1. Introduction
Gallium oxide (Ga
2O
3) has percolated through many recent technologies and emerged as a pivotal semiconductor in modern technological advancements, garnering significant attention across various scientific disciplines due to its exceptional properties [
1,
2]. With an ultra-wide bandgap of approximately 4.8 eV, a high-breakdown electric field exceeding 8 MV/cm, and an electron mobility of approximately 150 cm
2/V·s, Ga
2O
3 presents a compelling alternative for a range of applications, particularly in semiconductor research. Ga
2O
3’s cost-effectiveness and ease of manufacturing position it as a promising substitute for traditional materials such as silicon carbide (SiC) and gallium nitride (GaN) [
3,
4,
5,
6]. Ga
2O
3-based materials are some of the best ultra-wideband gap materials (UWBGs) and show great potential for safe and long-term sensing in harsh environments. Recently, Xinyi et al. showed the first thermal neutron detector based on a large-area (9 mm
2) p-NiO/β-Ga
2O
3 heterojunction diode [
7]. Additionally, the development of 3D-fabricated devices may lead to the efficient detection of alpha particles by their energy. The fabrication of 3D nanostructures using Ga
2O
3 is attracting attention for increasing the performance of devices, notably, in radiation, high-power electronics, and photonics applications, due to its high surface-to-volume ratio [
1,
6,
8].
The etching processes utilized in the fabrication of Ga
2O
3 devices are critical for optimizing device performance. Wet-etching techniques can etch along specific crystallographic directions, such as in (100), (010), and (
01) [
9,
10,
11,
12], which leads to etching in a slightly vertical direction [
12]. However, these techniques are limited by their inability to achieve high aspect ratios in micro- or nanostructures, which adversely affects the uniformity and reproducibility required for advanced electronic and photonic devices [
1]. These nanostructures facilitate photoenergy harvesting in optoelectronic sensors, e.g., UV detection for safety in petroleum sectors [
1,
2,
3,
4,
5], by amplifying photo-generated carriers with an increased junction area. Consequently, there is growing interest in developing alternative dry-etching processes for single-crystal Ga
2O
3 to assess chemical stability and identify appropriate etchants that can enhance etch rates effectively.
While wet etching offers advantages such as minimal surface damage, high selectivity, and throughput, it is constrained by several factors that include (i) uncontrollable isotropic etching, which makes it difficult to introduce micro- and nanostructures, and (ii) the disposal of significant quantities of toxic chemical solvents. In contrast, dry-etching techniques, particularly deep reactive-ion (DRI) etching, facilitate anisotropic etching, providing enhanced control and reproducibility. DRI etching is currently used to fabricate anisotropic nano- and microstructures and involves material removal facilitated by neutral gas atoms in a vacuum chamber, although research into the optimal gas chemistries and etching mechanisms specific to Ga
2O
3 remains ongoing [
1].
Despite the growing research and body of literature, a comprehensive understanding of Ga
2O
3 etching processes remains elusive, with numerous challenges associated with adjusting etching parameters [
10,
11,
13,
14]. Key variables influencing etching outcomes include the etch rate, selectivity, uniformity, directionality (isotropic versus anisotropic), surface quality, and reproducibility. Thus, researchers are actively seeking to establish optimal etching procedures that ensure chemical stability and enhance etch rates for Ga
2O
3.
In nanofabrication, dry etching involves gases or plasmas. It is a highly anisotropic, controlled process of the etching direction, with high uniformity [
15,
16,
17]. Accordingly, dry etching is an important step for forming patterns on the surface of Ga
2O
3-based materials. Dry etching is the process of implementing plasma or ion beams to fabricate 3D nanostructures on the surface of a semiconductor, which are highly desirable for optimizing the nanosensors’ detection mechanism. The fabrication of 3D nanostructures is greatly beneficial for increasing the charge detection rate, leading to faster response and increasing the devices’ sensitivity. They are considered very effective and ideal for high-performance sensing applications [
18].
However, one of the fabrication challenges encountered when dry etching Ga
2O
3-based materials is that they can have poor etch selectivity, which affects the difference between the etching rate of the Ga
2O
3 surface compared with the outside mask. This may cause the mask to be etched away before the structure is fully developed, thus ruining the entire process [
19]. In addition, some unwanted defects can form on the surface from the ion bombardment, causing a break in the bonds. This leads to surface roughness and below-surface defects from the etching process [
20].
