Ex Vivo Optical Coherence Tomography Analysis of Resected Human Bladder with a Forward-Looking Microelectromechanical Systems Mirror-Based Catheter
Abstract
1. Introduction
2. Materials and Methods
2.1. OCT System and Catheter Description
2.1.1. OCT System Set-Up
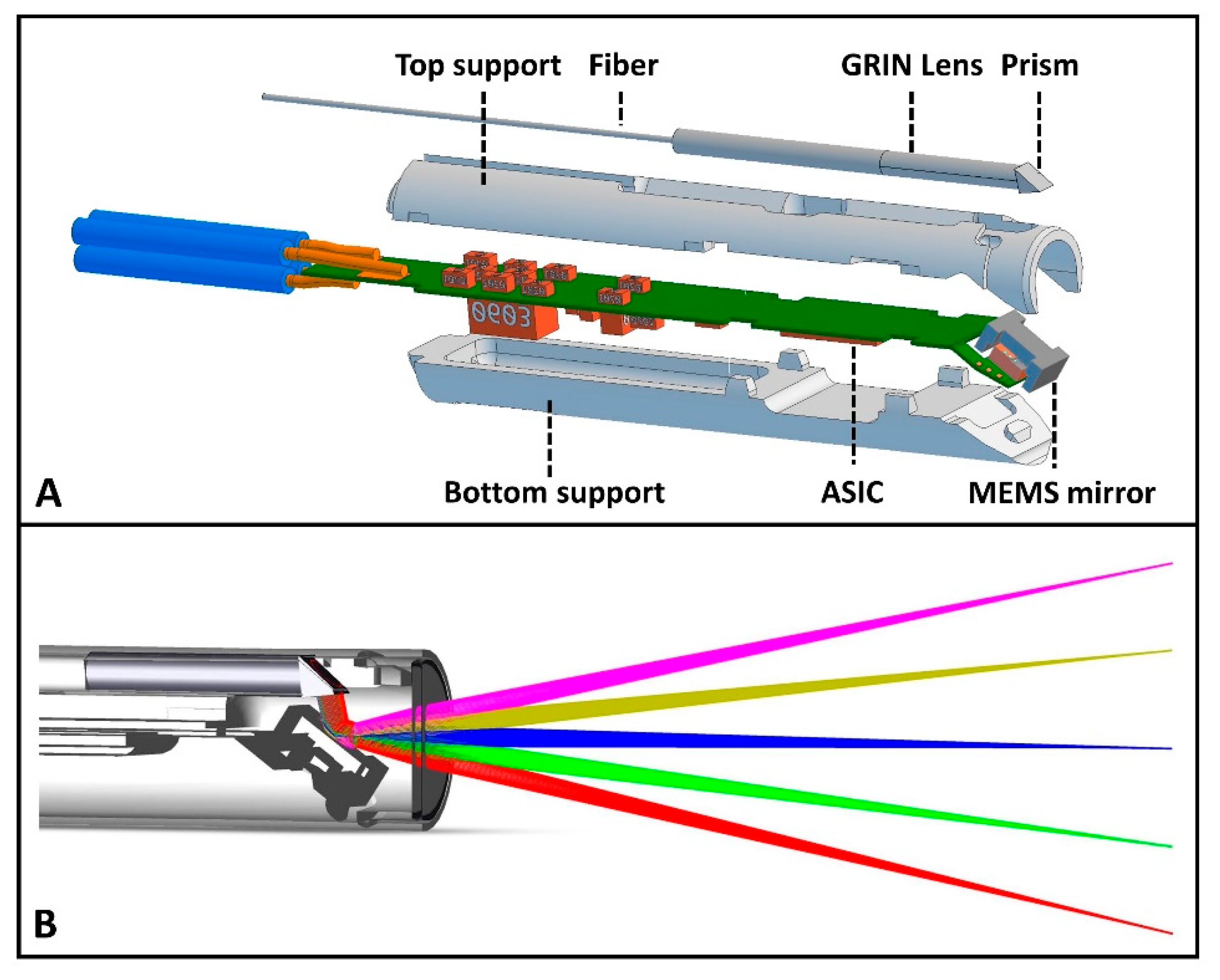
2.1.2. Design Properties of the MEMS-Based OCT Catheter
2.2. OCT System and Catheter Performance Analysis and Evaluation
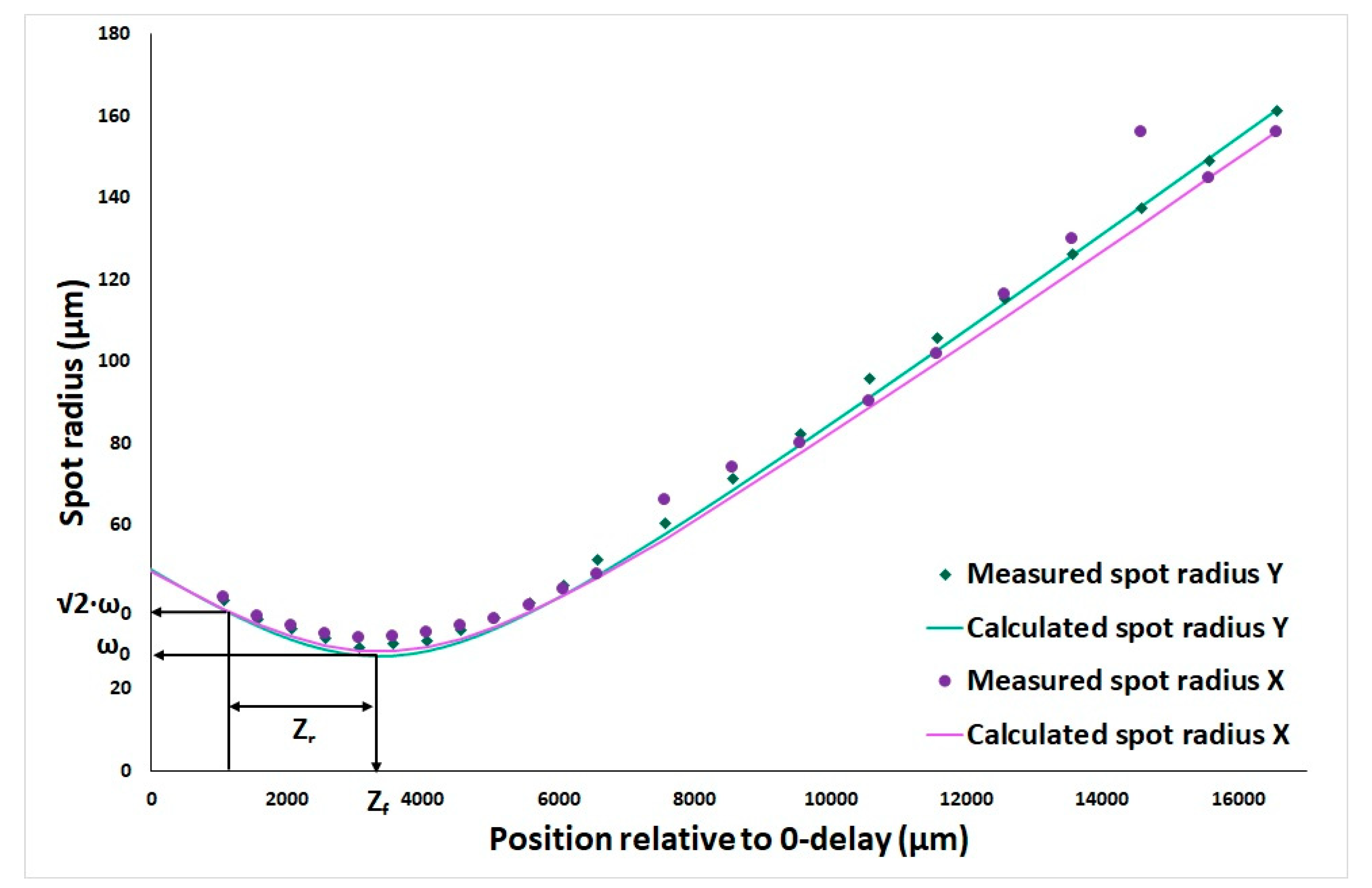
2.2.1. Waist and Rayleigh Length Determination
2.2.2. Cartesian Pixel Remapping
2.3. Ex Vivo Measurements on Bladder Specimens
2.3.1. Study Design
2.3.2. Study Population
2.3.3. Tissue Handling
2.3.4. OCT Image Analysis
3. Results
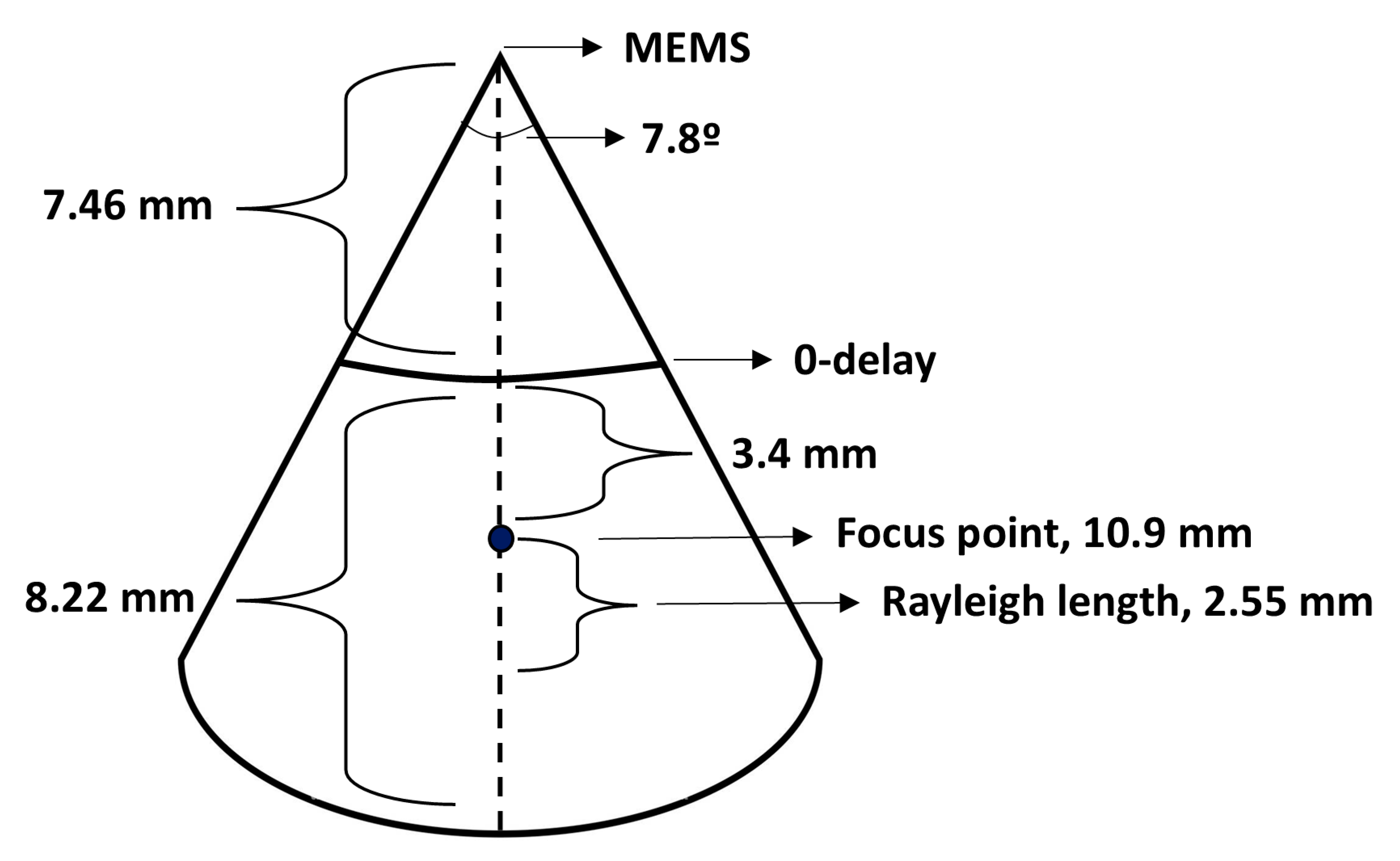
3.1. OCT System and Catheter Performance
3.2. Ex Vivo Measurements Results
3.2.1. Study Population
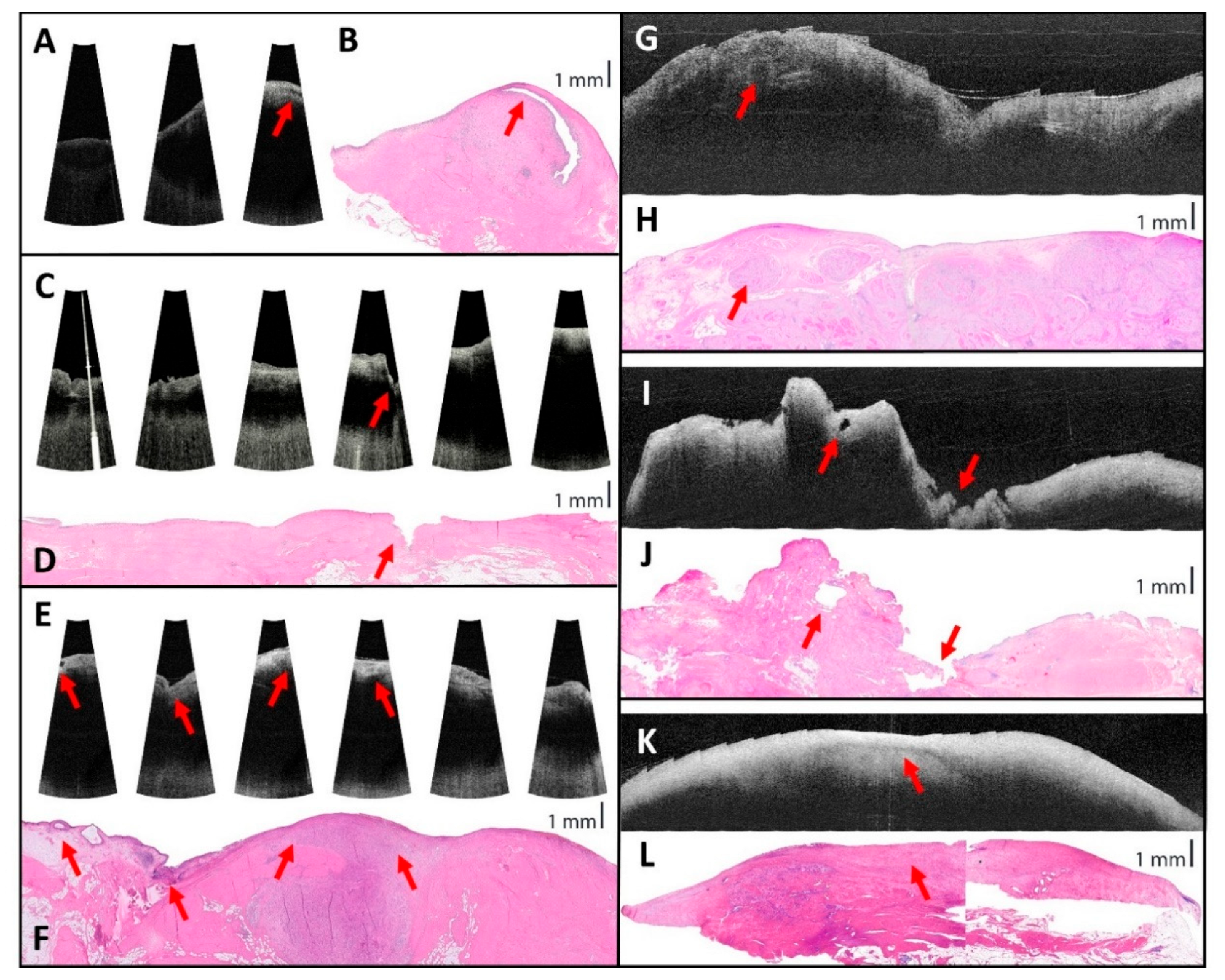
3.2.2. OCT Image Comparison to Histopathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASIC | Application-specific integrated circuit |
| BC | Bladder cancer |
| CLE | Confocal laser endomicroscopy |
| FOV | Field of view |
| FPS | Frames per second |
| GRIN | Gradient index |
| MEMS | Microelectromechanical systems |
| OCT | Optical coherence tomography |
| ROI | Region of interest |
| TURBT | Transurethral resection of a bladder tumor |
| VCSEL | Vertical-Cavity Surface-Emitting Laser |
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer: Lyon, France. 2024. Available online: https://gco.iarc.who.int/today (accessed on 20 February 2024).
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Escrig, J.L.D.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef]
