Indium Tin Oxide-Based Voltammetric Biosensor for the Detection of Antibodies Against the SARS-CoV-2 Virus Spike Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Other Materials
2.2. ITO Surface Modification with APTES
2.3. ITO and ITO/APTES Surface Characterization
2.4. Immobilization of SCoV-2
2.5. Coupling with Anti-rS
2.6. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mittal, A.; Khattri, A.; Verma, V. Structural and antigenic variations in the spike protein of emerging SARS-CoV-2 variants. PLoS Pathog. 2022, 18, e1010260. [Google Scholar] [CrossRef]
- Murin, C.D.; Wilson, I.A.; Ward, A.B. Antibody responses to viral infections: A structural perspective across three different enveloped viruses. Nat. Microbiol. 2019, 4, 734–747. [Google Scholar] [CrossRef]
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.J.; Jiang, S. The spike protein of SARS-CoV—A target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009, 7, 226–236. [Google Scholar] [CrossRef]
- Drobysh, M.; Ramanaviciene, A.; Viter, R.; Chen, C.-F.; Samukaite-Bubniene, U.; Ratautaite, V.; Ramanavicius, A. Biosensors for the determination of SARS-CoV-2 virus and diagnosis of COVID-19 infection. Int. J. Mol. Sci. 2022, 23, 666. [Google Scholar] [CrossRef]
- Plikusiene, I.; Maciulis, V.; Ramanaviciene, A.; Balevicius, Z.; Buzavaite-Verteliene, E.; Ciplys, E.; Slibinskas, R.; Simanavicius, M.; Zvirbliene, A.; Ramanavicius, A. Evaluation of kinetics and thermodynamics of interaction between immobilized SARS-CoV-2 nucleoprotein and specific antibodies by total internal reflection ellipsometry. J. Colloid Interface Sci. 2021, 594, 195–203. [Google Scholar] [CrossRef]
- Rabiee, N.; Fatahi, Y.; Ahmadi, S.; Abbariki, N.; Ojaghi, A.; Rabiee, M.; Radmanesh, F.; Dinarvand, R.; Bagherzadeh, M.; Mostafavi, E.; et al. Bioactive hybrid metal-organic framework (MOF)-based nanosensors for optical detection of recombinant SARS-CoV-2 spike antigen. Sci. Total Environ. 2022, 825, 153902. [Google Scholar] [CrossRef]
- Gomez-Gonzalez, E.; Barriga-Rivera, A.; Fernandez-Munoz, B.; Navas-Garcia, J.M.; Fernandez-Lizaranzu, I.; Munoz-Gonzalez, F.J.; Parrilla-Giraldez, R.; Requena-Lancharro, D.; Gil-Gamboa, P.; Rosell-Valle, C.; et al. Optical imaging spectroscopy for rapid, primary screening of SARS-CoV-2: A proof of concept. Sci. Rep. 2022, 12, 2356. [Google Scholar] [CrossRef]
