A Functional Magnetic Resonance Imaging Investigation of Hot and Cool Executive Functions in Reward and Competition
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Behavioural Tasks
2.2.1. Behaviour Rating Inventory of Executive Function—Adult Version (BRIEF-A)
2.2.2. The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ)
2.2.3. Go/No-Go Task
2.2.4. Balloon Analogue Risk Task (BART)
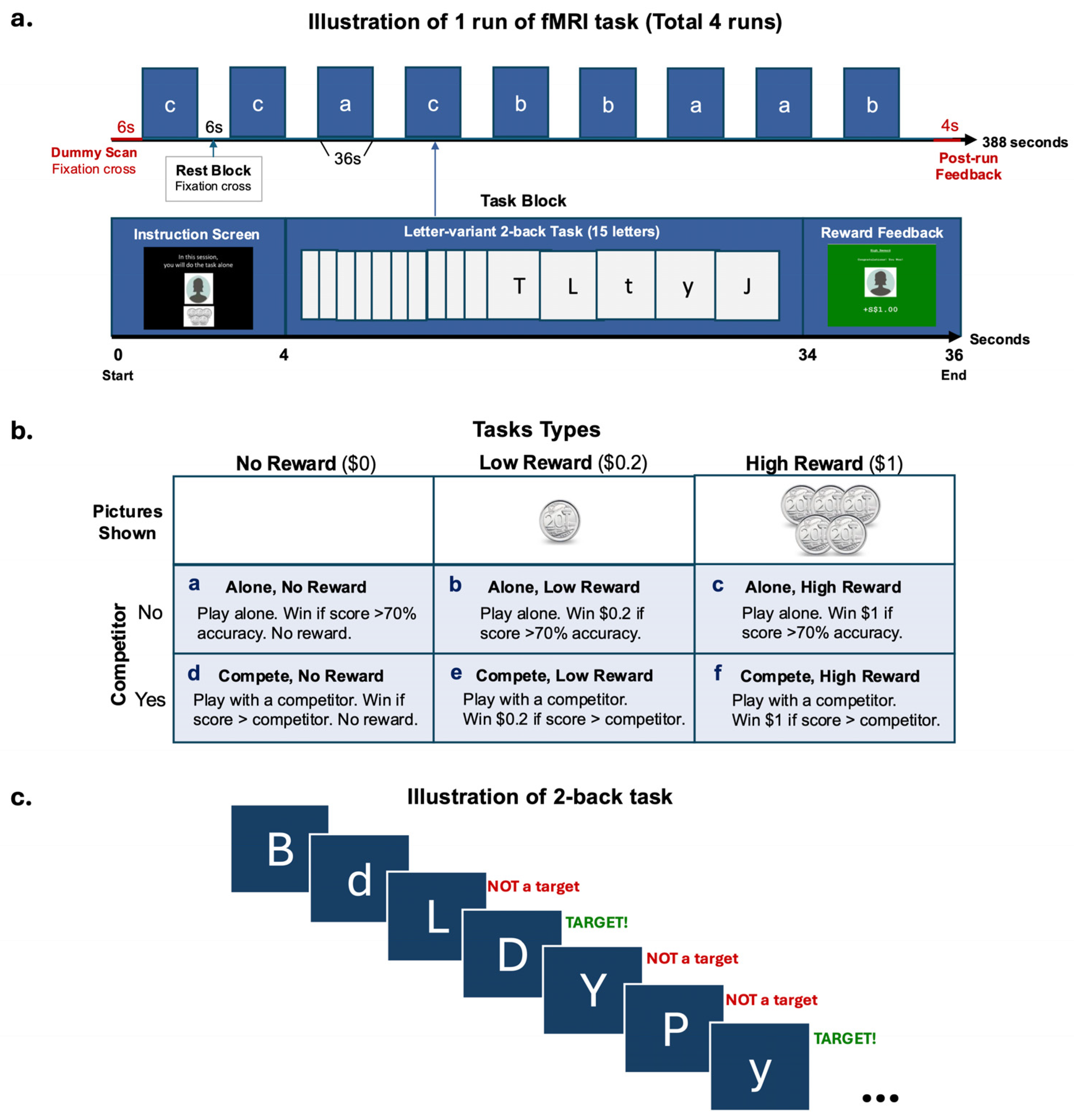
2.3. fMRI Task Design
2.4. Experimental Procedure
2.5. MRI Data Acquisition
2.6. Data Analysis
3. Results
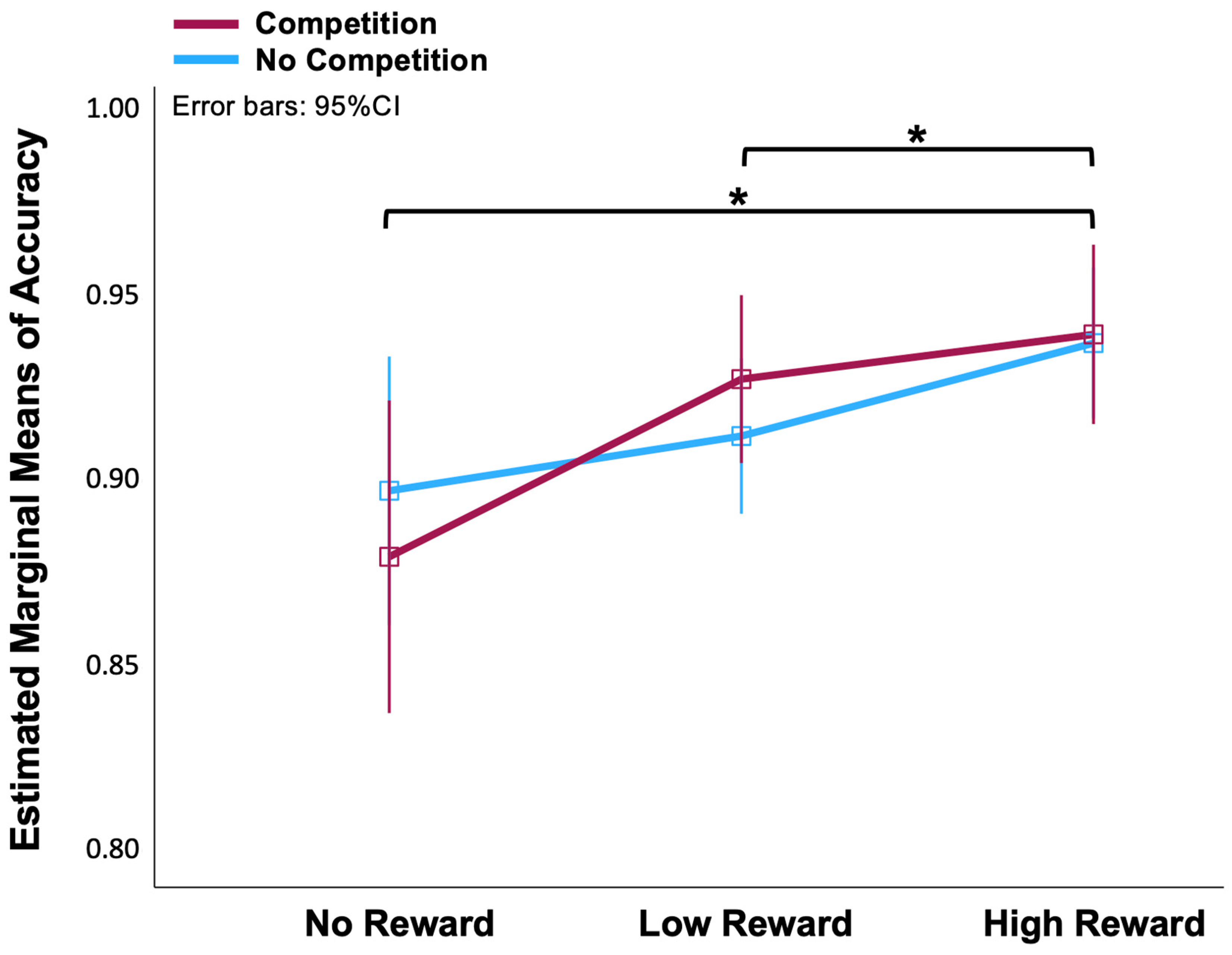
3.1. Behavioural Measures
3.2. fMRI Task
3.3. Debriefing
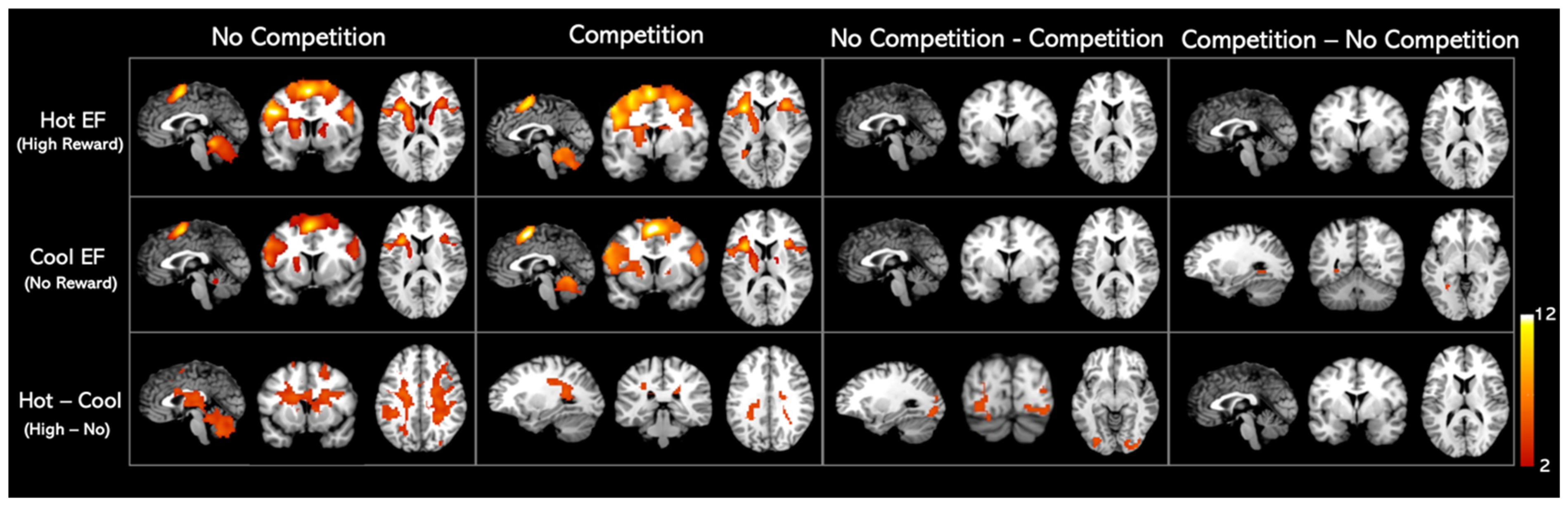
3.4. Neuroimaging Results
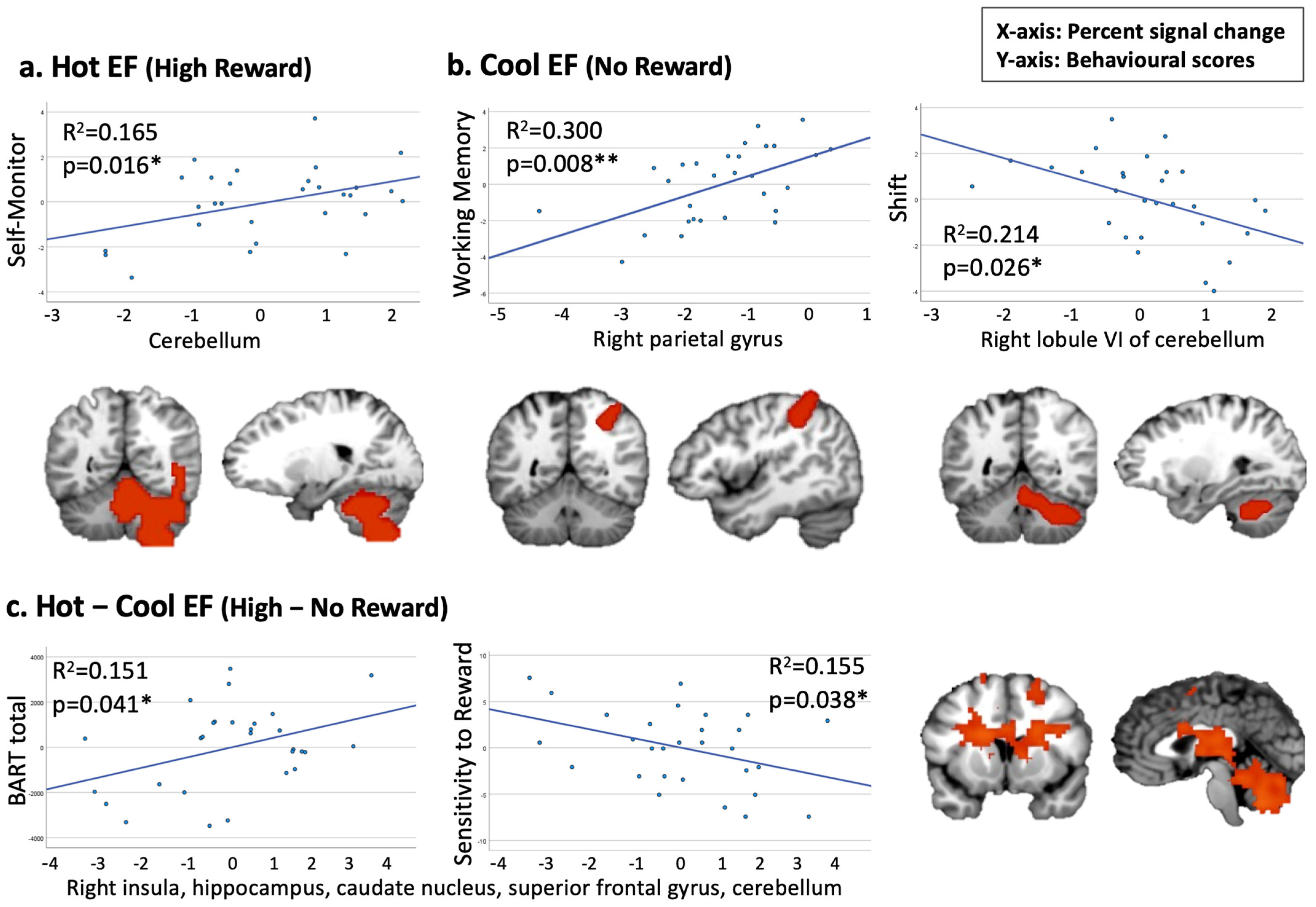
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scarmeas, N.; Stern, Y. Cognitive reserve and lifestyle. J. Clin. Exp. Neuropsychol. 2003, 25, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Law, K.M.Y.; Geng, S.; Li, T. Student enrollment, motivation and learning performance in a blended learning environment: The mediating effects of social, teaching, and cognitive presence. Comput. Educ. 2019, 136, 1–12. [Google Scholar] [CrossRef]
- Ansari, D. Culture and education: New frontiers in brain plasticity. Trends Cogn. Sci. 2012, 16, 93–95. [Google Scholar] [CrossRef]
- Pekrun, R.; Goetz, T.; Titz, W.; Perry, R.P. Academic Emotions in Students’ Self-Regulated Learning and Achievement: A Program of Qualitative and Quantitative Research. Educ. Psychol. 2002, 37, 91–105. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, W.; Chen, K.; Feng, S.; Ji, Y.; Shen, J.; Reiman, E.M.; Liu, Y. Arithmetic processing in the brain shaped by cultures. Proc. Natl. Acad. Sci. USA 2006, 103, 10775–10780. [Google Scholar] [CrossRef]
- Murayama, K.; FitzGibbon, L.; Sakaki, M. Process Account of Curiosity and Interest: A Reward-Learning Perspective. Educ. Psychol. Rev. 2019, 31, 875–895. [Google Scholar] [CrossRef]
- Tan, C.-C.; Chen, C.-M.; Lee, H.-M. Effectiveness of a digital pen-based learning system with a reward mechanism to improve learners’ metacognitive strategies in listening. Comput. Assist. Lang. Learn. 2020, 33, 785–810. [Google Scholar] [CrossRef]
- Brandl, F.; Le Houcq Corbi, Z.; Mulej Bratec, S.; Sorg, C. Cognitive reward control recruits medial and lateral frontal cortices, which are also involved in cognitive emotion regulation: A coordinate-based meta-analysis of fMRI studies. Neuroimage 2019, 200, 659–673. [Google Scholar] [CrossRef]
- Gossen, A.; Groppe, S.E.; Winkler, L.; Kohls, G.; Herrington, J.; Schultz, R.T.; Gründer, G.; Spreckelmeyer, K.N. Neural evidence for an association between social proficiency and sensitivity to social reward. Soc. Cogn. Affect. Neurosci. 2013, 9, 661–670. [Google Scholar] [CrossRef]
- Cubillo, A.; Makwana, A.B.; Hare, T.A. Differential modulation of cognitive control networks by monetary reward and punishment. Soc. Cogn. Affect. Neurosci. 2019, 14, 305–317. [Google Scholar] [CrossRef]
- Duverne, S.; Koechlin, E. Rewards and Cognitive Control in the Human Prefrontal Cortex. Cereb. Cortex 2017, 27, 5024–5039. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed]
- Zelazo, P.D. Executive Function and Psychopathology: A Neurodevelopmental Perspective. Annu. Rev. Clin. Psychol. 2020, 16, 431–454. [Google Scholar] [CrossRef] [PubMed]
- Kryza-Lacombe, M.; Christian, I.; Liuzzi, M.; Owen, C.; Hernandez, B.; Dougherty, L.; Wiggins, J. Executive functioning moderates neural reward processing in youth. Cogn. Affect. Behav. Neurosci. 2020, 21, 105–118. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, H. Hot and cool executive function in the development of behavioral problems in grade school. Dev. Psychopathol. 2024, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y. The Development of Internalizing and Externalizing Problems in Primary School: Contributions of Executive Function and Social Competence. Child Dev. 2021, 92, 889–903. [Google Scholar] [CrossRef]
- Colonna, S.; Eyre, O.; Agha, S.S.; Thapar, A.; van Goozen, S.; Langley, K. Investigating the associations between irritability and hot and cool executive functioning in those with ADHD. BMC Psychiatry 2022, 22, 166. [Google Scholar] [CrossRef]
- Yang, Y.; Shields, G.S.; Zhang, Y.; Wu, H.; Chen, H.; Romer, A.L. Child executive function and future externalizing and internalizing problems: A meta-analysis of prospective longitudinal studies. Clin. Psychol. Rev. 2022, 97, 102194. [Google Scholar] [CrossRef]
