Feasibility of Little Cherry/X-Disease Detection in Prunus avium Using Field Asymmetric Ion Mobility Spectrometry
Abstract
1. Introduction
2. Materials and Methods
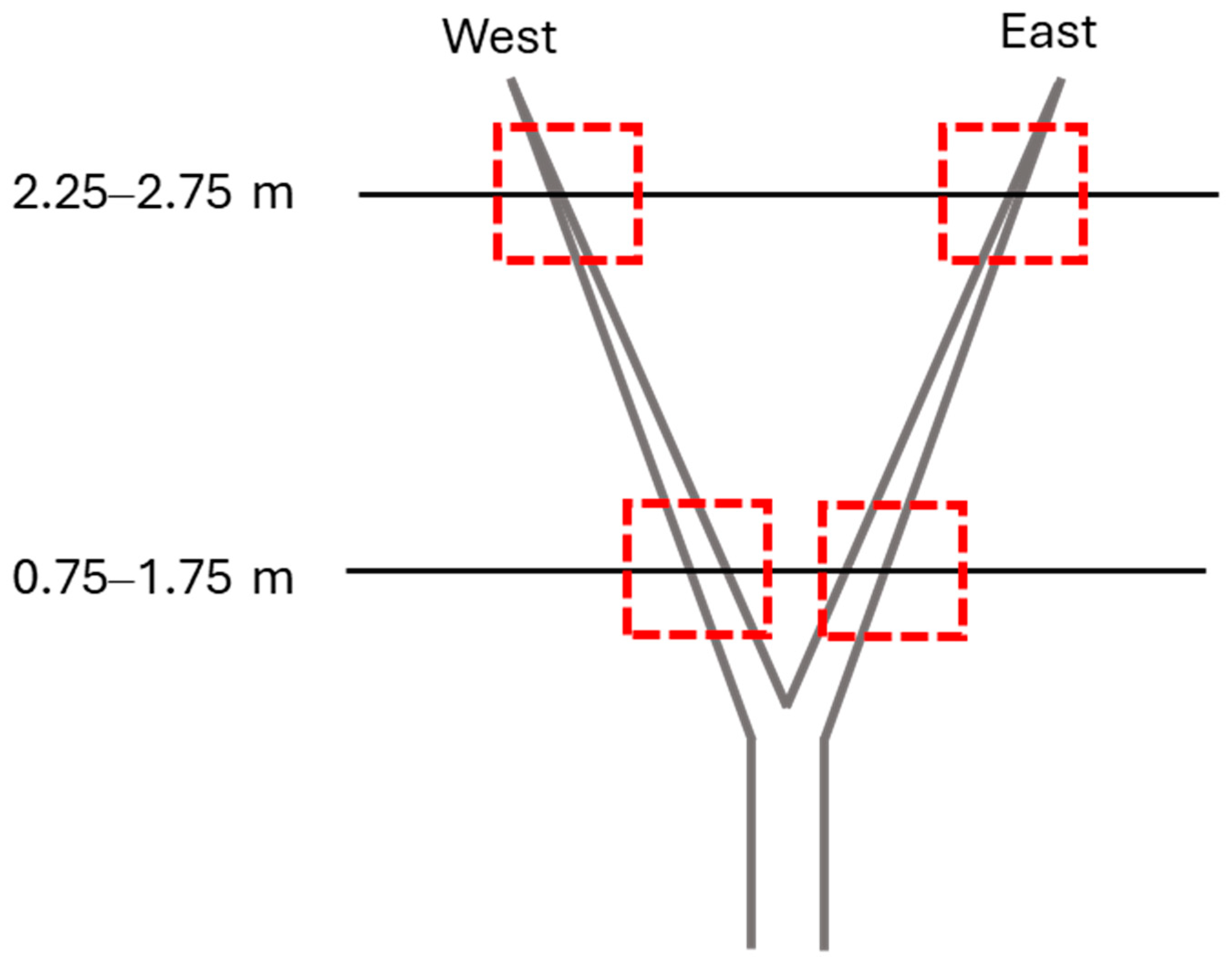
2.1. Field Sampling
2.2. Volatile Headspace Analysis
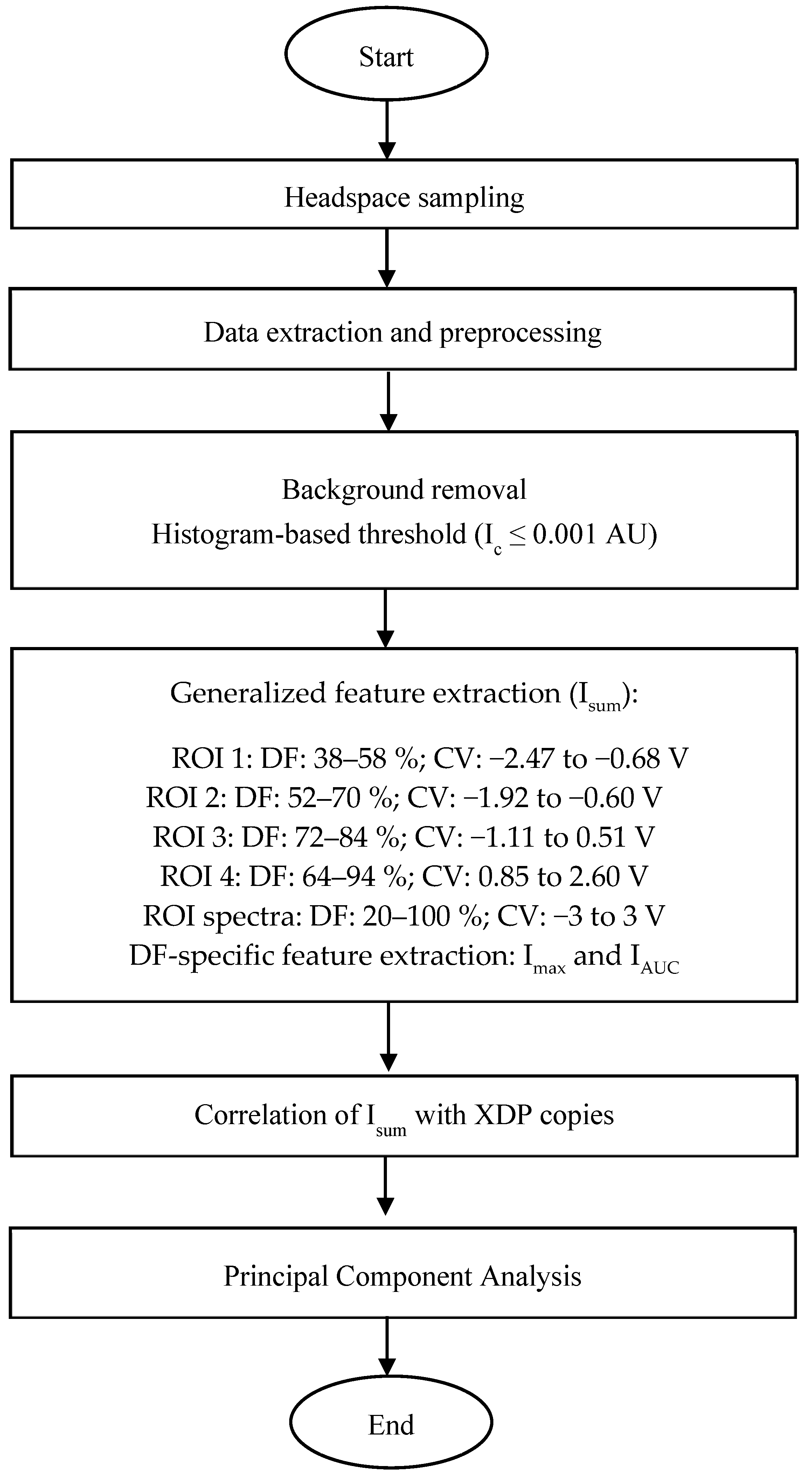
