A Wearable Internet of Things-Based Device for the Quantitative Assessment of Hand Tremors in Parkinson’s Disease: The ELENA Project
Abstract
1. Introduction
The ELENA Project: Overview of Functionality
2. Materials and Methods
2.1. Materials
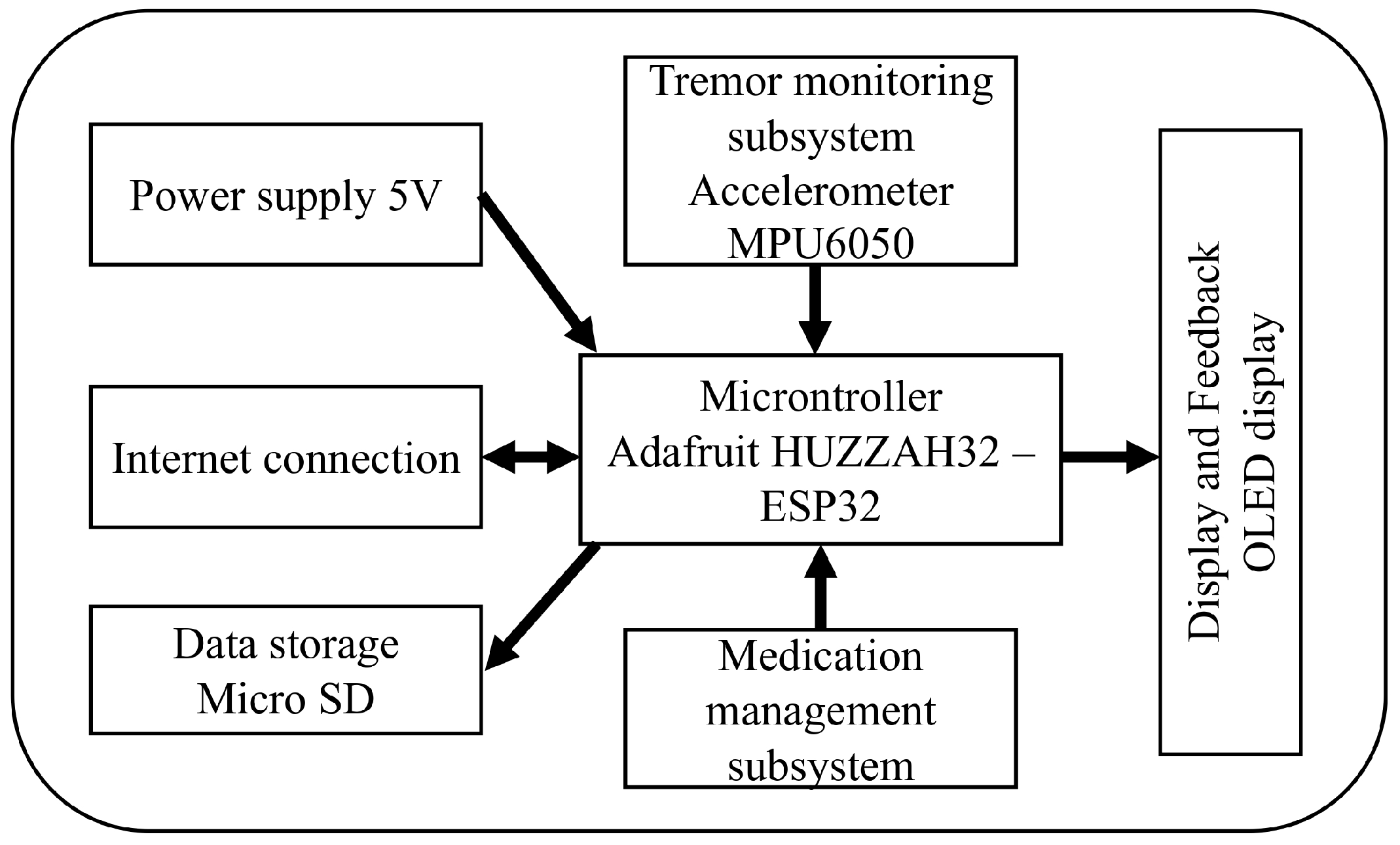
- Microcontroller: The system uses an Adafruit HUZZAH32—ESP32 Feather Board, which integrates Wi-Fi connectivity and provides sufficient processing power to handle data collection and transmission.
- Accelerometer: The MPU6050, a 6-degree-of-freedom (DoF) sensor that combines a 3-axis accelerometer and a 3-axis gyroscope, is used for precise tracking of upper limb movements.
- Data Storage: A micro SD card module is included for local storage of the tremor data collected, ensuring data retention even in case of connectivity issues.
- Display and Feedback: An OLED display (0.96” I2C 128 × 64 SSD1306) is used to provide real-time information to the patient. In addition, an auditory buzzer is integrated to issue alerts for medication reminders.
- Power Supply: The prototype is powered by a 3.7 V 2000 mAh lithium-ion battery, rechargeable via a USB connection for continuous operation.
- The following software and equipment were used in the design, programming, and calibration of the wearable system.
- Software for Programming the Wearable: The microcontroller was programmed using the Arduino IDE (Integrated Development Environment) 2.0.3, which supports C++ programming and allows easy integration of the accelerometer through libraries such as Adafruit’s MPU6050 and Wire.h for I2C communication. This setup facilitated data acquisition, storage, and communication to the cloud platform.
- Software for Constructing the Prototype: The physical design of the wearable device was created using CAD software (Tinkercad 2.0.1) for the initial prototyping and Fusion 360 for the final design. The prototype casing was 3D printed using PLA (polylactic acid) material, providing a lightweight and durable enclosure for the electronics.
- Calibration Equipment:
- –
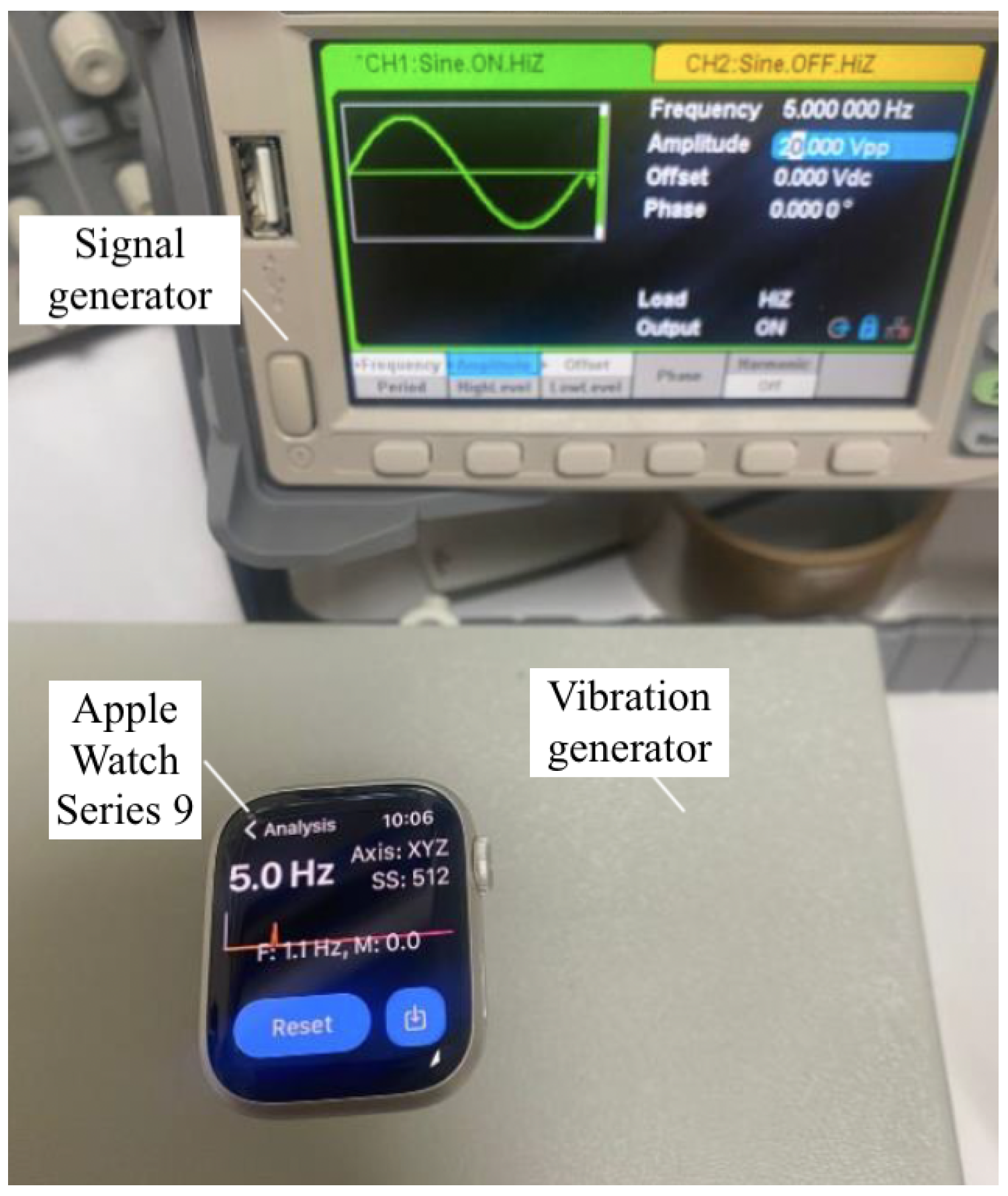
- Signal Generator and Vibration Generator: A combination of a signal generator (Siglent Technologies SDG2042X Arbitrary Waveform Function-Generator, 40 MHz) and a vibration generator was used to produce controlled sinusoidal signals across a range of frequencies. This setup allowed for the generation of known frequencies, which were essential for verifying the accuracy of the accelerometer’s measurements and for testing the system’s response to specific frequency inputs.
