1. Introduction
We obtain various types of information every day using the five senses and select appropriate actions in response. For example, we can simultaneously perceive multiple sensory inputs such as ‘video and sound’ or ‘color and scent’. We then integrate this information to select appropriate actions. While the five senses have been investigated in studies from various domains, the number of studies on multisensory integration, also referred to as crossmodal or multimodal integration, has increased in recent years [
1]. Both crossmodal and multimodal are terms related to multiple modalities, but they differ in meaning and usage. Crossmodal generally refers to situations where multisensory integration occurs through interactions between different modalities. Here, “interaction” means that two or more perceptual stimuli influence each other. Specifically, this includes cases where a stimulus in one modality affects the perception or cognition of another modality, as well as instances where such influences occur bidirectionally. On the other hand, multimodal refers to situations where multiple modalities coexist and are integrated without necessarily interacting with each other. In other words, the key difference between crossmodal and multimodal integration lies in whether the modalities influence one another during integration or remain independent. This study analyzes crossmodal perception, where interactions between different modalities influence perception.
Traditional crossmodal study has primarily focused on vision [
2]. However, in recent decades, crossmodal interactions involving other senses, such as olfaction, have also been discussed. Even within the scope of visual–olfactory crossmodal perception, numerous studies have been reported [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17]. Zellner et al. [
3] focused on the visual–olfactory crossmodal perception and investigated the effect of color on the perceived intensities of specific food scents. Their experiment involved participants evaluating the intensity of scents in a red liquid and a colorless liquid, each containing a strawberry scent. The results indicated a tendency for the colored liquid to be perceived as having a stronger scent than the colorless liquid. Gilbert et al. [
4] confirmed significant color associations for 20 different fragrances that remained stable, even when the experiment was repeated years later, suggesting a strong correlation between vision and olfaction. Miura et al. [
5] used color patches to investigate the effects of color–fragrance harmony on stress reduction. They found that harmonious color–fragrance combinations reduce stress more than disharmonious combinations. In addition, some studies have focused on the speeds of processing and discriminating scent. Demattè et al. [
6] presented participants with scents and color patches and instructed them to discriminate each stimulus as quickly and accurately as possible. The stimuli were presented under either a compatible condition (e.g., a strawberry scent with pink or a spearmint scent with turquoise) or an incompatible condition (e.g., a strawberry scent with turquoise or a spearmint scent with pink). Reaction times and identification accuracy were measured. The results showed that the more the color and scent were matched, the faster and more accurate the responses became. In addition, Demattè et al. [
7] showed that when the color and shape are congruent with a scent, the accuracy of scent discrimination increases, whereas incongruence leads to a decrease in accuracy. Various studies have demonstrated the existence of crossmodal associations between vision and olfaction.
While previous studies have relied primarily on subjective evaluations, recent studies have increasingly focused on objective analyses using physiological responses [
8,
9,
10,
11,
12,
13,
14,
15,
16,
17]. Österbauer et al. [
8] investigated the neurophysiological correlates of visual–olfactory crossmodal perception using functional magnetic resonance imaging (fMRI). Their experiment showed that the orbitofrontal cortex (OFC) and the caudal region of the insular cortex exhibited greater activation as the congruence between scent and color pairs increased. Furthermore, numerous studies have measured physiological responses using near-infrared spectroscopy (NIRS), which has a higher temporal resolution than fMRI [
9,
10,
11,
12,
13,
14,
15,
16,
17]. Compared to existing neuroimaging techniques, NIRS is relatively inexpensive, easy to set up, and does not require a specialized environment. Its high motion tolerance allows studies to be conducted in natural settings. Pinti et al. [
9] stated that NIRS allows the study of visual–olfactory crossmodal perception in natural environments, generating more reliable and ecologically valid data. NIRS measures brain activity at a maximum depth approximately halfway between the light source and detector [
10]. The maximum distance between the light source and detector to maintain detectable signals was 3 cm, resulting in a measurement depth of approximately 1.5 cm from the scalp surface. Therefore, it is challenging to use NIRS to measure the deep brain regions associated with olfactory perception, such as the piriform cortex, insular cortex, and hippocampus [
11]. However, among the regions associated with olfactory and visual perception, there are relatively shallow areas, such as the OFC, which have been investigated by recent studies using NIRS [
12,
13].
The connection between colored light and olfactory sensing is an emerging topic with important implications for sensor-based research and applications. Our study contributes to this area by demonstrating how NIRS can be utilized to detect the neural responses associated with multisensory integration. Understanding these interactions is crucial for the following reasons:
Investigating how different sensory modalities interact provides essential insights for developing advanced sensor systems. For example, our findings could inform the design of multimodal sensing technologies used in healthcare, smart environments, and human-computer interaction.
- 2.
Enhancing Sensor-Based Brain Activity Monitoring
By applying NIRS to study multisensory interactions, our work contributes to refining non-invasive brain sensing methodologies. These insights could support further developments in real-time brain monitoring, particularly in areas like neurofeedback, assistive technologies, and affective computing.
