Can the Efficacy of [18F]FDG-PET/CT in Clinical Oncology Be Enhanced by Screening Biomolecular Profiles?
Abstract
:1. Introduction
1.1. Positron Emission Tomography
1.2. 2-Deoxy-2-[18F]fluoroglucose ([18F]FDG)
1.3. Limitations: False Positives and Negatives
1.4. Clinical Importance
2. Factors Affecting the Clinical Efficacy of PET
2.1. Gross Features
2.1.1. Tumour Size
2.1.2. Tumour Grade
2.1.3. Cellularity
2.2. Molecular Features
2.2.1. Heterogeneity
2.2.2. Metabolism
2.2.3. Hypoxia
2.2.4. Angiogenesis
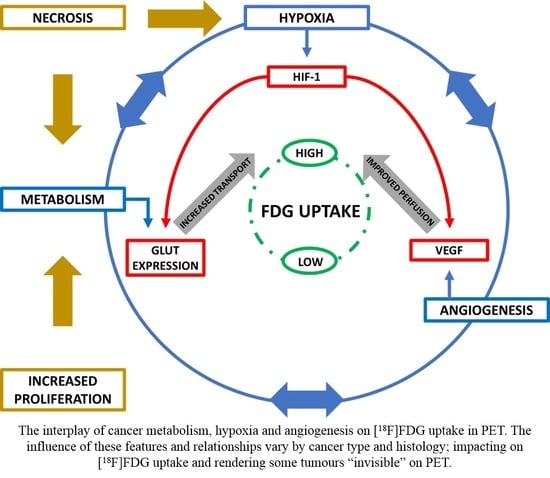
2.3. Interplay of Biological Features
2.4. Other Factors
2.4.1. P-glycoprotein
2.4.2. Tumour Suppressor Genes
2.4.3. Patient Factors
3. Optimising PET with Biomolecular Profiling
3.1. Stratification
3.2. Diagnosis and Predicting Prognosis
4. Profiling Specific Cancer Types
4.1. Oesophageal Cancer
4.2. Breast Cancer
4.3. Non-Small Cell Lung Cancer (NSCLC)
4.4. Glioma
4.5. Head and Neck Cancer
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Slobbe, P.; Windhorst, A.D.; Stigter-van Walsum, M.; Schuit, R.C.; Smit, E.F.; Niessen, H.G.; Solca, F.; Stehle, G.; van Dongen, G.A.; Poot, A.J. Development of [18f]afatinib as new tki-pet tracer for egfr positive tumours. Nucl. Med. Boil. 2014, 41, 749–757. [Google Scholar] [CrossRef]
- Tian, M.; Zhang, H.; Nakasone, Y.; Mogi, K.; Endo, K. Expression of glut-1 and glut-3 in untreated oral squamous cell carcinoma compared with fdg accumulation in a pet study. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.M.; Lee, H.J.; Goo, J.M.; Lee, H.Y.; Lee, J.J.; Chung, J.K.; Im, J.G. False positive and false negative fdg-pet scans in various thoracic diseases. Korean J. Radiol. 2006, 7, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Kubota, R.; Kubota, K.; Yamada, S.; Tada, M.; Ido, T.; Tamahashi, N. Active and passive mechanisms of [fluorine-18] fluorodeoxyglucose uptake by proliferating and prenecrotic cancer cells in vivo: A microautoradiographic study. J. Nucl. Med. 1994, 35, 1067–1075. [Google Scholar] [PubMed]
- Warning, K.; Hildebrandt, M.G.; Kristensen, B.; Ewertz, M. Utility of 18fdg-pet/ct in breast cancer diagnostics—A systematic review. Dan. Med. Bull. 2011, 58, A4289. [Google Scholar]
- Purohit, B.S.; Ailianou, A.; Dulguerov, N.; Becker, C.D.; Ratib, O.; Becker, M. Fdg-pet/ct pitfalls in oncological head and neck imaging. Insights Imaging 2014, 5, 585–602. [Google Scholar] [CrossRef] [PubMed]
- Jo, I.; Zeon, S.K.; Kim, S.H.; Kim, H.W.; Kang, S.H.; Kwon, S.Y.; Kim, S.J. Correlation of primary tumour fdg uptake with clinicopathologic prognostic factors in invasive ductal carcinoma of the breast. Nucl. Med. Mol. Imaging 2015, 49, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Balogova, S.; Huchet, V.; Kerrou, K.; Nataf, V.; Gutman, F.; Antoine, M.; Ruppert, A.M.; Prignon, A.; Lavolee, A.; Montravers, F.; et al. Detection of bronchioloalveolar cancer by means of pet/ct and 18f-fluorocholine and comparison with 18f-fluorodeoxyglucose. Nucl. Med. Commun. 2010, 31, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.C.; See, L.C.; Lai, C.H.; Yah-Huei, C.W.; Ng, K.K.; Ma, S.Y.; Lin, W.J.; Chen, J.T.; Chen, W.J.; Lai, C.R.; et al. 18f-fdg uptake in squamous cell carcinoma of the cervix is correlated with glucose transporter 1 expression. J. Nucl. Med. 2004, 45, 22–29. [Google Scholar]
- Society of Nuclear Medicine and Molecular Imaging. NCCN Practice Guidelines: Narrative Summary of Indications for FDG Pet and PET/CT; 2016. Available online: http://snmmi.files.cms-plus.com/images/NCCN%20Narrative%20Summary%20Feb%202016.pdf (accessed on 22 January 2019).
