Low Molecular Weight Chitosan-Coated PLGA Nanoparticles for Pulmonary Delivery of Tobramycin for Cystic Fibrosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PLGA and LMWC-PLGA NPs
2.3. Characterization of PLGA and LMWC-PLGA Nanoparticles
2.4. Drug Entrapment Efficiency and Loading Capacity
2.5. Investigation of the Mucoadhesive Properties of PLGA and LMWC-PLGA Nanoparticles
2.6. In Vitro Drug Release
2.7. Antimicrobial Activity of Tobramycin Nanoparticles
2.8. Preparation of Biofilms Using the Calgary Biofilm Device/MBEC Assay
3. Results
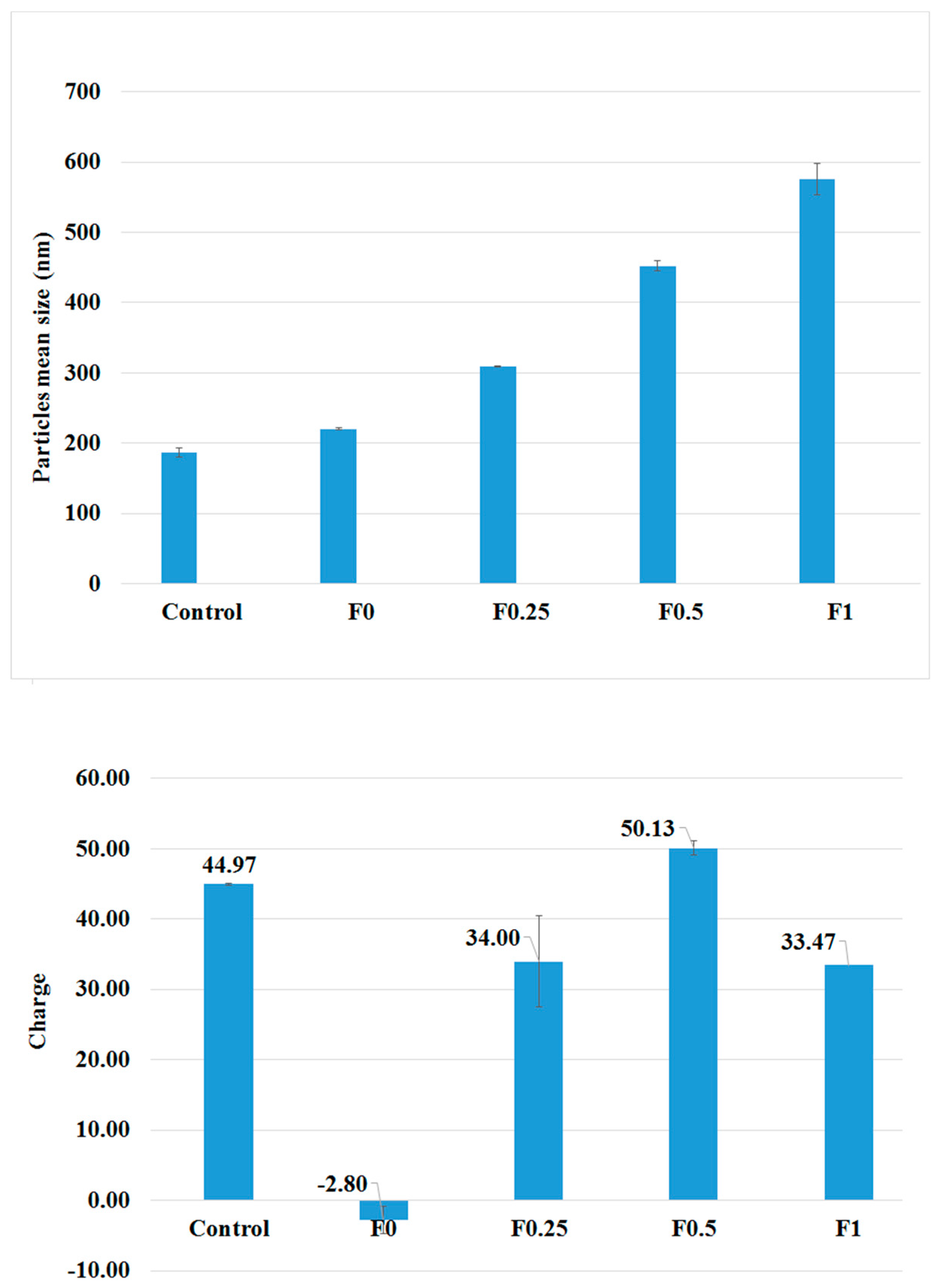
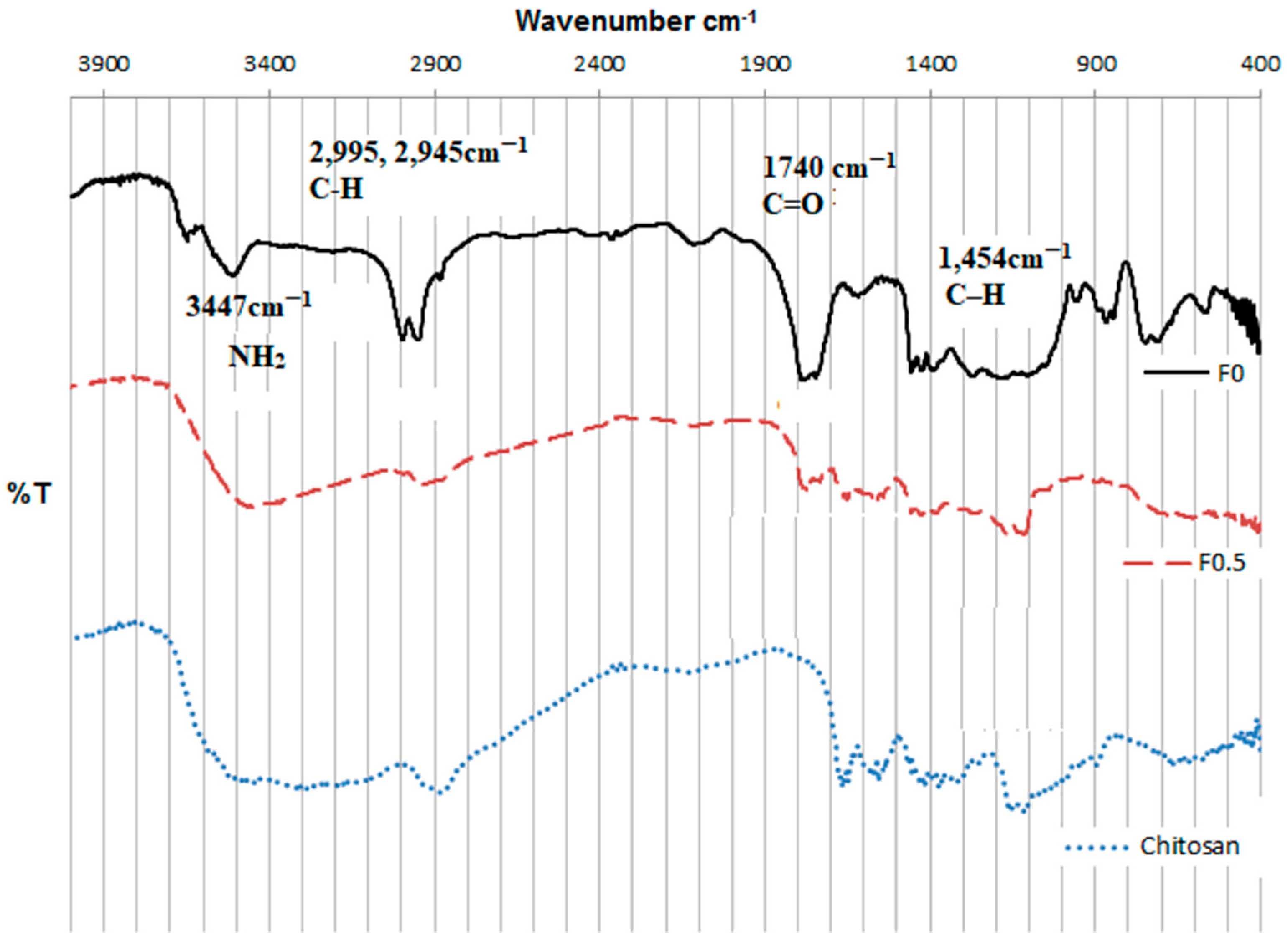
3.1. Characterization of PLGA and LMWC-PLGA Nanoparticles
3.2. Drug Entrapment Efficiency
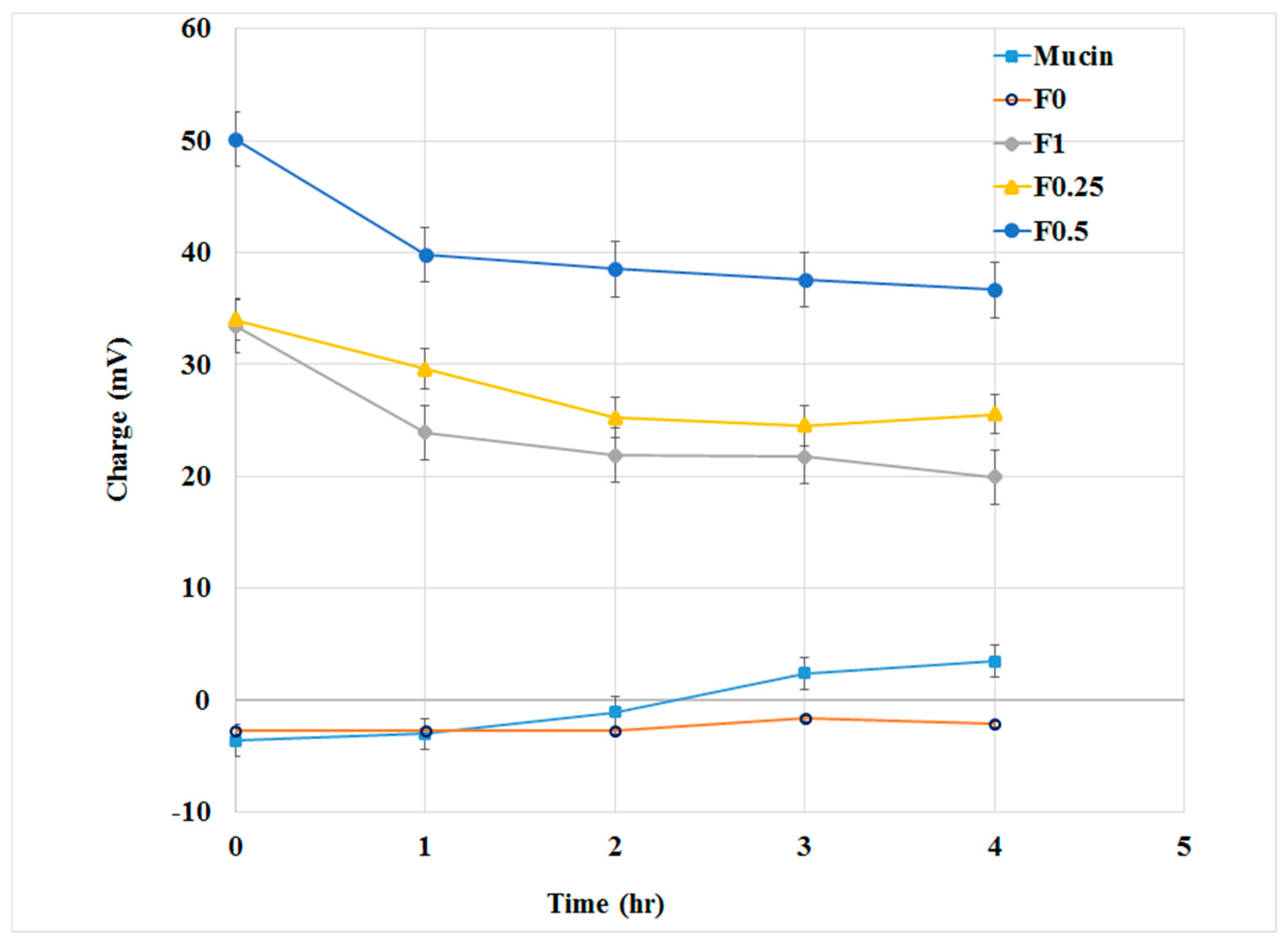
3.3. Mucoadhesive Properties of PLGA and LMWC-PLGA Nanoparticles
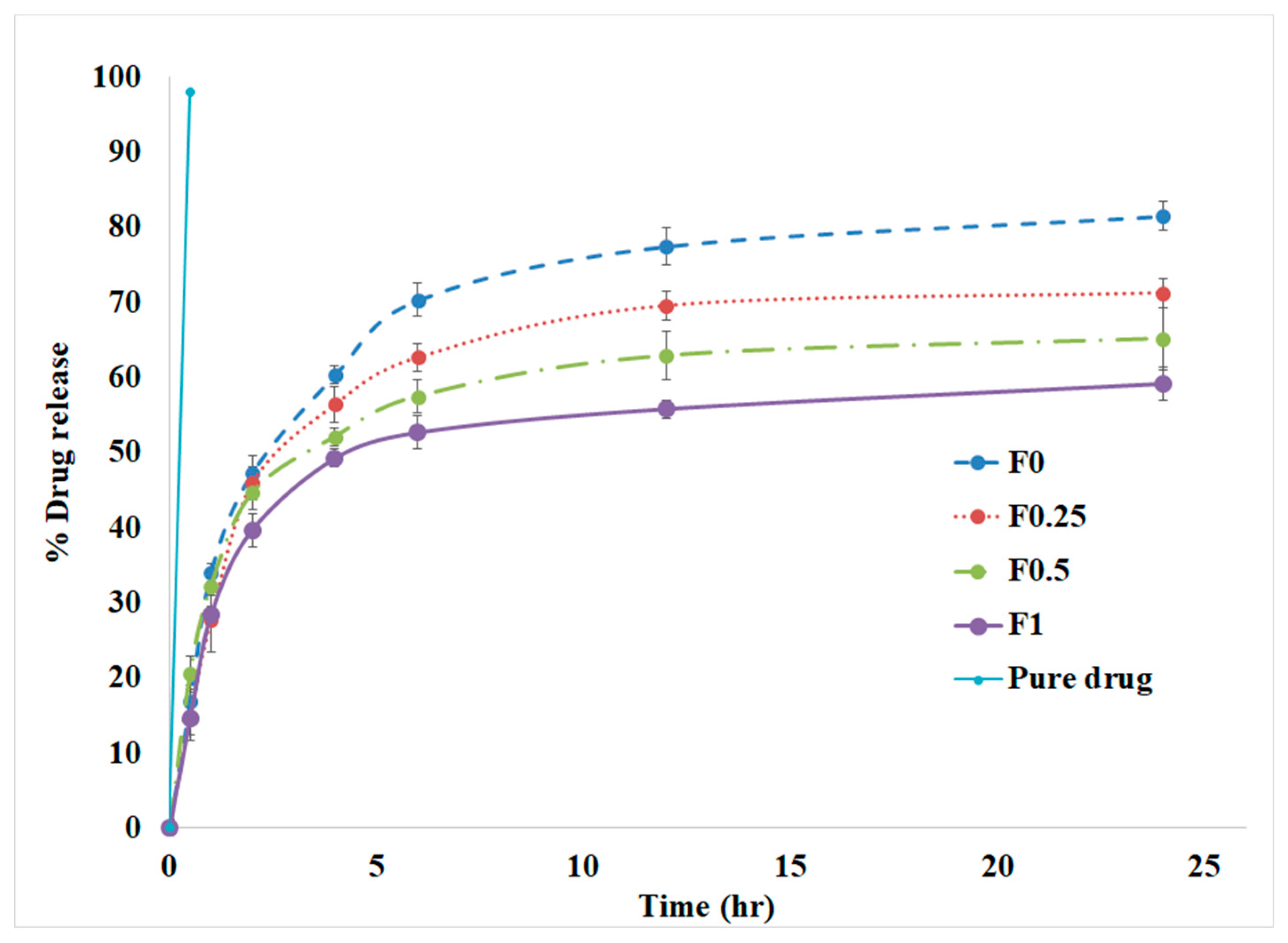
3.4. In Vitro Drug Release
3.5. Antimicrobial Activity of Tobramycin Nanoparticles
3.6. Effect of Tobramycin PLGA NPs on P. aeruginosa Biofilms
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, J.J.; Travis, S.M.; Greenberg, E.P.; Welsh, M.J. Cystic Fibrosis Airway Epithelia Fail to Kill Bacteria Because of Abnormal Airway Surface Fluid. Cell 1996, 85, 229–236. [Google Scholar] [CrossRef]
- Collins, F.S. Cystic fibrosis: Molecular biology and therapeutic implications. Science 1992, 256, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, L.S.; Yadav, K. Inhaled Formulation Design for the Treatment of Lung Infections. Curr. Pharm. Des. 2015, 21, 3875–3901. [Google Scholar] [CrossRef] [PubMed]
