New Insights on Hydration Monitoring in Elderly Patients by Interdigitated Wearable Sensors
Abstract
1. Introduction
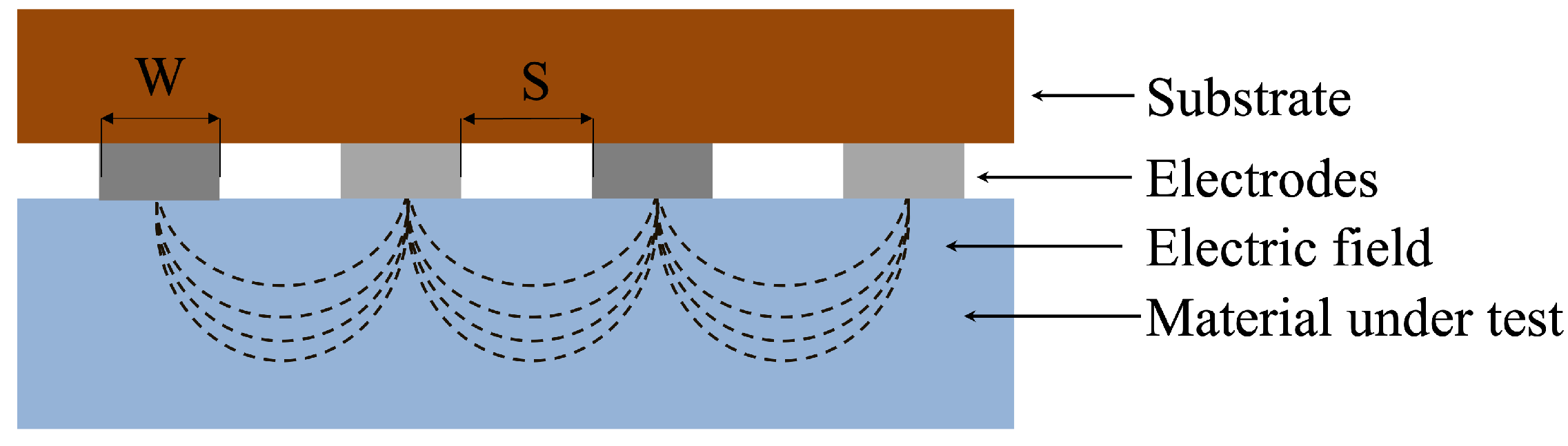
2. Interdigitated Electrodes for Hydration Monitoring
3. Materials and Methods
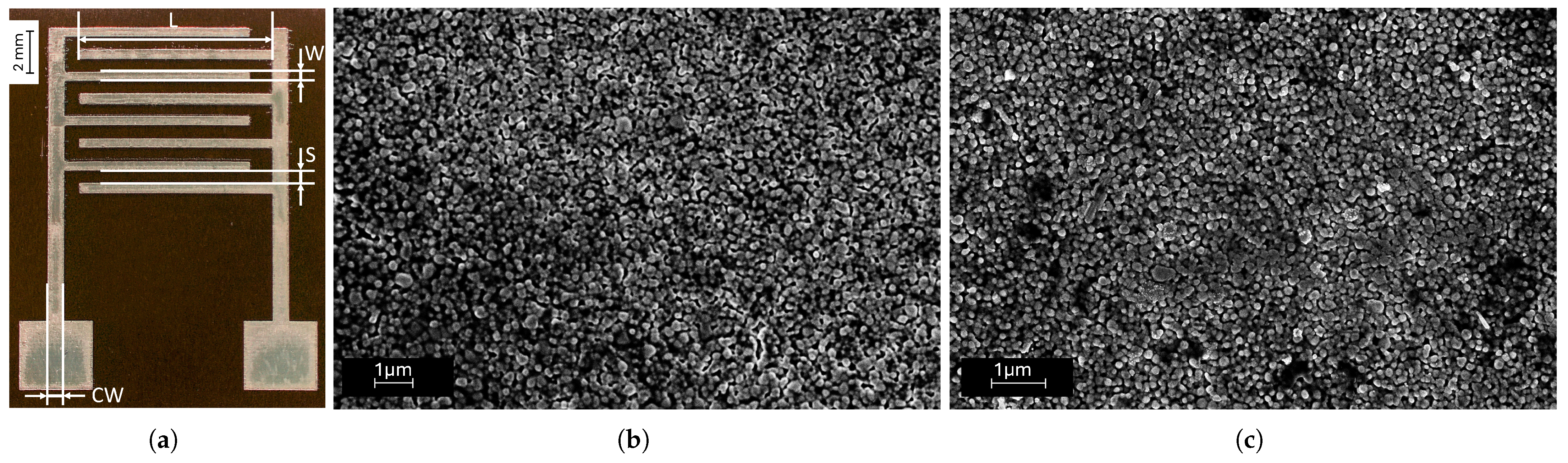
3.1. Printed Interdigitated Electrodes for Skin Hydration Monitoring
3.2. Scanning Electron Microscopy (SEM) Characterization
3.3. Electrochemical Impedance Spectroscopy Characterization
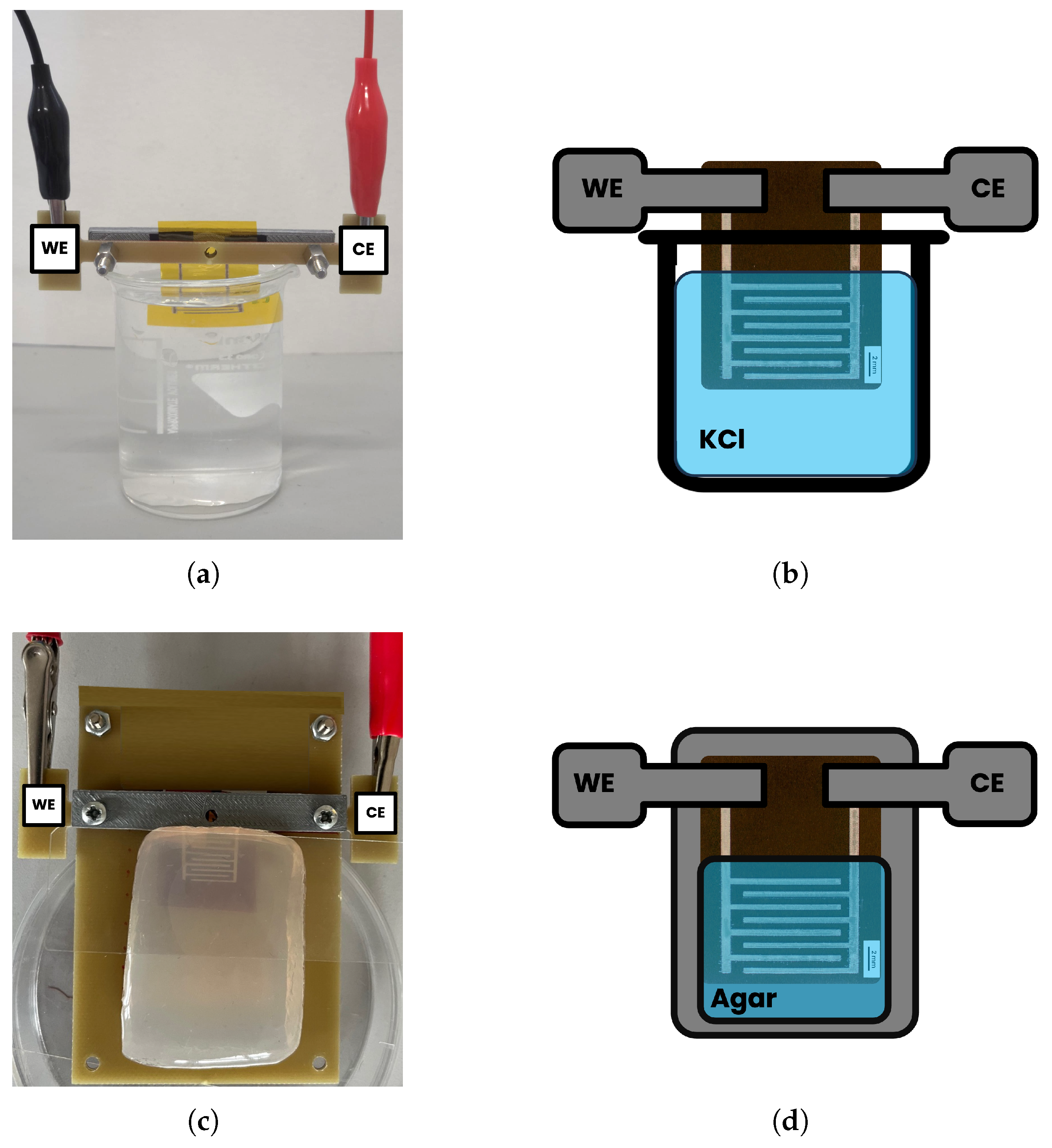
3.3.1. Electrochemical Impedance Measurements in Saline Solutions
3.3.2. Electrochemical Impedance Measurements on Agar Samples
4. Results and Discussion
4.1. Scanning Electron Microscopy Characterization
4.2. Electrochemical Impedance Measurements in Saline Solutions
4.3. Electrochemical Impedance Measurements on Agar Samples
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BIA | Bioelectrical Impedance Analysis |
| CE | Counter Electrode |
| EIS | Electrochemical Impedance Spectroscopy |
| IDE | Interdigitated sensor |
| MuSe | Multi-Sensor Wearable Device for Telemedicine |
| SEM | Scanning Electron Microscopy |
| WE | Working Electrode |
References
- Stradolini, F.; Tamburrano, N.; Modoux, T.; Tuoheti, A.; Demarchi, D.; Carrara, S. IoT for telemedicine practices enabled by an Android application with cloud system integration. In Proceedings of the 2018 IEEE International Symposium on Circuits and Systems (ISCAS), Florence, Italy, 27–30 May 2018; IEEE: New York, NY, USA, 2018; pp. 1–5. [Google Scholar]
- Soiza, R.L.; Hoyle, G.E.; Chua, M.P. Electrolyte and salt disturbances in older people: Causes, management and implications. Rev. Clin. Gerontol. 2008, 18, 143–158. [Google Scholar] [CrossRef]
- Bello, V.; Coghe, L.; Gerbasi, A.; Figus, E.; Dagliati, A.; Merlo, S. Machine learning-based approach towards identification of pharmaceutical suspensions exploiting speckle pattern images. Sensors 2024, 24, 6635. [Google Scholar] [CrossRef]