- Friedman, N.P.; Robbins, T.W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 2022, 47, 72–89. [Google Scholar] [CrossRef]
- Locke, H.S.; Braver, T.S. Motivational influences on cognitive control: Behavior, brain activation, and individual differences. Cogn. Affect. Behav. Neurosci. 2008, 8, 99–112. [Google Scholar] [CrossRef]
- Padmala, S.; Pessoa, L. Interactions between cognition and motivation during response inhibition. Neuropsychologia 2010, 48, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Herrera, P.M.; Van Meerbeke, A.V.; Speranza, M.; Cabra, C.L.; Bonilla, M.; Canu, M.; Bekinschtein, T.A. Expectation of reward differentially modulates executive inhibition. BMC Psychol. 2019, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Lertladaluck, K.; Chutabhakdikul, N.; Chevalier, N.; Moriguchi, Y. Effects of social and nonsocial reward on executive function in preschoolers. Brain Behav. 2020, 10, e01763. [Google Scholar] [CrossRef] [PubMed]
- Fröber, K.; Pittino, F.; Dreisbach, G. How sequential changes in reward expectation modulate cognitive control: Pupillometry as a tool to monitor dynamic changes in reward expectation. Int. J. Psychophysiol. 2020, 148, 35–49. [Google Scholar] [CrossRef]
- Engelmann, J.; Damaraju, E.; Padmala, S.; Pessoa, L. Combined effects of attention and motivation on visual task performance: Transient and sustained motivational effects. Front. Hum. Neurosci. 2009, 3, 342. [Google Scholar] [CrossRef]
- Beck, S.M.; Locke, H.S.; Savine, A.C.; Jimura, K.; Braver, T.S. Primary and secondary rewards differentially modulate neural activity dynamics during working memory. PLoS ONE 2010, 5, e9251. [Google Scholar] [CrossRef]
- Pochon, J.B.; Levy, R.; Fossati, P.; Lehericy, S.; Poline, J.B.; Pillon, B.; Le Bihan, D.; Dubois, B. The neural system that bridges reward and cognition in humans: An fMRI study. Proc. Natl. Acad. Sci. USA 2002, 99, 5669–5674. [Google Scholar] [CrossRef]
- Carver, C.S.; Johnson, S.L.; Joormann, J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: What depression has in common with impulsive aggression. Psychol. Bull. 2008, 134, 912–943. [Google Scholar] [CrossRef]
- Nejati, V.; Salehinejad, M.A.; Nitsche, M.A. Interaction of the Left Dorsolateral Prefrontal Cortex (l-DLPFC) and Right Orbitofrontal Cortex (OFC) in Hot and Cold Executive Functions: Evidence from Transcranial Direct Current Stimulation (tDCS). Neuroscience 2018, 369, 109–123. [Google Scholar] [CrossRef]
- Xin, F.; Lei, X. Competition between frontoparietal control and default networks supports social working memory and empathy. Soc. Cogn. Affect. Neurosci. 2015, 10, 1144–1152. [Google Scholar] [CrossRef]
- Schmidt, L.; Tusche, A.; Manoharan, N.; Hutcherson, C.; Hare, T.; Plassmann, H. Neuroanatomy of the vmPFC and dlPFC Predicts Individual Differences in Cognitive Regulation During Dietary Self-Control Across Regulation Strategies. J. Neurosci. 2018, 38, 5799–5806. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K.; Cohen, J.D. An Integrative Theory of Prefrontal Cortex Function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Moreno-López, L.; Stamatakis, E.A.; Fernández-Serrano, M.J.; Gómez-Río, M.; Rodríguez-Fernández, A.; Pérez-García, M.; Verdejo-García, A. Neural correlates of hot and cold executive functions in polysubstance addiction: Association between neuropsychological performance and resting brain metabolism as measured by positron emission tomography. Psychiatry Res. 2012, 203, 214–221. [Google Scholar] [CrossRef]
- Salehinejad, M.A.; Ghanavati, E.; Rashid, M.H.A.; Nitsche, M.A. Hot and cold executive functions in the brain: A prefrontal-cingular network. Brain Neurosci. Adv. 2021, 5, 23982128211007769. [Google Scholar] [CrossRef] [PubMed]
- Stuss, D.T. Functions of the Frontal Lobes: Relation to Executive Functions. J. Int. Neuropsychol. Soc. 2011, 17, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, Y. Relationship between cool and hot executive function in young children: A near-infrared spectroscopy study. Dev. Sci. 2022, 25, e13165. [Google Scholar] [CrossRef]
- Aharon, I.; Etcoff, N.; Ariely, D.; Chabris, C.F.; O’Connor, E.; Breiter, H.C. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron 2001, 32, 537–551. [Google Scholar] [CrossRef]
- Rademacher, L.; Krach, S.; Kohls, G.; Irmak, A.; Gründer, G.; Spreckelmeyer, K.N. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage 2010, 49, 3276–3285. [Google Scholar] [CrossRef]