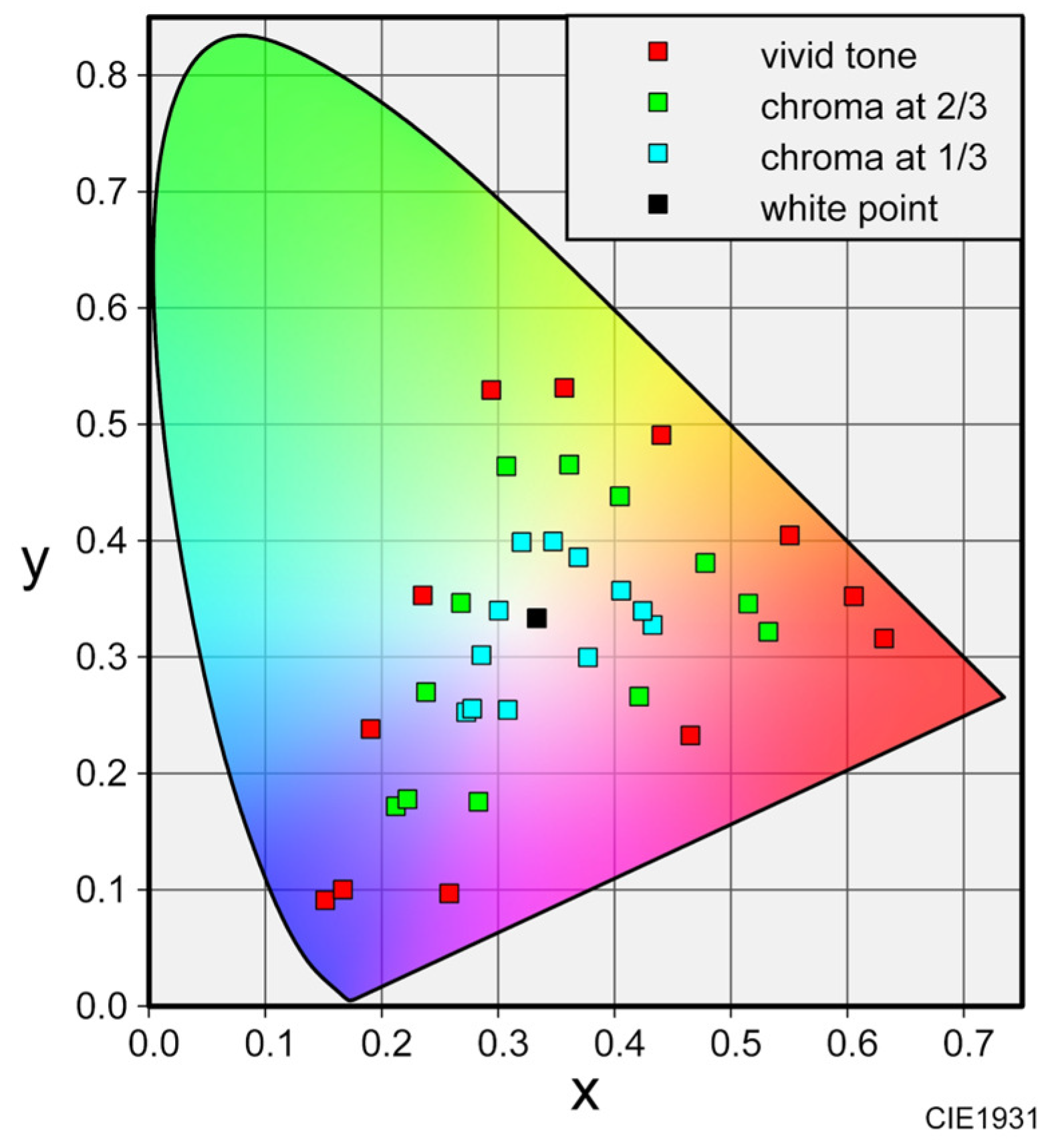
In previous studies on visual–olfactory crossmodal perception, various types of colors have been used as visual stimuli, such as colored liquids [
3,
14], color patches [
4,
5,
6,
7], and monitor displays [
16], all of which are small-scale visual stimuli of a few centimeters by a few centimeters, focusing on flat visual stimuli. However, since we live in environments with ambient lighting daily, spatial testing is important. Yamashita et al. [
16] and Oba et al. [
17] used physiological indicators to analyze the color–fragrance harmony in lighting environments. Colored light was used as the color stimulus for spatial examination, and an objective analysis was performed by measuring the OFC response to the stimuli. Yamashita et al. [
16] initially conducted a preliminary survey using five fragrances and 36 colored lights to select a ‘pleasant fragrance’ and ‘unpleasant fragrance’, as well as ‘harmonious colors’ and ‘disharmonious colors’ for each participant. The OFC response was measured under the four following conditions: (1) fragrance-only, (2) colored light-only, (3) harmonious crossmodal, and (4) disharmonious crossmodal presentations. The results showed that the highest OFC activation occurred during the fragrance-only presentation, with the left OFC showing greater activation than the right OFC. This suggested a relationship between pleasant emotions and the left OFC. Furthermore, Oba et al. [
17] analyzed whether differences in the lighting environment affected OFC activation and whether the left and right OFC activated differently based on pleasure or displeasure. They found that the OFC was more activated in a relaxing lighting environment closer to real-life conditions. Moreover, pleasant emotions were associated with greater activation of the left OFC, which supported the results of Yamashita et al. [
16]. However, these studies used a limited number of fragrances as stimuli, and these were generally perceived as pleasant by the participants. Therefore, the analysis focused on pleasant emotions related to the left OFC, making it challenging to analyze unpleasant emotions. This study aimed to analyze the pleasure–displeasure emotions evoked by color–fragrance harmony while also considering unpleasant emotions based on physiological indicators and focusing on the lateralization of brain function.