- van Hoogstraten, L.M.C.; Vrieling, A.; van der Heijden, A.G.; Kogevinas, M.; Richters, A.; Kiemeney, L.A. Global trends in the epidemiology of bladder cancer: Challenges for public health and clinical practice. Nat. Rev. Clin. Oncol. 2023, 20, 287–304. [Google Scholar] [CrossRef]
- Joyce, D.D.; Sharma, V.; Williams, S.B. Cost-Effectiveness and Economic Impact of Bladder Cancer Management: An Updated Review of the Literature. Pharmacoeconomics 2023, 41, 751–769. [Google Scholar] [CrossRef] [PubMed]
- Mossanen, M.; Gore, J.L. The burden of bladder cancer care: Direct and indirect costs. Curr. Opin. Urol. 2014, 24, 487–491. [Google Scholar] [CrossRef]
- Soukup, V.; Čapoun, O.; Cohen, D.; Hernández, V.; Babjuk, M.; Burger, M.; Compérat, E.; Gontero, P.; Lam, T.; MacLennan, S.; et al. Prognostic Performance and Reproducibility of the 1973 and 2004/2016 World Health Organization Grading Classification Systems in Non-muscle-invasive Bladder Cancer: A European Association of Urology Non-muscle Invasive Bladder Cancer Guidelines Panel Systematic Review. Eur. Urol. 2017, 72, 801–813. [Google Scholar] [CrossRef]
- Erikson, M.S.; Petersen, A.C.; Andersen, K.K.; Jacobsen, F.K.; Mogensen, K.; Hermann, G.G. Do repeated transurethral procedures under general anesthesia influence mortality in patients with non-invasive urothelial bladder cancer? A Danish national cohort study. Scand. J. Urol. 2020, 54, 281–289. [Google Scholar] [CrossRef]
