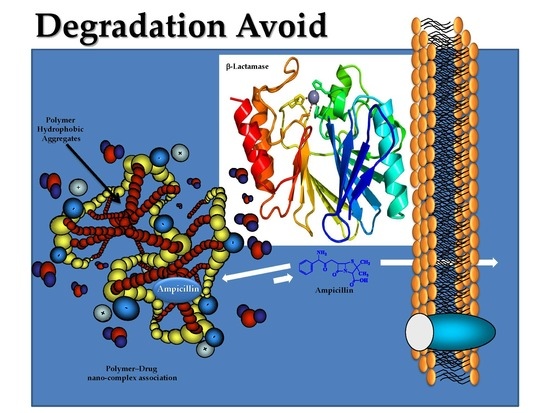
Application of Nanoparticle Technology to Reduce the Anti-Microbial Resistance through β-Lactam Antibiotic-Polymer Inclusion Nano-Complex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Obtention and FTIR Characterization of PAM-18Na Polymer
2.3. Preparation of Inclusion Nano-Complexes in Aqueous Media
2.4. Steady-State Fluorescence Assay
2.5. Characterization of Drug-Polymer Inclusion Complex
2.5.1. Thermal Characterization of the Polymer-Drug Blend
2.5.2. Association Efficiency
2.5.3. Zeta Potential and Size Measurements
2.5.4. Viscometric Measurements
2.6. Degradation Assays
2.6.1. Chemical Degradation Assay
2.6.2. Enzymatic Degradation Assay
2.6.3. Biological Degradation Assays
Ampicillin Susceptibility
β-Lactamase Production
Minimum Inhibitory Concentration (MIC)
2.7. Data Analysis
3. Results and Discussion
3.1. Obtention and FTIR Characterization of PAM-18Na Polymer
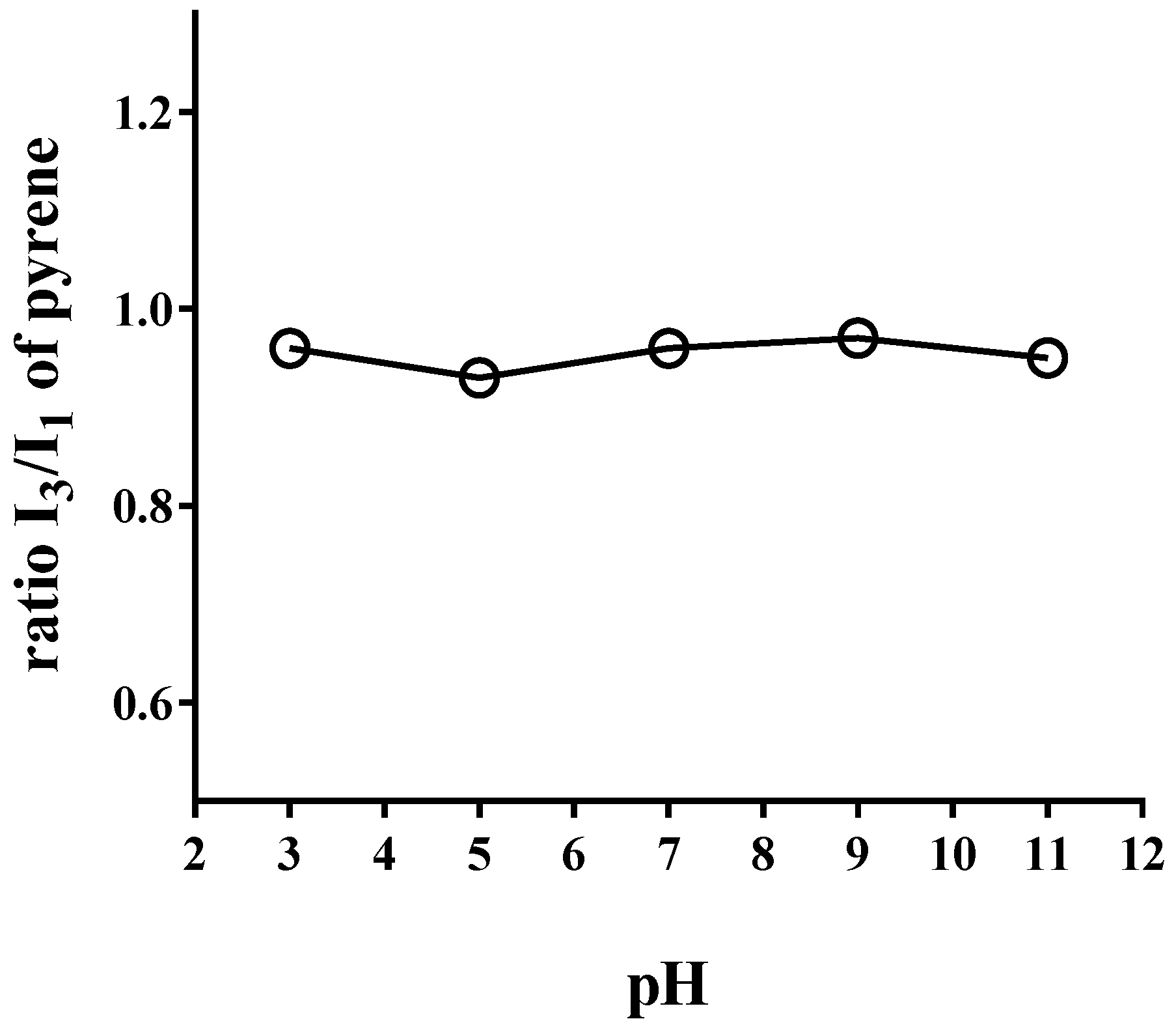
3.2. Steady-State Fluorescence Assay
3.3. Characterization of the Drug-Polymer Inclusion Complex
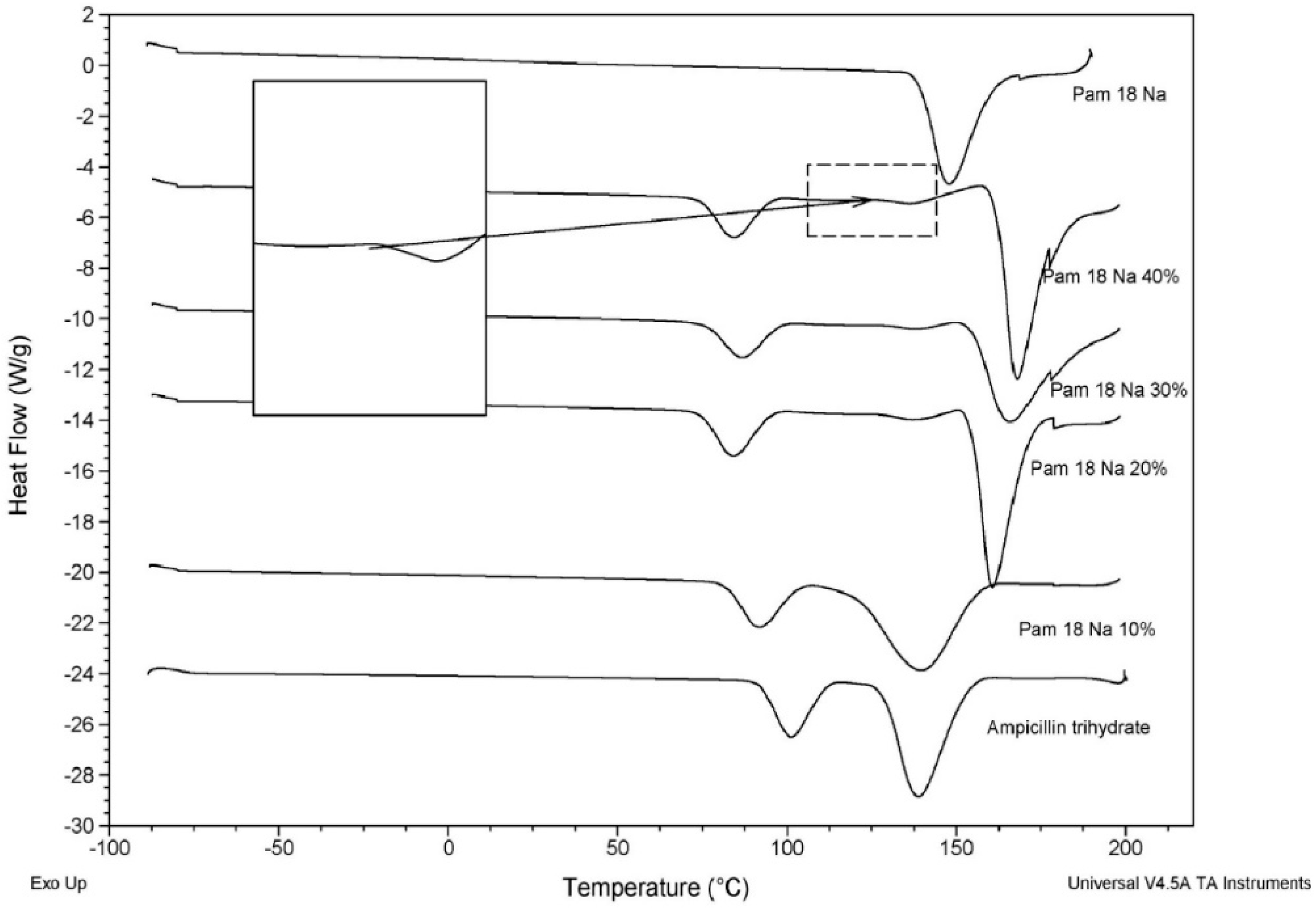
3.3.1. Thermal Characterization of the Polymer-Drug Blend
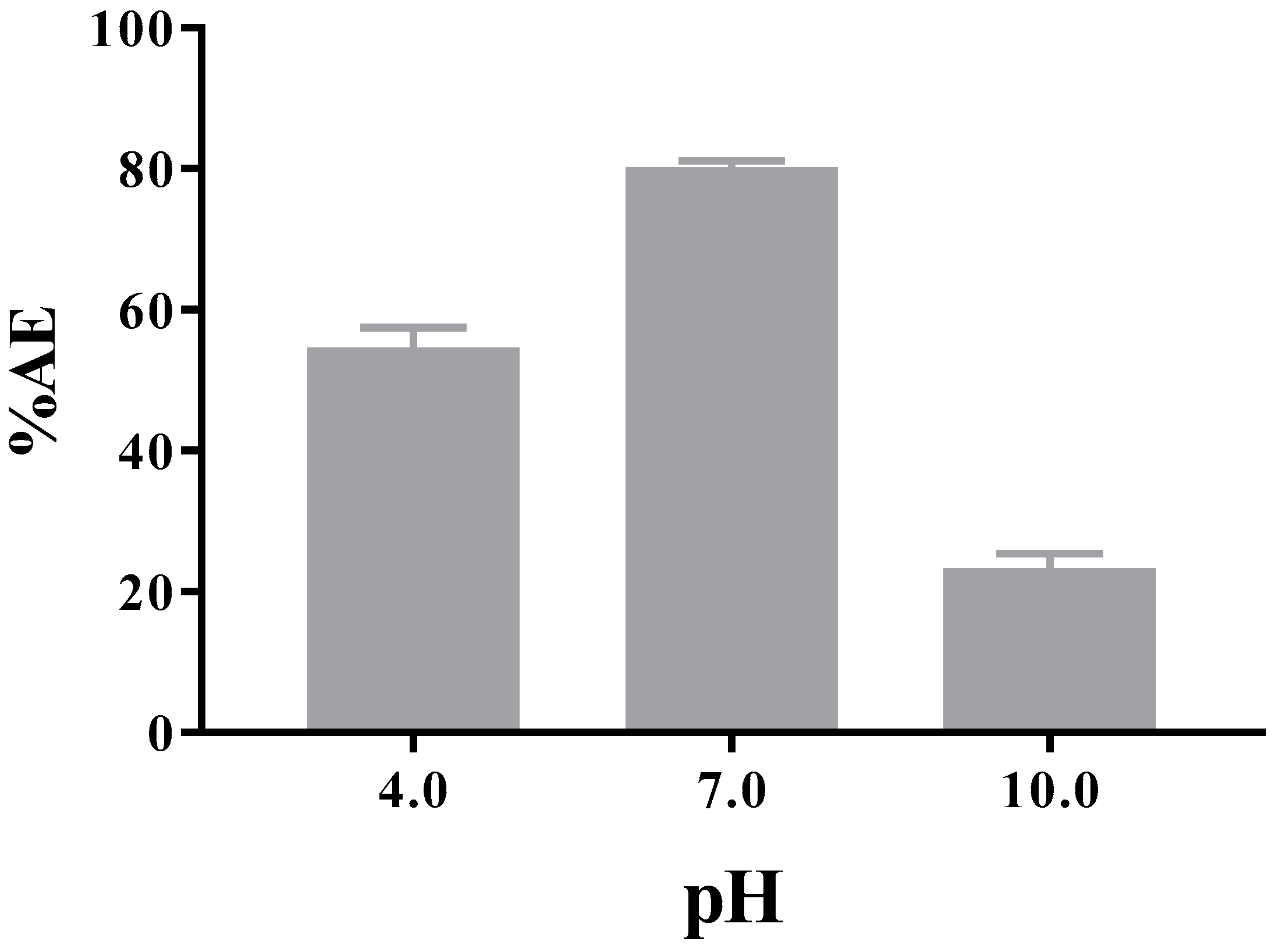
3.3.2. Encapsulation Efficiency
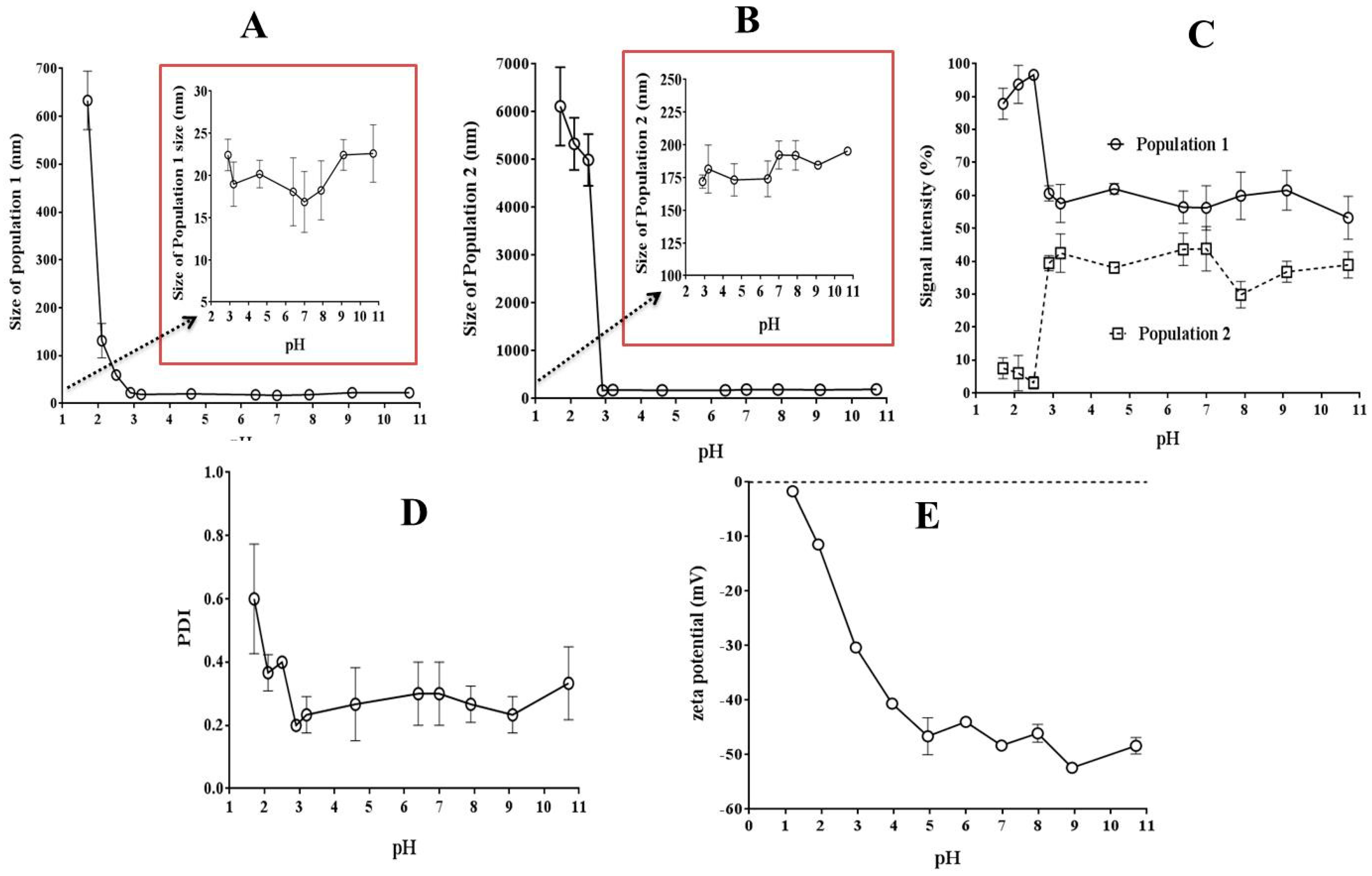
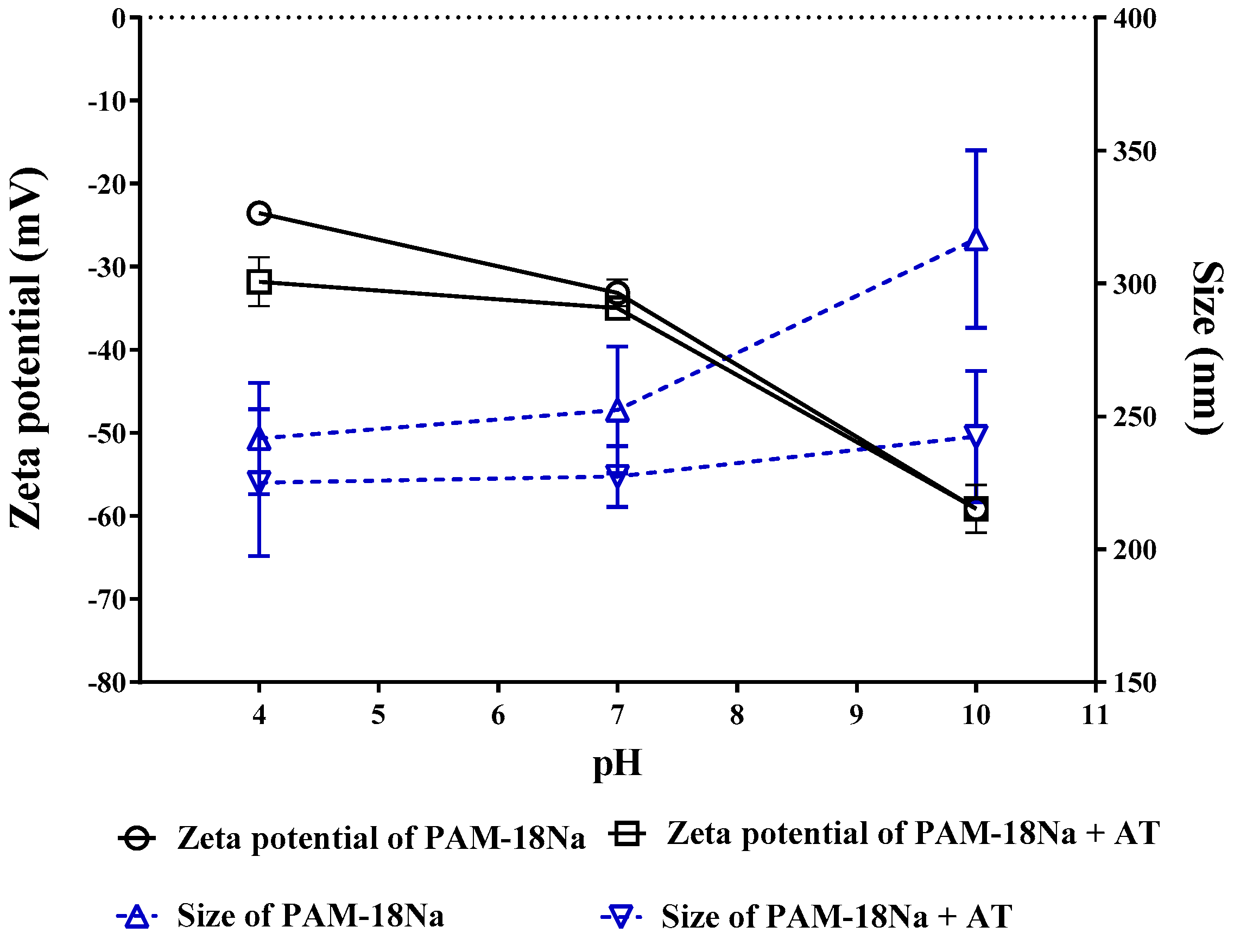
3.3.3. Zeta Potential and Size Measurements
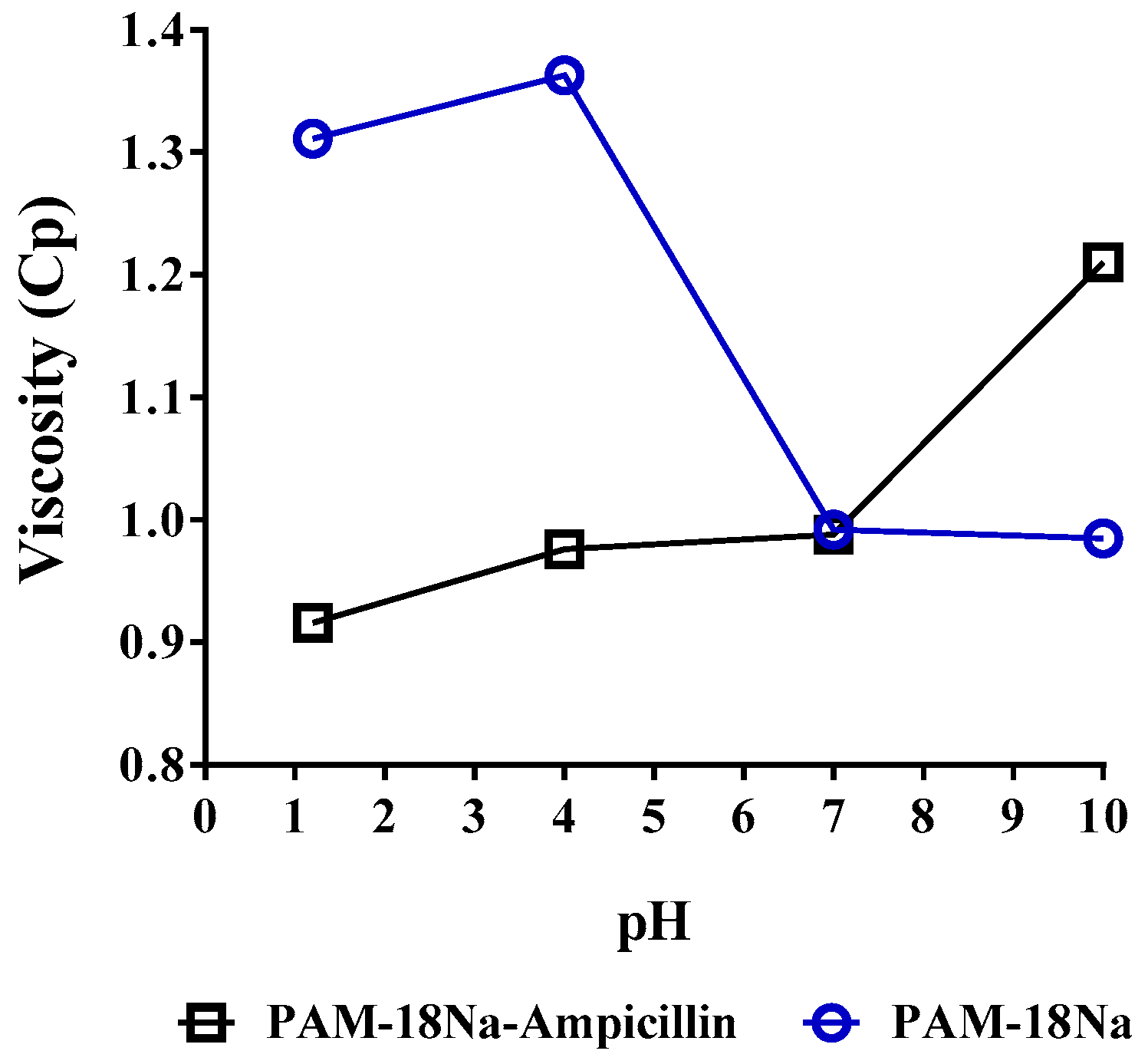
3.3.4. Viscometric Measurements
3.4. Degradation Assays
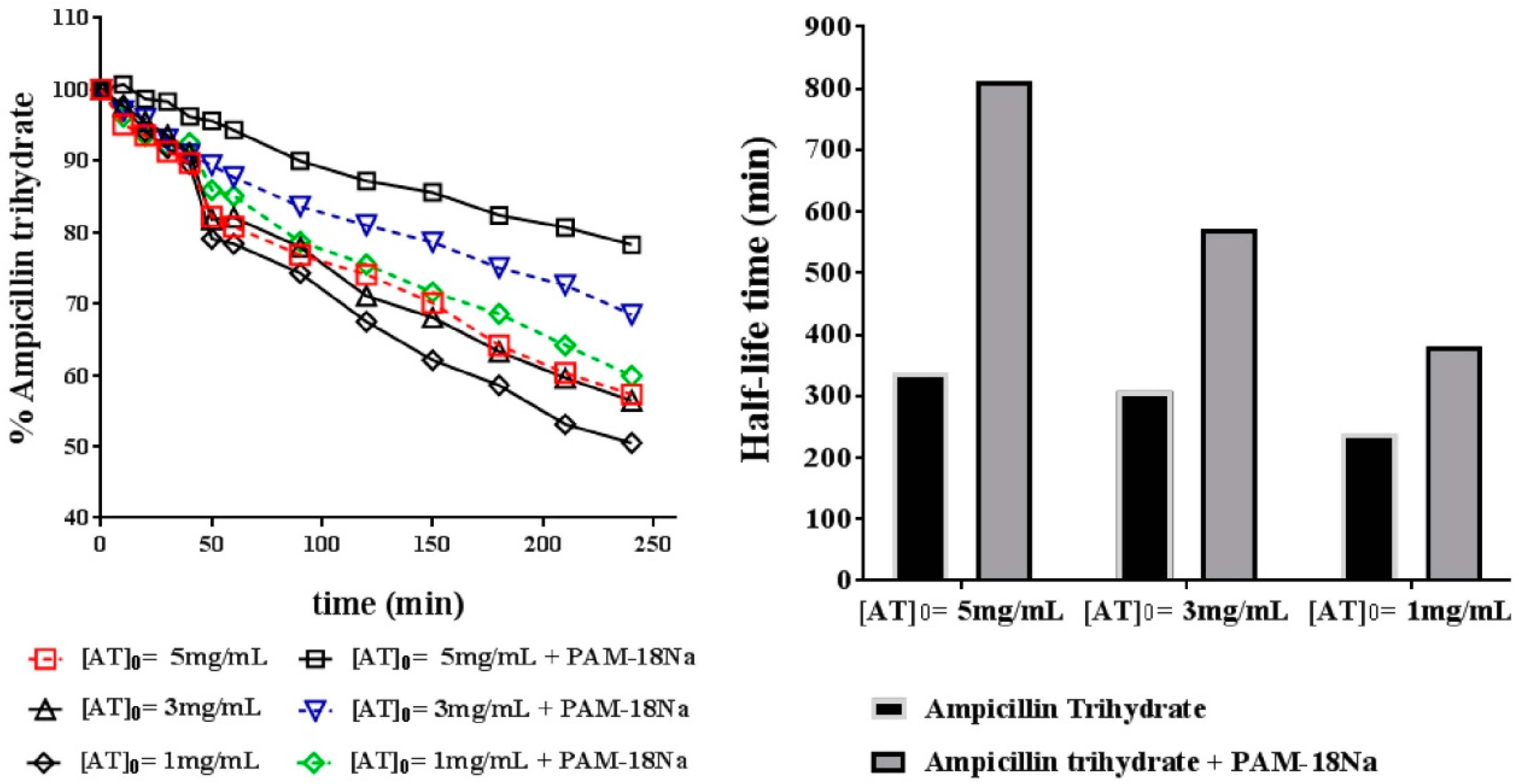
