Highly Selective Room-Temperature Blue LED-Enhanced NO2 Gas Sensors Based on ZnO-MoS2-TiO2 Heterostructures
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of ZnO-MoS2-TiO2 Heterostructures
2.2. Basic Material Characterizations
2.3. Gas Sensing Measurements
3. Results and Discussion
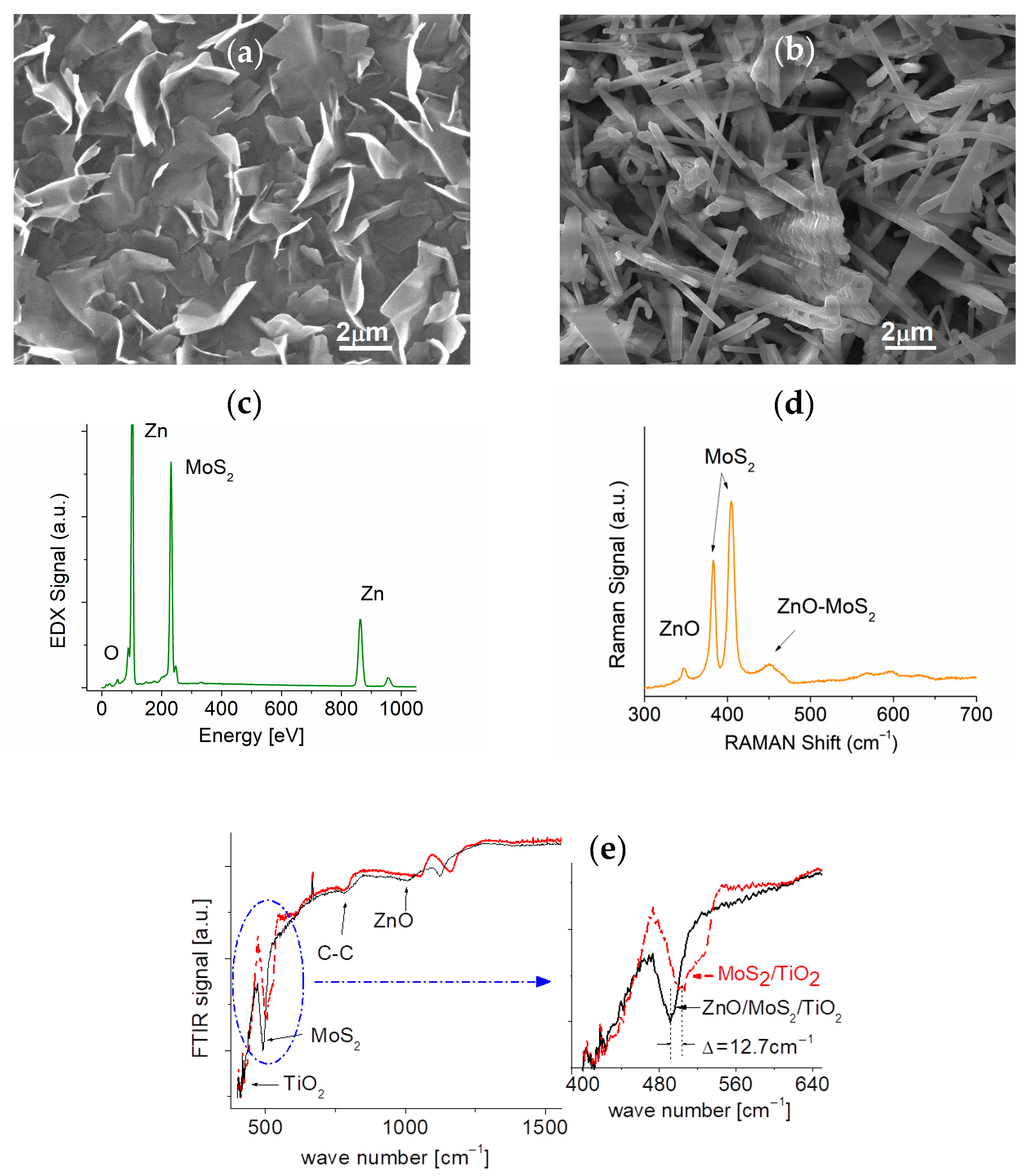
3.1. Morphologies of Surfaces of Binary and Ternary Samples
3.2. Composition and EDX Measurements
3.3. Raman and FTIR Measurements
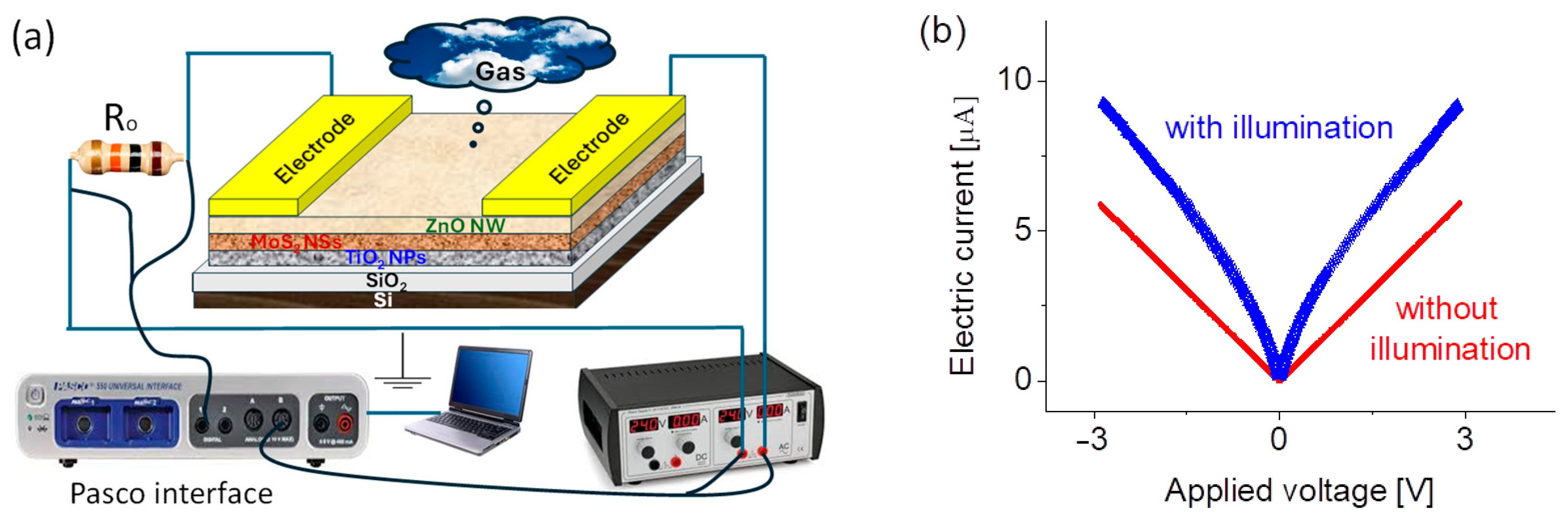
3.4. Fabrication of the Prototype and Its Basic Electrical Current–Voltage Properties
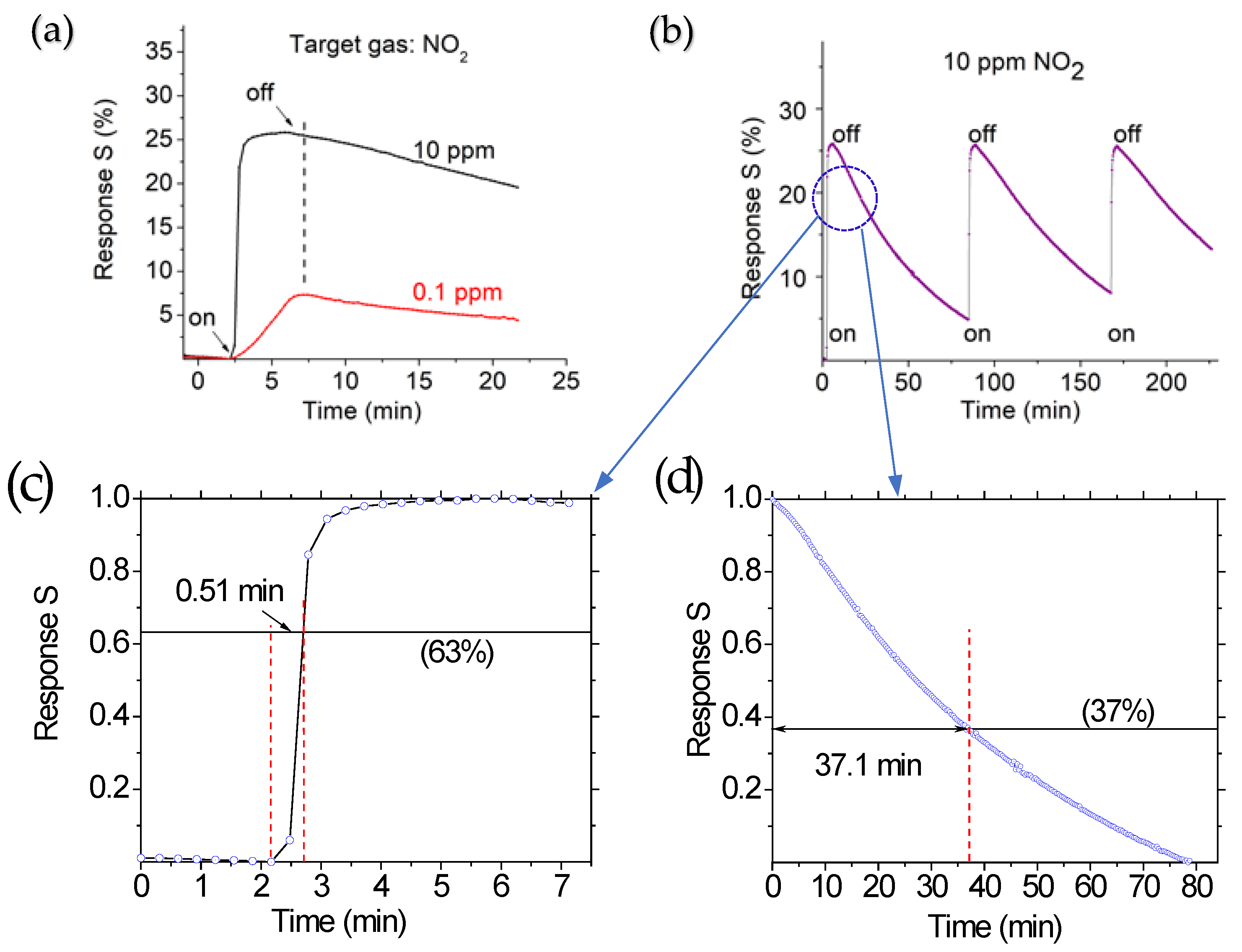
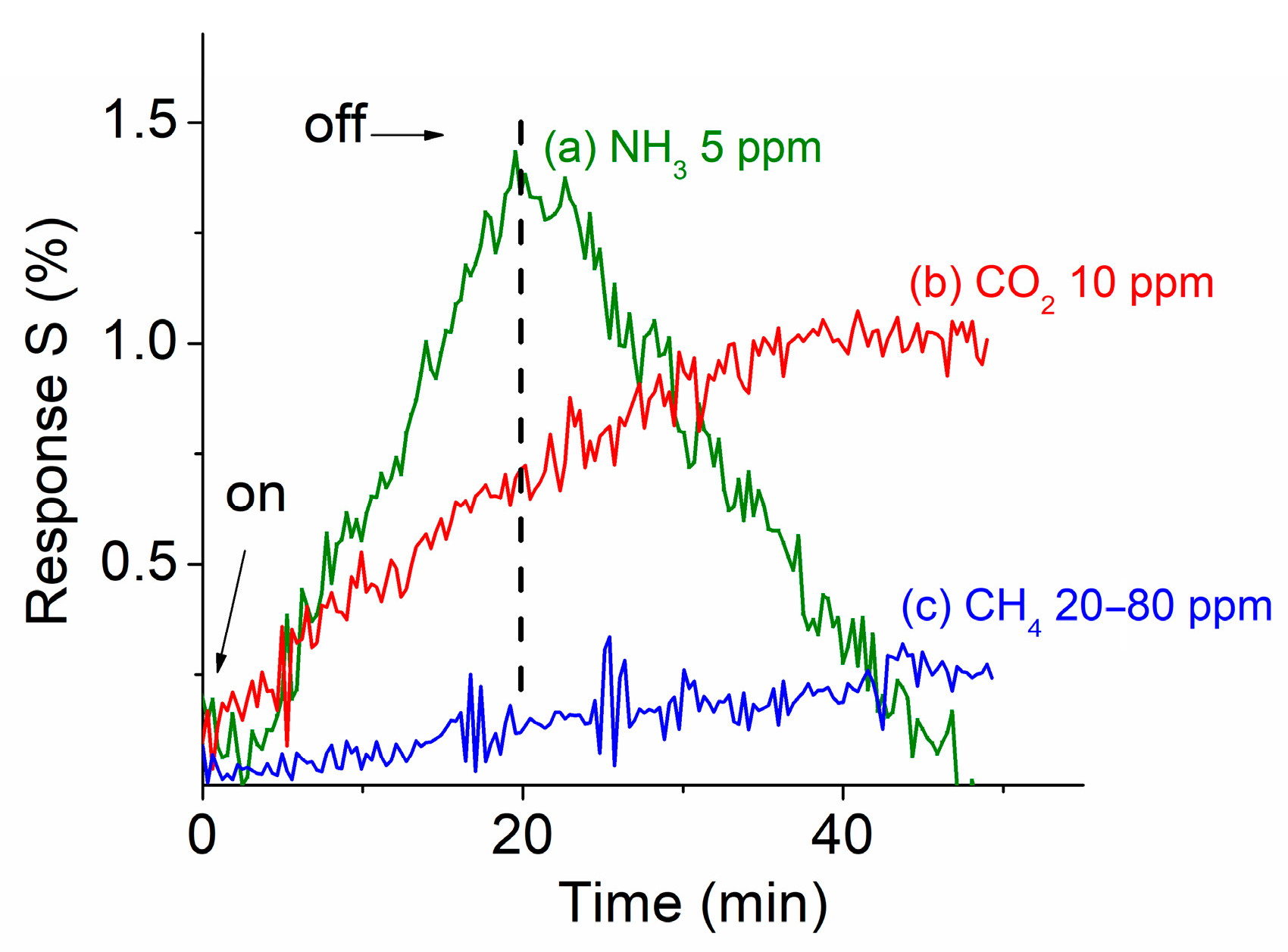
3.5. Sensor Performance
3.6. Sensing Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zong, B.; Wu, S.; Yang, Y.; Li, Q.; Tao, T.; Mao, S. Smart Gas Sensors: Recent Developments and Future Prospective. Nano-Micro Lett. 2025, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hou, J.; Hilal, M.; Kim, H.; Chen, Z.; Cui, Y.; Kim, J.-H.; Cai, Z. Development of High-Performance Ethanol Gas Sensors Based on La2O3 Nanoparticles-Embedded Porous SnO2 Nanofibers. Sensors 2024, 24, 6839. [Google Scholar] [CrossRef]
- Panda, S.; Mehlawat, S.; Dhariwal, N.; Kumar, A.; Sanger, A. Comprehensive review on gas sensors: Unveiling recent developments and addressing challenges. Mater. Sci. Eng. B 2024, 308, 117616. [Google Scholar] [CrossRef]
- Wu, Y.; Lei, M.; Xia, X. Research Progress of MEMS Gas Sensors: A Comprehensive Review of Sensing Materials. Sensors 2024, 24, 8125. [Google Scholar] [CrossRef]
- Ko, J.-K.; Park, I.-H.; Hong, K.; Kwon, K.C. Recent Advances in Chemoresistive Gas Sensors Using Two-Dimensional Materials. Nanomaterials 2024, 14, 1397. [Google Scholar] [CrossRef] [PubMed]
