Asymmetric Dimethylarginine Vibrational Spectroscopy Spectra and Density Functional Theory Model
Highlights
- First comprehensive vibrational characterization of isolated NG, NG-dimethylarginine (ADMA) using Raman and FT-IR spectroscopy.
- Experimental spectra were successfully correlated with DFT-based simulated spectra, achieving up to 86.67% vibrational band assignment for FT-IR and 54% for Raman spectra.
- Key vibrational modes such as N–H scissoring (1621 cm−1) and C=NH stretching (1667 cm−1) were confirmed through experimental and theoretical methods.
- The isolated ADMA molecule provided a clear spectral fingerprint, free from matrix effects, establishing a reference for future biomedical and diagnostic studies.
- This integrated experimental–computational approach supports the development of spectroscopic biomarkers for clinical diagnostics related to cardiovascular diseases.
Abstract
1. Introduction
2. Experimental
2.1. Spectral Normalization and Visualization
2.2. Feature Metrics
3. Results and Discussion
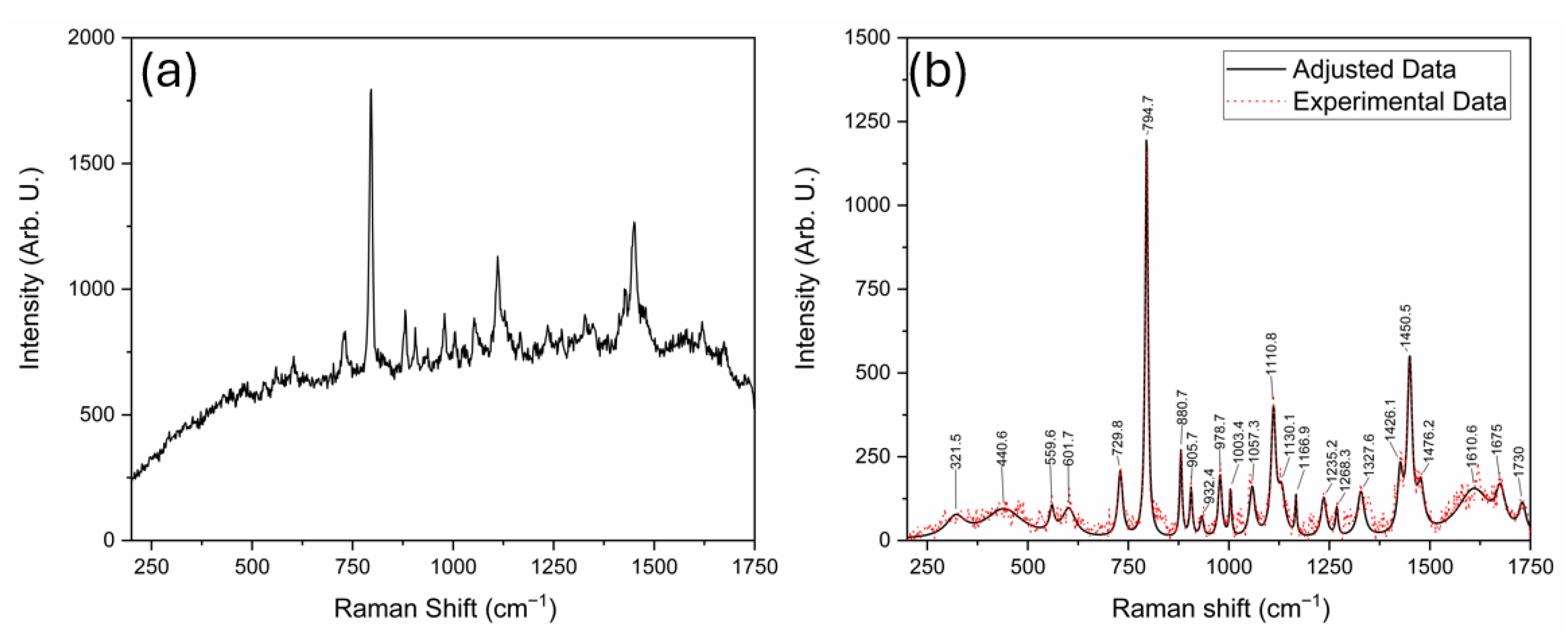
3.1. Raman Spectrum Analysis
| Simulated Bands | Bands Using a 785 nm Laser | Bands Using a 532 nm Laser | Assignments |
|---|---|---|---|
| 228.8 | 217.2 | ω C-H; ρ N-H | |
| 243 | 252.2 | τ N-H | |
| 270.7 | γ Molecular | ||
| 318.3 | 305.4 | 321.5 | γ Molecular |
| 360.8 | 336.2 | γ Molecular | |
| 411.5 | 419.2 | γ Molecular | |
| 428.8 | 440.6 | γ Molecular | |
| 492.8 | 488.5 | ρ Molecular | |
| 538.2 | 559.6 | ρ NH2; τ C-O, C=O | |
| 583.2 | 561.3 | δ N-C; γ C-H; ω N-H; ω C-O | |
| 631.3 | 610.4 | 601.7 | δ C-O; ρ C-H; γ Molecular |
| 736.2 | 726.5 | 729.8 | ν C-C; δ C-O; τ C-H |
| 796.5 | 796.1 | 794.7 | ω N-H, C-N; τ C-H |
| 846.7 | 881.7 | 880.7 | γ Molecular |
| 929.5 | 934.2 | 932.4 | ν C-C; ρ C-H; ω N-H |
| 966.6 | 976.9 | 978.7 | τ C-H; ν C-C; ν N-C; ν N-H, ν C-O |
| 1004.9 | 1003.4 | ν C-C; ρ N-H, C-H; ν C=N | |
| 1068.2 | 1053.1 | 1057.3 | τ N-H; ν C-N, C=N: ρ C-H |
| 1118.7 | 1108.1 | 1110.8 | γ Molecular |
| 1142.5 | 1166.9 | γ Molecular | |
| 1242.9 | 1256.2 | 1235.2 | τ N-H; τ C-H |
| 1308 | ω C-H; τ N-H, ν O-H, C-O | ||
| 1359.4 | 1326.2 | 1327.6 | γ Molecular |
| 1381.9 | γ Molecular | ||
| 1419.3 | 1426.1 | γ Molecular | |
| 1456.7 | 1449.2 | 1450.5 | δ C-H; τ N-H |
| 1473.6 | 1476.2 | δ C-H | |
| 1528.3 | ρ C-H; τ C-H; δ C-H; ν C-H | ||
| 1634.4 | 1610.6 | δ N-H; δ O-H; δ C-H | |
| 1698.7 | 1675 | ν C=NH; δ C-N-H; ν N-C; δ C-H |
