Investigation of Polymers as Matrix Materials for Application in Colorimetric Gas Sensors for the Detection of Ammonia
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Sensor Material Films
2.1.1. Reagents
2.1.2. Processing and Deposition of the Sensor Material
2.2. Laboratory Setup for Testing the Sensor Probes and Test Method
2.2.1. Calibration Gas Generator
2.2.2. Gas Measurement Chamber
2.2.3. Colorimetric Characterization
3. Results
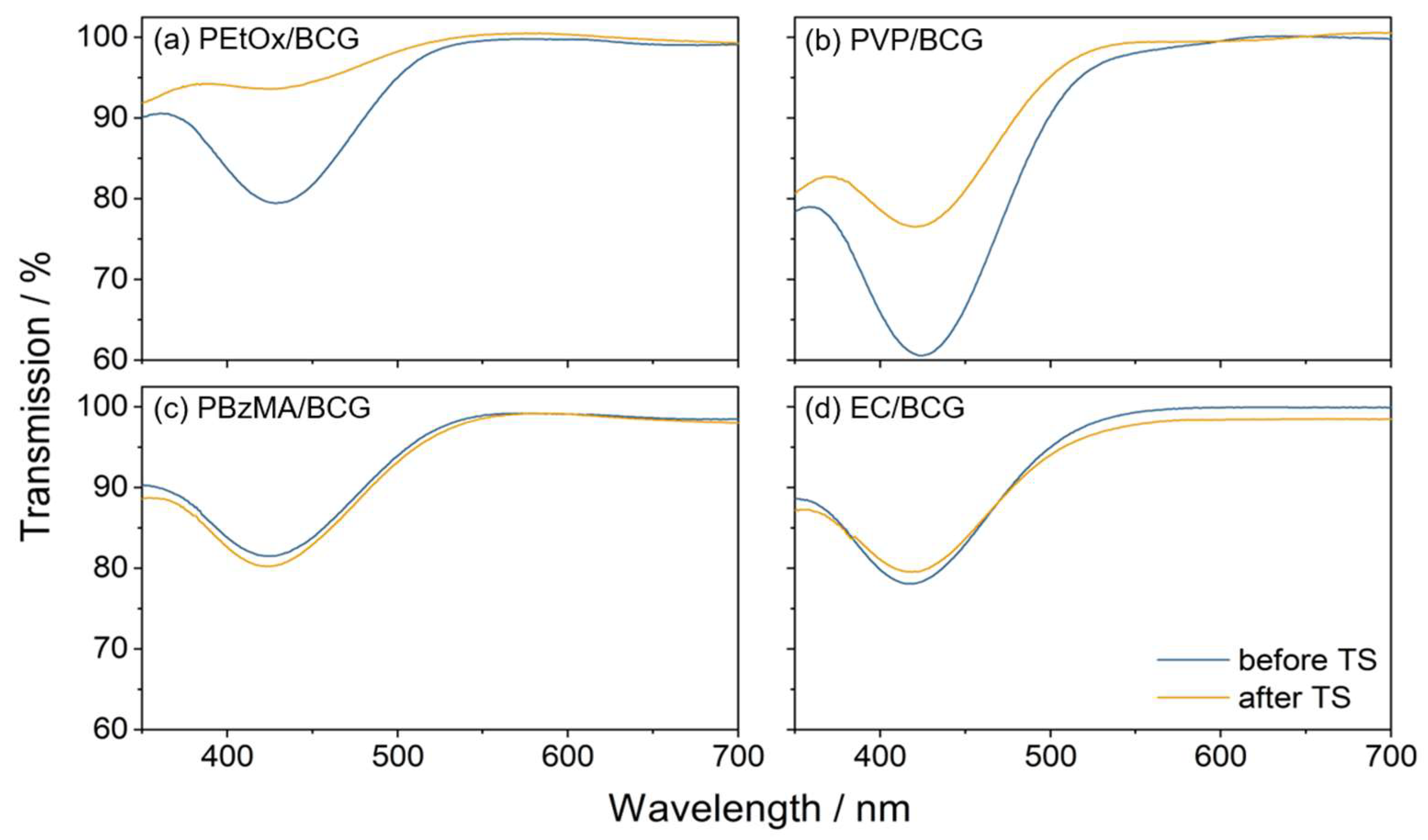
3.1. Shifting of Absorption Maxima
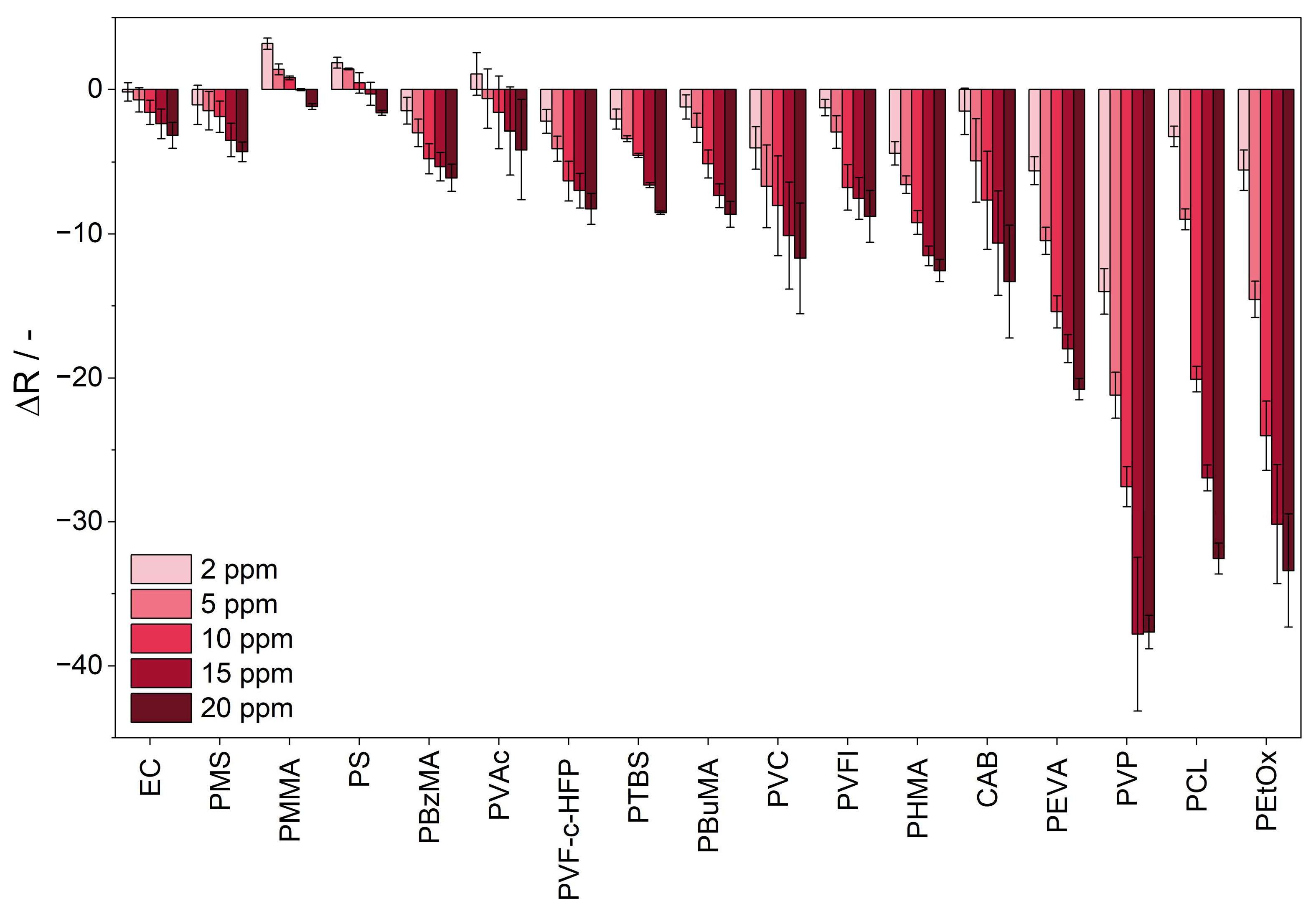
3.2. Response Towards Ammonia
3.3. Sensitivity
3.4. Response and Recovery Time
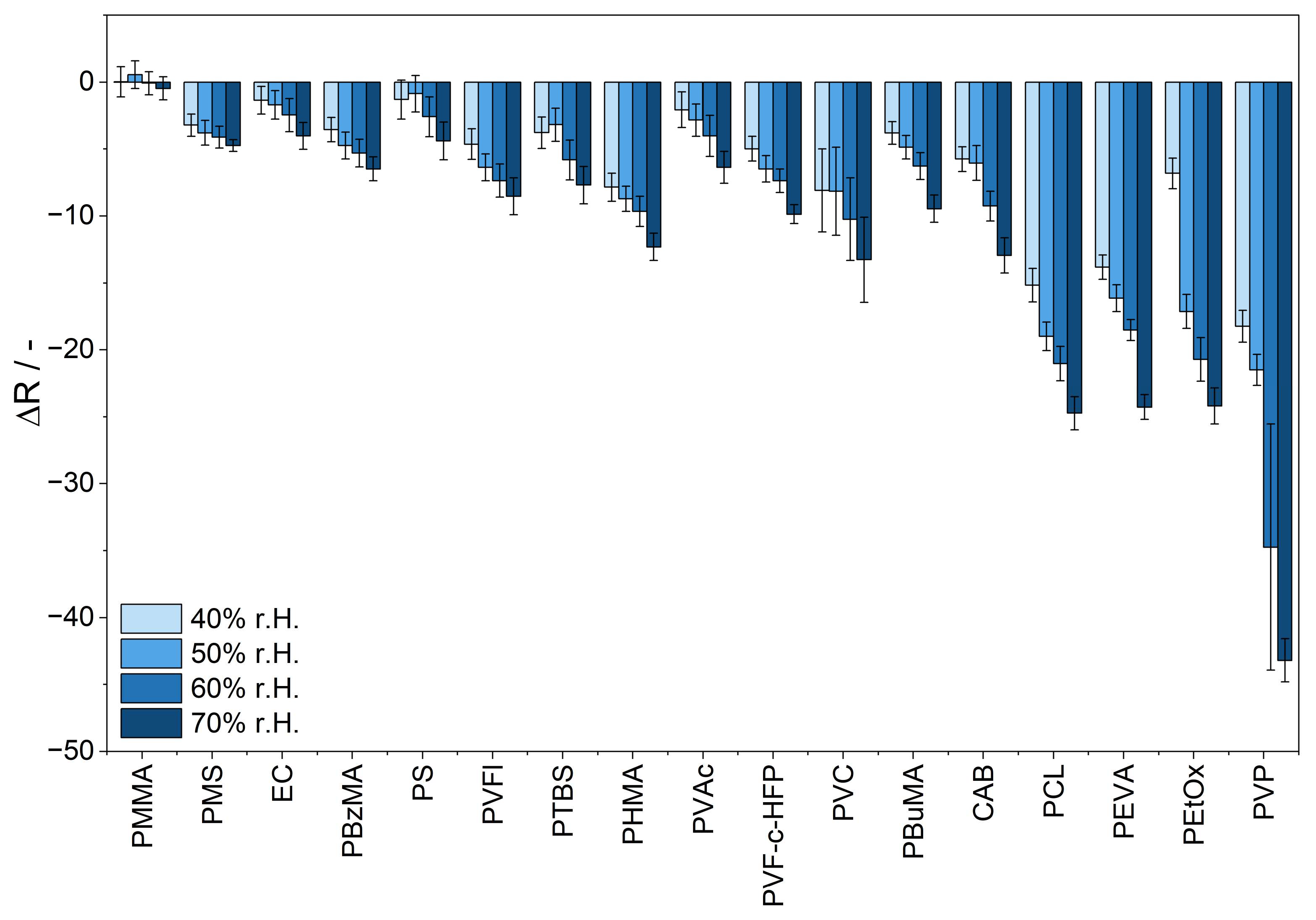
3.5. Influence of Humidity
3.6. Influence of Temperature
3.7. Stability of the Sensor Material
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Askim, J.R.; Suslick, K.S. The Optoelectronic Nose: Colorimetric and Fluorometric Sensor Arrays. Chem. Rev. 2019, 119, 231–292. [Google Scholar] [CrossRef]
- Wang, D.; Qian, L.; Zhang, F.; Mallires, K.; Tipparaju, V.V.; Yu, J.; Forzani, E.; Jia, C.; Yang, Q.; Tao, N.; et al. Multiplexed Chemical Sensing CMOS Imager. ACS Sens. 2022, 7, 3335–3342. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Bai, H.; Chiang, H.-S.; Karthik, M.; Li, S.-N.; Hsu, J.-N.; Shih, H.-Y. Development of regenerative dye impregnated mesoporous silica materials for assessing exposure to ammonia. J. Air Waste Manag. Assoc. 2012, 62, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Markovics, Á.; Nagy, G.; Kovács, B. Reflection-based sensor for gaseous ammonia. Sens. Actuators B Chem. 2009, 139, 252–257. [Google Scholar] [CrossRef]
- Tang, N.; Mu, L.; Qu, H.; Wang, Y.; Duan, X.; Reed, M.A. Smartphone-Enabled Colorimetric Trinitrotoluene Detection Using Amine-Trapped Polydimethylsiloxane Membranes. ACS Appl. Mater. Interfaces 2017, 9, 14445–14452. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, E.-H.; Lee, S.-W. A Flexible and Attachable Colorimetric Film Sensor for the Detection of Gaseous Ammonia. Biosensors 2022, 12, 664. [Google Scholar] [CrossRef]
- Feng, L.; Musto, C.J.; Suslick, K.S. A Simple and Highly Sensitive Colorimetric Detection Method for Gaseous Formaldehyde. J. Am. Chem. Soc. 2010, 132, 4046–4047. [Google Scholar] [CrossRef]