Our work contributes to the sensing field in two key ways:
We demonstrated the effectiveness of NIRS as a non-invasive tool to measure cortical activity related to the interaction between light and olfactory stimuli. This application showcases the potential of NIRS in advancing real-time sensory monitoring, expanding its use beyond traditional domains.
- 2.
Novelty in Sensing Approach
Although we did not develop a new sensor, our methodological approach integrates NIRS into a novel approach to explore sensory processing. Specifically, we applied NIRS to capture the subtle hemodynamic responses associated with crossmodal interactions, which could inspire further advancements in sensor-based neuroscience research.
3. Experiment II: Analysis of Functional Differences Between the Left and Right Orbitofrontal Cortex
In Experiment I, we conducted the study based on previous findings [
20,
21,
22] suggesting that the left OFC is associated with pleasant emotions, while the right OFC is associated with unpleasant emotions. Our results indicated that in the crossmodal presentation of a pleasant fragrance and its harmonious color, activation of the left OFC enhanced pleasant emotions. Conversely, in the crossmodal presentation of an unpleasant fragrance and its disharmonious color, activation of the right OFC may have enhanced unpleasant emotions. A recent crossmodal study [
23] has presented differing views on the functional differences between the left and right OFC in relation to pleasure and displeasure. Specifically, the left OFC is activated when the interaction between colored light and fragrance becomes more harmonious, promoting a crossmodal interaction and becoming dominant in crossmodal perception. In contrast, the right OFC shows a smaller effect from the interaction and is activated when the stimuli are perceived independently, in a more multimodal perception, highlighting this functional difference. Furthermore, studies have shown that even without directly smelling a fragrance, imagining the fragrance or recalling olfactory experiences can activate sensory regions related to olfactory perception, with stronger activation observed in the left OFC [