- Touw, D.J.; Brimicombe, R.W.; Hodson, M.E.; Heijerman, H.G.; Bakker, W. Inhalation of antibiotics in cystic fibrosis. Eur. Respir. J. 1995, 8, 1594–1604. [Google Scholar] [PubMed]
- Pilcer, G.; Vanderbist, F.; Amighi, K. Spray-dried carrier-free dry powder tobramycin formulations with improved dispersion properties. J. Pharm. Sci. 2009, 98, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Parlati, C.; Colombo, P.; Buttini, F.; Young, P.M.; Adi, H.; Ammit, A.J.; Traini, D. Pulmonary spray dried powders of tobramycin containing sodium stearate to improve aerosolization efficiency. Pharm. Res. 2009, 26, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Pai, V.B.; Nahata, M.C. Efficacy and safety of aerosolized tobramycin in cystic fibrosis. Pediatr. Pulmonol. 2001, 32, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Ratjen, F.; Döring, G.; Nikolaizik, W.H. Effect of inhaled tobramycin on early Pseudomonas aeruginosa colonisation in patients with cystic fibrosis. Lancet 2001, 358, 983–984. [Google Scholar] [CrossRef]
- Cheow, W.S.; Chang, M.W.; Hadinoto, K. Antibacterial Efficacy of Inhalable Antibiotic-Encapsulated Biodegradable Polymeric Nanoparticles Against E. coli Biofilm Cells. J. Biomed. Nanotechnol. 2010, 6, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, F.; d’Angelo, I.; Coletta, C.; d’Emmanuele di Villa Bianca, R.; Sorrentino, R.; Perfetto, B.; Tufano, M.A.; Miro, A.; La Rotonda, M.I.; Quaglia, F. Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: Modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J. Control. Release 2012, 157, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.P.; Labhasetwar, V.; Amidon, G.L.; Levy, R.J. Gastrointestinal uptake of biodegradable microparticles: Effect