- Courbat, J.; Briand, D.; Wöllenstein, J.; de Rooij, N.F. Polymeric foil optical waveguide with inkjet printed gas sensitive film for colorimetric sensing. Sens. Actuators B Chem. 2011, 160, 910–915. [Google Scholar] [CrossRef]
- Engel, L.; Tarantik, K.R.; Pannek, C.; Wöllenstein, J. Screen-Printed Sensors for Colorimetric Detection of Hydrogen Sulfide in Ambient Air. Sensors 2019, 19, 1182. [Google Scholar] [CrossRef]
- Henfling, M.; Gossner, U.; Kutter, C.; Hansch, W.; Trupp, S. Simultaneous optical and electrical CO2 detection in one sensitive film. Sens. Actuators B Chem. 2017, 241, 1256–1260. [Google Scholar] [CrossRef]
- Sun, X.; Hao, L.; Chen, L.; Guo, X.; Han, C.; Chen, J.; Jiao, W.; Wang, R.; He, X. Spray deposition of colorimetric H2 detector with Pd/MoO3 nanocomposites for rapid hydrogen leakage monitoring at room temperature. Appl. Surf. Sci. 2022, 599, 153878. [Google Scholar] [CrossRef]
- Korotcenkov, G. (Ed.) Handbook of Gas Sensor Materials: Properties, Advantages and Shortcomings for Applications Volume 2: New Trends and Technologies; Springer: New York, NY, USA, 2014; ISBN 978-1-4614-7388-6. [Google Scholar]
- Askim, J.R.; Mahmoudi, M.; Suslick, K.S. Optical sensor arrays for chemical sensing: The optoelectronic nose. Chem. Soc. Rev. 2013, 42, 8649–8682. [Google Scholar] [CrossRef] [PubMed]
- Courbat, J.; Briand, D.; Damon-Lacoste, J.; Wöllenstein, J.; de Rooij, N.F. Evaluation of pH indicator-based colorimetric films for ammonia detection using optical waveguides. Sens. Actuators B Chem. 2009, 143, 62–70. [Google Scholar] [CrossRef]
- Apostolidis, A. Combinatorial Approach for Development of Optical Gas Sensors—Concept and Application of High-Throughput Experimentation. Doctoral Dissertation, Universität Regensburg, Regensburg, Germany, 2005. [Google Scholar]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. (Eds.) Polymer Handbook, 4th ed.; Wiley: New York, NY, USA, 1999; ISBN 0-471-16628-6. [Google Scholar]
- Li, Q.; Xu, M.; Jiang, C.; Song, S.; Li, T.; Sun, M.; Chen, W.; Peng, H. Highly sensitive graphene-based ammonia sensor enhanced by electrophoretic deposition of MXene. Carbon 2023, 202, 561–570. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, C.; Zou, Y.; Xu, F.; Sun, L.; Xiang, C. Room-temperature humidity-resistant highly sensitive ammonia sensor based on a porous MXene/Na2Ti3O7 @polyaniline composite. Sens. Actuators B Chem. 2024, 405, 135323. [Google Scholar] [CrossRef]