- Plikusiene, I.; Maciulis, V.; Juciute, S.; Ramanavicius, A.; Balevicius, Z.; Slibinskas, R.; Kucinskaite-Kodze, I.; Simanavicius, M.; Balevicius, S.; Ramanaviciene, A. Investigation of SARS-CoV-2 nucleocapsid protein interaction with a specific antibody by combined spectroscopic ellipsometry and quartz crystal microbalance with dissipation. J. Colloid Interface Sci. 2022, 626, 113–122. [Google Scholar] [CrossRef]
- Li, J.; Wu, D.; Yu, Y.; Li, T.; Li, K.; Xiao, M.M.; Li, Y.; Zhang, Z.Y.; Zhang, G.J. Rapid and unamplified identification of COVID-19 with morpholino-modified graphene field-effect transistor nanosensor. Biosens. Bioelectron. 2021, 183, 113206. [Google Scholar] [CrossRef]
- Poghossian, A.; Jablonski, M.; Molinnus, D.; Wege, C.; Schoning, M.J. Field-Effect Sensors for Virus Detection: From Ebola to SARS-CoV-2 and Plant Viral Enhancers. Front. Plant Sci. 2020, 11, 598103. [Google Scholar] [CrossRef]
- Hwang, M.T.; Park, I.; Heiranian, M.; Taqieddin, A.; You, S.; Faramarzi, V.; Pak, A.A.; van der Zande, A.M.; Aluru, N.R.; Bashir, R. Ultrasensitive Detection of Dopamine, IL-6 and SARS-CoV-2 Proteins on Crumpled Graphene FET Biosensor. Adv. Mater. Technol. 2021, 6, 2100712. [Google Scholar] [CrossRef]
- Ventura, B.D.; Cennamo, M.; Minopoli, A.; Campanile, R.; Censi, S.B.; Terracciano, D.; Portella, G.; Velotta, R. Colorimetric Test for Fast Detection of SARS-CoV-2 in Nasal and Throat Swabs. ACS Sens. 2020, 5, 3043–3048. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, W.; Chen, F.; Ren, P. Highly sensitive and selective surface plasmon resonance biosensor for the detection of SARS-CoV-2 spike S1 protein. Analyst 2022, 147, 2809–2818. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, W.; Qin, Y.; Li, Y.; Liu, G.L.; Hu, W. Applying Machine Learning with Localized Surface Plasmon Resonance Sensors to Detect SARS-CoV-2 Particles. Biosensors 2022, 12, 173. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Li, T.; Fan, T.; Meng, C.; Li, C.; Kang, J.; Chai, L.; Hao, Y.; Tang, Y.; et al. A CRISPR/Cas12a-empowered surface plasmon resonance platform for rapid and specific diagnosis of the Omicron variant of SARS-CoV-2. Natl. Sci. Rev. 2022, 9, nwac104. [Google Scholar] [CrossRef]
