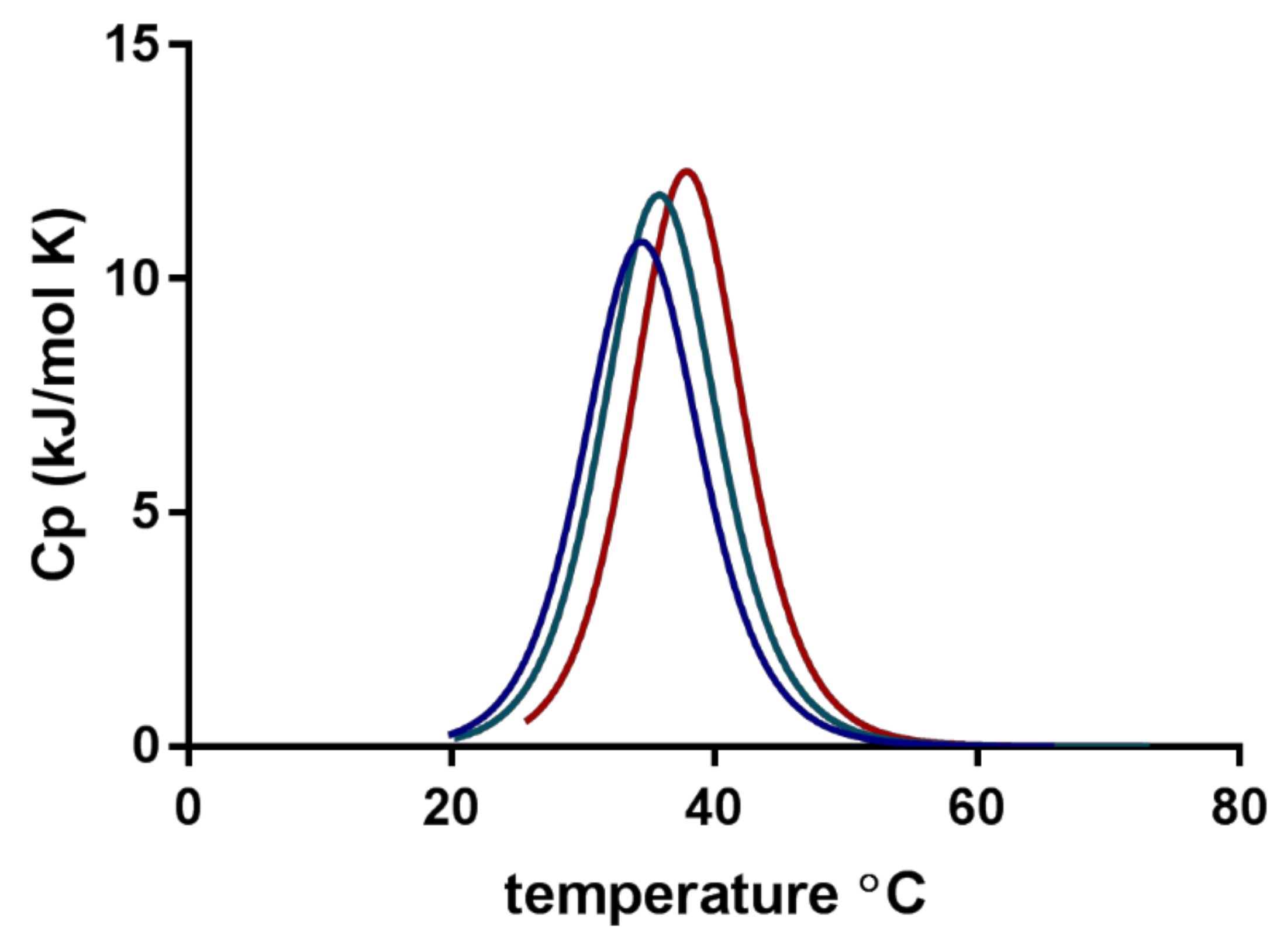
2.4. Nano DSC
2.5. Quantification by HPLC
2.6. Solubility Studies
2.7. Viscosity
2.8. Micellar Size, Polydispersity and Zeta Potential
2.9. Transmission Electron Microscopy (TEM)
2.10. In vitro Drug Release
2.11. Biocompatibility with Mucus-Producing HT29-MTX Cells
2.12. Preparation of Solid Dispersion
2.13. Statistical Analysis
3. Results
3.1. Isothermal Titration Calorimetry (ITC)
3.2. Nano DSC
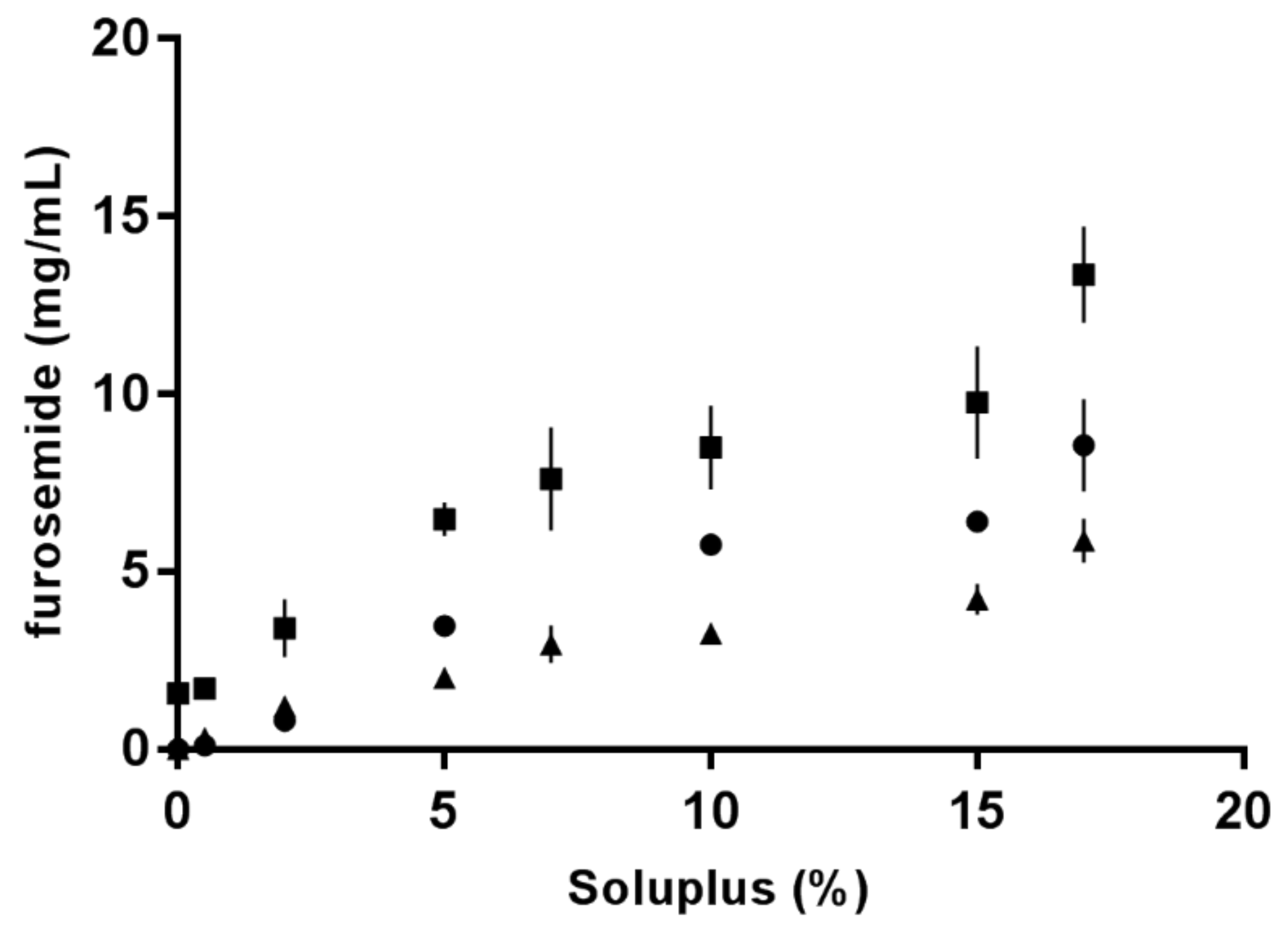
3.3. Solubility of Furosemide
3.4. Viscosity
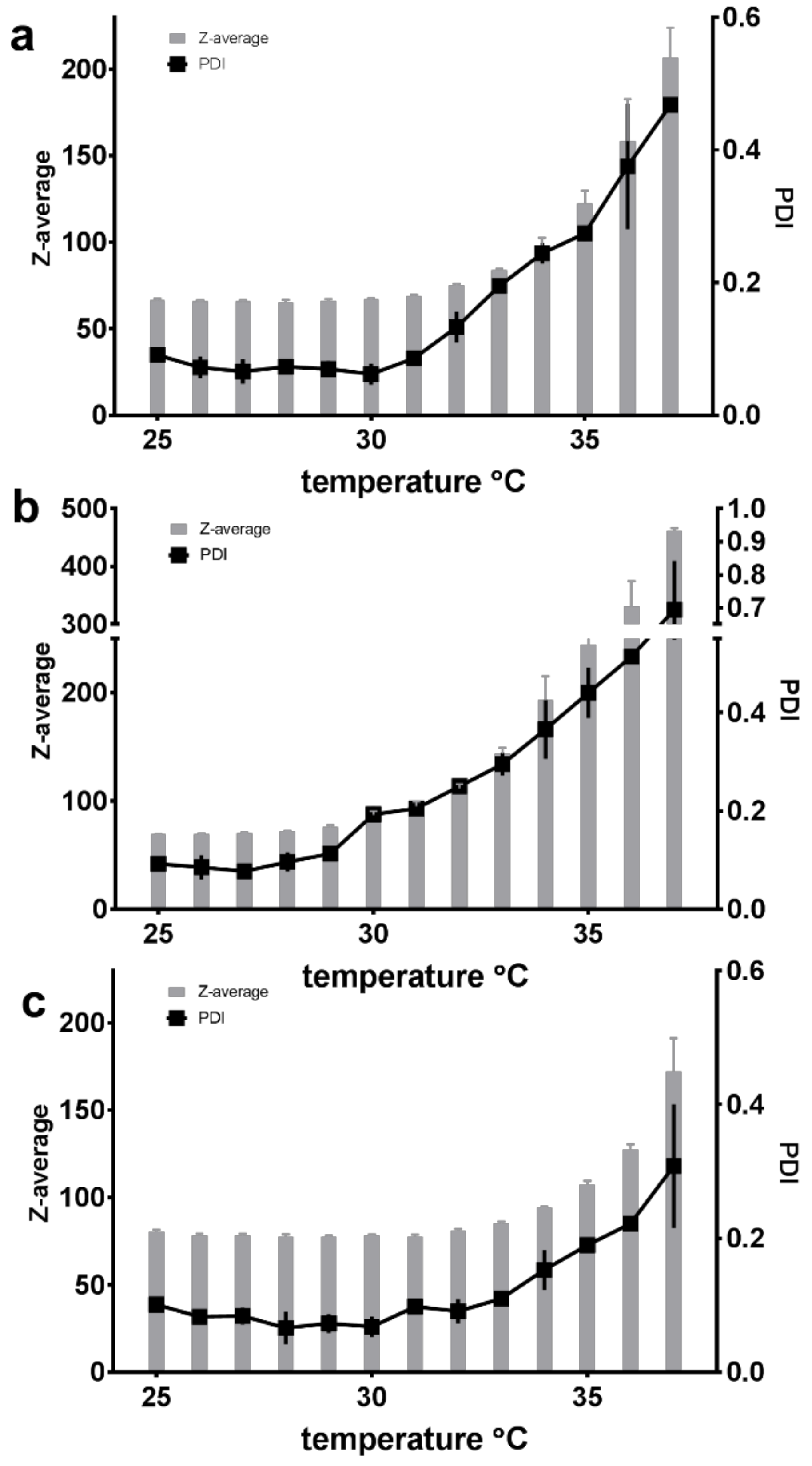
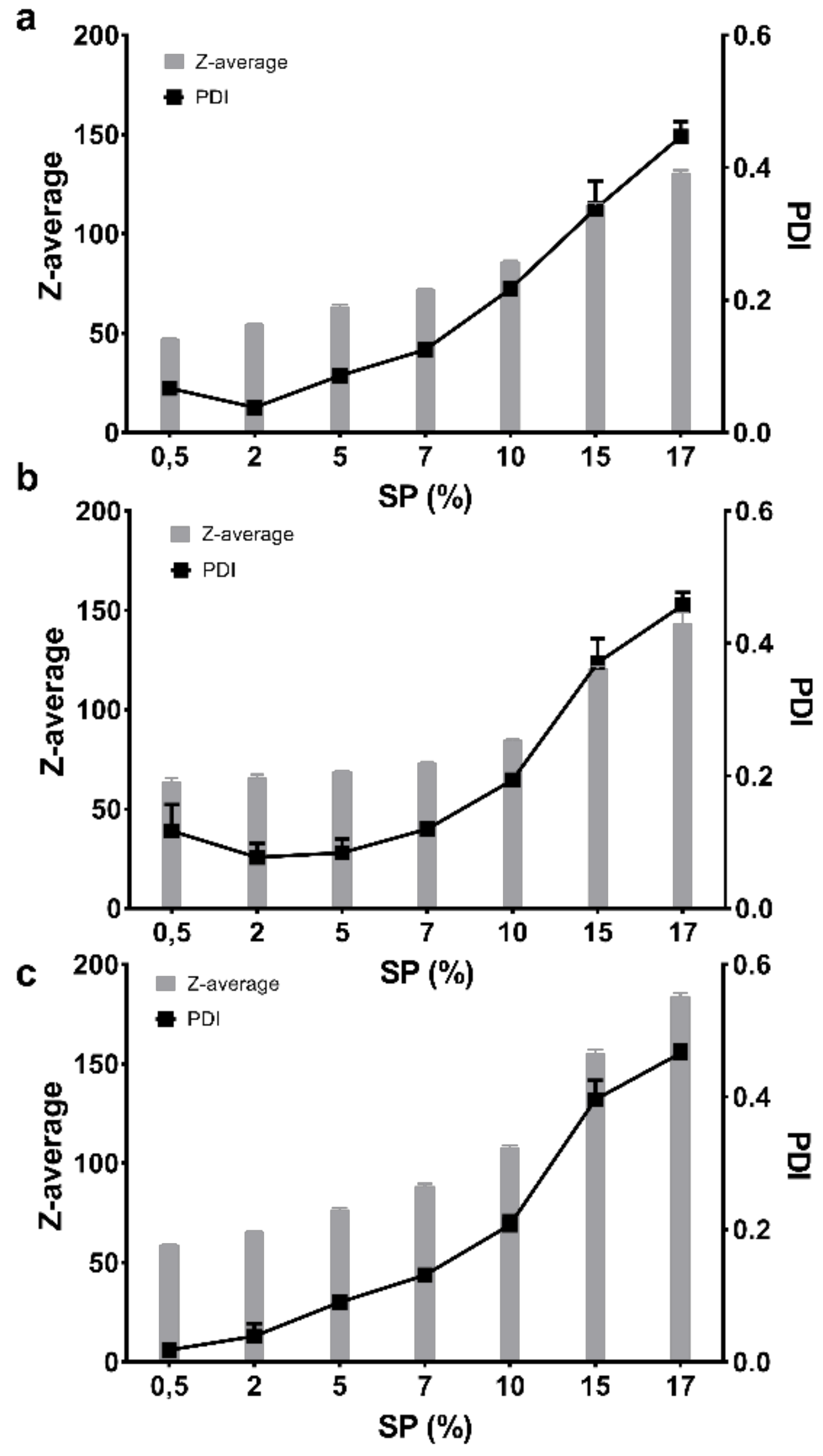
3.5. Micellar Size, Polydispersity and Zeta Potential
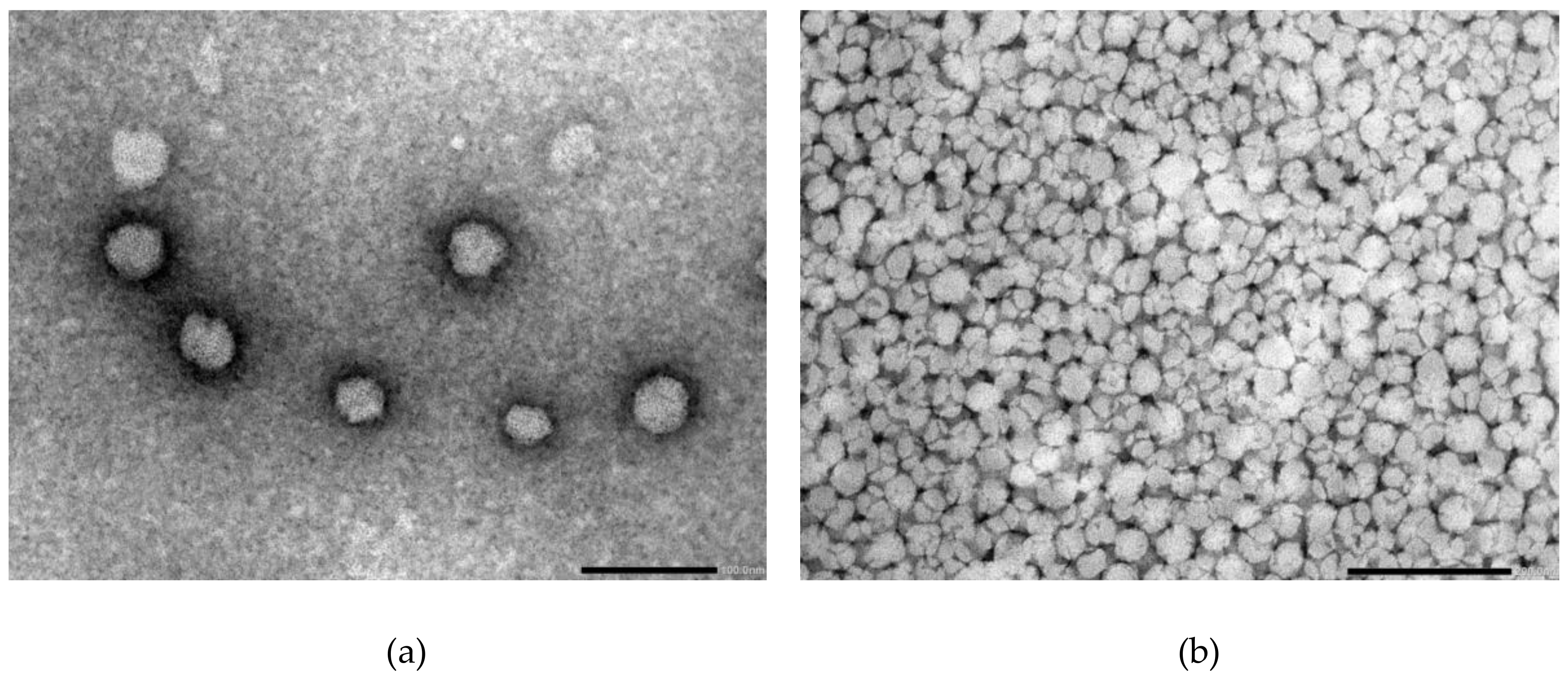
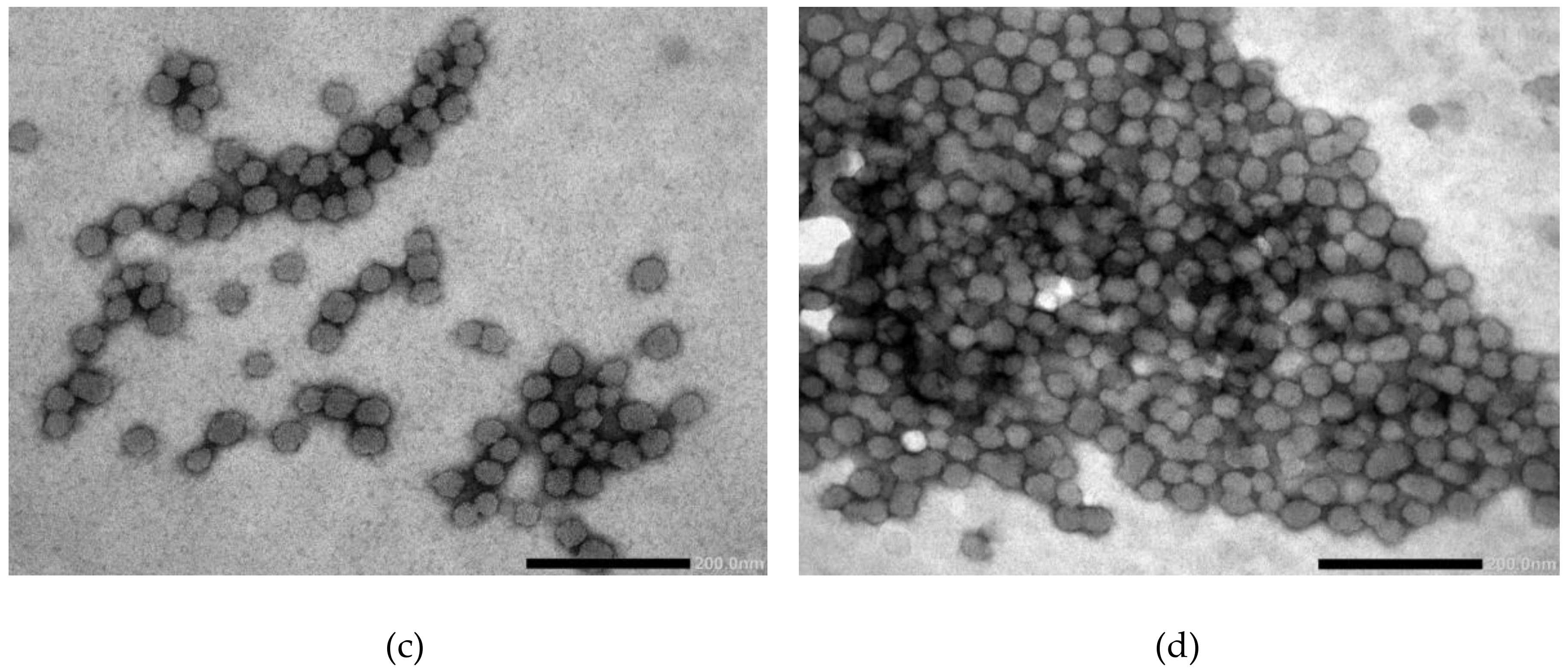
3.6. Transmission Electron Microscopy (TEM)
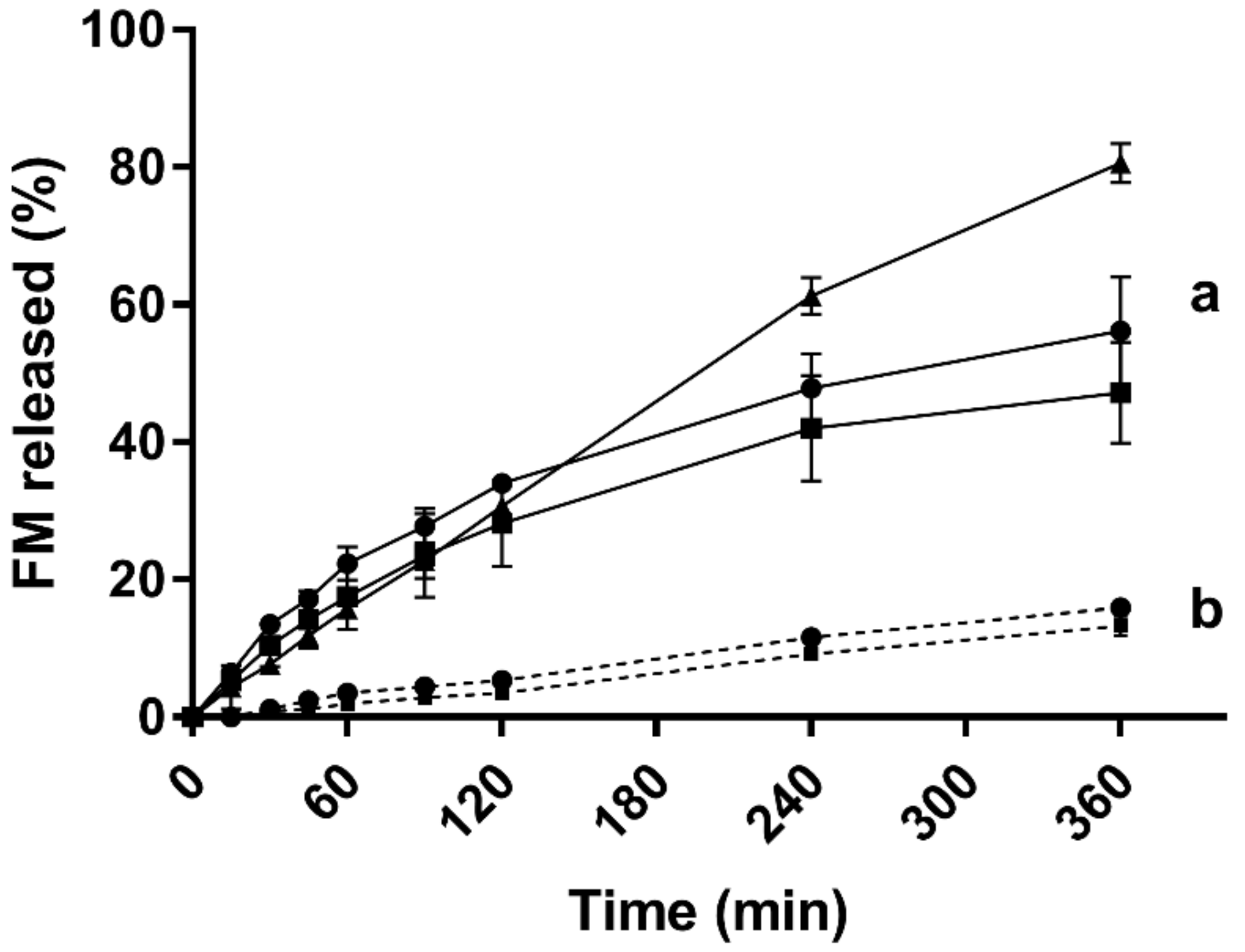
3.7. In vitro Drug Release
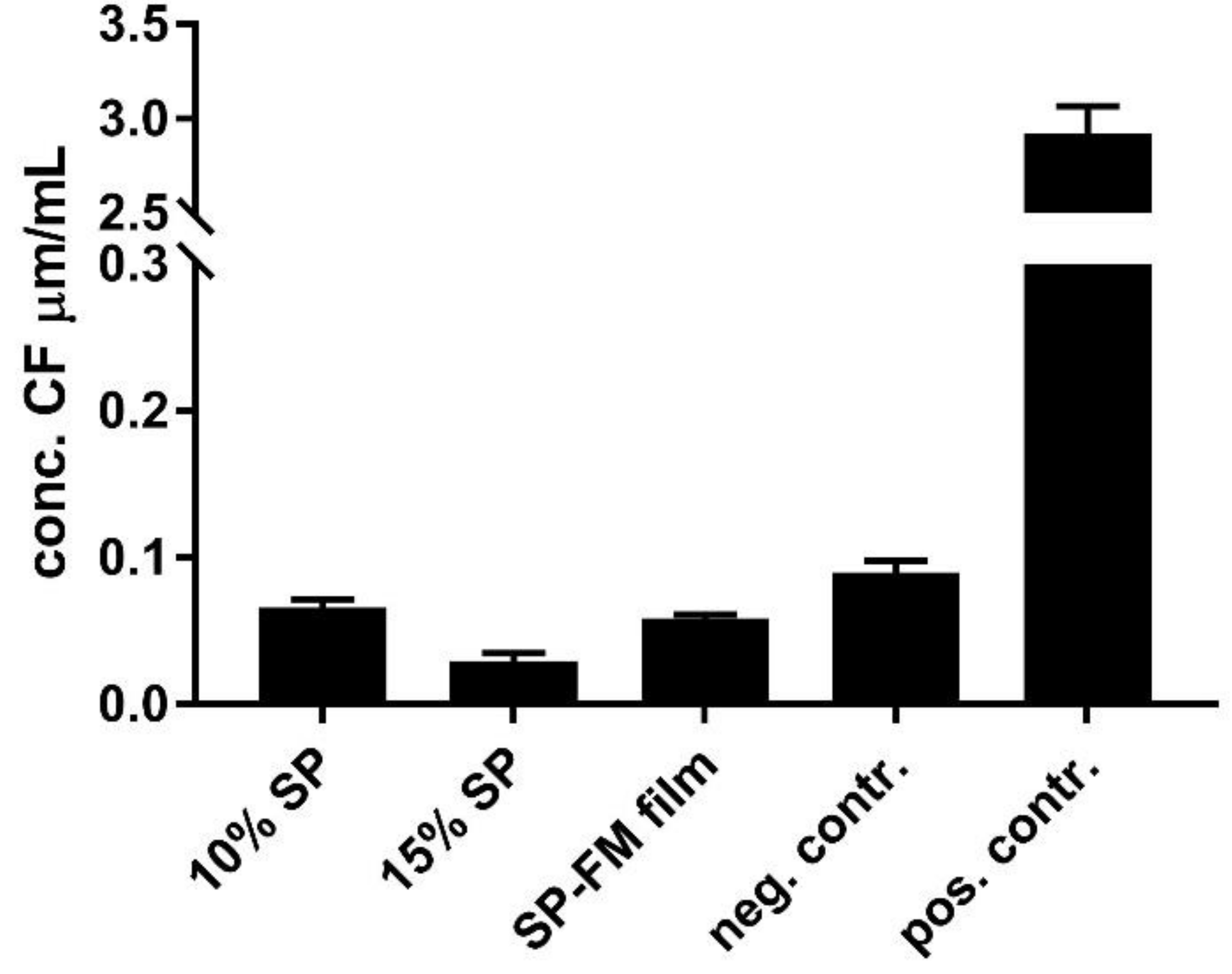