- Nguyen, C.K.; Taylor, P.D.; Zavabeti, A.; Alluhaybi, H.; Almalki, S.; Guo, X.; Irfan, M.; Kobaisi, M.A.; Ippolito, S.J.; Spencer, M.J.; et al. Instant-in-Air Liquid Metal Printed Ultrathin Tin Oxide for High-Performance Ammonia Sensors. Adv. Funct. Mater. 2024, 34, 2309342. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, Q.; Chen, C.; Wang, J.; Lin, B.; Liu, X.; Sun, X.; Liu, X.; Chen, L. A chemiresistive room temperature ammonia gas sensor based on self-assembled PPy/Zntpp. Sens. Actuators B Chem. 2024, 399, 134862. [Google Scholar] [CrossRef]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef]
- Li, C.; Tao, R.; Hou, J.; Wang, H.; Fu, C.; Luo, J. High-Performance Ammonia QCM Sensor Based on SnO2 Quantum Dots/Ti3C2Tx MXene Composites at Room Temperature. Nanomaterials 2024, 14, 1835. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Li, J.; Duan, Z.; Yang, Y.; Yuan, Z.; Jiang, Y.; Tai, H. Pd-Decorated ZnO Hexagonal Microdiscs for NH3 Sensor. Chemosensors 2024, 12, 43. [Google Scholar] [CrossRef]
- Shooshtari, M. Ammonia gas sensors based on multi-wall carbon nanofiber field effect transistors by using gate modulation. Colloids Surf. A Physicochem. Eng. Asp. 2025, 704, 135563. [Google Scholar] [CrossRef]
- Khachornsakkul, K.; Hung, K.-H.; Chang, J.-J.; Dungchai, W.; Chen, C.-H. A rapid and highly sensitive paper-based colorimetric device for the on-site screening of ammonia gas. Analyst 2021, 146, 2919–2927. [Google Scholar] [CrossRef]
- Cho, S.H.; Suh, J.M.; Eom, T.H.; Kim, T.; Jang, H.W. Colorimetric Sensors for Toxic and Hazardous Gas Detection: A Review. J. Electron. Mater. 2021, 17, 1–17. [Google Scholar] [CrossRef]
- Krishnan, S.T.; Son, K.H.; Kim, N.; Viswanath, B.; Kim, S.; An, J.H. Development of simple and sensitive hydrogel based colorimetric sensor array for the real-time quantification of gaseous ammonia. Mater. Sci. Eng. C 2017, 72, 583–589. [Google Scholar] [CrossRef]
- Hoang, A.T.; Cho, Y.B.; Park, J.-S.; Yang, Y.; Kim, Y.S. Sensitive naked-eye detection of gaseous ammonia based on dye-impregnated nanoporous polyacrylonitrile mats. Sens. Actuators B Chem. 2016, 230, 250–259. [Google Scholar] [CrossRef]