- Davies, A. Clinically assisted nutrition and hydration at the end of life. Clin. Med. 2025, 25, 100323. [Google Scholar] [CrossRef] [PubMed]
- Trenz, F.; Weigel, R.; Hagelauer, A. Methods for human dehydration measurement. Frequenz 2018, 72, 159–166. [Google Scholar] [CrossRef]
- Chiao, J.C.; Bing, S.; Chawang, K.; Crowe, B. Thirsty for a noninvasive wearable to detect dehydration: A review. IEEE Antennas Propag. Mag. 2024, 66, 66–76. [Google Scholar] [CrossRef]
- Armstrong, L.E. Hydration assessment techniques. Nutr. Rev. 2005, 63, S40–S54. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Soto, J.A.H.; Hacker, F.T.; Casa, D.J.; Kavouras, S.A.; Maresh, C.M. Urinary indices during dehydration, exercise, and rehydration. Int. J. Sport Nutr. Exerc. Metab. 1998, 8, 345–355. [Google Scholar] [CrossRef]
- Hooper, L.; Abdelhamid, A.; Ali, A.; Bunn, D.K.; Jennings, A.; John, W.G.; Kerry, S.; Lindner, G.; Pfortmueller, C.A.; Sjöstrand, F.; et al. Diagnostic accuracy of calculated serum osmolarity to predict dehydration in older people: Adding value to pathology laboratory reports. BMJ Open 2015, 5, e008846. [Google Scholar] [CrossRef] [PubMed]
- Popowski, L.A.; Oppliger, R.A.; Patrick Lambert, G.; Johnson, R.F.; Kim Johnson, A.; Gisolf, C. Blood and urinary measures of hydration status during progressive acute dehydration. Med. Sci. Sport. Exerc. 2001, 33, 747–753. [Google Scholar] [CrossRef]
- Cohen, R.; Fernie, G.; Roshan Fekr, A. Fluid intake monitoring systems for the elderly: A review of the literature. Nutrients 2021, 13, 2092. [Google Scholar] [CrossRef]
- Taylor, K.; Tripathi, A.K.; Jones, E.B. Adult dehydration. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2025. [Google Scholar]