- Akbari, E.; Jahanbin, K.; Afroozeh, A.; Yupapin, P.; Buntat, Z. Brief review of monolayer molybdenum disulfide application in gas sensors. Physical B 2018, 545, 510–518. [Google Scholar] [CrossRef]
- Shokri, A.; Salami, N. Gas sensor based on MoS2 monolayer. Sens. Actuators B Chem. 2016, 236, 378–385. [Google Scholar] [CrossRef]
- Kočí, M.; Izsák, T.; Vanko, G.; Sojková, M.; Hrdá, J.; Szabó, O.; Husák, M.; Végsö, K.; Varga, M.; Kromka, A. Improved Gas Sensing Capabilities of MoS2/Diamond Heterostructures at Room Temperature. ACS Appl. Mater. Interfaces 2023, 15, 34206–34214. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Joshi, N.; Zhao, S.; Long, H.; Zhou, L.; Ma, G.; Peng, B.; Oliveira, O.N., Jr.; Zettl, A.; Lin, L. NO2 gas sensors based on CVD tungsten diselenide monolayer. Appl. Surf. Sci. 2020, 529, 147110. [Google Scholar] [CrossRef]
- Li, X.; Wang, J. One-dimensional and two-dimensional synergized nanostructures for high-performing energy storage and conversion. InfoMat 2019, 2, 3–32. [Google Scholar] [CrossRef]
- Valentim, A.C.S.; da Silva, E.O.; da Silva, P.S.R.C.; Maria, D.S.G.; Tavares, I.B. Synergistic effect between hybrid nanoparticles of TiO2 and Nb2O5 in the nanostructured materials based on eva matrix. Polym. Test. 2018, 70, 111–116. [Google Scholar] [CrossRef]
- Ramirez-Torres, A.; Le, D.; Rahman, T.S. Effect of monolayer support on the electronic structure of single-layer MoS2. IOP Conf. Ser. Mater. Sci. Eng. 2015, 76, 012011. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mat. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hersam, M.C. Interface Characterization and Control of 2D Materials and Heterostructures. Adv. Mater. 