Early Detection and Monitoring of Nephrolithiasis: The Potential of Electrochemical Sensors
Abstract
1. Introduction
2. Mechanisms of Kidney Stone Formation and Associated Biomarkers
2.1. Stone Compositional Analysis
2.2. Roles of Biomarkers in Kidney Stone Formation
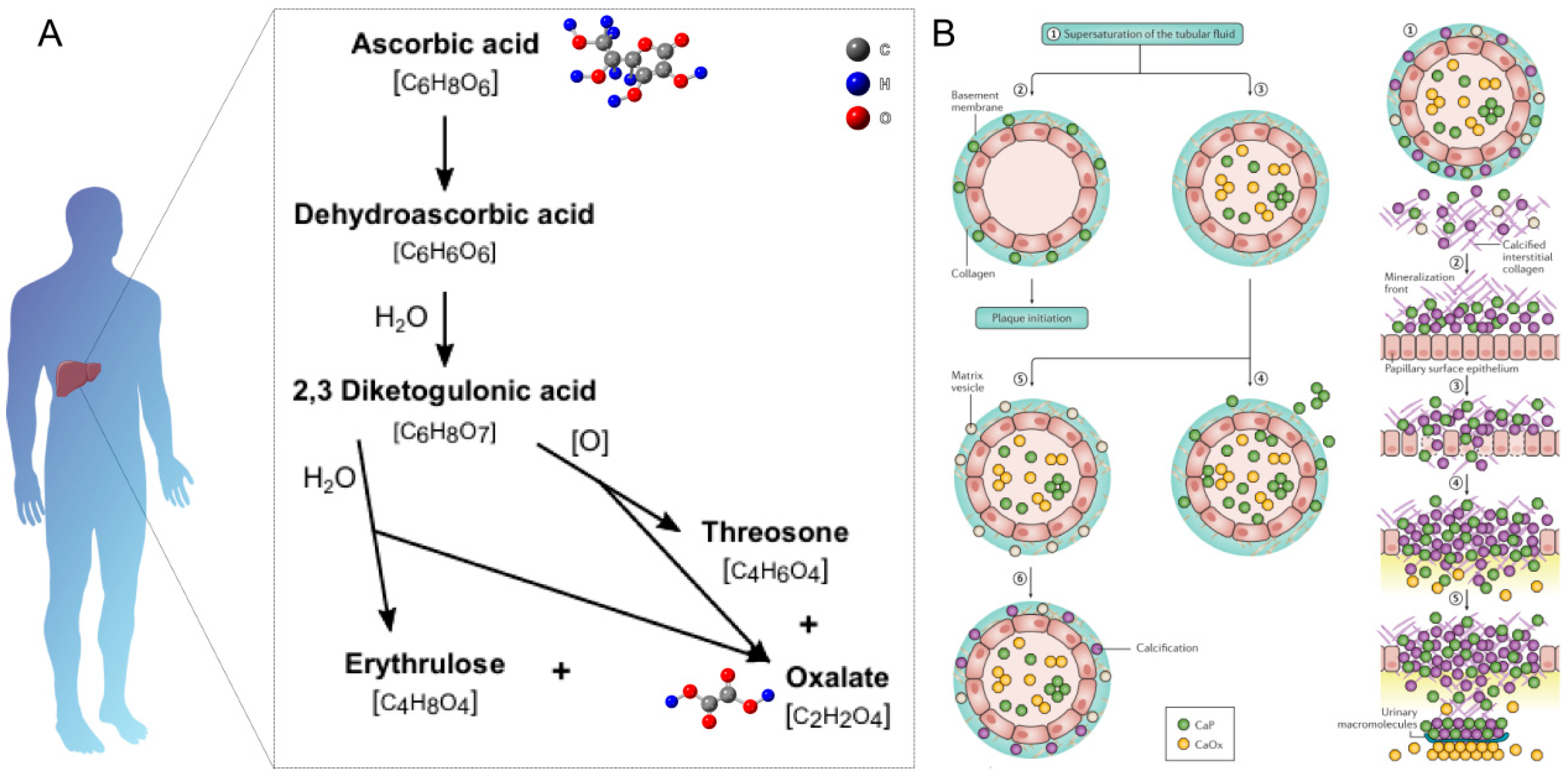
2.2.1. Oxalate
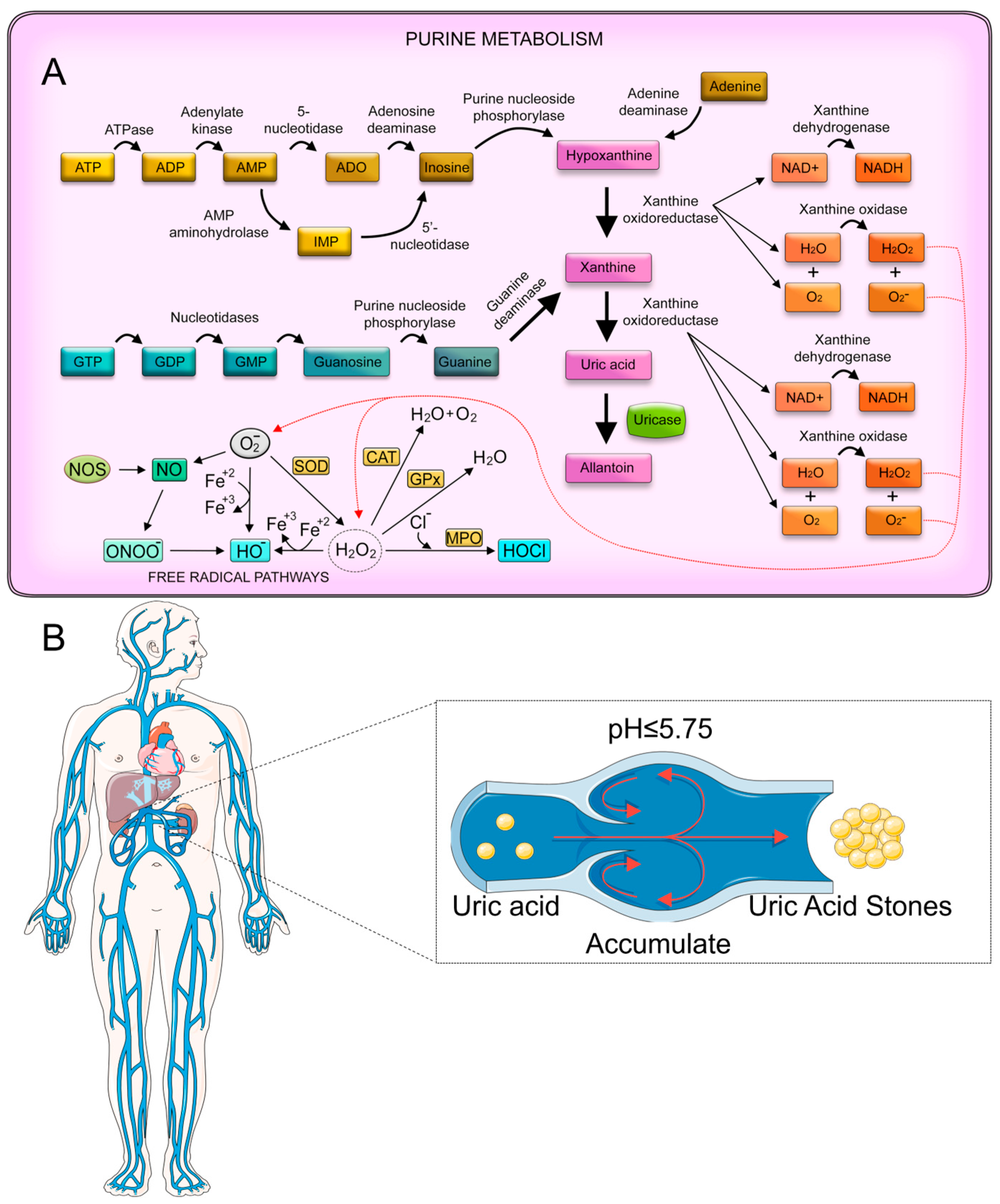
2.2.2. Uric Acid
2.2.3. Medical Detection of Relevant Metabolic Indicators
3. Electrochemical Sensors in Nephrolithiasis Detection Research
3.1. Oxalate and Uric Acid Detections
3.1.1. Oxalate Detection
3.1.2. Uric Acid Detection
4. Current Challenges and Barriers to Clinical Application
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mayans, L. Nephrolithiasis. Prim. Care Clin. Off. Pract. 2019, 46, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Bishop, K.; Momah, T.; Ricks, J. Nephrolithiasis. Prim. Care Clin. Off. Pract. 2020, 47, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Abufaraj, M.; Xu, T. Prevalence and Trends in Kidney Stone Among Adults in the USA: Analyses of National Health and Nutrition Examination Survey 2007–2018 Data. Eur. Urol. Focus 2021, 7, 1468–1475. [Google Scholar] [CrossRef]
- Chen, K.; Meskawi, M.; Miller, L.E.; Bhattacharyya, S.; Tailly, T.; Chew, B.H.; Bhojani, N. Trends in kidney stone prevalence among U.S. adults. Can. Urol. Assoc. J. 2024, 19, 58–60. [Google Scholar] [CrossRef]
- Alibrahim, H.; Swed, S.; Sawaf, B.; Alkhanafsa, M.; AlQatati, F.; Alzughayyar, T.; Abdeljawwad Abumunshar, N.A.; Alom, M.; Qafisheh, Q.; Aljunaidi, R.; et al. Kidney Stone Prevalence Among US Population: Updated Estimation from NHANES Data Set. JU Open Plus 2024, 2, e00115. [Google Scholar] [CrossRef]
- Alexander, R.T.; Hemmelgarn, B.R.; Wiebe, N.; Bello, A.; Morgan, C.; Samuel, S.; Klarenbach, S.W.; Curhan, G.C.; Tonelli, M. Kidney stones and kidney function loss: A cohort study. BMJ Br. Med. J. 2012, 345, e5287. [Google Scholar] [CrossRef]
- Bajwa, Z.H.; Gupta, S.; Warfield, C.A.; Steinman, T.I. Pain management in polycystic kidney disease. Kidney Int. 2001, 60, 1631–1644. [Google Scholar] [CrossRef]
- New, F.; Somani, B.K. A Complete World Literature Review of Quality of Life (QOL) in Patients with Kidney Stone Disease (KSD). Curr. Urol. Rep. 2016, 17, 88. [Google Scholar] [CrossRef]
- Tomczak, W.; Krajewski, W.; Chorbińska, J.; Nowak, Ł.; Grunwald, K.; Chełmoński, A.; Łaszkiewicz, J.; Małkiewicz, B.; Szydełko, T. Polish validation of the wisconsin stone quality of life questionnaire (POL-WISQoL). World J. Urol. 2024, 42, 590. [Google Scholar] [CrossRef]
- Raja, A.; Hekmati, Z.; Joshi, H.B. How Do Urinary Calculi Influence Health-Related Quality of Life and Patient Treatment Preference: A Systematic Review. J. Endourol. 2016, 30, 727–743. [Google Scholar] [CrossRef]
- French, W.W.; Scales, C.D.; Viprakasit, D.P.; Sur, R.L.; Friedlander, D.F. Predictors and Cost Comparison of Subsequent Urinary Stone Care at Index Versus Non-Index Hospitals. Urology 2022, 164, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Ghani, K.R.; Rojanasarot, S.; Cutone, B.; Bhattacharyya, S.K.; Krambeck, A.E. Economic burden of complicated ureteral stent removal in patients with kidney stone disease in the USA. J. Comp. Eff. Res. 2022, 11, 1253–1261. [Google Scholar] [CrossRef]
- Sáenz-Medina, J.; San Román, J.; Rodríguez-Monsalve, M.; Durán, M.; Carballido, J.; Prieto, D.; Gil Miguel, Á. Hospitalization Burden of Patients with Kidney Stones and Metabolic Comorbidities in Spain during the Period 2017–2020. Metabolites 2023, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Soucie, J.M.; Thun, M.J.; Coates, R.J.; McClellan, W.; Austin, H. Demographic and geographic variability of kidney stones in the United States. Kidney Int. 1994, 46, 893–899. [Google Scholar] [CrossRef]
- Hughes, P. Kidney stones epidemiology. Nephrology 2007, 12, S26–S30. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.M.; Cunha, T.D.S.; Curhan, G.C. Sex Differences and the Risk of Kidney Stones. Semin. Nephrol. 2022, 42, 230–235. [Google Scholar] [CrossRef]
- Curhan, G.C.; Willett, W.C.; Rimm, E.B.; Speizer, F.E.; Stampfer, M.J. Body size and risk of kidney stones. J. Am. Soc. Nephrol. 1998, 9, 1645–1652. [Google Scholar] [CrossRef]
- Johnson, C.M.; Wilson, D.M.; O’Fallon, W.M.; Malek, R.S.; Kurland, L.T. Renal stone epidemiology: A 25-year study in Rochester, Minnesota. Kidney Int. 1979, 16, 624–631. [Google Scholar] [CrossRef]
- Lieske, J.C.; Peña de la Vega, L.S.; Slezak, J.M.; Bergstralh, E.J.; Leibson, C.L.; Ho, K.L.; Gettman, M.T. Renal stone epidemiology in Rochester, Minnesota: An update. Kidney Int. 2006, 69, 760–764. [Google Scholar] [CrossRef]
- Robertson, W.G. Renal Stones in the Tropics. Semin. Nephrol. 2003, 23, 77–87. [Google Scholar] [CrossRef]
