Variation in Prescription Opioid Dispensing across Neighborhoods of Diverse Socioeconomic Disadvantages in Victoria, Australia
Abstract
1. Introduction
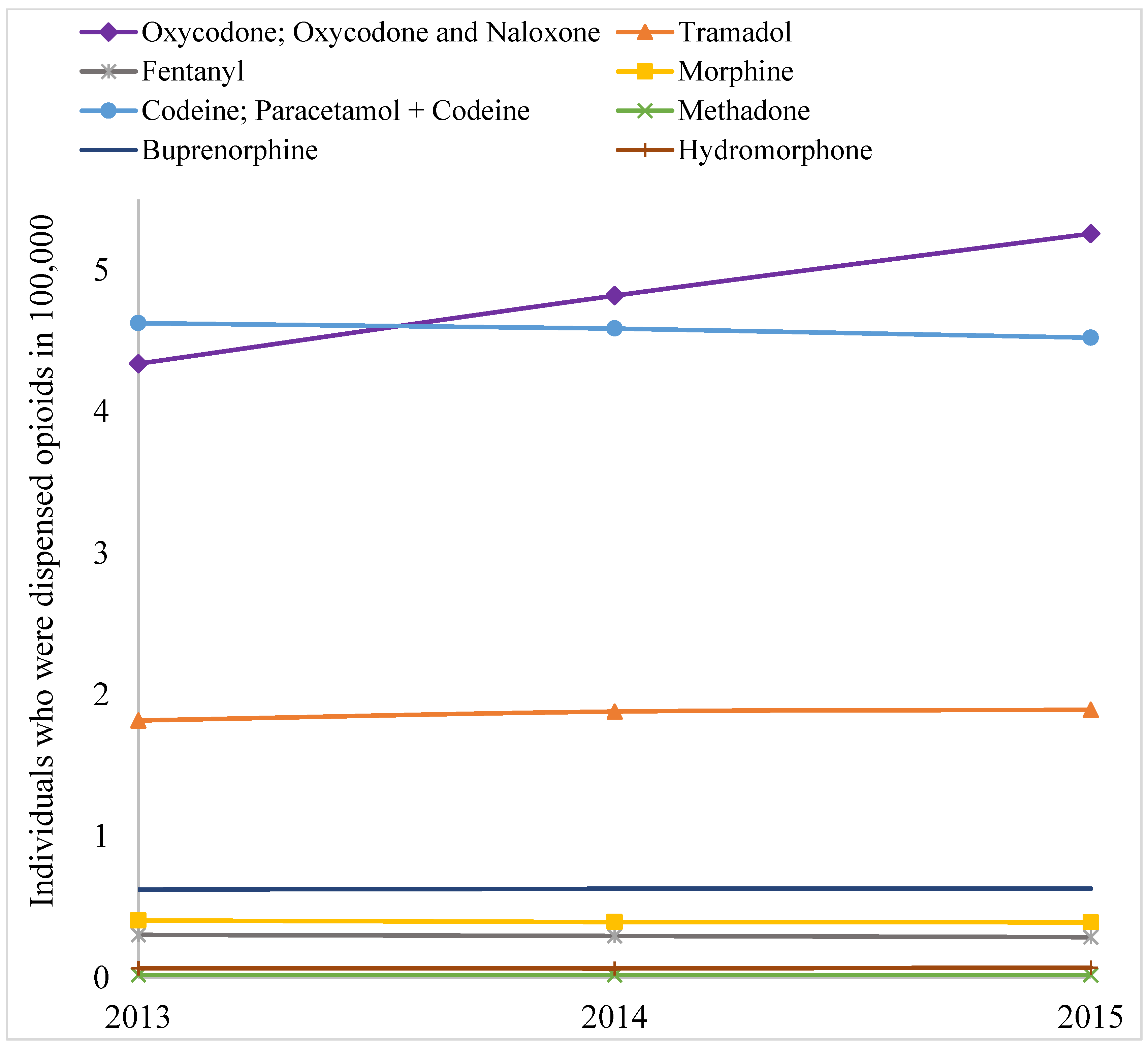
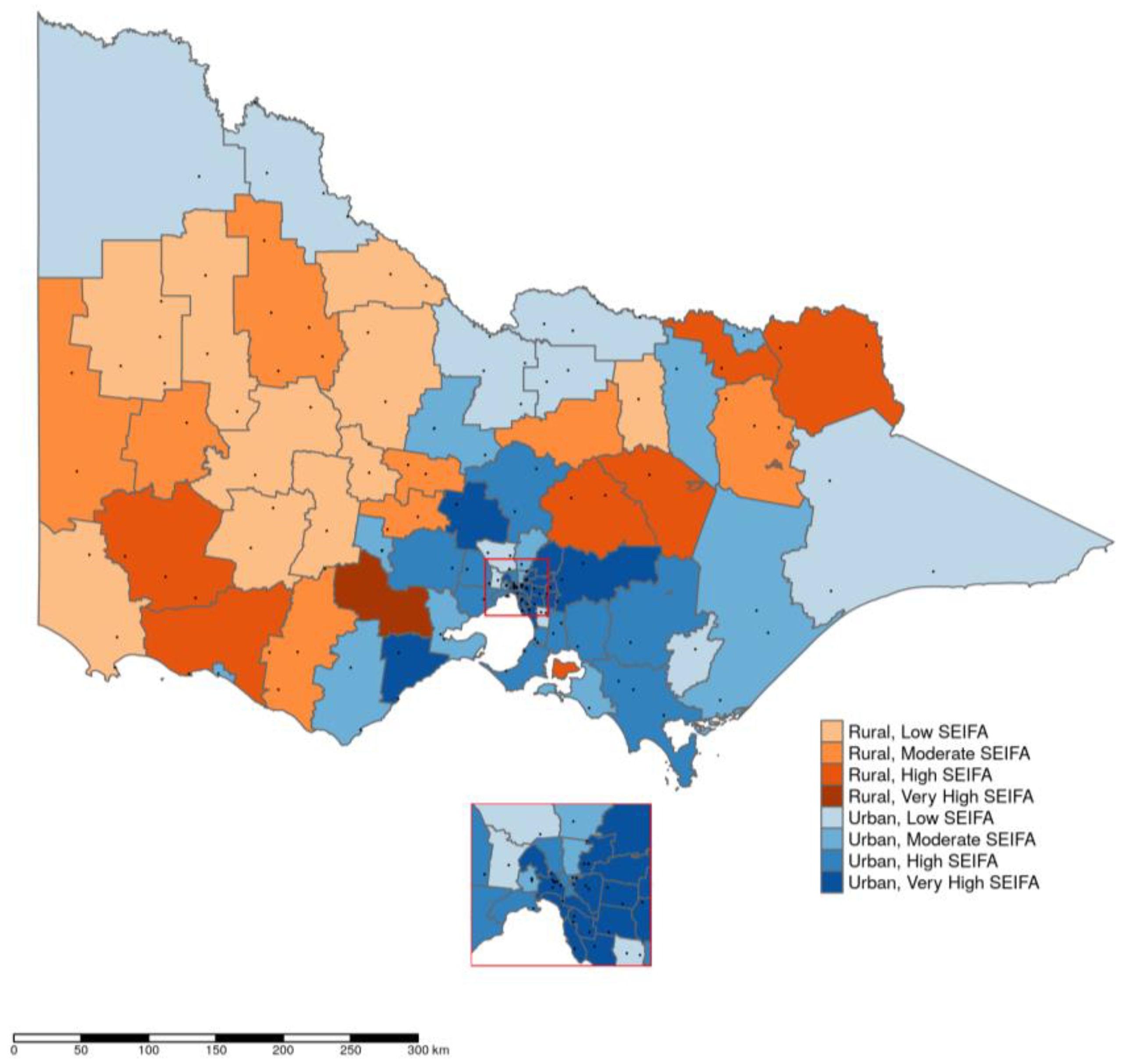
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Dispensing of Prescription Opioids in Australia
5.2. Dataset
5.3. Data Analysis
5.4. Ethics Approval and Consent to Participate
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schuckit, M.A. Treatment of opioid-use disorders. N. Engl. J. Med. 2016, 375, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Lembke, A. Why doctors prescribe opioids to known opioid abusers. N. Engl. J. Med. 2012, 367, 1580–1581. [Google Scholar] [CrossRef] [PubMed]
- Cicero, T.J.; Surratt, H.; Inciardi, J.A.; Munoz, A. Relationship between therapeutic use and abuse of opioid analgesics in rural, suburban, and urban locations in the United States. Pharmacoepidemiol. Drug Saf. 2007, 16, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Berecki-Gisolf, J.; Hassani-Mahmooei, B.; Clapperton, A.; McClure, R. Prescription opioid dispensing and prescription opioid poisoning: Population data from Victoria, Australia 2006 to 2013. Aust. New Z. J. Public Health 2017, 41, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Currow, D.C.; Phillips, J.; Clark, K. Using opioids in general practice for chronic non-cancer pain: An overview of current evidence. Med. J. Aust. 2016, 204, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Webb, T. During 2011–2015, 3601 People Died from an Opioid-Related Overdose—A Nearly Twofold Increase from 2001–2005. ABC News. 2017. Available online: http://www.abc.net.au/news/2017-11-24/deaths-from-prescription-drug-fentanyl-up-by-1800pc/9184396 (accessed on 24 November 2017).