3.2. FT-IR Spectrum Analysis
4. Applied Perspective and Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dowsett, L.; Higgins, E.; Alanazi, S.; Alshuwayer, N.A.; Leiper, F.C.; Leiper, J. ADMA: A Key Player in the Relationship between Vascular Dysfunction and Inflammation in Atherosclerosis. J. Clin. Med. 2020, 9, 3026. [Google Scholar] [CrossRef]
- Schulze, F.; Wesemann, R.; Schwedhelm, E.; Sydow, K.; Albsmeier, J.; Cooke, J.P.; Böger, R.H. Determination of asymmetric dimethylarginine (ADMA) using a novel ELISA assay. Clin. Chem. Lab. Med. 2004, 42, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Urinary Dimethylamine (DMA) and Its Precursor Asymmetric Dimethylarginine (ADMA) in Clinical Medicine, in the Context of Nitric Oxide (NO) and Beyond. J. Clin. Med. 2020, 9, 1843. [Google Scholar] [CrossRef] [PubMed]
- Sibal, L.; Agarwal, S.C.; Home, P.D.; Boger, R.H. The Role of Asymmetric Dimethylarginine (ADMA) in Endothelial Dys-function and Cardiovascular Disease. Curr. Cardiol. Rev. 2010, 6, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Leone, A.; Calver, A.; Collier, J.; Moncada, S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992, 339, 572–575. [Google Scholar] [CrossRef]
- Chen, B.-M.; Xia, L.-W.; Zhao, R.-Q. Determination of NG, NG-dimethylarginine in human plasma by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1997, 692, 467–471. [Google Scholar] [CrossRef]
- Braun, D.; Schlossmann, J.; Haen, E. An innovative, time- and cost-saving method for the quantification of asymmetric dimethylarginine in serum by high-performance liquid chromatography without evaporation. Sep. Sci. PLUS 2020, 3, 571–577. [Google Scholar] [CrossRef]
- Martens-Lobenhoffer, J.; Krug, O.; Bode-Böger, S.M. Determination of arginine and asymmetric dimethylarginine (ADMA) in human plasma by liquid chromatography/mass spectrometry with the isotope dilution technique. J. Mass Spectrom. 2004, 39, 1287–1294. [Google Scholar] [CrossRef]
- Valtonen, P.; Karppi, J.; Nyyssonen, K.; Valkonen, V.; Halonen, T.; Punnonen, K. Comparison of HPLC method and commercial ELISA assay for asymmetric dimethylarginine (ADMA) determination in human serum. J. Chromatogr. B 2005, 828, 97–102. [Google Scholar] [CrossRef]
- Crawford, A.; Silva, E.; York, K.; Li, C. Raman Spectroscopy: A Comprehensive Review; Department of Textile Engineering, Chemistry and Science North Carolina State University: Raleigh, NC, USA, 2019. [Google Scholar]