of particle size. Pharm. Res. 1996, 13, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Donovan, M.D. Large molecule and particulate uptake in the nasal cavity: The effect of size on nasal absorption. Adv. Drug Deliv. Rev. 1998, 29, 147–155. [Google Scholar] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Bivas-Benita, M.; Lin, M.Y.; Bal, S.M.; van Meijgaarden, K.E.; Franken, K.L.M.C.; Friggen, A.H.; Junginger, H.E.; Borchard, G.; Klein, M.R.; Ottenhoff, T.H.M. Pulmonary delivery of DNA encoding Mycobacterium tuberculosis latency antigen Rv1733c associated to PLGA–PEI nanoparticles enhances T cell responses in a DNA prime/protein boost vaccination regimen in mice. Vaccine 2009, 27, 4010–4017. [Google Scholar] [CrossRef] [PubMed]
- Nazarian, S.; Gargari, S.L.M.; Rasooli, I.; Hasannia, S.; Pirooznia, N. A PLGA-encapsulated chimeric protein protects against adherence and toxicity of enterotoxigenic Escherichia coli. Microbiol. Res. 2014, 169, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Dorati, R.; DeTrizio, A.; Spalla, M.; Migliavacca, R.; Pagani, L.; Pisani, S.; Chiesa, E.; Conti, B.; Modena, T.; Genta, I. Gentamicin Sulfate PEG-PLGA/PLGA-H Nanoparticles: Screening Design and Antimicrobial Effect Evaluation toward Clinic Bacterial Isolates. Nanomaterials 2018, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Dudhani, A.R.; Kosaraju, S.L. Bioadhesive chitosan nanoparticles: Preparation and characterization. Carbohydr. Polym. 2010, 81, 243–251. [Google Scholar] [CrossRef]
- Lehr, C.-M.; Bouwstra, J.A.; Schacht, E.H.; Junginger, H.E. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int. J. Pharm. 1992, 78, 43–48. [Google Scholar] [CrossRef]