26,
27]. Additionally, Broman et al. [
28] showed that the right hemisphere is involved in low-level olfactory processing and encoding based on perception, whereas the left hemisphere is associated with higher-order olfactory recognition and semantic interpretation, such as memory and the labeling of fragrances. These findings suggest that the functional differences between the left and right OFC may not be solely determined by ‘pleasure–displeasure’. Instead, another factor may be the extent to which crossmodal perception is enhanced by harmony (higher-order modality-integrated perception), as opposed to stimuli being merely perceived independently due to disharmony (lower-order modality-integrated perception or multimodal perception). In Experiment I, participants were not informed of the names of the fragrances during the stimulus selection phase. However, it has been reported that pleasant fragrances tend to be easier to associate with their corresponding objects, whereas unpleasant fragrances are more difficult to imagine. Given the findings [
23,
26,
27,
28], it is possible that this difference in imagery influenced the crossmodal perception of colored light and fragrance. Therefore, in
Section 3, we conduct a new NIRS experiment using fragrances that are familiar and easy to imagine for all participants, ensuring that both pleasant and unpleasant fragrances are equally accessible in terms of mental imagery. By analyzing the results, we aim to analyze whether the functional differences between the left and right OFC are influenced not only by the ‘pleasure–displeasure axis’ but also by the ‘crossmodal perception–multimodal perception axis’.
3.1. Selection of Experimental Stimuli and Experimental Procedure
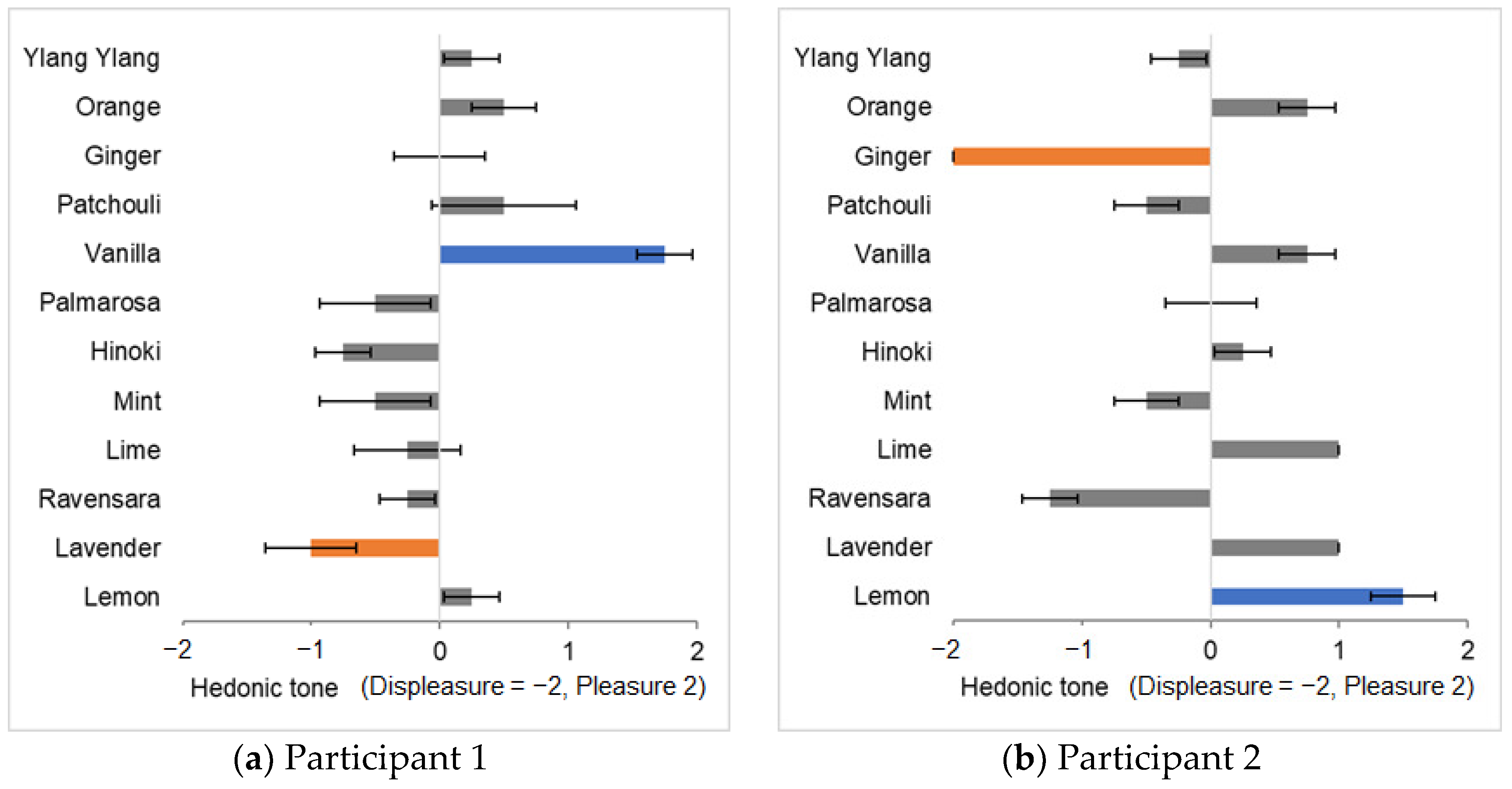