- Schlanger, L.E.; Bailey, J.L.; Sands, J.M. Electrolytes in the aging. Adv. Chronic Kidney Dis. 2010, 17, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Annuzzi, G.; Arpaia, P.; Cesaro, U.; Cuomo, O.; Frosolone, M.; Grassini, S.; Moccaldi, N.; Sannino, I. A customized bioimpedance meter for monitoring insulin bioavailability. In Proceedings of the 2020 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Dubrovnik, Croatia, 25–28 May 2020; IEEE: New York, NY, USA, 2020; pp. 1–5. [Google Scholar]
- Pinheiro, G.P.; Miranda, R.K.; Praciano, B.J.; Santos, G.A.; Mendonça, F.L.; Javidi, E.; da Costa, J.P.J.; de Sousa, R.T., Jr. Multi-sensor wearable health device framework for real-time monitoring of elderly patients using a mobile application and high-resolution parameter estimation. Front. Hum. Neurosci. 2022, 15, 750591. [Google Scholar] [CrossRef]
- Lewy, H. Wearable devices-from healthy lifestyle to active ageing. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; IEEE: New York, NY, USA, 2015; pp. 7748–7751. [Google Scholar]
- Todorov, A.R.; Dai, H.; Torah, R.N.; Ardern-Jones, M.R.; Beeby, S.P. Wearable interdigitated capacitive sensor with flexible analog front end for superficial skin hydration measurements. In Proceedings of the 2025 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Singapore, 22–25 June 2025. [Google Scholar]
- Todorov, A.R.; Dai, H.; Ko, E.Y.; Abdulkarim, L.S.; Chupreecha, N.; Fuller, J.; Corden, E.; Teo, Y.X.; Torah, R.N.; Ardern-Jones, M.R.; et al. Wearable System Using Printed Interdigitated Capacitive Sensor for Monitoring Atopic Dermatitis in Patients. IEEE Sens. J. 2025, 25, 37266–37275. [Google Scholar] [CrossRef]
- Bello, V.; Bodo, E.; Merlo, S. Speckle Pattern Acquisition and Statistical Processing for Analysis of Turbid Liquids. IEEE Trans. Instrum. Meas. 2023, 72, 1–4. [Google Scholar] [CrossRef]
- Kelman, Y.T.; Asraf, S.; Ozana, N.; Shabairou, N.; Zalevsky, Z. Optical tissue probing: Human skin hydration detection by speckle patterns analysis. Biomed. Opt. Express 2019, 10, 4874–4883. [Google Scholar] [CrossRef]
- Li, T.; Li, L.; Sun, H.; Xu, Y.; Wang, X.; Luo, H.; Liu, Z.; Zhang, T. Porous ionic membrane based flexible humidity sensor and its multifunctional applications. Adv. Sci. 2017, 4, 1600404. [Google Scholar] [CrossRef]
