Immediate Biomechanical Effects of Manual and Tool-Assisted Myofascial Release on the Erector Spinae Muscle
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
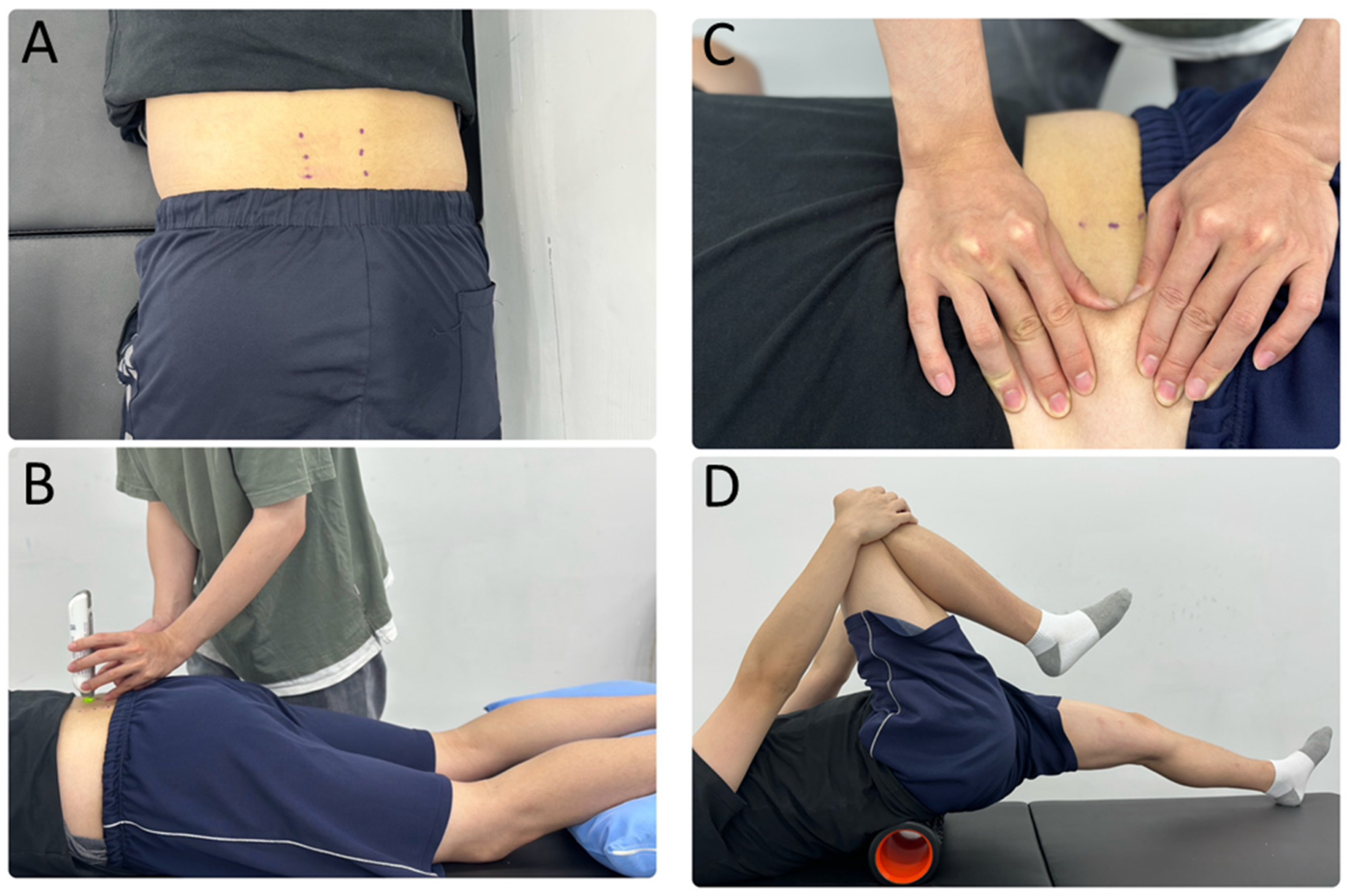
2.3. Myometric Parameter Measurement
2.4. Myofascial Release Intervention Procedures
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Mass index |
| EMG | Electromyography |
| MMR | Manual myofascial release |
| RCT | Randomized controlled trial |
| TMR | Tool-assisted myofascial release |
References
- Miyamori, T.; Saito, T.; Aoyagi, M.; Nozu, S.; Masui, Y.; Ishihara, Y.; Shimasaki, Y.; Yoshimura, M. Differences in the elastic modulus of the lumbar muscles between female athletes with and without low back pain. Clin. Biomech. 2023, 105, 105968. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, J.; Zhang, J.; Zhang, Z.; Wang, X. Quantifying the stiffness of lumbar erector spinae during different positions among participants with chronic low back pain. PLoS ONE 2022, 17, e0270286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Ng, G.Y.F.; Lee, W.C.; Fu, S.N. Increase in passive muscle tension of the quadriceps muscle heads in jumping athletes with patellar tendinopathy. Scand. J. Med. Sci. Sports 2017, 27, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Brocherie, F.; Millet, G.P.; Girard, O. Neuro-mechanical and metabolic adjustments to the repeated anaerobic sprint test in professional football players. Eur. J. Appl. Physiol. 2015, 115, 891–903. [Google Scholar] [CrossRef]
- Agoriwo, M.W.; Muckelt, P.E.; Yeboah, C.O.; Sankah, B.E.A.; Agyapong-Badu, S.; Akpalu, A.; Stokes, M. Feasibility and reliability of measuring muscle stiffness in Parkinson’s Disease using MyotonPRO device in a clinical setting in Ghana. Ghana. Med. J. 2022, 56, 78–85. [Google Scholar] [CrossRef]
- Jo, S.H.; Choi, H.J.; Cho, H.S.; Yoon, J.H.; Lee, W.Y. Effect of Core Balance Training on Muscle Tone and Balance Ability in Adult Men and Women. Int. J. Environ. Res. Public Health 2022, 19, 12190. [Google Scholar] [CrossRef]