- Engel, L.; Benito-Altamirano, I.; Tarantik, K.R.; Pannek, C.; Dold, M.; Prades, J.D.; Wöllenstein, J. Printed sensor labels for colorimetric detection of ammonia, formaldehyde and hydrogen sulfide from the ambient air. Sens. Actuators B Chem. 2021, 330, 129281. [Google Scholar] [CrossRef]
- Abilevitch, L.; Otorgust, G.; Mizrahi, L.; Dotan, A.; Amir, E. Flexible colorimetric sensor for ammonia detection based on polyurethane and bromocresol green. Polym. Bull. 2025, 82, 1531–1550. [Google Scholar] [CrossRef]
- Murugan, N.A.; Schrader, S.; Ågren, H. Role of Protonation State and Solvation on the pH Dependent Optical Properties of Bromocresol Green. J. Chem. Theory Comput. 2014, 10, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Fassi, S.; Bousnoubra, I.; Sehili, T.; Djebbar, K. Degradation of “bromocresol green” by direct UV photolysis, Acetone/UV and advanced oxidation processes (AOP’s) in homogeneous solution (H2O2/UV, S2O82−/UV).Comparative study. J. Mater. Environ. Sci. 2012, 3, 732–743. [Google Scholar]
- Nie, W.; Chen, Y.; Zhang, H.; Liu, J.; Peng, Z.; Li, Y. A novel colorimetric sensor array for real-time and on-site monitoring of meat freshness. Anal. Bioanal. Chem. 2022, 414, 6017–6027. [Google Scholar] [CrossRef]
- Schmitt, K.; Tarantik, K.R.; Pannek, C.; Wöllenstein, J. Colorimetric Materials for Fire Gas Detection—A Review. Chemosensors 2018, 6, 14. [Google Scholar] [CrossRef]
- Mazur, F.; Han, Z.; Tjandra, A.D.; Chandrawati, R. Digitalization of Colorimetric Sensor Technologies for Food Safety. Adv. Mater. 2024, 36, 2404274. [Google Scholar] [CrossRef]
- Zedler, M.; Tse, S.W.; Ruiz-Gonzalez, A.; Haseloff, J. Paper-Based Multiplex Sensors for the Optical Detection of Plant Stress. Micromachines 2023, 14, 314. [Google Scholar] [CrossRef]
- You, Z.; Zhao, M.; Lu, H.; Chen, H.; Wang, Y. Eye-Readable and Wearable Colorimetric Sensor Arrays for In Situ Monitoring of Volatile Organic Compounds. ACS Appl. Mater. Interfaces 2024, 16, 19359–19368. [Google Scholar] [CrossRef] [PubMed]
- EN ISO 6145-1:2019; DIN Deutsches Institut für Normung e. V. DIN EN ISO 6145-1:2020-02: Gas Analysis—Preparation of Calibration Gas Mixtures Using Dynamic Methods—Part 1: General Aspects (ISO 6145-1:2019). German version; Beuth Verlag GmbH: Berlin, Germany, 2020.