- Duan, Z.; Jiang, Y.; Yan, M.; Wang, S.; Yuan, Z.; Zhao, Q.; Sun, P.; Xie, G.; Du, X.; Tai, H. Facile, flexible, cost-saving, and environment-friendly paper-based humidity sensor for multifunctional applications. ACS Appl. Mater. Interfaces 2019, 11, 21840–21849. [Google Scholar] [CrossRef]
- Yeung, K.K.; Huang, T.; Hua, Y.; Zhang, K.; Yuen, M.M.; Gao, Z. Recent advances in electrochemical sensors for wearable sweat monitoring: A review. IEEE Sens. J. 2021, 21, 14522–14539. [Google Scholar] [CrossRef]
- Ji, W.; Zhu, J.; Wu, W.; Wang, N.; Wang, J.; Wu, J.; Wu, Q.; Wang, X.; Yu, C.; Wei, G.; et al. Wearable sweat biosensors refresh personalized health/medical diagnostics. Research 2021, 2021, 9757126. [Google Scholar] [CrossRef]
- Khalil, S.F.; Mohktar, M.S.; Ibrahim, F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef]
- Tahar, A.; Zrour, H.; Dupont, S.; Pozdzik, A. Non-invasive approaches to hydration assessment: A literature review. Urolithiasis 2024, 52, 132. [Google Scholar] [CrossRef]
- Jung, M.H.; Namkoong, K.; Lee, Y.; Koh, Y.J.; Eom, K.; Jang, H.; Jung, W.; Bae, J.; Park, J. Wrist-wearable bioelectrical impedance analyzer with miniature electrodes for daily obesity management. Sci. Rep. 2021, 11, 1238. [Google Scholar] [CrossRef]
- Huynh, T.H.; Jafari, R.; Chung, W.Y. A robust bioimpedance structure for smartwatch-based blood pressure monitoring. Sensors 2018, 18, 2095. [Google Scholar] [CrossRef]
- Morin, M.; Ruzgas, T.; Svedenhag, P.; Anderson, C.D.; Ollmar, S.; Engblom, J.; Björklund, S. Skin hydration dynamics investigated by electrical impedance techniques in vivo and in vitro. Sci. Rep. 2020, 10, 17218. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Myers, A.; Malhotra, A.; Lin, F.; Bozkurt, A.; Muth, J.F.; Zhu, Y. A wearable hydration sensor with conformal nanowire electrodes. Adv. Healthc. Mater. 2017, 6, 1601159. [Google Scholar] [CrossRef] [PubMed]