- Howles, S.A.; Thakker, R.V. Genetics of kidney stone disease. Nat. Rev. Urol. 2020, 17, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Harris, P.C.; Sas, D.J.; Lieske, J.C. The genetics of kidney stone disease and nephrocalcinosis. Nat. Rev. Nephrol. 2022, 18, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Konjengbam, H.; Meitei, S.Y. Association of kidney stone disease with dietary factors: A review. Anthropol. Rev. 2020, 83, 65–73. [Google Scholar] [CrossRef]
- Scales, C.D.; Smith, A.C.; Hanley, J.M.; Saigal, C.S. Prevalence of Kidney Stones in the United States. Eur. Urol. 2012, 62, 160–165. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Taylor, E.N.; Gambaro, G.; Curhan, G.C. Dietary and Lifestyle Risk Factors Associated with Incident Kidney Stones in Men and Women. J. Urol. 2017, 198, 858–863. [Google Scholar] [CrossRef]
- Cupisti, A.; D’Alessandro, C.; Samoni, S.; Meola, M.; Egidi, M.F. Nephrolithiasis and hypertension: Possible links and clinical implications. J. Nephrol. 2014, 27, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Zisman, A.L.; Evan, A.P.; Coe, F.L.; Worcester, E.M. Do kidney stone formers have a kidney disease? Kidney Int. 2015, 88, 1240–1249. [Google Scholar] [CrossRef]
- Di, X.; Liu, S.; Xiang, L.; Jin, X. Association between the systemic immune-inflammation index and kidney stone: A cross-sectional study of NHANES 2007-2018. Front. Immunol. 2023, 14, 1116224. [Google Scholar] [CrossRef]
- Roberson, N.P.; Dillman, J.R.; O’Hara, S.M.; DeFoor, W.R.; Reddy, P.P.; Giordano, R.M.; Trout, A.T. Comparison of ultrasound versus computed tomography for the detection of kidney stones in the pediatric population: A clinical effectiveness study. Pediatr. Radiol. 2018, 48, 962–972. [Google Scholar] [CrossRef]
- Rao, P.N. Imaging for kidney stones. World J. Urol. 2004, 22, 323–327. [Google Scholar] [CrossRef]
- Thomas, M. Clinical diagnosis of kidney stones. Nephrology 2007, 12, S1–S3. [Google Scholar] [CrossRef]
- Ray, A.A.; Ghiculete, D.; Pace, K.T.; Honey, R.J.D.A. Limitations to Ultrasound in the Detection and Measurement of Urinary Tract Calculi. Urology 2010, 76, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Viprakasit, D.P.; Sawyer, M.D.; Herrell, S.D.; Miller, N.L. Limitations of Ultrasonography in the Evaluation of Urolithiasis: A Correlation With Computed Tomography. J. Endourol. 2012, 26, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Wei, Q.; Long, H.; Yu, Z.; Deng, Z.; Meng, L.; Wang, J.; Luo, J.; Lin, C.-T.; Ma, L.; et al. Long-term stability of Au nanoparticle-anchored porous boron-doped diamond hybrid electrode for enhanced dopamine detection. Electrochim. Acta 2018, 271, 84–91. [Google Scholar] [CrossRef]
- Gee, C.-M.; Tseng, C.-C.; Wu, F.-Y.; Chang, H.-P.; Li, L.-J.; Hsieh, Y.-P.; Lin, C.-T.; Chen, J.-C. Flexible transparent electrodes made of electrochemically exfoliated graphene sheets from low-cost graphite pieces. Displays 2013, 34, 315–319. [Google Scholar] [CrossRef]
- Jadon, N.; Jain, R.; Sharma, S.; Singh, K. Recent trends in electrochemical sensors for multianalyte detection–A review. Talanta 2016, 161, 894–916. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wu, M.; Zheng, Y.; Zhang, P.; Ye, C.; Zhang, H.; Wang, K.; Su, W.; Chen, F.; Yu, J.; et al. Lycoris species identification and infrageneric relationship investigation via graphene enhanced electrochemical fingerprinting of pollen. Sens. Actuators B Chem. 2019, 298, 126836. [Google Scholar] [CrossRef]
- Loan, P.T.K.; Wu, D.; Ye, C.; Li, X.; Tra, V.T.; Wei, Q.; Fu, L.; Yu, A.; Li, L.-J.; Lin, C.-T. Hall effect biosensors with ultraclean graphene film for improved sensitivity of label-free DNA detection. Biosens. Bioelectron. 2018, 99, 85–91. [Google Scholar] [CrossRef]
- Deng, L.; Lai, G.; Fu, L.; Lin, C.-T.; Yu, A. Enzymatic deposition of gold nanoparticles at vertically aligned carbon nanotubes for electrochemical stripping analysis and ultrasensitive immunosensing of carcinoembryonic antigen. Analyst 2020, 145, 3073–3080. [Google Scholar] [CrossRef]
- Guo, P.; Wang, Y.; Chen, Z.; Jin, T.; Fu, L.; Lin, C.-T.; Lai, G. Voltammetric immunoassay of human IgG based on the release of cadmium(II) from CdS nanocrystals deposited on mesoporous silica nanospheres. Microchim. Acta 2018, 186, 15. [Google Scholar] [CrossRef]
- Shi, H.; Chen, F.; Zhao, S.; Ye, C.; Lin, C.-T.; Zhu, J.; Fu, L. Preparation of cassava fiber-iron nanoparticles composite for electrochemical determination of tea polyphenol. J. Food Meas. Charact. 2021, 15, 4711–4717. [Google Scholar] [CrossRef]