2.3. FAIMS Data Analysis
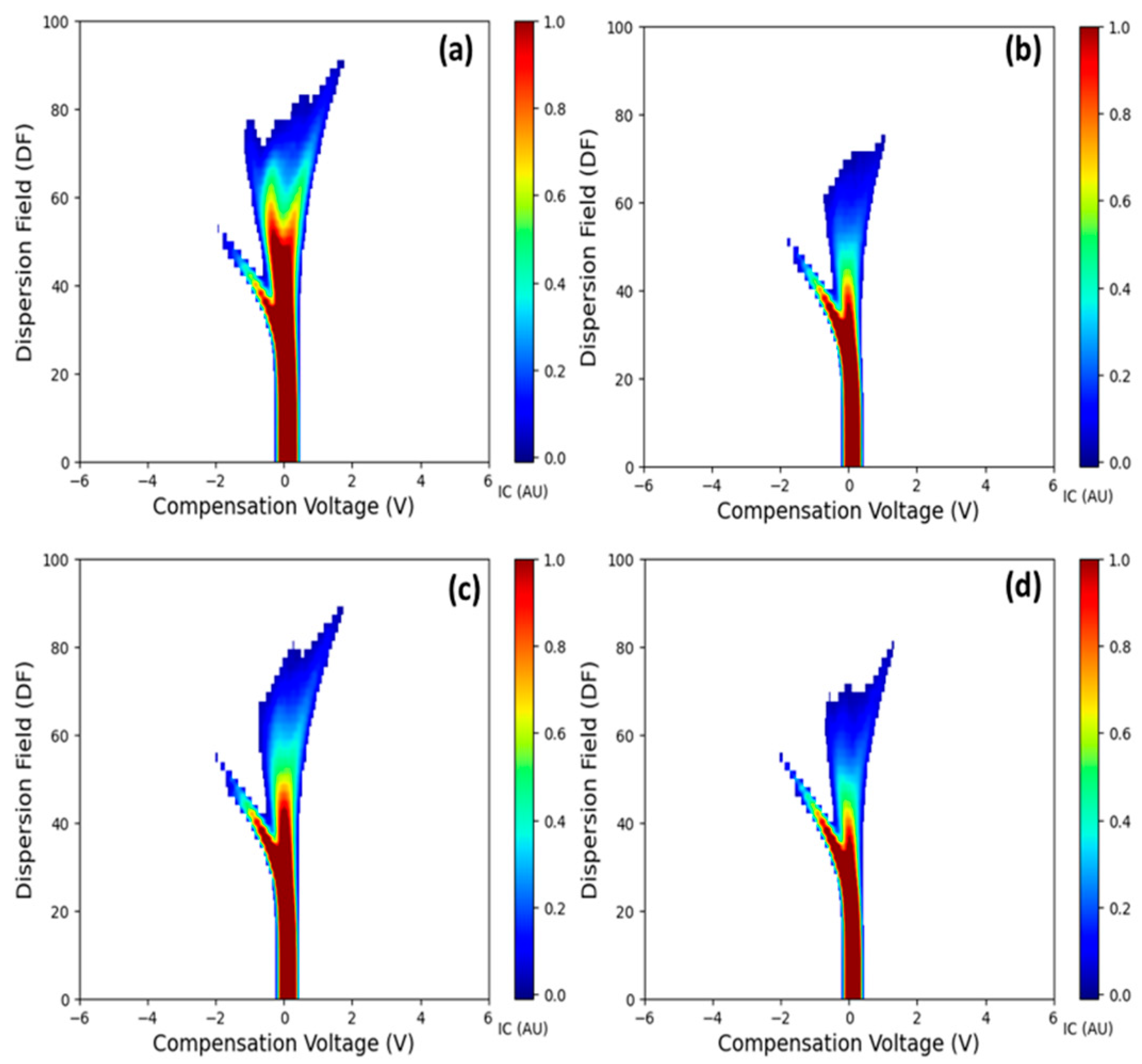
3. Results and Discussion
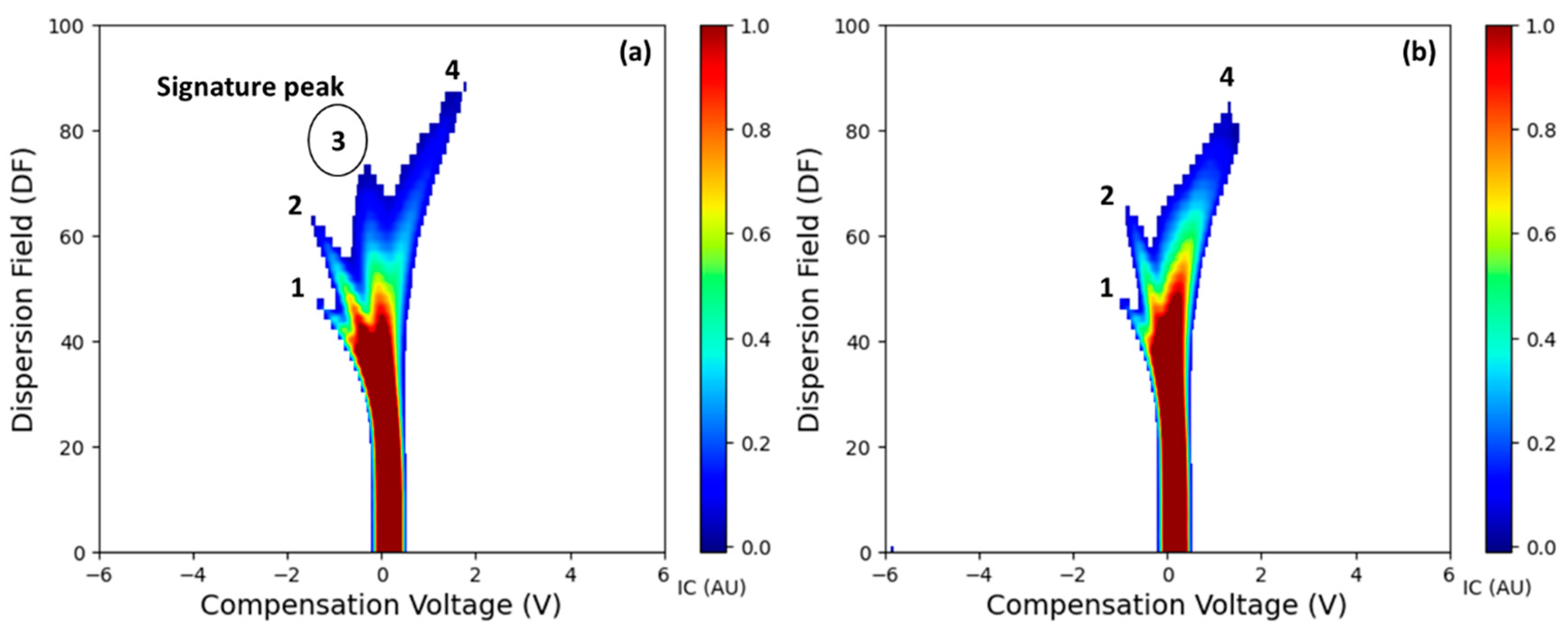
3.1. Salient FAIMS Spectra
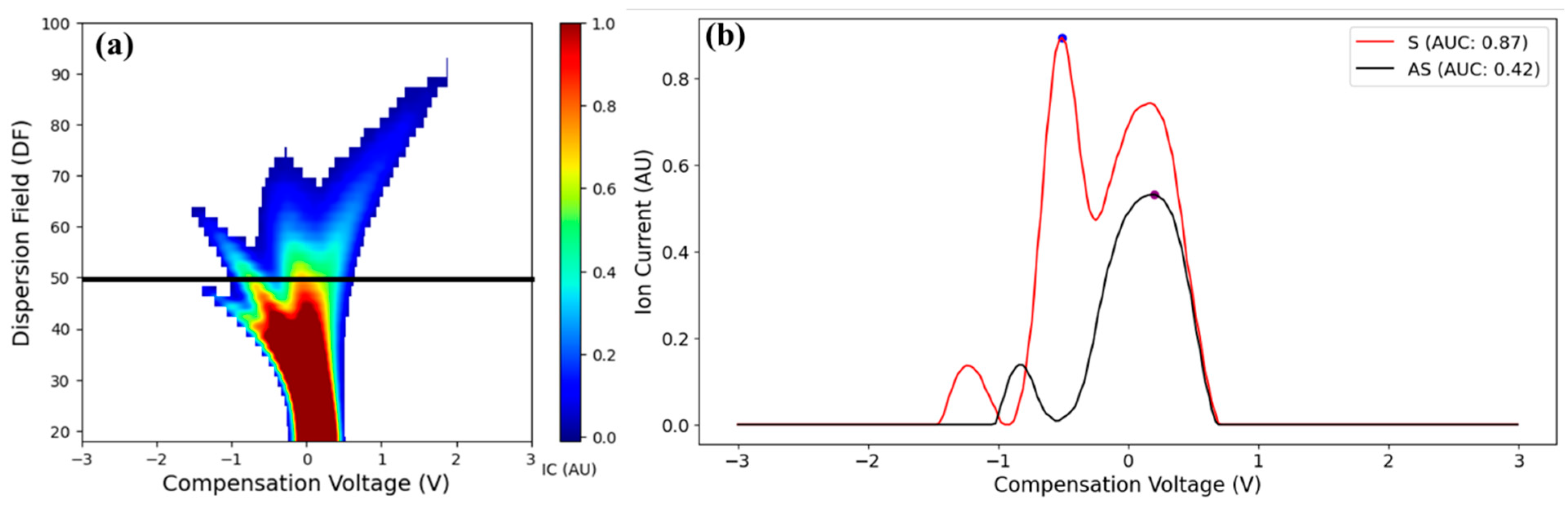
3.2. Feature Selection
3.3. Pattern Recognition
3.4. Spatial Distribution of VOCs
3.5. Validation with Other Host Species of LCD/X-Disease
4. Conclusions
- The FAIMS system detected differences in the VOC profiles between LCD/X-disease symptomatic and asymptomatic sweet cherry samples for ‘Benton’, ‘Cristalina’ and ‘Tieton’ cultivars. A distinct third ion current peak was identified as the potential signature feature potentially associated with the disease symptoms. Overall, symptomatic samples exhibited higher ion currents compared to the asymptomatic ones for features like Imax and IAUC across different DF intensities.