- Mills, A.; Wild, L.; Chang, Q. Plastic colorimetric film sensors for gaseous ammonia. Mikrochim. Acta 1995, 121, 225–236. [Google Scholar] [CrossRef]
- Kassal, P.; Šurina, R.; Vrsaljko, D.; Steinberg, I.M. Hybrid sol–gel thin films doped with a pH indicator: Effect of organic modification on optical pH response and film surface hydrophilicity. J. Sol-Gel Sci. Technol. 2014, 69, 586–595. [Google Scholar] [CrossRef]
- Finch, C.A. Hydrophilic polymers. In Specialty Polymers; Dyson, R.W., Ed.; Springer: Boston, MA, USA, 1987; pp. 65–82. ISBN 978-1-4615-7894-9. [Google Scholar]
- Sadeghi, M.; Semsarzadeh, M.A.; Barikani, M.; Ghalei, B. The effect of urethane and urea content on the gas permeation properties of poly(urethane-urea) membranes. J. Memb. Sci. 2010, 354, 40–47. [Google Scholar] [CrossRef]
- Yun, X.; Wang, Y.; Li, M.; Jin, Y.; Han, Y.; Dong, T. Application of permselective poly(ε-caprolactone) film for equilibrium-modified atmosphere packaging of strawberry in cold storage. J. Food Process Preserv. 2017, 41, e13247. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, Q.; Han, A.; Zhou, H.; Chen, R.; Guo, S. Fabrication of Poly(ε-caprolactone)-Based Biodegradable Packaging Materials with High Water Vapor Barrier Property. Ind. Eng. Chem. Res. 2020, 59, 22163–22172. [Google Scholar] [CrossRef]
- Ghanem, A.F.; Yassin, M.A.; Rabie, A.M.; Gouanvé, F.; Espuche, E.; Abdel Rehim, M.H. Investigation of water sorption, gas barrier and antimicrobial properties of polycaprolactone films contain modified graphene. J. Mater. Sci. 2021, 56, 497–512. [Google Scholar] [CrossRef]
- Corres, M.Á.; Mayor, Á.; Sangroniz, A.; Del Río, J.; Iriarte, M.; Etxeberria, A. Blends based on biodegradable poly(caprolactone) with outstanding barrier properties for packaging applications: The role of free volume and interactions. Eur. Polym. J. 2020, 135, 109869. [Google Scholar] [CrossRef]
- Kawakami, Y.; Sugisaka, T. Relationships between structure, permeability and glass transition temperature of oligodimethylsiloxanyl substituted polymers. J. Membr. Sci. 1990, 50, 189–205. [Google Scholar] [CrossRef]
- Mogri, Z.; Paul, D. Gas sorption and transport in poly(alkyl (meth)acrylate)s. I. Permeation properties. Polymer 2001, 42, 7765–7780. [Google Scholar] [CrossRef]
- Nunes-Pereira, J.; Ribeiro, S.; Ribeiro, C.; Gombek, C.J.; Gama, F.M.; Gomes, A.C.; Patterson, D.A.; Lanceros-Méndez, S. Poly(vinylidene fluoride) and copolymers as porous membranes for tissue engineering applications. Polym. Test. 2015, 44, 234–241. [Google Scholar] [CrossRef]
- Camacho, J.; Díez, E.; Díaz, I.; Ovejero, G. Hansen solubility parameter: From polyethylene and poly(vinyl acetate) homopolymers to ethylene–vinyl acetate copolymers. Polym. Int. 2017, 66, 1013–1020. [Google Scholar] [CrossRef]
- Wolinska-Grabczyk, A.; Kubica, P.; Jankowski, A. Effect of the acetate group content on gas permeation through membranes based on poly(ethylene-co-vinyl acetate) and its blends. J. Membr. Sci. 2013, 443, 227–236. [Google Scholar] [CrossRef]
- Chen, W.-L.; Chiu, H.-C.; Chu, H.; Hsieh, J.-C.; Chen, B.-X.; Tian, Y.-C.; Liau, S.-K.; Chan, M.-J.; Lu, C.-J.; Meng, H.-F.; et al. Electronic nano sponge breath ammonia sensors using hygroscopic polymers on vertical channel nano-porous structure. J. Mater. Chem. C 2021, 9, 12938–12950. [Google Scholar] [CrossRef]
- Yu, J.; Wang, D.; Tipparaju, V.V.; Tsow, F.; Xian, X. Mitigation of Humidity Interference in Colorimetric Sensing of Gases. ACS Sens. 2021, 6, 303–320. [Google Scholar] [CrossRef]
- Schoolaert, E.; Hoogenboom, R.; de Clerck, K. Colorimetric Nanofibers as Optical Sensors. Adv. Funct. Mater. 2017, 27, 1702646. [Google Scholar] [CrossRef]
- Sazali, N.; Ibrahim, H.; Jamaludin, A.S.; Mohamed, M.A.; Salleh, W.N.W.; Abidin, M.N.Z. Degradation and stability of polymer: A mini review. IOP Conf. Ser. Mater. Sci. Eng. 2020, 788, 12048. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- IMX477-AACK-C: Diagonal 7.857 mm (Type 1/2.3) 12.3Mega-Pixel CMOS Image Sensor with Square Pixel for Color Cameras. Available online: https://docs.arducam.com/Raspberry-Pi-Camera/Native-camera/source/IMX477-DS.pdf (accessed on 27 December 2024).