- Kurashina, W.; Iijima, Y.; Sasanuma, H.; Saito, T.; Takeshita, K. Evaluation of muscle stiffness in adhesive capsulitis with Myoton PRO. JSES Int. 2023, 7, 25–29. [Google Scholar] [CrossRef]
- Schneider, S.; Peipsi, A.; Stokes, M.; Knicker, A.; Abeln, V. Feasibility of monitoring muscle health in microgravity environments using Myoton technology. Med. Biol. Eng. Comput. 2015, 53, 57–66. [Google Scholar] [CrossRef]
- Bethers, A.H.; Swanson, D.C.; Sponbeck, J.K.; Mitchell, U.H.; Draper, D.O.; Feland, J.B.; Johnson, A.W. Positional release therapy and therapeutic massage reduce muscle trigger and tender points. J. Bodyw. Mov. Ther. 2021, 28, 264–270. [Google Scholar] [CrossRef]
- Behm, D.G.; Wilke, J. Do Self-Myofascial Release Devices Release Myofascia? Rolling Mechanisms: A Narrative Review. Sports Med. 2019, 49, 1173–1181. [Google Scholar] [CrossRef]
- Lopez-Torres, O.; Mon-Lopez, D.; Gomis-Marza, C.; Lorenzo, J.; Guadalupe-Grau, A. Effects of myofascial release or self-myofascial release and control position exercises on lower back pain in idiopathic scoliosis: A systematic review. J. Bodyw. Mov. Ther. 2021, 27, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, J.; Wang, X.; Wu, J.; Ren, Z. The effects of myofascial release technique for patients with low back pain: A systematic review and meta-analysis. Complement. Ther. Med. 2021, 59, 102737. [Google Scholar] [CrossRef]
- Dal Farra, F.; Risio, R.G.; Vismara, L.; Bergna, A. Effectiveness of osteopathic interventions in chronic non-specific low back pain: A systematic review and meta-analysis. Complement. Ther. Med. 2021, 56, 102616. [Google Scholar] [CrossRef] [PubMed]
- Ceca, D.; Elvira, L.; Guzman, J.F.; Pablos, A. Benefits of a self-myofascial release program on health-related quality of life in people with fibromyalgia: A randomized controlled trial. J. Sports Med. Phys. Fitness 2017, 57, 993–1002. [Google Scholar] [CrossRef]
- Chang, T.T.; Li, Z.; Zhu, Y.C.; Wang, X.Q.; Zhang, Z.J. Effects of Self-Myofascial Release Using a Foam Roller on the Stiffness of the Gastrocnemius-Achilles Tendon Complex and Ankle Dorsiflexion Range of Motion. Front. Physiol. 2021, 12, 718827. [Google Scholar] [CrossRef]