- Higashi, T.; Saga, T.; Nakamoto, Y.; Ishimori, T.; Fujimoto, K.; Doi, R.; Imamura, M.; Konishi, J. Diagnosis of pancreatic cancer using fluorine-18 fluorodeoxyglucose positron emission tomography (fdg pet)—Usefulness and limitations in “clinical reality”. Ann. Nucl. Med. 2003, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Adler, L.P.; Blair, H.F.; Makley, J.T.; Williams, R.P.; Joyce, M.J.; Leisure, G.; al-Kaisi, N.; Miraldi, F. Noninvasive grading of musculoskeletal tumours using pet. J. Nucl. Med. 1991, 32, 1508–1512. [Google Scholar]
- Berger, K.L.; Nicholson, S.A.; Dehdashti, F.; Siegel, B.A. Fdg pet evaluation of mucinous neoplasms: Correlation of fdg uptake with histopathologic features. Am. J. Roentgenol. 2000, 174, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.T.; Kim, Y.; Lee, K.S.; Yoon, S.B.; Cheon, E.M.; Kwon, O.J.; Rhee, C.H.; Han, J.; Shin, M.H. Localized form of bronchioloalveolar carcinoma: Fdg pet findings. Am. J. Roentgenol. 1998, 170, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K.; Ueda, Y.; Seki, H.; Yuasa, K.; Oguchi, M.; Noguchi, T.; Taniguchi, M.; Tonami, H.; Okimura, T.; Yamamoto, I. Fluorine-18-fdg pet imaging is negative in bronchioloalveolar lung carcinoma. J. Nucl. Med. 1998, 39, 1016–1020. [Google Scholar] [PubMed]
- Norikane, T.; Yamamoto, Y.; Maeda, Y.; Kudomi, N.; Matsunaga, T.; Haba, R.; Iwasaki, A.; Hoshikawa, H.; Nishiyama, Y. Correlation of (18)f-fluoromisonidazole pet findings with hif-1alpha and p53 expressions in head and neck cancer: Comparison with (18)f-fdg pet. Nucl. Med. Commun. 2014, 35, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Pugachev, A.; Ruan, S.; Carlin, S.; Larson, S.M.; Campa, J.; Ling, C.C.; Humm, J.L. Dependence of fdg uptake on tumor microenvironment. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Tamaki, N.; Honda, T.; Torizuka, T.; Kimura, T.; Inokuma, T.; Ohshio, G.; Hosotani, R.; Imamura, M.; Konishi, J. Expression of glucose transporters in human pancreatic tumors compared with increased fdg accumulation in pet study. J. Nucl. Med. 1997, 38, 1337–1344. [Google Scholar]
- Bos, R.; van Der Hoeven, J.J.; van Der Wall, E.; van Der Groep, P.; van Diest, P.J.; Comans, E.F.; Joshi, U.; Semenza, G.L.; Hoekstra, O.S.; Lammertsma, A.A.; et al. Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J. Clin. Oncol. 2002, 20, 379–387. [Google Scholar] [CrossRef]
- Kurokawa, T.; Yoshida, Y.; Kawahara, K.; Tsuchida, T.; Okazawa, H.; Fujibayashi, Y.; Yonekura, Y.; Kotsuji, F. Expression of glut-1 glucose transfer, cellular proliferation activity and grade of tumor correlate with [f-18]-fluorodeoxyglucose uptake by positron emission tomography in epithelial tumors of the ovary. Int. J. Cancer 2004, 109, 926–932. [Google Scholar] [CrossRef]
- Kunkel, M.; Reichert, T.E.; Benz, P.; Lehr, H.A.; Jeong, J.H.; Wieand, S.; Bartenstein, P.; Wagner, W.; Whiteside, T.L. Overexpression of glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer 2003, 97, 1015–1024. [Google Scholar] [CrossRef]