- The Guardian. Fatal Fentanyl Overdoses Rise as Australians Turn to More Potent Painkillers. 2017. Available online: https://www.theguardian.com/australia-news/2017/aug/31/fatal-fentanyl-overdoses-rise-as-australians-turn-to-more-potent-painkillers (accessed on 31 Auguest 2017).
- International Narcotics Control Board. Report of the International Narcotics Control Board for 2009; International Narcotics Control Board: Vienna, Austria, 2010. [Google Scholar]
- Therapeutic Goods Administration of Australian Government Department of Health. Prescription Strong (Schedule 8) Opioid Use and Misuse in Australia—Options for a Regulatory Response; Consultation Paper; TGA: Canberra, Australia, 2018.
- Manchikanti, L.; Kaye, A.M.; Kaye, A.D. Current state of opioid therapy and abuse. Curr. Pain Headache Rep. 2016, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, A.M.; Purdy, C.H.; Blondell, R.D. The epidemiologic association between opioid prescribing, non-medical use, and emergency department visits. J. Addict. Dis. 2008, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.S.; Cifuentes, M.; Verma, S.; Pransky, G. Geographic variation in opioid prescribing for acute, work-related, low back pain and associated factors: A multilevel analysis. Am. J. Ind. Med. 2009, 52, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Juurlink, D.; Moineddin, R.; Gozdyra, P.; Dhalla, I.; Paterson, M.; Mamdani, M. Geographical variation in opioid prescribing and opioid-related mortality in ontario. Healthc. Q. 2011, 14, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, J.S.; Green, T.C.; Cassidy, T.A.; Butler, S.F. Geographic information systems and pharmacoepidemiology: Using spatial cluster detection to monitor local patterns of prescription opioid abuse. Pharmacoepidemiol. Drug Saf. 2010, 19, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, S.; McRae, I.S.; Islam, M.M. How can geographical information systems and spatial analysis inform a response to prescription opioid misuse? A discussion in the context of existing literature. Curr. Drug Abuse Rev. 2015, 8, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Joynt, M.; Train, M.K.; Robbins, B.W.; Halterman, J.S.; Caiola, E.; Fortuna, R.J. The impact of neighborhood socioeconomic status and race on the prescribing of opioids in emergency departments throughout the united states. J. Gen. Int. Med. 2013, 28, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, M.; Bedson, J.; Jones, P.W.; Jordan, K.P. Pain medication management of musculoskeletal conditions at first presentation in primary care: Analysis of routinely collected medical record data. BMC Musculoskelet. Disord. 2014, 15, 418. [Google Scholar] [CrossRef] [PubMed]
- Blanch, B.; Pearson, S.A.; Haber, P.S. An overview of the patterns of prescription opioid use, costs and related harms in australia. Br. J. Clin. Pharmacol. 2014, 78, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; McRae, I.S.; Mazumdar, S.; Taplin, S.; McKetin, R. Prescription opioid analgesics for pain management in australia: Twenty years of dispensing. Int. Med. J. 2015, 46, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, L.; Gisev, N.; Cama, E.; Nielsen, S.; Larance, B.; Bruno, R. The extent and correlates of community-based pharmaceutical opioid utilisation in australia. Pharmacoepidemiol. Drug Saf. 2016, 25, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; McRae, I.S.; Mazumdar, S.; Simpson, P.; Wollersheim, D.; Fatema, K.; Butler, T. Prescription opioid dispensing in new south wales, australia: Spatial and temporal variation. BMC Pharmacol. Toxicol. 2018, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics. Characteristics of Bodily Pain in Australia. Available online: http://www.abs.gov.au/ausstats/abs@.Nsf/lookup/4841.0chapter12011 (accessed on 25 July 2012).