3.4.1. Chemical Stability
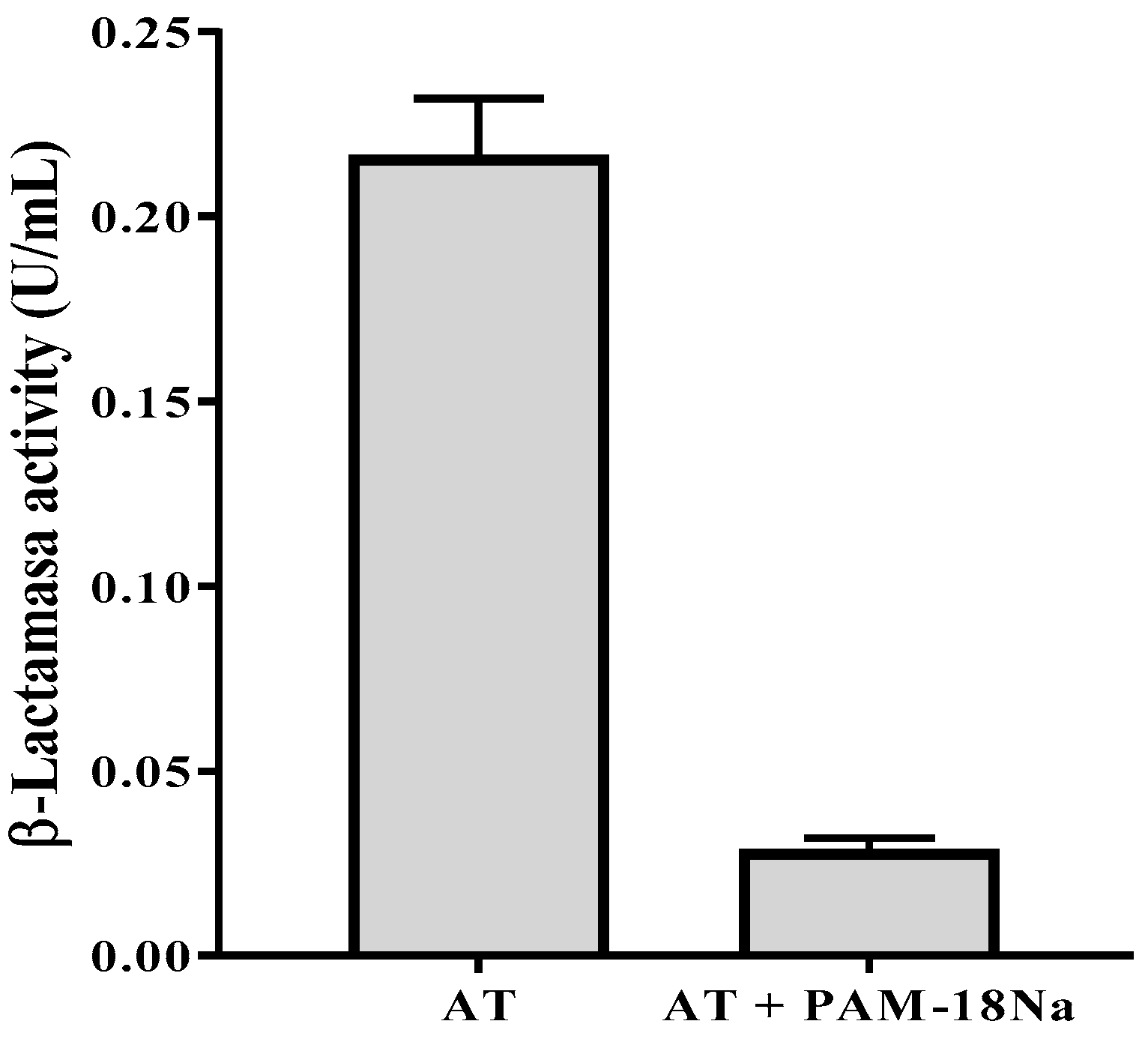
3.4.2. β-Lactamase Activity Assay
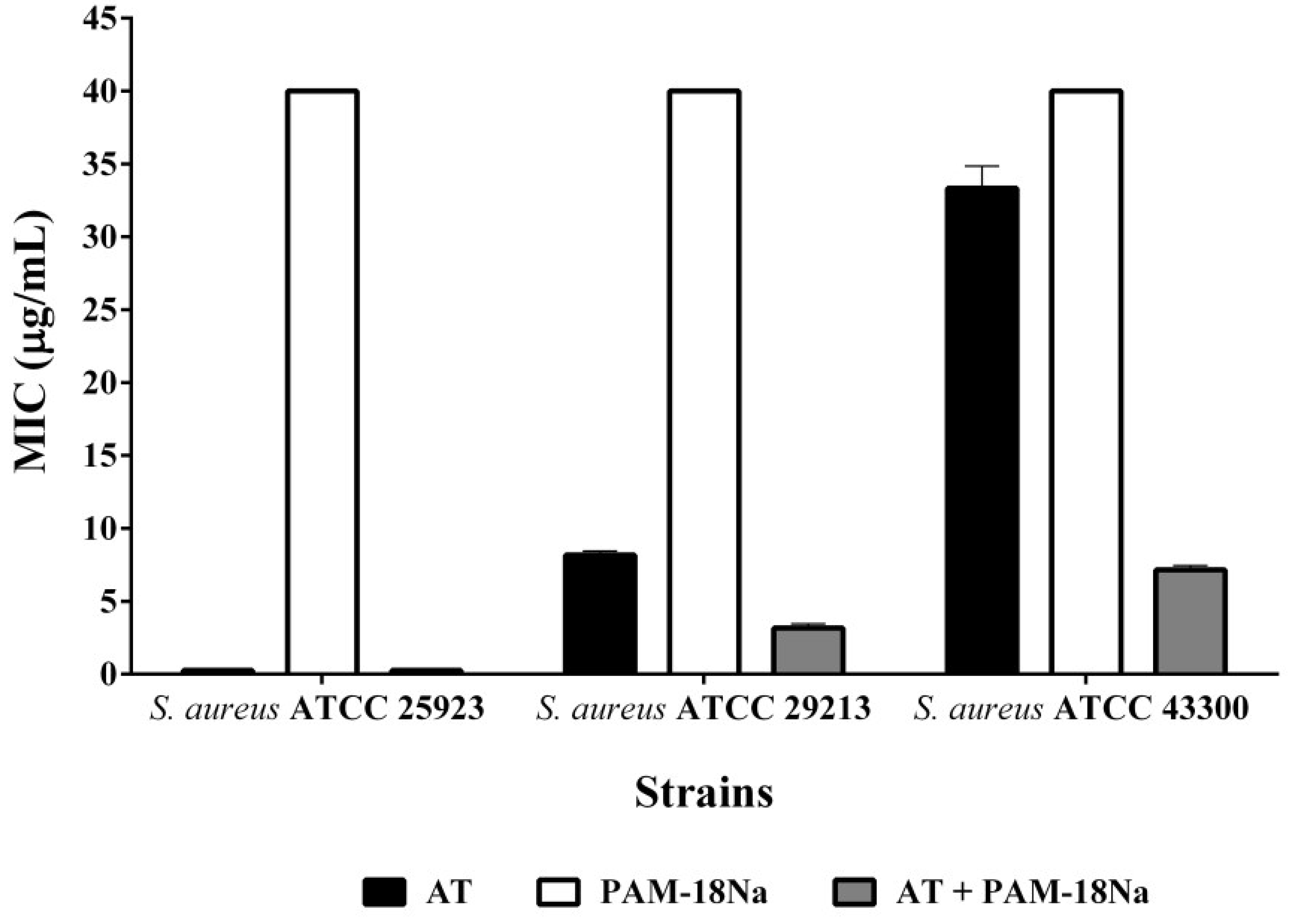
3.4.3. Antimicrobial Activity Assays
Strains Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fauci, A.; Marston, H. The Perpetual Challenge of Antimicrobial Resistance. J. Am. Med. Assoc. 2014, 311, 1853–1854. [Google Scholar] [CrossRef] [PubMed]
- Alanis, A.J. Resistance to Antibiotics: Are We in the Post-Antibiotic Era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.; Sanchez, S. Microbial drug discovery: 80 Years of progress. J. Antibiot. 2009, 62, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.; Mueller, L.; Polyakov, M.; Weinstock, S. Where have all the antibiotic patents gone. Nat. Biotechnol. 2006, 24, 1529–1531. [Google Scholar] [CrossRef] [PubMed]
- Naber, C.K. Staphylococcus aureus bacteremia: Epidemiology, pathophysiology, and management strategies. Clin. Infect. Dis. Oxf. J. 2009, 48, S231–S237. [Google Scholar] [CrossRef] [PubMed]
- Valaperta, R.; Tejada, M.; Frigerio, M. Staphylococcus aureus nosocomial infections: The role of a rapid and low-cost characterization for the establishment of a surveillance system. New Microbiol. 2010, 33, 223–232. [Google Scholar] [PubMed]
- Stratton, C.W. The clinical implications of ß-lactamases. Antimicrob. Infect. Dis. Newsl. 1996, 15, 67–72. [Google Scholar] [CrossRef]