We selected the same stimuli as in Experiment I. The same participants (age 23 ± 1 year) participated in this experiment. We selected familiar fragrances from the 12 essential oils used in Experiment I. Eight fragrances were selected: orange, ginger, vanilla, hinoki, mint, lime, lavender, and lemon. The ‘most pleasant’ and ‘most unpleasant’ fragrances were selected from this list following the same procedure as in
Section 2.3. Scent selection data for all participants are shown in
Figure S2 in the Supplemental Materials. This survey revealed that knowledge of the name of the fragrance affected the subjective hedonic tone judgment of many participants. This finding indicates that the imagery evoked by the names of fragrances significantly influences the pleasant–unpleasant evaluation of the fragrance. Harmonious and disharmonious colors were identified for the selected fragrances. As in Experiment I, the participants were informed of the definition of harmony prior to performing the evaluation experiment using the same experimental conditions as described in
Section 2.5. However, because they were aware of the names of the fragrances, we inferred that the participants evaluated harmony based on the semantic congruency between the fragrance and colored light.
3.2. Results and Discussion
3.2.1. Difference in Peak Values of oxyHb Concentration in the Left and Right Orbitofrontal Cortex
NIRS was used to measure OFC activation in response to the four types of presentations. The oxyHb values for each experiment are shown in
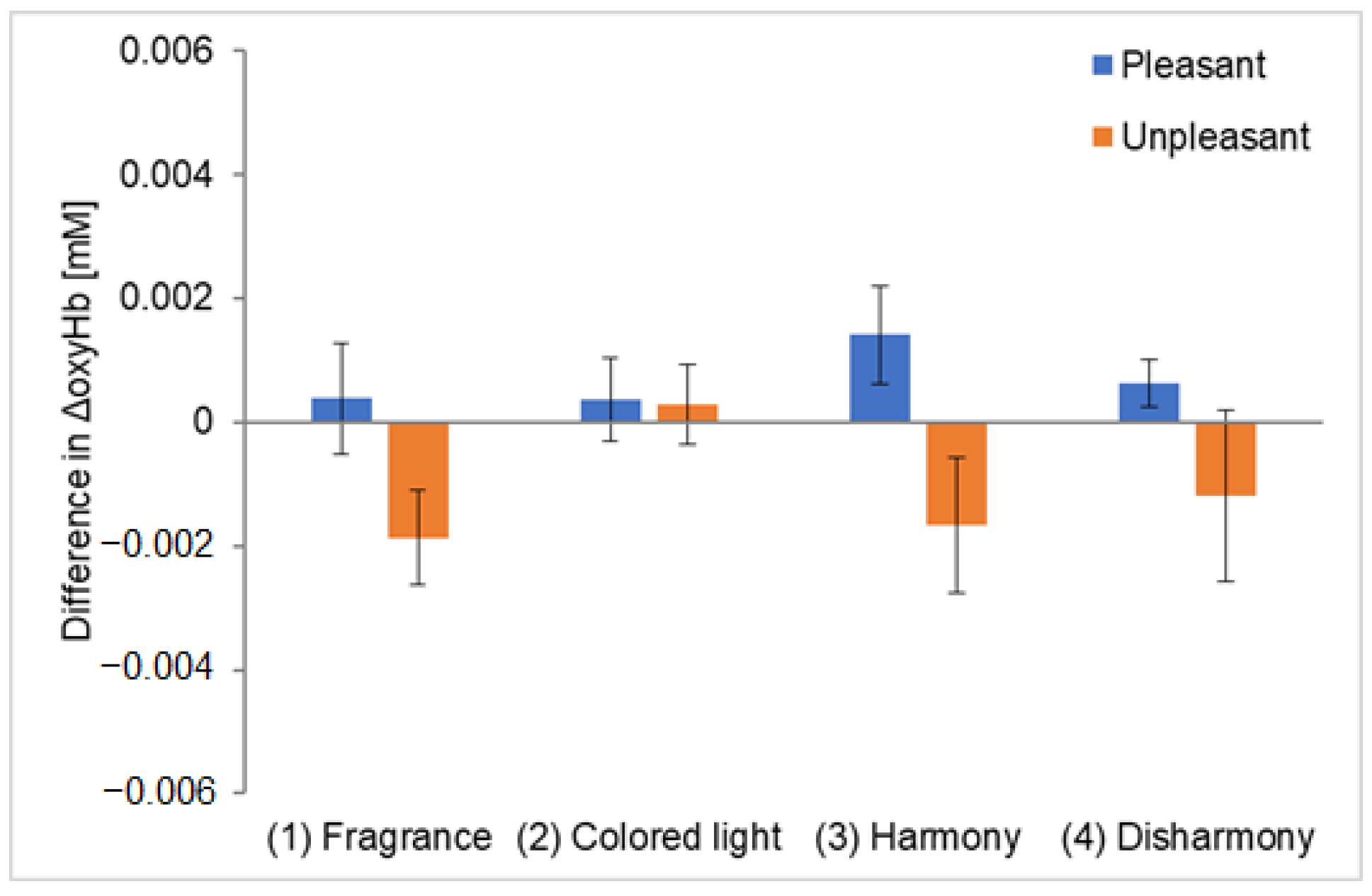
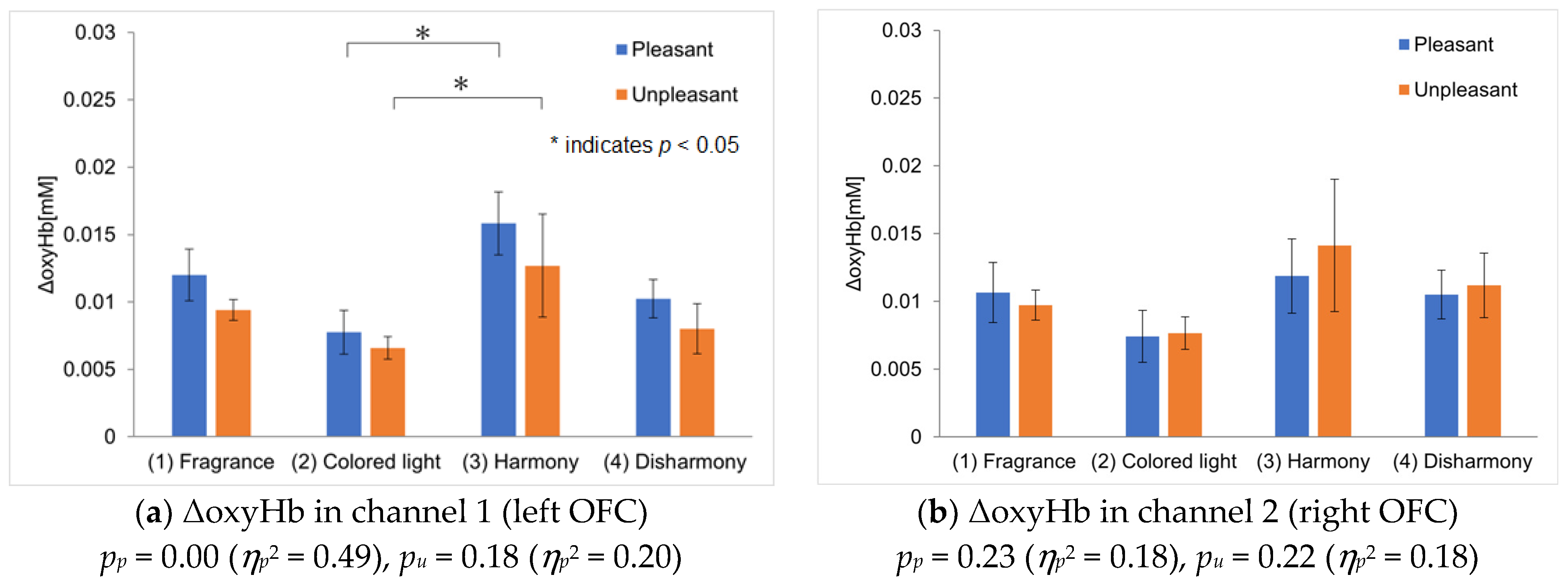
Figure 6; the vertical axis represents the average ΔoxyHb across all participants, while the error bars indicate the standard error of the mean ΔoxyHb of the participants. The ΔoxyHb for the colored light-only presentation is the average value obtained from responses to harmonious and disharmonious colors. Five outliers were removed using the Smirnov–Grubbs test at a 5% significance level, accounting for approximately 5% of the total data.