2018, 30, 1801586. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, X.; Luo, H.; Li, B.; Shi, J.; Fan, C.; Yang, J.; Zeng, M.; Zhou, Z.; Hu, N.; et al. Highly sensitive and recoverable room-temperature NO2 gas detection realized by 2D/0D MoS2/ZnS heterostructures with synergistic effects. Sens. Actuators B Chem. 2021, 347, 130608. [Google Scholar] [CrossRef]
- Li, Z.; Liao, Y.; Liu, Y.; Zeng, W.; Zhou, Q. Room temperature detection of nitrogen dioxide gas sensor based on Pt-modified MoSe2 nanoflowers: Experimental and theoretical analysis. Appl. Surf. Sci. 2023, 610, 155527. [Google Scholar] [CrossRef]
- Mirzaei, A.; Lee, M.H.; Safaeian, H.; Kim, T.U.; Kim, J.Y.; Kim, H.W.; Kim, S.S. Room Temperature Chemiresistive Gas Sensors Based on 2D MXenes. Sensors 2023, 23, 8829. [Google Scholar] [CrossRef]
- Kodan, S.; Kumar, A.; Sanger, A.; Arora, A.; Malik, V.K.; Chandra, R. Vertically aligned MoSe2-WS2 nanoworms heterojunction towards room temperature NO2 gas sensors. Sens. Actuators B Chem. 2024, 407, 135481. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, W.; Zhang, M.; Wang, Y.; Duan, Z.; Tan, C.; Bohao, L.; Ouyang, F.; Yuan, Z.; Tai, H.; et al. Edge-Enriched Mo2TiC2Tx/MoS2 Heterostructure with Coupling Interface for Selectively NO2 Monitoring. Adv. Funct. Mater. 2022, 32, 2203528. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Jiang, Y.; Duan, Z.; Yuan, Z.; Liu, B.; Huang, Q.; Zhao, Q.; Yang, Y.; Tai, H. Synergistic effect of charge transfer and interlayer swelling in V2CTx/SnS2 driving ultrafast and highly sensitive NO2 detection at room temperature. Sens. Actuators B Chem. 2024, 411, 135788. [Google Scholar] [CrossRef]