- –
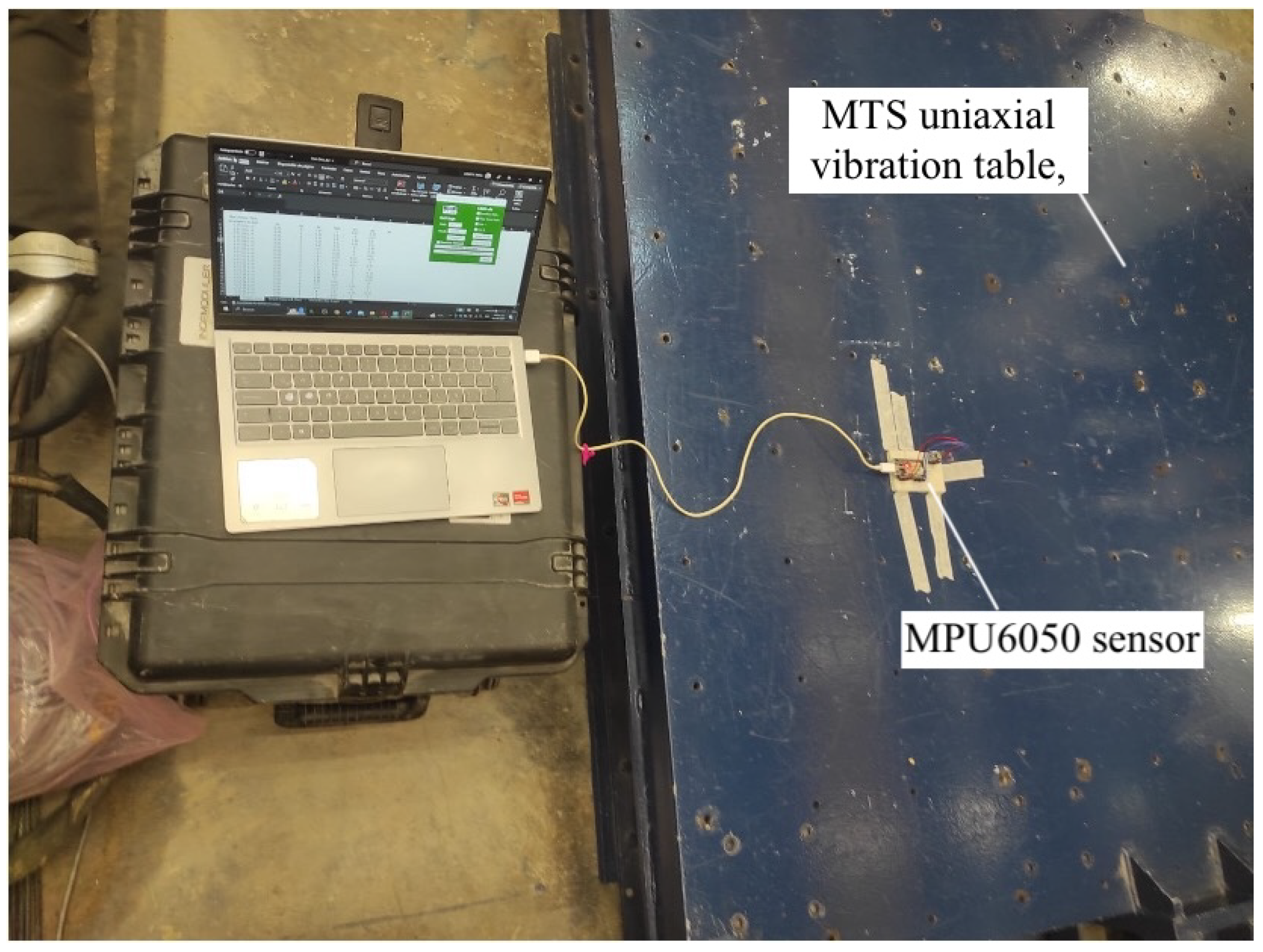
- Calibration Table: The calibration process used a MTS uniaxial vibration table (manufactured by Material Testing Systems, MTS), which has a platform size of 1.5 cm and moves in one direction (usually vertical); this is commonly used for seismic simulation. The table allowed for precise control over low-frequency and low-amplitude vibrations, which was necessary for testing the sensor’s response to controlled oscillations. This table was equipped with software to program specific frequencies and amplitudes, ensuring accurate calibration of the MPU6050 sensor.
- –
- Reference Watch: Apple Watch Series 9 (S9 41 SI AL SB SB SM GPS-CLA, 41 mm, Silver Aluminum case, Sport Band, GPS model) equipped with the Vibration Analysis application was used to validate and calibrate the prototype. The Apple Watch Series 9 contains built-in accelerometers capable of capturing motion data at sampling rates up to 100 Hz. The Vibration Analysis app leverages this capability to measure tremor frequency and amplitude accurately, providing a reliable benchmark for comparison with the developed wearable system.
2.2. Tremor Analysis Software
- Data Preprocessing: The raw accelerometer data were imported into MATLAB, where it was filtered using a Butterworth low-pass filter to remove high-frequency noise and improve the accuracy of tremor detection.
- Frequency analysis: Fast Fourier transform was applied to transform the time-domain data into the frequency domain, enabling the identification of the dominant tremor frequencies. The analysis focused on extracting tremor frequencies in the typical ranges of Parkinson’s disease (4–6 Hz for resting tremors).
- Data Visualization: MATLAB’s plotting functions were used to create visual representations of the tremor data, including time-series plots and power spectral density (PSD) graphs. These visualizations were uploaded to ThingSpeak for seamless data processing, real-time visualization, and improved tremor analysis, making it easier for healthcare providers to track and manage the progression of Parkinson’s disease symptoms in real time.
- These materials and tools enabled the development and precise calibration of the wearable system, ensuring its accuracy and reliability to monitor motor symptoms in patients with PD.