- Spreckelmeyer, K.N.; Krach, S.; Kohls, G.; Rademacher, L.; Irmak, A.; Konrad, K.; Kircher, T.; Gründer, G. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Soc. Cogn. Affect. Neurosci. 2009, 4, 158–165. [Google Scholar] [CrossRef]
- Strathearn, L.; Fonagy, P.; Amico, J.; Montague, P.R. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology 2009, 34, 2655–2666. [Google Scholar] [CrossRef]
- Hikosaka, O.; Isoda, M. Switching from automatic to controlled behavior: Cortico-basal ganglia mechanisms. Trends Cogn. Sci. 2010, 14, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.H.; Ostlund, S.B.; Balleine, B.W. Reward-guided learning beyond dopamine in the nucleus accumbens: The integrative functions of cortico-basal ganglia networks. Eur. J. Neurosci. 2008, 28, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Rusu, S.I.; Pennartz, C.M.A. Learning, memory and consolidation mechanisms for behavioral control in hierarchically organized cortico-basal ganglia systems. Hippocampus 2020, 30, 73–98. [Google Scholar] [CrossRef] [PubMed]
- Motzkin, J.C.; Baskin-Sommers, A.; Newman, J.P.; Kiehl, K.A.; Koenigs, M. Neural correlates of substance abuse: Reduced functional connectivity between areas underlying reward and cognitive control. Hum. Brain Mapp. 2014, 35, 4282–4292. [Google Scholar] [CrossRef] [PubMed]
- Bigliassi, M.; Filho, E. Functional significance of the dorsolateral prefrontal cortex during exhaustive exercise. Biol. Psychol. 2022, 175, 108442. [Google Scholar] [CrossRef]
- van den Bos, W.; Rodriguez, C.A.; Schweitzer, J.B.; McClure, S.M. Adolescent impatience decreases with increased frontostriatal connectivity. Proc. Natl. Acad. Sci. USA 2015, 112, E3765–E3774. [Google Scholar] [CrossRef]
- van den Bos, W.; Rodriguez, C.A.; Schweitzer, J.B.; McClure, S.M. Connectivity strength of dissociable striatal tracts predict individual differences in temporal discounting. J. Neurosci. 2014, 34, 10298–10310. [Google Scholar] [CrossRef]
- DiMenichi, B.C.; Tricomi, E. Increases in brain activity during social competition predict decreases in working memory performance and later recall. Hum. Brain Mapp. 2017, 38, 457–471. [Google Scholar] [CrossRef]
- Tsoi, L.; Dungan, J.; Waytz, A.; Young, L. Distinct neural patterns of social cognition for cooperation versus competition. Neuroimage 2016, 137, 86–96. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Zhang, X.; Zhang, D.; Tan, H.Y.; Yue, W.; Yan, H. Unsuppressed Striatal Activity and Genetic Risk for Schizophrenia Associated With Individual Cognitive Performance Under Social Competition. Schizophr. Bull. 2022, 48, 599–608. [Google Scholar] [CrossRef]
- Dichter, G.S.; Felder, J.N.; Green, S.R.; Rittenberg, A.M.; Sasson, N.J.; Bodfish, J.W. Reward circuitry function in autism spectrum disorders. Soc. Cogn. Affect. Neurosci. 2012, 7, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Kohls, G.; Schulte-Rüther, M.; Nehrkorn, B.; Müller, K.; Fink, G.R.; Kamp-Becker, I.; Herpertz-Dahlmann, B.; Schultz, R.T.; Konrad, K. Reward system dysfunction in autism spectrum disorders. Soc. Cogn. Affect. Neurosci. 2013, 8, 565–572. [Google Scholar] [CrossRef]
- Scott-Van Zeeland, A.A.; Dapretto, M.; Ghahremani, D.G.; Poldrack, R.A.; Bookheimer, S.Y. Reward processing in autism. Autism Res. 2010, 3, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, S.; Moessnang, C.; Bast, N.; Hohmann, S.; Aggensteiner, P.; Kaiser, A.; Tillmann, J.; Goyard, D.; Charman, T.; Ambrosino, S.; et al. Processing of social and monetary rewards in autism spectrum disorders. Br. J. Psychiatry 2023, 222, 100–111. [Google Scholar] [CrossRef]
- Leuker, C.; Pachur, T.; Hertwig, R.; Pleskac, T.J. Exploiting risk–reward structures in decision making under uncertainty. Cognition 2018, 175, 186–200. [Google Scholar] [CrossRef]
- Kuhnen, C.M.; Knutson, B. The neural basis of financial risk taking. Neuron 2005, 47, 763–770. [Google Scholar] [CrossRef]
- Hsu, M.; Bhatt, M.; Adolphs, R.; Tranel, D.; Camerer, C.F. Neural systems responding to degrees of uncertainty in human decision-making. Science 2005, 310, 1680–1683. [Google Scholar] [CrossRef]
- Pighin, S.; Bonini, N.; Hadjichristidis, C.; Schena, F.; Savadori, L. Decision making under stress: Mild hypoxia leads to increased risk-taking. Stress 2020, 23, 290–297. [Google Scholar] [CrossRef]
- Du, J.; Rolls, E.T.; Cheng, W.; Li, Y.; Gong, W.; Qiu, J.; Feng, J. Functional connectivity of the orbitofrontal cortex, anterior cingulate cortex, and inferior frontal gyrus in humans. Cortex 2020, 123, 185–199. [Google Scholar] [CrossRef]