Figure 6a and
Figure 6b show the analysis results for channel 1 and channel 2, corresponding to the left and right OFC, respectively. The ΔoxyHb measured on channel 1 was the highest during the crossmodal presentations of pleasant fragrances with harmonious colors, similar to the results of Experiment I (
Figure 6a). However, ΔoxyHb was also high during the crossmodal presentations of unpleasant fragrances with harmonious colors, a result different from that seen in Experiment I. This suggests that the participants might have imagined harmony more effectively for unpleasant fragrances in Experiment II, leading to dominant crossmodal perception and activation in the left OFC. Statistically significant differences were observed between the colored light-only and harmonious crossmodal presentations for both pleasant and unpleasant fragrances. For channel 2, while ΔoxyHb was slightly higher during the harmonious crossmodal presentation with pleasant fragrances, the difference was not significant (
Figure 6b). For unpleasant fragrances, ΔoxyHb peaked during a harmonious crossmodal presentation; however, in contrast to
Figure 6a, the error was large, and no statistically significant differences were observed between conditions. We have calculated a repeated-measures ANOVA and added values to the captions of
Figure 6a,b.
3.2.2. Differences in Difference in Peak Values of oxyHb Concentration Between the Left and Right Orbitofrontal Cortex
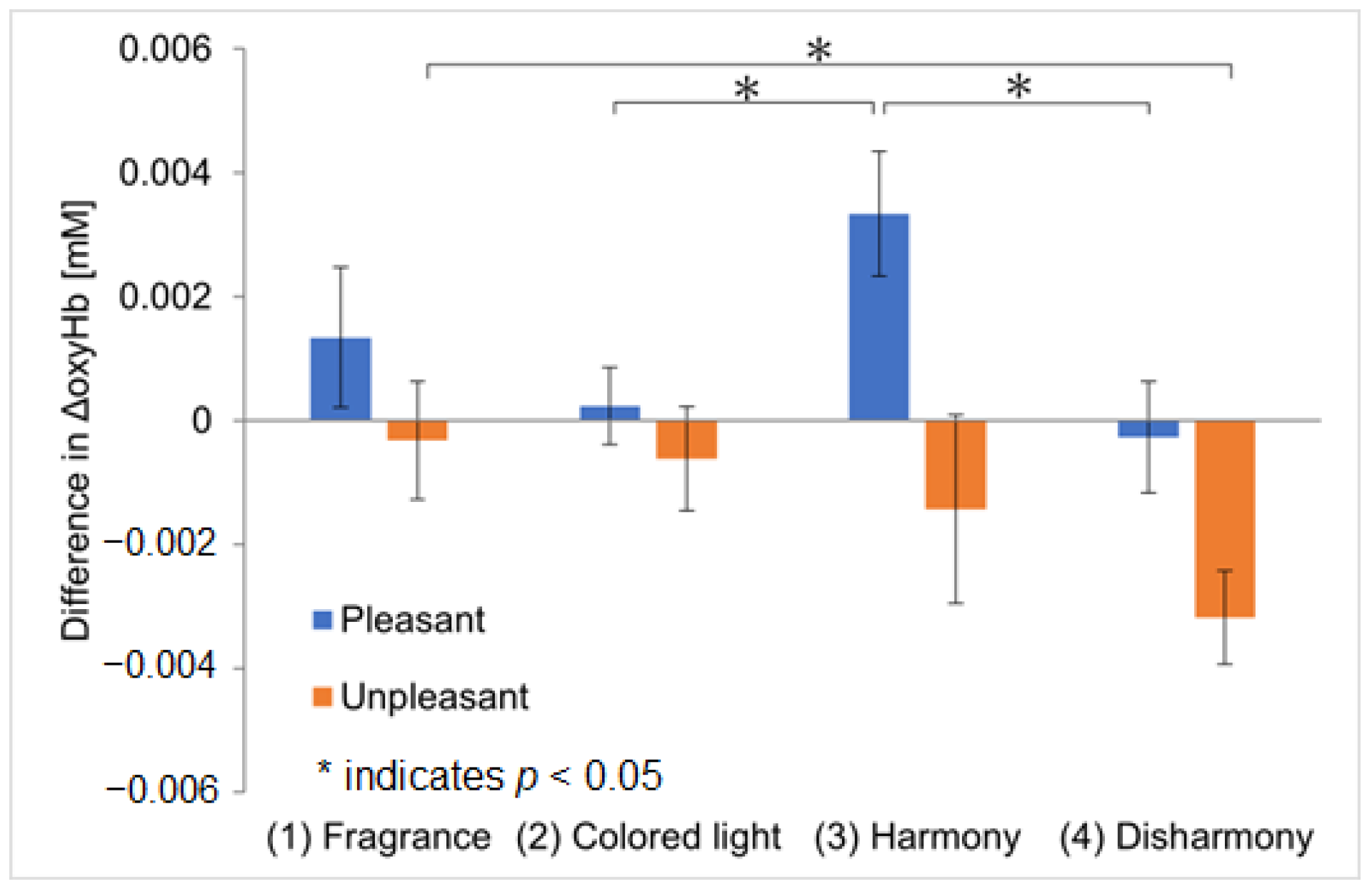
Figure 7 shows the results of the analysis of the ΔoxyHb differences between the left and right OFC, the vertical axis representing the difference in ΔoxyHb between channel 1 and channel 2, calculated using Equation (1). The error bars represent the standard error of the mean ΔoxyHb difference among the participants. The outliers described in
Section 3.2.1 were excluded from this analysis as well. For pleasant fragrances, the largest channel difference was observed during the crossmodal presentations with harmonious colors, confirming greater activation in the left OFC. This suggests that the greatest sense of pleasure was evoked during the crossmodal presentation with harmonious colors, yielding results similar to those of Experiment I. However, in contrast to Experiment I, significant differences were found between the ‘colored light-only and harmonious crossmodal’ and ‘harmonious and disharmonious crossmodal’ presentations, suggesting that knowing the names of the fragrances may enhance pleasure or create dominant crossmodal effects. For unpleasant fragrances, the difference in responses increased during the disharmonious crossmodal presentations, with greater activation in the right OFC. This result differs from that of Experiment I, indicating that the participants might have perceived unpleasant fragrances and disharmonious colored light as individual stimuli, leading to the activation of the right OFC. Therefore, Experiment II suggests that the left and right OFC were influenced not only by a ‘pleasure–displeasure axis’ but also by a ‘crossmodal perception–multimodal perception axis’.