2.3. Participants
- PD Group: This group consisted of 9 patients diagnosed with Parkinson’s disease by certified neurologists. The inclusion criteria for the PD group were as follows:
- –
- A confirmed diagnosis of Parkinson’s disease according to the clinical diagnostic criteria of the Brain Bank of the UK Parkinson’s Disease Society.
- –
- Age between 60 and 80 years of age.
- –
- Ability to provide informed consent and complete the study protocol.
- –
- Stable medication regimen for at least 30 days prior to the study.
- –
- No history of other neurological or musculoskeletal disorders that could interfere with motor assessments.
- Control Group: The control group included 9 participants of the same age without neurological conditions. The inclusion criteria for the control group were as follows:
- –
- Age between 60 and 80 years of age, matched as closely as possible to the PD group to control for age-related variability.
- –
- No history of neurological or musculoskeletal disorders.
- –
- No regular use of medications that affect the central nervous system.
- –
- Ability to provide informed consent and complete the study protocol.
2.4. Methods
2.4.1. Assessment Tools
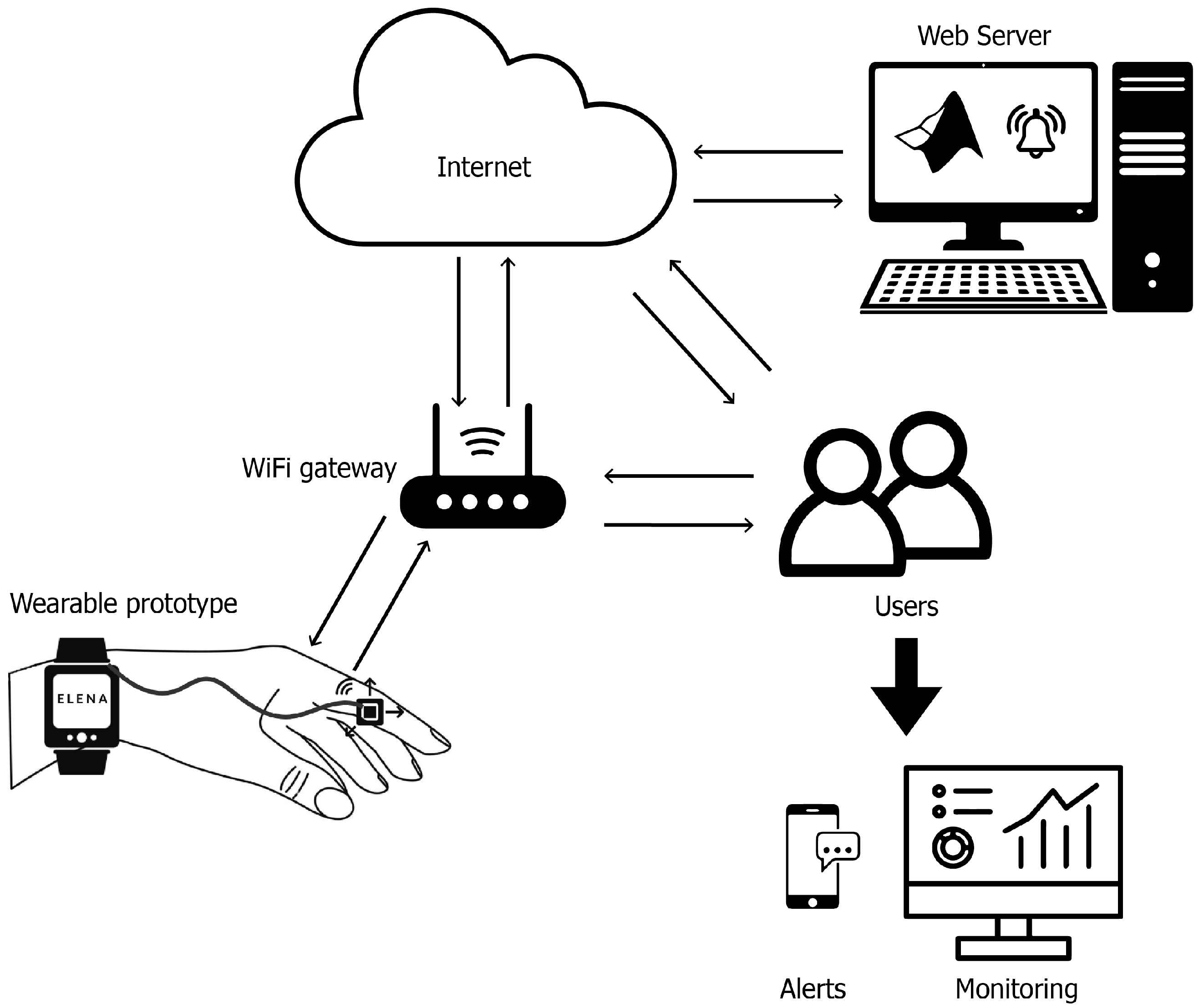
- Wrist-worn wearable prototype: This device consists of a microcontroller (Adafruit HUZZAH32—ESP32 Feather Board) housed inside a 3D-printed case and an accelerometer (MPU6050) positioned on the index finger to measure tremors in the upper extremities (see Figure 3). In addition, a glove was designed to securely hold the prototype, ensuring proper sensor placement and stability during use. The glove used to hold the wearable device was specifically designed to be lightweight, comfortable, and non-restrictive, ensuring minimal interference with natural tremor movements. Preliminary tests indicated no observable worsening or improvement of tremors due to the glove’s presence. This was confirmed through direct patient feedback and clinical observation during validation sessions.
- Data collection platform: The device was configured to store the collected data on a micro SD card and also transmit it to a cloud-based platform for visualization and analysis using MATLAB software. The monitoring system also provided real-time feedback to patients and their caregivers through an integrated OLED display and audible alerts.
2.4.2. Calibration Tests
- Sensor Calibration: This calibration was conducted using a six-position method on a calibration table, as seen in Figure 4.
- The MPU6050 sensor was mounted on a cube so it could be placed in six different static orientations. Each axis of the accelerometer was aligned parallel to the force of gravity in turn, verifying that it measured approximately 1 g. The table setup allowed the consistent and reliable positioning of the sensor to ensure accurate calibration. For further accuracy and testing, a uniaxial vibration table from MTS was used for more advanced calibration. The vibration table had a platform size of 1.5 cm and was designed for seismic simulation, which allowed for precise control over frequencies and amplitudes. This table provided a controlled environment to simulate low-frequency and low-amplitude movements that are similar to the conditions experienced during tremor symptoms. The frequencies and amplitudes were programmed using the MTS software provided with the table. This setup allowed us to test the sensor’s response to known vibrations, ensuring that it was calibrated correctly across all axes.
- 2.
- Signal Filtering: A Butterworth low-pass filter was applied in MATLAB to reduce noise from the accelerometer data, achieving a more accurate detection of tremor frequencies.
- 3.
- Accuracy Validation of the Prototype: To validate the accuracy of the prototype, a series of controlled laboratory tests were conducted comparing data collected from the MPU6050 sensor in the prototype to readings from an Apple Watch. The collected data, processed via MATLAB for frequency and amplitude analysis, were stored on the SD card and transmitted to the cloud. The validation process involved three main steps: preparation of equipment, data collection and analysis, and result comparison.
- Data Collection and Analysis: Both devices recorded vibration data across a range of frequencies. Data from the prototype were processed using MATLAB to analyze frequency and amplitude. Results were stored locally on an SD card and transmitted to the cloud for further processing.
- Comparison of Results: The analysis focused on the frequency values obtained from both devices. The key tests that were performed included the following:
- –
- Static Frequency Test: The frequencies were fixed and the results were compared for each frequency.
- –
- Dynamic Frequency Test: The vibration generator was set to vary the frequencies continuously.