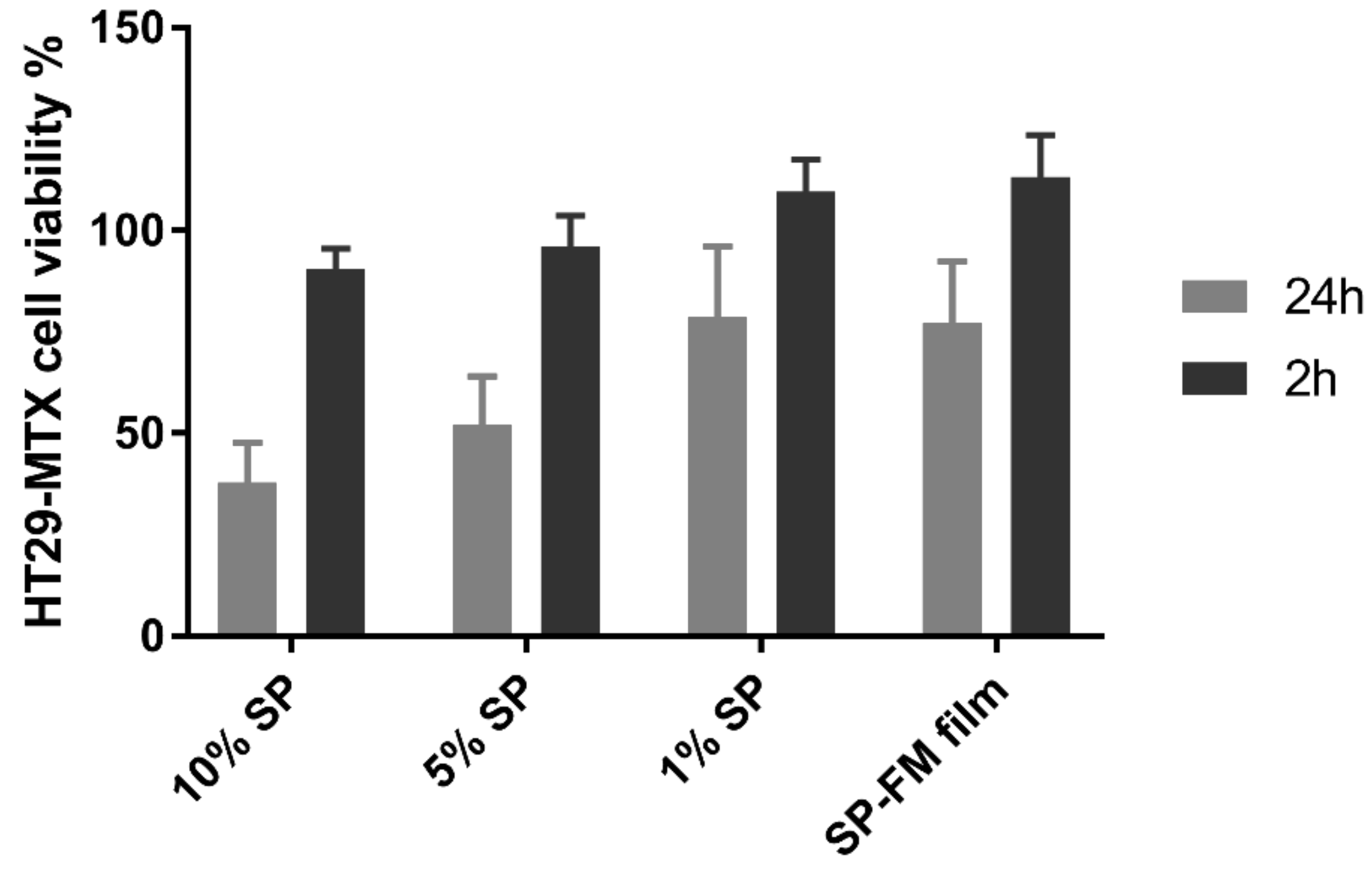
3.8. Biocompatibility with Mucus-Producing HT29-MTX Cells
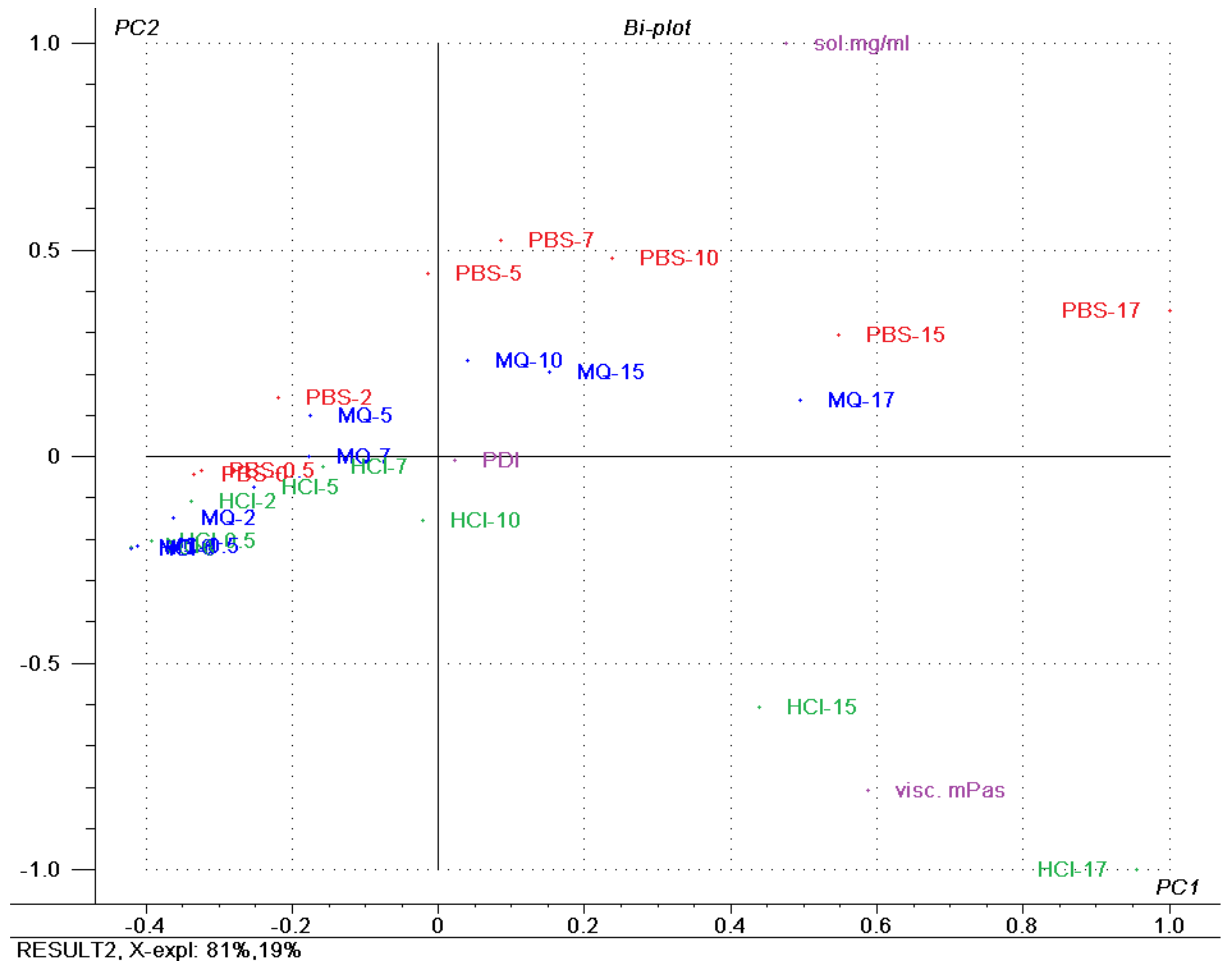
3.9. Principal Component Analysis (PCA)
4. Discussion
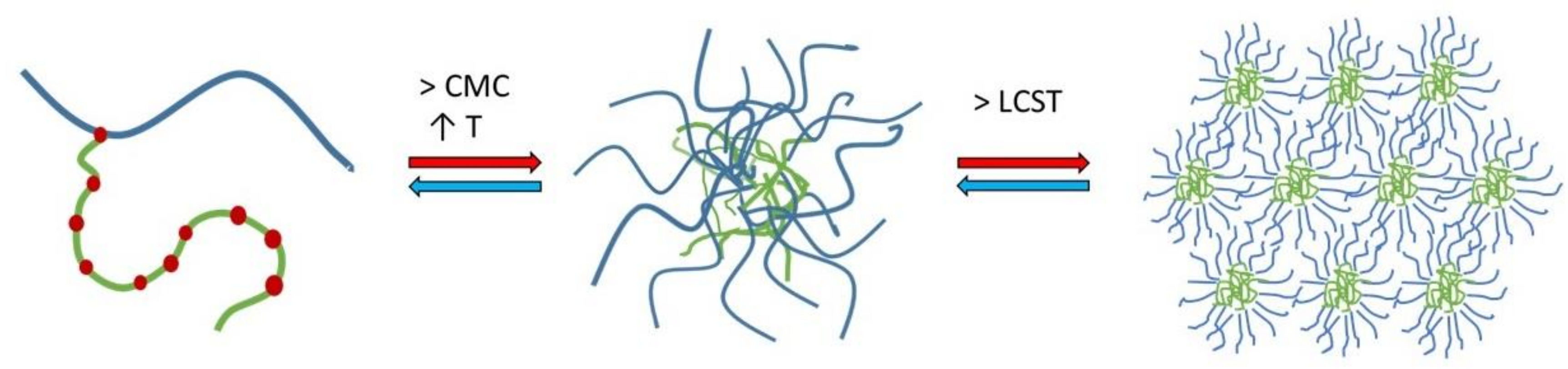
4.1. Micellisation Mechanism of Soluplus®
4.2. Concentration Dependency of Soluplus® Micellar Behaviour
4.3. Implications for Oral Drug Delievry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- BASF. Soluplus-Technical Information; The BASF Chemical Company-Pharma Ingredients and Services: Limburgerhof, Germany, 2010. [Google Scholar]
- Shi, N.-Q.; Lai, H.-W.; Zhang, Y.; Feng, B.; Xiao, X.; Zhang, H.-M.; Li, Z.-Q.; Qi, X.-R. On the inherent properties of Soluplus and its application in ibuprofen solid dispersions generated by microwave-quench cooling technology. Pharm. Dev. Technol. 2018, 23, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.-Q.; Zhang, Y.; Li, Y.; Lai, H.