- Tonello, S.; Zacchini, A.; Galli, A.; Golparvar, A.; Meimandi, A.; Peruzzi, G.; Pozzebon, A.; Lago, N.; Cester, A.; Giorgi, G.; et al. Design and in vitro characterization of a wearable multisensing system for hydration monitoring. IEEE Trans. Instrum. Meas. 2024, 73, 1–11. [Google Scholar] [CrossRef]
- Tonello, S.; Giorgi, G.; Narduzzi, C.; Fapanni, T.; Cantù, E.; Serpelloni, M.; Sardini, E.; Carrara, S. Preliminary study of a flexible printed multi-sensing platform for electromyography and lactate measuring during rehabilitation. In Proceedings of the 2021 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Lausanne, Switzerland, 23–25 June 2021; IEEE: New York, NY, USA, 2021; pp. 1–6. [Google Scholar]
- Dizon, A.; Orazem, M.E. On the impedance response of interdigitated electrodes. Electrochim. Acta 2019, 327, 135000. [Google Scholar] [CrossRef]
- Belabbaci, N.A.; Anaadumba, R.; Alam, M.A.U. Recent Advancements in Wearable Hydration-Monitoring Technologies: Scoping Review of Sensors, Trends, and Future Directions. JMIR mHealth uHealth 2025, 13, e60569. [Google Scholar] [CrossRef]
- Min, J.; Tu, J.; Xu, C.; Lukas, H.; Shin, S.; Yang, Y.; Solomon, S.A.; Mukasa, D.; Gao, W. Skin-interfaced wearable sweat sensors for precision medicine. Chem. Rev. 2023, 123, 5049–5138. [Google Scholar] [CrossRef]
- Es Sebar, L.; Angelini, E.; Grassini, S.; Iannucci, L.; Parvis, M. An op amp-less electrochemical impedance spectroscopy system. In Proceedings of the 2020 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Dubrovnik, Croatia, 25–28 May 2020; IEEE: New York, NY, USA, 2020; pp. 1–6. [Google Scholar]
- Iannucci, L.; Longombardo, S.R.; Lombardo, L.; Parvis, M.; Tonello, S.; Galli, A.; Grassini, S. Electrochemical Characterization of Flexible Interdigitated Electrodes for Hydration Monitoring. In Proceedings of the 2023 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Jeju, Republic of Korea, 14–16 June 2023; IEEE: New York, NY, USA, 2023; pp. 1–6. [Google Scholar]