- Fu, L.; Zheng, Y.; Zhang, P.; Zhang, H.; Wu, M.; Zhang, H.; Wang, A.; Su, W.; Chen, F.; Yu, J.; et al. An electrochemical method for plant species determination and classification based on fingerprinting petal tissue. Bioelectrochemistry 2019, 129, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zheng, Y.; Zhang, P.; Zhang, H.; Zhuang, W.; Zhang, H.; Wang, A.; Su, W.; Yu, J.; Lin, C.-T. Enhanced electrochemical voltammetric fingerprints for plant taxonomic sensing. Biosens. Bioelectron. 2018, 120, 102–107. [Google Scholar] [CrossRef]
- Fu, L.; Zheng, Y.; Zhang, P.; Zhang, H.; Xu, Y.; Zhou, J.; Zhang, H.; Karimi-Maleh, H.; Lai, G.; Zhao, S.; et al. Development of an electrochemical biosensor for phylogenetic analysis of Amaryllidaceae based on the enhanced electrochemical fingerprint recorded from plant tissue. Biosens. Bioelectron. 2020, 159, 112212. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Lin, C.-T.; Karimi-Maleh, H.; Chen, F.; Zhao, S. Plasmonic Nanoparticle-Enhanced Optical Techniques for Cancer Biomarker Sensing. Biosensors 2023, 13, 977. [Google Scholar] [CrossRef]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef]
- Rakesh Kumar, R.K.; Shaikh, M.O.; Chuang, C.-H. A review of recent advances in non-enzymatic electrochemical creatinine biosensing. Anal. Chim. Acta 2021, 1183, 338748. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Luo, F.; Wang, P.; Lin, Z. Miniaturized electrochemical sensors and their point-of-care applications. Chin. Chem. Lett. 2020, 31, 589–600. [Google Scholar] [CrossRef]
- Nemčeková, K.; Labuda, J. Advanced materials-integrated electrochemical sensors as promising medical diagnostics tools: A review. Mater. Sci. Eng. C 2021, 120, 111751. [Google Scholar] [CrossRef]
- Mahmudiono, T.; Olegovich Bokov, D.; Abdalkareem Jasim, S.; Kamal Abdelbasset, W.; Dinora, M.K. State-of-the-art of convenient and low-cost electrochemical sensor for food contamination detection: Technical and analytical overview. Microchem. J. 2022, 179, 107460. [Google Scholar] [CrossRef]
- Imani, S.; Bandodkar, A.J.; Mohan, A.M.V.; Kumar, R.; Yu, S.; Wang, J.; Mercier, P.P. A wearable chemical–electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 2016, 7, 11650. [Google Scholar] [CrossRef]
- Parmar, M.S. Kidney stones. BMJ 2004, 328, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Pearle, M.S.; Robertson, W.G.; Gambaro, G.; Canales, B.K.; Doizi, S.; Traxer, O.; Tiselius, H.G. Kidney stones. Nat. Rev. Dis. Primers 2016, 2, 16008. [Google Scholar] [CrossRef] [PubMed]
- Spivacow, F.R.; Del Valle, E.E.; Lores, E.; Rey, P.G. Kidney Stones: Composition, Frequency and Relation to Metabolic Diagnosis. Medicina 2016, 76, 343–348. [Google Scholar] [PubMed]
- Evan, A.P.; Worcester, E.M.; Coe, F.L.; Williams, J.; Lingeman, J.E. Mechanisms of human kidney stone formation. Urolithiasis 2015, 43, 19–32. [Google Scholar] [CrossRef]
- Moe, O.W. Kidney stones: Pathophysiology and medical management. Lancet 2006, 367, 333–344. [Google Scholar] [CrossRef]
- Ivanovski, O.; Drueke, T.B. A new era in the treatment of calcium oxalate stones? Kidney Int. 2013, 83, 998–1000. [Google Scholar] [CrossRef]
- Stamatelou, K.K.; Francis, M.E.; Jones, C.A.; Nyberg, L.M.; Curhan, G.C. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003, 63, 1817–1823. [Google Scholar] [CrossRef]
- Lieske, J.C.; Swift, H.; Martin, T.; Patterson, B.; Toback, F.G. Renal epithelial cells rapidly bind and internalize calcium oxalate monohydrate crystals. Proc. Natl. Acad. Sci. USA 1994, 91, 6987–6991. [Google Scholar] [CrossRef]
- Martin, X.; Smith, L.H.; Werness, P.G. Calcium oxalate dihydrate formation in urine. Kidney Int. 1984, 25, 948–952. [Google Scholar] [CrossRef]
- Tazzoli, V.; Domeneghetti, C. The crystal structures of whewellite and weddellite: Re-examination and comparison. Am. Mineral. 1980, 65, 327–334. [Google Scholar]
- Ma, Q.; Fang, L.; Su, R.; Ma, L.; Xie, G.; Cheng, Y. Uric acid stones, clinical manifestations and therapeutic considerations. Postgrad. Med. J. 2018, 94, 458–462. [Google Scholar] [CrossRef]
- Asplin, J.R. Uric acid stones. Semin. Nephrol. 1996, 16, 412–424. [Google Scholar]
- Becker, G.; Caring for Australians with Renal, I. The CARI guidelines. Kidney stones: Uric acid stones. Nephrol 2007, 12 (Suppl. S1), S21–S25. [Google Scholar] [CrossRef]
- Puig, J.G.; Torres, R.J.; de Miguel, E.; Sánchez, A.; Bailén, R.; Banegas, J.R. Uric acid excretion in healthy subjects: A nomogram to assess the mechanisms underlying purine metabolic disorders. Metabolism 2012, 61, 512–518. [Google Scholar] [CrossRef]
- Villa, L.; Giusti, G.; Knoll, T.; Traxer, O. Imaging for Urinary Stones: Update in 2015. Eur. Urol. Focus 2016, 2, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Moussa, M.; Papatsoris, A.G.; Abou Chakra, M.; Moussa, Y. Update on cystine stones: Current and future concepts in treatment. Intractable Rare Dis. Res. 2020, 9, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, A.; Goldfarb, D.S. Cystinuria. Semin. Nephrol. 2008, 28, 181–191. [Google Scholar] [CrossRef]