- Hurle, R.; Lazzeri, M.; Vanni, E.; Lughezzani, G.; Buffi, N.; Casale, P.; Saita, A.; Morenghi, E.; Forni, G.; Cardone, P.; et al. Active Surveillance for Low Risk Nonmuscle Invasive Bladder Cancer: A Confirmatory and Resource Consumption Study from the BIAS Project. J. Urol. 2018, 199, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Raj, S.; Russell, B.; Mensah, E.; Nair, R.; Thurairaja, R.; Khan, M.S.; Thomas, K.; Malde, S. Long-term outcomes of outpatient laser ablation for recurrent non-muscle invasive bladder cancer: A retrospective cohort study. BJUI Compass 2021, 3, 124–129. [Google Scholar] [CrossRef]
- Lindgren, M.S.; Hansen, E.; Azawi, N.; Nielsen, A.M.; Dyrskjøt, L.; Jensen, J.B. DaBlaCa-13 Study: Oncological Outcome of Short-Term, Intensive Chemoresection With Mitomycin in Nonmuscle Invasive Bladder Cancer: Primary Outcome of a Randomized Controlled Trial. J. Clin. Oncol. 2023, 41, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Liem, E.I.; Freund, J.E.; Savci-Heijink, C.D.; de la Rosette, J.J.; Kamphuis, G.M.; Baard, J.; Liao, J.C.; van Leeuwen, T.G.; de Reijke, T.M.; de Bruin, D.M. Validation of Confocal Laser Endomicroscopy Features of Bladder Cancer: The Next Step Towards Real-time Histologic Grading. Eur. Urol. Focus. 2020, 6, 81–87. [Google Scholar] [CrossRef]
- Lee, J.; Jeh, S.U.; Koh, D.H.; Chung, D.Y.; Kim, M.S.; Goh, H.J.; Lee, J.Y.; Choi, Y.D. Probe-Based Confocal Laser Endomicroscopy During Transurethral Resection of Bladder Tumors Improves the Diagnostic Accuracy and Therapeutic Efficacy. Ann. Surg. Oncol. 2019, 26, 1158–1165. [Google Scholar] [CrossRef]
- Beji, S.; Wrist Lam, G.; Østergren, P.B.; Toxvaerd, A.; Sønksen, J.; Fode, M. Diagnostic value of probe-based confocal laser endomicroscopy versus conventional endoscopic biopsies of non-muscle invasive bladder tumors: A pilot study. Scand. J. Urol. 2021, 55, 36–40. [Google Scholar] [CrossRef]