- Smith, D.V.; Ludwig, R.M.; Dennison, J.B.; Reeck, C.; Fareri, D.S. An fMRI Dataset on Social Reward Processing and Decision Making in Younger and Older Adults. Sci. Data 2024, 11, 158. [Google Scholar] [CrossRef]
- Camara, E.; Rodriguez-Fornells, A.; Münte, T.F. Functional connectivity of reward processing in the brain. Front. Hum. Neurosci. 2008, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.M.; Lance, C.E.; Isquith, P.K.; Fischer, A.S.; Giancola, P.R. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function-Adult version in healthy adults and application to attention-deficit/hyperactivity disorder. Arch. Clin. Neuropsychol. 2013, 28, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.M.; Isquith, P.K.; Gioia, G.A. Behavior Rating Inventory of Executive Function®—Adult Version (BRIEF-A). Arch. Clin. Neuropsychol. 2005. [Google Scholar]
- Dawson, E.L.; Shear, P.K.; Strakowski, S.M. Behavior regulation and mood predict social functioning among healthy young adults. J. Clin. Exp. Neuropsychol. 2012, 34, 297–305. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef]
- Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Rolls, E.T.; Huang, C.-C.; Lin, C.-P.; Feng, J.; Joliot, M. Automated anatomical labelling atlas 3. NeuroImage 2020, 206, 116189. [Google Scholar] [CrossRef]
- Brett, M.; Anton, J.-L.; Valabregue, R.; Poline, J.-B. Region of interest analysis using an SPM toolbox [abstract]. In Proceedings of the 8th International Conference on Functional Mapping of the Human Brain (Sendai), Sendai, Japan, 2–6 June 2002; Available on CD-ROM in NeuroImage. Volume 16, p. 497. [Google Scholar]
- Lee, S.-H.; Walker, Z.M.; Hale, J.B.; Chen, S.H.A. Frontal-subcortical circuitry in social attachment and relationships: A cross-sectional fMRI ALE meta-analysis. Behav. Brain Res. 2017, 325, 117–130. [Google Scholar] [CrossRef]
- du Boisgueheneuc, F.; Levy, R.; Volle, E.; Seassau, M.; Duffau, H.; Kinkingnehun, S.; Samson, Y.; Zhang, S.; Dubois, B. Functions of the left superior frontal gyrus in humans: A lesion study. Brain 2006, 129, 3315–3328. [Google Scholar] [CrossRef]
- Friederici, A.D.; Fiebach, C.J.; Schlesewsky, M.; Bornkessel, I.D.; von Cramon, D.Y. Processing Linguistic Complexity and Grammaticality in the Left Frontal Cortex. Cereb. Cortex 2006, 16, 1709–1717. [Google Scholar] [CrossRef]
- Niendam, T.A.; Laird, A.R.; Ray, K.L.; Dean, Y.M.; Glahn, D.C.; Carter, C.S. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 2012, 12, 241–268. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Bartolo, R.; Averbeck, B.B. Reward-related choices determine information timing and flow across macaque lateral prefrontal cortex. Nat. Commun. 2021, 12, 894. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Nie, C.; Zhang, Y.; Chen, Y.; Yang, T. Evidence accumulation for value computation in the prefrontal cortex during decision making. Proc. Natl. Acad. Sci. 2020, 117, 30728–30737. [Google Scholar] [CrossRef] [PubMed]
- Brissenden, J.A.; Adkins, T.J.; Hsu, Y.T.; Lee, T.G. Reward influences the allocation but not the availability of resources in visual working memory. J. Exp. Psychol. Gen. 2023, 152, 1825–1839. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C.; Kringelbach, M.L. Pleasure systems in the brain. Neuron 2015, 86, 646–664. [Google Scholar] [CrossRef]
- Richard, J.M.; Castro, D.C.; Difeliceantonio, A.G.; Robinson, M.J.; Berridge, K.C. Mapping brain circuits of reward and motivation: In the footsteps of Ann Kelley. Neurosci. Biobehav. Rev. 2013, 37, 1919–1931. [Google Scholar] [CrossRef]
- Catani, M.; Dell’Acqua, F.; Thiebaut de Schotten, M. A revised limbic system model for memory, emotion and behaviour. Neurosci. Biobehav. Rev. 2013, 37, 1724–1737. [Google Scholar] [CrossRef]
- Craig, A.D. Human feelings: Why are some more aware than others? Trends Cogn. Sci. 2004, 8, 239–241. [Google Scholar] [CrossRef]
- Insel, T.R.; Fernald, R.D. How the brain processes social information: Searching for the social brain. Annu. Rev. Neurosci. 2004, 27, 697–722. [Google Scholar] [CrossRef]
- Solomonov, N.; Victoria, L.W.; Lyons, K.; Phan, D.K.; Alexopoulos, G.S.; Gunning, F.M.; Flückiger, C. Social reward processing in depressed and healthy individuals across the lifespan: A systematic review and a preliminary coordinate-based meta-analysis of fMRI studies. Behav. Brain Res. 2023, 454, 114632. [Google Scholar] [CrossRef]