- Evan, A.P.; Coe, F.L.; Lingeman, J.E.; Shao, Y.; Matlaga, B.R.; Kim, S.C.; Bledsoe, S.B.; Sommer, A.J.; Grynpas, M.; Phillips, C.L.; et al. Renal crystal deposits and histopathology in patients with cystine stones. Kidney Int. 2006, 69, 2227–2235. [Google Scholar] [CrossRef][Green Version]
- Su, Y.; Hessou, E.P.; Colombo, E.; Belletti, G.; Moussadik, A.; Lucas, I.T.; Frochot, V.; Daudon, M.; Rouzière, S.; Bazin, D.; et al. Crystalline structures of l-cysteine and l-cystine: A combined theoretical and experimental characterization. Amino Acids 2022, 54, 1123–1133. [Google Scholar] [CrossRef]
- Saravakos, P.; Kokkinou, V.; Giannatos, E. Cystinuria: Current Diagnosis and Management. Urology 2014, 83, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Cruz-May, T.N.; Herrera, A.; Rodríguez-Hernández, J.; Basulto-Martínez, M.; Flores-Tapia, J.P.; Quintana, P. Structural and morphological characterization of kidney stones in patients from the Yucatan Maya population. J. Mol. Struct. 2021, 1235, 130267. [Google Scholar] [CrossRef]
- Kılınç, M.T.; Özkent, M.S.; Pişkin, M.M.; Göger, Y.E. Investigation of gaseous end products produced by thulium fiber laser lithotripsy of cystine, uric acid, and calcium oxalate monohydrate stones: A gas chromatographic and electron microscopic analysis. Urolithiasis 2024, 52, 125. [Google Scholar] [CrossRef]
- Chen, T.; Qian, B.; Zou, J.; Luo, P.; Zou, J.; Li, W.; Chen, Q.; Zheng, L. Oxalate as a potent promoter of kidney stone formation. Front. Med. 2023, 10, 1159616. [Google Scholar] [CrossRef] [PubMed]
- Ermer, T.; Nazzal, L.; Tio, M.C.; Waikar, S.; Aronson, P.S.; Knauf, F. Oxalate homeostasis. Nat. Rev. Nephrol. 2023, 19, 123–138. [Google Scholar] [CrossRef]
- Crivelli, J.J.; Mitchell, T.; Knight, J.; Wood, K.D.; Assimos, D.G.; Holmes, R.P.; Fargue, S. Contribution of Dietary Oxalate and Oxalate Precursors to Urinary Oxalate Excretion. Nutrients 2021, 13, 62. [Google Scholar] [CrossRef]
- Poore, R.E.; Hurst, C.H.; Assimos, D.G.; Holmes, R.P. Pathways of hepatic oxalate synthesis and their regulation. Am. J. Physiol.-Cell Physiol. 1997, 272, C289–C294. [Google Scholar] [CrossRef]
- Moya-Garzon, M.D.; Rodriguez-Rodriguez, B.; Martin-Higueras, C.; Franco-Montalban, F.; Fernandes, M.X.; Gomez-Vidal, J.A.; Pey, A.L.; Salido, E.; Diaz-Gavilan, M. New salicylic acid derivatives, double inhibitors of glycolate oxidase and lactate dehydrogenase, as effective agents decreasing oxalate production. Eur. J. Med. Chem. 2022, 237, 114396. [Google Scholar] [CrossRef]
- Holmes, R.P.; Goodman, H.O.; Assimos, D.G. Contribution of dietary oxalate to urinary oxalate excretion. Kidney Int. 2001, 59, 270–276. [Google Scholar] [CrossRef]
- Robertson, W.G.; Scurr, D.S.; Bridge, C.M. Factors influencing the crystallisation of calcium oxalate in urine-critique. J. Cryst. Growth 1981, 53, 182–194. [Google Scholar] [CrossRef]
- Hoppe, B. An update on primary hyperoxaluria. Nat. Rev. Nephrol. 2012, 8, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Al-Atar, U.; Bokov, A.A.; Marshall, D.; Teichman, J.M.H.; Gates, B.D.; Ye, Z.-G.; Branda, N.R. Mechanism of Calcium Oxalate Monohydrate Kidney Stones Formation: Layered Spherulitic Growth. Chem. Mater. 2010, 22, 1318–1329. [Google Scholar] [CrossRef]
- Maruyama, M.; Sawada, K.P.; Tanaka, Y.; Okada, A.; Momma, K.; Nakamura, M.; Mori, R.; Furukawa, Y.; Sugiura, Y.; Tajiri, R.; et al. Quantitative analysis of calcium oxalate monohydrate and dihydrate for elucidating the formation mechanism of calcium oxalate kidney stones. PLoS ONE 2023, 18, e0282743. [Google Scholar] [CrossRef] [PubMed]
- Sakhaee, K. Epidemiology and clinical pathophysiology of uric acid kidney stones. J. Nephrol. 2014, 27, 241–245. [Google Scholar] [CrossRef]
- Savio, L.E.B.; Leite-Aguiar, R.; Alves, V.S.; Coutinho-Silva, R.; Wyse, A.T.S. Purinergic signaling in the modulation of redox biology. Redox Biol. 2021, 47, 102137. [Google Scholar] [CrossRef] [PubMed]
- Copur, S.; Demiray, A.; Kanbay, M. Uric acid in metabolic syndrome: Does uric acid have a definitive role? Eur. J. Intern. Med. 2022, 103, 4–12. [Google Scholar] [CrossRef]
- Fathallah-Shaykh, S.A.; Cramer, M.T. Uric acid and the kidney. Pediatr. Nephrol. 2014, 29, 999–1008. [Google Scholar] [CrossRef]
- Zhang, W.-z. Chapter Five-Uric acid en route to gout. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2023; Volume 116, pp. 209–275. [Google Scholar]
- Grases, F.; Villacampa, A.I.; Costa-Bauzá, A.; Söhnel, O. Uric acid calculi: Types, etiology and mechanisms of formation. Clin. Chim. Acta 2000, 302, 89–104. [Google Scholar] [CrossRef]