- Bodmeier, R.; Chen, H. Evaluation of Biodegradable Poly(lactide) Pellets Prepared by Direct Compression. J. Pharm. Sci. 1989, 78, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Al-Nemrawi, N.K.; Dave, R.H. Effect of Formulation Variables on Poly(lactic-co-glycolic acid) Nanoparticle Properties: A Factorial Design Study. J. Pharm. Sci. Pharmacol. 2015, 2, 1–10. [Google Scholar] [CrossRef]
- Surassmo, S.; Saengkrit, N.; Ruktanonchai, U.R.; Suktham, K.; Woramongkolchai, N.; Wutikhun, T.; Puttipipatkhachorn, S. Surface modification of PLGA nanoparticles by carbopol to enhance mucoadhesion and cell internalization. Colloids Surf. B Biointerfaces 2015, 130, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Russ, H.; McCleary, D.; Katimy, R.; Montana, J.L.; Miller, R.B.; Krishnamoorthy, R.; Davis, C.W. Development and Validation of a Stability-Indicating HPLC Method for the Determination of Tobramycin and Its Related Substances in an Ophthalmic Suspension. J. Liq. Chromatogr. Relat. Technol. 1998, 21, 2165–2181. [Google Scholar] [CrossRef]
- De Campos, A.M.; Diebold, Y.; Carvalho, E.L.S.; Sánchez, A.; Alonso, M.J. Chitosan Nanoparticles as New Ocular Drug Delivery Systems: In Vitro Stability, in Vivo Fate, and Cellular Toxicity. Pharm. Res. 2004, 21, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Rençber, S.; Karavana, S.Y.; Yılmaz, F.F.; Eraç, B.; Nenni, M.; Özbal, S.; Pekçetin, Ç.; Gurer-Orhan, H.; Hoşgör-Limoncu, M.; Güneri, P.; et al. Development, characterization, and in vivo assessment of mucoadhesive nanoparticles containing fluconazole for the local treatment of oral candidiasis. Int. J. Nanomed. 2016, 11, 2641–2653. [Google Scholar] [CrossRef] [PubMed]
