1. Introduction
Microelectrode arrays (MEAs) constitute an important class of tools for cellular electrophysiology, where the electrical properties of electrogenic cells, e.g., neurons or cardiomyocytes, are of central interest. MEAs typically employ multiple closely spaced microscale electrodes capable of recording electrical signals from biological cells. Due to their increasingly diverse and promising applications including basic neuroscience and cardiac research, brain-computer interfaces, drug screening, and precision medicine, efforts in the past few decades have continuously advanced the technology in terms of the number of electrodes, number of parallel recordings, spatio-temporal resolution, signal-to-noise ratio, co-integration of a multitude of sensing and stimulating modalities, ease of use, and throughput [
1,
2,
3]. These advancements have been realized via refinements of all components constituting MEA technology including array design, integrated circuitry, off-chip hardware, and software. While all these components contribute to the final performance of a MEA system, the design of the microelectrodes that make up the array remains crucial. Consequently, electrode engineering, mostly via micro-nano-fabrication techniques, has become an increasingly important path to innovate these systems.
Microelectrode designs and fabrication processes spanning a large number of different materials [
4,
5,
6,
7,
8], shapes [
9,
10,
11,
12,
13], and sizes [
14,
15,
16] have been reported and extensively reviewed [
17,
18,
19,
20]. The large number of design choices, while allowing for diverse possibilities of novel and performant functional structures, nonetheless, comes at the expense of engineers having to optimize over an increasingly expansive parameter space. This issue is further complicated by a lack of adequate simulation tools to aid design. Consequently, during early-stage development when the number of possible design candidates remains high, electrode engineers have to iteratively fabricate and test multiple designs to empirically screen and optimize.