- Galea, S.; Vlahov, D. Social determinants and the health of drug users: Socioeconomic status, homelessness, and incarceration. Public Health Rep. 2002, 117 (Suppl. 1), S135–S145. [Google Scholar] [PubMed]
- Allin, S.; Laporte, A. Socioeconomic status and the use of medicines in the ontario public drug program. Can. Public Policy 2011, 37, 563–576. [Google Scholar] [CrossRef]
- Prunuske, J.P.; St Hill, C.A.; Hager, K.D.; Lemieux, A.M.; Swanoski, M.T.; Anderson, G.W.; Lutfiyya, M.N. Opioid prescribing patterns for non-malignant chronic pain for rural versus non-rural us adults: A population-based study using 2010 namcs data. BMC Health Serv. Res. 2014, 14, 563. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, C.A.; Carrie, A.G.; Grymonpre, R.E.; Metge, C.J.; St John, P. Access and intensity of use of prescription analgesics among older manitobans. Can. J. Clin. Pharmacol. 2009, 16, e322–330. [Google Scholar] [PubMed]
- Simoni-Wastila, L. The use of abusable prescription drugs: The role of gender. J. Womens Health Gend. Based Med. 2000, 9, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Prescription Painkiller Overdoses: A Growing Epidemic, Especially among Women. 2017. Available online: https://www.cdc.gov/vitalsigns/prescriptionpainkilleroverdoses/index.html (accessed on 18 November 2017).
- Back, S.E.; Payne, R.L.; Wahlquist, A.H.; Carter, R.E.; Stroud, Z.; Haynes, L.; Hillhouse, M.; Brady, K.T.; Ling, W. Comparative profiles of men and women with opioid dependence: Results from a national multisite effectiveness trial. Am. J. Drug Alcohol. Abuse 2011, 37, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Back, S.E.; Lawson, K.M.; Singleton, L.M.; Brady, K.T. Characteristics and correlates of men and women with prescription opioid dependence. Addict. Behav. 2011, 36, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, A.; Croft, P.; Langford, R.M.; Donaldson, L.J.; Jones, G.T. Prevalence of chronic pain in the UK: A systematic review and meta-analysis of population studies. BMJ Open 2016, 6, e010364. [Google Scholar] [CrossRef] [PubMed]
- Lembke, A. Why Doctors Prescribe Opioids to Patients They Know Are Abusing Them? Scope. Stanford Medicine: Stanford, CA, USA, 2012; Available online: http://scopeblog.stanford.edu/2012/10/25/why-doctors-prescribe-opioids-to-patients-they-know-are-abusing-them/ (accessed on 4 November 2016).
- Van Zee, A. The promotion and marketing of oxycontin: Commercial triumph, public health tragedy. Am. J. Public Health 2009, 99, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Bruno, R.; Degenhardt, L.; Stoove, M.A.; Fischer, J.A.; Carruthers, S.J.; Lintzeris, N. The sources of pharmaceuticals for problematic users of benzodiazepines and prescription opioids. Med. J. Aust. 2013, 199, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, A.; Bruno, R.; Larance, B.; Burns, L. Prescription of opioid analgesics and related harms in australia. Med. J. Aust. 2011, 195, 280–284. [Google Scholar] [CrossRef] [PubMed]
- NPS Medicinewise. Complications with Oxycodone and Naloxone. 2017. Available online: https://www.nps.org.au/australian-prescriber/articles/complications-with-oxycodone-and-naloxone (accessed on 1 August 2017).
- NPS Medicinewise. Targin 10/5 mg Modified Release Tablets. Available online: https://www.nps.org.au/medical-info/medicine-finder/targin-10-5-mg-modified-release-tablets (accessed on October 2017).