- Kurata, T.; Oguri, T.; Isobe, T.; Ishioka, S.; Yamakido, M. Differential expression of facilitative glucose transporter (glut) genes in primary lung cancers and their liver metastases. Jpn. J. Cancer Res. GANN 1999, 90, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, M.; Sloof, G.W.; Hoekstra, O.S.; Ten Kate, F.J.; Meijer, G.A.; Reitsma, J.B.; Boellaard, R.; van Lanschot, J.J.; Molthoff, C.F. 18fdg uptake in oesophageal adenocarcinoma: Linking biology and outcome. J. Cancer Res. Clin. Oncol. 2008, 134, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Tsukamoto, E.; Kuge, Y.; Kanegae, K.; Zhao, S.; Hikosaka, K.; Hosokawa, M.; Kohanawa, M.; Tamaki, N. Fdg uptake and glucose transporter subtype expressions in experimental tumor and inflammation models. J. Nucl. Med. 2001, 42, 1551–1555. [Google Scholar] [PubMed]
- Denko, N.C. Hypoxia, hif1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer 2008, 8, 705–713. [Google Scholar] [CrossRef]
- Dearling, J.L.; Flynn, A.A.; Sutcliffe-Goulden, J.; Petrie, I.A.; Boden, R.; Green, A.J.; Boxer, G.M.; Begent, R.H.; Pedley, R.B. Analysis of the regional uptake of radiolabeled deoxyglucose analogs in human tumor xenografts. J. Nucl. Med. 2004, 45, 101–107. [Google Scholar] [PubMed]
- Aronen, H.J.; Pardo, F.S.; Kennedy, D.N.; Belliveau, J.W.; Packard, S.D.; Hsu, D.W.; Hochberg, F.H.; Fischman, A.J.; Rosen, B.R. High microvascular blood volume is associated with high glucose uptake and tumor angiogenesis in human gliomas. Clin. Cancer Res. 2000, 6, 2189–2200. [Google Scholar] [PubMed]
- Laking, G.; Price, P. Radionuclide imaging of perfusion and hypoxia. Eur. J. Nucl. Med. Mol. Imaging 2010, 37 (Suppl. 1), S20–S29. [Google Scholar] [CrossRef] [PubMed]
- De Bruyne, S.; Van Damme, N.; Smeets, P.; Ferdinande, L.; Ceelen, W.; Mertens, J.; Van de Wiele, C.; Troisi, R.; Libbrecht, L.; Laurent, S.; et al. Value of dce-mri and fdg-pet/ct in the prediction of response to preoperative chemotherapy with bevacizumab for colorectal liver metastases. Br. J. Cancer 2012, 106, 1926–1933. [Google Scholar] [CrossRef]
- Colavolpe, C.; Chinot, O.; Metellus, P.; Mancini, J.; Barrie, M.; Bequet-Boucard, C.; Tabouret, E.; Mundler, O.; Figarella-Branger, D.; Guedj, E. Fdg-pet predicts survival in recurrent high-grade gliomas treated with bevacizumab and irinotecan. Neuro Oncol. 2012, 14, 649–657. [Google Scholar] [CrossRef]
- Goshen, E.; Davidson, T.; Zwas, S.T.; Aderka, D. Pet/ct in the evaluation of response to treatment of liver metastases from colorectal cancer with bevacizumab and irinotecan. Technol. Cancer Res. Treat. 2006, 5, 37–43. [Google Scholar] [CrossRef]
- Crippa, F.; Seregni, E.; Agresti, R.; Chiesa, C.; Pascali, C.; Bogni, A.; Decise, D.; De Sanctis, V.; Greco, M.; Daidone, M.G.; et al. Association between [18f]fluorodeoxyglucose uptake and postoperative histopathology, hormone receptor status, thymidine labelling index and p53 in primary breast cancer: A preliminary observation. Eur. J. Nucl. Med. 1998, 25, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Schulte, M.; Brecht-Krauss, D.; Heymer, B.; Guhlmann, A.; Hartwig, E.; Sarkar, M.R.; Diederichs, C.G.; Schultheiss, M.; Kotzerke, J.; Reske, S.N. Fluorodeoxyglucose positron emission tomography of soft tissue tumours: Is a non-invasive determination of biological activity possible? Eur. J. Nucl. Med. 1999, 26, 599–605. [Google Scholar] [CrossRef]