- Mediavilla, J.R.; Chen, L.; Mathema, B.; Kreiswirth, B.N. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA). Curr. Opin. Microbiol. 2012, 15, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Enright, M.C.; Robinson, D.A.; Randle, G.; Feil, E.J.; Grundmann, H.; Spratt, B.G. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc. Natl. Acad. Sci. USA 2002, 99, 7687–7692. [Google Scholar] [CrossRef] [PubMed]
- Grema, H.A. Methicillin Resistant Staphylococcus aureus (MRSA): A Review. Adv. Anim. Vet. Sci. 2015, 3, 79–98. [Google Scholar] [CrossRef]
- Papp-Wallace, K.M.; Bethel, C.R.; Gootz, T.D.; Shang, W.; Stroh, J.; Lau, W.; McLeod, D.; Price, L.; Marfat, A.; Distler, A.; et al. Inactivation of a class A and a class C β-lactamase by 6β-(hydroxymethyl)penicillanic acid sulfone. Biochem. Pharmacol. 2012, 83, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Fast, W.; Sutton, L.D. Metallo-β-lactamase: Inhibitors and reporter substrates. Biochim. Biophys. Acta Proteins Proteom. 2013, 1834, 1648–1659. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Lin, J. Beta-lactamase induction and cell wall metabolism in Gram-negative bacteria. Front. Microbiol. 2013, 4, 128. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, K.; Balcewich, M.; Mark, B.; Vocadlo, D. Small molecule inhibitors of a glycoside hydrolase attenuate inducible AmpC-mediated beta-lactam resistance. J. Biol. Chem. 2007, 282, 21382–21391. [Google Scholar] [CrossRef] [PubMed]
- Kalhapure, R.S.; Suleman, N.; Mocktar, C.; Seedat, N.; Govender, T. Nanoengineered drug delivery systems for enhancing antibiotic therapy. J. Pharm. Sci. 2015, 104, 872–905. [Google Scholar] [CrossRef] [PubMed]