- Laffaye, G.; Da Silva, D.T.; Delafontaine, A. Self-Myofascial Release Effect With Foam Rolling on Recovery After High-Intensity Interval Training. Front. Physiol. 2019, 10, 1287. [Google Scholar] [CrossRef]
- Yokochi, M.; Nakamura, M.; Iwata, A.; Kaneko, R.; Konrad, A.; Yamada, N. Efficacy of self-care foam rolling intervention on muscle function and pain conducted by postoperative patients who underwent total knee arthroplasty from the second to the third postoperative week. J. Bodyw. Mov. Ther. 2024, 40, 1177–1180. [Google Scholar] [CrossRef]
- Beardsley, C.; Škarabot, J. Effects of self-myofascial release: A systematic review. J. Bodyw. Mov. Ther. 2015, 19, 747–758. [Google Scholar] [CrossRef]
- Ianieri, G.; Saggini, R.; Marvulli, R.; Tondi, G.; Aprile, A.; Ranieri, M.; Benedetto, G.; Altini, S.; Lancioni, G.E.; Goffredo, L.; et al. New approach in the assessment of the tone, elasticity and the muscular resistance: Nominal scales vs MYOTON. Int. J. Immunopathol. Pharmacol. 2009, 22 (Suppl. S3), 21–24. [Google Scholar] [CrossRef]
- Ditroilo, M.; Hunter, A.M.; Haslam, S.; De Vito, G. The effectiveness of two novel techniques in establishing the mechanical and contractile responses of biceps femoris. Physiol. Meas. 2011, 32, 1315–1326. [Google Scholar] [CrossRef]
- Bizzini, M.; Mannion, A.F. Reliability of a new, hand-held device for assessing skeletal muscle stiffness. Clin. Biomech. 2003, 18, 459–461. [Google Scholar] [CrossRef]
- Koterba, J.; Saulicz, E. Reliability of measurement of neck and back muscle mechanical properties using MyotonPRO: A systematic review. J. Bodyw. Mov. Ther. 2025, 44, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Lettner, J.; Królikowska, A.; Ramadanov, N.; Oleksy, Ł.; Hakam, H.T.; Becker, R.; Prill, R. Evaluating the Reliability of MyotonPro in Assessing Muscle Properties: A Systematic Review of Diagnostic Test Accuracy. Medicina 2024, 60, 851. [Google Scholar] [CrossRef] [PubMed]