- Smith, T.A. Influence of chemoresistance and p53 status on fluoro-2-deoxy-d-glucose incorporation in cancer. Nucl. Med. Biol. 2010, 37, 51–55. [Google Scholar] [CrossRef]
- Seo, S.; Hatano, E.; Higashi, T.; Nakajima, A.; Nakamoto, Y.; Tada, M.; Tamaki, N.; Iwaisako, K.; Kitamura, K.; Ikai, I.; et al. P-glycoprotein expression affects 18f-fluorodeoxyglucose accumulation in hepatocellular carcinoma in vivo and in vitro. Int. J. Oncol. 2009, 34, 1303–1312. [Google Scholar]
- Berkers, C.R.; Maddocks, O.D.; Cheung, E.C.; Mor, I.; Vousden, K.H. Metabolic regulation by p53 family members. Cell Metab. 2013, 18, 617–633. [Google Scholar] [CrossRef]
- Groheux, D.; Giacchetti, S.; Moretti, J.L.; Porcher, R.; Espie, M.; Lehmann-Che, J.; de Roquancourt, A.; Hamy, A.S.; Cuvier, C.; Vercellino, L.; et al. Correlation of high 18f-fdg uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 426–435. [Google Scholar] [CrossRef]
- Sasaki, M.; Sugio, K.; Kuwabara, Y.; Koga, H.; Nakagawa, M.; Chen, T.; Kaneko, K.; Hayashi, K.; Shioyama, Y.; Sakai, S.; et al. Alterations of tumor suppressor genes (rb, p16, p27 and p53) and an increased fdg uptake in lung cancer. Ann. Nucl. Med. 2003, 17, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.C.; Turkington, T.G.; Wilson, J.M.; Wong, T.Z. A systematic review of the factors affecting accuracy of suv measurements. Am. J. Roentgenol. 2010, 195, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Bares, R.; Klever, P.; Hauptmann, S.; Hellwig, D.; Fass, J.; Cremerius, U.; Schumpelick, V.; Mittermayer, C.; Bull, U. F-18 fluorodeoxyglucose pet in vivo evaluation of pancreatic glucose metabolism for detection of pancreatic cancer. Radiology 1994, 192, 79–86. [Google Scholar] [CrossRef]
- Friess, H.; Langhans, J.; Ebert, M.; Beger, H.G.; Stollfuss, J.; Reske, S.N.; Buchler, M.W. Diagnosis of pancreatic cancer by 2[18f]-fluoro-2-deoxy-d-glucose positron emission tomography. Gut 1995, 36, 771–777. [Google Scholar] [CrossRef]
- Zimny, M.; Bares, R.; Fass, J.; Adam, G.; Cremerius, U.; Dohmen, B.; Klever, P.; Sabri, O.; Schumpelick, V.; Buell, U. Fluorine-18 fluorodeoxyglucose positron emission tomography in the differential diagnosis of pancreatic carcinoma: A report of 106 cases. Eur. J. Nucl. Med. 1997, 24, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Diederichs, C.G.; Staib, L.; Glatting, G.; Beger, H.G.; Reske, S.N. Fdg pet: Elevated plasma glucose reduces both uptake and detection rate of pancreatic malignancies. J. Nucl. Med. 1998, 39, 1030–1033. [Google Scholar]