Vertical devices such as β-Ga2O3 trench metal-oxide semiconductor (MOS) diodes and fin field-effect transistors employ deep-etching techniques to mitigate the intense electric fields in surface areas. However, the process of deep etching can lead to plasma damage due to inductively coupled plasma (ICP) reactive-ion etching (RIE). Publications dedicated exclusively to the fabrication of high-aspect-ratio nanostructures using wide-bandgap materials such as Ga2O3 are lacking. These structures are particularly valuable in the development of nanosensors, promising improved sensing mechanisms through increased profile depth, which is anticipated to enhance performance in extreme environmental conditions.
In this study, we present the development of a deep ion-etching process for β-Ga
2O
3, using BCl
3-based inductively coupled plasma (ICP) reactive-ion etching (RIE). Unlike [
21,
22], which discuss mixtures for moderate depths, our pure BCl
3 ICP-RIE with Ar-interrupted cycles and SUEX overcomes small-substrate lithography barriers, achieving unprecedented 6.97 µm depths for vertical devices. Several device applications have been obtained using BCl
3-driven deep ion etching of β-Ga
2O
3, such as field-effect transistors [
23] and Schottky barrier diodes [
24]. Unlike prior work on etching or wet-etching techniques [
10,
11,
13,
14,
25], this research systematically investigated the chemical stability of Ga
2O
3 (010) and (
) planes, optimizing etching parameters to enhance etch depth, selectivity, and surface quality.
2. Materials and Methods
2.1. Surface Fabrication by Photolithography
In this study, 5.00 mm × 5.00 mm Sn-doped Ga2O3 substrates, sourced from Tamura Corporation in Tokyo, Japan, were employed for the anisotropic etching procedure. Sn-doped substrates with distinct orientations, specifically cleaved Ga2O3 (010) and Ga2O3 (), were used. Ga2O3 Sn doping was achieved at 0.03%. A photolithography mask was designed and fabricated by the vendor. The experiments were conducted using advanced fabrication and characterization equipment available at the nanofabrication lab (Center for Nano and Micro Manufacturing (CNM2)) at the University of California, Davis. The equipment used in this process included a wet bench for photoresist development and substrate cleaning, a Sky 335R6 Heated Roller Laminator (Oregon Laminations Company, Portland, OR, USA) for laminating the SUEX dry film, an EVG 620 Mask Aligner (Irvine, CA, USA) for exposing the photomask patterns, and a Dektak XT (Bruker Corporation, Billerica, MA, USA) for analyzing the step height of the photoresist and Ga2O3. Additionally, a PlasmaTherm Apex SLR RIE/ICP (Bruker Corporation, Billerica, MA, USA) was used for etching Ga2O3, a Carl Zeiss Axiotron Microscope (Zeiss Global Group Inc., Dublin, CA, USA) for optical inspection, an FEI Nova NanoSEM 430 (Bridge Tronic Global, Irvine, CA, USA) for microscopy analysis, a hotplate for heated resist stripping, and a programmable vacuum oven for post-exposure baking.