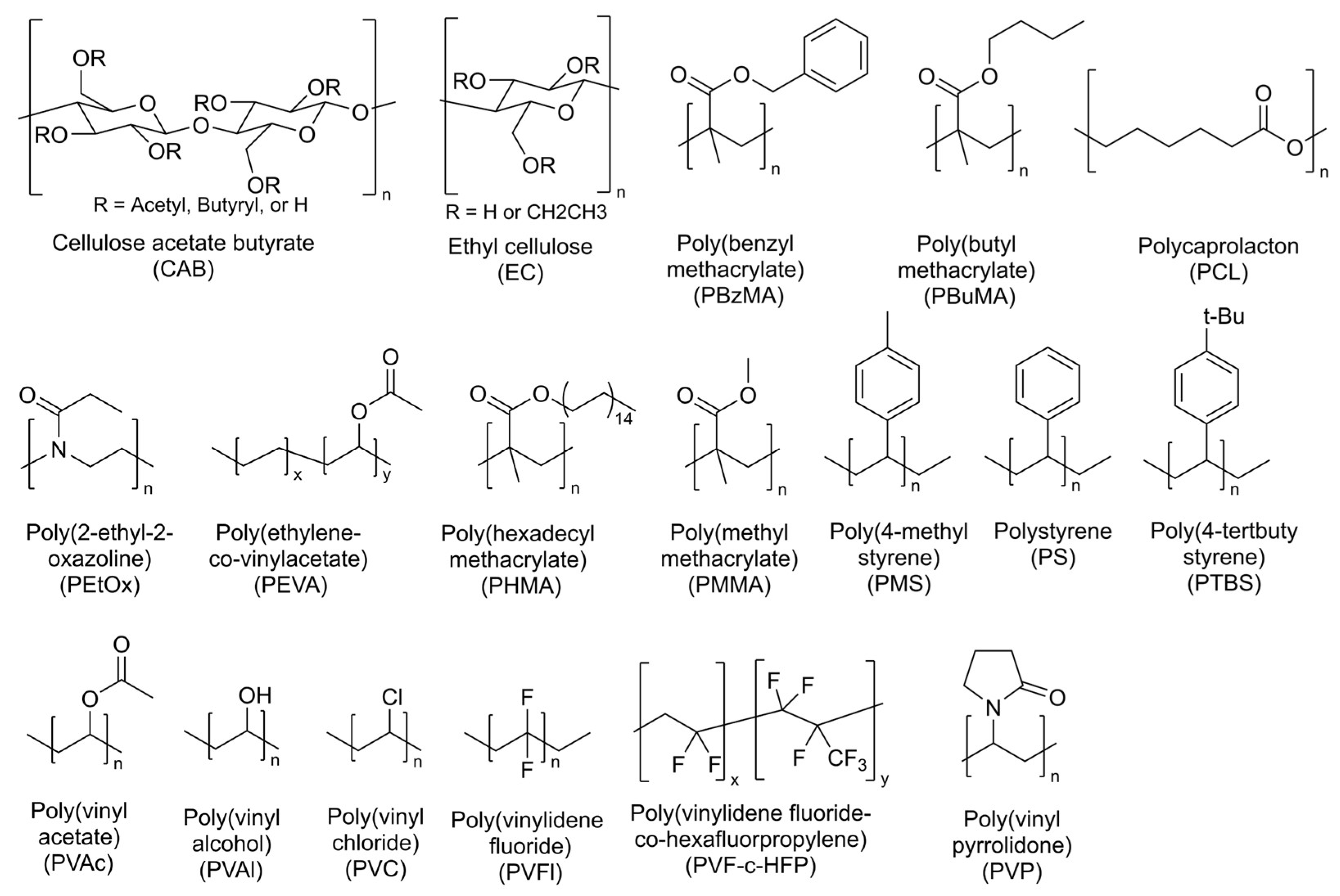
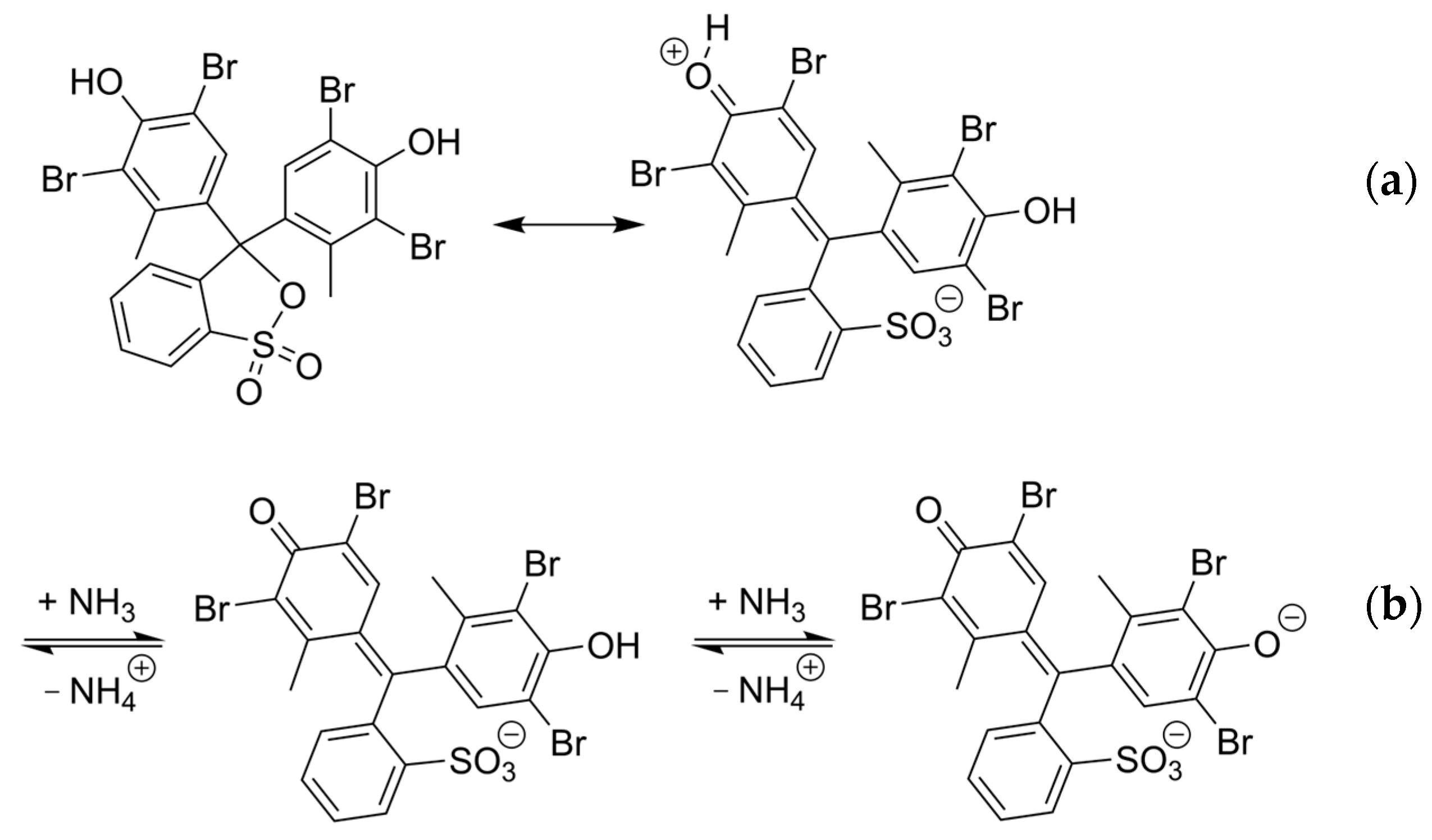

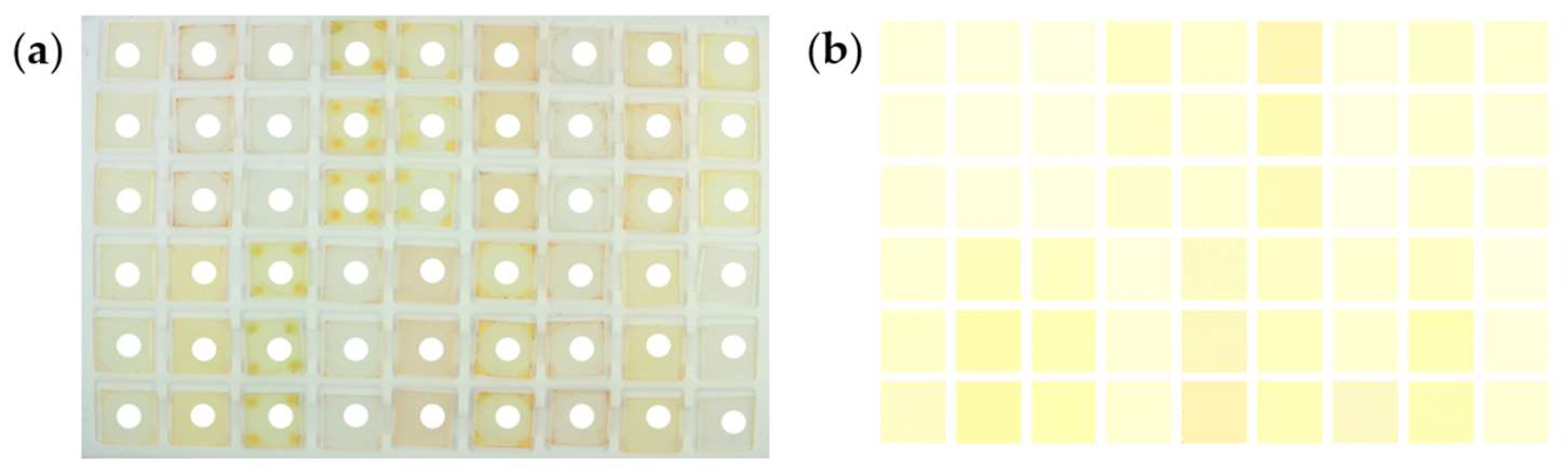
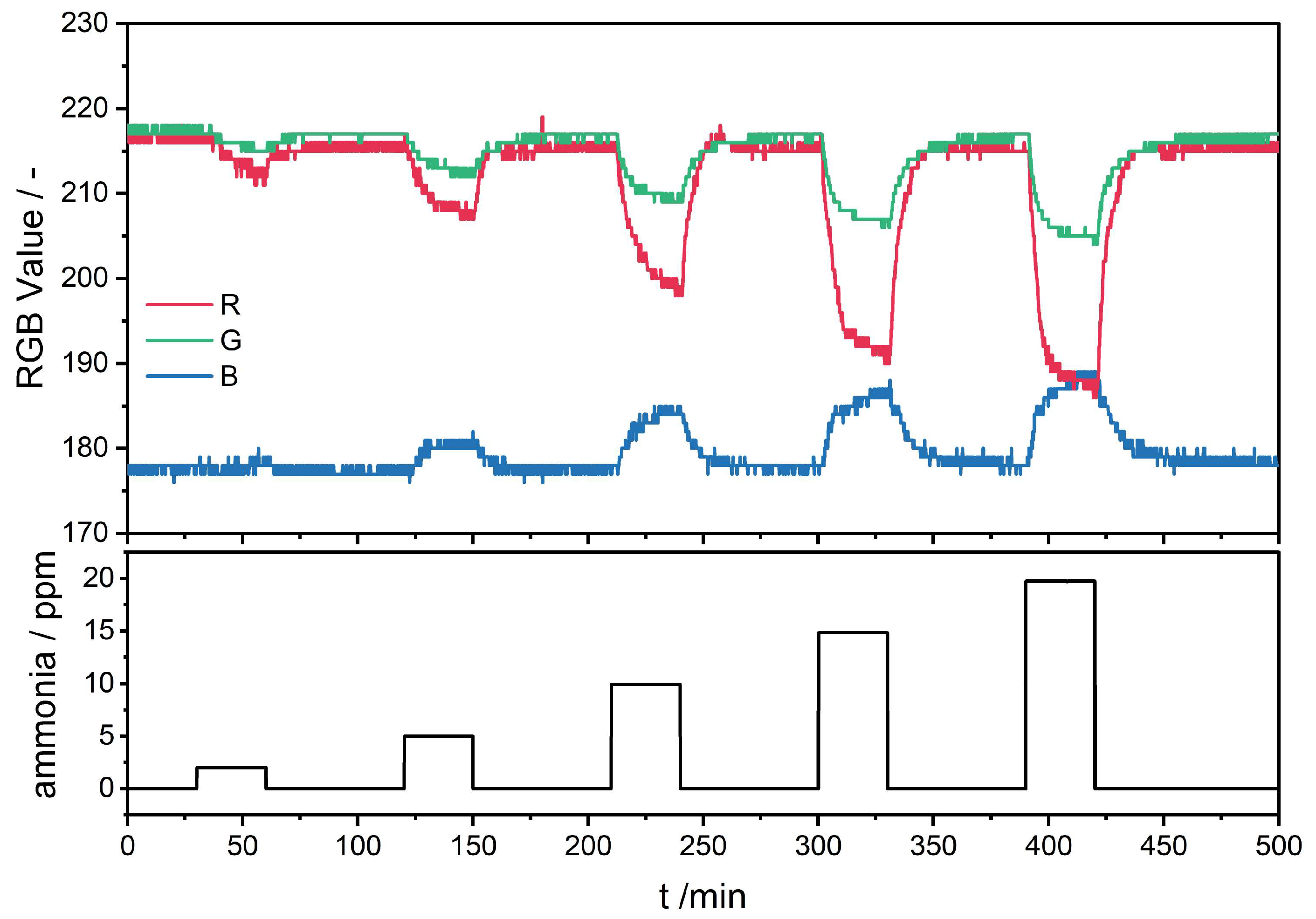


| Polymer | Supplier | Molar Mass | Solvent |
|---|---|---|---|
| Ethyl cellulose | Sigma-Aldrich | cp 10 | Toluene |
| Cellulose acetate butyrate | Sigma-Aldrich | 30,000 | DCM |
| Polystyrene | Carl Roth | 320,000 | Toluene |
| Polycaprolactone | Sigma-Aldrich | 80,000 | DCM |
| Poly (4-methylstyrene) | Sigma-Aldrich | 72,000 | Toluene |
| Poly (4-tert-buthylstyrene) | Sigma-Aldrich | 50,000–100,000 | Toluene |
| Poly methyl methacrylate | Sigma-Aldrich | 350,000 | Toluene |
| Poly (benzyl methacrylate) | Sigma-Aldrich | 100,000 | Toluene |
| Poly (butyl methacrylate) | Sigma-Aldrich | 337,000 | Toluene |
| Poly (hexadecyl methacrylate) | Sigma-Aldrich | 200,000 | Toluene |
| Polyvinyl acetate | Carl Roth | 55,000–70,000 | Toluene |
| Poly (ethylene-co-vinyl acetate) | Sigma-Aldrich | n.a. | DCM |
| Poly (vinylidene fluoride) | Thermo Fischer | n.a. | DMF |
| Poly(vinylidene fluoride-co-hexa-fluoropropylene) | Sigma-Aldrich | 400,000 | DMF |
| Polyvinyl alcohol | Sigma-Aldrich | 160,000 | Water |
| Polyvinyl chloride | Carl Roth | 21,000 | THF |
| Polyvinyl pyrrolidone | Carl Roth | 900,000–1,200,000 | Water |