- Ohigashi, T.; Kozakai, N.; Mizuno, R.; Miyajima, A.; Murai, M. Endocytoscopy: Novel endoscopic imaging technology for in-situ observation of bladder cancer cells. J. Endourol. 2006, 20, 698–701. [Google Scholar] [CrossRef]
- Sung, H.H.; Scherr, D.S.; Slaton, J.; Liu, H.; Feeny, K.L.; Lingley-Papadopoulos, C.; Gearheart, J.; Zara, J.M.; Lerner, S.P. Phase II multi-center trial of optical coherence tomography as an adjunct to white light cystoscopy for intravesical real time imaging and staging of bladder cancer. Urol. Oncol. 2021, 39, 434.e23–434.e29. [Google Scholar] [CrossRef] [PubMed]
- Karl, A.; Stepp, H.; Willmann, E.; Buchner, A.; Hocaoglu, Y.; Stief, C.; Tritschler, S. Optical coherence tomography for bladder cancer—ready as a surrogate for optical biopsy? Results of a prospective mono-centre study. Eur. J. Med. Res. 2010, 15, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, E.; Kirillin, M.; Feldchtein, F.; Vitkin, A.; Sergeeva, E.; Zagaynova, E.; Streltzova, O.; Shakhov, B.; Gubarkova, E.; Gladkova, N. Differential diagnosis of human bladder mucosa pathologies in vivo with cross-polarization optical coherence tomography. Biomed. Opt. Express 2015, 6, 1464–1476. [Google Scholar] [CrossRef] [PubMed]
- Zha, W.L.; Cheng, Y.; Yu, W.; Zhang, X.B.; Shen, A.G.; Hu, J.M. HPLC assisted Raman spectroscopic studies on bladder cancer. Laser Phys. Lett. 2015, 12, 045701. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Broderick, N.G.R.; Xu, W. Low-resolution fiber-optic Raman spectroscopy for bladder cancer diagnosis: A comparison study of varying laser power, integration time, and classification methods. J. Raman Spectrosc. 