- Shipp, D.W.; Sinjab, F.; Notingher, I. Raman spectroscopy: Techniques and applications in the life sciences. Adv. Opt. Photonics 2017, 9, 315–428. [Google Scholar] [CrossRef]
- Rostron, P.; Gerber, D. Raman Spectroscopy, a review. Int. J. Eng. Tech. Res. 2016, 6, 50–64. [Google Scholar]
- Berthomieu, C.; Hienerwadel, R. Fourier transform infrared (FTIR) spectroscopy. Photosynth. Res. 2009, 101, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, S. The biochemistry, measurement and current clinical significance of asymmetric dimethylarginine. Ann. Clin. Biochem. Int. J. Lab. Med. 2010, 47, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Torres, E.; DiLabio, G.A. A (nearly) Universally Applicable Method for Modeling Noncovalent Interactions Using B3LYP. J. Phys. Chem. Lett. 2012, 3, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.M.L.; Kesharwani, M.K. Assessment of CCSD(T)-F12 Approximations and Basis Sets for Harmonic Vibrational Frequencies. J. Chem. Theory Comput. 2014, 10, 2085–2090. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Mostafapour, S.; Dörfer, T.; Heinke, R.; Rösch, P.; Popp, J.; Bocklitz, T. Investigating the effect of different pre-treatment methods on Raman spectra recorded with different excitation wavelengths. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 302, 123100. [Google Scholar] [CrossRef]
- Ju, Y.; Neumann, O.; Bajomo, M.; Zhao, Y.; Nordlander, P.; Halas, N.J.; Patel, A. Identifying Surface-Enhanced Raman Spectra with a Raman Library Using Machine Learning. ACS Nano 2023, 17, 21251–21261. [Google Scholar] [CrossRef]
- Quatela, A.; Miloudi, L.; Tfayli, A.; Baillet-Guffroy, A. In vivo Raman Microspectroscopy: Intra- and Intersubject Variability of Stratum Corneum Spectral Markers. Ski. Pharmacol. Physiol. 2016, 29, 102–109. [Google Scholar] [CrossRef]
- Samuel, A.Z.; Mukojima, R.; Horii, S.; Ando, M.; Egashira, S.; Nakashima, T.; Iwatsuki, M.; Takeyama, H. On Selecting a Suitable Spectral Matching Method for Automated Analytical Applications of Raman Spectroscopy. ACS Omega 2021, 6, 2060–2065. [Google Scholar] [CrossRef]
- Malyshev, V.; Michota-Kamińska, A.; Shao, S.; D’SOuza, F.; Noworyta, K. Determination of Asymmetric Dimethylarginine by Using Organic Semiconductor-Based Molecularly Imprinted Polymer Film. ECS J. Solid State Sci. Technol. 2018, 7, Q3189–Q3195. [Google Scholar] [CrossRef]