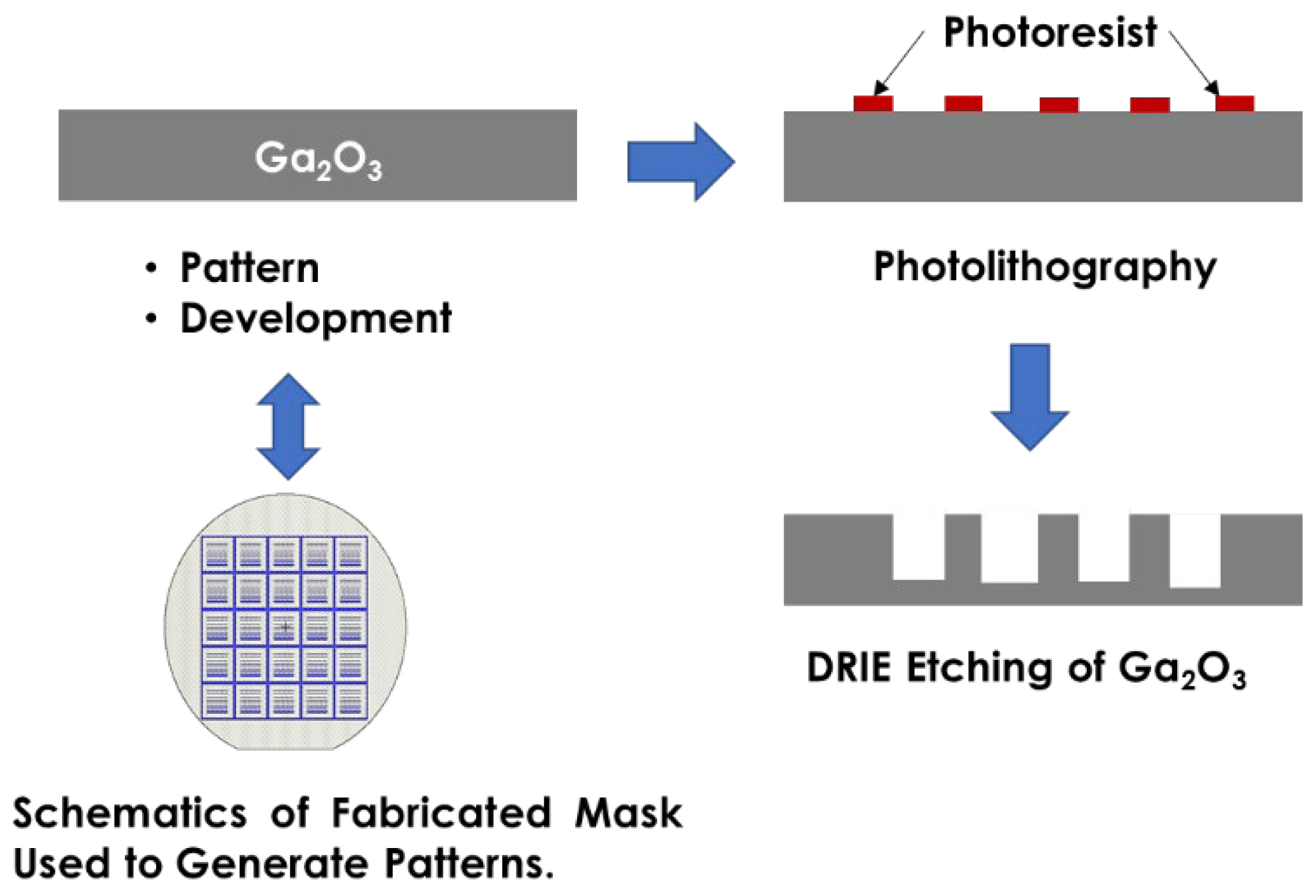
Figure 1 presents a flowchart of the fabrication process of the etched Ga
2O
3 substrate. The etching process for Ga
2O
3 substrates necessitates thorough substrate cleaning. Initially, substrates of Sn-doped Ga
2O
3 (010) and (
) were prepared. A sequential ultrasonication process was employed to clean the substrates, utilizing acetone for 5 min, isopropanol for 5 min, deionized (DI) water for rinsing, and drying with nitrogen. After cleaning, we employed a negative photoresist, Futurrex NR9–1500PY (Futurrex, Inc., Franklin, NJ, USA), which was applied to the substrates via spin coating, and a SUEX K40 dry film (DJ MicroLaminates, Inc., Sudbury, MA, USA) that was laminated directly onto the surface.
The negative photoresist Futurrex NR9–1500PY was applied to the substrates through spin coating at a speed of 3000 rpm for 45 s. Given the requirement for a thicker resist in the etching process, the thickness of the photoresist was adjusted by varying the spin coater speed from 1500 rpm to 300 rpm, over durations ranging from 2 to 45 s. This adjustment also enabled control over the density and alignment of the Ga2O3 substrate.
Subsequently, a soft bake was conducted at 150 °C for 2 min to enhance photoresist adhesion. The substrates were then aligned and exposed using the EVG 620 at an energy dose of 400 mJ/cm
2 in a vacuum contact. A counter dummy Ga
2O
3 was used to achieve good planarization between the photomask and the sample. A post-exposure bake was performed at 100 °C for 2 min to further stabilize the photoresist patterns. The surface features were then examined using an optical microscope. Following this, deep reactive ion etching (DRIE) was employed to selectively remove undesired areas, thereby achieving the targeted shapes and openings in the Ga
2O
3 substrates (
Figure 2).