- Thursfield, V.; Farrugia, H. Cancer in Victoria: Statistics & Trends 2016; Cancer Council Victoria: Melbourne, Australia, 2017. [Google Scholar]
- Australian Bureau of Statistics. Socio Economic Indexes for Areas (SEIFA). 2018. Available online: http://www.abs.gov.au/ausstats/abs@.nsf/lookup/2033.0.55.001main+features100042011 (accessed on 28 March 2013).
- Islam, M.M.; McRae, I.S. An inevitable wave of prescription drug monitoring programs in the context of prescription opioids: Pros, cons and tensions. BMC Pharmacol. Toxicol. 2014, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Bruno, R. Implementing real-time prescription drug monitoring: Are we ready? Drug Alcohol. Rev. 2014, 33, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Ogeil, R.P.; Heilbronn, C.; Lloyd, B.; Lubman, D.I. Prescription drug monitoring in australia: Capacity and coverage issues. Med. J. Aust. 2016, 204, 148. [Google Scholar] [CrossRef] [PubMed]
- Griggs, C. 2014–2015 Apha Policy Committee Report. Integrated Nationwide Prescription Drug Monitoring Program. 2015. Available online: https://www.pharmacist.com/sites/default/files/files/prescription%20drug%20monitoring%20programs.pdf (accessed on 26 August 2016).
- Piantadosi, S.; Byar, D.P.; Green, S.B. The ecological fallacy. Am. J. Epidemiol. 1988, 127, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Australian Government Department of Health. The Pharmaceutical Benefit Scheme. 2016. Available online: http://www.pbs.gov.au/info/about-the-pbs (accessed on 18 August 2016).
- World Health Organization. Guidelines for Atc Classification and Ddd Assignment. Oslo: Who Collaborating Centre for Drug Statistics Methodology. 2017. Available online: https://www.whocc.no/ddd/definition_and_general_considera/ (accessed on 30 July 2018).
- Royston, P. Ptrend: Stata Module for Trend Analysis for Proportions. 2002. Available online: http://econpapers.repec.org/repec:boc:bocode:s426101 (accessed on 29 October 2014).
- Department of Infrastructure and Regional Development (DIRD). Local Government National Report, 2013–14; DIRD: Canberra, Australia, 2015. Available online: http://regional.Gov.Au/local/publications/reports/2013_2014/infra2466_lgnr_2013-14.Pdf (accessed on 1 September 2018).
- Naing, N.N. Easy way to learn standardization: Direct and indirect methods. Malays. J. Med. Sci. 2000, 7, 10–15. [Google Scholar] [PubMed]
- Wickham, H. Tidyverse: Easily Install and Load the ‘Tidyverse’. R Package Version 1.2.1. Available online: https://rdrr.io/cran/tidyverse/ (accessed on 14 November 2017).
- R Core Team. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2011; Available online: http://www.R-project.org/ (accessed on 2 July 2018).
- Tennekes, M. Tmap: Thematic maps in r. J. Stat. Softw. 2018, 84, 1–39. [Google Scholar] [CrossRef]
- Data & Statistical Services; Princeton University. Interpretation (Regression with Logarithms). Available online: https://www.princeton.edu/~otorres/Stata/inference.htm (accessed on 12 September 2018).