| Poly (2-ethyl-2-oxazoline) | Thermo Fischer | 200,000 | Water |
| Sensor Matrix | λmax (Protonated Form)/nm | λmax (Deprotonated Form)/nm |
|---|---|---|
| PVAc | 415 | 617 |
| PMMA | 416 | 610 |
| CAB | 419 | 611 |
| EC | 419 | 628 |
| PS | 420 | 618 |
| PBzMA | 424 | 628 |
| PMS | 424 | 624 |
| PCL | 424 | 618 |
| PBuMA | 425 | 617 |
| PTBS | 425 | 618 |
| PVP | 425 | 629 |
| PVAl | 429 | 627 |
| PEtOx | 429 | 628 |
| PHMA | 452 | 636 |
| Sensor Matrix | Response Time/Min | Recovery Time/Min |
|---|---|---|
| PMMA | n.d. | n.d. |
| PS | n.d. | n.d. |
| EC | 2.5 | 11.5 |
| PMS | 2.7 | 14.1 |
| PBzMA | 2.8 | 13.6 |
| PVF-c-HFP | 3.8 | 19.4 |
| PVFl | 3.8 | 23.4 |
| PVAc | 4.2 | 10.6 |
| PTBS | 5.5 | 32.2 |
| PHMA | 5.7 | 24.1 |
| PBuMA | 6.0 | 25.1 |
| CAB | 6.8 | 23.4 |
| PEVA | 7.5 | 26.3 |
| PVC | 8.0 | 24.1 |
| PVP | 8.0 | 31.7 |
| PCL | 10.5 | 16.8 |
| PEtOx | 12.0 | 17.7 |
| PVAl | >30 | 62.3 |
| Polymer | Sensitivity | Response Time | Regeneration Time | Humidity | Temperature | Stability |
|---|---|---|---|---|---|---|
| EC | 0 | 100 | 96 | 92 | 98 | 98 |
| PMS | 2 | 100 | 84 | 97 | 95 | 94 |
| PMMA | 2 | n.d. | n.d. | 100 | 96 | 96 |
| PS | 2 | n.d. | n.d. | 89 | 100 | 95 |
| PBzMA | 5 | 97 | 86 | 91 | 79 | 100 |
| PVAc | 8 | 83 | 100 | 85 | 85 | 80 |
| PVF-c-HFP | 10 | 86 | 59 | 83 | 84 | 95 |
| PTBS | 13 | 69 | 52 | 86 | 94 | 89 |