- Jiang, M.; Dong, T.; Han, C.; Liu, L.; Zhang, T.; Kang, Q.; Wang, P.; Zhou, F. Regenerable and high-throughput surface plasmon resonance assay for rapid screening of anti-SARS-CoV-2 antibody in serum samples. Anal. Chim. Acta 2022, 1208, 339830. [Google Scholar] [CrossRef]
- Kumar, N.; Shetti, N.P.; Jagannath, S.; Aminabhavi, T.M. Electrochemical sensors for the detection of SARS-CoV-2 virus. Chem. Eng. J. 2022, 430, 132966. [Google Scholar] [CrossRef]
- Liustrovaite, V.; Drobysh, M.; Rucinskiene, A.; Baradoke, A.; Ramanaviciene, A.; Plikusiene, I.; Samukaite-Bubniene, U.; Viter, R.; Chen, C.-F.; Ramanavicius, A. Towards an electrochemical immunosensor for the detection of antibodies against SARS-CoV-2 spike protein. J. Electrochem. Soc. 2022, 169, 037523. [Google Scholar] [CrossRef]
- Rashed, M.Z.; Kopechek, J.A.; Priddy, M.C.; Hamorsky, K.T.; Palmer, K.E.; Mittal, N.; Valdez, J.; Flynn, J.; Williams, S.J. Rapid detection of SARS-CoV-2 antibodies using electrochemical impedance-based detector. Biosens. Bioelectron. 2021, 171, 112709. [Google Scholar] [CrossRef]
- Tabrizi, M.A.; Fernández-Blázquez, J.P.; Medina, D.M.; Acedo, P. An ultrasensitive molecularly imprinted polymer-based electrochemical sensor for the determination of SARS-CoV-2-RBD by using macroporous gold screen-printed electrode. Biosens. Bioelectron. 2021, 196, 113729. [Google Scholar] [CrossRef]
- Drobysh, M.; Liustrovaite, V.; Baradoke, A.; Viter, R.; Chen, C.-F.; Ramanavicius, A.; Ramanaviciene, A. Determination of rSpike protein by specific antibodies with screen-printed carbon electrode modified by electrodeposited gold nanostructures. Biosensors 2022, 12, 593. [Google Scholar] [CrossRef]
- Oliveira, M.E.; Lopes, B.V.; Rossato, J.H.H.; Maron, G.K.; Gallo, B.B.; La Rosa, A.B.; Balboni, R.D.C.; Alves, M.L.F.; Ferreira, M.R.A.; da Silva Pinto, L.; et al. Electrochemical Biosensor Based on Laser-Induced Graphene for COVID-19 Diagnosing: Rapid and Low-Cost Detection of SARS-CoV-2 Biomarker Antibodies. Surfaces 2022, 5, 187–201. [Google Scholar] [CrossRef]