- Yin, L.; Yu, T.; Guo, W.; Liu, J.; Song, H.; He, H.; Lin, Y. Green, efficient, and controllable preparation of In2O3 uniformly modified MoS2 nanoflowers for methanol detection. Appl. Phys. Lett. 2025, 126, 051602. [Google Scholar] [CrossRef]
- Liu, Y. Development of In2O3-Based Sensing Materials for NO2 Detection at Room Temperature. Ph.D. Thesis, University of Calgary, Calgary, AB, Canada, 2023. [Google Scholar]
- Peng, X.; Han, Y.; Zhang, Q.; Feng, P.; Jia, P.; Cui, H.; Wang, L.; Duan, S. Performance Improvement of MoS2 Gas Sensor at Room Temperature. IEEE Trans. Electron Devices 2022, 68, 4644–4650. [Google Scholar] [CrossRef]
- Peng, X.; Chen, J.; Wang, S.; Wang, L.; Duan, S.; Feng, P.; Chu, J. High-temperature operation of v-MoS2 nanowalls/TiO2 photodetectors with excellent performances. Appl. Surf. Sci. 2022, 599, 153904. [Google Scholar] [CrossRef]
- Aldalbahi, A.; Wang, Z.B.; Ahamad, T.; Alshehri, S.M.; Feng, P.X. Two-Step Facile Preparation of 2D MoS2/ZnO Nanocomposite p-n Junctions with Enhanced Photoelectric Performance. Int. J. Photoenergy 2021, 2021, 1884293. [Google Scholar] [CrossRef]
- Zhou, A.F.; Flores, S.Y.; Pacheco, E.; Peng, X.; Zhang, S.G.; Feng, P.X. Ternary TiO2/MoS2/ZnO hetero-nanostructure based multifunctional sensing devices. Discov. Nano 2024, 19, 157. [Google Scholar] [CrossRef]
- Nalwa, H.S. A review of molybdenum disulfide (MoS2) based photodetectors: From ultra-broadband, self-powered to flexible devices. RSC Adv. 2020, 10, 30529–30602. [Google Scholar] [CrossRef]
- Feng, P.X.; Zhang, H.X.; Peng, X.Y.; Sajjad, M.; Chu, J. A novel compact design of calibration equipment for gas and thermal sensors. Rev. Sci. Instrum. 2011, 82, 043303. [Google Scholar] [CrossRef]