-W.; Xiao, X.; Feng, B.; Qi, X.-R. Self-micellizing solid dispersions enhance the properties and therapeutic potential of fenofibrate: Advantages, profiles and mechanisms. Int. J. Pharm. 2017, 528, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Barmpalexis, P.; Karagianni, A.; Kachrimanis, K. Molecular simulations for amorphous drug formulation: Polymeric matrix properties relevant to hot-melt extrusion. Eur. J. Pharm. Sci. 2018, 119, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Solanki, N.; Gupta, S.S.; Serajuddin, A.T.M. Rheological analysis of itraconazole-polymer mixtures to determine optimal melt extrusion temperature for development of amorphous solid dispersion. Eur. J. Pharm. Sci. 2018, 111, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Dian, L.; Yu, E.; Chen, X.; Wen, X.; Zhang, Z.; Qin, L.; Wang, Q.; Li, G.; Wu, C. Enhancing oral bioavailability of quercetin using novel soluplus polymeric micelles. Nanoscale Res. Lett. 2014, 9, 684. [Google Scholar] [CrossRef] [Green Version]
- Al-Zoubi, N.; Odah, F.; Obeidat, W.; Al-Jaberi, A.; Partheniadis, I.; Nikolakakis, I. Evaluation of Spironolactone Solid Dispersions Prepared by Co-Spray Drying With Soluplus® and Polyvinylpyrrolidone and Influence of Tableting on Drug Release. J. Pharm. Sci. 2018, 107, 2385–2398. [Google Scholar] [CrossRef]
- Tanida, S.; Kurokawa, T.; Sato, H.; Kadota, K.; Tozuka, Y. Evaluation of the micellization mechanism of an amphipathic graft copolymer with enhanced solubility of ipriflavone. Chem. Pharm. Bull. 2016, 64, 68–72. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, C.; Wang, Y.; Xu, Y.; Zhao, J.; Gao, M.; Ding, Y.; Peng, J.; Li, L. Development and evaluation of a novel drug delivery: Soluplus®/TPGS mixed micelles loaded with piperine in vitro and in vivo. Drug Dev. Ind. Pharm. 2018, 44, 1409–1416. [Google Scholar] [CrossRef]