- Arenas, T.; Mora, C.; Salamanca, C.H.; Jaramillo, M.C. Actividad del (2E)-3-(2, 3-dimetoxifenil)-1-(4-metilfenil) prop-2-en-1-ona en presencia del poli(ácido maleico-co-2-vinil-pirrolidona) sobre un aislamiento clínico de Staphylococcus aureus productor de β-lactamasas. Iatreia 2012, 25, 12–19. [Google Scholar]
- Xiong, M.H.; Bao, Y.; Yang, X.Z.; Zhu, Y.H.; Wang, J. Delivery of antibiotics with polymeric particles. Adv. Drug Deliv. Rev. 2014, 78, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Bezzaoucha, F.; Lochon, P.; Jonquières, A.; Fischer, A.; Brembilla, A.; Aïnad-Tabet, D. New amphiphilic polyacrylamides: Synthesis and characterisation of pseudo-micellar organisation in aqueous media. Eur. Polym. J. 2007, 43, 4440–4452. [Google Scholar] [CrossRef]
- Salamanca, C.; Urbano, B.; Olea, A.F. Potential drug delivery system: Study of the association of a model nitroimidazole drug with aggregates of amphiphilic polymers on aqueous solution. Braz. J. Pharm. Sci. 2011, 47, 6. [Google Scholar] [CrossRef]
- Wang, Y.; Grayson, S.M. Approaches for the preparation of non-linear amphiphilic polymers and their applications to drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 852–865. [Google Scholar] [CrossRef] [PubMed]