- Habboush, S.; Rojas, S.; Rodríguez, N.; Rivadeneyra, A. The role of interdigitated electrodes in printed and flexible electronics. Sensors 2024, 24, 2717. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X. A focused review on the flexible wearable sensors for sports: From kinematics to physiologies. Micromachines 2022, 13, 1356. [Google Scholar] [CrossRef]
- Assalve, G.; Lunetti, P.; Di Cagno, A.; De Luca, E.W.; Aldegheri, S.; Zara, V.; Ferramosca, A. Advanced wearable devices for monitoring sweat biochemical markers in athletic performance: A comprehensive review. Biosensors 2024, 14, 574. [Google Scholar] [CrossRef]
- Teixeira, E.; Fonseca, H.; Diniz-Sousa, F.; Veras, L.; Boppre, G.; Oliveira, J.; Pinto, D.; Alves, A.J.; Barbosa, A.; Mendes, R.; et al. Wearable devices for physical activity and healthcare monitoring in elderly people: A critical review. Geriatrics 2021, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Galli, A.; Giorgi, G.; Narduzzi, C.; Peruzzi, G.; Pozzebon, A.; Tonello, S. Iot technologies for active ageing: An overview of the elderly dehydration case. In Proceedings of the 2022 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Messina, Italy, 22–24 June 2022; IEEE: New York, NY, USA, 2022; pp. 1–6. [Google Scholar]
- Pirovano, P.; Dorrian, M.; Shinde, A.; Donohoe, A.; Brady, A.J.; Moyna, N.M.; Wallace, G.; Diamond, D.; McCaul, M. A wearable sensor for the detection of sodium and potassium in human sweat during exercise. Talanta 2020, 219, 121145. [Google Scholar] [CrossRef]
- Orazem, M.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons, INC.: Hoboken, NJ, USA, 2008; pp. 1–523. [Google Scholar] [CrossRef]
- Yilmaz, T.; Foster, R.; Hao, Y. Broadband tissue mimicking phantoms and a patch resonator for evaluating noninvasive monitoring of blood glucose levels. IEEE Trans. Antennas Propag. 2014, 62, 3064–3075. [Google Scholar] [CrossRef]