- PCA revealed clustering in the FAIMS data, suggesting potential differentiation between infection levels and sample types.
- VOC profiles varied across cultivars, possibly due to intrinsic biological differences and varying pathogen titer levels. Despite most cherry cultivars being heavily inbred, the cultivars examined here are not closely related, suggesting that these results may be broadly applicable to all cherry cultivars [49].
- The FAIMS spectra for LCD/X-disease infected samples were distinct from AS samples of other host Prunus species, such as peach and nectarines. This further confirms the diagnostic potential of the FAIMS system.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Long, L.E.; Núñez-Elisea, R.; Cahn, H. Evaluation of sweet cherry cultivars and advanced selections adapted to the pacific northwest usa. Acta Hortic. 2008, 795, 255–260. [Google Scholar] [CrossRef]
- Chavoshi, M.; Watkins, C.; Oraguizie, B.; Zhao, Y.; Iezzoni, A.; Oraguize, N. Phenotyping Protocol for Sweet Cherry (Prunus avium L.) to Facilitate an Understanding of Trait Inheritance. J. Am. Pomol. Soc. 2014, 68, 125–134. [Google Scholar]
- Wright, A.A.; Shires, M.K.; Beaver, C.; Bishop, G.; DuPont, S.T.; Naranjo, R.; Harper, S. Effect of ‘Candidatus Phytoplasma pruni’ Infection on Sweet Cherry Fruit. Phytopathology 2021, 111, 2195–2202. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.A.; Shires, M.; Molnar, C.; Bishop, G.; Johnson, A.; Frias, C.; Harper, S.J. Titer and Distribution of ‘Candidatus Phytoplasma pruni’ in Prunus avium. Phytopathology 2022, 112, 1406–1412. [Google Scholar] [CrossRef]
- Harper, S.J.; Northfield, T.D.; Nottingham, L.B.; DuPont, S.T.; Thompson, A.A.; Sallato, B.V.; Serban, C.F.; Shires, M.K.; Wright, A.A.; Catron, K.A.; et al. Recovery Plan for X-Disease in Stone Fruit Caused by ‘Candidatus Phytoplasma pruni’. Plant Health Prog. 2023, 24, 258–295. [Google Scholar] [CrossRef]
- Mekuria, T.A.; Zhang, S.; Eastwell, K.C. Rapid and sensitive detection of Little cherry virus 2 using isothermal reverse transcription-recombinase polymerase amplification. J. Virol. Methods 2014, 205, 24–30. [Google Scholar] [CrossRef]
- Molnar, C.; DuPont, T.; Thompson, A. Estimated Impact of X-disease and Little Cherry Disease in Washington and Oregon from 2015 to 2020. J. Ext. 2022, 60, 17. [Google Scholar] [CrossRef]
- Foster, W.R.; Lott, T.B. “Little Cherry,” A Virus Disease. Sci. Agric. 1947, 27, 1–6. [Google Scholar] [CrossRef]
- Galinato, S.P.; Gallardo, R.K.; Beers, E.H.; Bixby-Brosi, A.J. Developing a Management Strategy for Little Cherry Disease: The Case of Washington State. Plant Dis. 2019, 103, 2184–2190. [Google Scholar] [CrossRef]
- Eastwell, K.C.; Bernardy, M.G.; Li, T.S.C. Comparison between woody indexing and a rapid hybridisation assay for the diagnosis of little cherry disease in cherry trees. Ann. Appl. Biol. 1996, 128, 269–277. [Google Scholar] [CrossRef]