- Barraza, R.G.; Olea, A.F.; Valdebenito, C.E.; Dougnac, V.; Fuentes, I. Solubilization of p-nitrophenol in aggregates formed by hydrophobically modified polyelectrolytes. J. Colloid Interface Sci. 2004, 275, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, C.; Barraza, R.G.; Acevedo, B.; Olea, A.F. Hydrophobically modified polyelectrolytes as potential drugs reservoirs of N-alkyl-nitroimidazoles. J. Chil. Chem. Soc. 2007, 52. [Google Scholar] [CrossRef]
- Anton, P.; Laschewsky, A. Solubilization by polysoaps. Colloid Polym. Sci. 1994, 272, 1118–1128. [Google Scholar] [CrossRef]
- Bayoudh, S.; Laschewsky, A.; Wischerhoff, E. Amphiphilic hyperbranched polyelectrolytes: A new type of polysoap. Colloid Polym. Sci. 1999, 277, 519–527. [Google Scholar] [CrossRef]
- Olea, A.F.; Barraza, R.; Fuentes, I.; Acevedo, B. Solubilization of Phenols by Intramolecular Micelles Formed by Copolymers of Maleic Acid and Olefins. Macromolecules 2002, 35, 1049–1053. [Google Scholar] [CrossRef]
- Mitchell, S.M.; Ullman, J.L.; Teel, A.L.; Watts, R.J. PH and temperature effects on the hydrolysis of three β-lactam antibiotics: Ampicillin, cefalotin and cefoxitin. Sci. Total Environ. 2014, 466–467, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Galleni, M.; Frere, J. A survey of the kinetic parameters of class C beta-lactamases. Penicillins. Biochem. J. 1988, 255, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Michaux, C.; Massant, J.; Kerff, F. Crystal structure of a cold-adapted class C β-lactamase. FEBS J. 2012, 275, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. M02-A11. Performance Standards for Antimicrobial Disk Susceptibility Tests; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Marshall, S.A.; Sutton, L.D.; Jones, R.N. Evaluation of S1 chromogenic cephalosporin??