- Adrián-Ventura, J.; Costumero, V.; Parcet, M.A.; Ávila, C. Linking personality and brain anatomy: A structural MRI approach to Reinforcement Sensitivity Theory. Soc. Cogn. Affect. Neurosci. 2019, 14, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Davidow, J.Y.; Foerde, K.; Galván, A.; Shohamy, D. An Upside to Reward Sensitivity: The Hippocampus Supports Enhanced Reinforcement Learning in Adolescence. Neuron 2016, 92, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yoon, H.; Kim, H.; Hamann, S. Individual differences in sensitivity to reward and punishment and neural activity during reward and avoidance learning. Soc. Cogn. Affect. Neurosci. 2015, 10, 1219–1227. [Google Scholar] [CrossRef]
- Delgado, M.R.; Stenger, V.A.; Fiez, J.A. Motivation-dependent Responses in the Human Caudate Nucleus. Cereb. Cortex 2004, 14, 1022–1030. [Google Scholar] [CrossRef]
- Oyama, K.; Hori, Y.; Mimura, K.; Nagai, Y.; Eldridge, M.A.G.; Saunders, R.C.; Miyakawa, N.; Hirabayashi, T.; Hori, Y.; Inoue, K.I.; et al. Chemogenetic Disconnection between the Orbitofrontal Cortex and the Rostromedial Caudate Nucleus Disrupts Motivational Control of Goal-Directed Action. J. Neurosci. 2022, 42, 6267–6275. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Wang, Z.; Wang, M.; Dong, G.H. Males are more sensitive to reward and less sensitive to loss than females among people with internet gaming disorder: fMRI evidence from a card-guessing task. BMC Psychiatry 2020, 20, 357. [Google Scholar] [CrossRef]
- Wang, Q.; Lv, C.; He, Q.; Xue, G. Dissociable fronto-striatal functional networks predict choice impulsivity. Brain Struct. Funct. 2020, 225, 2377–2386. [Google Scholar] [CrossRef]
- Votinov, M.; Myznikov, A.; Zheltyakova, M.; Masharipov, R.; Korotkov, A.; Cherednichenko, D.; Habel, U.; Kireev, M. The Interaction Between Caudate Nucleus and Regions Within the Theory of Mind Network as a Neural Basis for Social Intelligence. Front. Neural Circuits 2021, 15, 727960. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, B.; Zhong, X.; Hou, L.; Zhang, M.; Yang, M.; Wu, Z.; Chen, X.; Mai, N.; Zhou, H.; et al. Static and dynamic functional connectivity variability of the anterior-posterior hippocampus with subjective cognitive decline. Alzheimers Res. Ther. 2022, 14, 122. [Google Scholar] [CrossRef]
- Chen, J.; Wu, S.; Li, F. Cognitive Neural Mechanism of Backward Inhibition and Deinhibition: A Review. Front. Behav. Neurosci. 2022, 16, 846369. [Google Scholar] [CrossRef]
- Klingberg, T.; Forssberg, H.; Westerberg, H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J. Cogn. Neurosci. 2002, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.J.; Marois, R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature 2004, 428, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Vallesi, A.; Visalli, A.; Gracia-Tabuenca, Z.; Tarantino, V.; Capizzi, M.; Alcauter, S.; Mantini, D.; Pini, L. Fronto-parietal homotopy in resting-state functional connectivity predicts task-switching performance. Brain Struct. Funct. 2022, 227, 655–672. [Google Scholar] [CrossRef]
- Berger, A.; Sadeh, M.; Tzur, G.; Shuper, A.; Kornreich, L.; Inbar, D.; Cohen, I.J.; Michowiz, S.; Yaniv, I.; Constantini, S.; et al. Task switching after cerebellar damage. Neuropsychology 2005, 19, 362–370. [Google Scholar] [CrossRef]
- Baumann, O.; Mattingley, J.B. Cerebellum and Emotion Processing. Adv. Exp. Med. Biol. 2022, 1378, 25–39. [Google Scholar] [CrossRef]
- Van Overwalle, F.; Baetens, K.; Mariën, P.; Vandekerckhove, M. Social cognition and the cerebellum: A meta-analysis of over 350 fMRI studies. Neuroimage 2014, 86, 554–572. [Google Scholar] [CrossRef]
- Leggio, M.; Olivito, G. Topography of the cerebellum in relation to social brain regions and emotions. Handb. Clin. Neurol. 2018, 154, 71–84. [Google Scholar] [CrossRef]
- Ciapponi, C.; Li, Y.; Osorio Becerra, D.A.; Rodarie, D.; Casellato, C.; Mapelli, L.; D’Angelo, E. Variations on the theme: Focus on cerebellum and emotional processing. Front. Syst. Neurosci. 2023, 17, 1185752. [Google Scholar] [CrossRef]
- Peterburs, J.; Desmond, J.E. The role of the human cerebellum in performance monitoring. Curr. Opin. Neurobiol. 2016, 40, 38–44. [Google Scholar] [CrossRef]
- Greening, S.G.; Finger, E.C.; Mitchell, D.G. Parsing decision making processes in prefrontal cortex: Response inhibition, overcoming learned avoidance, and reversal learning. Neuroimage 2011, 54, 1432–1441. [Google Scholar] [CrossRef]