The necessity of empirical testing during the early-stage design of electrodes incurs significant cost and represents a non-trivial barrier to new electrode development. Therefore, cost-effective hardware solutions aiding new electrode design and development also become increasingly important. In this paper, the preliminary results of which were presented at the Eurosensors conference [
21], we first identify what constitutes a useful set of tests for the early-stage screening of array-based electrophysiological recording electrodes and describe the current challenges in implementing these tests. We then address the needs and challenges by implementing a cost-effective, compact, and easy-to-assemble testbed hardware solution that seeks to facilitate a lower entry barrier for and more efficient development of new array-based electrophysiological microelectrodes. Finally, we experimentally demonstrate the testbed with different electrodes microfabricated on 60-electrode passive arrays. At significantly lower costs than existing solutions, the platform can perform electrochemical impedance spectroscopy (EIS) with an accuracy comparable to that of commercial equipment, enabling the resolution of impedance differences between microscale electrodes. Additionally, the platform is demonstrated to record from in vitro cell cultures at signal-to-noise ratios comparable to those of high-cost MEA recording systems, which facilitated the extraction of spike amplitudes from neurons as a test metric.
3. Fabrication, Packaging, and Interfacing for Electrodes Under Test
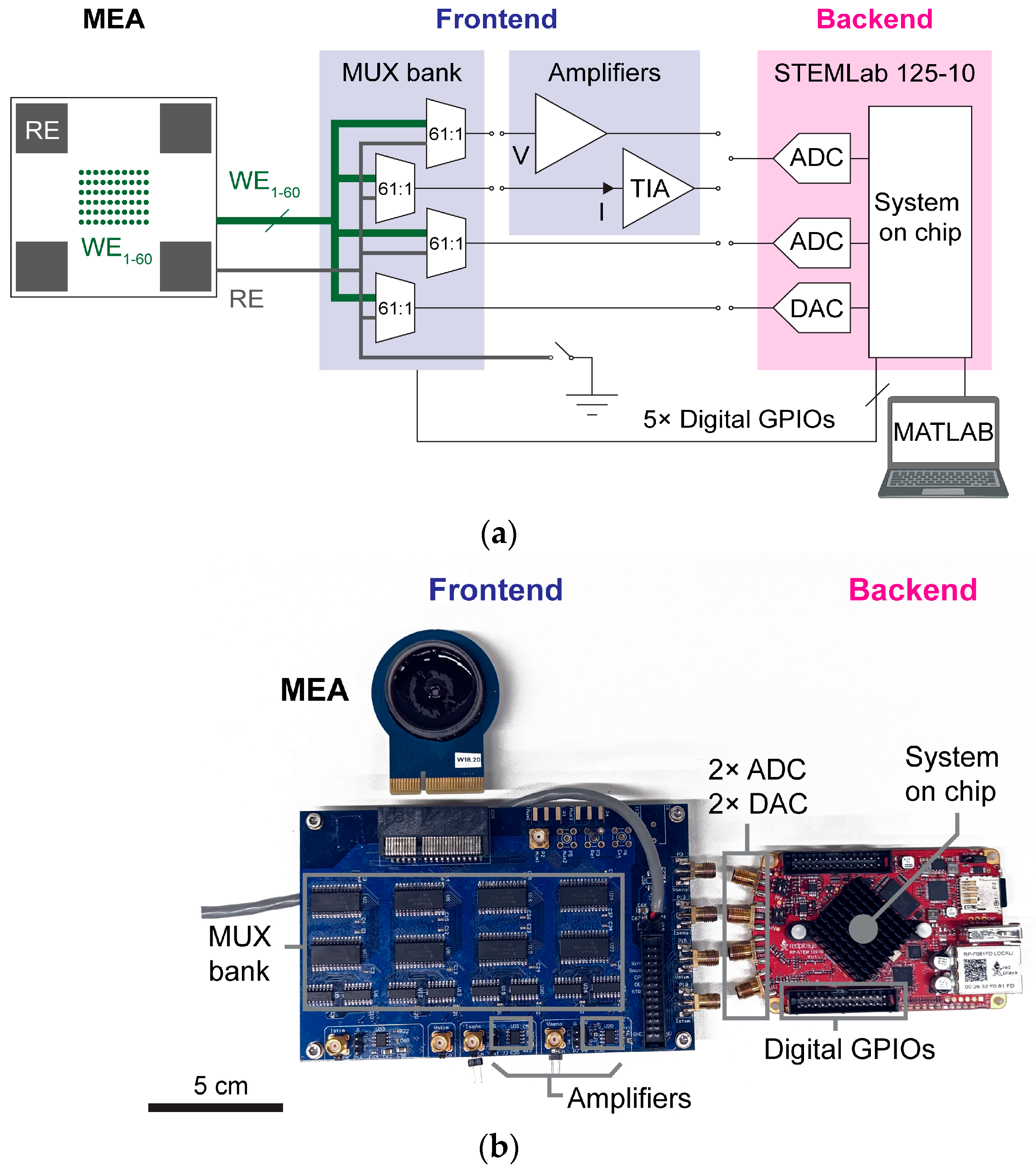
In order to interface the testbed platform frontend to microscale electrodes, we microfabricated passive MEA chips hosting several electrode designs to be tested. These chips were packaged onto an adapter printed circuit board (PCB), which was then connected to the frontend board via a PCIe socket. The packaged devices could be plated with in vitro cell cultures for ER testing.
Depending on the specific electrode designs that developers wish to test, the MEA chip layout and package described in this section may have to be adapted but generally should serve as a versatile test vehicle template for interfacing various electrodes under test to the testbed. The template supports the testing of up to 60 array-based electrophysiological microelectrodes.