- Liu, H.-L.; Guo, H.; Yang, T.; Zhang, Z.; Kumamoto, Y.; Shen, C.-C.; Hsu, Y.-T.; Li, L.-J.; Saito, R.; Kawata, S. Anomalous lattice vibrations of monolayer MoS2 probed by ultraviolet Raman scattering. Phys. Chem. Chem. Phys. 2015, 17, 14561–14568. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous Lattice Vibrations of Single- and Few-Layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Decremps, F.; Porres, J.P.; Saitta, A.M.; Chervin, J.C.; Polian, A. High-pressure Raman spectroscopy study of wurtzite Zno. Phys. Rev. B 2002, 65, 092101. [Google Scholar] [CrossRef]
- Ribut, S.H.; Abdullah, C.A.C.; Yusoff, M.Z.M. Investigations of structural and optical properties of zinc oxide thin films growth on various substrates. Results Phys. 2019, 13, 102146. [Google Scholar] [CrossRef]
- Zhuo, R.F.; Feng, H.T.; Liang, Q.; Liu, J.Z.; Chen, J.T.; Yan, D.; Feng, J.J.; Li, H.J.; Cheng, S.; Geng, B.S.; et al. Morphology-controlled synthesis, growth mechanism, optical and microwave absorption properties of ZnO nanocombs. J. Phys. D Appl. Phys. 2008, 41, 185405. [Google Scholar] [CrossRef]
- Flores, S.Y.; Gonzalez-Espiet, J.; Cintrón, J.; Villanueva, N.D.J.; Camino, F.E.; Kisslinger, K.; Cruz, D.M.P.; Rivera, R.D.; Fonseca, L.F. Fluorinated Iron and Cobalt Phthalocyanine Nanowire Chemiresistors for Environmental Gas Monitoring at Parts-per-Billion Levels. ACS Appl. Nano Mater. 2022, 5, 4688–4699. [Google Scholar] [CrossRef]
- Gould, R.D.; Ibrahim, N.A. The electrical response of evaporated phthalocyanine thin films on exposure to NO2. Thin Solid Film. 2001, 398, 432–437. [Google Scholar] [CrossRef]