- Wilke, J.; Niemeyer, P.; Niederer, D.; Schleip, R.; Banzer, W. Influence of Foam Rolling Velocity on Knee Range of Motion and Tissue Stiffness: A Randomized, Controlled Crossover Trial. J. Sport. Rehabil. 2019, 28, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Urrejola-Contreras, G.P.; Martínez, J.M.; Rodríguez-Bagó, M.; Ronda, E. Myotonometry in machinery operators and its relationship with postural ergonomic risk. Ann. Work. Expo. Health 2024, 68, 605–616. [Google Scholar] [CrossRef]
- Qu, G.; Wang, H.; Zhou, G.; Liu, H. Effects of two-week machine massage on muscle properties in adolescent wrestlers. Front. Physiol. 2023, 14, 1129836. [Google Scholar] [CrossRef]
- Korhonen, R.K.; Vain, A.; Vanninen, E.; Viir, R.; Jurvelin, J.S. Can mechanical myotonometry or electromyography be used for the prediction of intramuscular pressure? Physiol. Meas. 2005, 26, 951–963. [Google Scholar] [CrossRef]
- Feng, Y.N.; Li, Y.P.; Liu, C.L.; Zhang, Z.J. Assessing the elastic properties of skeletal muscle and tendon using shearwave ultrasound elastography and MyotonPRO. Sci. Rep. 2018, 8, 17064. [Google Scholar] [CrossRef]
- Lohr, C.; Braumann, K.-M.; Reer, R.; Schroeder, J.; Schmidt, T. Reliability of tensiomyography and myotonometry in detecting mechanical and contractile characteristics of the lumbar erector spinae in healthy volunteers. Eur. J. Appl. Physiol. 2018, 118, 1349–1359. [Google Scholar] [CrossRef]
- Pruyn, E.C.; Watsford, M.L.; Murphy, A.J. Validity and reliability of three methods of stiffness assessment. J. Sport. Health Sci. 2016, 5, 476–483. [Google Scholar] [CrossRef]
- Wong, K.K.; Chai, H.M.; Chen, Y.J.; Wang, C.L.; Shau, Y.W.; Wang, S.F. Mechanical deformation of posterior thoracolumbar fascia after myofascial release in healthy men: A study of dynamic ultrasound imaging. Musculoskelet. Sci. Pract. 2017, 27, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, K.; Takei, H.; Usa, H.; Mitomo, S.; Ogawa, D. Comparative analysis of ultrasound changes in the vastus lateralis muscle following myofascial release and thermotherapy: A pilot study. J. Bodyw. Mov. Ther. 2015, 19, 327–336. [Google Scholar] [CrossRef]