- Silva, L.R.G.; Stefano, J.S.; Orzari, L.O.; Brazaca, L.C.; Carrilho, E.; Marcolino-Junior, L.H.; Bergamini, M.F.; Munoz, R.A.A.; Janegitz, B.C. Electrochemical Biosensor for SARS-CoV-2 cDNA Detection Using AuPs-Modified 3D-Printed Graphene Electrodes. Biosensors 2022, 12, 622. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, F.; Xie, W.; Zhou, T.C.; OuYang, J.; Jin, L.; Li, H.; Zhao, C.Y.; Zhang, L.; Wei, J.; et al. Ultrasensitive supersandwich-type electrochemical sensor for SARS-CoV-2 from the infected COVID-19 patients using a smartphone. Sens. Actuators B Chem. 2021, 327, 128899. [Google Scholar] [CrossRef]
- Yang, B.; Zeng, X.; Zhang, J.; Kong, J.; Fang, X. Accurate identification of SARS-CoV-2 variant delta using graphene/CRISPR-dCas9 electrochemical biosensor. Talanta 2022, 249, 123687. [Google Scholar] [CrossRef]
- Hatamluyi, B.; Rezayi, M.; Amel Jamehdar, S.; Rizi, K.S.; Mojarrad, M.; Meshkat, Z.; Choobin, H.; Soleimanpour, S.; Boroushaki, M.T. Sensitive and specific clinically diagnosis of SARS-CoV-2 employing a novel biosensor based on boron nitride quantum dots/flower-like gold nanostructures signal amplification. Biosens. Bioelectron. 2022, 207, 114209. [Google Scholar] [CrossRef]
- Raziq, A.; Kidakova, A.; Boroznjak, R.; Reut, J.; Öpik, A.; Syritski, V. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021, 178, 113029. [Google Scholar] [CrossRef]
- Mojsoska, B.; Larsen, S.; Olsen, D.A.; Madsen, J.S.; Brandslund, I.; Alatraktchi, F.A. Rapid SARS-CoV-2 Detection Using Electrochemical Immunosensor. Sensors 2021, 21, 390. [Google Scholar] [CrossRef]
- Fabiani, L.; Saroglia, M.; Galatà, G.; De Santis, R.; Fillo, S.; Luca, V.; Faggioni, G.; D’Amore, N.; Regalbuto, E.; Salvatori, P.; et al. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens. Bioelectron. 2021, 171, 112686. [Google Scholar] [CrossRef]
- Ameku, W.A.; Provance, D.W.; Morel, C.M.; De-Simone, S.G. Rapid Detection of Anti-SARS-CoV-2 Antibodies with a Screen-Printed Electrode Modified with a Spike Glycoprotein Epitope. Biosensors 2022, 12, 272. [Google Scholar] [CrossRef]
- Liv, L.; Yener, M.; Coban, G.; Can, S.A. Electrochemical biosensing platform based on hydrogen bonding for detection of the SARS-CoV-2 spike antibody. Anal. Bioanal. Chem. 2022, 414, 1313–1322. [Google Scholar] [CrossRef]
- Souza, D.d.; Machado, S.A.S.; Avaca, L.A. Square wave voltammetry. Part I: Theoretical aspects. Química Nova 2003, 26, 81–89. [Google Scholar] [CrossRef]
- Chen, A.; Shah, B. Electrochemical sensing and biosensing based on square wave voltammetry. Anal. Methods 2013, 5, 2158–2173. [Google Scholar] [CrossRef]