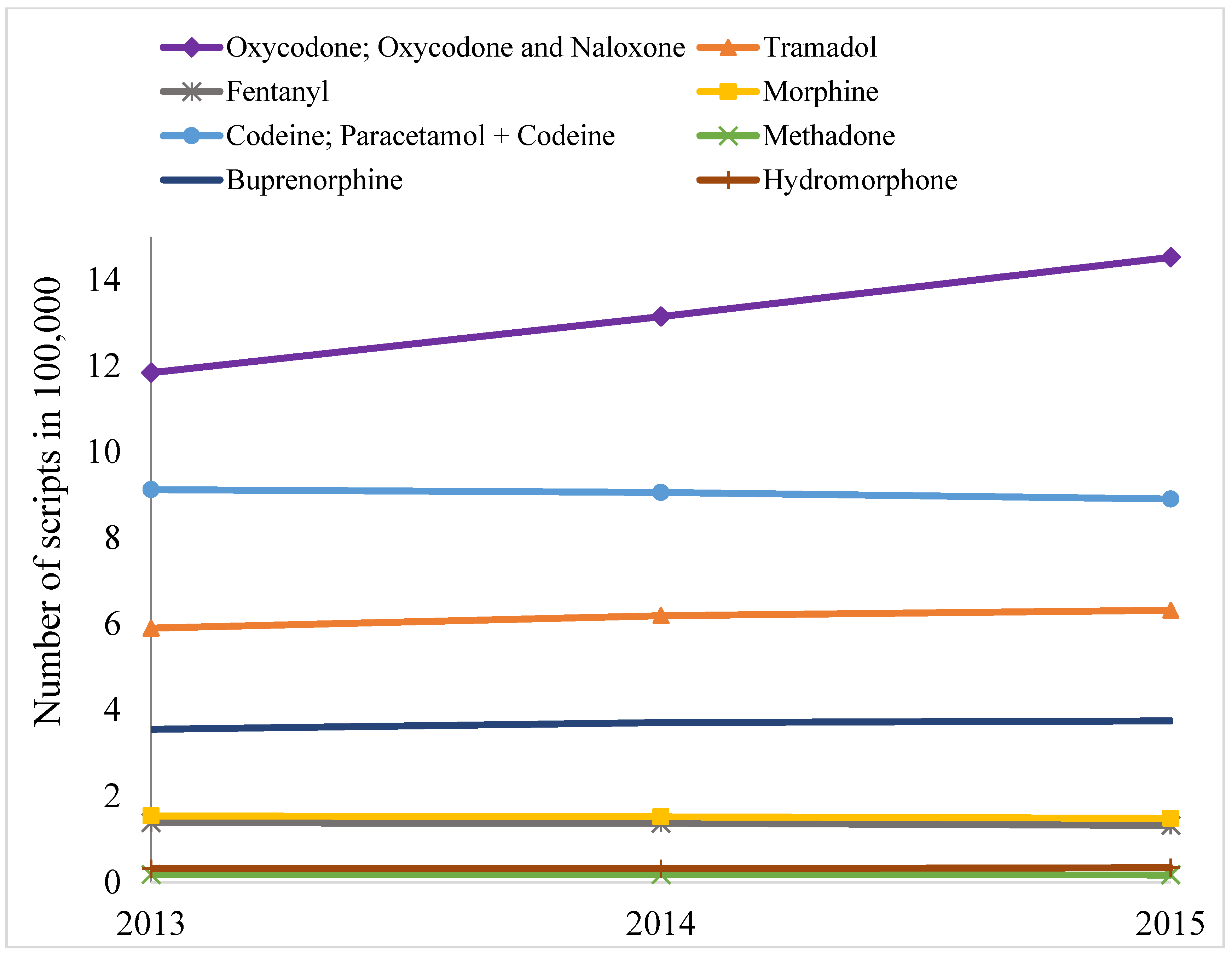

| Generic Drugs | 2013 | 2014 | 2015 |
|---|---|---|---|
| Tramadol | 2.74 | 2.79 | 2.76 |
| Codeine and derivatives § | 6.16 | 6.04 | 5.76 |
| Oxycodone and derivatives ¶ | 3.10 | 3.12 | 3.18 |
| Fentanyl | 1.02 | 0.96 | 0.89 |
| Morphine (& derivatives) | 1.03 | 0.93 | 0.85 |
| Buprenorphine | 0.68 | 0.70 | 0.70 |
| Hydromorphone | 0.32 | 0.30 | 0.33 |
| Methadone | 0.30 | 0.28 | 0.27 |
| Tapentadol | - | 0.05 | 0.22 |
| Year | Quantity Dispensed in Terms of DDD/1000 People/Day | |||||||
|---|---|---|---|---|---|---|---|---|
| Low SEIFA | Moderate SEIFA | High SEIFA | Very High SEIFA | |||||
| Men | Women | Men | Women | Men | Women | Men | Women | |
| 2013 | 18.92 | 22.25 | 16.95 | 20.69 | 14.12 | 17.76 | 8.71 | 11.96 |
| 2014 | 18.56 | 22.34 | 16.69 | 20.76 | 13.80 | 17.84 | 8.52 | 11.66 |
| 2015 | 18.43 | 22.13 | 16.46 | 20.86 | 13.57 | 17.51 | 8.24 | 11.45 |
| Year | Variable | SEIFA Index | |||
|---|---|---|---|---|---|
| Low | Moderate | High | Very High | ||
| 2013 | Population (in 100,000) | 9.50 | 11.22 | 15.06 | 21.56 |
| Number of individuals who were dispensed prescription opioids (in 100,000) | 2.36 | 2.66 | 3.31 | 3.75 | |
| Proportion of population that were dispensed prescription opioids (in %) | 24.84 | 23.71 | 21.98 | 17.39 | |
| Amount of prescription opioids used (in DDD/1000 people/day) | 20.58 | 18.84 | 15.95 | 10.36 | |
| 2014 | Population (in 100,000) | 9.61 | 11.43 | 15.46 | 21.88 |
| Number of individuals who were dispensed prescription opioids (in 100,000) | 2.49 | 2.78 | 3.50 | 3.88 | |
| Proportion of population that were dispensed prescription opioids (in %) | 25.91 | 24.32 | 22.64 | 17.73 | |
| Amount of prescription opioids used (in DDD/1000 people/day) | 20.45 | 18.74 | 15.84 | 10.12 | |
| 2015 | Population (in 100,000) | 9.70 | 11.63 | 15.86 | 22.18 |
| Number of individuals who were dispensed prescription opioids (in 100,000) | 2.57 | 2.90 | 3.67 | 4.00 | |