- Moumen, A.; Konar, R.; Zappa, D.; Teblum, E.; Nessim, G.D.; Comini, E. Robust Room-Temperature NO2 Sensors from Exfoliated 2D Few-Layered CVD-Grown Bulk Tungsten Di-selenide (2H-WSe2). ACS Appl. Mater. Interfaces 2021, 13, 4316–4329. [Google Scholar] [CrossRef]
- Moumen, A.; Konar, R.; Zappa, D.; Teblum, E.; Nessim, G.D.; Comini, E. Room-Temperature NO2 Sensing of CVD-Modified WS2–WSe2 He-terojunctions. ACS Appl. Nano Mater. 2023, 6, 7323–7329. [Google Scholar] [CrossRef]
- Kok, S.E.; Kian, M.Y. Overcoming Long Recovery Time of Metal-Oxide Gas Sensor With Certainty Factor Sensing Algorithm. Int. J. Smart Sens. Intell. Syst. 2020, 7, 1–6. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Choi, J. Improved recovery of NO2 sensors using heterojunctions between transition metal dichalcogenides and ZnO nanoparticles. Micro Nano Syst. Lett. 2023, 11, 5. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, R. Recent Advances in MoS2 and Its Derivatives-Based Two-Dimensional Gas Sensors. ECS J. Solid State Sci. Technol. 2022, 11, 097003. [Google Scholar] [CrossRef]
- Hau, H.H.; Duong, T.T.H.; Man, N.K.; Nga, T.T.V.; Xuan, C.T.; Le, D.T.T.; Van Toan, N.; Hung, C.M.; Van Duy, N.; Van Hieu, N.; et al. Enhanced NO2 gas-sensing performance at room temperature using exfoliated MoS2 nanosheets. Sens. Actuators A Phys. 2021, 332, 113137. [Google Scholar] [CrossRef]
- Feng, P.X.; Chavez, E.; Malca, C. Super Stable Pollution Gas Sensor Based on Functionalized 2D Boron Nitride Nanosheet Materials for High Humidity Environments. Chemosensors 2018, 6, 49. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhao, Q.; Duan, Z.; Xie, C.; Duan, X.; Li, S.; Ye, Z.; Jiang, Y.; Tai, H. Ag2Te nanowires for humidity-resistant trace-level NO2 detection at room temperature. Sens. Actuators B Chem. 2022, 363, 131790. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Long, X.; Cao, J.; Xin, X.; Guan, X.; Peng, J.; Zheng, X. Gas Sensors Based on Mechanically Exfoliated MoS2 Nanosheets for Room-Temperature NO2 Detection. Sensors 2019, 19, 2123. [Google Scholar] [CrossRef]
- Doubi, Y.; Hartiti, B.; Siadat, M.; Nkuissi, H.J.T.; Labrim, H.; Fadili, S.; Tahri, M.; Thevenin, P.; Losson, E. The effect of experimental process on properties of pure TiO2 nanostructure for fast NO2 gas sensors. Appl. Phys. A 2022, 128, 463. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, X.; Chen, J.; Bermak, A.; Fan, Z. A fast-response/recovery ZnO hierarchical nanostructure-based gas sensor with ultra-high room-temperature output response. Sens. Actuators B Chem. 2015, 206, 764–771. [Google Scholar] [CrossRef]
- Han, Y.; Huang, D.; Ma, Y.; He, G.; Hu, J.; Zhang, J.; Hu, N.; Su, Y.; Zhou, Z.; Zhang, Y.; et al. Design of hetero-nanostructures on MoS2 nanosheets to boost NO2 room-temperature sensing. ACS Appl. Mater. Interfaces 2018, 10, 22640–22649. [Google Scholar] [CrossRef]
- Choi, H.-J.; Kwon, S.-H.; Lee, W.-S.; Im, K.-G.; Kim, T.-H.; Noh, B.-R.; Park, S.; Oh, S.; Kim, K.-K. Ultraviolet Photoactivated Room Temperature NO2 Gas Sensor of ZnO Hemitubes and Nanotubes Covered with TiO2 Nanoparticles. Nanomaterials 2020, 10, 46. [Google Scholar] [CrossRef]
- Zhou, B.; Peng, X.; Chu, J.; Malca, C.; Diaz, L.; Zhou, A.F.; Feng, P.X. Type II ZnO-MoS2 Heterostructure-Based Self-Powered UV-MIR Ultra-Broadband p-n Photodetectors. Molecules 2025, 30, 1063. [Google Scholar] [CrossRef]
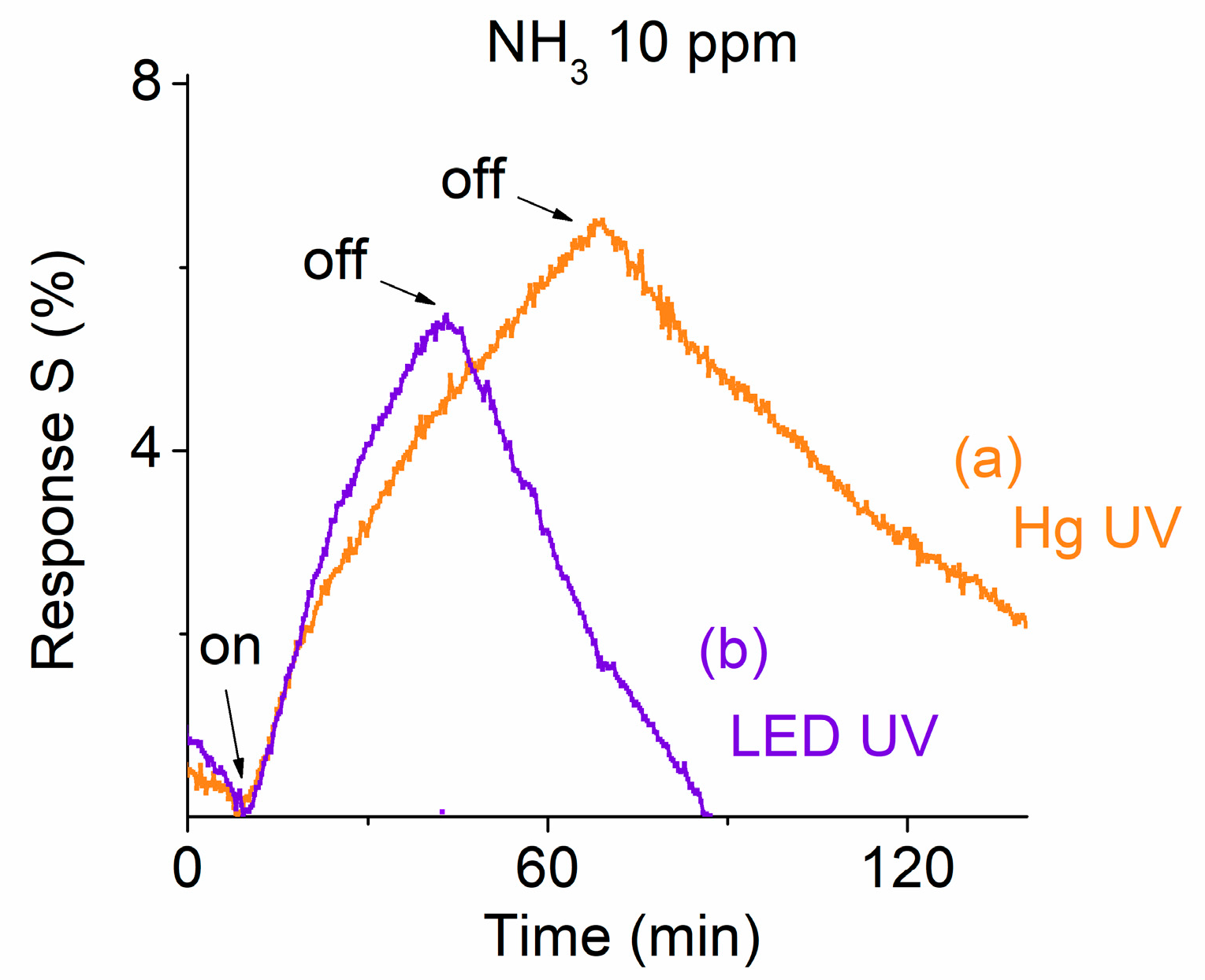