We also employed an alternative approach by laminating SUEX K40 directly onto the Ga2O3 () substrates, with a thickness of 38.26 μm, as measured with Dektak. The substrates were then aligned and exposed using the EVG 620 at an energy dose of 700 mJ/cm2 in a vacuum contact. A counter dummy Ga2O3 was used to achieve good planarization between the photomask and the sample. A post-exposure bake was performed at 80 °C for 1 h and then cooled down overnight.
The Ga2O3 samples were etched using PlasmaTherm Apex SLR RIE/ICP. In the dry-etching process, BCl3 ran at 20 sccm for 5 min. The etching step included multiple loops with an inductively coupled plasma (ICP) power of 400 W and the power of RIE of 100 W. The cooling steps included 40 sccm Ar at 90 mTorr for 1 min. Finally, the photoresist was stripped overnight with a Futurrex RR41 resist stripper at 110 °C and 120 °C for NR9–1500PY and SUEX dry film, respectively. Finally, the samples were analyzed by using DektakXT and field-emission scanning electron microscopy (FEI 430 Nova NanoSEM).
2.2. Structural and Surface Morphology Characterizations
DektakXT was used to measure the depth profile and to prove the precise thickness of thin films across the wafer surface. In addition, the substrates were observed using optical microscopy and scanning electron microscopy, which provided images of the sample topography by scanning the surface with a high-energy beam of electrons.
3. Results
3.1. Surface Morphology
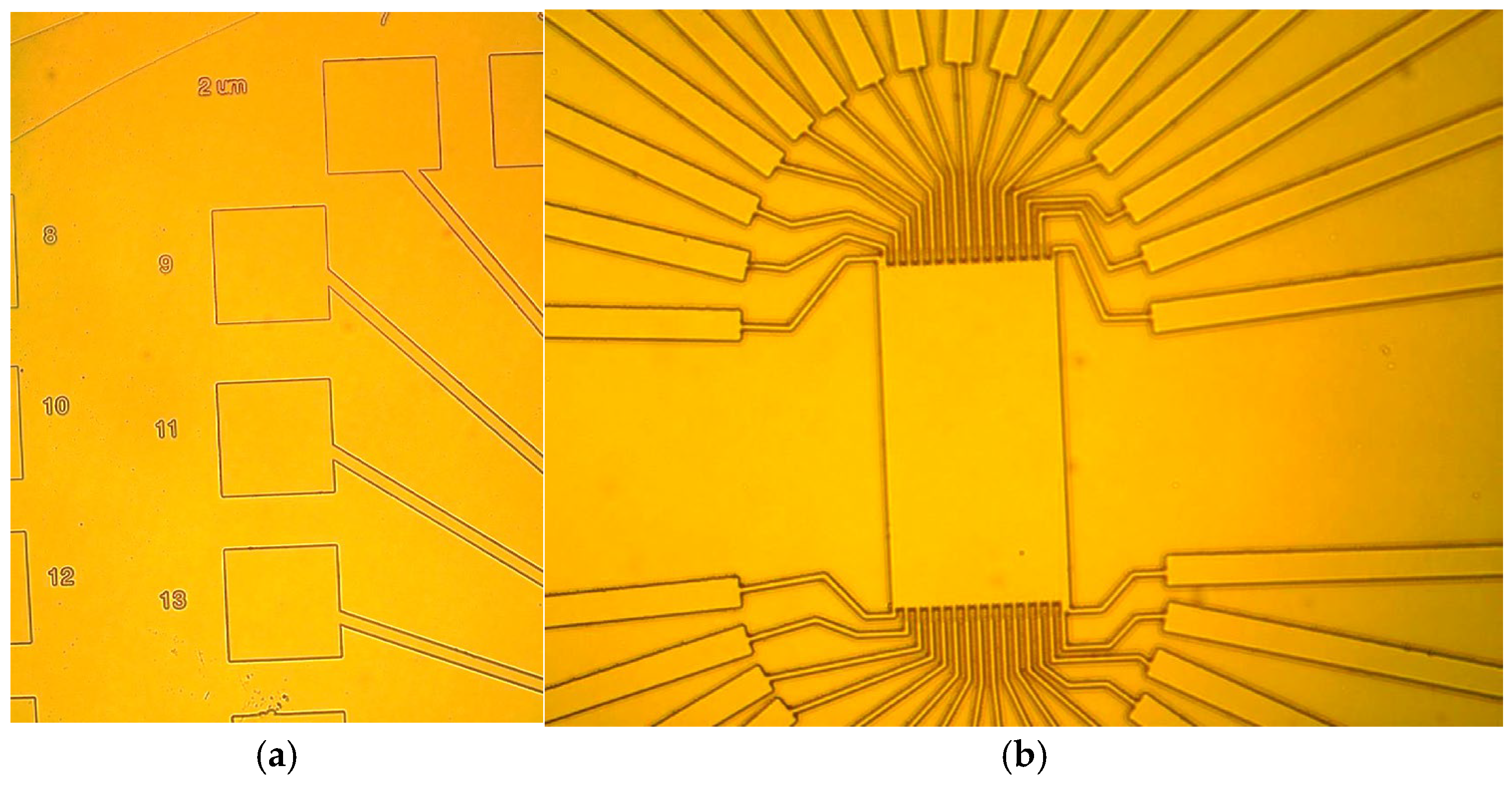
The surface features were first examined using an optical microscope, confirming the introduction of patterns. The characterization of optical images in surface lithography also requires high-resolution imaging techniques to ascertain the fidelity and precision of pattern transfer on Ga2O3 substrates.
Figure 3 and
Figure 4 show the optical response of the lithographically patterned surfaces, with clear differentiation of intended patterns of 2.41 μm and 9.75 μm (
Figure 3b), confirming the successful introduction of specific geometric configurations.