- Zerwekh, J.E.; Drake, E.; Gregory, J.; Griffith, D.; Hofmann, A.F.; Menon, M.; Pak, C.Y. Assay of urinary oxalate: Six methodologies compared. Clin. Chem. 1983, 29, 1977–1980. [Google Scholar] [CrossRef]
- Hansen, E.H.; Winther, S.K.; Gundstrup, M. Enzymatic Assay of Oxalate in Urine by Flow Injection Analysis Using Immobilized Oxalate Oxidase and Chemiluminescence Detection. Anal. Lett. 1994, 27, 1239–1253. [Google Scholar] [CrossRef]
- Kovar, K.A.; el Bolkiny, M.N.; Rink, R.; Hamid, M.A. An enzymatic assay for the colorimetric and fluorimetric determination of uric acid in sera. Arch. Pharm. 1990, 323, 235–237. [Google Scholar] [CrossRef]
- Jen, J.F.; Hsiao, S.L.; Liu, K.H. Simultaneous determination of uric acid and creatinine in urineby an eco-friendly solvent-free high performance liquid chromatographic method. Talanta 2002, 58, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Ghafar-Zadeh, E. Wireless Integrated Biosensors for Point-of-Care Diagnostic Applications. Sensors 2015, 15, 3236–3261. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, S.; Ali, M.A.; Anand, P.; Agrawal, V.V.; John, R.; Maji, S.; Malhotra, B.D. Microfluidic-integrated biosensors: Prospects for point-of-care diagnostics. Biotechnol. J. 2013, 8, 1267–1279. [Google Scholar] [CrossRef]
- Noah, N.M.; Ndangili, P.M. Current Trends of Nanobiosensors for Point-of-Care Diagnostics. J. Anal. Methods Chem. 2019, 2019, 2179718. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Adarakatti, P.S.; Banks, C.E. Electroanalytical Overview: The Electroanalytical Detection of Oxalate. Sens. Actuators Rep. 2023, 6, 100176. [Google Scholar] [CrossRef]
- Ma, L.; Zeng, Q.; Zhang, M.; Wang, L.; Cheng, F. Direct determination of oxalic acid by a bare platinum electrode contrasting a platinum nanoparticles-modified glassy carbon electrode. J. Exp. Nanosci. 2016, 11, 1242–1252. [Google Scholar] [CrossRef]
- Hu, K.; Chen, X.; Song, X.; Wu, Y.; Huang, K.; Chen, P. Carbon dots and MnO2 nanosheet nanocomposites sensing platform for sensitive detection of oxalate in urine samples of urolithiasis patients. Talanta 2024, 266, 124976. [Google Scholar] [CrossRef] [PubMed]
- Maiyalagan, T.; Kannan, P.; Jonsson-Niedziolka, M.; Niedziolka-Jonsson, J. Tungsten carbide nanotubes supported platinum nanoparticles as a potential sensing platform for oxalic acid. Anal. Chem. 2014, 86, 7849–7857. [Google Scholar] [CrossRef]
- Nagarajan, R.D.; Sundramoorthy, A.K. One-pot electrosynthesis of silver nanorods/graphene nanocomposite using 4-sulphocalix[4]arene for selective detection of oxalic acid. Sens. Actuators B Chem. 2019, 301, 127132. [Google Scholar] [CrossRef]
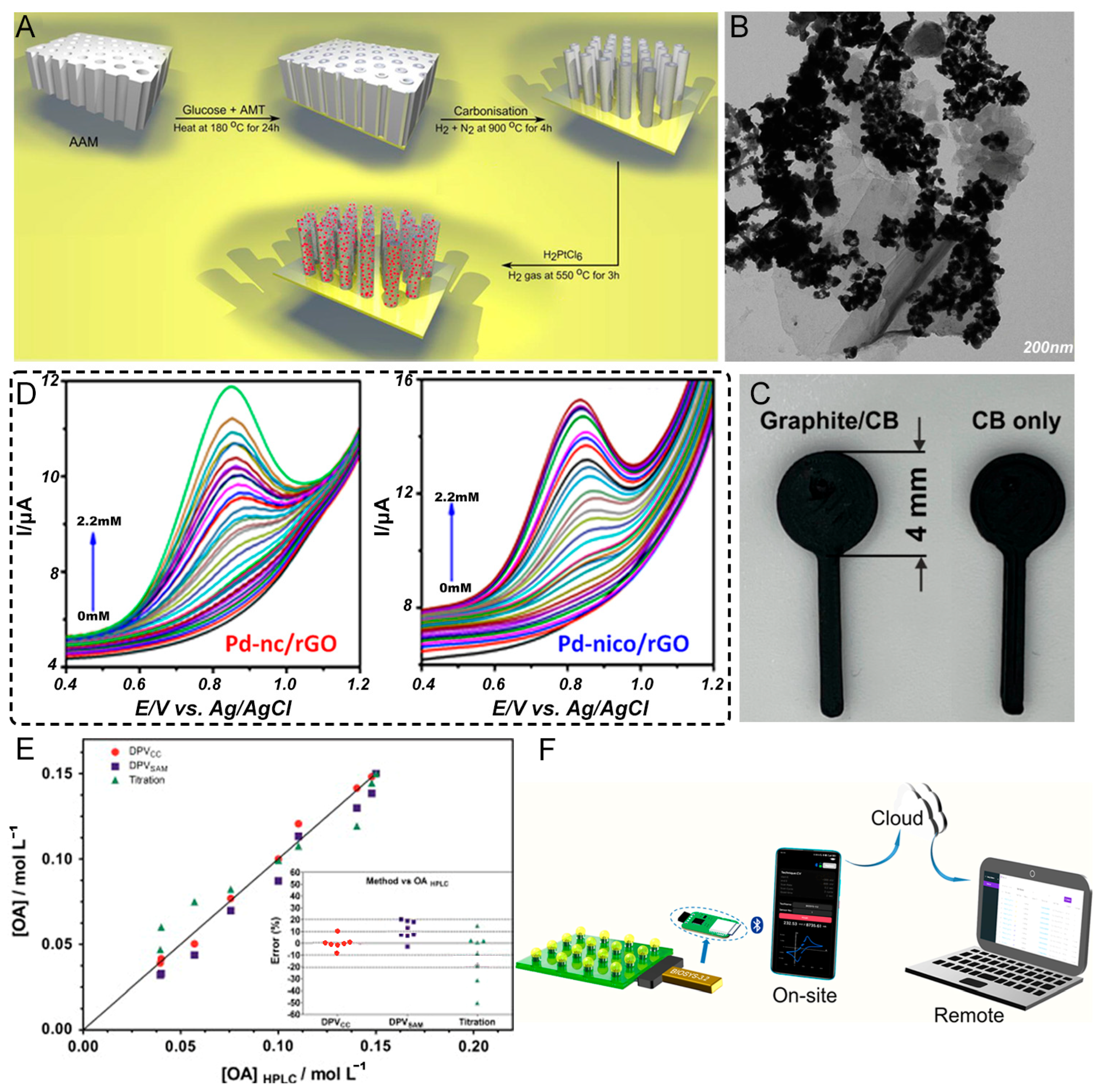
- Arantes, I.V.S.; Crapnell, R.D.; Bernalte, E.; Whittingham, M.J.; Paixao, T.; Banks, C.E. Mixed Graphite/Carbon Black Recycled PLA Conductive Additive Manufacturing Filament for the Electrochemical Detection of Oxalate. Anal. Chem. 2023, 95, 15086–15093. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhang, S.; Zhang, H.; Dong, Y.; Yao, P.; Du, Y.; Song, P.; Gong, X.; Xu, W. Metal−support interaction in single-atom electrocatalysts: A perspective of metal oxide supports. eScience 2024, 4, 100269. [Google Scholar] [CrossRef]
- Ferro, S.; Martínez-Huitle, C.A.; De Battisti, A. Electroxidation of oxalic acid at different electrode materials. J. Appl. Electrochem. 2010, 40, 1779–1787. [Google Scholar] [CrossRef]
- Šljukić, B.; Baron, R.; Compton, R.G. Electrochemical Determination of Oxalate at Pyrolytic Graphite Electrodes. Electroanalysis 2007, 19, 918–922. [Google Scholar] [CrossRef]
- Kesavan, L.; Kalekar, A.M.; Damlin, P.; Kvarnström, C. Reduced graphene oxide supported palladium nano-shapes for electro-oxidation of oxalic acid. J. Electroanal. Chem. 2019, 847, 113167. [Google Scholar] [CrossRef]
- Araújo, E.G.; Oliveira, G.R.; Santos, E.V.; Martínez-Huitle, C.A.; Panizza, M.; Fernandes, N.S. Applicability of electroanalysis for monitoring oxalic acid (OA) concentration during its electrochemical oxidation. J. Electroanal. Chem. 2013, 701, 32–35. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, J.; Li, G.; Chen, Y.; Xu, T.; Zhang, X. Wireless USB-like electrochemical platform for individual electrochemical sensing in microdroplets. Anal. Chim. Acta 2022, 1197, 339526. [Google Scholar] [CrossRef]
- Hussain, S.; Abbas Zaidi, S.; Vikraman, D.; Kim, H.-S.; Jung, J. Facile preparation of tungsten carbide nanoparticles for an efficient oxalic acid sensor via imprinting. Microchem. J. 2020, 159, 105404. [Google Scholar] [CrossRef]