- Zhao, S.; Kuge, Y.; Nakada, K.; Mochizuki, T.; Takei, T.; Okada, F.; Tamaki, N. Effect of steroids on [18f]fluorodeoxyglucose uptake in an experimental tumour model. Nucl. Med. Commun. 2004, 25, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, B.A.; Starmans, M.H.; Leijenaar, R.T.; Dubois, L.J.; van der Kogel, A.J.; Kaanders, J.H.; Boutros, P.C.; Lambin, P.; Bussink, J. Systematic analysis of 18f-fdg pet and metabolism, proliferation and hypoxia markers for classification of head and neck tumors. BMC Cancer 2014, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Rege, S.; Safa, A.A.; Chaiken, L.; Hoh, C.; Juillard, G.; Withers, H.R. Positron emission tomography: An independent indicator of radiocurability in head and neck carcinomas. Am. J. Clin. Oncol. 2000, 23, 164–169. [Google Scholar] [CrossRef]
- Sepesi, B.; Raymond, D.P.; Polomsky, M.; Watson, T.J.; Litle, V.R.; Jones, C.E.; Hu, R.; Qiu, X.; Peters, J.H. Does the value of pet-ct extend beyond pretreatment staging? An analysis of survival in surgical patients with esophageal cancer. J. Gastrointest. Surg. 2009, 13, 2121–2127. [Google Scholar] [CrossRef]
- Suzuki, A.; Xiao, L.; Hayashi, Y.; Macapinlac, H.A.; Welsh, J.; Lin, S.H.; Lee, J.H.; Bhutani, M.S.; Maru, D.M.; Hofstetter, W.L.; et al. Prognostic significance of baseline positron emission tomography and importance of clinical complete response in patients with esophageal or gastroesophageal junction cancer treated with definitive chemoradiotherapy. Cancer 2011, 117, 4823–4833. [Google Scholar] [CrossRef]
- Minn, H.; Lapela, M.; Klemi, P.J.; Grenman, R.; Leskinen, S.; Lindholm, P.; Bergman, J.; Eronen, E.; Haaparanta, M.; Joensuu, H. Prediction of survival with fluorine-18-fluoro-deoxyglucose and pet in head and neck cancer. J. Nucl. Med. 1997, 38, 1907–1911. [Google Scholar]
- Chatterton, B.E.; Ho Shon, I.; Baldey, A.; Lenzo, N.; Patrikeos, A.; Kelley, B.; Wong, D.; Ramshaw, J.E.; Scott, A.M. Positron emission tomography changes management and prognostic stratification in patients with oesophageal cancer: Results of a multicentre prospective study. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 354–361. [Google Scholar] [CrossRef]
- Gillies, R.S.; Middleton, M.R.; Han, C.; Marshall, R.E.; Maynard, N.D.; Bradley, K.M.; Gleeson, F.V. Role of positron emission tomography-computed tomography in predicting survival after neoadjuvant chemotherapy and surgery for oesophageal adenocarcinoma. Br. J. Surg. 2012, 99, 239–245. [Google Scholar] [CrossRef]
- Meyers, B.F.; Downey, R.J.; Decker, P.A.; Keenan, R.J.; Siegel, B.A.; Cerfolio, R.J.; Landreneau, R.J.; Reed, C.E.; Balfe, D.M.; Dehdashti, F.; et al. The utility of positron emission tomography in staging of potentially operable carcinoma of the thoracic esophagus: Results of the american college of surgeons oncology group z0060 trial. J. Thorac. Cardiovasc. Surg. 2007, 133, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Cerfolio, R.J.; Bryant, A.S. Maximum standardized uptake values on positron emission tomography of esophageal cancer predicts stage, tumor biology and survival. Ann. Thorac. Surg. 2006, 82, 391–394; discussion 394–395. [Google Scholar] [CrossRef] [PubMed]