- Avgoustakis, K.; Beletsi, A.; Panagi, Z.; Klepetsanis, P.; Karydas, A.G.; Ithakissios, D.S. PLGA–mPEG nanoparticles of cisplatin: In vitro nanoparticle degradation, in vitro drug release and in vivo drug residence in blood properties. J. Control. Release 2002, 79, 123–135. [Google Scholar] [CrossRef]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New Technology for Rapid Determination of Antibiotic Susceptibilities of Bacterial Biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [PubMed]
- Parveen, S.; Sahoo, S.K. Long circulating chitosan/PEG blended PLGA nanoparticle for tumor drug delivery. Eur. J. Pharmacol. 2011, 670, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Kong, L. Chitosan-Modified PLGA Nanoparticles with Versatile Surface for Improved Drug Delivery. AAPS PharmSciTech 2013, 14, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Taetz, S.; Nafee, N.; Beisner, J.; Piotrowska, K.; Baldes, C.; Mürdter, T.E.; Huwer, H.; Schneider, M.; Schaefer, U.F.; Klotz, U.; Lehr, C.-M. The influence of chitosan content in cationic chitosan/PLGA nanoparticles on the delivery efficiency of antisense 2′-O-methyl-RNA directed against telomerase in lung cancer cells. Eur. J. Pharm. Biopharm. 2009, 72, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Nafee, N.; Taetz, S.; Schneider, M.; Schaefer, U.F.; Lehr, C.-M. Chitosan-coated PLGA nanoparticles for DNA/RNA delivery: Effect of the formulation parameters on complexation and transfection of antisense oligonucleotides. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Katas, H.; Alpar, H.O. Development and characterisation of chitosan nanoparticles for siRNA delivery. J. Control. Release 2006, 115, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Dhir, R.K.; Dyer, T.D. Modern Concrete Materials: Binders, Additions and Admixtures: Proceedings of the International Conference Held at the University of Dundee, Scotland, UK, 8–10 September 1999; Thomas Telford Ltd.: London, UK, 1999; ISBN 978-0-7277-2822-7. [Google Scholar]
- Abdel-Wahhab, M.A.; Abdel-Wahhab, K.G.; Mannaa, F.A.; Hassan, N.S.; Safar, R.; Diab, R.; Foliguet, B.; Ferrari, L.; Rihn, B.H. Uptake of Eudragit Retard L (Eudragit® RL) Nanoparticles by Human THP-1 Cell Line and Its Effects on Hematology and Erythrocyte Damage in Rats. Materials 2014, 7, 1555–1572. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, R.S.; Chandasana, H.; Chhonker, Y.S.; Rathi, C.; Kumar, D.; Mitra, K.; Shukla, P.K. Mucoadhesive nanoparticles for prolonged ocular delivery of natamycin: In vitro and pharmacokinetics studies. Int. J. Pharm. 2012, 432, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Yoncheva, K.; Vandervoort, J.; Ludwig, A. Development of mucoadhesive poly(lactide-co-glycolide) nanoparticles for ocular application. Pharm. Dev. Technol. 2011, 16, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Al-Nemrawi, N.K.; Dave, R.H. Formulation and characterization of acetaminophen nanoparticles in orally disintegrating films. Drug Deliv. 2016, 23, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Takeuchi, H.; Hino, T.; Kunou, N.; Kawashima, Y. In vitro drug release behavior of d,l-lactide/glycolide copolymer (PLGA) nanospheres with nafarelin acetate prepared by a novel spontaneous emulsification