We initially used NR78–8000P (Futurrex, Inc., Franklin, NJ, USA) as a photoresist, which led to poor patterning because of its thickness and ineffective wafer edge cooling. Despite BCl3’s good selectivity, the photoresist burned during a 30-min etch, and a hardbake step worsened the removal issues of the photoresist. For improved lithography, a counter piece was needed to ensure proper planarization of the photomask to the Ga2O3 piece, preventing uneven UV exposure. To address the resist burning, the hardbake step was removed, a thinner resist (NR9–1500P at 1.7 µm) was used, and the etch was segmented into 5-min increments with Ar cooling in between.
3.2. Etching Parameters
Etching parameters in lithography are crucial for achieving the desired results, particularly when creating deep-etched features. Factors that collectively determine the efficiency, rate, and quality of the dry-etching process for β-Ga2O3 include gas composition, ICP power, bias power, cooling water temperature, chamber pressure, and mask material.
We analyzed the effects of the crystal structure and the etching time and rate on the etched features of Ga2O3 using our etching process to improve the pattern transfer and minimize the undesired effects, such as undercutting or roughness.
3.2.1. Crystal Orientation
Crystal orientation significantly influences etching outcomes in photolithography due to variations in atomic arrangements and bonding strength within the crystal lattice. Different crystallographic planes exhibit distinct etch rates, leading to anisotropic etching, where certain directions are preferentially etched over others. This anisotropy affects the fidelity of the final pattern, potentially causing issues such as unintended sidewall angles or undercuts. Therefore, understanding and controlling the crystal orientation of Ga2O3 is crucial for optimizing etching processes and achieving precise lithographic results.
Anam et al. [
26] analyzed the structural, thermal, and electronic properties of two-dimensional gallium oxide (β-Ga
2O
3) using a first-principles design. The atomic arrangement in the (010) and (
) surfaces of Ga
2O
3 is different (
Figure 5), which affects the etching profile. The (010) plane has four Ga
2O
3 in its unit cell, with Ga atoms having only a tetrahedral coordination geometry. The atomically thin-layered structure in the (
) direction has slightly different structural parameters from the rest of the predicted 2D allotropes of β-Ga
2O
3. It has a 1 to 1 Ga and O ratio, with six Ga atoms and six O atoms in its unit cell. Therefore, the fidelity of the etched pattern is affected by the stability of the surface structure.