4. Additional Experiment: Correlation Between Hedonic Tone and Degree of Harmony in Subjective Evaluation
The results of Experiment I suggest that when a pleasant fragrance and its harmonious color are crossmodally perceived, the left OFC is activated, potentially enhancing pleasant emotions. In contrast, when an unpleasant fragrance and its disharmonious color are crossmodally perceived, the right OFC is activated, potentially enhancing unpleasant emotions. In our experiments in the previous sections, NIRS was used to objectively analyze the recall of pleasant and unpleasant emotions while considering the functional differences of the left and right OFC, as reported in the literature [
20,
21,
22]. However, it was not possible to verify whether the participants judged pleasure or displeasure subjectively. Therefore, as an additional experiment, the relationship between the subjective hedonic tone and the degree of harmony in the crossmodal perception of colored light and fragrance was investigated.
First, an experiment was conducted where the subjective hedonic tone of the crossmodal perception of two types of pleasant and unpleasant fragrances selected in 3.2 and 36 lighting colors generated in 3.1 was evaluated. There were six of the eight participants who participated in Experiment I (age 23 ± 1 year). The participants simultaneously perceived the colored light and fragrance and rated the hedonic tone of the experience on an 11-point scale. The experimental environment was the same as in
Section 2.4. Using the evaluation results of hedonic tone and the degree of harmony evaluation results from
Section 2.3, a scatter plot showing their correlation is shown in
Figure 8a shows the results for the pleasant fragrance, and (b) shows the results for the unpleasant fragrance. The vertical axis represents the degree of harmony, and the horizontal axis represents the hedonic tone. Additionally, the closer the score is to ‘5’, the more harmonious or pleasant it is, while the closer it is to ‘−5’, the more disharmonious or unpleasant it is. The correlation coefficients for each are noted in the lower right of the scatter plots. The density of points due to overlapping is shown using a heatmap based on Kernel Density Estimation.
In the case of the pleasant fragrance shown in
Figure 8a, a high positive correlation of 0.71 was confirmed between the hedonic tone and harmony in the crossmodal perception of colored light and fragrance. This indicates that for the pleasant fragrance, colored lights with higher harmony were perceived as more pleasant, while those with lower harmony suppressed or induced displeasure. This result supports the findings of Experiment I, where the left OFC was activated when a pleasant fragrance and its harmonious color were crossmodally perceived, enhancing pleasant emotions. In contrast, for the unpleasant fragrance shown in
Figure 8b, the correlation coefficient was 0.41, which is lower than for the pleasant fragrance but still indicates a positive correlation. This suggests that for the unpleasant fragrance, colored lights with lower harmony were perceived as more unpleasant, while those with higher harmony suppressed or induced pleasure. This result also supports the findings of Experiment I, where the right OFC was activated when an unpleasant fragrance and its disharmonious color were crossmodally perceived, enhancing unpleasant emotions. The slightly lower correlation for the unpleasant fragrance can be attributed to the difference in how one participant perceived harmony, as mentioned in
Section 2.6.2. This participant perceived the combination of the unpleasant fragrance and its harmonious color as more unpleasant, which may have influenced the negative correlation. When excluding this participant, the correlation coefficient was 0.55.
In Experiment II, it was suggested that the functional differences between the left and right OFC are also influenced by the ‘crossmodal perception–multimodal perception axis’. Focusing on the degree of harmony between the colored light and fragrance, it was found that when the degree of harmony is high, semantic crossmodal perception becomes dominant, resulting in higher-order modality-integrated perception. In contrast, when the degree of harmony is low, the stimuli are not semantically linked and are processed merely as independent stimuli, resulting in lower-order modality-integrated perception. Based on this, it can be inferred that for the pleasant fragrance, higher harmony in the colored light enhances pleasant emotions, indicating that crossmodal perception leads to pleasure. Conversely, for the unpleasant fragrance, lower harmony in the colored light enhances unpleasant emotions, suggesting that multimodal perception leads to displeasure. Therefore, it can be concluded that the ‘pleasant–unpleasant axis’ and the ‘crossmodal perception–multimodal perception axis’ are interrelated in the functional differences between the left and right OFC.