-lactamase disk assay tested against Gram-positive anaerobes, coagulase-negative staphylococci, Prevotella spp. and Enterococcus spp. Diagn. Microbiol. Infect. Dis. 1995, 22, 353–355. [Google Scholar] [CrossRef]
- Yu, S.; Vosbeek, A.; Corbella, K.; Severson, J.; Schesser, J.; Sutton, L.D. A chromogenic cephalosporin for β-lactamase inhibitor screening assays. Anal. Biochem. 2012, 428, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. M07-A9. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32. [Google Scholar]
- Kalyanasundaram, K.; Thomas, J.K. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J. Am. Chem. Soc. 1977, 99, 2039–2044. [Google Scholar] [CrossRef]
- W. Binana-Limbele, R.Z. Fluorescence probing of microdomains in aqueous solutions of polysoaps. 1. Use of pyrene to study the conformational state of polysoaps and their comicellization with cationic surfactants. Macromolecules 1987, 20, 1331–1335. [Google Scholar] [CrossRef]
- Salamanca, C.H.; Castillo, D.F.; Villada, J.D.; Rivera, G.R. Physicochemical characterization of in situ drug-polymer nanocomplex formed between zwitterionic drug and ionomeric material in aqueous solution. Mater. Sci. Eng. C 2017, 72. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. CLSI Document M100-S24. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Turos, E.; Reddy, G.; Greenhalgh, K. Penicillin-Bound Polyacrylate Nanoparticles: Restoring the Activity of β-Lactam Antibiotics Against MRSA. Bioorg. Med. Chem. Lett. 2007, 17, 3468–3472. [Google Scholar] [CrossRef] [PubMed]
- Turos, E.; Shim, J.-Y.; Wang, Y.; Greenhalgh, K.; Reddy, G.S.K.; Dickey, S.; Lim, D.V. Antibiotic-conjugated polyacrylate nanoparticles: New opportunities for development of anti-MRSA agents. Bioorg. Med. Chem. Lett. 2007, 17, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Colzi, I.; Troyan, A.N.; Perito, B.; Casalone, E.; Romoli, R.; Pieraccini, G.; Škalko-Basnet, N.; Adessi, A.; Rossi, F.; Gonnelli, C.; et al. Antibiotic delivery by liposomes from prokaryotic microorganisms: Similia cum similis works better. Eur. J. Pharm. Biopharm. 2015, 94, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Muppidi, K.; Pumerantz, A.; Wang, J.; Betageri, G. Development and stability studies of novel liposomal vancomycin formulations. Arch. ISRN Pharm. 2012, 2012, 636743. [Google Scholar] [CrossRef] [PubMed]
- Salvage, J.P.; Rose, S.F.; Phillips, G.J.; Hanlon, G.W.; Lloyd, A.W.; Ma, I.Y.; Armes, S.P.; Billingham, N.C.; Lewis, A.L. Novel biocompatible phosphorylcholine-based self-assembled nanoparticles for drug delivery. J. Control. Release 2005, 104, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tang, Y.; Billingham, N.C.; Armes, S.P. Synthesis of Biocompatible, Stimuli-Responsive, Physical Gels Based on ABA Triblock Copolymers. Biomacromolecules 2003, 4, 864–868. [Google Scholar] [CrossRef] [PubMed]