- Eastwell, K.C.; Bernardy, M.G. Partial Characterization of a Closterovirus Associated with Apple Mealybug-Transmitted Little Cherry Disease in North America. Phytopathology 2001, 91, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Theilmann, D.A.; Willis, L.G.; Bosch, B.-J.; Forsythe, I.J.; Li, Q. The Baculovirus Transcriptional Transactivator ie0 Produces Multiple Products by Internal Initiation of Translation. Virology 2001, 290, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Rott, M.E.; Jelkmann, W. Detection and Partial Characterization of a Second Closterovirus Associated with Little Cherry Disease, Little cherry virus-2. Phytopathology 2001, 91, 261–267. [Google Scholar] [CrossRef]
- Pourreza, A.; Lee, W.S.; Ehsani, R.; Schueller, J.K.; Raveh, E. An optimum method for real-time in-field detection of Huanglongbing disease using a vision sensor. Comput. Electron. Agric. 2015, 110, 221–232. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, H.; Ciechanowska, I.; Spaner, D. Application of infrared thermal imaging for the rapid diagnosis of crop disease. IFAC-Pap. 2018, 51, 424–430. [Google Scholar] [CrossRef]
- Mishra, P.; Polder, G.; Vilfan, N. Close Range Spectral Imaging for Disease Detection in Plants Using Autonomous Platforms: A Review on Recent Studies. Curr. Robot. Rep. 2020, 1, 43–48. [Google Scholar] [CrossRef]
- Tholl, D.; Boland, W.; Hansel, A.; Loreto, F.; Röse, U.S.R.; Schnitzler, J. Practical approaches to plant volatile analysis. Plant J. 2006, 45, 540–560. [Google Scholar] [CrossRef]
- Kafle, G.K.; Khot, L.R.; Sankaran, S.; Bahlol, H.Y.; Tufariello, J.A.; Hill, H.H. State of ion mobility spectrometry and applications in agriculture: A review. Eng. Agric. Environ. Food 2016, 9, 346–357. [Google Scholar] [CrossRef]
- Mayer, C.J.; Vilcinskas, A.; Gross, J. Phytopathogen Lures Its Insect Vector by Altering Host Plant Odor. J. Chem. Ecol. 2008, 34, 1045–1049. [Google Scholar] [CrossRef]
- Sugio, A.; Hogenhout, S.A. The genome biology of phytoplasma: Modulators of plants and insects. Curr. Opin. Microbiol. 2012, 15, 247–254. [Google Scholar] [CrossRef]
- Aksenov, A.A.; Novillo, A.V.G.; Sankaran, S.; Fung, A.G.; Pasamontes, A.; Martinelli, F.; Cheung, W.H.K.; Ehsani, R.; Dandekar, A.M.; Davis, C.E. Volatile Organic Compounds (VOCs) for Noninvasive Plant Diagnostics. In ACS Symposium Series; Beck, J.J., Coats, J.R., Duke, S.O., Koivunen, M.E., Eds.; American Chemical Society: Washington DC, USA, 2013; Volume 1141, pp. 73–95. [Google Scholar] [CrossRef]
- Aksenov, A.A.; Pasamontes, A.; Peirano, D.J.; Zhao, W.; Dandekar, A.M.; Fiehn, O.; Ehsani, R.; Davis, C.E. Detection of Huanglongbing Disease Using Differential Mobility Spectrometry. Anal. Chem. 2014, 86, 2481–2488. [Google Scholar] [CrossRef]
- Rid, M.; Mesca, C.; Ayasse, M.; Gross, J. Apple Proliferation Phytoplasma Influences the Pattern of Plant Volatiles Emitted Depending on Pathogen Virulence. Front. Ecol. Evol. 2016, 3, 152. [Google Scholar] [CrossRef]