| PBuMA | 16 | 63 | 63 | 81 | 84 | 93 |
| PVC | 18 | 42 | 36 | 81 | 78 | 99 |
| PHMA | 18 | 67 | 36 | 85 | 84 | 87 |
| PVFl | 19 | 86 | 39 | 87 | 81 | 87 |
| CAB | 33 | 54 | 39 | 74 | 89 | 79 |
| PEVA | 40 | 47 | 26 | 62 | 79 | 82 |
| PVP | 68 | 42 | 0 | 0 | 0 | 0 |
| PCL | 98 | 0 | 71 | 65 | 21 | 89 |
| PEtOx | 100 | 12 | 66 | 32 | 41 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, S.; Henfling, M.; Trupp, S. Investigation of Polymers as Matrix Materials for Application in Colorimetric Gas Sensors for the Detection of Ammonia. Sensors 2025, 25, 2829. https://doi.org/10.3390/s25092829
Hoffmann S, Henfling M, Trupp S. Investigation of Polymers as Matrix Materials for Application in Colorimetric Gas Sensors for the Detection of Ammonia. Sensors. 2025; 25(9):2829. https://doi.org/10.3390/s25092829
Chicago/Turabian StyleHoffmann, Sonja, Michael Henfling, and Sabine Trupp. 2025. "Investigation of Polymers as Matrix Materials for Application in Colorimetric Gas Sensors for the Detection of Ammonia" Sensors 25, no. 9: 2829. https://doi.org/10.3390/s25092829
APA StyleHoffmann, S., Henfling, M., & Trupp, S. (2025). Investigation of Polymers as Matrix Materials for Application in Colorimetric Gas Sensors for the Detection of Ammonia. Sensors, 25(9), 2829. https://doi.org/10.3390/s25092829