- –
- Error Analysis: Repeated measurements were taken to calculate the percentage error for the prototype.
2.4.3. Clinical Tasks and Procedure
- Resting State Measurements: Each participant completed four resting tests for each hand:
- Sitting with the hand and forearm resting on the legs for 1 min.
- Sitting with the upper extremity extended without support for 1 min.
- Standing with the hand and forearm resting on the legs for 1 min.
- Standing with the upper extremity extended without support for 1 min.
- Active Movement Tests: Participants performed two active tasks with each hand:
- Writing their name for 1 min.
- Simulating a waving gesture for 1 min.
2.4.4. Tremor Frequency Calculation
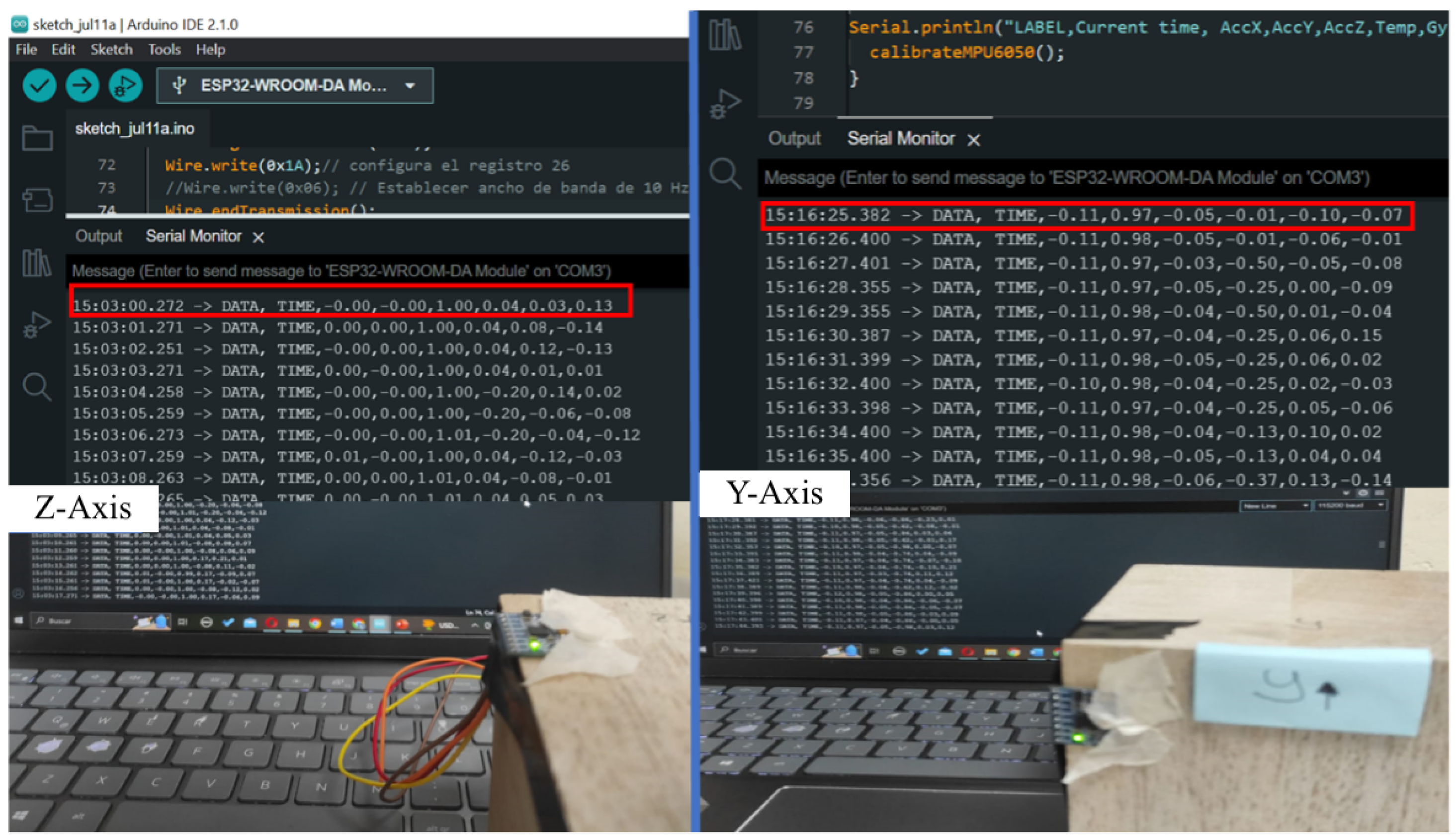
- Data Collection: Acceleration data along the X, Y, and Z axes were recorded at a sampling rate of 55.56 Hz for multiple test sessions per patient. The chosen sampling frequency was based on the typical frequency range of Parkinsonian tremors, which generally fall between 3 and 6 Hz, with some components potentially as low as 1 Hz and as high as 12 Hz. According to the Nyquist criterion, a minimum sampling rate of at least twice the maximum expected frequency is required to accurately capture the signal without aliasing. Our selected sampling rate of 55.56 Hz satisfies this condition and provides sufficient resolution for accurate tremor frequency analysis while maintaining efficient data storage and power consumption on the wearable system.
- Preprocessing: The recorded data were processed to correct missing values, remove trends, apply a filter to eliminate low-frequency noise, normalize the signals, and convert acceleration values to standard units. Low-frequency components often arise from postural shifts, baseline drift due to sensor movement, or voluntary gross motor activity such as hand repositioning. For example, during tasks involving writing or arm extension, we observed slow baseline shifts and large-amplitude voluntary motions that produced dominant components below 1 Hz. These components could obscure the tremor-related frequency content and were effectively reduced using a high-pass Butterworth filter (cutoff frequency = 1 Hz), improving the accuracy of tremor frequency estimation.
- Frequency Analysis: A Fast Fourier Transform was applied to the processed acceleration data to extract the dominant frequency components. The analysis considered the energy distribution across frequency bands to identify the most prominent frequency range of tremors. Since the MPU6050 sensor captures acceleration along three orthogonal axes (x, y, z), the power spectral density (PSD) was computed independently for each axis. The axis exhibiting the highest spectral peak was used to identify the dominant tremor frequency. Additionally, the total energy of the tremor signal was calculated by summing the squared acceleration values across all three axes within each window. This approach provides a more comprehensive measure of tremor intensity, accounting for multidirectional movement components and enhancing robustness against variability in axis orientation.
- Results Storage: The extracted frequency components and amplitude values were stored locally and on the cloud for further statistical analysis.
3. Results
3.1. Results of the Calibration Tests
3.2. Results of the Accuracy Validations Tests
3.3. Results of the Clinical Tasks and Procedure
4. Discussion
5. Future Work
5.1. Limitations
5.2. Emerging Technologies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Parkinson’s Foundation. Tremor. Available online: https://www.parkinson.org/understanding-parkinsons/movement-symptoms/tremor (accessed on 14 April 2025).