- Huang, X.; Cheng, H.; Chen, K.; Zhang, Y.; Zhang, Y.; Liu, Y.; Zhu, C.; Ouyang, S.c.; Kong, G.W.; Yu, C.; et al. Epidermal impedance sensing sheets for precision hydration assessment and spatial mapping. IEEE Trans. Biomed. Eng. 2013, 60, 2848–2857. [Google Scholar] [CrossRef]
- Li, S.; Xiao, X.; Zhang, X. Hydration status in older adults: Current knowledge and future challenges. Nutrients 2023, 15, 2609. [Google Scholar] [CrossRef] [PubMed]
- Sahmel, J.; Ramachandran, G. Comparison of hydration index, percent hydration, and trans-epidermal water loss measurements for dermal exposure and risk assessment. Ann. Work Expo. Health 2022, 66, 907–922. [Google Scholar] [CrossRef]
- Eda, N.; Nakamura, N.; Inai, Y.; Sun, Z.; Sone, R.; Watanabe, K.; Akama, T. Changes in the skin characteristics associated with dehydration and rehydration. Eur. J. Sport Sci. 2023, 23, 552–560. [Google Scholar] [CrossRef]
- Ding, X.; Hernandez-Serrano, A.I.; Young, J.J.; Pickwell-MacPherson, E. Variation of skin hydration profile with biophysical factors and lifestyle revealed by in vivo terahertz sensing. Biomed. Opt. Express 2024, 15, 5180–5198. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Vega Diaz, N.; Talluri, A.; Nescolarde, L. Classification of hydration in clinical conditions: Indirect and direct approaches using bioimpedance. Nutrients 2019, 11, 809. [Google Scholar] [CrossRef] [PubMed]
- Gidado, I.M.; Qassem, M.; Triantis, I.F.; Kyriacou, P.A. Review of advances in the measurement of skin hydration based on sensing of optical and electrical tissue properties. Sensors 2022, 22, 7151. [Google Scholar] [CrossRef] [PubMed]
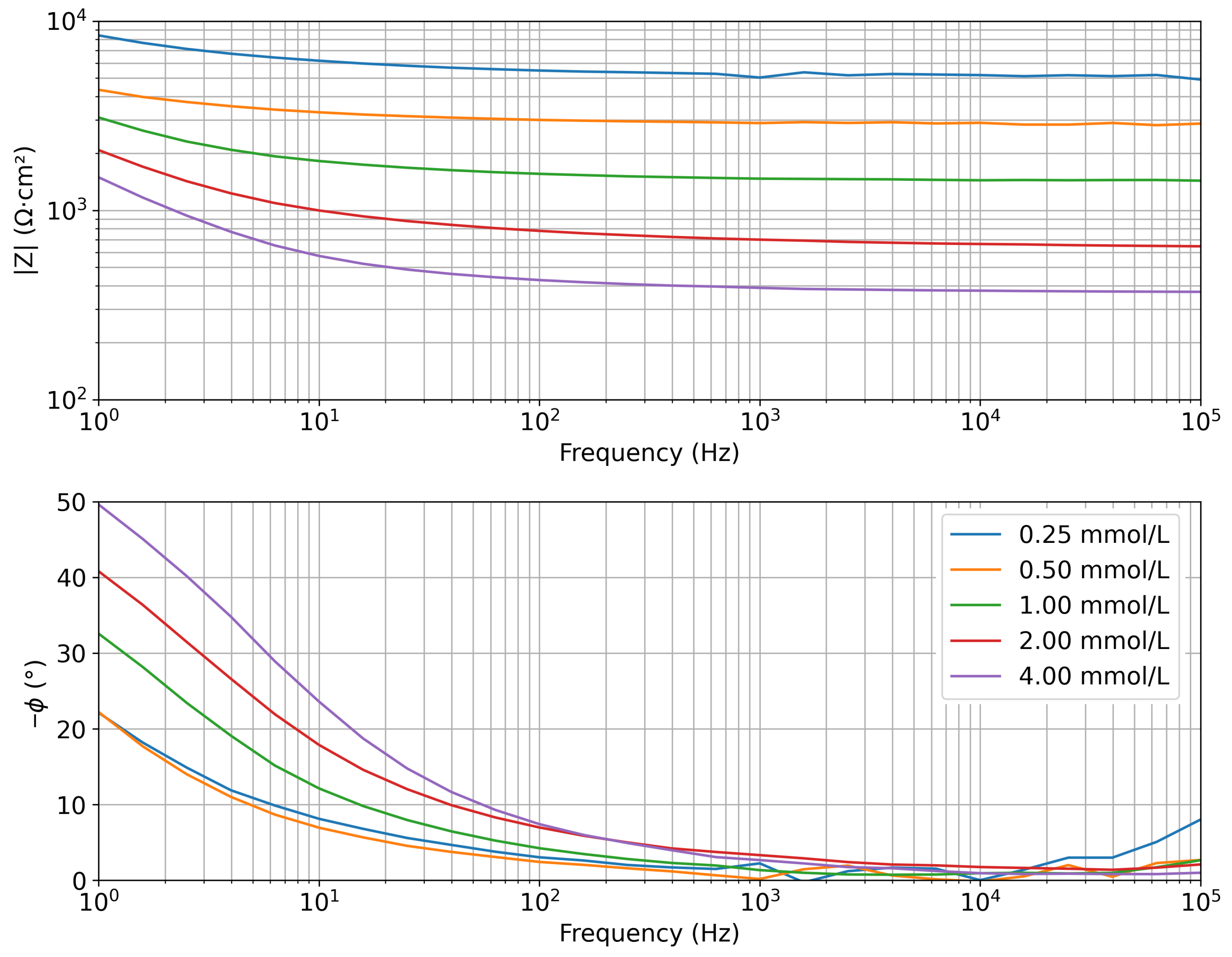
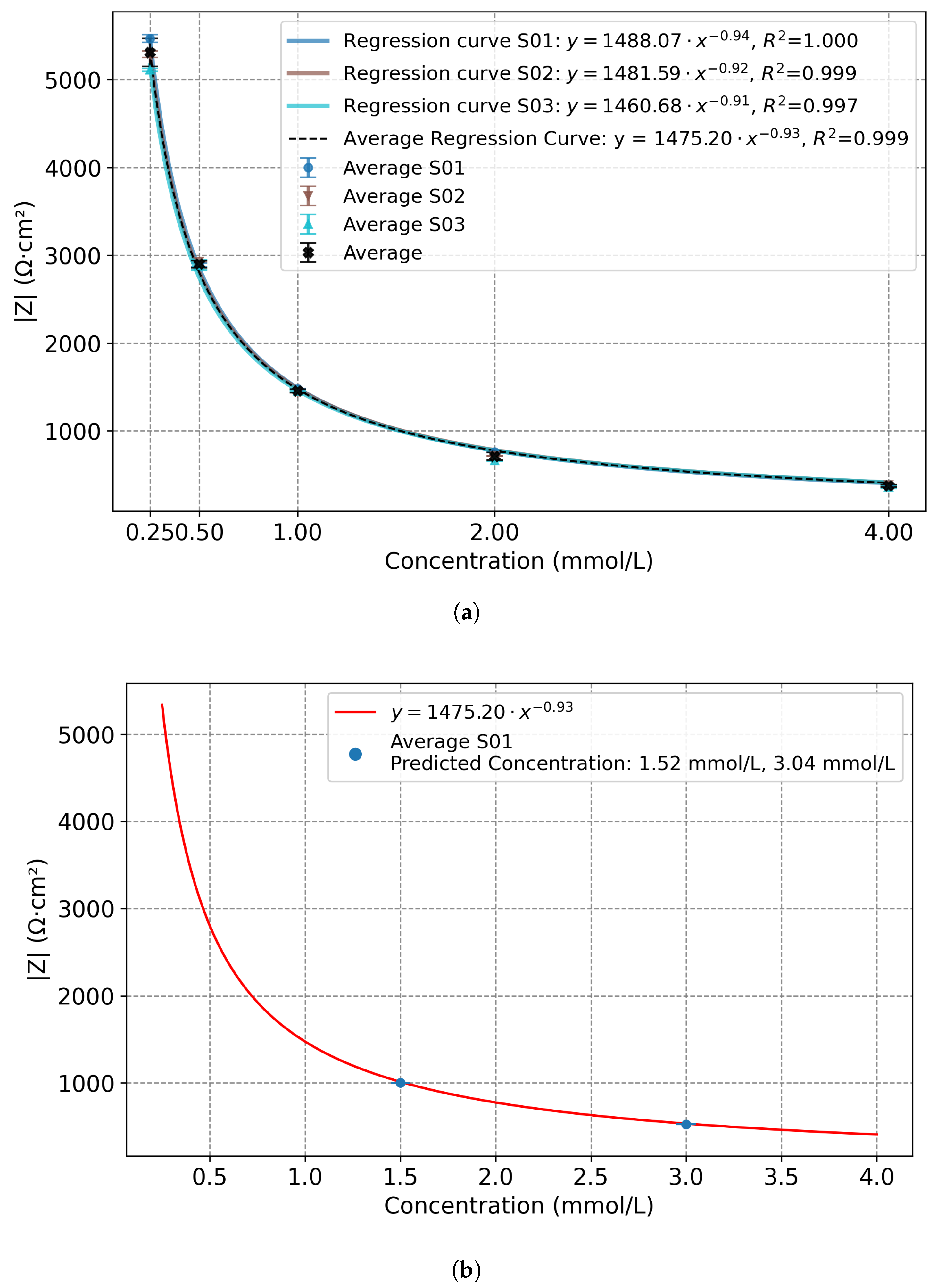
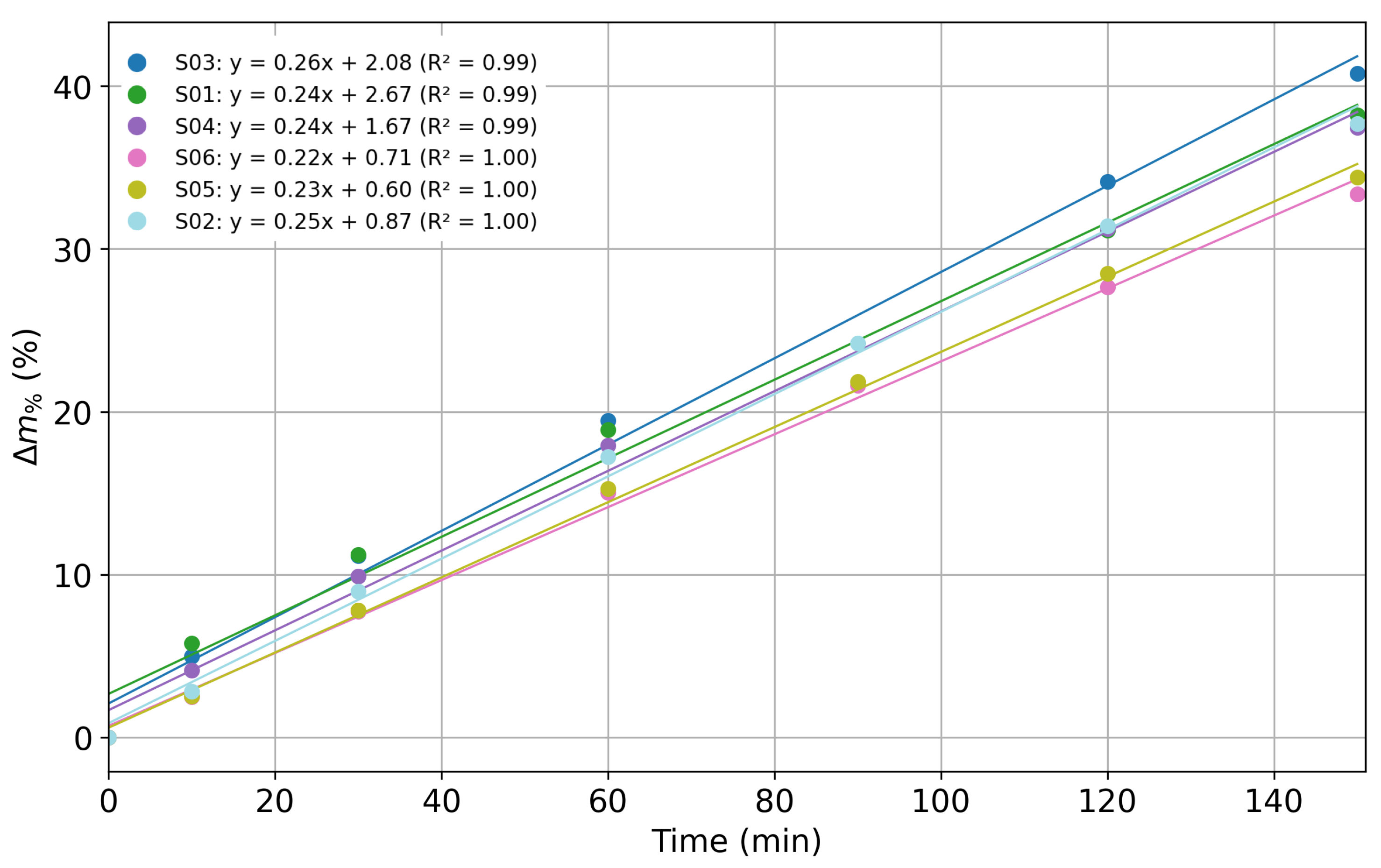
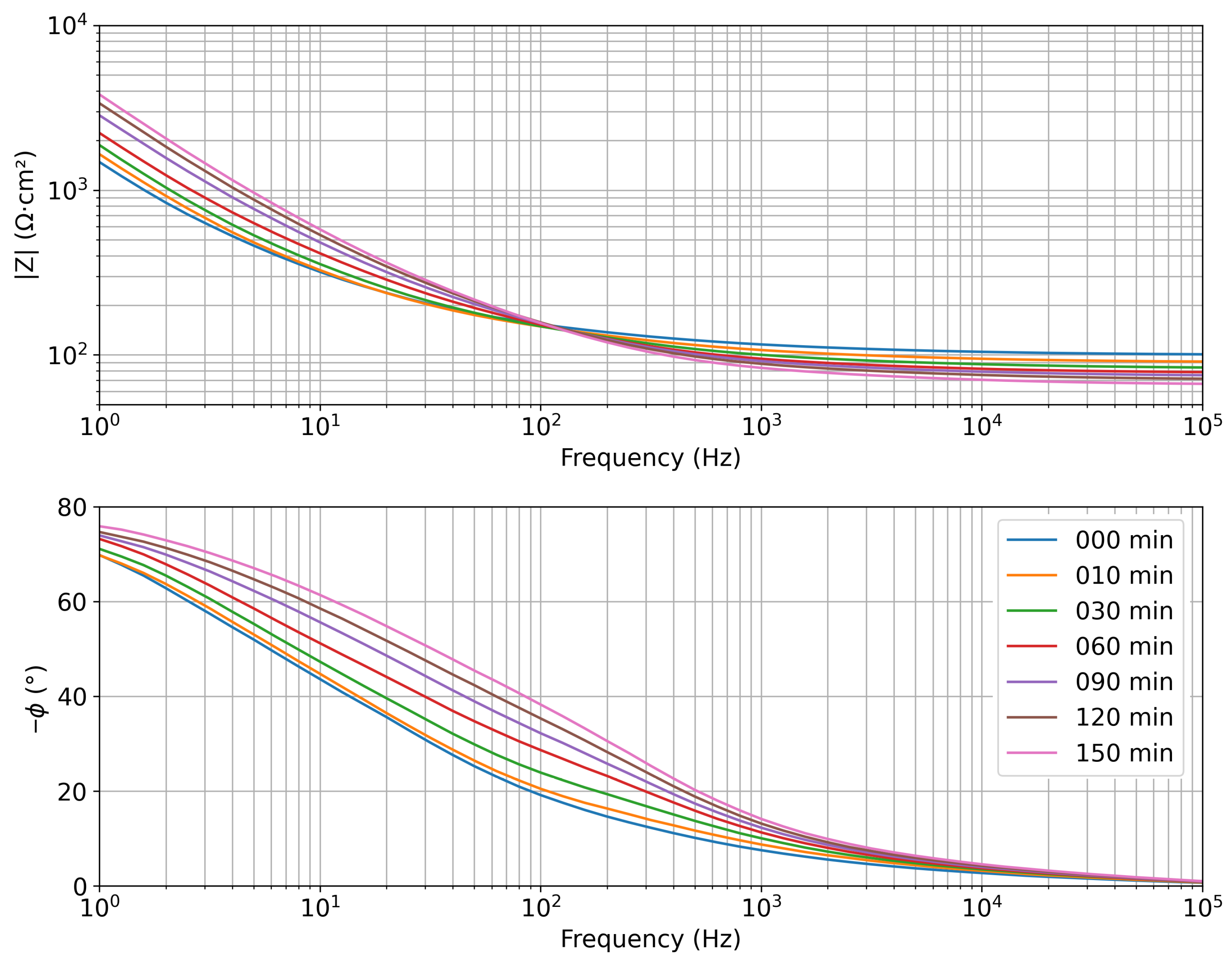
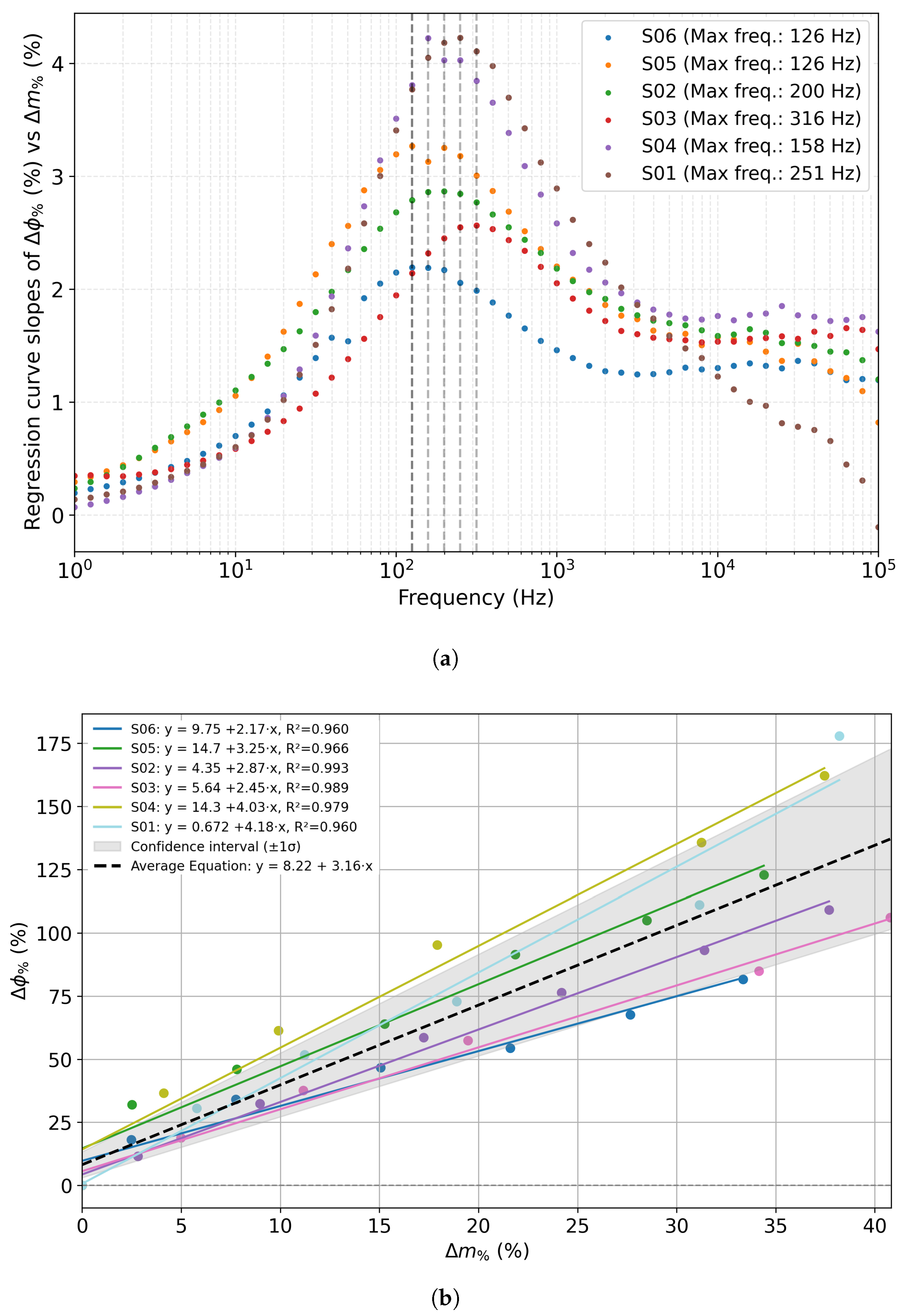
| Concentration (mmol/L) | |Z| Mean (Ω·cm2) | Std. Dev. (Ω·cm2) |
|---|---|---|
| 0.25 | 5300 | 160 |
| 0.50 | 2900 | 42 |