- Remijnse, P.L.; Nielen, M.M.; Uylings, H.B.; Veltman, D.J. Neural correlates of a reversal learning task with an affectively neutral baseline: An event-related fMRI study. Neuroimage 2005, 26, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Lu, Z.; Levin, I.P.; Bechara, A. The impact of prior risk experiences on subsequent risky decision-making: The role of the insula. Neuroimage 2010, 50, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Galvan, A.; Fuligni, A.J.; Lieberman, M.D.; Telzer, E.H. Longitudinal Changes in Prefrontal Cortex Activation Underlie Declines in Adolescent Risk Taking. J. Neurosci. 2015, 35, 11308. [Google Scholar] [CrossRef] [PubMed]
- Quan, P.; He, L.; Mao, T.; Fang, Z.; Deng, Y.; Pan, Y.; Zhang, X.; Zhao, K.; Lei, H.; Detre, J.A.; et al. Cerebellum anatomy predicts individual risk-taking behavior and risk tolerance. Neuroimage 2022, 254, 119148. [Google Scholar] [CrossRef]
- Porcelli, A.J.; Delgado, M.R. Stress and Decision Making: Effects on Valuation, Learning, and Risk-taking. Curr. Opin. Behav. Sci. 2017, 14, 33–39. [Google Scholar] [CrossRef]
- Xu, S.; Pan, Y.; Wang, Y.; Spaeth, A.M.; Qu, Z.; Rao, H. Real and hypothetical monetary rewards modulate risk taking in the brain. Sci. Rep. 2016, 6, 29520. [Google Scholar] [CrossRef]
- Cohen, L.; Dehaene, S. Specialization within the ventral stream: The case for the visual word form area. Neuroimage 2004, 22, 466–476. [Google Scholar] [CrossRef]
- Seghier, M.L. The Angular Gyrus: Multiple Functions and Multiple Subdivisions. Neurosci. 2012, 19, 43–61. [Google Scholar] [CrossRef]
- Spagna, A.; Hajhajate, D.; Liu, J.; Bartolomeo, P. Visual mental imagery engages the left fusiform gyrus, but not the early visual cortex: A meta-analysis of neuroimaging evidence. Neurosci. Biobehav. Rev. 2021, 122, 201–217. [Google Scholar] [CrossRef]
| Measures | Mean (Standard Deviation) |
|---|---|
| Age (years) | 25.55 (4.54) |
| BART Total (points) | 7777.59 (1937.21) |
| BRIEF-A Inhibit * | 51.45 (9.04) |
| BRIEF-A Shift * | 55 (8.73) |
| BRIEF-A Emotional Control * | 50.33 (9.31) |
| BRIEF-A Self-Monitor * | 47.3 (8.65) |
| BRIEF-A Working Memory * | 53.94 (9.21) |
| BRIEF-A Reward | 10.72 (4.17) |
| Go/No-Go Overall Accuracy | 96.7% (2.6%) |
| Condition | Cluster | Volume | Cluster p(FWE) | T | x | y | z | Label |
|---|---|---|---|---|---|---|---|---|
| Hot EF (High reward—Rest) | ||||||||
| No Compete | 1 | 7270 | 4.16 × 10−11 | 15.87 | −39 | −42 | 45 | Left parietal gyrus excluding supramarginal and angular gyrus |
| 14.98 | −27 | −3 | 57 | Left superior frontal gyrus–dorsolateral | ||||
| 14.28 | −30 | 18 | 9 | Left insula | ||||
| 13.95 | −42 | 3 | 30 | Left precentral gyrus | ||||
| 13.82 | −6 | 0 | 57 | Left supplementary motor area | ||||
| 12.13 | −54 | −24 | 45 | Left postcentral gyrus | ||||
| 12.12 | −45 | −36 | 42 | Left parietal gyrus excluding supramarginal and angular gyrus | ||||
| 12.10 | −27 | −57 | 48 | Left superior parietal gyrus | ||||
| 11.91 | 30 | 18 | 6 | Right insula | ||||
| 11.41 | −45 | 0 | 42 | Left precentral gyrus | ||||
| 10.46 | 18 | 0 | 63 | Right superior frontal gyrus–dorsolateral | ||||
| 10.27 | 24 | −6 | 51 | Right precentral gyrus | ||||
| 8.91 | 45 | 3 | 33 | Right precentral gyrus | ||||
| 8.55 | 9 | 15 | 45 | Right supplementary motor area | ||||
| 2 | 608 | 0.000001 | 10.97 | −45 | −69 | −3 | Left middle occipital gyrus | |
| 8.38 | −42 | −51 | −30 | Left crus I of cerebellar hemisphere | ||||
| 7.65 | −27 | −54 | −30 | Left crus VI of cerebellar hemisphere | ||||
| 6.48 | −42 | −84 | −6 | Left inferior occipital gyrus | ||||
| 6.14 | −33 | −90 | −9 | Left inferior occipital gyrus | ||||
| 3 | 2416 | 2.22 × 10−16 | 6.62 | 0 | −45 | −15 | Lobule III of vermis | |
| 9.05 | 42 | −54 | −33 | Right crus I of cerebellar hemisphere | ||||
| 9.03 | 30 | −48 | −33 | Right lobule VI of cerebellar hemisphere | ||||
| 9.02 | 18 | −66 | −48 | Right lobule VIII of cerebellar hemisphere | ||||
| 8.74 | 39 | −60 | −30 | Right crus I of cerebellar hemisphere | ||||
| 8.71 | 24 | −57 | −27 | Right lobule VI of cerebellar hemisphere | ||||
| 8.70 | 12 | −72 | −45 | Right lobule VII of cerebellar hemisphere | ||||
| 7.96 | 15 | −48 | −21 | Right lobule IV–V of cerebellar hemisphere | ||||
| 7.39 | 9 | −69 | −24 | Right lobule VI of cerebellar hemisphere | ||||
| 7.17 | 6 | −66 | −27 | Lobule VII of vermis | ||||
| 7.05 | 51 | −69 | −6 | Right inferior temporal gyrus | ||||
| 6.58 | −24 | −66 | −51 | Left lobule VIII of cerebellar hemisphere | ||||