- Ibrahim, A.; Zhou, Y.; Jenkins, M.E.; Trejos, A.L.; Naish, M.D. Real-time voluntary motion prediction and Parkinson’s tremor reduction using deep neural networks. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Daniel, S.E.; Blankson, S.; Lees, A.J. A clinicopathologic study of 100 cases of Parkinson’s disease. Arch. Neurol. 1993, 50, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.E.; Loewenson, R.B.; Resch, J.A.; Baker, A.B. Parkinson’s disease: Clinical analysis of 100 patients. Neurology 1973, 23, 783. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.L.; Dinesh, K.; Snyder, C.W.; Xiong, M.; Tarolli, C.G.; Sharma, S.; Dorsey, E.R.; Sharma, G. A real-world study of wearable sensors in Parkinson’s disease. npj Park. Dis. 2021, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A.; Smith, W.W. Gene–environment interactions in Parkinson’s disease. Park. Relat. Disord. 2007, 13, S309–S315. [Google Scholar] [CrossRef]
- Elble, R.; Koller, W. Parkinson tremor. In Tremor; John Hopkins University Press: Baltimore, MA, USA, 1990; pp. 118–119. [Google Scholar]
- Marsden, C. Origins of normal and pathological tremor. In Movement Disorders: Tremor; Springer: Berlin/Heidelberg, Germany, 1984; pp. 37–84. [Google Scholar]
- Findley, L.; Gresty, M.; Halmagyi, G. Tremor, the cogwheel phenomenon and clonus in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1981, 44, 534–546. [Google Scholar] [CrossRef]
- Goetz, C.G.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stebbins, G.T.; Stern, M.B.; Tilley, B.C.; Dodel, R.; Dubois, B.; et al. Movement disorder society-sponsored revision of the unified parkinson’s disease rating scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov. Disord. 2007, 22, 41–47. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement disorder society-sponsored revision of the Unified parkinson’s disease rating scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Connolly, B.S.; Lang, A.E. Pharmacological treatment of Parkinson disease: A review. JAMA J. Am. Med. Assoc. 2014, 311, 1670–1683. [Google Scholar] [CrossRef]
- Nutt, J.G.; Wooten, G.F. Diagnosis and initial management of Parkinson’s disease. N. Engl. J. Med. 2005, 353, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.; Yiannikas, C.; Morris, J.; Einstein, R.; Jackson, D.; Byth, K. Postural tremor of Parkinson’s disease. Clin. Neuropharmacol. 1994, 17, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, H.H. Updates in the medical management of Parkinson disease. Clevel. Clin. J. Med. 2012, 79, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Lozano, A.; Kim, Y.; Hutchison, W.; Sime, E.; Halket, E.; Lang, A. Double-blind evaluation of subthalamic nucleus deep brain stimulation in advanced Parkinson’s disease. Neurology 1998, 51, 850–855. [Google Scholar] [CrossRef]
- Hariz, M.; Blomstedt, P. Deep brain stimulation for Parkinson’s disease. J. Intern. Med. 2022, 292, 764–778. [Google Scholar] [CrossRef]
- Pasluosta, C.F.; Gassner, H.; Winkler, J.; Klucken, J.; Eskofier, B.M. An emerging era in the management of Parkinson’s disease: Wearable technologies and the internet of things. IEEE J. Biomed. Health Inform. 2015, 19, 1873–1881. [Google Scholar] [CrossRef]
- Delrobaei, M.; Memar, S.; Pieterman, M.; Stratton, T.W.; McIsaac, K.; Jog, M. Towards remote monitoring of Parkinson’s disease tremor using wearable motion capture systems. J. Neurol. Sci. 2018, 384, 38–45. [Google Scholar] [CrossRef]
- Tzallas, A.T.; Tsipouras, M.G.; Rigas, G.; Tsalikakis, D.G.; Karvounis, E.C.; Chondrogiorgi, M.; Psomadellis, F.; Cancela, J.; Pastorino, M.; Waldmeyer, M.T.A.; et al. PERFORM: A system for monitoring, assessment and management of patients with Parkinson’s disease. Sensors 2014, 14, 21329–21357. [Google Scholar] [CrossRef]