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A review of advanced techniques for detecting plant diseases. Comput. Electron. Agric. 2010, 72, 1–13. [Google Scholar] [CrossRef]
- Li, Z.; Paul, R.; Ba Tis, T.; Saville, A.C.; Hansel, J.C.; Yu, T.; Ristaino, J.B.; Wei, Q. Non-invasive plant disease diagnostics enabled by smartphone-based fingerprinting of leaf volatiles. Nat. Plants 2019, 5, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D.; Hossain, O.; Weinhold, A.; Röse, U.S.R.; Wei, Q. Trends and applications in plant volatile sampling and analysis. Plant J. 2021, 106, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, F.; Scalenghe, R.; Davino, S.; Panno, S.; Scuderi, G.; Ruisi, P.; Villa, P.; Stroppiana, D.; Boschetti, M.; Goulart, L.R.; et al. Advanced methods of plant disease detection. A review. Agron. Sustain. Dev. 2015, 35, 1–25. [Google Scholar] [CrossRef]
- Gomez-Gonzalez, S.; Ruiz-Jimenez, J.; Priego-Capote, F.; Luque de Castro, M.D. Qualitative and quantitative sugar profiling in olive fruits, leaves, and stems by gas chromatography-tandem mass spectrometry (GC-MS/MS) after ultrasound-assisted leaching. J. Agric. Food Chem. 2010, 58, 12292–12299. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, Z.; Zeng, D.; Xie, C. Metal-oxide-semiconductor based gas sensors: Screening, preparation, and integration. Phys. Chem. Chem. Phys. 2017, 19, 6313–6329. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Cellini, A.; Biondi, E.; Blasioli, S.; Rocchi, L.; Farneti, B.; Braschi, I.; Savioli, S.; Rodriguez-Estrada, M.T.; Biasioli, F.; Spinelli, F. Early detection of bacterial diseases in apple plants by analysis of volatile organic compounds profiles and use of electronic nose: Early detection of bacterial diseases by volatile organic compounds profiling. Ann. Appl. Biol. 2016, 168, 409–420. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, N.; Zhou, D.; Sun, Y.; Sun, K.; Pan, L.; Tu, K. Discrimination and growth tracking of fungi contamination in peaches using electronic nose. Food Chem. 2018, 262, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Kothawade, G.S.; Chandel, A.K.; Khot, L.R.; Sankaran, S.; Bates, A.A.; Schroeder, B.K. Field asymmetric ion mobility spectrometry for pre-symptomatic rot detection in stored Ranger Russet and Russet Burbank potatoes. Postharvest Biol. Technol. 2021, 181, 111679. [Google Scholar] [CrossRef]
- Shvartsburg, A.A.; Li, F.; Tang, K.; Smith, R.D. High-Resolution Field Asymmetric Waveform Ion Mobility Spectrometry Using New Planar Geometry Analyzers. Anal. Chem. 2006, 78, 3706–3714. [Google Scholar] [CrossRef]
- Neal, M. Machine Learning Techniques for the Early Detection of Cancer Using Volatile Organic Compounds. Ph.D. Thesis, University of Warwick, Coventry, UK, 2019. Available online: https://wrap.warwick.ac.uk/137225/ (accessed on 24 February 2023).