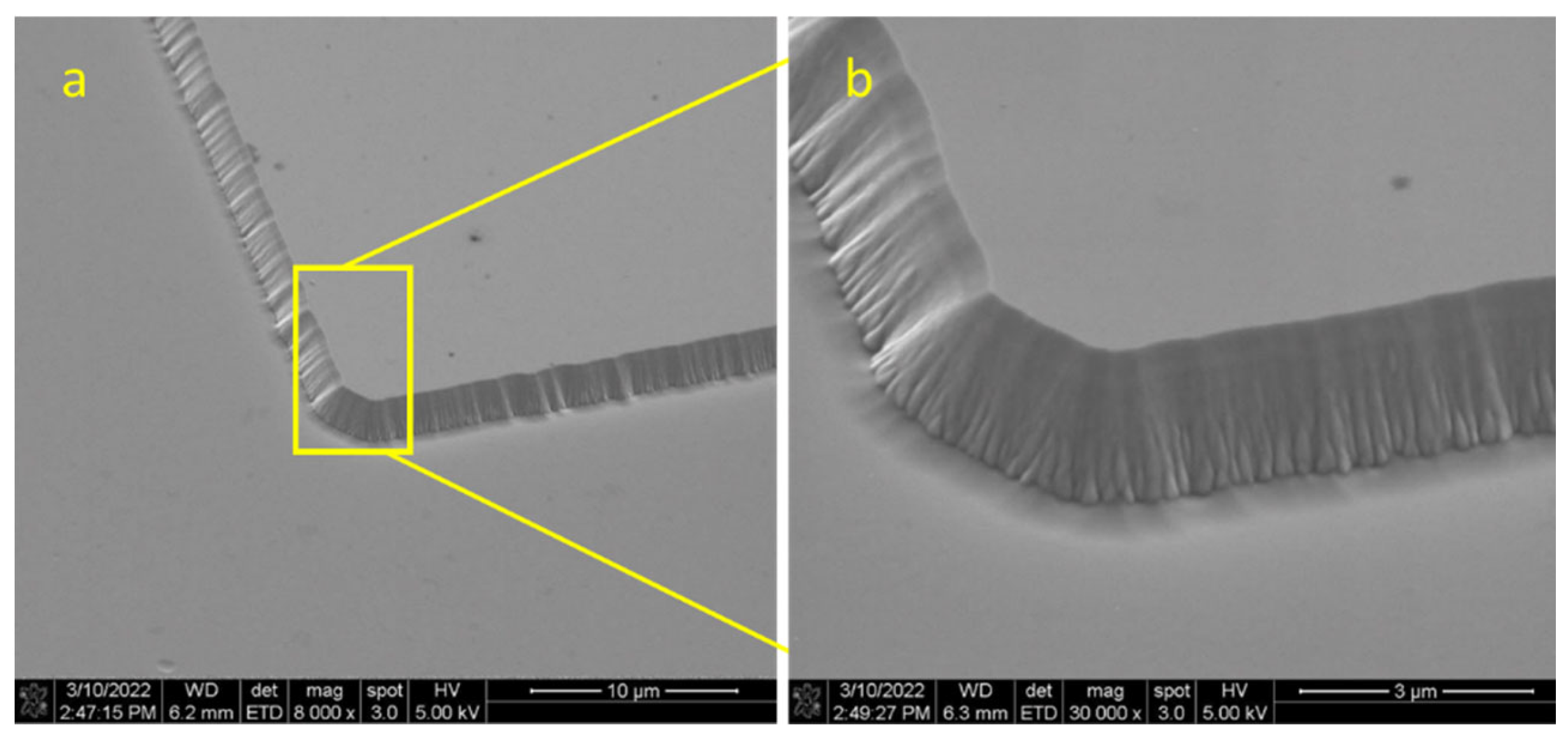
The atomic arrangement in different directions on the Ga
2O
3 (010) and Ga
2O
3 (
) surfaces is shown in the morphology of the Ga
2O
3 substrate after dry etching, as presented in
Figure 6 The SEM image of the Ga
2O
3 substrate at a low magnification (
Figure 6a) shows the etched pattern that is clearly visible and recognizable. At a higher magnification (
Figure 6b,c), the SEM images were distinctly different in the edge and corner profiles, although both the (010) and (
) orientations were etched at 6.7 nm/min for 30 min. The surface structure of Ga
2O
3 (010) is more stable than Ga
2O
3 (
) [
26]. This difference in the surface energy and stability is clearly seen in
Figure 6b,c, where the (010)-oriented Ga
2O
3 has well-defined corners compared with Ga
2O
3 (
).