- Rigas, G.; Tzallas, A.T.; Tsipouras, M.G.; Bougia, P.; Tripoliti, E.E.; Baga, D.; Fotiadis, D.I.; Tsouli, S.G.; Konitsiotis, S. Assessment of tremor activity in the Parkinson’s disease using a set of wearable sensors. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 478–487. [Google Scholar] [CrossRef]
- Bazgir, O.; Habibi, S.A.H.; Palma, L.; Pierleoni, P.; Nafees, S. A classification system for assessment and home monitoring of tremor in patients with Parkinson’s disease. J. Med. Signals Sens. 2018, 8, 65–72. [Google Scholar] [CrossRef]
- Espay, A.J.; Hausdorff, J.M.; Sánchez-Ferro, Á.; Klucken, J.; Merola, A.; Bonato, P.; Paul, S.S.; Horak, F.B.; Vizcarra, J.A.; Mestre, T.A.; et al. A roadmap for implementation of patient-centered digital outcome measures in Parkinson’s disease obtained using mobile health technologies. Mov. Disord. 2019, 34, 657–663. [Google Scholar] [CrossRef]
- Lipsmeier, F.; Taylor, K.I.; Kilchenmann, T.; Wolf, D.; Scotland, A.; Schjodt-Eriksen, J.; Cheng, W.Y.; Fernandez-Garcia, I.; Siebourg-Polster, J.; Jin, L.; et al. Evaluation of smartphone-based testing to generate exploratory outcome measures in a phase 1 Parkinson’s disease clinical trial. Mov. Disord. 2018, 33, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Peña, L.; Collado, E.; Sáez, Y. Explorando los avances y desafios en la monitorización continua de los temblores para la enfermedad de Parkinson: Una revisión sistemática. I+D Tecnológico 2024, 20, 115–128. [Google Scholar] [CrossRef]
- Mughal, H.; Javed, A.R.; Rizwan, M.; Almadhor, A.S.; Kryvinska, N. Parkinson’s disease management via wearable sensors: A systematic review. IEEE Access Pract. Innov. Open Solut. 2022, 10, 35219–35237. [Google Scholar] [CrossRef]
- Adams, J.L.; Dinesh, K.; Xiong, M.; Tarolli, C.G.; Sharma, S.; Sheth, N.; Aranyosi, A.; Zhu, W.; Goldenthal, S.; Biglan, K.M.; et al. Multiple wearable sensors in Parkinson and Huntington disease individuals: A pilot study in clinic and at home. Digit. Biomark. 2017, 1, 52–63. [Google Scholar] [CrossRef]
- Pierleoni, P.; Palma, L.; Belli, A.; Pernini, L. A real-time system to aid clinical classification and quantification of tremor in Parkinson’s disease. In Proceedings of the IEEE-EMBS International Conference on Biomedical and Health Informatics (BHI), Valencia, Spain, 1–4 June 2014; pp. 113–116. [Google Scholar]
- Woods, A.M.; Nowostawski, M.; Franz, E.A.; Purvis, M. Parkinson’s disease and essential tremor classification on mobile device. Pervasive Mob. Comput. 2014, 13, 1–12. [Google Scholar] [CrossRef]
- LeMoyne, R.; Mastroianni, T.; Cozza, M.; Coroian, C.; Grundfest, W. Implementation of an iPhone for characterizing Parkinson’s disease tremor through a wireless accelerometer application. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 4954–4958. [Google Scholar]
- Keijsers, N.; Horstink, M.; Van Hilten, J.; Hoff, J.; Gielen, C. Detection and assessment of the severity of Levodopa-induced dyskinesia in patients with Parkinson’s disease by neural networks. Mov. Disord. Off. J. Mov. Disord. Soc. 2000, 15, 1104–1111. [Google Scholar] [CrossRef]
- Schrag, A.; Schelosky, L.; Scholz, U.; Poewe, W. Reduction of parkinsonian signs in patients with Parkinson’s disease by dopaminergic versus anticholinergic single-dose challenges. Mov. Disord. Off. J. Mov. Disord. Soc. 1999, 14, 252–255. [Google Scholar] [CrossRef]
- de Araújo, A.C.A.; Santos, E.G.D.R.; De Sà, K.S.G.; Furtado, V.K.T.; Santos, F.A.; De Lima, R.C.; Krejcova, L.V.; Santos-Lobato, B.L.; Pinto, G.H.L.; Cabral, A.d.S.; et al. Hand resting tremor assessment of healthy and patients with Parkinson’s disease: An exploratory machine learning study. Front. Bioeng. Biotechnol. 2020, 8, 778. [Google Scholar] [CrossRef]