- Gong, Y.; Fang, S.; Zheng, Y.; Guo, H.; Yang, F. Tetra-cyanostilbene macrocycle: An effective “turn-on” fluorescence sensor for oxalic acid in aqueous media. J. Photochem. Photobiol. A Chem. 2023, 435, 114307. [Google Scholar] [CrossRef]
- Raoof, J.B.; Chekin, F.; Ehsani, V. Palladium-doped mesoporous silica SBA-15 modified in carbon-paste electrode as a sensitive voltammetric sensor for detection of oxalic acid. Sens. Actuators B Chem. 2015, 207, 291–296. [Google Scholar] [CrossRef]
- Chen, X.; Cai, Z.; Huang, Z.; Oyama, M.; Jiang, Y.; Chen, X. Non-enzymatic oxalic acid sensor using platinum nanoparticles modified on graphene nanosheets. Nanoscale 2013, 5, 5779–5783. [Google Scholar] [CrossRef]
- Akhond, M.; Absalan, G.; Tafakori, A.; Ershadifar, H. Simultaneous Determination of Thiocyanate and Oxalate in Urine using a Carbon Ionic Liquid Electrode Modified with TiO2-Fe Nanoparticles. Anal. Bioanal. Chem. Res. 2016, 3, 73–86. [Google Scholar]
- Zafar, M.A.; Liu, Y.; Allende, S.; Jacob, M.V. Electrochemical sensing of oxalic acid using silver nanoparticles loaded nitrogen-doped graphene oxide. Carbon Trends 2022, 8, 100188. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, Y.; You, Z.; Sha, H.; Gong, S.; Liu, J.; Sun, W. Sensitive electrochemical determination of oxalic acid in spinach samples by a graphene-modified carbon ionic liquid electrode. Ionics 2015, 21, 877–884. [Google Scholar] [CrossRef]
- Venkadesh, A.; Mathiyarasu, J.; Radhakrishnan, S. Electrochemical Enzyme-free Sensing of Oxalic Acid Using an Amine-mediated Synthesis of CuS Nanosphere. Anal. Sci. 2021, 37, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Radhakrishnan, S.; Jayaseelan, S.S.; Kim, B.-S. Non-enzymatic Electrochemical Oxidation Based on AuNP/PPy/rGO Nanohybrid Modified Glassy Carbon Electrode as a Sensing Platform for Oxalic Acid. Electroanalysis 2016, 28, 2626–2632. [Google Scholar] [CrossRef]
- Ahmar, H.; Fakhari, A.R.; Nabid, M.R.; Rezaei, S.J.T.; Bide, Y. Electrocatalytic oxidation of oxalic acid on palladium nanoparticles encapsulated on polyamidoamine dendrimer-grafted multi-walled carbon nanotubes hybrid material. Sens. Actuators B Chem. 2012, 171–172, 611–618. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, C.; Pu, W.; Zhang, J. Determination of oxalic acid in spinach with carbon nanotubes-modified electrode. Food Chem. 2009, 114, 1523–1528. [Google Scholar] [CrossRef]
- Alizadeh, T.; Nayeri, S. Graphite/Ag/AgCl nanocomposite as a new and highly efficient electrocatalyst for selective electroxidation of oxalic acid and its assay in real samples. Mater. Sci. Eng. C 2019, 100, 826–836. [Google Scholar] [CrossRef]
- Luo, X.; Chen, L.; Yang, J.; Li, S.; Li, M.; Mo, Q.; Li, Y.; Li, X. Electrochemically simultaneous detection of ascorbic acid, sulfite and oxalic acid on Pt-Pd nanoparticles/chitosan/nitrogen doped graphene modified glassy carbon electrode: A method for drug quality control. Microchem. J. 2021, 169, 106623. [Google Scholar] [CrossRef]
- Lakshmi, D.; Whitcombe, M.J.; Davis, F.; Sharma, P.S.; Prasad, B.B. Electrochemical Detection of Uric Acid in Mixed and Clinical Samples: A Review. Electroanalysis 2011, 23, 305–320. [Google Scholar] [CrossRef]
- Dryhurst, G. Primary Products of Electrochemical Oxidation of Uric Acid in Aqueous and Methanolic Solution. J. Electrochem. Soc. 1971, 118, 699. [Google Scholar] [CrossRef]
- Shakkthivel, P.; Chen, S.-M. Simultaneous determination of ascorbic acid and dopamine in the presence of uric acid on ruthenium oxide modified electrode. Biosens. Bioelectron. 2007, 22, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Slaughter, G. Electrochemical carbon-based sensors for non-enzymatic uric acid sensing. Microchem. J. 2025, 208, 112331. [Google Scholar] [CrossRef]
- Jain, S.; Verma, S.; Singh, S.P.; Sharma, S.N. An electrochemical biosensor based on novel butylamine capped CZTS nanoparticles immobilized by uricase for uric acid detection. Biosens. Bioelectron. 2019, 127, 135–141. [Google Scholar] [CrossRef]
- Sun, M.; Cui, C.; Chen, H.; Wang, D.; Zhang, W.; Guo, W. Enzymatic and Non-Enzymatic Uric Acid Electrochemical Biosensors: A Review. ChemPlusChem 2023, 88, e202300262. [Google Scholar] [CrossRef]
- Noma, S.A.A. Investigation of improved uricase release and kinetic parameters through dual affected responsive nanopolymers. Process Biochem. 2023, 131, 52–58. [Google Scholar] [CrossRef]
- Savk, A.; Özdil, B.; Demirkan, B.; Nas, M.S.; Calimli, M.H.; Alma, M.H.; Inamuddin; Asiri, A.M.; Şen, F. Multiwalled carbon nanotube-based nanosensor for ultrasensitive detection of uric acid, dopamine, and ascorbic acid. Mater. Sci. Eng. C 2019, 99, 248–254. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Xin, J.; Ma, H.; Pang, H.; Tan, L.; Wang, X. A non-enzymatic voltammetric xanthine sensor based on the use of platinum nanoparticles loaded with a metal-organic framework of type MIL-101(Cr). Application to simultaneous detection of dopamine, uric acid, xanthine and hypoxanthine. Microchim. Acta 2018, 186, 9. [Google Scholar] [CrossRef]