- Sert, B.; Elverisli, G.B.; Atilgan, E. Effectiveness of structured myofascial release in the treatment of primary dysmenorrhea. Eur. J. Obstet. Gynecol. Reprod. Biol. 2025, 311, 114025. [Google Scholar] [CrossRef]
- Devantery, K.; Morin, M.; Grimard, J.; Gaudreault, N. Effects of a Myofascial Technique on the Stiffness and Thickness of the Thoracolumbar Fascia and Lumbar Erector Spinae Muscles in Adults with Chronic Low Back Pain: A Randomized before-and-after Experimental Study. Bioengineering 2023, 10, 332. [Google Scholar] [CrossRef]
- Bialosky, J.E.; Bishop, M.D.; Price, D.D.; Robinson, M.E.; George, S.Z. The mechanisms of manual therapy in the treatment of musculoskeletal pain: A comprehensive model. Man. Ther. 2009, 14, 531–538. [Google Scholar] [CrossRef]
- Szikszay, T.M.; Adamczyk, W.M.; Carvalho, G.F.; Dolotov, D.; Erdmann, R.; Heitkamp, H.; Jung, A.; Luebke, L.; Rogosch, K.; Luedtke, K. Association between myofascial trigger point therapy and conditioned pain modulation. J. Bodyw. Mov. Ther. 2024, 38, 73–80. [Google Scholar] [CrossRef]
- Ajimsha, M.S.; Al-Mudahka, N.R.; Al-Madzhar, J.A. Effectiveness of myofascial release: Systematic review of randomized controlled trials. J. Bodyw. Mov. Ther. 2015, 19, 102–112. [Google Scholar] [CrossRef]
- Morales-Artacho, A.J.; Lacourpaille, L.; Guilhem, G. Effects of warm-up on hamstring muscles stiffness: Cycling vs foam rolling. Scand. J. Med. Sci. Sports 2017, 27, 1959–1969. [Google Scholar] [CrossRef]
- Heiß, R.; Mayer, I.; Hüttel, M.; Lutter, C.; Forst, R.; Hoppe, M.; Freiwald, J.; Roemer, F.; Hotfiel, T. Evaluation of Tissue Stiffness in Athletes with Different Experience in Foam Rolling Assessed by Acoustic Radiation Force Impulse Elastography. Semin. Musculoskelet. Radiol. 2019, 23, S1–S6. [Google Scholar] [CrossRef]
- Ishida, H.; Suehiro, T. Immediate effects of foam rolling on lateral thigh soft tissue movement: A pilot study. J. Bodyw. Mov. Ther. 2025, 42, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Kozlenia, D.; Domaradzki, J. Acute Effect of Short Intensive Self-Myofascial Release on Jump Performance in Amateur Athletes: A Randomized Cross-Over Study. Int. J. Environ. Res. Public Health 2022, 19, 16816. [Google Scholar] [CrossRef]