- Hardy, M.; Chu, H.O.M. Laser wavelength selection in Raman spectroscopy. Analyst 2025, 150, 1986–2008. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, B.; Foley, S.; Cardey, B.; Enescu, M. An experimental and theoretical study of the amino acid side chain Raman bands in proteins. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Cordero, E.; Korinth, F.; Stiebing, C.; Krafft, C.; Schie, I.W.; Popp, J. Evaluation of Shifted Excitation Raman Difference Spectroscopy and Comparison to Computational Background Correction Methods Applied to Biochemical Raman Spectra. Sensors 2017, 17, 1724. [Google Scholar] [CrossRef]
- Bhunia, S.; Srivastava, S.K.; Materny, A.; Ojha, A.K. A vibrational and conformational characterization of arginine at different pH values investigated using Raman spectroscopy combined with DFT calculations. J. Raman Spectrosc. 2016, 47, 1073–1085. [Google Scholar] [CrossRef]
- Larkin, P.J. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Colthup, N.B.; Daly, L.H.; Wiberley, S.E. Introduction to Infrared and Raman Spectroscopy, 3rd ed.; Academic Press: Boston, MA, USA, 1990. [Google Scholar]
- Lejaeghere, K.; Van Speybroeck, V.; Van Oost, G.; Cottenier, S. Error Estimates for Solid-State Density-Functional Theory Predictions: An Overview by Means of the Ground-State Elemental Crystals. Crit. Rev. Solid State Mater. Sci. 2014, 39, 1–24. [Google Scholar] [CrossRef]
- Gangwar, R.K.; Pathak, A.K.; Chiavaioli, F.; Bakar, M.H.A.; Kamil, Y.M.; Mahdi, M.A.; Singh, V.K. Optical fiber SERS sensors: Unveiling advances, challenges, and applications in a miniaturized technology. Coord. Chem. Rev. 2024, 510, 215861. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, Q.; Cong, S.; Zhao, Z. SERS Materials with Small-Molecule Sensitivity for Biological Diagnosis. Anal. Sens. 2024, 4, e202300067. [Google Scholar] [CrossRef]
- Tkachenko, K.; González-Saíz, J.M.; Calvo, A.C.; Osta, R.; Pizarro, C. Comparative Blood Profiling Based on ATR-FTIR Spectroscopy and Chemometrics for Differential Diagnosis of Patients with Amyotrophic Lateral Sclerosis—Pilot Study. Biosensors 2024, 14, 526. [Google Scholar] [CrossRef]



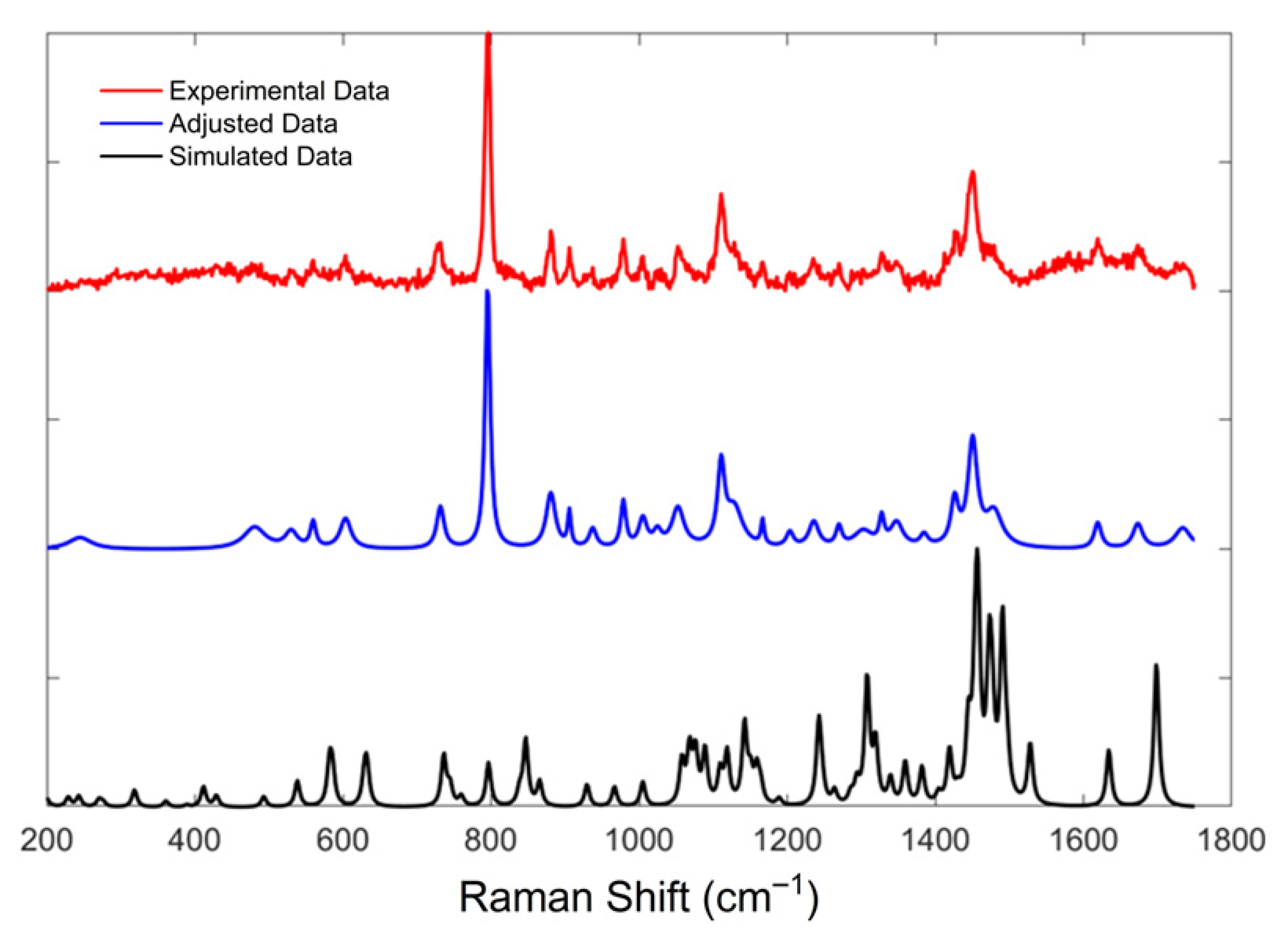

| Feature Metrics | Experimental vs. Adjusted | Adjusted vs. Simulated | Experimental Data vs. Simulated | |
|---|---|---|---|---|
| Cosine | 0.9365 | 0.5688 | 0.5601 | |
| Correlation | 0.9021 | 0.3646 | 0.3078 | |