2020, 51, 323–334. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Broderick, N.; Liu, Y.; Zhou, Y.; Han, J.; Xu, W. Identification and characterization of bladder cancer by low-resolution fiber-optic Raman spectroscopy. J. Biophotonics 2018, 11, e201800016. [Google Scholar] [CrossRef]
- Fujimoto, J.; Swanson, E. The Development, Commercialization, and Impact of Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT1–OCT13. [Google Scholar] [CrossRef]
- de Boer, J.F.; Leitgeb, R.; Wojtkowski, M. Twenty-five years of optical coherence tomography: The paradigm shift in sensitivity and speed provided by Fourier domain OCT [Invited]. Biomed. Opt. Express 2017, 8, 3248–3280. [Google Scholar] [CrossRef] [PubMed]
- Muller, B.G.; van Kollenburg, R.A.A.; Swaan, A.; Zwartkruis, E.C.H.; Brandt, M.J.; Wilk, L.S.; Almasian, M.; Schreurs, A.W.; Faber, D.J.; Rozendaal, L.R.; et al. Needle-based optical coherence tomography for the detection of prostate cancer: A visual and quantitative analysis in 20 patients. J. Biomed. Opt. 2018, 23, 086001. [Google Scholar] [CrossRef]
- Li, J.; de Groot, M.; Helderman, F.; Mo, J.; Daniels, J.M.A.; Grünberg, K.; Sutedja, T.G.; de Boer, J.F. High speed miniature motorized endoscopic probe for optical frequency domain imaging. Opt. Express 2012, 20, 24132–24138. [Google Scholar] [CrossRef]
- Chen, Y.; Burnes, D.L.; de Bruin, M.; Mujat, M.; de Boer, J.F. Three-dimensional pointwise comparison of human retinal optical property at 845 and 1060 nm using optical frequency domain imaging. J. Biomed. Opt. 2009, 14, 024016. [Google Scholar] [CrossRef]