- Rizk, N.; Downey, R.J.; Akhurst, T.; Gonen, M.; Bains, M.S.; Larson, S.; Rusch, V. Preoperative 18[f]-fluorodeoxyglucose positron emission tomography standardized uptake values predict survival after esophageal adenocarcinoma resection. Ann. Thorac. Surg. 2006, 81, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Baer, S.; Casaubon, L.; Schwartz, M.R.; Marcogliese, A.; Younes, M. Glut3 expression in biopsy specimens of laryngeal carcinoma is associated with poor survival. Laryngoscope 2002, 112, 393–396. [Google Scholar] [CrossRef]
- Van Westreenen, H.L.; Heeren, P.A.; van Dullemen, H.M.; van der Jagt, E.J.; Jager, P.L.; Groen, H.; Plukker, J.T. Positron emission tomography with f-18-fluorodeoxyglucose in a combined staging strategy of esophageal cancer prevents unnecessary surgical explorations. J. Gastrointest. Surg. 2005, 9, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; Smith, P.W.; Brix, W.K.; Wick, M.R.; Theodosakis, N.; Swenson, B.R.; Kozower, B.D.; Jones, D.R. Correlations between selected tumor markers and fluorodeoxyglucose maximal standardized uptake values in esophageal cancer. Eur. J. Cardio-Thorac. Surg. 2009, 35, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Schreurs, L.M.; Smit, J.K.; Pavlov, K.; Pultrum, B.B.; Pruim, J.; Groen, H.; Hollema, H.; Plukker, J.T. Prognostic impact of clinicopathological features and expression of biomarkers related to (18)f-fdg uptake in esophageal cancer. Ann. Surg. Oncol. 2014, 21, 3751–3757. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Chauhan, A.; Zhuang, H.; Chandra, P.; Schnall, M.; Alavi, A. Clinicopathologic factors associated with false negative fdg-pet in primary breast cancer. Breast Cancer Res. Treat. 2006, 98, 267–274. [Google Scholar] [CrossRef]
- Yap, C.S.; Schiepers, C.; Fishbein, M.C.; Phelps, M.E.; Czernin, J. Fdg-pet imaging in lung cancer: How sensitive is it for bronchioloalveolar carcinoma? Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Marom, E.M.; Aloia, T.A.; Moore, M.B.; Hara, M.; Herndon, J.E., 2nd; Harpole, D.H., Jr.; Goodman, P.C.; Patz, E.F., Jr. Correlation of fdg-pet imaging with glut-1 and glut-3 expression in early-stage non-small cell lung cancer. Lung Cancer 2001, 33, 99–107. [Google Scholar] [CrossRef]
- Nihashi, T.; Dahabreh, I.J.; Terasawa, T. Diagnostic accuracy of pet for recurrent glioma diagnosis: A meta-analysis. Am. J. Neuroradiol. 2013, 34, 944–950, s941-911. [Google Scholar] [CrossRef] [PubMed]
- Cher, L.M.; Murone, C.; Lawrentschuk, N.; Ramdave, S.; Papenfuss, A.; Hannah, A.; O’Keefe, G.J.; Sachinidis, J.I.; Berlangieri, S.U.; Fabinyi, G.; et al. Correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in gliomas using 18f-fluoromisonidazole, 18f-fdg pet and immunohistochemical studies. J. Nucl. Med. 2006, 47, 410–418. [Google Scholar] [PubMed]