- RoyChoudhury, S.; Umasankar, Y.; Hutcheson, J.D.; Lev-Tov, H.A.; Kirsner, R.S.; Bhansali, S. Uricase Based Enzymatic Biosensor for Non-invasive Detection of Uric Acid by Entrapment in PVA-SbQ Polymer Matrix. Electroanalysis 2018, 30, 2374–2385. [Google Scholar] [CrossRef]
- Zenasni, M.; Quintero-Jaime, A.; Salinas-Torres, D.; Benyoucef, A.; Morallón, E. Electrochemical synthesis of composite materials based on titanium carbide and titanium dioxide with poly(N-phenyl-o-phenylenediamine) for selective detection of uric acid. J. Electroanal. Chem. 2021, 895, 115481. [Google Scholar] [CrossRef]
- Joshi, A.; Slaughter, G. Multiwalled carbon nanotubes supported Fe nanostructured interfaces for electrochemical detection of uric acid. Microchem. J. 2024, 204, 110934. [Google Scholar] [CrossRef]
- Liu, L.; Liu, L.; Wang, Y.; Ye, B.-C. A novel electrochemical sensor based on bimetallic metal–organic framework-derived porous carbon for detection of uric acid. Talanta 2019, 199, 478–484. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Y.; Zhu, R.; Chen, Y.; Liu, X.; Li, M.; Yang, L.; Wang, Y.; Wang, D. Fiber based organic electrochemical transistor integrated with molecularly imprinted membrane for uric acid detection. Talanta 2022, 238, 123055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, C.; Reis, N.M.; Chen, W.; Liang, B.; Liu, Z. A portable microfluidic electrochemical sensor with nonlinear fit strategy for wide-range uric acid detection. Microchem. J. 2024, 203, 110908. [Google Scholar] [CrossRef]
- Chen, F.; Wang, J.; Chen, L.; Lin, H.; Han, D.; Bao, Y.; Wang, W.; Niu, L. A Wearable Electrochemical Biosensor Utilizing Functionalized Ti3C2Tx MXene for the Real-Time Monitoring of Uric Acid Metabolite. Anal. Chem. 2024, 96, 3914–3924. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, Y.; Chen, Y.; Yang, M.; Zhao, S.; Qi, N.; Wang, Y.; Huo, D.; Hou, C. Hemin-Functionalized Microfluidic Chip with Dual-Electric Signal Outputs for Accurate Determination of Uric Acid. ACS Appl. Mater. Interfaces 2022, 14, 41369–41378. [Google Scholar] [CrossRef]
- Chakkarapani, L.D.; Arumugam, S.; Brandl, M. Layer-by-layer sensor architecture of polymers and nanoparticles for electrochemical detection of uric acid in human urine samples. Mater. Today Chem. 2021, 22, 100561. [Google Scholar] [CrossRef]
- Xie, X.; Mo, G.; Hu, B. Electrochemical assembling of nano-MOFs at carbon fiber for the high-performance uric acid sensing. Sens. Actuators B Chem. 2023, 393, 134263. [Google Scholar] [CrossRef]
- Iyyappan, E.; Samuel Justin, S.J.; Wilson, P.; Palaniappan, A. Nanoscale Hydroxyapatite for Electrochemical Sensing of Uric Acid: Roles of Mesopore Volume and Surface Acidity. ACS Appl. Nano Mater. 2020, 3, 7761–7773. [Google Scholar] [CrossRef]
- Wang, Q.; Si, H.; Zhang, L.; Li, L.; Wang, X.; Wang, S. A fast and facile electrochemical method for the simultaneous detection of epinephrine, uric acid and folic acid based on ZrO2/ZnO nanocomposites as sensing material. Anal. Chim. Acta 2020, 1104, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Peng, Z.; Huo, W.; Li, Y.; Liu, S.; Kang, L.; Wu, X.; Dai, L.; Wang, L.; Jun, S.C.; et al. Stabilizing Zn Metal Anode Through Regulation of Zn Ion Transfer and Interfacial Behavior with a Fast Ion Conductor Protective Layer. Small 2023, 19, e2303963. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Guo, H.; Xue, R.; Wang, M.; Zhao, X.; Fan, T.; Yang, W.; Xu, M.; Yang, W. Electrochemical sensor based on covalent organic frameworks-MWCNT-NH2/AuNPs for simultaneous detection of dopamine and uric acid. J. Electroanal. Chem. 2021, 880, 114932. [Google Scholar] [CrossRef]
- de Fátima Giarola, J.; César Pereira, A. Development and Application of a Sensor Based on Carbonaceous Materials and Cobalt Phthalocyanine Composite for Electrochemical Determination of Uric Acid. Electroanalysis 2016, 28, 1348–1355. [Google Scholar] [CrossRef]
- Quan, C.; Chen, W.; Yang, M.; Hou, Y. Electrochemical sensor using cobalt oxide-modified porous carbon for uric acid determination. Microchim. Acta 2023, 190, 401. [Google Scholar] [CrossRef]