- de Bruin, D.M.; Burnes, D.L.; Loewenstein, J.; Chen, Y.; Chang, S.; Chen, T.C.; Esmaili, D.D.; de Boer, J.F. In Vivo three-dimensional imaging of neovascular age-related macular degeneration using optical frequency domain imaging at 1050 nm. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4545–4552. [Google Scholar] [CrossRef]
- Attendu, X.; Ruis, R.M.; Boudoux, C.; van Leeuwen, T.G.; Faber, D.J. Simple and robust calibration procedure for k-linearization and dispersion compensation in optical coherence tomography. J. Biomed. Opt. 2019, 24, 056001. [Google Scholar] [CrossRef] [PubMed]
- HSL-1 Flexible Scan Rate & Range Model at 1060nm (VCSEL). Available online: https://ois.santec.com/products/lasers-for-swept-source-oct/flexible-scan-rate-model#block-167670 (accessed on 22 November 2024).
- Yang, X.; Chin, L.; Klyen, B.R.; Shavlakadze, T.; McLaughlin, R.A.; Grounds, M.D.; Sampson, D.D. Quantitative assessment of muscle damage in the mdx mouse model of Duchenne muscular dystrophy using polarization-sensitive optical coherence tomography. J. Appl. Physiol. 2013, 115, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Colt, H.; Murgu, S.D.; Ahn, Y.C.; Brenner, M. Multimodality bronchoscopic [corrected] imaging of tracheopathica osteochondroplastica. J. Biomed. Opt. 2009, 14, 034035, Erratum in J. Biomed. Opt. 2009, 14, 049801. [Google Scholar] [CrossRef]
- Ren, H.; Waltzer, W.C.; Bhalla, R.; Liu, J.; Yuan, Z.; Lee, C.S.D.; Darras, F.; Schulsinger, D.; Adler, H.L.; Kim, J.; et al. Diagnosis of bladder cancer with microelectromechanical systems-based cystoscopic optical coherence tomography. Urology 2009, 74, 1351–1357. [Google Scholar] [CrossRef]