| 4 | 647 | 7.67 × 10−7 | 9.59 | 39 | −42 | 48 | Right parietal gyrus excluding supramarginal and angular gyrus | |
| 5.38 | 27 | −60 | 54 | Right superior parietal gyrus | ||||
| 5.16 | 15 | −63 | 54 | Right precuneus | ||||
| 4.45 | 30 | −66 | 27 | Right superior occipital gyrus | ||||
| Compete | 1 | 6621 | 1.72 × 10−10 | 15.01 | −39 | −42 | 45 | Left parietal gyrus excluding supramarginal and angular gyrus |
| 12.82 | −6 | 0 | 60 | Left supplementary motor area | ||||
| 12.79 | −27 | −57 | 48 | Left superior parietal gyrus | ||||
| 12.5 | −27 | −3 | 57 | Left superior frontal gyrus–dorsolateral | ||||
| 12.46 | −45 | 0 | 30 | Left precentral gyrus | ||||
| 12.39 | −30 | 15 | 9 | Left insula | ||||
| 12.32 | −3 | 3 | 57 | Left supplementary motor area | ||||
| 12.06 | −24 | −6 | 60 | Left superior frontal gyrus–dorsolateral | ||||
| 11.66 | −48 | −3 | 45 | Left precentral gyrus | ||||
| 11.43 | −6 | 9 | 51 | Left supplementary motor area | ||||
| 9.9 | 24 | −3 | 54 | Right precentral gyrus | ||||
| 9.26 | 15 | 3 | 57 | Right supplementary motor area | ||||
| 7.92 | −39 | −54 | −30 | Left crus I of cerebellar hemisphere | ||||
| 2 | 1719 | 8.22 × 10−14 | 9.19 | 18 | −51 | −24 | Right lobule IV–V of cerebellar hemisphere | |
| 9.16 | 30 | −48 | −30 | Right lobule VI of cerebellar hemisphere | ||||
| 9.08 | 3 | −57 | −12 | Lobule IV–V of vermis | ||||
| 8.75 | 27 | −57 | −27 | Right lobule VI of cerebellar hemisphere | ||||
| 8.43 | 12 | −72 | −45 | Right lobule VIII of cerebellar hemisphere | ||||
| 8.1 | 0 | −45 | −21 | Lobule III of vermis | ||||
| 8.02 | 18 | −63 | −48 | Right lobule VIII of cerebellar hemisphere | ||||
| 7.74 | 0 | −45 | −15 | Lobule III of vermis | ||||
| 7.42 | 3 | −66 | −33 | Lobule III of vermis | ||||
| 6.37 | −21 | −69 | −51 | Right lobule VIII of cerebellar hemisphere | ||||
| 3 | 591 | 8.56 × 10−7 | 8.93 | 36 | −45 | 48 | Right parietal gyrus excluding supramarginal and angular gyrus | |
| 7.75 | 45 | −36 | 48 | Right parietal gyrus excluding supramarginal and angular gyrus | ||||
| 4 | 187 | 0.004 | 8.05 | 45 | −66 | −6 | Right inferior temporal gyrus | |
| 6.10 | 45 | −81 | −6 | Right inferior occipital gyrus | ||||
| Cool EF (No reward—Rest) | ||||||||
| No compete | 1 | 4056 | 3.38 × 10−10 | 14.61 | −6 | 0 | 57 | Left supplementary motor area |
| 11.35 | −27 | −3 | 57 | Left superior frontal gyrus–dorsolateral | ||||
| 9.99 | −30 | 18 | 9 | Left insula | ||||
| 9.85 | −45 | −33 | 45 | Left parietal gyrus excluding supramarginal and angular gyrus | ||||
| 9.57 | −54 | −21 | 48 | Left postcentral gyrus | ||||
| 9.48 | −42 | 0 | 30 | Left precentral gyrus | ||||
| 9.31 | −48 | 0 | 42 | Left precentral gyrus | ||||
| 9.06 | −39 | −42 | 45 | Left parietal gyrus excluding supramarginal and angular gyrus | ||||
| 7.87 | −27 | −54 | 45 | Left parietal gyrus excluding supramarginal and angular gyrus | ||||
| 7.03 | −54 | −18 | 24 | Left postcentral gyrus | ||||
| 7.01 | −48 | −30 | 60 | Left postcentral gyrus | ||||
| 6.81 | 18 | 0 | 63 | Right superior frontal gyrus–dorsolateral | ||||
| 5.76 | −39 | 27 | 33 | Left middle frontal gyrus | ||||
| 2 | 473 | 0.00003 | 7.07 | 30 | −48 | −30 | Right lobule VI of cerebellar hemisphere | |
| 7.00 | 42 | −54 | −33 | Right crus I of cerebellar hemisphere | ||||
| 6.76 | 39 | −60 | −30 | Right crus I of cerebellar hemisphere | ||||
| 5.87 | 15 | −51 | −21 | Right lobule IV–V of cerebellar hemisphere | ||||
| 4.90 | 6 | −54 | −15 | Lobule IV–V of vermis | ||||
| 4.58 | 0 | −45 | −15 | Lobule III of vermis | ||||
| 3 | 297 | 0.001 | 7.02 | 21 | −66 | −51 | Right lobule VIII of cerebellar hemisphere | |
| 6.97 | 24 | −69 | −54 | Right lobule VIII of cerebellar hemisphere | ||||
| 4.97 | 6 | −78 | −45 | Right lobule VIIB of cerebellar hemisphere | ||||
| 4 | 176 | 0.009 | 7.00 | −42 | −69 | −3 | Left middle occipital gyrus | |
| 5.64 | −42 | −84 | −6 | Left inferior occipital gyrus | ||||
| 5 | 376 | 0.0002 | 6.80 | 30 | 18 | 9 | Right insula | |
| 6.12 | 48 | 3 | 30 | Right precentral gyrus | ||||
| 6.02 | 51 | 6 | 21 | Right precentral gyrus | ||||
| 6 | 280 | 0.001 | 6.40 | 48 | −33 | 48 | Right parietal gyrus excluding supramarginal and angular gyrus | |
| 5.92 | 39 | −42 | 48 | Right parietal gyrus excluding supramarginal and angular gyrus | ||||
| 4.78 | 45 | −45 | 60 | Right superior parietal gyrus | ||||
| 4.61 | 30 | −54 | 45 | Right parietal gyrus excluding supramarginal and angular gyrus | ||||