solvent diffusion method. J. Pharm. Sci. 1994, 83, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Seju, U.; Kumar, A.; Sawant, K.K. Development and evaluation of olanzapine-loaded PLGA nanoparticles for nose-to-brain delivery: In vitro and in vivo studies. Acta Biomater. 2011, 7, 4169–4176. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Bernier, S.P.; Kuchma, S.L.; Hammond, J.H.; Hasan, F.; O’Toole, G.A. Aminoglycoside resistance of Pseudomonas aeruginosa biofilms modulated by extracellular polysaccharide. Int. Microbiol. 2010, 13, 207–212. [Google Scholar] [PubMed]
- Sabaeifard, P.; Abdi-Ali, A.; Soudi, M.R.; Gamazo, C.; Irache, J.M. Amikacin loaded PLGA nanoparticles against Pseudomonas aeruginosa. Eur. J. Pharm. Sci. 2016, 93, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Döring, G.; Conway, S.P.; Heijerman, H.G.; Hodson, M.; Høiby, N.; Smyth, A.; Touw, D. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: A European consensus. Eur. Respir. J. 2000, 16, 749–767. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.W. Biofilms and antibiotic therapy: Is there a role for combating bacterial resistance by the use of novel drug delivery systems? Adv. Drug Deliv. Rev. 2005, 57, 1539–1550. [Google Scholar] [CrossRef] [PubMed]

| Component | Formulation | ||||
|---|---|---|---|---|---|
| Control | F0 | F0.25 | F0.5 | F1 | |
| Tobramycin | - | 200 | 200 | 200 | 200 |
| Low Molecular Weight Chitosan (LMWC) | 100 | - | 50 | 100 | 200 |
| Poly(vinyl alcohol) (PVA) | 80 | 80 | 80 | 80 | 80 |
| Poly(lactic-co-glycolic acid) (PLGA) 50:50 | 100 | 100 | 100 | 100 | 100 |
| Formulae | Size (nm) | PDI | Zeta Potential (mV) | EE (%) | LC (%) |