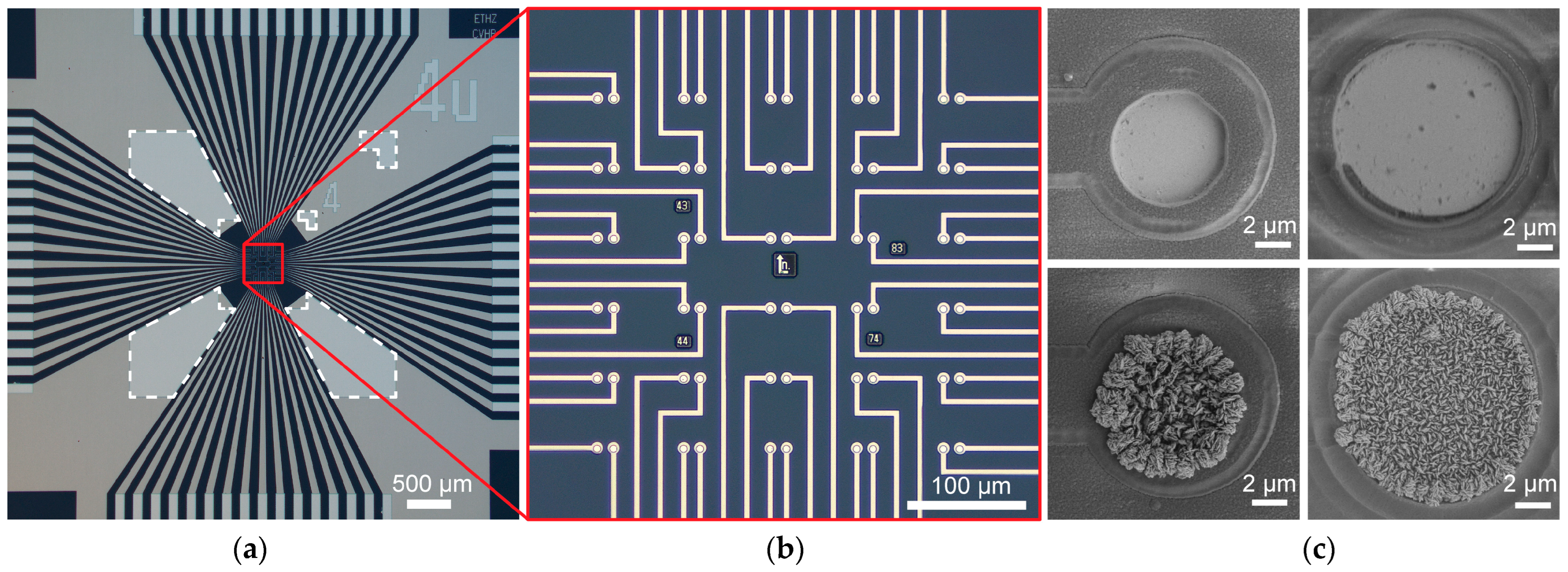
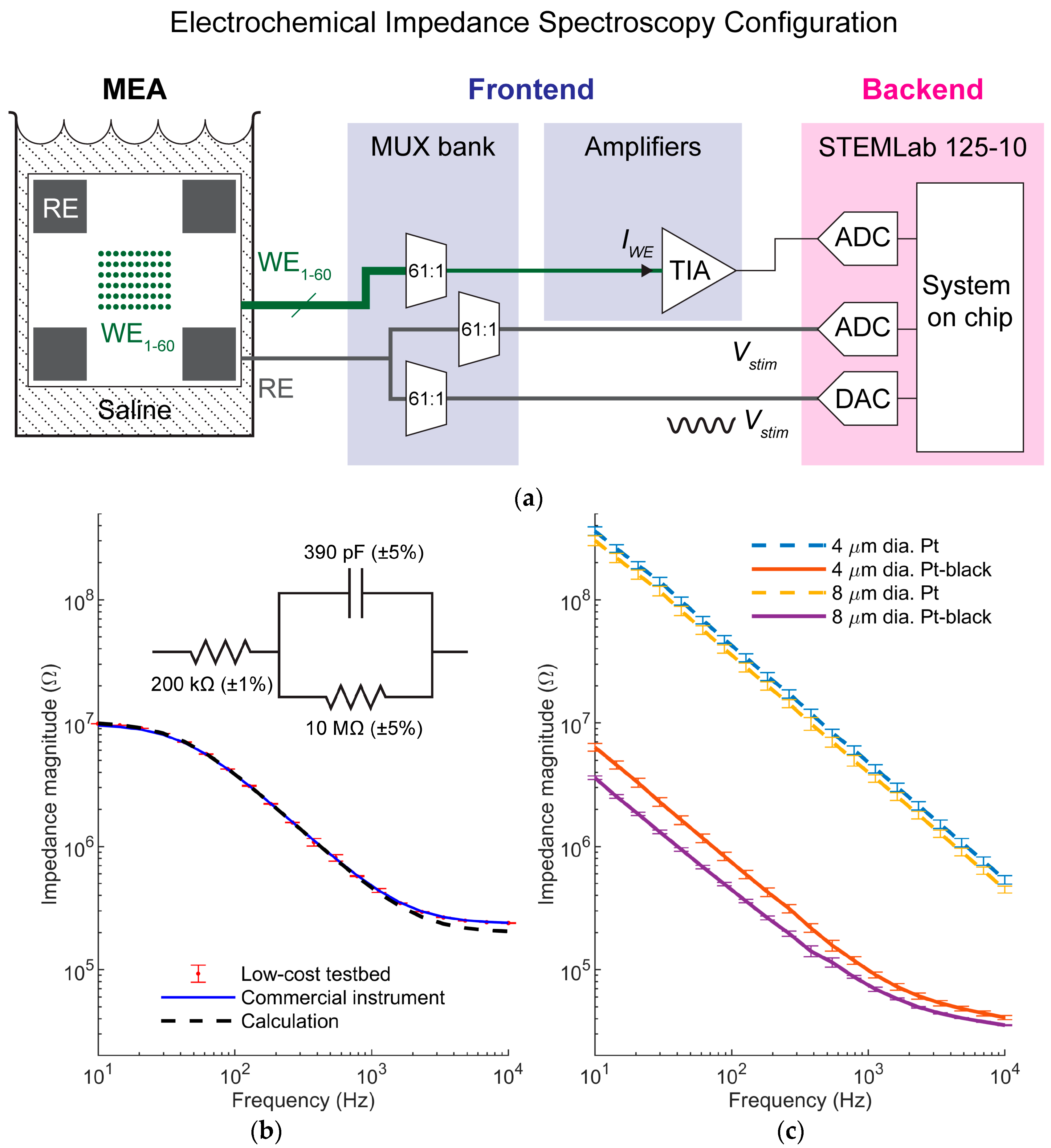
For specific electrodes under test in this paper, we microfabricated circular planar electrodes with photomask openings of 4 µm and 8 µm diameters. Electrodes were made of either platinum or highly porous platinum, known as platinum-black (Pt-black) (
Figure 2c). Both high- and low-impedance materials, as well as a small difference in electrode size, were used to effectively show the impedance range and resolution of the platform. Microelectrodes were fabricated on a passive MEA featuring an array of 60 working electrodes (WEs) and 1 large reference electrode (RE) on a glass substrate with SiO
2 as surface passivation (
Figure 2a,b). The sensing array covered an area of 310 × 300 μm
2 with 30 pairs of electrodes, each with an electrode center-to-center pitch of 14 µm.
Microelectrode arrays were microfabricated using standard cleanroom techniques. Fused silica wafers (100 mm in diameter, 380 µm thick) were used as starting substrates. A layer of 500 nm thick Si3N4 was first deposited using a Plasmalab 80Plus plasma-enhanced chemical vapor deposition system (Oxford Instruments, Abingdon, UK). Negative photoresist AZ nLOF 2020 (Merck, Darmstadt, Germany) was spin-coated at a spin speed of 5000 rpm. The wafer was then baked at 110 °C for 60 s and exposed with an MA/BA8 