Hogan et al. [
21] investigated the dry etching of β-Ga
2O
3 and found that the etch rate on (100)-oriented Ga
2O
3 was significantly lower than on the (010) and (
) planes. This difference was attributed to the presence of surface oxygen anions and a lower concentration of dangling bonds on the (100) surface.
For wet etching, they used H
3PO
4, which is considered an efficient wet etchant on (100)-oriented β-Ga
2O
3 [
11]. However, the etching process led to isotropic side etching. Despite the slower etch rate on the (010)-oriented Ga
2O
3, it may be the better choice for achieving high-aspect-ratio micro- or nanostructures. Notably, groove-shaped pits were observed on etched β-Ga
2O
3 (010) single crystals [
12]. The (010) orientation of Ga
2O
3 tends to promote the formation of deeper micro- or nano-features on the surface, with the etch pit formation influenced by voids and dislocation defects.
In the context of two-dimensional (2D) β-Ga
2O
3 allotropes, the energy characteristics of the (010) and (
) planes reveal distinct differences in stability and electronic properties, which may influence their suitability in various semiconductor technologies. Potential future integration includes UV-responsive nanosensors utilizing a 4.8 eV bandgap for advanced photoenergy applications. This study examined the energetic stability of these planes and identified the structural configurations responsible for their unique electronic behaviors using particle swarm optimization combined with density functional theory (DFT) calculations [
26]. The (010) plane exhibited a more favorable energetic profile compared with the (
) orientation, primarily due to strong bonding interactions and lower surface energy. This atomic arrangement in the (010) plane promotes optimal π-bonding, which enhances structural integrity and facilitates better electronic transport properties. In contrast, while the (
) plane remains stable, it has a higher energy configuration that could lead to increased electronic localization and reduced carrier mobility, potentially affecting device performance.
Band structure calculations, using both generalized gradient approximation (GGA) and the local density approximation (LDA–1/2), show that the (010) plane maintained a larger indirect band gap of approximately 4 eV [
27]. This property is particularly advantageous for applications in transparent conductive oxides and semiconductor devices, as it supports improved light absorption and minimizes recombination losses.
In conclusion, the comparative analysis of the (010) and () planes of β-Ga2O3 highlights the importance of structural orientation in determining both the fidelity of the etch patterns and the electronic and optical characteristics of 2D materials.
3.2.2. Etching Time
Etching time is another crucial parameter studied for ensuring accurate and reliable photolithographic outcomes. It is well known that if the etching process is insufficient, it can lead to incomplete pattern transfer, resulting in unwanted material remaining on the substrate. This not only compromises the patterns’ integrity but can also diminish the features’ resolution. Meanwhile, an excessive etching time can lead to over-etching, which may cause a loss of critical dimensions, distort the intended feature profiles, and introduce surface roughness, ultimately degrading the material quality. Adding BCl
3 to the Cl
2/BCl
3 gas mixture can improve the etching rate of β-Ga
2O
3 to reduce the surface roughness after the etching reaction of BCl
3/β-Ga
2O
3. As a result, the surface damage of β-Ga
2O
3 has been decreased after post-wet chemical treatments [
22]. In the deep-etching process between BCl
3 plasma and Ga
2O
3, the reaction of the etching mechanism leads to the dissociation of chloride radicals (Cl
−) and the formation of volatile Ga-Cl species, which are absorbed and remove the undesired material. The oxygen molecules in Ga
2O
3 are removed and replaced by chloride (Cl) to react with Ga molecules. However, B atoms could form a non-volatile compound that stays on the etched surface as a residue. Therefore, it would be impossible for boron to be an effective incorporation in increasing the etching mechanism.
Figure 7 and
Figure 8 show a top-view SEM image of the etched sidewall on Ga
2O
3 (
), with 1.65 µm after 5 h of etching (
Figure 7), and 6.97 µm after 24 h of etching (
Figure 8). Depths were averaged from profilometer scans at five locations per sample to confirm uniformity, with the maximum recorded value of 6.97 µm. With further optimization of the lithography and etch recipes, the surface can be cleaned, the sidewall angle can be made steeper, and the surface roughness can be reduced.