- Manyak, M.J.; Gladkova, N.D.; Makari, J.H.; Schwartz, A.M.; Zagaynova, E.V.; Zolfaghari, L.; Zara, J.M.; Iksanov, R.; Feldchtein, F.I. Evaluation of superficial bladder transitional-cell carcinoma by optical coherence tomography. J. Endourol. 2005, 19, 570–574. [Google Scholar] [CrossRef]
- Goh, A.C.; Tresser, N.J.; Shen, S.S.; Lerner, S.P. Optical coherence tomography as an adjunct to white light cystoscopy for intravesical real-time imaging and staging of bladder cancer. Urology 2008, 72, 133–137, Erratum in Urology 2009, 73, 217. [Google Scholar] [CrossRef]
- Schmidbauer, J.; Remzi, M.; Klatte, T.; Waldert, M.; Mauermann, J.; Susani, M.; Marberger, M. Fluorescence cystoscopy with high-resolution optical coherence tomography imaging as an adjunct reduces false-positive findings in the diagnosis of urothelial carcinoma of the bladder. Eur. Urol. 2009, 56, 914–919. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | Simulated Value | Experimental Value |
|---|---|---|
| Beam path angle | 30 degrees | Varying * |
| Lateral scan range | 5 mm | N/A |
| Focus point from MEMS mirror (water) | 10.3 mm | N/A ** |
| Focus point from MEMS mirror (air) | 7.7 mm | 10.9 mm |
| Rayleigh length (water) | 2.64 mm | N/A |
| Rayleigh length (air) | 2.0 mm | 2.55 mm |
| Distance to zero delay | N/A | 7.5 mm |
| Axial scan range | N/A | 8.22 mm |
| Axial resolution after dispersion compensation (in air) | N/A | 11 µm |
| Lateral resolution in focus (ω0) | N/A | 28.6 µm |
| Output power at catheter tip | N/A | 6 mW |
| Sweep frequency | N/A | 200 kHz |
| Coherence length | N/A | >100 m [26] |