Gen4 mask aligner (SUSS MicroTec, Garching, Germany) using an i-line source at a dose of 40–50 mJ/cm2. Following a post-exposure bake of 110 °C for 60 s, the wafer was developed for 90 s using an AZ 726 MIF developer (Merck, Darmstadt, Germany). A film stack of Ti-Pt (20/150 nm) was deposited using a BAK UNI evaporator (Evatec, Trubbach, Switzerland). After stripping of the photoresist using a TechniStrip NI555 stripping solution (Technic, Cranston, RI, USA), the metal lift-off process was completed with the Ti-Pt layer patterned to form electrode arrays, tracks, and bond pads. For surface passivation, ~1.2 µm thick SiO2 film was deposited using a Plasmalab 80Plus plasma-enhanced chemical vapor deposition system. A second photolithography sequence to define the electrode and bond pad openings was carried out with the same type of photoresist and exposure parameters mentioned in the previous step. Using this photoresist mask, the passivation SiO2 was etched through using reactive ion etching (CHF3/CF4/O2) in a Plasmalab 100 system (Oxford Instruments, Abingdon, UK). Photoresist was stripped using O2 plasma in a GIGAbatch asher (PVA TePla, Wettenberg, Germany). A blanket SiO2 etch was used to ensure over-etching reduced the SiO2 film to a final thickness of 1.0 μm. MEA chips were diced from the wafer using a DAD3240 dicer (DISCO, Tokyo, Japan).