- Laborda, E.; Molina, A.; Martínez-Ortiz, F.; Compton, R.G. Electrode modification using porous layers. Maximising the analytical response by choosing the most suitable voltammetry: Differential Pulse vs Square Wave vs Linear sweep voltammetry. Electrochim. Acta 2012, 73, 3–9. [Google Scholar] [CrossRef]
- Uslu, B.; Ozkan, S.A. Electroanalytical Methods for the Determination of Pharmaceuticals: A Review of Recent Trends and Developments. Anal. Lett. 2011, 44, 2644–2702. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, R.; Radhapyari, K.; Jadon, N.; Agarwal, S. Voltammetric techniques for the assay of pharmaceuticals--a review. Anal. Biochem. 2011, 408, 179–196. [Google Scholar] [CrossRef]
- Khan, M.Z.H.; Harkin-Jones, E. Effect of ITO surface properties on SAM modification: A review toward biosensor application. Cogent Eng. 2016, 3, 1170097. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y. AFM and impedance spectroscopy characterization of the immobilization of antibodies on indium-tin oxide electrode through self-assembled monolayer of epoxysilane and their capture of Escherichia coli O157:H7. Biosens. Bioelectron. 2005, 20, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, M.; Can, K.; Ersoz, M. Immobilization of albumin on indium-tin oxide (ITO) surface via isocyanate linkage. J. Electroanal. Chem. 2009, 633, 228–234. [Google Scholar] [CrossRef]
- Gundogdu, A.; Aydin, E.B.; Sezginturk, M.K. A novel electrochemical immunosensor based on ITO modified by carboxyl-ended silane agent for ultrasensitive detection of MAGE-1 in human serum. Anal. Biochem. 2017, 537, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Yagati, A.K.; Lee, T.; Min, J.; Choi, J.W. Electrochemical performance of gold nanoparticle-cytochrome c hybrid interface for H2O2 detection. Colloids Surf. B Biointerfaces 2012, 92, 161–167. [Google Scholar] [CrossRef]
- Canbaz, M.C.; Sezginturk, M.K. Fabrication of a highly sensitive disposable immunosensor based on indium tin oxide substrates for cancer biomarker detection. Anal. Biochem. 2014, 446, 9–18. [Google Scholar] [CrossRef]
- Rao, X.; Guyon, C.; Ognier, S.; Da Silva, B.; Chu, C.; Tatoulian, M.; Hassan, A.A. High density gold nanoparticles immobilized on surface via plasma deposited APTES film for decomposing organic compounds in microchannels. Appl. Surf. Sci. 2018, 439, 272–281. [Google Scholar] [CrossRef]