Following the etching process and subsequent treatment with Futurrex RR41 (110–120 °C overnight), we evaluated piranha cleaning (H2SO4–H2O2; 5 min) and O2 plasma ashing (200 W; 2 min) protocols. The plasma-ashing process ensures the removal of the photoresist from the etched substrates. Consequently, this process reduced surface residue by approximately 70%; however, complete removal was not achieved, and harsher conditions pose a risk of surface degradation. For harsh sensors, this residue may enhance passivation.
3.2.3. Etching Rate
The etching rate in DRIE directly impacts the depth, aspect ratio, and quality of the etched features. A balanced etching rate is essential for deep etching: too fast a rate can lead to isotropic etching, rough surfaces, and a poor sidewall profile; too slow a rate can lead to long process times, surface non-uniformity, and reduced throughput. When comparing pure BCl
3 and Cl
2 etching, pure BCl
3 was much more effective than pure Cl
2 [
21]. The etch rate using mixtures of BCl
3 and Cl
2 was roughly related to the weighted average of each etchant, indicating minimal interaction between the etch species. Specifically, BCl
3 produced higher etch rates compared with Cl
2, and the addition of Cl
2 to BCl
3 did not significantly enhance the etch rate [
21,
22].
The 24-h total process time included 211 cycles of 5-min BCl
3 etching (1055 min; ~17.6 h) with 1-minute Ar cooling steps per cycle (~3.5 h), resulting in an etch depth of 6.96 μm for (
) Ga
2O
3. The etch rate of 6.7 nm/min represents the rate during active BCl
3 etching periods, while the reported process durations (e.g., 5 h and 24 h) incorporate the full cyclic procedure, including 1-min Ar cooling steps per cycle, leading to a slightly lower effective average rate of ~6.6 nm/min in extended runs. The etch rate for SUEX K40 during the same 5 min cycle was 2.44 nm/min (12.2 nm/cycle for 211 cycles). The Ga
2O
3-to-SUEX K40 selectivity was 2.75:1.
Table 1 shows different etching rates obtained in the literature compared with our work.
A consistent etching rate is crucial for achieving uniform feature sizes and depths across the entire wafer. However, the pattern angle can affect the steepness of the sidewalls in certain regions. As shown in
Figure 9, surface features positioned at different angles exhibit varying degrees of sidewall roughness, which may also be linked to the underlying crystal orientation. Additionally, some areas show a ‘stepped’ appearance, as though multiple lithographic steps were performed, though this is not the case. The sidewall quality, including roughness and steepness, varies across features placed at specific angles, which may be influenced by crystal orientation. This suggests that crystalline orientation plays a role in determining the steepness and roughness of the sidewalls, even when the etching rate remains constant.
4. Conclusions
This study introduced an innovative deep ion-etching process for β-Ga2O3, achieving a remarkable etch depth of 6.97 µm on Ga2O3 () substrates, a significant advancement over previously reported etching techniques. The novelty of this work lies in the successful implementation of a BCl3-based inductively coupled plasma (ICP) reactive-ion etching (RIE) process, optimized with a SUEX K40 dry film photoresist to overcome lithographic challenges associated with small substrates (<5.00 mm × 5.00 mm). This approach eliminates edge bead issues, ensuring intimate photomask contact and enabling high-resolution patterning of features as small as <5.00 µm. The achieved etch rate of 6.70 nm/min, coupled with a Ga2O3 to SUEX K40 selectivity of 2.75:1, represents an advanced step in fabricating high-aspect-ratio nanostructures for vertical Ga2O3 devices.
Furthermore, this research provides novel insights into the influence of crystallographic orientation on sidewall quality, revealing that the (010) plane’s stability enhances pattern fidelity compared with the () plane.
While further optimization of etch parameters is needed to enhance sidewall verticality and smoothness, these results mark a significant leap forward in Ga2O3 nanofabrication. By enabling deep etching for applications in optoelectronic devices and harsh-environment nanosensors, this work not only expands the understanding of β-Ga2O3’s potential in 2D material systems but also sets a new benchmark for precision and scalability in wide-bandgap semiconductor processing, opening avenues for innovative electronic and energy technologies.
This research was motivated by the need for efficient fabrication processes, particularly for applications requiring sensors to operate in harsh environments, owing to their low leakage current density in power devices.