- Koruk, H.; Rajagopal, S. A Comprehensive Review on the Viscoelastic Parameters Used for Engineering Materials, Including Soft Materials, and the Relationships between Different Damping Parameters. Sensors 2024, 24, 6137. [Google Scholar] [CrossRef]
- Schleip, R. Fascial plasticity—A new neurobiological explanation: Part 1. J. Bodyw. Mov. Ther. 2003, 7, 11–19. [Google Scholar] [CrossRef]
- Schleip, R. Fascial plasticity—A new neurobiological explanation Part 2. J. Bodyw. Mov. Ther. 2003, 7, 104–116. [Google Scholar] [CrossRef]
- Hirose, N.; Yoshimura, A.; Akiyama, K.; Furusho, A. Sex and pressure effects of foam rolling on acute range of motion in the hamstring muscles. PLoS ONE 2025, 20, e0319148. [Google Scholar] [CrossRef]
| Group | ||
|---|---|---|
| MMR | TMR | |
| Female | ||
| Number | 10 | 6 |
| Age (years) | 21.10 ± 2.60 | 21.00 ± 0.00 |
| Weight (kg) | 50.80 ± 5.49 | 53.67 ± 3.93 |
| Height (m) | 1.59 ± 0.06 | 1.58 ± 0.06 |
| BMI | 20.10 ± 1.64 | 21.53 ± 1.62 |
| Male | ||
| Number | 5 | 9 |
| Age (years) | 20.40 ± 2.19 | 21.67 ± 1.58 |
| Weight (kg) | 70.60 ± 10.78 | 67.89 ± 9.61 |
| Height (m) | 1.75 ± 0.05 | 1.73 ± 0.06 |
| BMI | 22.95 ± 2.49 | 21.49 ± 2.43 |
| Elasticity | Stiffness | Tone | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | df | F | p | η2 | df | F | p | η2 | df | F | p | η2 |
| Between-subjects effects | ||||||||||||
| Gender | 1 | 2.284 | 0.143 | 0.081 | 1 | 5.503 | 0.027 * | 0.175 | 1 | 4.329 | 0.047 * | 0.143 |
| Group | 1 | 0.322 | 0.575 | 0.012 | 1 | 0.266 | 0.611 | 0.010 | 1 | 2.420 | 0.132 | 0.085 |
| Gender × Group | 1 | 0.063 | 0.804 | 0.002 | 1 | 1.339 | 0.258 | 0.049 | 1 | 2.774 | 0.108 | 0.096 |
| Error | 26 | 26 | 26 | |||||||||
| Within-subjects effects | ||||||||||||
| Time | 1 | 3.058 | 0.092 | 0.105 | 1 | 18.772 | <0.001 † | 0.419 | 1 | 2.575 | 0.121 | 0.090 |
| Time × Gender | 1 | 0.015 | 0.903 | 0.001 | 1 | 1.137 | 0.296 | 0.042 | 1 | 0.746 | 0.396 | 0.028 |
| Time × Group | 1 | 7.023 | 0.014 * | 0.213 | 1 | 10.107 | 0.004 † | 0.280 | 1 | 12.643 | 0.001 † | 0.327 |