- El-Said, W.A.; Al-Bogami, A.S.; Alshitari, W. Synthesis of gold nanoparticles@reduced porous graphene-modified ITO electrode for spectroelectrochemical detection of SARS-CoV-2 spike protein. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 264, 120237. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Senturk, H.; Yildiz, E.; Maral, M. Impedimetric Detection Based on Label-Free Immunoassay Developed for Targeting Spike S1 Protein of SARS-CoV-2. Diagnostics 2022, 12, 1992. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeong, S.; Sarawut, S.; Kim, H.; Son, S.U.; Lee, S.; Rabbani, G.; Kwon, H.; Lim, E.K.; Ahn, S.N.; et al. An immunosensor based on a high performance dual-gate oxide semiconductor thin-film transistor for rapid detection of SARS-CoV-2. Lab Chip 2022, 22, 899–907. [Google Scholar] [CrossRef]
- Chang, H.; Jiang, M.; Zhu, Q.; Liu, A.; Wu, Y.; Li, C.; Ji, X.; Gong, L.; Li, S.; Chen, Z.; et al. A novel photoelectrochemical immunosensor based on TiO2@Bi2WO6 hollow microspheres and Ag2S for sensitive detection of SARS-CoV-2 nucleocapsid protein. Microchem. J. 2022, 182, 107866. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, T.; Luo, T.; Luo, X.; Yan, F.; Tang, W.; Liu, J. Bipolar silica nanochannel array confined electrochemiluminescence for ultrasensitive detection of SARS-CoV-2 antibody. Biosens. Bioelectron. 2022, 215, 114563. [Google Scholar] [CrossRef]
- Hwang, D.-K.; Misra, M.; Lee, Y.-E.; Baek, S.-D.; Myoung, J.-M.; Lee, T.I. The role of Ar plasma treatment in generating oxygen vacancies in indium tin oxide thin films prepared by the sol-gel process. Appl. Surf. Sci. 2017, 405, 344–349. [Google Scholar] [CrossRef]
- Sypabekova, M.; Hagemann, A.; Rho, D.; Kim, S. Review: 3-Aminopropyltriethoxysilane (APTES) Deposition Methods on Oxide Surfaces in Solution and Vapor Phases for Biosensing Applications. Biosensors 2022, 13, 36. [Google Scholar] [CrossRef]
- Drobysh, M.; Liustrovaite, V.; Baradoke, A.; Rucinskiene, A.; Ramanaviciene, A.; Ratautaite, V.; Viter, R.; Chen, C.-F.; Plikusiene, I.; Samukaite-Bubniene, U.; et al. Electrochemical determination of interaction between SARS-CoV-2 spike protein and specific antibodies. Int. J. Mol. Sci. 2022, 23, 6768. [Google Scholar] [CrossRef]