The difference between crossmodal and multimodal perception can be viewed neuroscientifically as a difference in the following actions [
29].
Crossmodal perception involves interactions between different sensory modalities, wherein information from one sense influences the perception of another. This process may engage multiple brain regions, such as:
The orbitofrontal cortex and insula, which integrate sensory inputs to form coherent perceptual experiences.
The superior temporal sulcus, which plays a key role in integrating audiovisual stimuli.
The intraparietal sulcus, which coordinates attentional resources across the senses.
The piriform cortex and visual association area information are linked, and the sense of smell is enhanced by vision. Crossmodal processing might be characterized by dynamic interactions between these regions and often requires more extensive neural integration than unimodal processing.
By contrast, multimodal perception refers to the parallel processing of multiple sensory inputs without necessarily integrating them into a unified experience. This process often relies more on independent sensory-specific brain regions. Primary sensory cortices (e.g., the visual, entorhinal, and piriform cortices) process different sensory inputs separately. Higher-order association areas may still interact, but the level of integration is lower than that of crossmodal perception.
5. Conclusions
This study focused on the lateralization of brain function in the left and right OFC by analyzing the pleasure–displeasure evoked by harmony between colored light and fragrance. Two experiments were conducted, each following the processes of stimulus generation, selection, and NIRS measurement during stimulus presentation. In Experiment I, 36 colored lights and 12 fragrances were used as stimuli. A selection survey was used to have participants rate the hedonic tones of 12 fragrances without knowing their names—of which the most pleasant and unpleasant fragrances were selected. The participants then rated the degree of harmony between the selected fragrances and colored lights, leading to the selection of ‘harmonious’ and ‘disharmonious’ color–fragrance combinations. Each participant was presented with two different fragrances and four colored lights as the experimental stimuli. Based on these selected stimuli, NIRS measurements of OFC activity were performed under the four following presentation conditions: fragrance-only, colored light-only, harmonious crossmodal, and disharmonious crossmodal presentations. The NIRS device measured changes in the oxyHb concentrations in the left and right OFC. Moving averages were applied to the measured data, and the difference in oxyHb concentration (ΔoxyHb) before and after stimulus presentation was calculated as a response to each stimulus. We found that the ΔoxyHb associated with channel 1 was the largest during the crossmodal presentation with harmonious colors. This suggests that the participants felt more pleasant when they perceived the harmonious color simultaneously with the pleasant fragrance rather than when only smelling the pleasant fragrance. The ΔoxyHb measured on channel 2 was the largest during the crossmodal presentation with disharmonious colors. This suggests that participants felt more displeasure when they perceived the disharmonious color simultaneously with the unpleasant fragrance rather than when they only smelled the unpleasant fragrance. In Experiment II, eight familiar fragrances were selected from the original list of twelve fragrances, and the same procedures as in Experiment I were followed to select the ‘most pleasant’ and ‘most unpleasant’ fragrances, as well as the ‘harmonious’ and ‘disharmonious’ colors associated with them. The NIRS measurements in Experiment II showed that ΔoxyHb was highest in channel 1 for the pleasant and unpleasant fragrances in the harmonious crossmodal presentation, suggesting that the left OFC is more active when crossmodal effects are dominant. An additional experiment showed a positive correlation between hedonic tones and the degree of harmony, indicating that harmonious colors evoke pleasure, whereas disharmonious colors evoke displeasure. This suggests that the lateralization of brain function in the left and right OFC arises from the interaction between the ‘pleasure–displeasure axis’ and ‘crossmodal perception–multimodal perception axis’.
Although our work is rooted in experimental psychophysics, its significance extends to the field of sensing technology, particularly to the application and optimization of NIRS for studying multisensory interactions. To better highlight this aspect, we have refined our discussion to emphasize the following points:
Our study employed NIRS in a novel way to examine the psychophysical correlates of crossmodal interactions between light and olfactory stimuli. Unlike conventional applications of NIRS, we specifically investigated its use for detecting subtle hemodynamic responses associated with sensory integration.
- 2.
Contribution to Sensor-Based Research
Although we did not design a new sensor, our approach introduces an innovative application of existing sensing technology to a relatively unexplored research domain. By demonstrating how NIRS can be effectively utilized in multisensory studies, our study lays the groundwork for further developments in sensor-based neuroscience and biomedical engineering.
Future studies should explore different methods for analyzing OFC responses. In this study, moving averages were applied to the measurement data, and oxyHb was used as the response to each stimulus. Further analyses that focus on deoxygenated hemoglobin using GLM-based analyses or a combination of these methods may allow for a more objective analysis of the physiological responses. Furthermore, the fragrances used in this study were essential oils, which may have limited the range of displeasure tested in this analysis. Using more intense and unpleasant fragrances could potentially reveal a more distinct lateralization of brain function in the OFC. Moreover, as mentioned in
Section 2.3, differences in the color presentation method may influence the harmony perceived by participants; this requires experimental demonstration. While the sample size of eight participants is relatively small, it is required for larger follow-up studies to validate our results. Although gender differences were not a primary focus of this study, we recognize their potential influence on sensory perception and neural responses. Also, future research should incorporate a more diverse sample to examine potential gender-related variations. A recent study has proposed a method for understanding odor descriptions in multimodal contexts, where odor information is communicated indirectly through text and images [
30]. An analysis of the relevance of those multimodal studies is also an issue.