| Proportion of population that were dispensed prescription opioids (in %) | 26.49 | 24.94 | 23.14 | 18.03 | |
| Amount of prescription opioids used (in DDD/1000 people/day) | 20.28 | 18.68 | 15.56 | 9.87 | |
| Variables | B * | P | 95% Confidence Interval |
|---|---|---|---|
| SEIFA index level | |||
| Very high | 1.00 | - | - |
| High | 1.21 | <0.01 | 1.17–1.24 |
| Moderate | 1.48 | <0.01 | 1.44–1.53 |
| Low | 1.60 | <0.01 | 1.55–1.65 |
| Sex | |||
| Men | 1.00 | - | - |
| Women | 1.14 | <0.01 | 1.12–1.17 |
| Age group (years) | |||
| 0–19 | 1.00 | - | - |
| 20–44 | 4.81 | <0.01 | 4.60–5.04 |
| 45–64 | 11.05 | <0.01 | 10.55–11.57 |
| 65+ | 18.69 | <0.01 | 17.79–19.64 |
| Urbanization | |||
| Urban | 1.00 | - | - |
| Rural | 2.43 | <0.01 | 2.36–2.51 |
| Year | |||
| 2013 | 1.00 | - | - |
| 2014 | 0.96 | <0.01 | 0.94–0.99 |
| 2015 | 0.98 | 0.28 | 0.96–1.01 |
| Cancer prevalence | 1.00 | <0.01 | 1.00–1.00 |
| Constant | 1.02 | 0.38 | 0.97–1.07 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.M.; Wollersheim, D. Variation in Prescription Opioid Dispensing across Neighborhoods of Diverse Socioeconomic Disadvantages in Victoria, Australia. Pharmaceuticals 2018, 11, 116. https://doi.org/10.3390/ph11040116
Islam MM, Wollersheim D. Variation in Prescription Opioid Dispensing across Neighborhoods of Diverse Socioeconomic Disadvantages in Victoria, Australia. Pharmaceuticals. 2018; 11(4):116. https://doi.org/10.3390/ph11040116
Chicago/Turabian StyleIslam, M Mofizul, and Dennis Wollersheim. 2018. "Variation in Prescription Opioid Dispensing across Neighborhoods of Diverse Socioeconomic Disadvantages in Victoria, Australia" Pharmaceuticals 11, no. 4: 116. https://doi.org/10.3390/ph11040116
APA StyleIslam, M. M., & Wollersheim, D. (2018). Variation in Prescription Opioid Dispensing across Neighborhoods of Diverse Socioeconomic Disadvantages in Victoria, Australia. Pharmaceuticals, 11(4), 116. https://doi.org/10.3390/ph11040116