For packaging, dies were attached and wire-bonded to adapter PCBs, which provided metal wirings to connect each electrode of the array to the system frontend. The adapter PCB also featured a 3 mm diameter through-hole, which acted as a viewport for observing cells cultured on top of the array using an inverted microscope. After wire-bonding, polycarbonate rings were affixed onto the adapter PCB, and MEA chips were encapsulated by epoxy, leaving only the array exposed, forming a well chamber (
Figure 1b).
Pt-black was grown on the electrodes of selected chips by electrodeposition. The chamber was filled with a solution of chloroplatinic acid hexahydrate (7 mM, Merck, Darmstadt, Germany), lead acetate (0.3 mM, Merck, Darmstadt, Germany), and DI water. A platinum wire was immersed in the solution and formed the anode. All the array electrodes were shorted and formed the cathode. A voltage of 1.3 V was applied between the two terminals for ~22 s for microelectrodes fabricated with 4 µm diameter mask openings or ~26 s for microelectrodes fabricated with 8 µm diameter mask openings. Additional deposition time could optionally be added based on visual inspection of Pt-black coatings.
5. Electrophysiological Recording Test
While EIS is useful for testing electrodes decoupled from the rest of the recording chain, different biological cell cultures or tissues may exhibit different responses to electrode materials and geometries. Biocompatibility and adhesion affinity between the cells and the electrodes contribute to cell-electrode coupling performance. Therefore, the platform was designed to perform a second test, namely electrophysiological recording (ER), in which the platform was configured to record signals of interest from different cell types and plating protocols. This test was aimed at verifying that electrophysiological signals could be obtained from electrodes under test and yielded signals or detectable spike amplitudes.
It should be noted that the platform delivered this functionality as an efficient test to aid electrode designers to evaluate cell-electrode compatibility and was not intended as a replacement for a full MEA recording system. Specifically, the testbed enabled looping through all electrodes in the array and recording from one electrode at a time. For each electrode, the recording was performed for the entire specified duration before moving to the next electrode in the array. Additionally, due to the buffer size limitations of the off-the-shelf STEMLab, recording for each electrode was performed repeatedly in segments of continuous 1.07 s of measurements. These design choices allowed the system to optimize for cost and ease of assembly while remaining sufficient to provide useful information on electrode performances in the presence of target cell and tissue types. To demonstrate ER functionality, we used Pt-Black electrodes fabricated with 8 µm diameter mask openings to record EAPs from primary rat cortical neurons.
5.1. Electrophysiological Recording Configuration