- Xing, X.; Luo, N.; Li, S.; Zhou, L.; Song, C.; Liu, J. Identification and classification of Parkinsonian and essential tremors for diagnosis using machine learning algorithms. Front. Neurosci. 2022, 16, 701632. [Google Scholar] [CrossRef]
- Batista, L.P.; Ureña, C.; Collado, E.; Sáez, Y. Initial validation of ELENA: A low-cost device for tremor monitoring in Parkinson’s patients. In Proceedings of the LACCEI, Hybrid, Costa Rica, 17–19 July 2024. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, P.; Lueth, T.C. Quantitative assessment of parkinsonian tremor based on an inertial measurement unit. Sensors 2015, 15, 25055–25071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Z.; Zeng, M.; Li, X.; Wang, Z.; Pan, G. Tremor-based discrimination of Parkinson’s disease and essential tremor using a wearable sensor: A comparative study. Sci. Rep. 2022, 12, 4318. [Google Scholar] [CrossRef]


| Patient | Age | Gender | Years Diagnosed | Medication | Dominant Hand |
|---|---|---|---|---|---|
| 1 | 65 | Male | 5 | Levodopa carbidopa ½ tab, Pramipexol 1 mg ½ tab | Right |
| 2 | 75 | Male | 8 | Levodopa carbidopa ½ tab, Pramipexol 1 mg ½ tab | Right |
| 3 | 60 | Male | 4 | None | Right |
| 4 | 73 | Female | 7 | Levodopa carbidopa | Right |
| 5 | 68 | Female | 10 | Levodopa carbidopa ¼ tab | Right |
| 6 | 68 | Female | 9 | Levodopa, Pramipexol | Right |
| 7 | 77 | Female | 12 | Levodopa carbidopa | Right |
| 8 | 62 | Male | 2 | Pramipexol 0.25 mg | Right |
| 9 | 66 | Male | 6 | Pramipexol 0.25 mg | Right |
| Control | Age | Gender | Dominant Hand | Medication |
|---|---|---|---|---|
| 1 | 64 | Male | Right | None |
| 2 | 70 | Female | Right | None |
| 3 | 62 | Male | Right | None |
| 4 | 65 | Female | Right | None |
| 5 | 67 | Male | Right | None |
| 6 | 68 | Female | Right | None |
| 7 | 72 | Male | Right | None |
| 8 | 63 | Female | Right | None |
| 9 | 69 | Male | Right | None |
| Condition | Metric | Prototype ELENA | Apple Watch | Deviation (%) |
|---|---|---|---|---|
| Static Testing | Frequency Range (Hz) | 1–10 | 3–10 (Detectable) | <0.5 |
| Dynamic Testing | Frequency Range (Hz) | 1–12 | 3–12 (Detectable) | <0.5 |
| Error Analysis | Average Frequency Error (Hz) | 0.02 | Baseline | - |
| Error Analysis | Percentage Error (%) | −0.37 | Baseline | - |
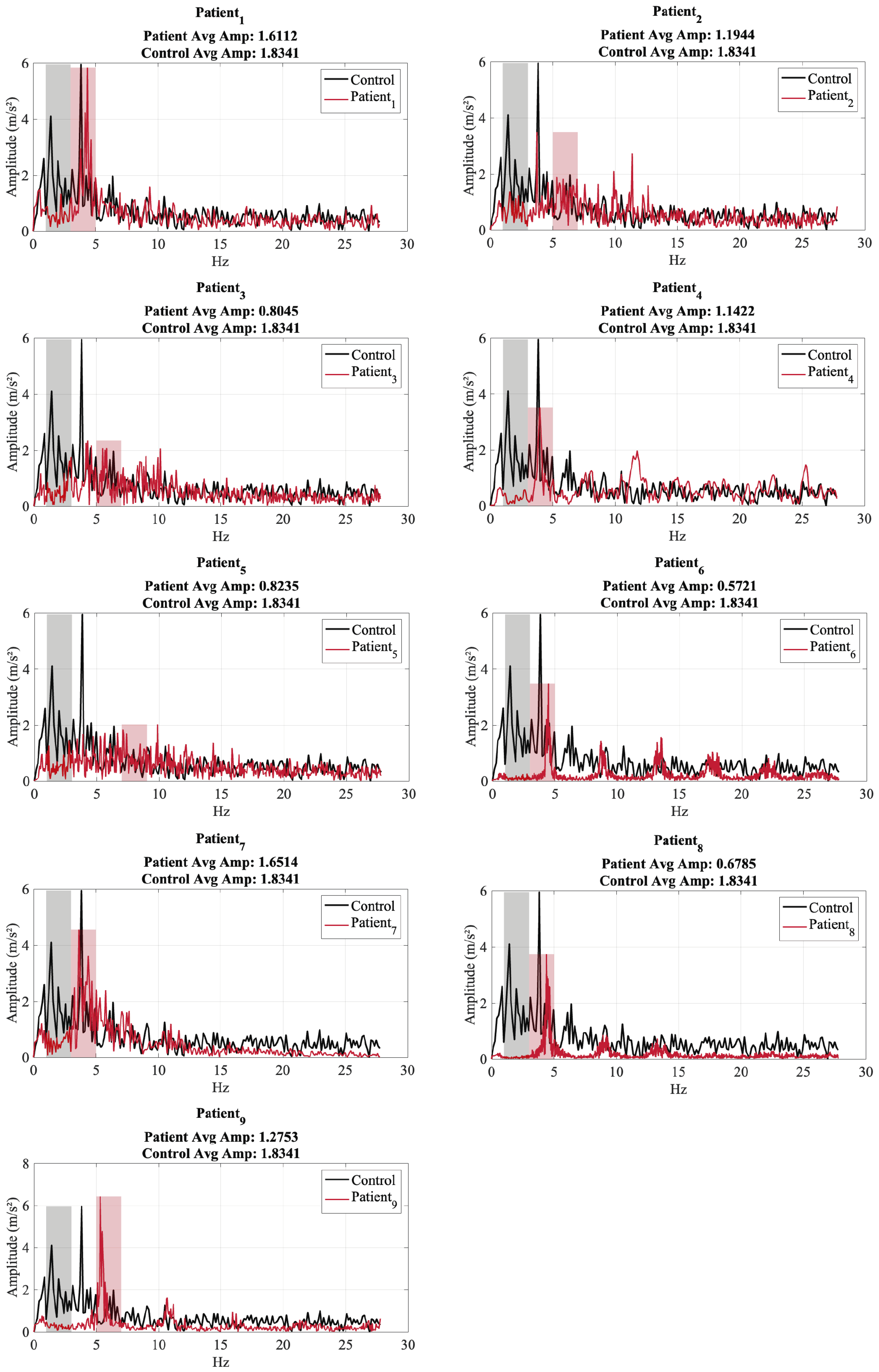
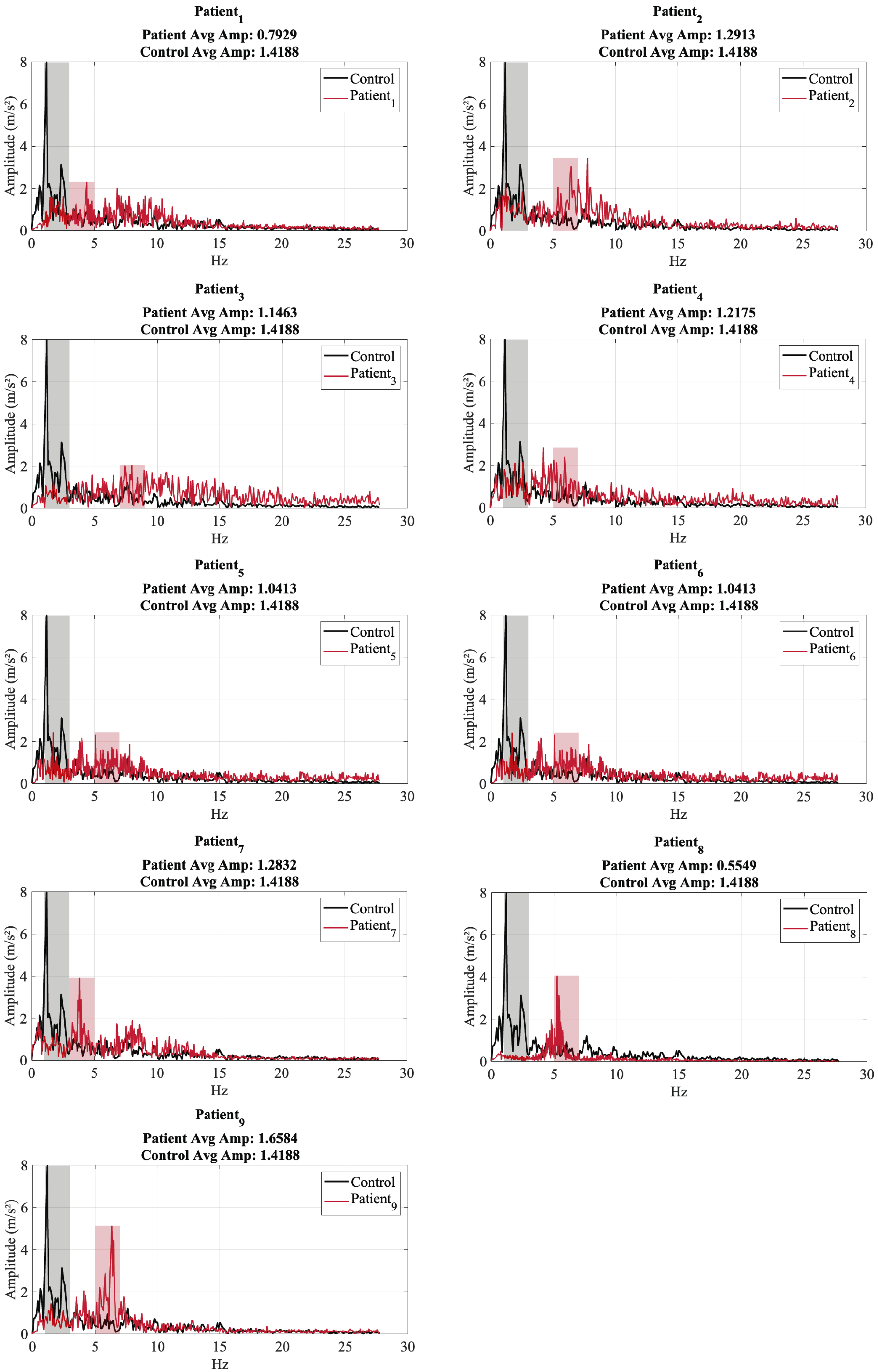
| Patient ID | Test ID | Age (Years) | Years Diagnosed | Dominant Freq. X (Hz) | Dominant Freq. Y (Hz) | Dominant Freq. Z (Hz) | Energy Concentration Range (Hz) | Avg. Amplitude Dominant Range (m/s2) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 65 | 5 | 4.3519 | 4.3519 | 4.3519 | 3.0–5.0 | 1.6112 |
| 1 | 2 | 65 | 5 | 4.4049 | 4.3599 | 4.4049 | 3.0–5.0 | 0.7929 |
| 1 | 3 | 65 | 5 | 5.8124 | 6.0827 | 7.2317 | 5.0–7.0 | 1.3023 |
| 1 | 4 | 65 | 5 | 1.8769 | 7.2573 | 1.9394 | 1.0–3.0 | 1.0741 |
| 1 | 5 | 65 | 5 | 0.8148 | 0.5926 | 0.8148 | 1.0–3.0 | 1.2816 |
| 1 | 6 | 65 | 5 | 0.8418 | 0.9620 | 0.8418 | 1.0–3.0 | 1.9874 |
| 2 | 1 | 75 | 8 | 3.7177 | 11.2935 | 3.7177 | 5.0–7.0 | 1.1944 |
| 2 | 2 | 75 | 8 | 7.7614 | 6.1275 | 3.7582 | 5.0–7.0 | 1.2913 |
| 2 | 3 | 75 | 8 | 6.5899 | 6.1728 | 8.2583 | 5.0–7.0 | 1.7209 |
| 2 | 4 | 75 | 8 | 5.6081 | 5.6081 | 5.8710 | 5.0–7.0 | 1.7848 |
| 2 | 5 | 75 | 8 | 0.6536 | 0.6536 | 2.3693 | 1.0–3.0 | 1.4575 |
| 2 | 6 | 75 | 8 | NaN | NaN | NaN | NaN | NaN |
| 3 | 1 | 60 | 4 | 4.3062 | 2.8506 | 9.9466 | 5.0–7.0 | 0.8045 |
| 3 | 2 | 60 | 4 | 7.9697 | 7.5828 | 8.8982 | 7.0–9.0 | 1.1463 |
| 3 | 3 | 60 | 4 | 7.0535 | 4.2176 | 7.0535 | 7.0–9.0 | 0.9343 |
| 3 | 4 | 60 | 4 | NaN | NaN | NaN | NaN | NaN |
| 3 | 5 | 60 | 4 | NaN | NaN | NaN | NaN | NaN |