- Wu, H.; Wang, K.; Wang, H.; Chen, F.; Huang, W.; Chen, Y.; Chen, J.; Tao, J.; Wen, X.; Xiong, S. Novel self-assembled tacrolimus nanoparticles cross-linking thermosensitive hydrogels for local rheumatoid arthritis therapy. Colloids Surf. B Biointerfaces 2017, 149, 97–104. [Google Scholar] [CrossRef]
- Varela-Garcia, A.; Concheiro, A.; Alvarez-Lorenzo, C. Soluplus micelles for acyclovir ocular delivery: Formulation and cornea and sclera permeability. Int. J. Pharm. 2018, 552, 39–47. [Google Scholar] [CrossRef]
- Taveira, S.F.; Varela-Garcia, A.; dos Santos Souza, B.; Marreto, R.N.; Martin-Pastor, M.; Concheiro, A.; Alvarez-Lorenzo, C. Cyclodextrin-based poly (pseudo) rotaxanes for transdermal delivery of carvedilol. Carbohydr. Polym. 2018, 200, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Park, K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 2003, 92, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Tomlins, P.; Sahota, T. Thermoresponsive gels. Gels 2017, 3, 4. [Google Scholar] [CrossRef]
- Granero, G.; Longhi, M.; Mora, M.; Junginger, H.; Midha, K.; Shah, V.; Stavchansky, S.; Dressman, J.; Barends, D. Biowaiver monographs for immediate release solid oral dosage forms: Furosemide. J. Pharm. Sci. 2010, 99, 2544–2556. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, M.L.; Campbell, S.A.; Erdman, A.G.; Haynes, C.L.; Wolf, S.M.; McCullough, J. The big picture on nanomedicine: The state of investigational and approved nanomedicine products. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Riché, E.; Carrié, A.; Andin, N.; Mabic, S. High-purity water and pH. Am. Lab. 2006, 38, 22. [Google Scholar]
- Ph.Eur. In European Pharmacopoeia, 8.4 ed.; The European Directorate for the Quality of Medicines: Strasbourg, France, 2015.