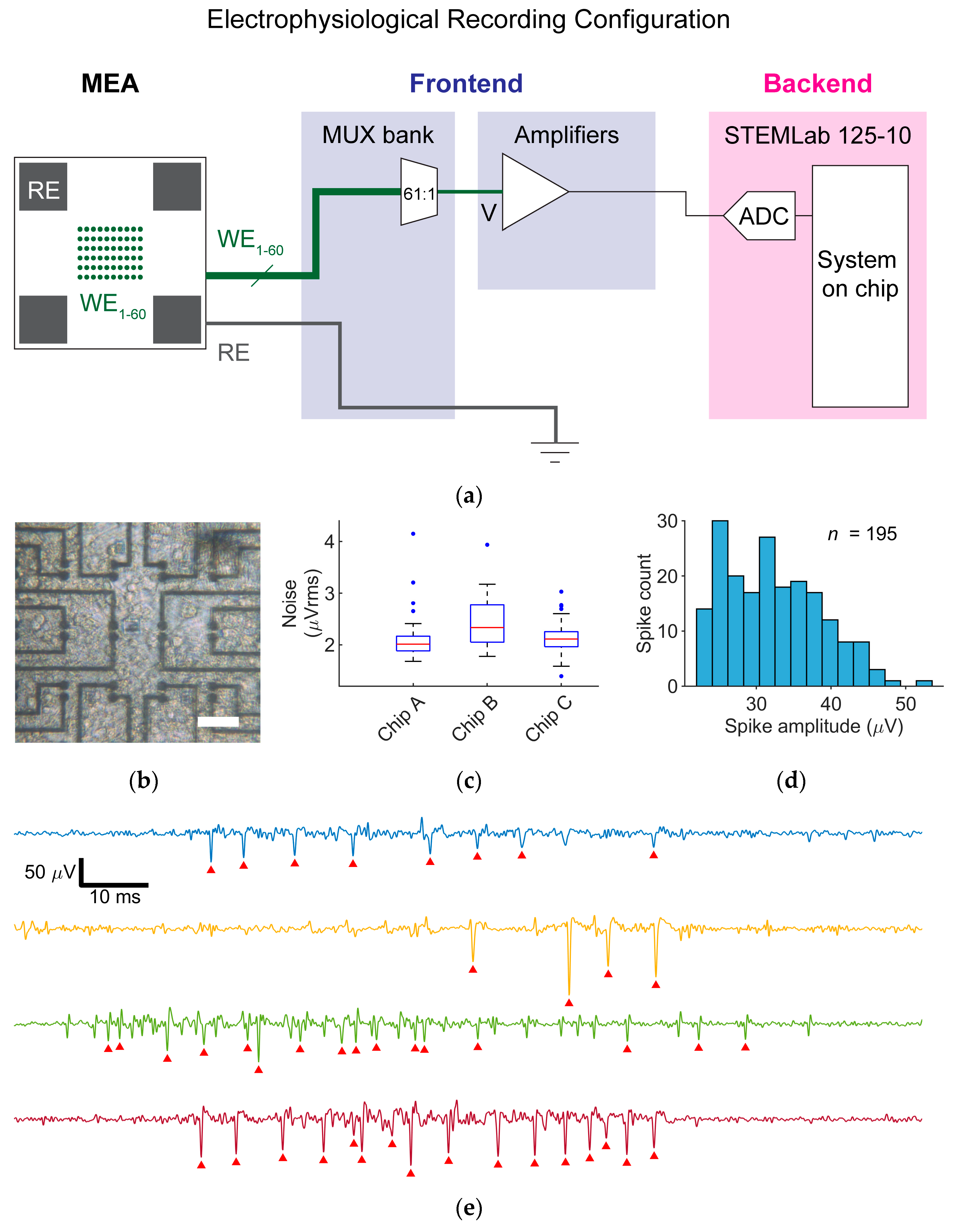
For the ER mode, one working electrode was routed via the MUX bank to the voltage amplifier (
Figure 4a). Output from the amplifier was recorded by an ADC on the STEMLab. The reference electrode was grounded. The maximum continuous recording duration was limited by the data acquisition buffer size,
Buffer = 16,384 samples, of the STEMLab. For a sampling frequency
fs, the system could record a maximum duration of
before having to save the data and repeating the data acquisition for any total (non-continuous) duration. Using the MUX Bank, the recording could be repeated for any set of electrodes in sequence. By looping through the electrodes one at a time, electrodes detecting electrophysiological activities could be identified. Active electrodes of interest could then be recorded for a longer total duration, from which spike amplitude information could be extracted and reported.
5.2. Recording of Neural Extracellular Action Potentials
Rat cortical neurons were plated on MEA devices featuring Pt-black electrodes with an 8 µm mask opening diameter (
Figure 2c, bottom right). Before neuron plating, devices were sterilized in 70% ethanol for 40 min and washed three times with sterile deionized (DI) water. The exposed arrays on the devices were then first coated with 7 µL of 0.05% (
v/
v) polyethylenimine (Merck, Darmstadt, Germany) in borate buffer (Thermo Fisher Scientific, Waltham, MA, USA) and kept in an incubator for 40 min at 37 °C. After rinsing three times with DI water, the arrays were subsequently coated with 7 μL of 0.02 mg mL
−1 laminin (Merck, Darmstadt, Germany) in Neurobasal medium (Thermo Fisher Scientific, Waltham, MA, USA) and kept in an incubator for at least 35 min. Cortices of E18 Wistar rat embryos were harvested in ice-cold Hanks’ balanced salt solution (Thermo Fisher Scientific, Waltham, MA, USA) and then dissociated in trypsin with 0.25% ethylenediaminetetraacetate (Thermo Fisher Scientific, Waltham, MA, USA). After cell counting, laminin was removed and 7000 cells were seeded onto the arrays. The devices were then incubated at 37 °C for ~40 min before 2 mL of plating medium was added. The plating medium was taken from a solution of 450 mL Neurobasal medium, 50 mL horse serum (Thermo Fisher Scientific, MA, Waltham, USA), 1.25 mL GlutaMAX (Thermo Fisher Scientific, Waltham, MA, USA), and 10 mL B-27 (Thermo Fisher Scientific, Waltham, MA, USA). Twice a week, 50% of the culture medium was exchanged with a growth medium. The growth medium was taken from a solution of 49 mL BrainPhys medium (STEMCELL Technologies, Vancouver, BC, Canada), 1 mL SM1 (STEMCELL Technologies, Vancouver, BC, Canada), and 100 µL Penicillin–Streptomycin (Thermo Fisher Scientific, Waltham, MA, USA). The cultures were maintained in an incubator at 37 °C and 5% CO
2. All recordings were performed at 37 °C. A representative image of a neuronal culture on an array taken through the backside viewport of the PCB is shown in
Figure 4b.