| 3 | 6 | 60 | 4 | 0.6270 | 3.5114 | 7.3990 | 3.0–5.0 | 0.9048 |
| 4 | 1 | 73 | 7 | 3.9683 | 3.9683 | 4.0431 | 3.0–5.0 | 1.1422 |
| 4 | 2 | 73 | 7 | 4.2301 | 5.4992 | 1.9741 | 5.0–7.0 | 1.2175 |
| 4 | 3 | 73 | 7 | 4.1249 | 8.1976 | 4.1249 | 3.0–5.0 | 1.0849 |
| 4 | 4 | 73 | 7 | 5.6096 | 5.6096 | 4.1227 | 5.0–7.0 | 1.0899 |
| 4 | 5 | 73 | 7 | NaN | NaN | NaN | NaN | NaN |
| 4 | 6 | 73 | 7 | 0.7944 | 0.6355 | 1.4829 | 1.0–3.0 | 0.6899 |
| 5 | 1 | 68 | 10 | 9.8692 | 3.8257 | 5.5999 | 7.0 - 9.0 | 0.8235 |
| 5 | 2 | 68 | 10 | 1.6920 | 5.6402 | 6.3922 | 5.0 - 7.0 | 1.0413 |
| 5 | 3 | 68 | 10 | 5.6004 | 8.4379 | 4.6296 | 5.0 - 7.0 | 1.2352 |
| 5 | 4 | 68 | 10 | 2.9380 | 4.3403 | 1.9364 | 1.0 - 3.0 | 1.1354 |
| 5 | 5 | 68 | 10 | 1.9960 | 5.4336 | 2.0515 | 1.0 - 3.0 | 1.0606 |
| 5 | 6 | 68 | 10 | 0.6578 | 0.6072 | 9.4111 | 1.0 - 3.0 | 0.7640 |
| 6 | 1 | 68 | 9 | 4.4767 | 4.4579 | 4.4579 | 3.0–5.0 | 0.5721 |
| 6 | 2 | 68 | 9 | 1.6920 | 5.6402 | 6.3922 | 5.0–7.0 | 1.0413 |
| 6 | 3 | 68 | 9 | 5.7319 | 5.7319 | 5.7319 | 5.0–7.0 | 1.1137 |
| 6 | 4 | 68 | 9 | NaN | NaN | NaN | NaN | NaN |
| 6 | 5 | 68 | 9 | NaN | NaN | NaN | NaN | NaN |
| 6 | 6 | 68 | 9 | 0.7663 | 5.3640 | 0.6705 | 5.0–7.0 | 0.9140 |
| 7 | 1 | 77 | 12 | 3.6353 | 5.3132 | 7.2707 | 3.0 - 5.0 | 1.6510 |
| 7 | 2 | 77 | 12 | 3.8354 | 4.2422 | 3.7192 | 3.0 - 5.0 | 1.2830 |
| 7 | 3 | 77 | 12 | 5.1743 | 5.1743 | 4.4374 | 3.0 - 5.0 | 0.5210 |
| 7 | 4 | 77 | 12 | 4.8864 | 5.2132 | 4.9642 | 5.0 - 7.0 | 0.5980 |
| 7 | 5 | 77 | 12 | NaN | NaN | NaN | NaN | NaN |
| 7 | 6 | 77 | 12 | 4.2735 | 2.1368 | 4.2735 | 3.0 - 5.0 | 8.9833 |
| 8 | 1 | 62 | 2 | 4.3851 | 4.4657 | 4.4174 | 3.0–5.0 | 0.6785 |
| 8 | 2 | 62 | 2 | 5.2426 | 4.6135 | 5.2426 | 5.0–7.0 | 0.5549 |
| 8 | 3 | 62 | 2 | 5.0403 | 5.0563 | 5.0403 | 3.0–5.0 | 0.4965 |
| 8 | 4 | 62 | 2 | 5.2104 | 5.2104 | 5.2104 | 5.0–7.0 | 0.6632 |
| 8 | 5 | 62 | 2 | NaN | NaN | NaN | NaN | NaN |
| 8 | 6 | 62 | 2 | NaN | NaN | NaN | NaN | NaN |
| 9 | 1 | 66 | 6 | 5.3168 | 5.4796 | 5.5339 | 5.0–7.0 | 1.2753 |
| 9 | 2 | 66 | 6 | 6.3301 | 5.4364 | 5.4364 | 5.0–7.0 | 1.6584 |
| 9 | 3 | 66 | 6 | 5.6926 | 5.6926 | 5.7453 | 5.0–7.0 | 0.7262 |
| 9 | 4 | 66 | 6 | 6.0171 | 6.0171 | 6.0171 | 5.0–7.0 | 1.1919 |
| 9 | 5 | 66 | 6 | 2.6953 | 4.0704 | 5.7206 | 5.0–7.0 | 0.9993 |
| 9 | 6 | 66 | 6 | 1.1111 | 1.1111 | 1.0101 | 1.0–3.0 | 1.9980 |
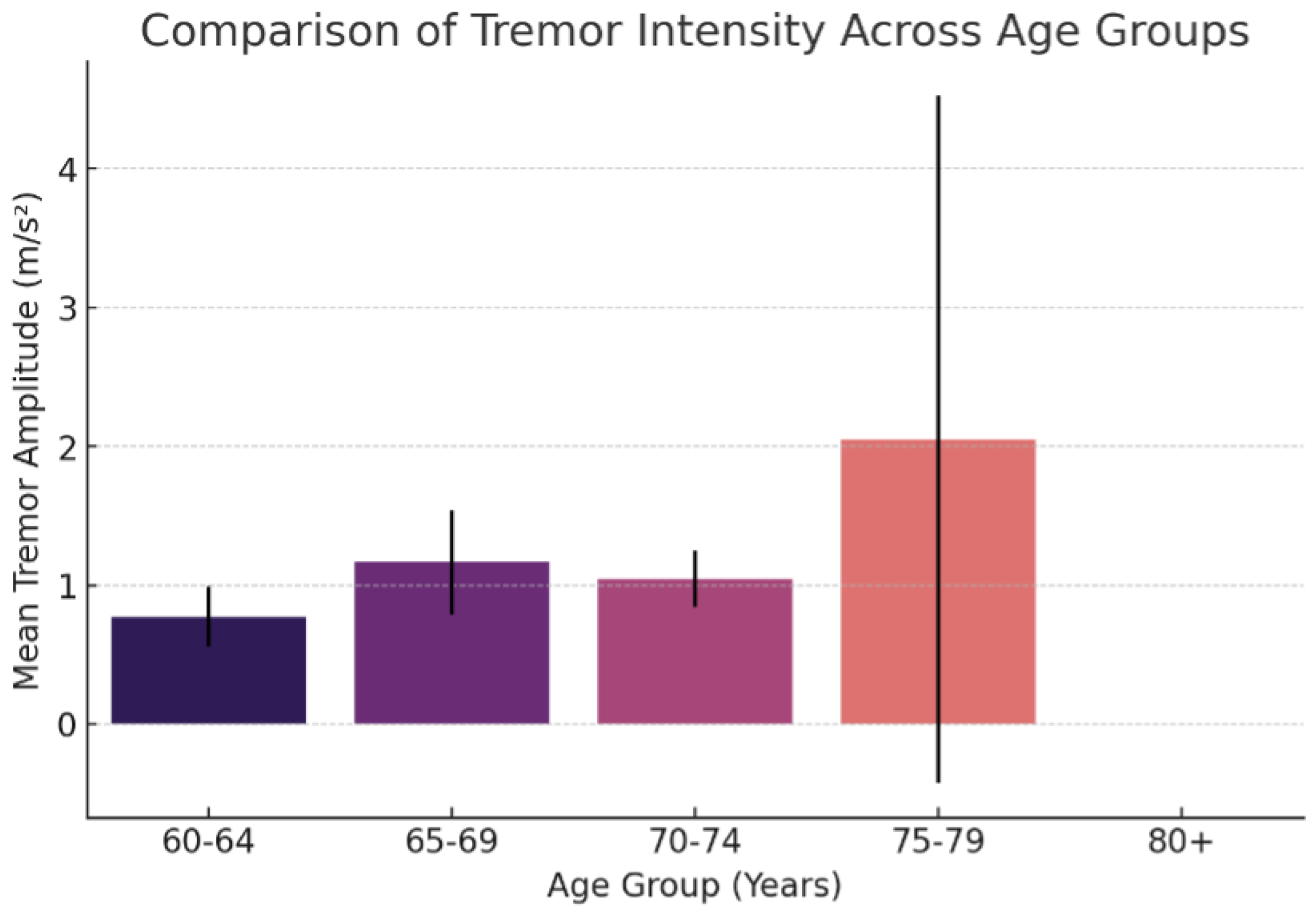
| Age Group | Mean Tremor Amplitude (m/s2) | Standard Deviation |
|---|---|---|
| 60–64 | 0.7729 | 0.2167 |
| 65–69 | 1.1636 | 0.3759 |
| 70–74 | 1.0449 | 0.2055 |
| 75–79 | 2.0487 | 2.4743 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saez, Y.; Ureña, C.; Valenzuela, J.; García, A.; Collado, E. A Wearable Internet of Things-Based Device for the Quantitative Assessment of Hand Tremors in Parkinson’s Disease: The ELENA Project. Sensors 2025, 25, 2763. https://doi.org/10.3390/s25092763
Saez Y, Ureña C, Valenzuela J, García A, Collado E. A Wearable Internet of Things-Based Device for the Quantitative Assessment of Hand Tremors in Parkinson’s Disease: The ELENA Project. Sensors. 2025; 25(9):2763. https://doi.org/10.3390/s25092763
Chicago/Turabian StyleSaez, Yessica, Cristian Ureña, Julia Valenzuela, Antony García, and Edwin Collado. 2025. "A Wearable Internet of Things-Based Device for the Quantitative Assessment of Hand Tremors in Parkinson’s Disease: The ELENA Project" Sensors 25, no. 9: 2763. https://doi.org/10.3390/s25092763
APA StyleSaez, Y., Ureña, C., Valenzuela, J., García, A., & Collado, E. (2025). A Wearable Internet of Things-Based Device for the Quantitative Assessment of Hand Tremors in Parkinson’s Disease: The ELENA Project. Sensors, 25(9), 2763. https://doi.org/10.3390/s25092763