| Compete | 1 | 5661 | 3.39 × 10−10 | 14.6 | −3 | 3 | 57 | Left supplementary motor area |
| 14.38 | −3 | 9 | 54 | Left supplementary motor area | ||||
| 14.28 | −6 | 0 | 60 | Left supplementary motor area | ||||
| 13.83 | −9 | −3 | 63 | Left supplementary motor area | ||||
| 12.67 | −27 | −3 | 54 | Left superior frontal gyrus–dorsolateral | ||||
| 11.32 | −33 | 15 | 9 | Left insula | ||||
| 10.71 | −45 | −3 | 42 | Left precentral gyrus | ||||
| 10.51 | −48 | −33 | 48 | Left postcentral gyrus | ||||
| 10.34 | −45 | 0 | 30 | Left precentral gyrus | ||||
| 9.9 | 27 | 0 | 51 | Right precentral gyrus | ||||
| 9.78 | −54 | 6 | 30 | Left precentral gyrus | ||||
| 9.64 | −36 | −42 | 48 | Left parietal gyrus excluding supramarginal and angular gyrus | ||||
| 9.32 | −51 | 3 | 21 | Left precentral gyrus | ||||
| 9.06 | −27 | −57 | 48 | Left superior parietal gyrus | ||||
| 2 | 1229 | 1.3 × 10−11 | 9.20 | 30 | −51 | −30 | Right lobule VI of cerebellar hemisphere | |
| 8.13 | 3 | −57 | −12 | Lobule IV–V of vermis | ||||
| 8.01 | 39 | −60 | −30 | Right crus I of cerebellar hemisphere | ||||
| 7.76 | 18 | −60 | −45 | Right lobule VIII of cerebellar hemisphere | ||||
| 7.64 | 21 | −66 | −48 | Right lobule VIII of cerebellar hemisphere | ||||
| 6.11 | 12 | −45 | −24 | Right lobule III of cerebellar hemisphere | ||||
| 4.11 | 3 | −75 | −30 | Lobule VII of vermis | ||||
| 3 | 190 | 0.002 | 9.17 | −39 | −54 | −30 | Left crus I of cerebellar hemisphere | |
| 4 | 450 | 0.000006 | 7.66 | 51 | −33 | 48 | Right parietal gyrus excluding supramarginal and angular gyrus | |
| 7.49 | 48 | −36 | 51 | Right parietal gyrus excluding supramarginal and angular gyrus | ||||
| 7.37 | 36 | −42 | 45 | Right parietal gyrus excluding supramarginal and angular gyrus | ||||
| 5.89 | 30 | −54 | 45 | Right parietal gyrus excluding supramarginal and angular gyrus | ||||
| 4.06 | 15 | −63 | 54 | Right precuneus | ||||
| 5 | 228 | 0.001 | 7.62 | −42 | −69 | 0 | Left middle occipital gyrus | |
| 4.61 | −42 | −84 | −6 | Left inferior occipital gyrus | ||||
| 6 | 170 | 0.004 | 6.97 | 45 | −66 | −6 | Right inferior temporal gyrus | |
| 5.71 | 42 | −84 | −6 | Right inferior occipital gyrus | ||||
| 5.54 | 48 | −78 | −3 | Right inferior occipital gyrus | ||||
| Hot—Cool EF | ||||||||
| No Compete | 1 | 12,249 | 0.0003 | 7.87 | 5.67 | 33 | 3 | Right insula |
| 7.06 | 30 | −36 | 3 | Right hippocampus | ||||
| 6.66 | −15 | 15 | 12 | Left caudate nucleus | ||||
| 6.6 | −12 | −15 | 18 | Left ventral lateral nucleus | ||||
| 6.48 | −30 | −51 | 60 | Left superior parietal gyrus | ||||
| 6.42 | 24 | −9 | 60 | Right superior frontal gyrus–dorsolateral | ||||
| 6.19 | −21 | −57 | 63 | Left superior parietal gyrus | ||||
| Compete | 1 | 392 | 0.001 | 4.78 | 18 | −54 | 45 | Right precuneus |
| 3.59 | 15 | 12 | 21 | Right caudate nucleus | ||||
| No Compete—Compete | 1 | 387 | 0.0004 | 5.27 | 33 | −87 | −6 | Right inferior occipital gyrus |
| 5.23 | 27 | −90 | 9 | Right middle occipital gyrus | ||||
| 4.53 | 21 | −81 | −6 | Right lingual gyrus | ||||
| 4.35 | 36 | −57 | −18 | Right fusiform gyrus | ||||
| 3.97 | 30 | −69 | −18 | Right fusiform gyrus | ||||
| 2 | 216 | 0.007 | 4.86 | −30 | −78 | −6 | Left fusiform gyrus | |
| 4.41 | −24 | −87 | 15 | Left middle occipital gyrus | ||||
| 4.25 | −21 | −90 | 12 | Left superior occipital gyrus | ||||
| 3.84 | −21 | −78 | −18 | Left lobule VI of cerebellar hemisphere | ||||
| 3.77 | −12 | −90 | 15 | Left cuneus | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-Y.; Fung, H.; Wang, Y.; Ho, R.C.-M.; Chen, S.-H.A. A Functional Magnetic Resonance Imaging Investigation of Hot and Cool Executive Functions in Reward and Competition. Sensors 2025, 25, 806. https://doi.org/10.3390/s25030806
Lin H-Y, Fung H, Wang Y, Ho RC-M, Chen S-HA. A Functional Magnetic Resonance Imaging Investigation of Hot and Cool Executive Functions in Reward and Competition. Sensors. 2025; 25(3):806. https://doi.org/10.3390/s25030806
Chicago/Turabian StyleLin, Hsin-Yu, Hoki Fung, Yifan Wang, Roger Chun-Man Ho, and Shen-Hsing Annabel Chen. 2025. "A Functional Magnetic Resonance Imaging Investigation of Hot and Cool Executive Functions in Reward and Competition" Sensors 25, no. 3: 806. https://doi.org/10.3390/s25030806
APA StyleLin, H.-Y., Fung, H., Wang, Y., Ho, R. C.-M., & Chen, S.-H. A. (2025). A Functional Magnetic Resonance Imaging Investigation of Hot and Cool Executive Functions in Reward and Competition. Sensors, 25(3), 806. https://doi.org/10.3390/s25030806