- Lesuffleur, T.; Porchet, N.; Aubert, J.-P.; Swallow, D.; Gum, J.R.; Kim, Y.S.; Real, F.X.; Zweibaum, A. Differential expression of the human mucin genes MUC1 to MUC5 in relation to growth and differentiation of different mucus-secreting HT-29 cell subpopulations. J. Cell Sci. 1993, 106, 771–783. [Google Scholar] [PubMed]
- Bouchemal, K.; Agnely, F.; Koffi, A.; Ponchel, G. A concise analysis of the effect of temperature and propanediol-1, 2 on Pluronic F127 micellization using isothermal titration microcalorimetry. J. Colloid Interface Sci. 2009, 338, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Bouchemal, K.; Agnely, F.; Koffi, A.; Djabourov, M.; Ponchel, G. What can isothermal titration microcalorimetry experiments tell us about the self-organization of surfactants into micelles? J. Mol. Recognit. 2010, 23, 335–342. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Esbensen, K.H.; Guyot, D.; Westad, F.; Houmoller, L.P. Multivariate data Analysis: In Practice: An Introduction to Multivariate Data Analysis and Experimental Design; Multivariate Data Analysis. Camo Process AS: Oslo, Norway, 2002. [Google Scholar]
- Higuchi, T.; Connors, K. Phase-solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–122. [Google Scholar]
- He, Z.; Alexandridis, P. Micellization thermodynamics of Pluronic P123 (EO20PO70EO20) amphiphilic block copolymer in aqueous ethylammonium nitrate (EAN) solutions. Polymers 2017, 10, 32. [Google Scholar] [CrossRef]
- Hardung, H.; Djuric, D.; Ali, S. Combining HME & solubilization: Soluplus®—The solid solution. Drug Deliv. Technol. 2010, 10, 20–27. [Google Scholar]
- Cespi, M.; Casettari, L.; Palmieri, G.F.; Perinelli, D.R.; Bonacucina, G. Rheological characterization of polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol graft copolymer (Soluplus®) water dispersions. Colloid Polym. Sci. 2014, 292, 235–241. [Google Scholar] [CrossRef]
- Bernabeu, E.; Gonzalez, L.; Cagel, M.; Gergic, E.P.; Moretton, M.A.; Chiappetta, D.A. Novel Soluplus®—TPGS mixed micelles for encapsulation of paclitaxel with enhanced in vitro cytotoxicity on breast and ovarian cancer cell lines. Colloids Surf. B Biointerfaces 2016, 140, 403–411. [Google Scholar] [CrossRef]
- Yu, H.; Xia, D.; Zhu, Q.; Zhu, C.; Chen, D.; Gan, Y. Supersaturated polymeric micelles for oral cyclosporine A delivery. Eur. J. Pharm. Biopharm. 2013, 85, 1325–1336. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of poly (ethylene oxide)-poly (propylene oxide)-poly (ethylene oxide) triblock copolymers in aqueous solutions: Thermodynamics of copolymer association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Salah, I.; Shamat, M.A.; Cook, M. Soluplus solutions as thermothickening materials for topical drug delivery. J. Appl. Polym. Sci. 2018, 46915. [Google Scholar] [CrossRef]
- Keating, A.V.; Soto, J.; Tuleu, C.; Forbes, C.; Zhao, M.; Craig, D.Q. Solid state characterisation and taste masking efficiency evaluation of polymer based extrudates of isoniazid for paediatric administration. Int. J. Pharm. 2018, 536, 536–546. [Google Scholar] [CrossRef]
- Owens III, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.L.; Mrsny, R.J. Transcellular uptake mechanisms of the intestinal epithelial barrier Part one. Pharm. Sci. Technol. Today 1999, 2, 144–151. [Google Scholar] [CrossRef]