- Grönroos, T.J.; Lehtiö, K.; Söderström, K.-O.; Kronqvist, P.; Laine, J.; Eskola, O.; Viljanen, T.; Grénman, R.; Solin, O.; Minn, H. Hypoxia, blood flow and metabolism in squamous-cell carcinoma of the head and neck: Correlations between multiple immunohistochemical parameters and pet. BMC Cancer 2014, 14, 876. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, G.B.; Vogelius, I.R.; Rasmussen, J.H.; Schumaker, L.; Ioffe, O.; Cullen, K.; Fischer, B.M.; Therkildsen, M.H.; Specht, L.; Bentzen, S.M. Immunohistochemical biomarkers and fdg uptake on pet/ct in head and neck squamous cell carcinoma. Acta Oncol. 2015, 54, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kaida, H.; Kawahara, A.; Hattori, S.; Kurata, S.; Hayakawa, M.; Hirose, Y.; Uchida, M.; Kage, M.; Fujita, H.; et al. The relationship between glut-1 and vascular endothelial growth factor expression and 18f-fdg uptake in esophageal squamous cell cancer patients. Clin. Nucl. Med. 2012, 37, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Uchida, M.; Kwang-Lee, K.; Kitamura, N.; Yoshimura, T.; Sasabe, E.; Yamamoto, T. Correlation of metabolism/hypoxia markers and fluorodeoxyglucose uptake in oral squamous cell carcinomas. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.; Lee, J.M.; Ki, K.D.; Choi, Y.J.; Seol, H.J.; Lee, S.K.; Huh, C.Y.; Kim, G.Y.; Lim, S.J. Correlation between fdg uptake by pet/ct and the expressions of glucose transporter type 1 and hexokinase ii in cervical cancer. Int. J. Gynecol. Cancer 2012, 22, 654–658. [Google Scholar] [CrossRef]
- Van Baardwijk, A.; Dooms, C.; van Suylen, R.J.; Verbeken, E.; Hochstenbag, M.; Dehing-Oberije, C.; Rupa, D.; Pastorekova, S.; Stroobants, S.; Buell, U.; et al. The maximum uptake of (18)f-deoxyglucose on positron emission tomography scan correlates with survival, hypoxia inducible factor-1alpha and glut-1 in non-small cell lung cancer. Eur. J. Cancer 2007, 43, 1392–1398. [Google Scholar] [CrossRef]
- Takebayashi, R.; Izuishi, K.; Yamamoto, Y.; Kameyama, R.; Mori, H.; Masaki, T.; Suzuki, Y. [18f]fluorodeoxyglucose accumulation as a biological marker of hypoxic status but not glucose transport ability in gastric cancer. J. Exp. Clin. Cancer Res. 2013, 32, 34. [Google Scholar] [CrossRef]
- Izuishi, K.; Yamamoto, Y.; Sano, T.; Takebayashi, R.; Nishiyama, Y.; Mori, H.; Masaki, T.; Morishita, A.; Suzuki, Y. Molecular mechanism underlying the detection of colorectal cancer by 18f-2-fluoro-2-deoxy-d-glucose positron emission tomography. J. Gastrointest. Surg. 2012, 16, 394–400. [Google Scholar] [CrossRef]
- Hamada, K.; Tomita, Y.; Qiu, Y.; Zhang, B.; Ueda, T.; Myoui, A.; Higuchi, I.; Yoshikawa, H.; Aozasa, K.; Hatazawa, J. 18f-fdg-pet of musculoskeletal tumors: A correlation with the expression of glucose transporter 1 and hexokinase II. Ann. Nucl. Med. 2008, 22, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.K.; Lee, W.W.; Park, S.Y.; Kim, H.; Kim, S.E. Relationship between fdg uptake and expressions of glucose transporter type 1, type 3 and hexokinase-ii in reed-sternberg cells of hodgkin lymphoma. Oncol. Res. 2009, 17, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Kim, I.J.; Kim, S.S.; Kim, S.J.; Lee, C.H.; Kim, Y.K. Relationship between biological marker expression and fluorine-18 fluorodeoxyglucose uptake in incidentally detected thyroid cancer. Cancer Biother. Radiopharm. 2010, 25, 309–315. [Google Scholar] [CrossRef] [PubMed]
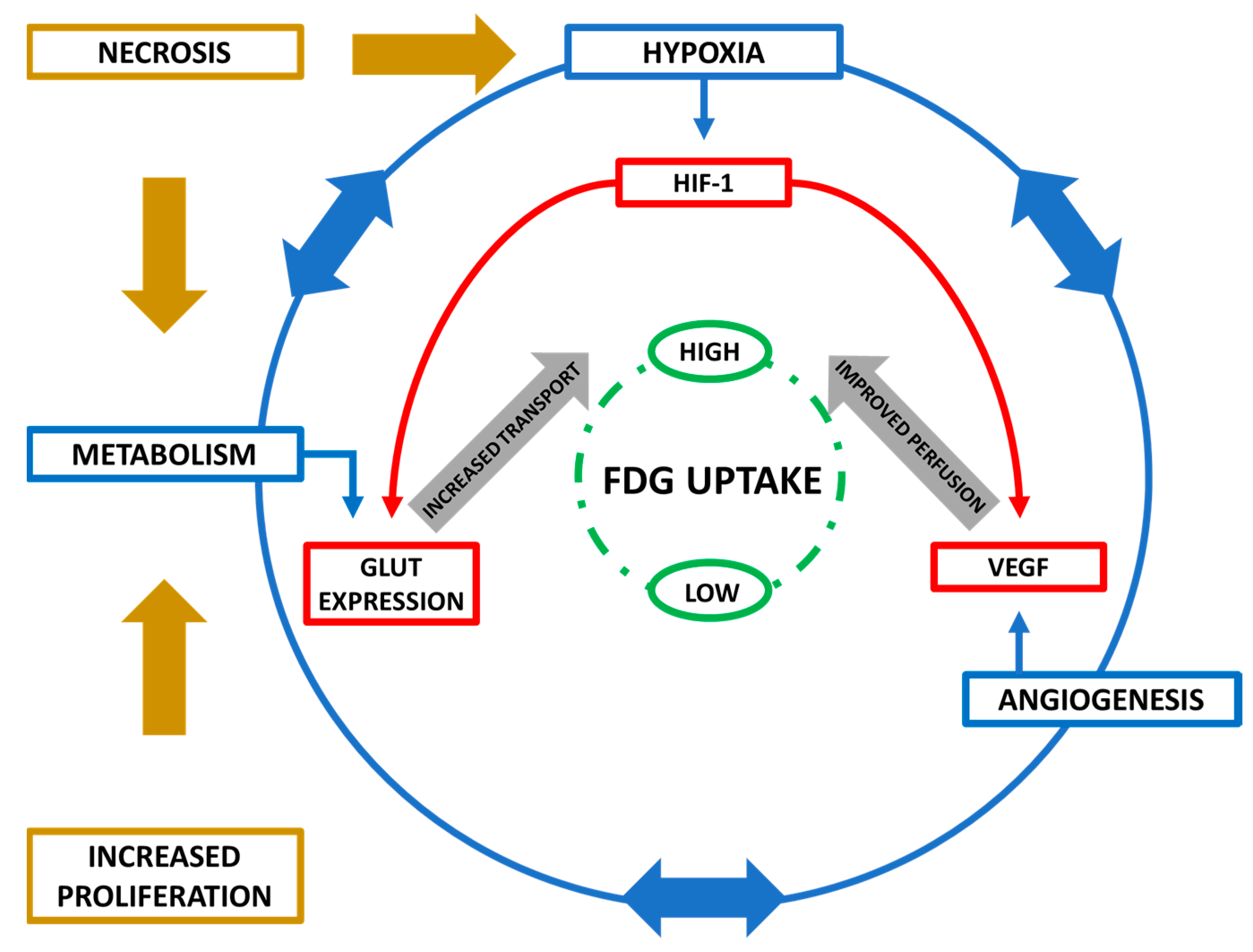
| Target Genes | Metabolic Function |
|---|---|