| Strain | Average Diameter (mm) | Standard Deviation |
|---|---|---|
| S. aureus ATCC 25923 (sensitive) | 33.99 | 0.52 |
| S. aureus ATCC 29213 (resistant) | 20.15 | 0.93 |
| S. aureus ATCC 43300 (resistant) | 12.19 | 0.51 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamanca, C.H.; Yarce, C.J.; Roman, Y.; Davalos, A.F.; Rivera, G.R. Application of Nanoparticle Technology to Reduce the Anti-Microbial Resistance through β-Lactam Antibiotic-Polymer Inclusion Nano-Complex. Pharmaceuticals 2018, 11, 19. https://doi.org/10.3390/ph11010019
Salamanca CH, Yarce CJ, Roman Y, Davalos AF, Rivera GR. Application of Nanoparticle Technology to Reduce the Anti-Microbial Resistance through β-Lactam Antibiotic-Polymer Inclusion Nano-Complex. Pharmaceuticals. 2018; 11(1):19. https://doi.org/10.3390/ph11010019
Chicago/Turabian StyleSalamanca, Constain H., Cristhian J. Yarce, Yony Roman, Andrés F. Davalos, and Gustavo R. Rivera. 2018. "Application of Nanoparticle Technology to Reduce the Anti-Microbial Resistance through β-Lactam Antibiotic-Polymer Inclusion Nano-Complex" Pharmaceuticals 11, no. 1: 19. https://doi.org/10.3390/ph11010019
APA StyleSalamanca, C. H., Yarce, C. J., Roman, Y., Davalos, A. F., & Rivera, G. R. (2018). Application of Nanoparticle Technology to Reduce the Anti-Microbial Resistance through β-Lactam Antibiotic-Polymer Inclusion Nano-Complex. Pharmaceuticals, 11(1), 19. https://doi.org/10.3390/ph11010019