- Semjonov, K.; Lust, A.; Kogermann, K.; Laidmäe, I.; Maunu, S.L.; Hirvonen, S.-P.; Yliruusi, J.; Nurk, G.; Lust, E.; Heinämäki, J. Melt-electrospinning as a method to improve the dissolution and physical stability of a poorly water-soluble drug. Eur. J. Pharm. Sci. 2018, 121, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Lesuffleur, T.; Barbat, A.; Dussaulx, E.; Zweibaum, A. Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res. 1990, 50, 6334–6343. [Google Scholar] [PubMed]
- Pontier, C.; Pachot, J.; Botham, R.; Lenfant, B.; Arnaud, P. HT29-MTX and Caco-2/TC7 monolayers as predictive models for human intestinal absorption: Role of the mucus layer. J. Pharm. Sci. 2001, 90, 1608–1619. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, M.I.; Hagesaether, E.; Smistad, G.; Hiorth, M. An in vitro study of mucoadhesion and biocompatibility of polymer coated liposomes on HT29-MTX mucus-producing cells. Int. J. Pharm. 2016, 498, 225–233. [Google Scholar] [CrossRef]
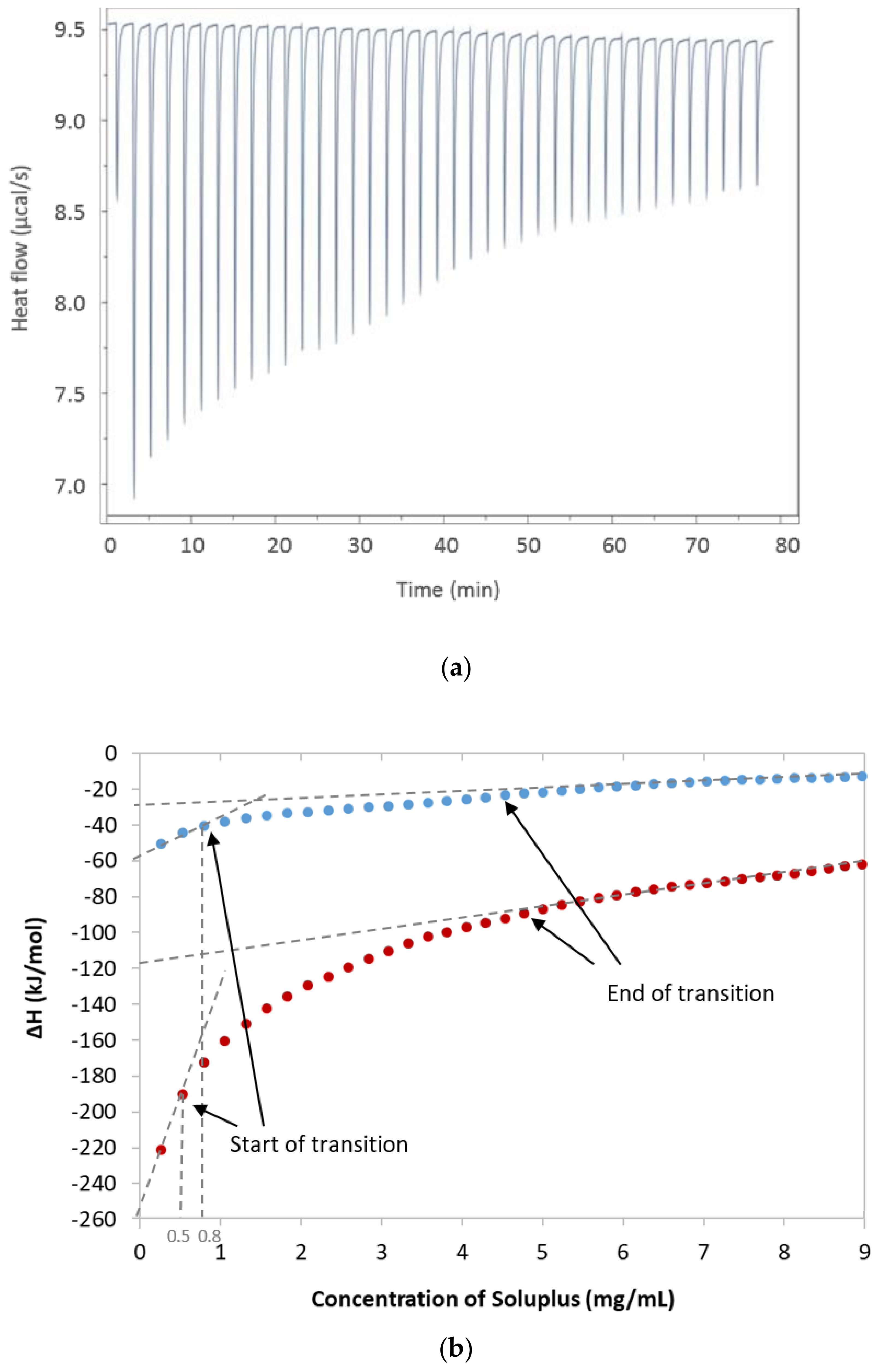
| Temperature (°C) | Sample Cell | CMC (mg/mL) | ΔHmic (KJ·mol-1) | TΔSmic (KJ·mol−1) | ΔGmic (KJ·mol−1) |
|---|---|---|---|---|---|
| 25 25 | Milli-Q water 0.065 mM FM | 0.8 0.5 | 26.3 ± 2.1 132.3 ± 4.6 | 55.7 168.7 | −29.4 −30.6 |
| 37 | Milli-Q water | 0.5 | 12.6 ± 0.1 | 44.4 | −31.8 |
| Test Medium | Furosemide (mg/mL) | Tm (°C) | ΔH (kJ·mol−1) |
|---|---|---|---|
| PBS pH 7.4 | - | 33.5 ± 0.3 | 176.0 ± 93.0 |
| PBS pH 7.4 | 0.05 | 32.8 ± 0.6 | 158.5 ± 99.1 |
| Milli-Q water | - | 36.0 ± 0.1 | 145.6 ± 72.2 |
| Milli-Q water | 0.05 | 35.7 ± 0.2 | 139.5 ± 21.8 |
| 0.1 M HCl | - | 38.2 ± 0.5 | 116.7 ± 23.0 |
| 0.1 M HCl | 0.05 | 37.8 ± 0.6 | 174.6 ± 44.8 |
| Conc. of Soluplus (% w/w) | Viscosity (mPa·s) | ||
|---|---|---|---|
| Milli-Q water | PBS pH 7.4 | 0.1 M HCl | |
| 0 | 0.96 ± 0.06 | 0.98 ± 0.01 | 0.97 ± 0.04 |
| 0.5 | 1.02 ± 0.01 | 1.03 ± 0.01 | 1.15 ± 0.14 |
| 2 | 1.17 ± 0.03 | 1.22 ± 0.03 | 1.23 ± 0.01 |
| 5 | 1.79 ± 0.02 | 1.76 ± 0.02 | 1.84 ± 0.04 |
| 7 | 2.21 ± 0.02 | 2.32 ± 0.04 | 2.49 ± 0.04 |
| 10 | 3.16 ± 0.01 | 3.87 ± 0.04 | 4.25 ± 0.06 |
| 15 | 4.28 ± 0.04 | 7.44 ± 0.54 | 10.33 ± 0.18 |
| 17 | 7.65 ± 0.03 | 11.26 ± 0.78 | 16.64 ± 0.47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alopaeus, J.F.; Hagesæther, E.; Tho, I. Micellisation Mechanism and Behaviour of Soluplus®–Furosemide Micelles: Preformulation Studies of an Oral Nanocarrier-Based System. Pharmaceuticals 2019, 12, 15. https://doi.org/10.3390/ph12010015
Alopaeus JF, Hagesæther E, Tho I. Micellisation Mechanism and Behaviour of Soluplus®–Furosemide Micelles: Preformulation Studies of an Oral Nanocarrier-Based System. Pharmaceuticals. 2019; 12(1):15. https://doi.org/10.3390/ph12010015
Chicago/Turabian StyleAlopaeus, Julia F., Ellen Hagesæther, and Ingunn Tho. 2019. "Micellisation Mechanism and Behaviour of Soluplus®–Furosemide Micelles: Preformulation Studies of an Oral Nanocarrier-Based System" Pharmaceuticals 12, no. 1: 15. https://doi.org/10.3390/ph12010015
APA StyleAlopaeus, J. F., Hagesæther, E., & Tho, I. (2019). Micellisation Mechanism and Behaviour of Soluplus®–Furosemide Micelles: Preformulation Studies of an Oral Nanocarrier-Based System. Pharmaceuticals, 12(1), 15. https://doi.org/10.3390/ph12010015