- Rahmati, Z.; Roushani, M.; Hosseini, H.; Choobin, H. An electrochemical immunosensor using SARS-CoV-2 spike protein-nickel hydroxide nanoparticles bio-conjugate modified SPCE for ultrasensitive detection of SARS-CoV-2 antibodies. Microchem. J. 2021, 170, 106718. [Google Scholar] [CrossRef] [PubMed]
- Drobysh, M.; Ramanavicius, A.; Baradoke, A. Polyaniline-based electrochemical immunosensor for the determination of antibodies against SARS-CoV-2 spike protein. Sci. Total Environ. 2023, 862, 160700. [Google Scholar] [CrossRef]
- Liustrovaite, V.; Drobysh, M.; Ratautaite, V.; Ramanaviciene, A.; Rimkute, A.; Simanavicius, M.; Dalgediene, I.; Kucinskaite-Kodze, I.; Plikusiene, I.; Chen, C.-F.; et al. Electrochemical biosensor for the evaluation of monoclonal antibodies targeting the N protein of SARS-CoV-2 virus. Sci. Total Environ. 2024, 924, 171042. [Google Scholar] [CrossRef] [PubMed]
- Samper, I.C.; Sanchez-Cano, A.; Khamcharoen, W.; Jang, I.; Siangproh, W.; Baldrich, E.; Geiss, B.J.; Dandy, D.S.; Henry, C.S. Electrochemical Capillary-Flow Immunoassay for Detecting Anti-SARS-CoV-2 Nucleocapsid Protein Antibodies at the Point of Care. ACS Sens. 2021, 6, 4067–4075. [Google Scholar] [CrossRef]
- Yakoh, A.; Pimpitak, U.; Rengpipat, S.; Hirankarn, N.; Chailapakul, O.; Chaiyo, S. Paper-based electrochemical biosensor for diagnosing COVID-19: Detection of SARS-CoV-2 antibodies and antigen. Biosens. Bioelectron. 2021, 176, 112912. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, A.; Afaghi, P.; Ghandi, K. Catalysis on Nanostructured Indium Tin Oxide Surface for Fast and Inexpensive Probing of Antibodies during Pandemics. Catalysts 2021, 11, 191. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Guo, W.; Zhang, T.; Kang, Q.; Wang, P.; Zhou, F.; Yang, L. Flow injection analysis coupled with photoelectrochemical immunoassay for simultaneous detection of anti-SARS-CoV-2-spike and anti-SARS-CoV-2-nucleocapsid antibodies in serum samples. Anal. Chim. Acta 2023, 1280, 341857. [Google Scholar] [CrossRef]

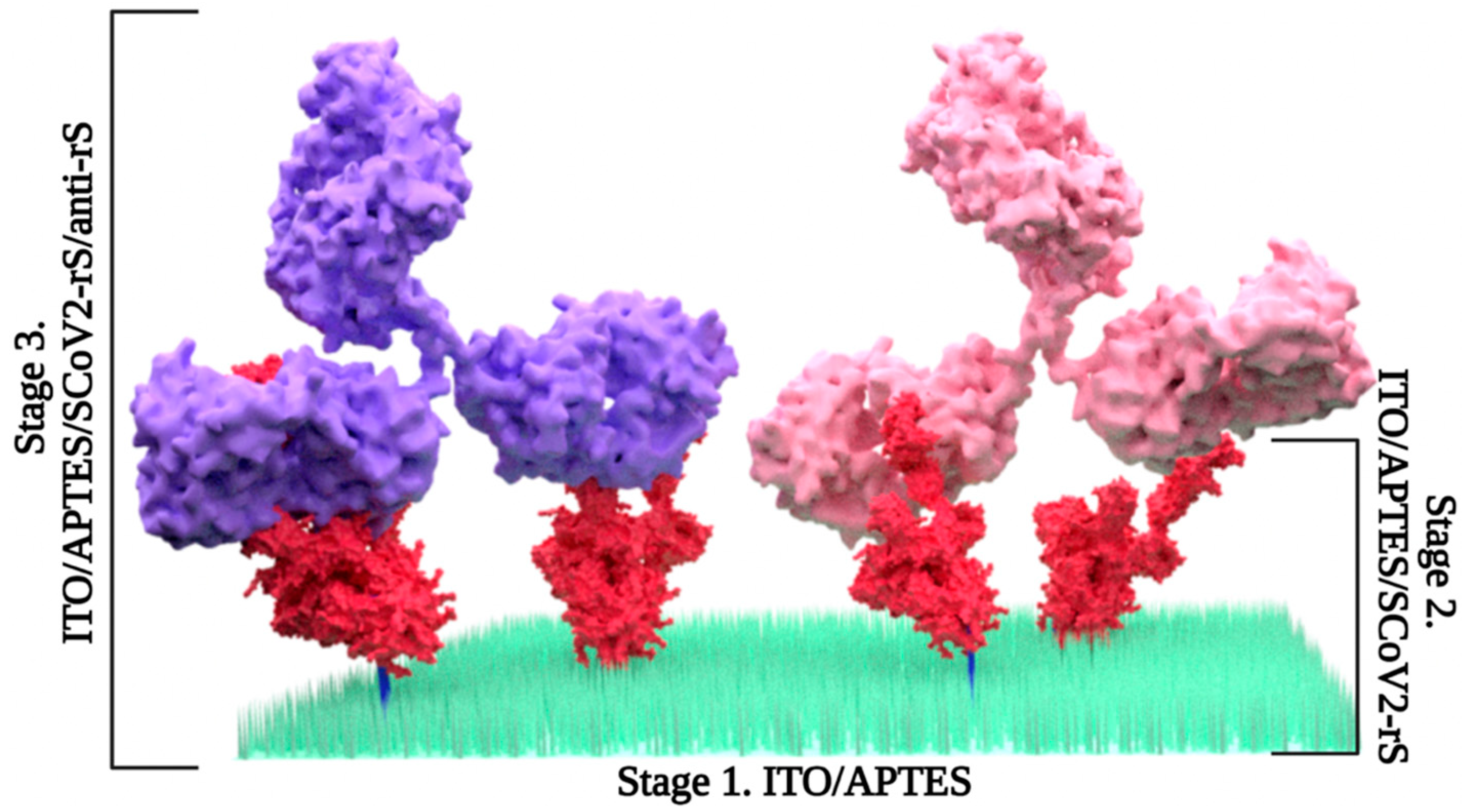
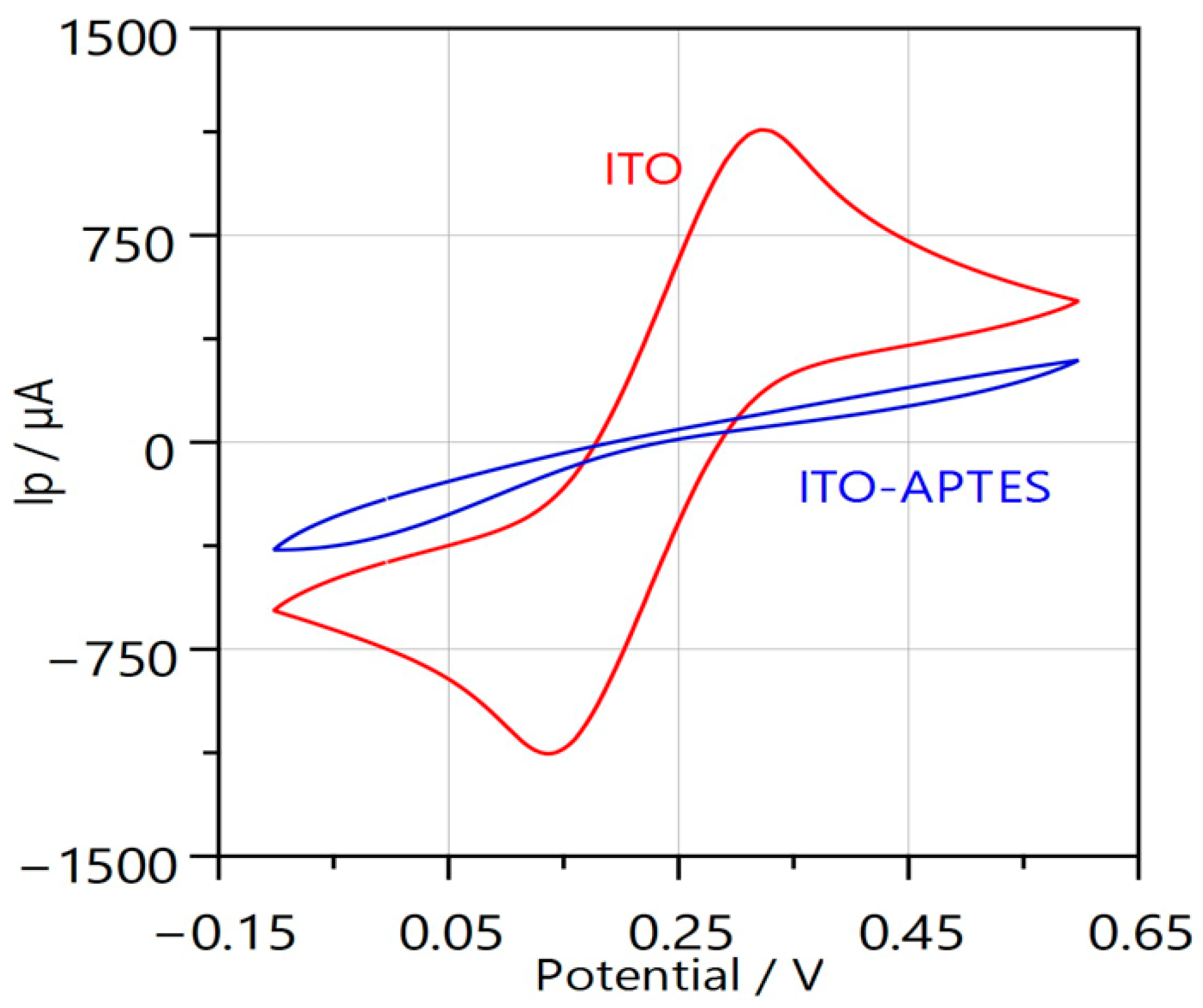
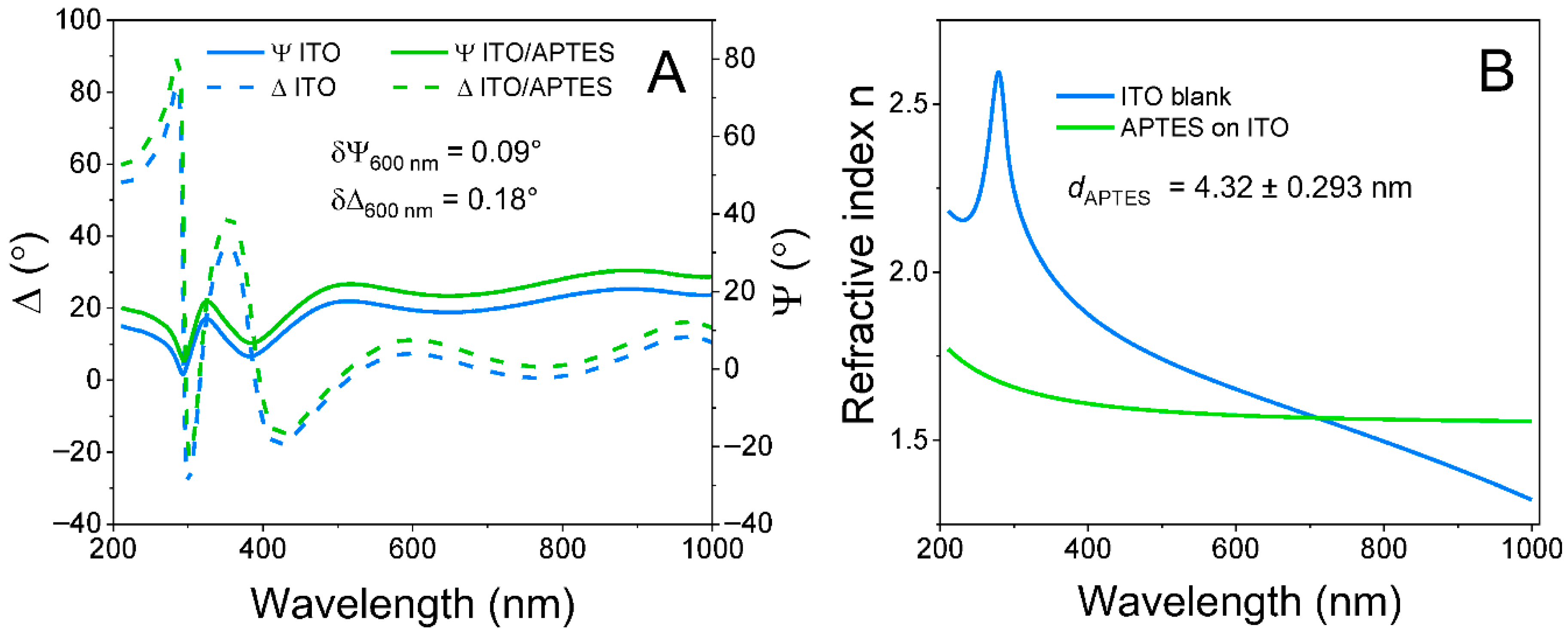
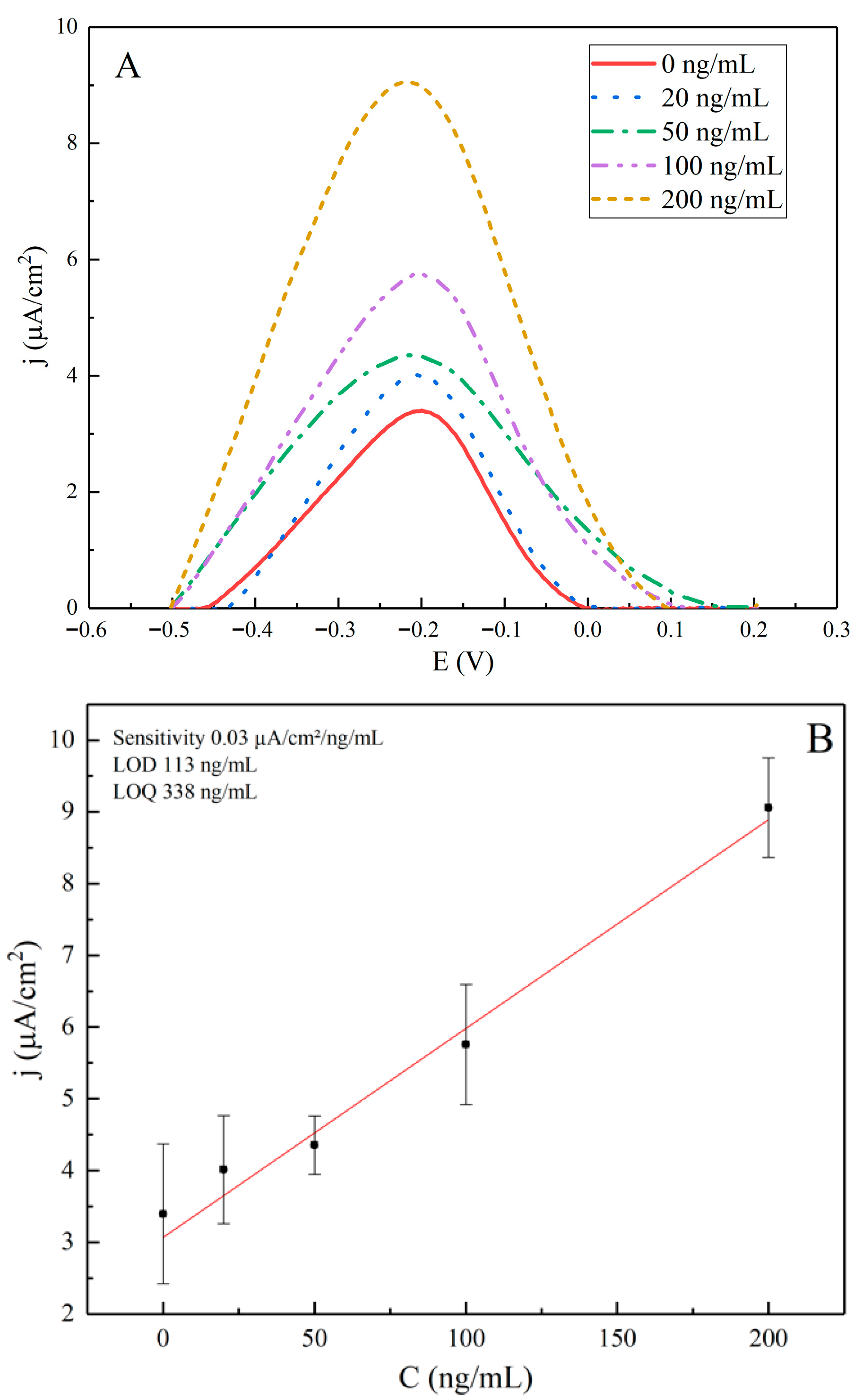
| [anti-rS], ng/mL | j, µA/cm2 |