| Frames per second rate (after averaging and interlacing) | N/A | 45–55 Hz |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remmelink, M.J.; Bloemen, P.R.; van der Voorn, P.; Attendu, X.; van den Elzen, R.M.; Nieuwenhuijzen, J.A.; Oddens, J.R.; van Leeuwen, T.G.; de Bruin, D.M. Ex Vivo Optical Coherence Tomography Analysis of Resected Human Bladder with a Forward-Looking Microelectromechanical Systems Mirror-Based Catheter. Sensors 2025, 25, 5794. https://doi.org/10.3390/s25185794
Remmelink MJ, Bloemen PR, van der Voorn P, Attendu X, van den Elzen RM, Nieuwenhuijzen JA, Oddens JR, van Leeuwen TG, de Bruin DM. Ex Vivo Optical Coherence Tomography Analysis of Resected Human Bladder with a Forward-Looking Microelectromechanical Systems Mirror-Based Catheter. Sensors. 2025; 25(18):5794. https://doi.org/10.3390/s25185794
Chicago/Turabian StyleRemmelink, Marinka J., Paul R. Bloemen, Patrick van der Voorn, Xavier Attendu, Richard M. van den Elzen, Jakko A. Nieuwenhuijzen, Jorg R. Oddens, Ton G. van Leeuwen, and Daniel M. de Bruin. 2025. "Ex Vivo Optical Coherence Tomography Analysis of Resected Human Bladder with a Forward-Looking Microelectromechanical Systems Mirror-Based Catheter" Sensors 25, no. 18: 5794. https://doi.org/10.3390/s25185794
APA StyleRemmelink, M. J., Bloemen, P. R., van der Voorn, P., Attendu, X., van den Elzen, R. M., Nieuwenhuijzen, J. A., Oddens, J. R., van Leeuwen, T. G., & de Bruin, D. M. (2025). Ex Vivo Optical Coherence Tomography Analysis of Resected Human Bladder with a Forward-Looking Microelectromechanical Systems Mirror-Based Catheter. Sensors, 25(18), 5794. https://doi.org/10.3390/s25185794