For all recordings from neuronal cultures, the sampling rate was set to 15.26 kHz, which, as described previously regarding the buffer size of the STEMLab, allowed for a maximum continuous recording duration of 1.07 s. For each chip, all 60 electrodes were recorded for a total (non-continuous) duration of 10.7 s each to scan for electrodes detecting electrophysiological activity. Active electrodes of interest were then selected and recorded for up to 10 min. Input-referred signals were computed by adjusting the ADC output by the gain (× 430) of the voltage amplifier. Signal traces were then bandpass-filtered over 300–5000 Hz. Input-referred noise (Vnoise,rms) was estimated for each electrode by taking the standard deviation of all filtered voltage traces recorded by the electrode. Noise data was obtained in the presence of active cell cultures to include contributions from electrode, instrument-related, and biological noise sources. Signal attenuation across the electrode was assumed to be small for this range of electrode impedance and hence neglected in calculations. EAPs were detected and counted using a spike detection threshold of 10 × Vnoise,rms.
Specifically, we recorded from six MEAs with 8 µm diameter Pt-black electrodes on days in vitro (DIV) 30–31, three of which showed electrophysiological activities. Input-referred noise distributions for three chips with active cell cultures are displayed in
Figure 4c and show reasonable electrode-to-electrode and chip-to-chip variability. Among the three chips, 40/180 electrodes were detecting electrical activity. Representative voltage traces containing EAPs, recorded from different electrodes on three chips, are shown in
Figure 4e. From a representative longer recording of 10 min from an active electrode, EAPs were extracted with a mean amplitude of 32.3 ± 6.4 µV. A histogram of EAP amplitudes is shown in
Figure 4d. The amplitudes of detected EAPs in this case verified the compatibility and adhesion affinity of a typical primary neuronal culture with the specific size and material choice of the electrodes under test. The capability to resolve a large number of EAP signals at signal-to-noise ratios > 10—well within the typical performance of higher-cost full MEA recording systems [
3]—demonstrates the cost-effectiveness of the platform for the purpose of capturing spike amplitude statistics as test metrics.
6. Conclusions
We designed and demonstrated a low-cost, compact, and easy-to-assemble multimodal testbed for array-based electrophysiological electrodes. At a fraction of the costs of comparable commercial equipment, the system could accurately resolve impedance differences between electrodes of different microscale sizes and material choices across a frequency range relevant to many types of electrophysiological signals. The platform was also used to record EAPs from rat cortical neurons with signal-to-noise ratios comparable to those obtained with higher-cost MEA recording systems. By cost-effectively enabling these two tests, the testbed can aid the early-stage development of electrophysiological microelectrodes and lower the entry barrier to MEA development.
While we have proposed the two aforementioned tests as broadly useful for electrophysiological electrodes, there might be additional testing requirements, which are important for specific MEA functionalities and applications and are not included here. For example, in case the MEA needs to be implantable, the stability of electrodes against degradation over time is a major consideration. While long-term stability testing is not included here, the platform can be readily incorporated into an accelerated aging procedure, where impedance can be tracked over time under conditions of interest as a way to predict reliability during chronic applications. Additionally, the modular design of the platform also addresses the need for testing versatility. The platform can be extended by additional instruments or wiring configurations. For example, a voltage waveform generator can be connected and routed to electrodes to perform electroporation. Lastly, when considered in conjunction with other reports for low-cost methods for making MEAs [
25,
26,
27], our solution may also contribute effectively to bringing cellular electrophysiology and MEA technology to a broader community of not only device and application developers but also educators and students.