|---|---|
| 0 | 4.09 ± 0.98 |
| 20 | 3.48 ± 0.75 |
| 50 | 4.07 ± 0.41 |
| 100 | 5.17 ± 0.84 |
| 200 | 8.57 ± 0.69 |
| Electrode | Sensing Element | Method | Redox Probe | LOD | |
|---|---|---|---|---|---|
| SPCE | Spike glycoprotein | CV, DPV | [Fe(CN)6]4−/3− | 0.27 nM, 0.14 nM | [21] |
| SPCE | Spike glycoprotein | DPV | [Fe(CN)6]4−/3− | 0.30 aM | [52] |
| SPCE | Spike glycoprotein | Electrochemical impedance spectroscopy (EIS) | - | 0.42 nM | [53] |
| SPCE | Nucleocapsid protein | EIS, SWV | [Fe(CN)6]4−/3− | 16 pM, 47 pM | [54] |
| SPCE | Nucleocapsid protein | Chronoamperometry | - | 13 pM | [55] |
| Paper-based SPCE | Receptor-binding domain | SWV | [Fe(CN)6]4−/3− | 6.40 pM | [56] |
| ITO | Spike glycoprotein | Electrical Resistive Sensing | - | ~0.50 nM | [57] |
| ITO | Spike glycoprotein, Nucleocapsid protein | Photoelectrochemical | - | 1.18 nM, 0.65 nM | [58] |
| ITO | Spike glycoprotein | SWV | - | 0.75 nM | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zvirzdine, G.; Drobysh, M.; Ramanaviciene, A.; Ratautaite, V.; Zukauskas, S.; Stanciauskaite, M.; Plikusiene, I.; Ramanavicius, A. Indium Tin Oxide-Based Voltammetric Biosensor for the Detection of Antibodies Against the SARS-CoV-2 Virus Spike Protein. Sensors 2025, 25, 6737. https://doi.org/10.3390/s25216737
Zvirzdine G, Drobysh M, Ramanaviciene A, Ratautaite V, Zukauskas S, Stanciauskaite M, Plikusiene I, Ramanavicius A. Indium Tin Oxide-Based Voltammetric Biosensor for the Detection of Antibodies Against the SARS-CoV-2 Virus Spike Protein. Sensors. 2025; 25(21):6737. https://doi.org/10.3390/s25216737
Chicago/Turabian StyleZvirzdine, Greta, Maryia Drobysh, Almira Ramanaviciene, Vilma Ratautaite, Sarunas Zukauskas, Migle Stanciauskaite, Ieva Plikusiene, and Arunas Ramanavicius. 2025. "Indium Tin Oxide-Based Voltammetric Biosensor for the Detection of Antibodies Against the SARS-CoV-2 Virus Spike Protein" Sensors 25, no. 21: 6737. https://doi.org/10.3390/s25216737
APA StyleZvirzdine, G., Drobysh, M., Ramanaviciene, A., Ratautaite, V., Zukauskas, S., Stanciauskaite, M., Plikusiene, I., & Ramanavicius, A. (2025). Indium Tin Oxide-Based Voltammetric Biosensor for the Detection of Antibodies Against the SARS-CoV-2 Virus Spike Protein. Sensors, 25(21), 6737. https://doi.org/10.3390/s25216737