| Simulated Bands | FT-IR Bands | Assignments |
|---|---|---|
| 583.1 | 571.8 | γ Molecular |
| 629.6 | 633 | ω O-H; ρ C-H; δ C-H; ν N-H |
| 736.1 | 725.6 | ν C-C; ρ N-H; δ C-O; ω C-H |
| 796.5 | ω N-H; τ C-H | |
| 846.8 | 840 | τ C-H; δ N=C-N, O=C-O; ν C-N, C-C; ω N-H |
| 929.5 | 933 | ν C-C; τ C-H; ω N-H, C-H |
| 1005 | γ Molecular; ω N-H | |
| 1057.8 | 1067.9 | γ Molecular; ω N-H, C-H |
| 1150.7 | 1107.4 | γ Molecular |
| 1163.7 | γ Molecular; τ N-H | |
| 1246.6 | 1229.9 | γ Molecular; ω N-H |
| 1339.5 | γ Molecular; τ N-H | |
| 1359.3 | γ Molecular; ω N-H | |
| 1381.9 | γ Molecular; ω N-H | |
| 1404.5 | 1409.2 | ω C-H; τ N-H; ν C-H |
| 1490.7 | 1488.8 | δ C-H; ν C-N |
| 1528.2 | ρ C-H, N-H; δ N-C | |
| 1634.4 | 1621.8 | δ N-H; ρ C-H |
| 1698.7 | 1667.6 | ν C-N, C=N; δ C-H; ρ C-H |
| 1838.9 | ν C=N; ρ N-H, C-H; δ O-H, O-C | |
| 2882.6 | ω C-H | |
| 2983.6 | ν C-H | |
| 3057.2 | 3032 | ν C-H |
| 3103.3 | ω C-H | |
| 3162.5 | 3175.7 | ν C-H |
| 3705.5 | ν O-H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canul-Solis, L.P.; Rodríguez-Aranda, M.d.C.; Rivera-Pérez, E.; Ortiz-Dosal, A.; Guevara, E.; Martínez-Ruiz, E.O.; Ortiz-Dosal, L.C.; Reyes-Reyes, A.; Kolosovas-Machuca, E.S. Asymmetric Dimethylarginine Vibrational Spectroscopy Spectra and Density Functional Theory Model. Sensors 2025, 25, 6818. https://doi.org/10.3390/s25226818
Canul-Solis LP, Rodríguez-Aranda MdC, Rivera-Pérez E, Ortiz-Dosal A, Guevara E, Martínez-Ruiz EO, Ortiz-Dosal LC, Reyes-Reyes A, Kolosovas-Machuca ES. Asymmetric Dimethylarginine Vibrational Spectroscopy Spectra and Density Functional Theory Model. Sensors. 2025; 25(22):6818. https://doi.org/10.3390/s25226818
Chicago/Turabian StyleCanul-Solis, Luis Pablo, Ma. del Carmen Rodríguez-Aranda, Emmanuel Rivera-Pérez, Alejandra Ortiz-Dosal, Edgar Guevara, Erick Osvaldo Martínez-Ruiz, Luis Carlos Ortiz-Dosal, Adán Reyes-Reyes, and Eleazar Samuel Kolosovas-Machuca. 2025. "Asymmetric Dimethylarginine Vibrational Spectroscopy Spectra and Density Functional Theory Model" Sensors 25, no. 22: 6818. https://doi.org/10.3390/s25226818
APA StyleCanul-Solis, L. P., Rodríguez-Aranda, M. d. C., Rivera-Pérez, E., Ortiz-Dosal, A., Guevara, E., Martínez-Ruiz, E. O., Ortiz-Dosal, L. C., Reyes-Reyes, A., & Kolosovas-Machuca, E. S. (2025). Asymmetric Dimethylarginine Vibrational Spectroscopy Spectra and Density Functional Theory Model. Sensors, 25(22), 6818. https://doi.org/10.3390/s25226818