| 1.00 | 1456 | 20 |
| 2.00 | 711 | 43 |
| 4.00 | 376 | 14 |
| Sensor | Max. Slope (%/%) | Frequency (Hz) | R2 |
|---|---|---|---|
| S01 | 4.23 | 252 | 0.960 |
| S02 | 2.88 | 200 | 0.993 |
| S03 | 2.60 | 316 | 0.992 |
| S04 | 4.22 | 158 | 0.974 |
| S05 | 3.27 | 126 | 0.969 |
| S06 | 2.20 | 126 | 0.963 |
| Average | 3.23 | 196.2 | 0.975 |
| Sensor | Slope (%/%) | R2 | Deviation% from Slope Average Value |
|---|---|---|---|
| S01 | 4.18 | 0.960 | 32.4 |
| S02 | 2.87 | 0.993 | 9.2 |
| S03 | 2.45 | 0.989 | 22.4 |
| S04 | 4.03 | 0.979 | 27.5 |
| S05 | 3.25 | 0.966 | 2.9 |
| S06 | 2.17 | 0.960 | 31.2 |
| Average | 3.16 | 0.975 | 20.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Es Sebar, L.; Bonaldo, S.; Cristaldi, L.; Franchin, L.; Grassini, S.; Iannucci, L.; Lombardo, L.; Mineo, C.; Neviani, A.; Restelli, L.; et al. New Insights on Hydration Monitoring in Elderly Patients by Interdigitated Wearable Sensors. Sensors 2025, 25, 7081. https://doi.org/10.3390/s25227081
Es Sebar L, Bonaldo S, Cristaldi L, Franchin L, Grassini S, Iannucci L, Lombardo L, Mineo C, Neviani A, Restelli L, et al. New Insights on Hydration Monitoring in Elderly Patients by Interdigitated Wearable Sensors. Sensors. 2025; 25(22):7081. https://doi.org/10.3390/s25227081
Chicago/Turabian StyleEs Sebar, Leila, Stefano Bonaldo, Loredana Cristaldi, Lara Franchin, Sabrina Grassini, Leonardo Iannucci, Luca Lombardo, Chiara Mineo, Andrea Neviani, Lorenzo Restelli, and et al. 2025. "New Insights on Hydration Monitoring in Elderly Patients by Interdigitated Wearable Sensors" Sensors 25, no. 22: 7081. https://doi.org/10.3390/s25227081
APA StyleEs Sebar, L., Bonaldo, S., Cristaldi, L., Franchin, L., Grassini, S., Iannucci, L., Lombardo, L., Mineo, C., Neviani, A., Restelli, L., Sannino, I., Tonello, S., & Svelto, C. (2025). New Insights on Hydration Monitoring in Elderly Patients by Interdigitated Wearable Sensors. Sensors, 25(22), 7081. https://doi.org/10.3390/s25227081