- Sinha, R.; Khot, L.R.; Schroeder, B.K. FAIMS based sensing of Burkholderia cepacia caused sour skin in onions under bulk storage condition. J. Food Meas. Charact. 2017, 11, 1578–1585. [Google Scholar] [CrossRef]
- Sinha, R.; Khot, L.R.; Schroeder, B.K.; Si, Y. Rapid and non–destructive detection of Pectobacterium carotovorum causing soft rot in stored potatoes through volatile biomarkers sensing. Crop Prot. 2017, 93, 122–131. [Google Scholar] [CrossRef]
- Sinha, R.; Khot, L.R.; Schroeder, B.K.; Sankaran, S. FAIMS based volatile fingerprinting for real-time postharvest storage infections detection in stored potatoes and onions. Postharvest Biol. Technol. 2018, 135, 83–92. [Google Scholar] [CrossRef]
- Kothawade, G.S.; Sankaran, S.; Bates, A.A.; Schroeder, B.K.; Khot, L.R. Feasibility of Volatile Biomarker-Based Detection of Pythium Leak in Postharvest Stored Potato Tubers Using Field Asymmetric Ion Mobility Spectrometry. Sensors 2020, 20, 7350. [Google Scholar] [CrossRef] [PubMed]
- Lauri, P.E. Developments in high density cherries in france: Integration of tree architecture and manipulation. Acta Hortic. 2005, 667, 285–292. [Google Scholar] [CrossRef]
- Wright, A.A.; Shires, M.K.; Harper, S.J. Titer and distribution of little cherry virus 2 in Prunus avium. Arch. Virol. 2021, 166, 1415–1419. [Google Scholar] [CrossRef]
- Gallinger, J.; Zikeli, K.; Zimmermann, M.R.; Görg, L.M.; Mithöfer, A.; Reichelt, M.; Seemüller, E.; Gross, J.; Furch, A.C.U. Specialized 16SrX phytoplasmas induce diverse morphological and physiological changes in their respective fruit crops. PLoS Pathog. 2021, 17, e1009459. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; McFarlane, M.; Daulton, E.; Skinner, J.; O’Connell, N.; Wurie, S.; Chambers, S.; Nwokolo, C.; Bardhan, K.; Savage, R.; et al. Non-invasive exhaled volatile organic biomarker analysis to detect inflammatory bowel disease (IBD). Dig. Liver Dis. 2016, 48, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Vautz, W.; Franzke, J.; Zampolli, S.; Elmi, I.; Liedtke, S. On the potential of ion mobility spectrometry coupled to GC pre-separation—A tutorial. Anal. Chim. Acta 2018, 1024, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wei, H.-R.; Wang, J.-W.; Zong, X.-J.; Zhu, D.-Z.; Liu, Q.-Z. Phytoplasmas change the source–sink relationship of field-grown sweet cherry by disturbing leaf function. Physiol. Mol. Plant Pathol. 2015, 92, 22–27. [Google Scholar] [CrossRef]
- Falchi, R.; Bonghi, C.; Drincovich, M.F.; Famiani, F.; Lara, M.V.; Walker, R.P.; Vizzotto, G. Sugar metabolism in stone fruit: Source-sink relationships and environmental and agronomical effects. Front. Plant Sci. 2020, 11, 573982. [Google Scholar] [CrossRef]
- Hartikainen, K.; Nerg, A.-M.; Kivimäenpää, M.; Kontunen-Soppela, S.; Mäenpää, M.; Oksanen, E.; Rousi, M.; Holopainen, T. Emissions of volatile organic compounds and leaf structural characteristics of European aspen (Populus tremula) grown under elevated ozone and temperature. Tree Physiol. 2009, 29, 1163–1173. [Google Scholar] [CrossRef]
- Blande, J.D.; Holopainen, J.K.; Niinemets, Ü. Plant volatiles in polluted atmospheres: Stress responses and signal degradation. Plant Cell Environ. 2014, 37, 1892–1904. [Google Scholar] [CrossRef]
- Choi, C.; Kappel, F. Inbreeding, coancestry, and founding clones of sweet cherries from North America. J. Am. Soc. Hortic. Sci. 2004, 129, 535–543. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kothawade, G.S.; Khot, L.R.; Chandel, A.K.; Molnar, C.; Harper, S.J.; Wright, A.A. Feasibility of Little Cherry/X-Disease Detection in Prunus avium Using Field Asymmetric Ion Mobility Spectrometry. Sensors 2025, 25, 2034. https://doi.org/10.3390/s25072034
Kothawade GS, Khot LR, Chandel AK, Molnar C, Harper SJ, Wright AA. Feasibility of Little Cherry/X-Disease Detection in Prunus avium Using Field Asymmetric Ion Mobility Spectrometry. Sensors. 2025; 25(7):2034. https://doi.org/10.3390/s25072034
Chicago/Turabian StyleKothawade, Gajanan S., Lav R. Khot, Abhilash K. Chandel, Cody Molnar, Scott J. Harper, and Alice A. Wright. 2025. "Feasibility of Little Cherry/X-Disease Detection in Prunus avium Using Field Asymmetric Ion Mobility Spectrometry" Sensors 25, no. 7: 2034. https://doi.org/10.3390/s25072034
APA StyleKothawade, G. S., Khot, L. R., Chandel, A. K., Molnar, C., Harper, S. J., & Wright, A. A. (2025). Feasibility of Little Cherry/X-Disease Detection in Prunus avium Using Field Asymmetric Ion Mobility Spectrometry. Sensors, 25(7), 2034. https://doi.org/10.3390/s25072034