|---|---|---|---|---|---|
| Control | 187.00 ± 6.19 | 0.206 ±0.014 | +45.00 ± 4.30 | NA | NA |
| F0 | 220.70 ± 1.77 | 0.194 ±0.002 | −2.80 ± 0.10 | 85.34% | 45.92% |
| F0.25 | 309.57 ± 1.12 | 0.295 ±0.011 | +34.00 ± 1.90 | 88.47% | 43.15% |
| F0.5 | 451.80 ± 7.19 | 0.297 ±0.106 | +50.13 ± 6.50 | 87.20% | 37.33% |
| F1 | 575.77 ± 2.67 | 0.319 ±0.130 | +33.47 ± 1.00 | 83.74% | 30.87% |
| Formula | MIC (μg/mL) | MBEC (μg/mL) |
|---|---|---|
| Tobramycin | 1 | 7.8 |
| F0 | 128.15 | 512 |
| F0.25 | 32.25 | 250 |
| F0.5 | 4.95 | 15.6 |
| F1 | 2.9 | 125 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Nemrawi, N.K.; Alshraiedeh, N.H.; Zayed, A.L.; Altaani, B.M. Low Molecular Weight Chitosan-Coated PLGA Nanoparticles for Pulmonary Delivery of Tobramycin for Cystic Fibrosis. Pharmaceuticals 2018, 11, 28. https://doi.org/10.3390/ph11010028
Al-Nemrawi NK, Alshraiedeh NH, Zayed AL, Altaani BM. Low Molecular Weight Chitosan-Coated PLGA Nanoparticles for Pulmonary Delivery of Tobramycin for Cystic Fibrosis. Pharmaceuticals. 2018; 11(1):28. https://doi.org/10.3390/ph11010028
Chicago/Turabian StyleAl-Nemrawi, Nusaiba K., Nid’’A H. Alshraiedeh, Aref L. Zayed, and Bashar M. Altaani. 2018. "Low Molecular Weight Chitosan-Coated PLGA Nanoparticles for Pulmonary Delivery of Tobramycin for Cystic Fibrosis" Pharmaceuticals 11, no. 1: 28. https://doi.org/10.3390/ph11010028
APA StyleAl-Nemrawi, N. K., Alshraiedeh, N. H., Zayed, A. L., & Altaani, B. M. (2018). Low Molecular Weight Chitosan-Coated PLGA Nanoparticles for Pulmonary Delivery of Tobramycin for Cystic Fibrosis. Pharmaceuticals, 11(1), 28. https://doi.org/10.3390/ph11010028