- Veera Manohara Reddy, Y.; Sravani, B.; Agarwal, S.; Gupta, V.K.; Madhavi, G. Electrochemical sensor for detection of uric acid in the presence of ascorbic acid and dopamine using the poly(DPA)/SiO2@Fe3O4 modified carbon paste electrode. J. Electroanal. Chem. 2018, 820, 168–175. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, S.; Singh, A.; Jyoti; Pawan; Kaur, J.D.; Kaur, H.; Mohan, B.; Rana, S. Graphene oxide functionalized organosilanes for electrochemical detection of uric acid in urine and water. Mater. Chem. Phys. 2024, 319, 129347. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, X.; Yu, L.; He, S.; Zhu, R.; Meng, Z. Constructing “off–on” luminescence biosensor based on fluorescence resonance energy transfer for autofluorescence-free detection of uric acid and glucose. Inorg. Chem. Commun. 2025, 174, 113889. [Google Scholar] [CrossRef]
- Li, C.; Xu, Q.; Zhu, J.; Luo, T.; Yang, M.; Dai, H.; Kosinova, M.L.; Zhang, S.; Tu, R. Laser chemical vapor deposition of nitrogen-doped SiC electrode for electrochemical detection of uric acid. Surf. Interfaces 2024, 51, 104704. [Google Scholar] [CrossRef]
- Jiang, Z.; Dong, H.; Zhou, Y.; Chen, Y.; Hao, W.; Zhang, Z.; Hao, Y.; Liu, L.; Wang, X.; Xu, M. Simultaneous and Ratiometric Electrochemical Determination of Uric Acid and Hypoxanthine Based on In Situ Carbonized Polydopamine Graphene Paper. ACS Appl. Nano Mater. 2023, 6, 9268–9275. [Google Scholar] [CrossRef]
- Li, S.-N.; Zhang, J.; Liu, Y.-Q.; Zhou, K.-P.; Jiang, X.-Y.; Yu, J.-G. Cobalt vanadate intertwined in carboxylated multiple-walled carbon nanotubes for simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid. Talanta 2025, 282, 127038. [Google Scholar] [CrossRef] [PubMed]
- Murugan, N.; Jerome, R.; Preethika, M.; Sundaramurthy, A.; Sundramoorthy, A.K. 2D-titanium carbide (MXene) based selective electrochemical sensor for simultaneous detection of ascorbic acid, dopamine and uric acid. J. Mater. Sci. Technol. 2021, 72, 122–131. [Google Scholar] [CrossRef]


| Sensor | Linear Detection Range (μM) | Limit of Detection (μM) | Real Sample | Ref. |
| Pd nanocube@rGO | 49.5–10,000 | 50 | [106] | |
| Ag nanorod@rGO | 3000–30,000 | 40 | tap water | [101] |
| CuS nanospheres | 50–700 | 35.6 | [116] | |
| TiO2–Fe NP/ionic liquid | 500–3000 | 23 | urine | [113] |
| Au NP–polypyrrole@rGO | 50–7000 | 20 | [117] | |
| Pd NP@functional CNTs | 30–5000 | 20 | spinach | [118] |
| CNTs | 50–150 | 12 | spinach | [119] |
| Pt NP@rGO | 100–50,000 | 10 | spinach | [112] |
| TGM organic fluorescent probe | 0–0.16 | 3.86 | vegetable | [110] |
| Graphite@Ag–AgCl | 10–750 | 3.7 | urine | [120] |
| Ag NP@n-doped rGO | 10–300 | 2 | [114] | |
| Pt–Pd NP@n-doped rGO | 1.5–500 | 0.84 | [121] | |
| N-CD-MnO2 NSs | 1–50 | 0.69 | urine | [99] |
| rGO/ionic liquid | 8–6000 | 0.48 | spinach | [115] |
| Pd NP@molecular sieve (SBA-15) | 10–140 | 0.40 | onion and tomato | [111] |
| Pt NP@WC nanotubes | 0.012–0.125 | 0.012 | tomato | [100] |
| Sensor | Linear Detection Range (μM) | Limit of Detection (μM) | Real Sample | Ref. |
| CoPc-MWCNT | 125–4000 | 260 | urine | [145] |
| ZnGa2O4: Mn2+@MnO2 fluorescent | 20–50 | 1.3 | serum | [149] |
| CNCo | 2–110 | 0.83 | serum | [134] |
| N-doped SiC | 3.7–125 | 0.77 | urine | [150] |
| PyTS@Ti3C2Tx | 5–100 | 0.48 | sweat | [137] |
| He@CNT/alk-Ti3C2Tx/CHIP | 1–1000 | 0.41 | urine | [138] |
| Poly(DPA)/SiO2@Fe3O4/CPE | 1.2–8.2 | 0.4 | urine | [147] |
| COF-NH2-MWCNT-Au | 0.3–200 | 0.29 | urine | [145] |
| ZrO2/ZnO | 10–2400 | 0.29 | serum | [143] |
| Methylene blue-cPDA/graphene paper | 0.6–350 | 0.2 | serum | [151] |
| CoV/MWCNT-COOH | 1–100 | 0.1 | urine | [152] |
| Co-N/C@ MWCNT | 1–40 | 0.09 | serum | [146] |
| Ti-C-Tx | 0.5–4; 100–1500 | 0.075 | urine | [153] |
| GO-Silane | 2.5–80 | 0.065 | urine | [148] |
| n-HA/CPE | 0.068–50 | 0.05 | urine | [141] |
| SN-ZIF/CF | 0.01–2800 | 0.0056 | serum | [140] |
| PAMAM/MWCNT-AgNP/PNR | 0.016–2500 | 0.005 | urine | [139] |
| MIP/PEDOT/carbon fiber | 0.001–500 | 0.001 | artificial urine | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, K.; Zhao, N.; Shi, P.; Sun, Z.; Ye, C.; Fu, L.; Dai, D.; Chu, W.; Cai, T.; Tsai, H.-S.; et al. Early Detection and Monitoring of Nephrolithiasis: The Potential of Electrochemical Sensors. Sensors 2025, 25, 2547. https://doi.org/10.3390/s25082547
Sun K, Zhao N, Shi P, Sun Z, Ye C, Fu L, Dai D, Chu W, Cai T, Tsai H-S, et al. Early Detection and Monitoring of Nephrolithiasis: The Potential of Electrochemical Sensors. Sensors. 2025; 25(8):2547. https://doi.org/10.3390/s25082547
Chicago/Turabian StyleSun, Kaiqiang, Ningbin Zhao, Peizheng Shi, Zhuang Sun, Chen Ye, Li Fu, Dan Dai, Wubo Chu, Tao Cai, Hsu-Sheng Tsai, and et al. 2025. "Early Detection and Monitoring of Nephrolithiasis: The Potential of Electrochemical Sensors" Sensors 25, no. 8: 2547. https://doi.org/10.3390/s25082547
APA StyleSun, K., Zhao, N., Shi, P., Sun, Z., Ye, C., Fu, L., Dai, D., Chu, W., Cai, T., Tsai, H.-S., & Lin, C.-T. (2025). Early Detection and Monitoring of Nephrolithiasis: The Potential of Electrochemical Sensors. Sensors, 25(8), 2547. https://doi.org/10.3390/s25082547