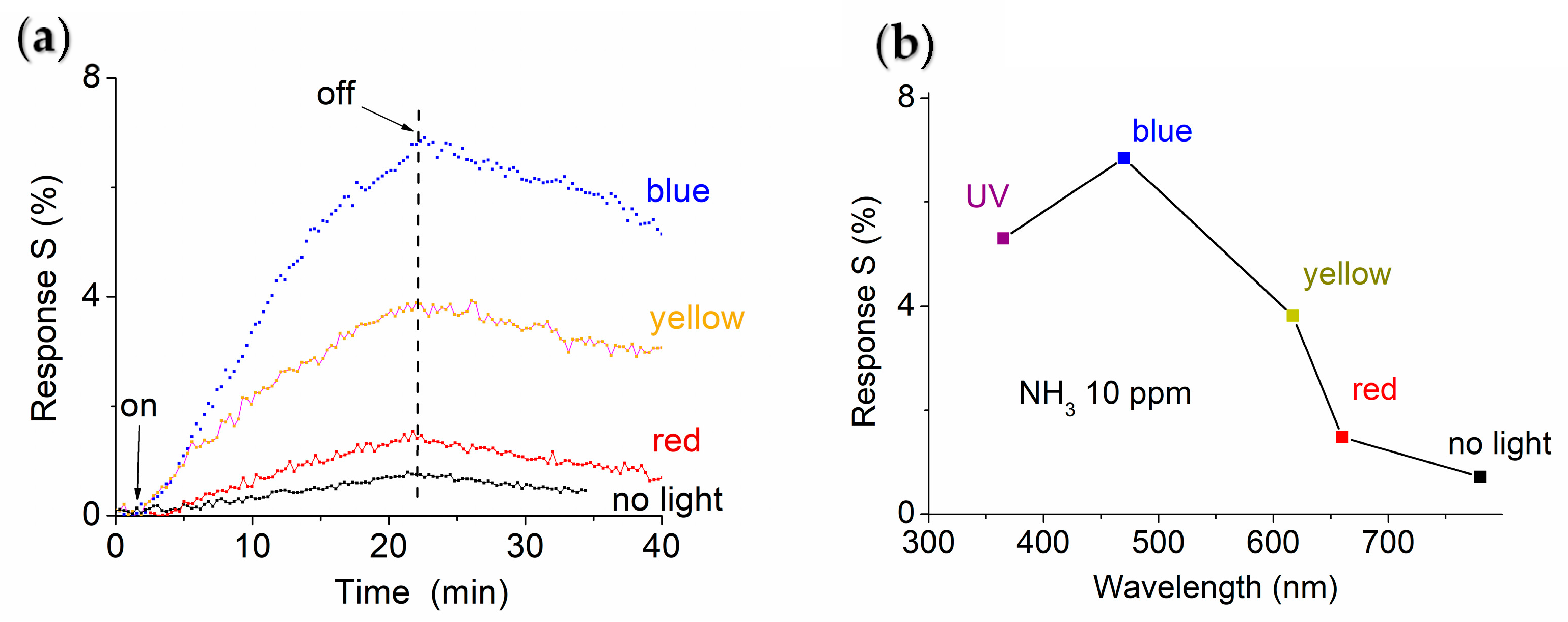
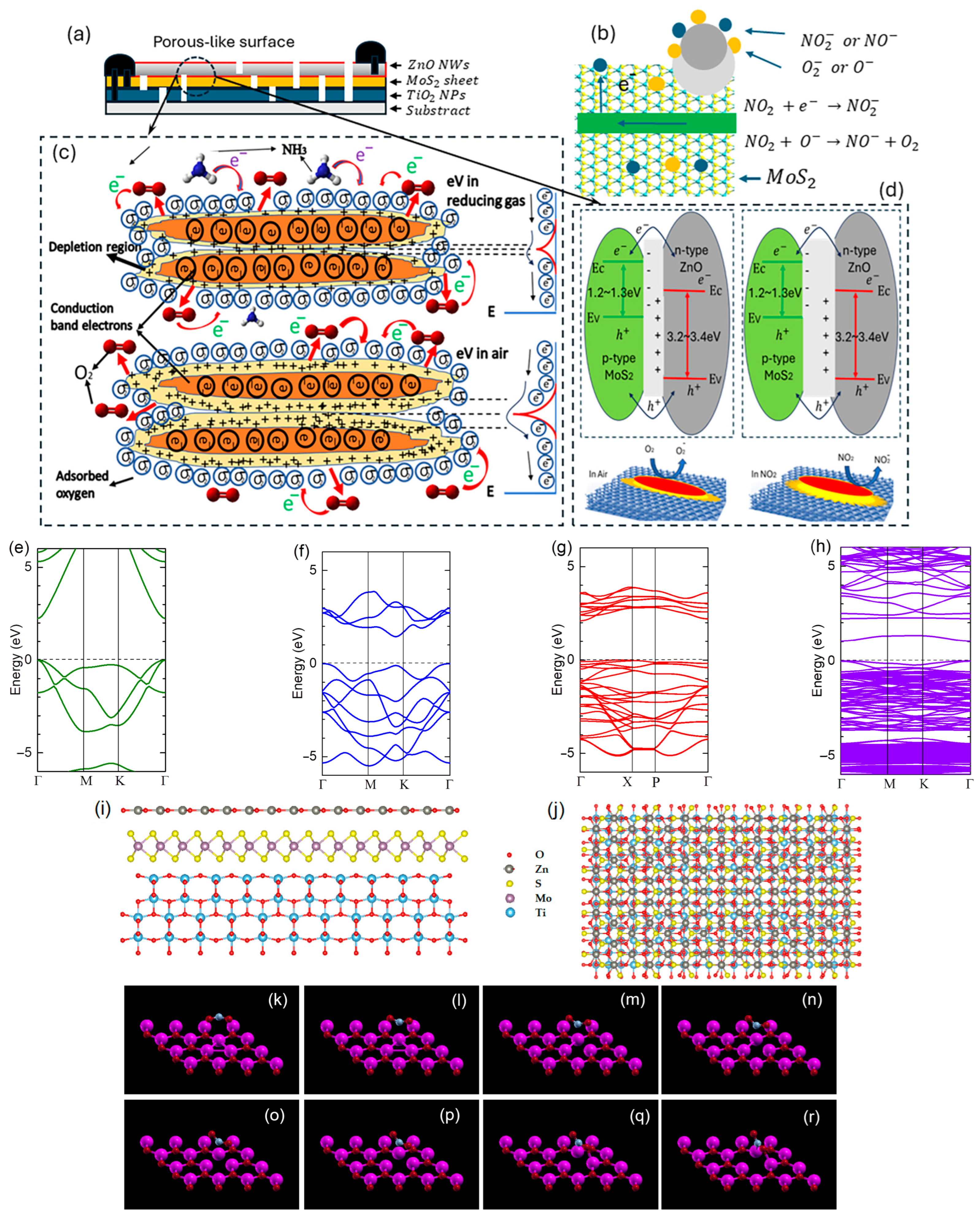
| Material | LOD [ppm] | Response Time | Recovery Time | Ref. |
|---|---|---|---|---|
| MoS2 | 5 | ~80 s | 2 s | [47] |
| TiO2 | 10 | 35 s | 18 s | [48] |
| ZnO | 5 | 20 min | 15 min | [49] |
| ZnO-MoS2 | 0.5 | >40 s | - | [50] |
| TiO2-ZnO | 5 | 26/36 s | 224/271 s | [51] |
| ZnO-MoS2-TiO2 | 0.1 | 31 s | 37 min | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores, S.Y.; Pacheco, E.; Malca, C.; Peng, X.; Chen, Y.; Zhou, B.; Pinero, D.M.; Diaz-Vazquez, L.M.; Zhou, A.F.; Feng, P.X. Highly Selective Room-Temperature Blue LED-Enhanced NO2 Gas Sensors Based on ZnO-MoS2-TiO2 Heterostructures. Sensors 2025, 25, 1781. https://doi.org/10.3390/s25061781
Flores SY, Pacheco E, Malca C, Peng X, Chen Y, Zhou B, Pinero DM, Diaz-Vazquez LM, Zhou AF, Feng PX. Highly Selective Room-Temperature Blue LED-Enhanced NO2 Gas Sensors Based on ZnO-MoS2-TiO2 Heterostructures. Sensors. 2025; 25(6):1781. https://doi.org/10.3390/s25061781
Chicago/Turabian StyleFlores, Soraya Y., Elluz Pacheco, Carlos Malca, Xiaoyan Peng, Yihua Chen, Badi Zhou, Dalice M. Pinero, Liz M. Diaz-Vazquez, Andrew F. Zhou, and Peter X. Feng. 2025. "Highly Selective Room-Temperature Blue LED-Enhanced NO2 Gas Sensors Based on ZnO-MoS2-TiO2 Heterostructures" Sensors 25, no. 6: 1781. https://doi.org/10.3390/s25061781
APA StyleFlores, S. Y., Pacheco, E., Malca, C., Peng, X., Chen, Y., Zhou, B., Pinero, D. M., Diaz-Vazquez, L. M., Zhou, A. F., & Feng, P. X. (2025). Highly Selective Room-Temperature Blue LED-Enhanced NO2 Gas Sensors Based on ZnO-MoS2-TiO2 Heterostructures. Sensors, 25(6), 1781. https://doi.org/10.3390/s25061781