| GLUT-1/GLUT-3 | Cellular Glucose Entry |
| HKII | Phosphorylation |
| PGI, PFK1, Aldolase, TPI, GAPDH, PGK, PGM, enolase, PK, PFKFB1-4 | Glycolysis |
| LDHA | Pyruvate>Lactate Conversion |
| MCT4 | Cellular Lactate Removal |
| PDK1, MXI1 | Decreased Mitochondrial Activity |
| COX4I2, Lon Protease | O2 Consumption in Hypoxia |
| Cancer Type | [18F]FDG Uptake Association | Biomarker | Function | Reference |
|---|---|---|---|---|
| Oesophageal SCC | + + − + 0 0 | HK-I HK-II * HK-II VEGF VEGF KI67 | Metabolism Metabolism Metabolism Angiogenesis Angiogenesis Proliferation | [19] [19] [58] [66] [57,66] [23] |
| Oesophageal AC | + − 0 0 0 0 | GLUT-1 HK-II HIF-1α VEGF P53 Ki67 | Metabolism Metabolism Hypoxia Angiogenesis TSG Proliferation | [23,57] [58] [58] [23] [58] [58] |
| Breast | + + 0 0 0 0 | GLUT-1 HK-1 HK-II ** HK-III HIF-1α VEGF | Metabolism Metabolism Metabolism Metabolism Hypoxia Angiogenesis | [19] [19] [19] [19] [19] [19] |
| Head and Neck | − + + | GLUT-1 GLUT-3 VEGF | Metabolism Metabolism Angiogenesis | [2,21,64] [2] [64] |
| Oral SCC | + + + + | GLUT-1 ** GLUT-3 ** HK-II HIF-1α | Metabolism Metabolism Metabolism Hypoxia | [2,21,67] [2,21] [67] [67] |
| Cervical | + + | GLUT-1 HK-II | Metabolism Metabolism | [9,68] [68] |
| Pancreatic | + | GLUT-1 | Metabolism | [18] |
| Ovarian | + | GLUT-1 | Metabolism | [20] |
| NSCLC | + + 0 + 0 | GLUT-1 GLUT-3 GLUT-3 HIF-1α Ki-67 | Metabolism Metabolism Metabolism Hypoxia Proliferation | [61,69] [61] [69] [69] [69] |
| Glioma | + | VEGF | Angiogenesis | [63] |
| Gastric | 0 0 + 0 | GLUT-1 HKII HIF-1α PCNA | Metabolism Metabolism Hypoxia Proliferation | [70] [70] [70] [70] |
| Colorectal | + 0 | HIF-1α PCNA | Hypoxia Proliferation | [71] [71] |
| Musculoskeletal | + + | GLUT-1 HK-II | Metabolism Metabolism | [72] [72] |
| Hodgkin’s Lymphoma | + 0 0 | GLUT-1 GLUT-3 HK-II | Metabolism Metabolism Metabolism | [73] [73] [73] |
| Thyroid | 0 0 0 + | GLUT-1 GLUT-3 HK-II VEGF | Metabolism Metabolism Metabolism Angiogenesis | [74] [74] [74] [74] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Neill, H.; Malik, V.; Johnston, C.; Reynolds, J.V.; O’Sullivan, J. Can the Efficacy of [18F]FDG-PET/CT in Clinical Oncology Be Enhanced by Screening Biomolecular Profiles? Pharmaceuticals 2019, 12, 16. https://doi.org/10.3390/ph12010016
O’Neill H, Malik V, Johnston C, Reynolds JV, O’Sullivan J. Can the Efficacy of [18F]FDG-PET/CT in Clinical Oncology Be Enhanced by Screening Biomolecular Profiles? Pharmaceuticals. 2019; 12(1):16. https://doi.org/10.3390/ph12010016
Chicago/Turabian StyleO’Neill, Hazel, Vinod Malik, Ciaran Johnston, John V Reynolds, and Jacintha O’Sullivan. 2019. "Can the Efficacy of [18F]FDG-PET/CT in Clinical Oncology Be Enhanced by Screening Biomolecular Profiles?" Pharmaceuticals 12, no. 1: 16. https://doi.org/10.3390/ph12010016
APA StyleO’Neill, H., Malik, V., Johnston, C., Reynolds, J. V., & O’Sullivan, J. (2019). Can the Efficacy of [18F]FDG-PET/CT in Clinical Oncology Be Enhanced by Screening Biomolecular Profiles? Pharmaceuticals, 12(1), 16. https://doi.org/10.3390/ph12010016