| Time × Gender × Group | 1 | 0.901 | 0.351 | 0.033 | 1 | 0.005 | 0.943 | 0.000 | 1 | 0.719 | 0.404 | 0.027 |
| Error (Time) | 26 | 26 | 26 | |||||||||
| Pre-Intervention | Post-Intervention | Percentage Change (%) | a Comparison Between Pre and Post-Intervention | |||||
|---|---|---|---|---|---|---|---|---|
| Myoton | Group | n | Mean ± SD | Mean ± SD | Mean ± SD | df | T | p Value |
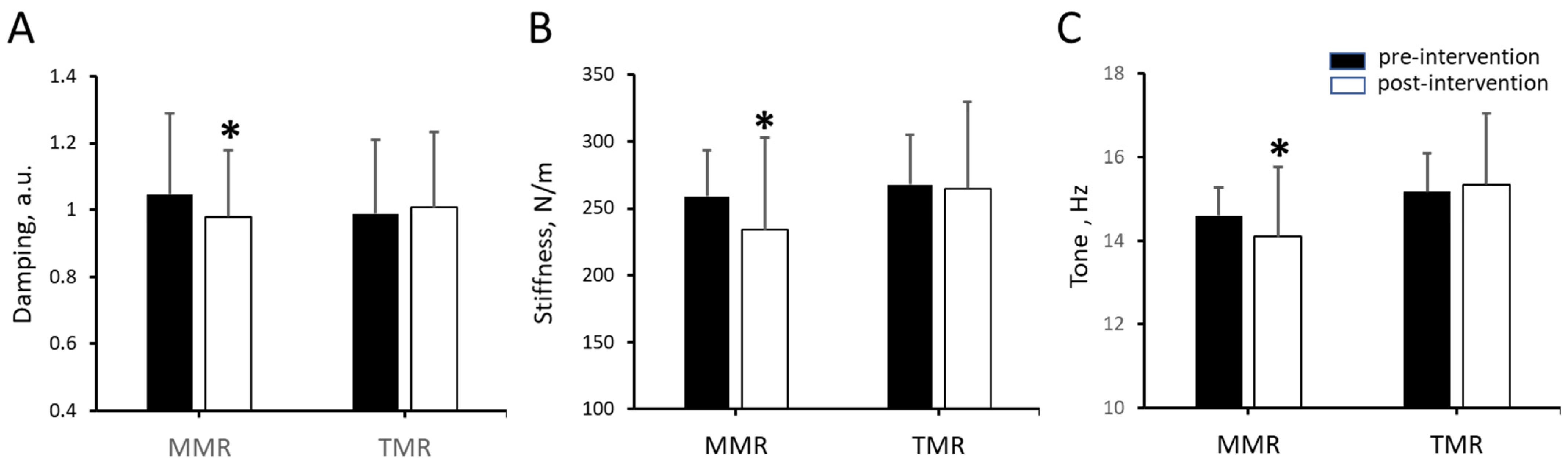
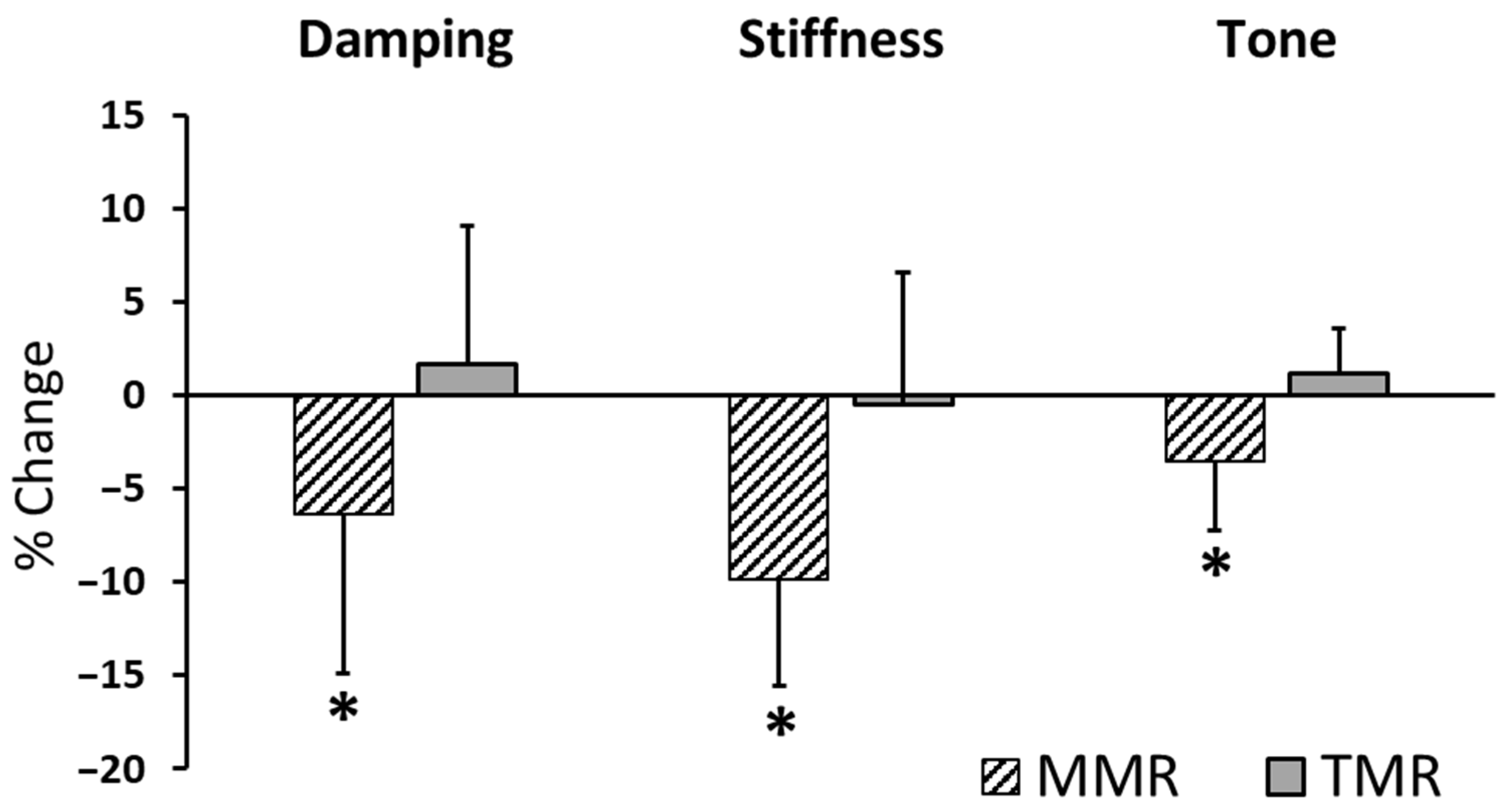
| Elasticity | MMR | 15 | 1.046 ± 0.242 | 0.979 ± 0.222 | −6.389 ± 8.514 | 14 | 2.747 | 0.016 |
| (logarithmic decrement) | TMR | 15 | 0.988 ± 0.200 | 1.006 ± 0.227 | 1.715 ± 7.402 | 14 | −0.980 | 0.344 |
| b p value | 0.482 | 0.738 | < 0.0125 | |||||
| Stiffness | MMR | 15 | 258.831 ± 34.495 | 233.847 ± 37.19 | −9.813 ±5.769 | 14 | 6.589 | <0.0125 |
| (N/m) | TMR | 15 | 267.650 ± 68.849 | 264.628 ± 65.44 | −0.519 ± 7.089 | 14 | 0.652 | 0.525 |
| b p value | 0.661 | 0.127 | <0.0125 | |||||
| Tone | MMR | 15 | 14.597 ± 0.691 | 14.092 ± 0.922 | −3.489 ± 3.730 | 14 | 3.639 | <0.0125 |
| (Hz) | TMR | 15 | 15.168 ± 1.678 | 15.340 ± 1.701 | 1.191 ± 2.411 | 14 | −1.835 | 0.088 |
| b p value | 0.233 | 0.019 | <0.0125 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, Y.-L.; Lin, H.-Y.; Chien, A. Immediate Biomechanical Effects of Manual and Tool-Assisted Myofascial Release on the Erector Spinae Muscle. Sensors 2025, 25, 7021. https://doi.org/10.3390/s25227021
Hsieh Y-L, Lin H-Y, Chien A. Immediate Biomechanical Effects of Manual and Tool-Assisted Myofascial Release on the Erector Spinae Muscle. Sensors. 2025; 25(22):7021. https://doi.org/10.3390/s25227021
Chicago/Turabian StyleHsieh, Yueh-Ling, Heng-Yi Lin, and Andy Chien. 2025. "Immediate Biomechanical Effects of Manual and Tool-Assisted Myofascial Release on the Erector Spinae Muscle" Sensors 25, no. 22: 7021. https://doi.org/10.3390/s25227021
APA StyleHsieh, Y.-L., Lin, H.-Y., & Chien, A. (2025). Immediate Biomechanical Effects of Manual and Tool-Assisted Myofascial Release on the Erector Spinae Muscle. Sensors, 25(22), 7021. https://doi.org/10.3390/s25227021







